Abstract
Refractory wound is a severe complication that leads to limb amputation in diabetes. Endothelial nitric oxide synthase (eNOS) plays a key role in normal wound repair but is uncoupled in streptozotocin (STZ)-induced type 1 diabetes because of reduced cofactor tetrahydrobiopterin (BH4). We tested the hypothesis that overexpression of GTP cyclohydrolase I (GTPCH I), the rate-limiting enzyme for de novo BH4 synthesis, retards NOS uncoupling and accelerates wound healing in STZ mice. Blood glucose levels were significantly increased in both male endothelium-specific GTPCH I transgenic mice (Tg-GCH; via a tie-2 promoter) and wild-type (WT) littermates 5 days after STZ regimen. A full-thickness excisional wound was created on mouse dorsal skin by a 4-mm punch biopsy. Wound closure was delayed in STZ mice, which was rescued in STZ Tg-GCH mice. Cutaneous BH4 level was significantly reduced in STZ mice vs. WT mice, which was maintained in STZ Tg-GCH mice. In STZ mice, constitutive NOS (cNOS) activity and nitrite levels were decreased compared with WT mice, paralleled by increased superoxide anion (O2−) level and inducible NOS (iNOS) activity. In STZ Tg-GCH mice, nitrite level and cNOS activity were potentiated and O2− level and iNOS activity were suppressed compared with STZ mice. Thus endothelium-specific BH4 overexpression accelerates wound healing in type 1 diabetic mice by enhancing cNOS activity and suppressing oxidative stress.
Keywords: tetrahydrobiopterin
impaired wound healing in diabetic patients is a serious complication that often leads to amputation with limited treatment regimens (34). Various factors contribute to delayed diabetic wound healing, including inflammatory response, decreased quantity of granulation tissue, peripheral neuropathy, and reduced wound angiogenesis (4, 11). Nitric oxide (NO) is a critical signaling molecule of angiogenesis and serves as a key autocrine and paracrine mediator in skin homeostasis (39). A decrease in cutaneous endothelial nitric oxide synthase (eNOS) contributes to impaired diabetic wound healing (17, 32, 36). Conversely, enhancing wound NO bioavailability by supplementation of its precursor l-arginine or topical application of NO donors accelerates diabetic wound healing (40–42). Our previous study (20) demonstrated that cutaneous gene therapy of eNOS restores delayed diabetic wound healing with a concomitant augmentation of eNOS protein and activity, as well as suppression of wound superoxide anion (O2−) level.
Tetrahydrobiopterin (BH4) is an essential cofactor of eNOS (8), maintaining its stability by binding to the heme moiety of the enzyme as well as participating in the oxidation of l-arginine to l-citrulline and NO formation (38). A reduced level of BH4 results in eNOS uncoupling and the consequent production of O2− instead of NO (6). In diabetes BH4 is decreased, and sepiapterin supplementation elevates BH4 and restores NO production (22, 33). eNOS uncoupling was found in both heart and aortic endothelial cells of streptozotocin (STZ)-induced diabetic mice, with concomitant heart and endothelial dysfunction (25, 30). The intracellular BH4 level is controlled by its de novo synthesis pathway from guanosine triphosphate (GTP), and GTP cyclohydrolase I (GTPCH I) is the first and rate-limiting enzyme (37). In vitro overexpression of GTPCH I by gene transfer could reverse diabetes-induced BH4 deficiency and restore the ability of endothelial cells to produce NO (21). In vivo studies have demonstrated that an increase in BH4 level is able to inhibit vascular oxidative stress and ameliorate eNOS function in diabetes in a transgenic mouse model with endothelium-targeted GTPCH I overexpression (Tg-GCH) (2, 5). However, little information is available regarding how endogenous BH4 influences wound healing in diabetes when eNOS becomes uncoupled.
In the present study, we used Tg-GCH mice to test the hypothesis that BH4 can rescue the delayed wound healing in type 1 diabetes by suppressing oxidative stress. Our results demonstrate that endothelium-specific overexpression of GTPCH I accelerates wound healing in STZ-induced type 1 diabetic mice through enhanced constitutive NOS (cNOS) activity and suppressed oxidative stress.
MATERIALS AND METHODS
Induction of type 1 diabetes.
Wild-type (WT) C57BL/6 male mice were obtained from Charles River Breeding Laboratories (Portage, MI). Tg-GCH mice of C57BL/6 background were bred in house. WT and Tg-GCH mice at 10–12 wk of age (20–25 g) were rendered diabetic by intraperitoneal injection of 60 mg/kg STZ (Sigma-Aldrich) in 50 mM sodium citrate (pH 4.5) daily for 5 days (20, 27). Control mice were treated with daily injections of citrate buffer. Blood glucose was measured from the mouse tail vein with a OneTouch blood glucose meter (Lifescan). Once blood glucose level reached >250 mg/dl, daily measurements followed for 1 wk before experiments. All animal procedures were approved by the Institutional Animal Care and Use Committee.
Full-thickness excisional wound.
One week after blood glucose reached 250 mg/dl (13), STZ mice and citrate-treated control mice were anesthetized with a halothane-oxygen vapor mixture (1.0–1.5%), and the dorsum was clipped free of hair. A full-thickness excisional wound was created on the dorsomedial back of each animal with a 4-mm punch biopsy (Acuderm). Full-thickness skin was removed, exposing the underlying muscle, and then the wound was covered with a bioclusive transparent dressing (Johnson & Johnson). Wound closure rate was measured by tracing the wound area every other day onto the bioclusive dressing. The tracings were digitized, and the areas were calculated in blinded fashion with the use of a computerized algorithm (Sigma Scan; Jandel Scientific), as we described previously (20).
Superoxide measurement.
Cutaneous O2− level was quantified by lucigenin-enhanced chemiluminescence on wound closure as we described previously (18, 20). Wound tissue clipped free of hair was cut into 2 × 2-mm pieces and placed in polypropylene tubes containing 5 μM lucigenin in 1 ml of modified Krebs solution. Tubes were read in a luminometer (TD-20/20, Turner Designs) at 10 min after initiation of the reaction. O2− level was expressed as nanomoles per minute per milligram of tissue. In addition, the O2−-sensitive fluorescent dye dihydroethidium (DHE) was used to evaluate in situ production of O2− on wound closure as we described previously (18, 20). Unfixed frozen skin tissues were cut into 30-μm sections and placed on glass slides. Slides were incubated with 1 μM DHE in a light-protected, humidified chamber at 37°C for 30 min and then coverslipped. Tissue sections were then visualized with a Zeiss 210 confocal microscope, and fluorescence was detected with a 590-nm long-pass filter. Images were collected and stored digitally.
BH4 measurement.
Cutaneous BH4 level was measured by high-performance liquid chromatography (HPLC) with fluorescence detection after iodine oxidation in acidic or alkaline conditions as we previously described (43). Briefly, tissues were homogenized in cold extract buffer [in mM: 50 Tris·HCl pH 7.4, 1 dithiothreitol (DTT), 1 ethylenediaminetetraacetic acid (EDTA)] and centrifuged at 16,000 g for 15 min at 4°C. Protein was removed by adding 10 μl of a 1:1 mixture of 1.5 M HClO4 and 2 M H3PO4 to 90 μl of extracts, followed by centrifugation. To determine total biopterin [BH4, dihydropterin (BH2), and oxidized biopterin] by acid oxidation, 10 μl of 1% iodine in 2% KI solution was added to the 90 μl of protein-free supernatant. To determine BH2 and oxidized biopterin by alkali oxidation, 10 μl of 1 M NaOH was added to 80 μl of extract and then 10 μl of 1% iodine in 2% KI solution was added. Samples were incubated at room temperature for 1 h in the dark. Alkaline oxidation samples were then acidified with 20 μl of 1 M H3PO4. Iodine was reduced by adding 5 μl of fresh ascorbic acid (20 mg/ml). Samples of 50 μl were injected into a 250-mm-long, 4.6-mm-inner diameter Spherisorb ODS-1 column (5-μm particle size; Alltech Associates) isocratically eluted with a methanol-water (5:95, vol/vol) mobile phase running at a flow rate of 1.0 ml/min. Fluorescence detection (350 nm excitation, 450 nm emission) was performed with a fluorescence detector (RF10AXL, Shimadzu). BH4 concentrations, expressed as picomoles per milligram of protein, were calculated by subtracting BH2 plus oxidized biopterin from total biopterins.
Radioimmunoassay of NOS activity.
Skin tissues were homogenized and centrifuged, and supernatants were subjected to NOS activity assay with a commercial NOS radioimmunoassay (RIA) kit (Calbiochem) as we described previously (20). Briefly, aliquots of supernatants were incubated with [3H]arginine (10 μM final arginine, 62 Ci/mM; Amersham) in the presence of 1 mmol/l NADPH, 3 μmol/l BH4, 600 μmol/l CaCl2, 1 μmol/l flavin adenine dinucleotide, and 1 μmol/l flavin mononucleotide in a final volume of 50 μl for 60 min at room temperature. The reaction was quenched with 400 μl of stop buffer. Experiments were also performed in the presence of either EGTA (5.0 mM) or NG-nitro-l-arginine (l-NNA; 1 mM). After resin was added, the reaction mixtures were transferred to spin cups and centrifuged. l-[3H]citrulline content in eluate was determined by liquid scintillation counting. Samples of buffer containing l-[3H]arginine in the absence of skin tissue served as background counts, which were subtracted from all measurements. l-NNA was used to block all NOS activity. EGTA was added to the reaction mixture to eliminate calcium and estimate inducible nitric oxide synthase (iNOS) activity. cNOS [eNOS and neuronal NOS (nNOS)] activity was then determined by taking the difference between total NOS activity and iNOS activity, as we previously described (20). The results were normalized to protein content as measured by the bicinchoninic acid (BCA) assay (Pierce). Enzyme activities were expressed as picomoles of l-[3H]citrulline produced per minute per milligram of total protein.
Nitrite measurement.
Wound tissue was cut into small pieces on wound closure and used to determine nitrite level with Griess reagents (Sigma Chemical) as we described previously (20). After incubation with Eagle's minimal essential medium (EMEM) in a CO2 incubator for 24 h, wound tissue was weighed and the NO stable metabolite nitrite concentration in EMEM was determined.
Statistical analysis.
Data are expressed as means ± SE. The significance of differences between groups was evaluated by unpaired Student's t-test. When more than two treatment groups were compared, one-way ANOVA with Newman-Keuls test was used. A probability level of P < 0.05 was considered statistically significant.
RESULTS
Induction of diabetes in mice.
As shown in Table 1, STZ administration induced diabetes in mice as evidenced by hyperglycemia [blood glucose levels: 392 ± 10 vs. 168 ± 4 mg/dl in WT mice (P < 0.05) and 369 ± 33 vs. 166 ± 4 mg/dl in Tg-GCH mice (P < 0.05)].
Table 1.
General characteristics of Tg-GCH mice and WT littermates 1 wk after induction of diabetes with STZ (diabetic) or after buffer injection (control)
| WT |
Tg-GCH | |||
|---|---|---|---|---|
| Control (n = 6–10) | Diabetic (n = 8–10) | Control (n = 4–6) | Diabetic (n = 6–8) | |
| Body weight, g | ||||
| 0 wk | 24.78±0.96 | 23.49±1.32 | 20.74±1.14 | 21.50±1.29 |
| 1 wk after injection | 24.80±0.50 | 23.50±3.00 | 22.60±1.80 | 22.70±0.50 |
| Blood glucose levels, mg/dl | ||||
| 0 wk | 173.50±3.92 | 167.50±3.61 | 149.43±3.46 | 165.80±4.56 |
| 1 wk after injection | 159.00±5.04 | 392.00±9.51* | 156.00±5.75 | 369.00±33.27* |
Values are means ± SE for n mice. Diabetic status was induced by daily injection of streptozotocin (STZ) for 5 days (60 mg/kg ip); control mice were treated with daily injections of citrate buffer. Tg-GCH, GTP cyclohydrolase I-overexpressing transgenic mice; WT, wild-type mice.
P < 0.001, compared with untreated mice.
Effects of GTPCH I overexpression on wound healing in diabetic mice.
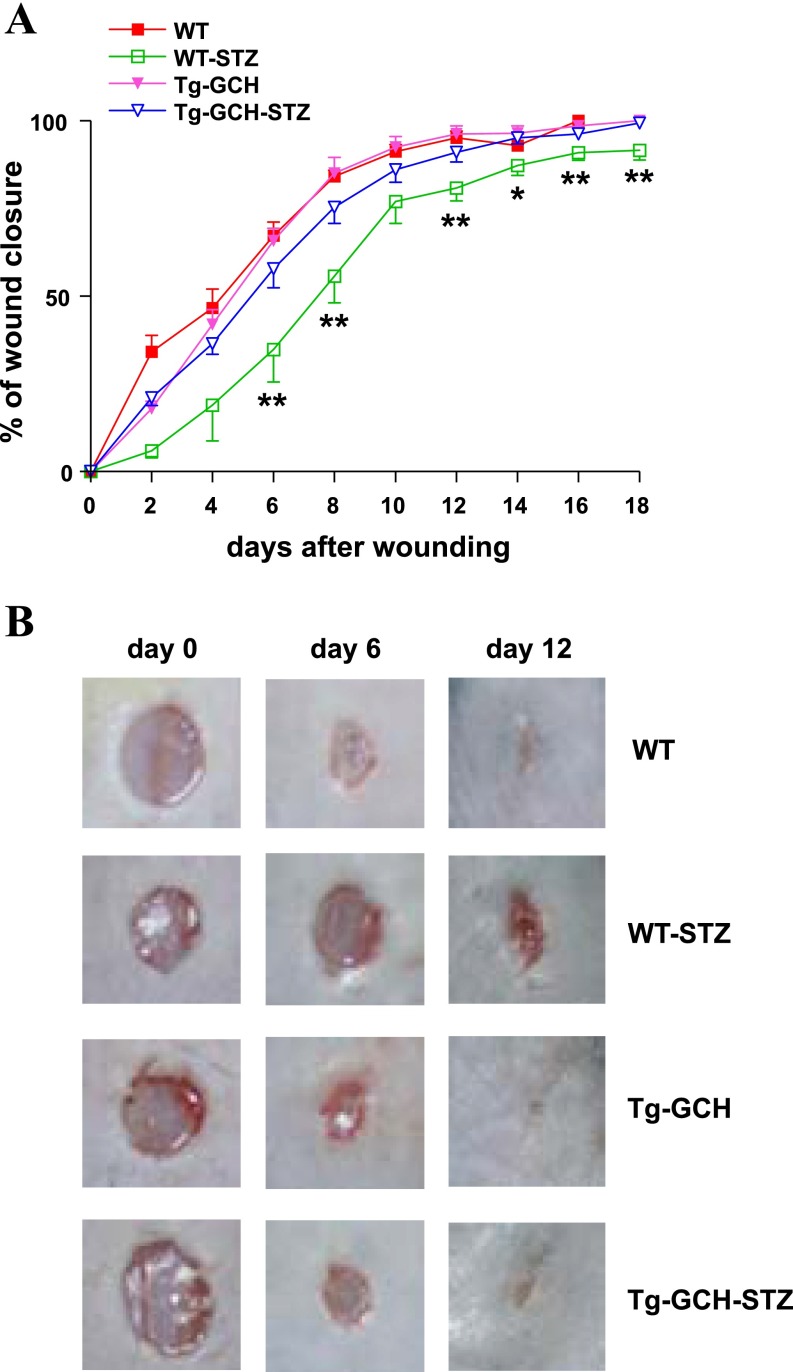
As shown in Fig. 1A, compared with control mice the rate of wound closure was significantly delayed in STZ mice (67.23 ± 3.84% vs. 34.82 ± 9.27% on day 6; P < 0.01). The percentage of wound closure in STZ Tg-GCH mice was significantly higher than that in WT STZ mice beginning on day 6 (57.75 ± 5.35% vs. 34.82 ± 9.27%; P < 0.05) and continuing through day 18 (Fig. 1B). There was no difference in wound healing between WT and Tg-GCH mice (67.23 ± 3.84% vs. 65.80 ± 3.59% on day 6; P > 0.05).
Fig. 1.
A: wound closure rate in wild-type (WT) and streptozotocin (STZ)-induced type 1 diabetic mice with or without GTP cyclohydrolase (GTPCH) I transgene expression (Tg-GCH). Data are expressed as means ± SE; n = 4–6/group. *P < 0.05, **P < 0.01 vs. other groups. B: representative wounds on days 0, 6, and 12 after injury are shown for each group.
Overexpression of GTPCH I decreases cutaneous O2− level in diabetic mice.
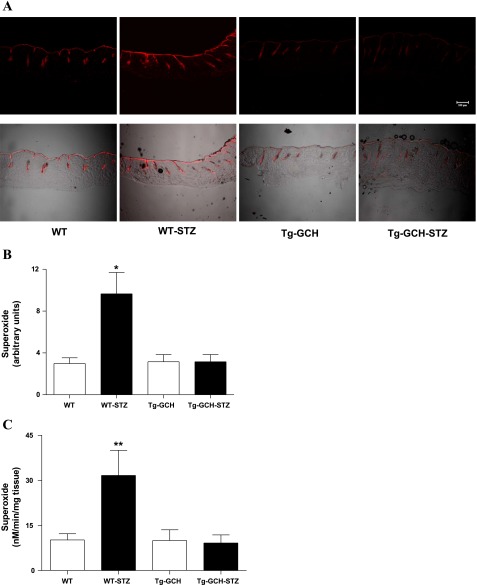
To determine the effects of endothelium-specific GTPCH I overexpression on cutaneous oxidative stress in diabetes, we estimated cutaneous O2− levels, using two complementary methods. In situ detection of O2− by lucigenin-enhanced chemiluminescence showed that cutaneous ethidium fluorescence in WT STZ mice was increased compared with control mice (P < 0.01) (Fig. 2C). The cutaneous O2− level in STZ-induced Tg-GCH diabetic mice was significantly attenuated compared with that of WT STZ mice (Fig. 2C). Consistently, DHE staining revealed that the increased cutaneous O2− level in diabetic mice was diminished by GTPCH I overexpression in Tg-GCH mice (Fig. 2, A and B).
Fig. 2.
Effects of GTPCH I on cutaneous O2− level. A and B: in situ detection of cutaneous O2− level with fluorescent confocal microscopy. Red fluorescence occurs when dihydroethidium is oxidized to ethidium bromide by O2−, and red signal intensity reflects O2− level. Representative sections of skin tissue (30 μm) are shown. A, top: fluorescence image. A, bottom: fluorescence image superimposed with transmittal image. B: statistical data of fluorescence measurement. Data are expressed as means ± SE; n = 4/group. *P < 0.05 vs. other groups. C: cutaneous O2− was measured by lucigenin-enhanced chemiluminescence. Data are expressed as means ± SE; n = 6/group. **P < 0.01 vs. other groups.
Effects of GTPCH I overexpression on cutaneous BH4 level.
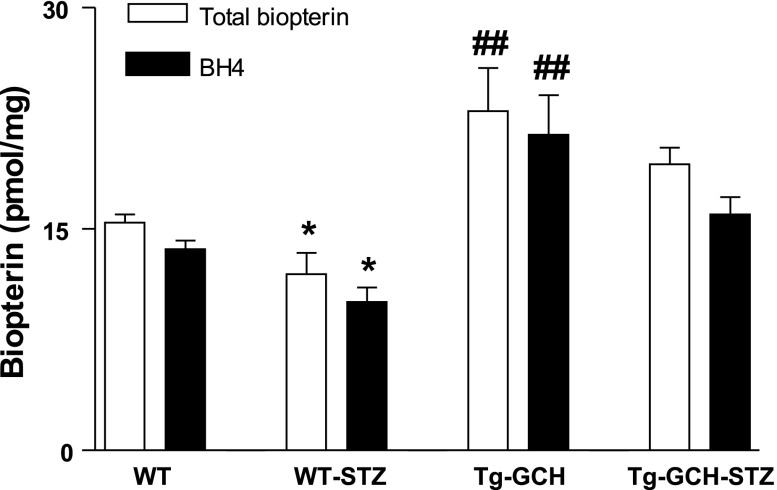
We next examined the impact of GTPCH I on cutaneous BH4 level with HPLC (Fig. 3). Cutaneous BH4 level was significantly reduced in WT STZ mice compared with nondiabetic control mice. Endothelium-targeted GTPCH I overexpression resulted in a significant increase of cutaneous BH4 level in Tg-GCH mice, approximately threefold higher compared with WT mice. BH4 level in STZ Tg-GCH mice was significantly elevated compared with that of WT STZ mice (P < 0.01) (Fig. 3).
Fig. 3.
Cutaneous total biopterin and tetrahydrobiopterin (BH4) level in WT and STZ-induced diabetic mice with or without GTPCH I transgene expression. Data are expressed as means ± SE; n = 4–6/group. *P < 0.05 vs. other groups; ##P < 0.01 vs. WT mice.
Effects of GTPCH I overexpression on NOS activity in diabetic mice.
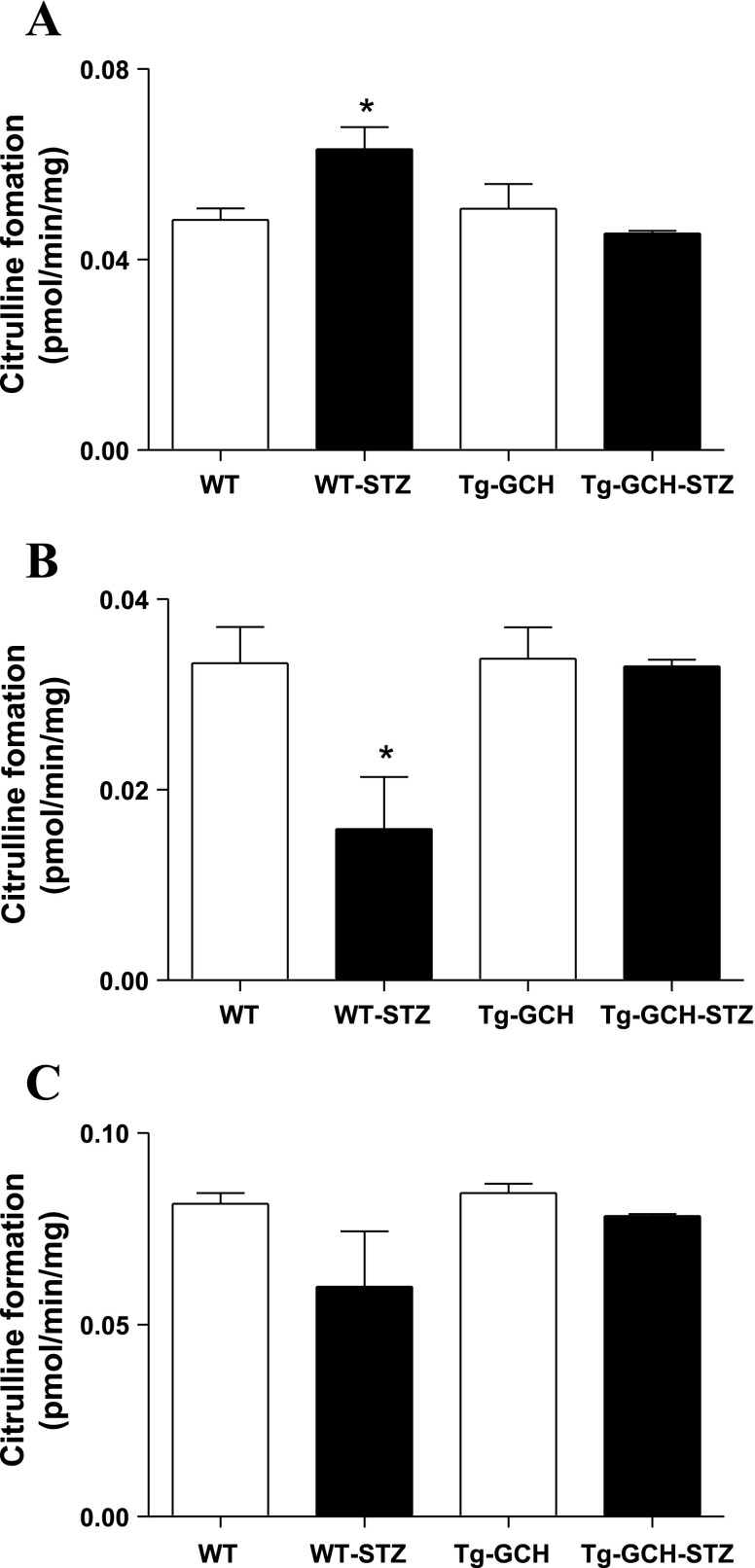
To further evaluate the relationship between BH4 level and NOS activity, we examined whether cNOS and iNOS activity were altered by STZ-induced diabetes and GTPCH I overexpression. In WT mice, cutaneous cNOS activity was decreased after the STZ regimen. Endothelium-targeted GTPCH I overexpression reversed STZ-evoked reduction of cNOS activity (Fig. 4B; n = 4–6, P < 0.05). In contrast, STZ treatment resulted in a significant increase of iNOS activity in WT mice, which was retarded by GTPCH I overexpression in STZ Tg-GCH mice (Fig. 4A; n = 4–6, P < 0.05). In addition, there was no significant difference in cNOS and iNOS activities between WT and Tg-GCH mice (Fig. 4, A–C; n = 4–6, P > 0.05).
Fig. 4.
Cutaneous inducible nitric oxide synthase (iNOS; A), constitutive NOS [cNOS; B: calcium-dependent endothelial NOS (eNOS) and neuronal NOS (nNOS)], and total NOS (cNOS and iNOS) enzymatic activities as determined by measuring l-[3H]arginine-to-l-[3H]citrulline conversion. Data are expressed as means ± SE; n = 4–6/group. *P < 0.05 vs. other groups.
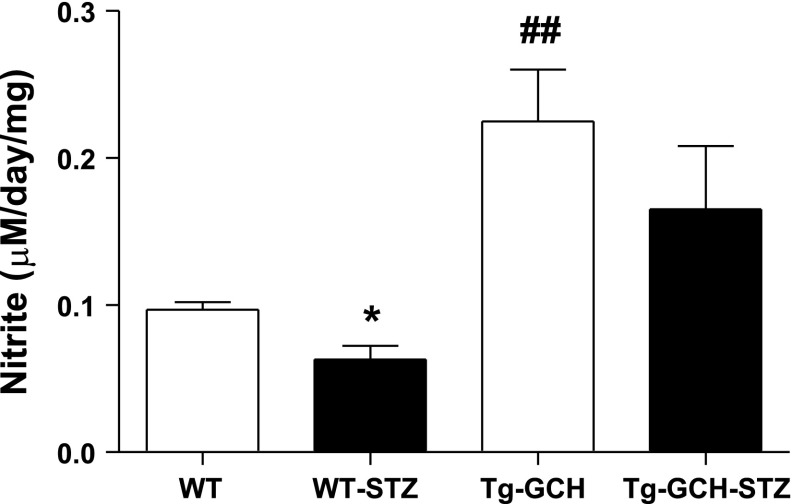
Effects of GTPCH I overexpression on NO level in diabetic mice.
We next estimated cutaneous NO level by measuring its stable metabolite nitrite. The cutaneous nitrite level was significantly reduced in STZ mice compared with nondiabetic control mice. Endothelium-targeted GTPCH I overexpression resulted in a significant increase of cutaneous nitrite level in Tg-GCH mice compared with WT mice (P < 0.01). Nitrite level in STZ Tg-GCH mice was significantly enhanced compared with that in WT STZ mice (P < 0.01) (Fig. 5).
Fig. 5.
Cutaneous nitrite level measured by Griess reaction on wound closure. Data are expressed as means ± SE; n = 4–6/group. *P < 0.05 vs. other groups, ##P < 0.01 vs. WT mice.
DISCUSSION
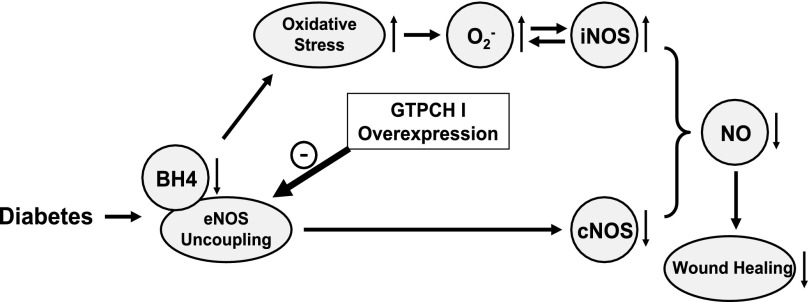
The present study provides first evidence that in vivo endothelium-targeted overexpression of GTPCH I in STZ-induced type 1 diabetic mice 1) ameliorated the wound healing delay, 2) significantly increased cutaneous BH4 and decreased O2− levels, and 3) restored the decreased cutaneous cNOS activity (Fig. 6).
Fig. 6.
Schematic illustration of possible mechanisms in GTPCH I regulation of oxidative stress and wound healing in type 1 diabetes. Endothelium-targeted overexpression of GTPCH I ameliorated the wound healing delay by increasing cutaneous BH4 level and decreasing O2− level. In addition, endothelium-targeted GTPCH I overexpression restored cNOS and iNOS activities to normal with elevated NO level in cutaneous tissue of diabetic mice.
The intracellular BH4 level depends on a balance of its de novo synthesis and degradation. BH4 de novo synthesis uses GTP as the precursor, and GTPCH I is the first and rate-limiting enzyme (37). The importance of GTPCH I in BH4 level and eNOS activity has been explored both in vivo and in vitro. Our previous study (43) showed that in vitro gene transfer of human GTPCH I could restore arterial GTPCH I activity and BH4 level, with subsequent reduction of O2− level, augmentation of basal NO level, and improvement of endothelium-dependent relaxation. Establishment of an in vivo model with endothelium-specific GTPCH I overexpression provides an efficient approach to investigate the role of endogenous BH4 in endothelium (2). In Tg-GCH mice, BH4 level was found to be two- to fourfold higher in organs with a high proportion of endothelial cells such as lungs and aorta but not increased in plasma or tissues such as liver (2). In the present study, our data showed that BH4 level was increased ∼1.5-fold in the skin of Tg-GCH mice.
NO serves as a key mediator in skin homeostasis (19). It promotes processes central to wound healing including angiogenesis (9), migration and proliferation of fibroblasts, epithelial cells, keratinocytes, endothelial cells, and the mobilization of endothelial progenitor cells (13). It is well established that all three NOS isoforms are expressed in skin. Expression of nNOS has been observed in keratinocytes and melanocytes; eNOS can be detected in keratinocytes of the basal epidermal layer, dermal fibroblasts, endothelial capillaries, and eccrine glands; and iNOS can be induced in keratinocytes, fibroblasts, Langerhans, and endothelial cells (12, 19). Recent studies including ours have demonstrated that cutaneous eNOS expression, cNOS activity, and NO level are significantly decreased in STZ-induced type 1 diabetes (20, 31, 36). In vivo cutaneous gene therapy of eNOS on the wounds of diabetic mice was able to restore eNOS protein and NO level and accelerate wound healing in STZ-induced diabetic mice (20). In the present study, we found that there were decreased cutaneous cNOS activity and nitrite levels in STZ-induced diabetic mice; however, iNOS activity was found to be increased in STZ diabetic mice, and this was reversed by endothelium-specific GTPCH I overexpression. In support of this possibility, increased iNOS activity has been reported in both chronic venous ulcers and diabetic foot ulcers (1, 16). Furthermore, studies have shown that endothelial cNOS and iNOS are regulated differently in STZ-induced diabetes, in that prolonged hyperglycemia led to a downregulation of eNOS but at the same time resulted in upregulation of iNOS in the heart, aorta, and kidneys, as well as the sciatic nerve (3). Vascular endothelium was shown to be the main site of altered eNOS as well as iNOS expression in these tissues (3). Consistently, increased iNOS expression and decreased eNOS expression exist in cardiovascular tissues, in parallel with endothelial dysfunction in STZ-induced diabetes (24). Although the underlying mechanisms are not completely understood, hyperglycemia-induced PKC activation appears to be responsible for the augmented iNOS expression.
Diabetes manifests in a significant decrease of BH4 in vascular tissue or cultured endothelial cells (5, 33). In the absence of a sufficient BH4 level, eNOS becomes uncoupled from arginine oxidation and produces O2− rather than NO. O2− inactivates NO, forming peroxynitrite, which in turn can oxidize BH4 within minutes at a physiologically relevant concentration. Importantly, overproduction of peroxynitrite could increase iNOS through NF-κB activation in endothelial cells (7). In addition, with the use of the O2− spin trap DEPMPO and the NO spin trap Fe-MGD complex, it has been proven that BH4 cofactor deficient iNOS is not a NO-producing enzyme but a O2−-producing enzyme (15). These studies are in agreement with our present findings, and together they suggest that in diabetes a depleted BH4 level contributes to decreased cNOS activity but increased O2− production that may lead to potentiated iNOS activity (28). Therefore, differential regulation of cNOS and iNOS activities may result in a lower cutaneous NO level in STZ mice. In diabetic Tg-GCH mice, the cutaneous O2− level was markedly reduced compared with WT mice, but the cutaneous BH4 level was maintained to restore cNOS activity and suppress oxidative stress, resulting in improved wound healing. Studies have shown that diabetes evoked impairment of endothelium-dependent relaxation in arteries, which was alleviated in iNOS-deficient mice (14).
Our present data showed that the increase of endothelial BH4 level had no effects on basal iNOS and eNOS activities in Tg-GCH mice, and there was no difference in wound healing between WT and Tg mice. In contrast, GTPCH I overexpression evoked a significant increase in nitrite level in Tg-GCH mice compared with WT mice. One possibility is that under normal conditions the cutaneous NO level is sufficient with enough BH4, and thus a further increase in BH4 level does not make a significant difference in NO production. In contrast, as a potent reducing molecule, BH4 increased by GTPCH I overexpression could readily reduce oxidative stress in diabetes, resulting in abrogated oxidation of NO as shown in STZ-treated Tg-GCH mice. Despite the high concentration of BH4 in the reaction mixture of the NOS activity assay, cNOS activity was found to be significantly reduced in the skin of STZ mice, which is consistent with our previous findings (20). Previous studies have shown that maximal NOS activity depends not only on BH4 availability but also on the relative concentration of BH2, because BH2 can depress NOS activity (29). Thus GTPCH I overexpression may increase cNOS activity by maintaining an optimal BH4-to-BH2 ratio in the reaction mixture.
Oxidative stress has been proposed as an important pathogenic factor in diabetic wound complications (35). Accordingly, antioxidants have been reported to accelerate diabetic wound healing. Vitamin E supplementation enhances the wound healing process in diabetic rats (23). Curcumin, which is known to exert antioxidant effects, also improves wound healing by decreasing reactive oxygen species and modulating collagen formation (26). Unlike exogenous antioxidants, BH4 may ameliorate delayed wound healing in diabetes in two processes; besides alleviating cutaneous oxidative stress, it could restore normal cNOS and iNOS activities. Consistent with this notion, endothelium-specific overexpression of GTPCH I rescues endothelial dysfunction in STZ-induced type 1 diabetes and DOCA-salt hypertension; both exhibit typical eNOS uncoupling (2, 10). Our findings may provide a mechanistic basis for augmentation of endogenous BH4 levels by targeting GTPCH I as a rational therapeutic strategy to recouple eNOS and suppress oxidative stress on wound healing in type 1 diabetes.
In summary, the results of the present study demonstrate that endothelium-targeted overexpression of GTPCH I accelerates wound healing in type 1 diabetes by increasing cutaneous BH4 level and suppressing oxidative stress (Fig. 6). These findings may provide a mechanistic basis for targeting the endogenous GTPCH/BH4 pathway as a potential therapeutic strategy to combat endothelial dysfunction and refractory wound healing in diabetes.
GRANTS
This work was supported in part by National Institute of General Medical Sciences Grant R01-GM-077352 and American Diabetes Association Research Award 7-08-RA-23 (to A. F. Chen).
REFERENCES
- 1.Abd-El-Aleem SA, Ferguson MW, Appleton I, Kairsingh S, Jude EB, Jones K, McCollum CN, Ireland GW. Expression of nitric oxide synthase isoforms and arginase in normal human skin and chronic venous leg ulcers. J Pathol 191: 434–442, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Alp NJ, Mussa S, Khoo J, Cai S, Guzik T, Jefferson A, Goh N, Rockett KA, Channon KM. Tetrahydrobiopterin-dependent preservation of nitric oxide-mediated endothelial function in diabetes by targeted transgenic GTP-cyclohydrolase I overexpression. J Clin Invest 112: 725–735, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bojunga J, Dresar-Mayert B, Usadel KH, Kusterer K, Zeuzem S. Antioxidative treatment reverses imbalances of nitric oxide synthase isoform expression and attenuates tissue-cGMP activation in diabetic rats. Biochem Biophys Res Commun 316: 771–780, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest 117: 1219–1222, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai S, Khoo J, Mussa S, Alp NJ, Channon KM. Endothelial nitric oxide synthase dysfunction in diabetic mice: importance of tetrahydrobiopterin in eNOS dimerisation. Diabetologia 48: 1933–1940, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Channon KM Tetrahydrobiopterin: regulator of endothelial nitric oxide synthase in vascular disease. Trends Cardiovasc Med 14: 323–327, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Cooke CL, Davidge ST. Peroxynitrite increases iNOS through NF-κB and decreases prostacyclin synthase in endothelial cells. Am J Physiol Cell Physiol 282: C395–C402, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Cosentino F, Luscher TF. Tetrahydrobiopterin and endothelial nitric oxide synthase activity. Cardiovasc Res 43: 274–278, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Donnini S, Ziche M. Constitutive and inducible nitric oxide synthase: role in angiogenesis. Antioxid Redox Signal 4: 817–823, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Du YH, Guan YY, Alp NJ, Channon KM, Chen AF. Endothelium-specific GTP cyclohydrolase I overexpression attenuates blood pressure progression in salt-sensitive low-renin hypertension. Circulation 117: 1045–1054, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Falanga V Wound healing and its impairment in the diabetic foot. Lancet 366: 1736–1743, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Frank S, Kampfer H, Wetzler C, Pfeilschifter J. Nitric oxide drives skin repair: novel functions of an established mediator. Kidney Int 61: 882–888, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Gallagher KA, Liu ZJ, Xiao M, Chen H, Goldstein LJ, Buerk DG, Nedeau A, Thom SR, Velazquez OC. Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1alpha. J Clin Invest 117: 1249–1259, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gunnett CA, Heistad DD, Faraci FM. Gene-targeted mice reveal a critical role for inducible nitric oxide synthase in vascular dysfunction during diabetes. Stroke 34: 2970–2974, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Huisman A, Vos I, van Faassen EE, Joles JA, Grone HJ, Martasek P, van Zonneveld AJ, Vanin AF, Rabelink TJ. Anti-inflammatory effects of tetrahydrobiopterin on early rejection in renal allografts: modulation of inducible nitric oxide synthase. FASEB J 16: 1135–1137, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Jude EB, Boulton AJ, Ferguson MW, Appleton I. The role of nitric oxide synthase isoforms and arginase in the pathogenesis of diabetic foot ulcers: possible modulatory effects by transforming growth factor beta1. Diabetologia 42: 748–757, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Kampfer H, Pfeilschifter J, Frank S. Expression and activity of arginase isoenzymes during normal and diabetes-impaired skin repair. J Invest Dermatol 121: 1544–1551, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Li L, Fink GD, Watts SW, Northcott CA, Galligan JJ, Pagano PJ, Chen AF. Endothelin-1 increases vascular superoxide via endothelinA-NADPH oxidase pathway in low-renin hypertension. Circulation 107: 1053–1058, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Luo JD, Chen AF. Nitric oxide: a newly discovered function on wound healing. Acta Pharmacol Sin 26: 259–264, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Luo JD, Wang YY, Fu WL, Wu J, Chen AF. Gene therapy of endothelial nitric oxide synthase and manganese superoxide dismutase restores delayed wound healing in type 1 diabetic mice. Circulation 110: 2484–2493, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Meininger CJ, Cai S, Parker JL, Channon KM, Kelly KA, Becker EJ, Wood MK, Wade LA, Wu G. GTP cyclohydrolase I gene transfer reverses tetrahydrobiopterin deficiency and increases nitric oxide synthesis in endothelial cells and isolated vessels from diabetic rats. FASEB J 18: 1900–1902, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Meininger CJ, Marinos RS, Hatakeyama K, Martinez-Zaguilan R, Rojas JD, Kelly KA, Wu G. Impaired nitric oxide production in coronary endothelial cells of the spontaneously diabetic BB rat is due to tetrahydrobiopterin deficiency. Biochem J 349: 353–356, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Musalmah M, Nizrana MY, Fairuz AH, NoorAini AH, Azian AL, Gapor MT, Wan Ngah WZ. Comparative effects of palm vitamin E and alpha-tocopherol on healing and wound tissue antioxidant enzyme levels in diabetic rats. Lipids 40: 575–580, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Nagareddy PR, Xia Z, McNeill JH, MacLeod KM. Increased expression of iNOS is associated with endothelial dysfunction and impaired pressor responsiveness in streptozotocin-induced diabetes. Am J Physiol Heart Circ Physiol 289: H2144–H2152, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Oak JH, Cai H. Attenuation of angiotensin II signaling recouples eNOS and inhibits nonendothelial NOX activity in diabetic mice. Diabetes 56: 118–126, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Panchatcharam M, Miriyala S, Gayathri VS, Suguna L. Curcumin improves wound healing by modulating collagen and decreasing reactive oxygen species. Mol Cell Biochem 290: 87–96, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Park L, Raman KG, Lee KJ, Lu Y, Ferran LJ Jr, Chow WS, Stern D, Schmidt AM. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat Med 4: 1025–1031, 1998. [DOI] [PubMed] [Google Scholar]
- 28.Pieper GM Enhanced, unaltered and impaired nitric oxide-mediated endothelium-dependent relaxation in experimental diabetes mellitus: importance of disease duration. Diabetologia 42: 204–213, 1999. [DOI] [PubMed] [Google Scholar]
- 29.Prabhakar SS Tetrahydrobiopterin reverses the inhibition of nitric oxide by high glucose in cultured murine mesangial cells. Am J Physiol Renal Physiol 281: F179–F188, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Ren J, Duan J, Thomas DP, Yang X, Sreejayan N, Sowers JR, Leri A, Kajstura J, Gao F, Anversa P. IGF-I alleviates diabetes-induced RhoA activation, eNOS uncoupling, and myocardial dysfunction. Am J Physiol Regul Integr Comp Physiol 294: R793–R802, 2008. [DOI] [PubMed] [Google Scholar]
- 31.Schaffer MR, Tantry U, Efron PA, Ahrendt GM, Thornton FJ, Barbul A. Diabetes-impaired healing and reduced wound nitric oxide synthesis: a possible pathophysiologic correlation. Surgery 121: 513–519, 1997. [DOI] [PubMed] [Google Scholar]
- 32.Schaffer MR, Tantry U, Gross SS, Wasserburg HL, Barbul A. Nitric oxide regulates wound healing. J Surg Res 63: 237–240, 1996. [DOI] [PubMed] [Google Scholar]
- 33.Shinozaki K, Kashiwagi A, Nishio Y, Okamura T, Yoshida Y, Masada M, Toda N, Kikkawa R. Abnormal biopterin metabolism is a major cause of impaired endothelium-dependent relaxation through nitric oxide/O2− imbalance in insulin-resistant rat aorta. Diabetes 48: 2437–2445, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med 341: 738–746, 1999. [DOI] [PubMed] [Google Scholar]
- 35.Soneja A, Drews M, Malinski T. Role of nitric oxide, nitroxidative and oxidative stress in wound healing. Pharmacol Rep 57, Suppl: 108–119, 2005. [PubMed] [Google Scholar]
- 36.Stallmeyer B, Anhold M, Wetzler C, Kahlina K, Pfeilschifter J, Frank S. Regulation of eNOS in normal and diabetes-impaired skin repair: implications for tissue regeneration. Nitric Oxide 6: 168–177, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Thony B, Auerbach G, Blau N. Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem J 347: 1–16, 2000. [PMC free article] [PubMed] [Google Scholar]
- 38.Vasquez-Vivar J, Kalyanaraman B, Martasek P. The role of tetrahydrobiopterin in superoxide generation from eNOS: enzymology and physiological implications. Free Radic Res 37: 121–127, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Witte MB, Barbul A. Role of nitric oxide in wound repair. Am J Surg 183: 406–412, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Witte MB, Kiyama T, Barbul A. Nitric oxide enhances experimental wound healing in diabetes. Br J Surg 89: 1594–1601, 2002. [DOI] [PubMed] [Google Scholar]
- 41.Witte MB, Thornton FJ, Tantry U, Barbul A. l-Arginine supplementation enhances diabetic wound healing: involvement of the nitric oxide synthase and arginase pathways. Metabolism 51: 1269–1273, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Yamasaki K, Edington HD, McClosky C, Tzeng E, Lizonova A, Kovesdi I, Steed DL, Billiar TR. Reversal of impaired wound repair in iNOS-deficient mice by topical adenoviral-mediated iNOS gene transfer. J Clin Invest 101: 967–971, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zheng JS, Yang XQ, Lookingland KJ, Fink GD, Hesslinger C, Kapatos G, Kovesdi I, Chen AF. Gene transfer of human guanosine 5′-triphosphate cyclohydrolase I restores vascular tetrahydrobiopterin level and endothelial function in low renin hypertension. Circulation 108: 1238–1245, 2003. [DOI] [PubMed] [Google Scholar]