Abstract
This review summarizes recent advances in our understanding of the pre- and posttranscriptional mechanisms that regulate leptin production and secretion in adipocytes. Basal leptin production is proportional to the status of energy stores, i.e., fat cell size, and this is mainly regulated by alterations in leptin mRNA levels. Leptin mRNA levels are regulated by hormones, including glucocorticoids and catecholamines, but little is known about the transcriptional mechanisms involved. Leptin synthesis and secretion is also acutely modulated in response to hormones such as insulin and the availability of metabolic fuels. Acute variations in leptin production over a time course of minutes to hours are mediated at the levels of both translation and secretion. Increases in amino acids and insulin after a meal activate the mammalian target of rapamycin (mTOR) pathway, leading to an increase in specific rates of leptin biosynthesis. Cross-talk among mTOR, PKA, and AMP-activated protein kinase pathways appears to integrate hormonal and nutrient signals that regulate leptin mRNA translation, at least in part through mechanisms involving its 5′- and 3′-untranslated regions. In addition, the rate of leptin secretion from preformed stores in response to hormonal cues is also regulated. Insulin stimulates, and adrenergic agonists inhibit, leptin secretion, and this likely contributes to variations in the magnitude of nutrition-related leptin excursions and oscillations. Overall, the study of leptin production has contributed to a deepening understanding of leptin biology and, more broadly, to our understanding of the cellular and molecular mechanisms by which the adipocyte integrates hormonal and nutrient signals to regulate adipokine production.
Keywords: adipose tissue, adrenergic, feeding, insulin, obesity
leptin is a 16-kda protein encoded by the obese (lep) gene that is expressed and secreted mainly by adipocytes. When energy stores are low, a fall in leptin decreases thermogenesis, promotes fatty acid (FA) over glucose oxidation, and decreases the activity of the hypothalamic pituitary adrenal and gonadal axes (1). Leptin also modulates the activities of the hematopoietic (5) and immune (52) systems, as well as angiogenesis (7).
The importance of the leptin system in regulating body fat in animals and humans is dramatically demonstrated by the extreme obesity caused by leptin deficiency (53, 93). In leptin-sensitive lean individuals, short-term increases in circulating leptin in response to feeding promote satiety. However, with chronic overfeeding and obesity, resistance to leptin action develops. Thus the ability of the adipocyte to appropriately alter leptin production to match the level of leptin resistance may be critical to limiting weight gain. Subtle alterations in the ability of the adipocyte to regulate basal and meal-induced leptin release, as well as its pulsatile pattern of secretion, may therefore establish a new “settling point”, with a defense of a body weight at an obese level. Although leptin administration may not promote weight loss in dieters, it helps maintain weight loss achieved by dieting (68). Thus unraveling the basic mechanisms regulating leptin production and secretion may point to new therapeutic approaches for treating obesity or its complications.
Chronic Changes in Nutritional Status Regulate Leptin at the Level of Gene Expression
Early studies established that the leptin mRNA expression is increased in obesity (46, 50) and that long-term alterations in food intake, e.g., starvation for 48 h or overfeeding occur in close association with variations in leptin mRNA levels, suggesting mainly transcriptional control (28, 71). Numerous descriptive studies demonstrate that the chronic hormonal milieus associated with a positive energy balance, i.e., elevated insulin and high local glucocorticoids (GC), increase leptin production by increasing leptin mRNA levels, whereas signals associated with starvation, such as catecholamines released by activation of the sympathetic nervous system, decrease leptin mRNA (20, 62).
The mechanisms regulating long-term alterations in leptin gene expression in response to changes in the level of obesity/fat cell size or hormonal signals remain unclear. Available in vitro studies are not consistent with a major effect of insulin on leptin gene transcription. Insulin increases leptin promoter activity only after a very long treatment, 48 h, in 3T3-L1 cells (54). Furthermore, incubation of rat adipocytes with insulin for 1–2 h does not affect leptin mRNA levels, yet it increases leptin release, suggesting that insulin regulates leptin production through posttranscriptional mechanisms (8, 40, 66). In support of this interpretation, actinomycin D does not affect insulin stimulation of leptin release from isolated rat adipocytes (8). In vivo, insulin infusion into starved rats increases leptin mRNA expression in rodents after 2.5–4 h of infusion (71, 94), but this could be due to a secondary event, such as a lowering of catabolic signals including cAMP. Consistent with this idea, activation of β-adrenergic receptors (AR) decreases leptin mRNA levels over the long term (43, 64, 91).
GC increase leptin mRNA levels. In vitro incubation of rat adipocytes with a synthetic GC, dexamethasone, increases leptin mRNA level and release as early as 2 h (8). Our laboratory (58) and others (36, 56) find that, in humans, oral GCs increase leptin mRNA and serum leptin levels by approximately twofold, 24–48 h after administration. Coadministration of a pulse of GCs with a meal or insulin increases serum leptin after 5–7 h, but it is not known whether this is because of an increase in leptin mRNA (34, 35). Long-term culture of human adipose tissue with dexamethasone for 1–7 days maintains initial leptin mRNA expression levels (39, 69). Furthermore, culture with the combination of insulin and GC increases leptin mRNA levels additively or synergistically, depending on depot (8, 39, 69, 77), suggesting that these two hormones are major regulators of leptin levels in humans.
Tumor necrosis factor-α (TNFα), a proinflammatory cytokine, decreases leptin expression over the long term (80, 82). TNFα acutely increases leptin production by adipocytes (82). In human adipose tissue, TNFα increases leptin mRNA only when added together with dexamethasone, and the mechanism involves an activation of p38 MAPK (80). Interleukin-6, another inflammatory cytokine, also increases leptin production when added in the presence of dexamethasone, but the mechanism is not yet established (81). Thus the increase in local cortisol and inflammatory cytokines in adipose tissue may contribute to higher leptin mRNA levels in obesity and contribute to higher leptin levels observed after endotoxin administration (19).
Neuropeptides may have a role in regulating leptin production. Melanin-concentrating hormone stimulates leptin release from isolated rat adipocytes (9). The mechanism appears to involve an increase in leptin promoter activity, as shown in 3T3-L1 adipocytes through MAPK and mammalian target of rapamycin (mTOR)/S6 kinase pathways (10). Neuropeptide Y (NPY) is released by sympathetic neurons that innervate adipose tissue and may also be expressed by human adipocytes (32). Kos et al. (32) reported that NPY decreases leptin release in isolated human adipocytes. This result was unexpected because NPY exerts an antilipolytic effect via an inhibition of cAMP production and is known to decrease leptin. In contrast to these results, Serradeil-Le Gal et al. (73) found that NPY increases leptin production by human adipocytes (33). Further work is needed to clarify the importance of NPY, its receptors, and the cellular signaling pathways by which they fine tune leptin expression in response to a stress (33). Taken together, available evidence indicates that the balance of nutrition-induced alterations in hormonal and neural signals regulates leptin production and mRNA levels, at least in part at the level of leptin transcription.
Very little is known about mechanisms regulating leptin gene transcription. Like other adipocyte genes that are induced during differentiation, CCAAT/enhancer-binding protein (C/EBP)α increases leptin expression by increasing promoter activity (29). The stimulatory effect of GC on leptin mRNA expression appears to occur at least in part by modulating leptin transcription. However, other than the identification of a putative GC response element in the promoter of leptin gene (25), little is known how GCs increase leptin mRNA. A recent study by Zeigerer et al. (89) showed that the addition of a peroxisome proliferator-activated receptor (PPAR)-γ agonist during differentiation of 3T3-L1 increased leptin expression; however, this may have been secondary to a general effect on differentiation. In mature adipocytes, PPAR-γ agonists actually decrease leptin promoter activity and leptin production (18, 29, 75). No PPAR response element in the leptin promoter has yet been identified; however, PPAR-γ agonists antagonize the C/EBPα-mediated activation of leptin promoter activity (29), and this mechanism may explain the effect of PPAR-γ agonists to decrease leptin expression. Identification of the sequences responsible for the hormonal regulation of leptin mRNA transcription and the cell- or tissue-specific expression of leptin remains an important gap in our knowledge of leptin biology.
Progress in our understanding of the regulation of leptin expression has been slowed by the lack of cell culture lines that express high levels of leptin and respond robustly to hormonal signals. The classic 3T3-L1 adipocyte cell line expresses very low levels of leptin mRNA. Although an alternate method of differentiation can increase leptin mRNA expression and permit analysis of the regulation of leptin secretion (76, 89), the levels are still far below those observed in adipose tissue in vivo. After transplantation of 3T3-F442A preadipocyte into mice, leptin mRNA levels achieve normal in vivo levels, suggesting the importance of factors that are missing ex vivo (51). Additionally, although insulin and GC are known to increase leptin levels in intact fragments of adipose tissue additively or synergistically (8, 39, 69, 77), the fact that this is not observed in 3T3-L1 (63) or other cell culture models represents a significant obstacle to mechanistic studies of relevance to the human adipocyte.
Posttranscriptional Mechanisms Dominate the Short-Term Nutritional Regulation of Leptin Production
Studies from several laboratories demonstrate that insulin does not affect leptin mRNA levels yet increases leptin release twofold after 1–2 h of incubation (8, 38, 40, 66). Our studies in rat adipose tissue used biosynthetic labeling to directly demonstrate the translational control of leptin by feeding, starvation, insulin, and adrenergic agonists (40, 65). Short-term starvation (14 h) decreases relative rates of leptin biosynthesis despite little or no effect on mRNA levels. The decline in leptin translation is more marked in younger/leaner compared with older/obese rats. In vitro, insulin increases rates of leptin biosynthesis by approximately two- to threefold in adipose tissue of younger rats (6–7 wk old) without affecting leptin mRNA levels and has a smaller effect (up to 1.5-fold) in older/more obese rats (12–14 wk old). The magnitude of the insulin effect on leptin mRNA translation is also greater in starved compared with fed rats (40, 65), most likely attributable to the lower baseline. Consistent with the importance of the translational control in the nutritional regulation of leptin expression, more leptin mRNA is associated with polysome fractions in adipose tissue from fed compared with starved rats. Furthermore, in vitro insulin treatment increases leptin mRNA association with translationally active polysome fractions (40).
Activation of the sympathetic nervous system may antagonize the insulin stimulation of leptin translation. β-AR agonists decrease leptin synthesis and secretion within hours without affecting leptin mRNA levels (17, 65). Addition of isoproterenol, a β-AR agonist, to incubations of rat adipose tissue acutely blocks the insulin stimulation of relative rates of leptin biosynthesis without affecting leptin mRNA levels (65). Thus the counterregulation of leptin biosynthesis by insulin and catecholamines has the potential to fine tune the rate of leptin production over a short time course, 1 h or less.
Mechanisms Regulating Leptin Translation
Alterations in translational initiation or efficiency could mediate changes in leptin mRNA translation with feeding or insulin. Leptin mRNA of both human and mouse includes a short 5′-untranslated region (UTR) that lacks a 5′-terminal oligopyrimidine but is predicted to have conserved secondary structures that may affect translation (66). Using reporter constructs transfected into 3T3-L1 adipocytes, we found that the 5′-UTR of leptin mRNA increases translation of luciferase by two- to threefold without affecting its mRNA level. Consistent with this result, Kandror's group also found that the mouse leptin 5′-UTR increases translation of a bicistronic reporter transfected into human embryonic kidney (HEK)-293 cells by about 50% (14). Small differences in experimental protocols or cell types studied may explain the different magnitude of the effects. It is also possible that a factor that is expressed uniquely in adipocytes enhances leptin translation via interaction with the 5′-UTR of leptin.
Leptin mRNA includes a relatively long 3′-UTR (∼3 kb) with multiple potential structural motifs (Ref. 93 and our unpublished results). We noted potential adenosine-uridine-rich elements within the leptin 3′-UTR, including a UUAUUUAUU monomer and several AUUUA repeats that are conserved between species and are known to regulate mRNA stability as well as translational efficiency. Using reporter constructs, we demonstrated that the 3′-UTR of human leptin mRNA markedly decreases translational efficiency (40). There appear to be multiple negative elements in 3′-UTR of leptin since constructs containing a shorter length of 3′-UTR are less effective at inhibiting reporter expression. Studies are needed to define the cis and trans elements responsible for this inhibitory translational control. Gantt et al. (21) showed that an RNA-binding protein (RBP), HuR, binds to glucose transporter 1 and leptin mRNA in adipocytes. We also detected HuR binding to leptin mRNA (our unpublished observation) and are presently examining whether this and other RBPs regulate leptin mRNA translation or stability and whether nutritional or hormonal status can affect their binding to leptin mRNA. We postulate that the regulation of RBP expression or availability may coordinate regulation of multiple adipokines.
Both the 5′- and 3′-UTRs play a role in mediating the stimulatory effect of insulin on leptin translation. Although insulin does not specifically increase the translation of constructs containing only the 5′-UTR (14, 40), it does stimulate translation of luciferase in constructs that include the inhibitory 3′-UTR (40). Thus available evidence suggests that insulin derepresses leptin translation through a mechanism that involves an interaction of the 5′- and 3′-UTRs. Chakrabarti et al. (14) also showed that the mouse 5′-UTR is not sufficient for the stimulation of leptin translation by insulin or nutrients present in DMEM (i.e., glucose and amino acids). They also demonstrated that insulin provokes a small stimulation of the translation of the bicistronic reporter construct in HEK-293 cells regardless of the presence of the leptin 5′-UTR. In our experiments using 3T3-L1 adipocytes, insulin caused a small but consistent increase in both control and 5′-UTR reporter constructs that is likely explained by its well-known effect on general protein synthesis. Unraveling the novel mechanisms by which 5′- and 3′-UTRs of leptin specifically regulate insulin-stimulated leptin synthesis, over and beyond its effects on general protein synthesis, is a key question for future research. Furthermore, central to understanding shifts in leptin production with fasting and refeeding, it will be important to investigate the mechanisms by which activation of the cAMP pathway antagonizes insulin-induced luciferase activity (P. Brauner and S. Fried, unpublished observations).
The possibility that leptin mRNA stability is regulated has not been addressed. Leptin, like other cytokine mRNAs, includes several adenosine-uridine-rich elements that are known to regulate their half life (90). We may not have seen any variations in our leptin UTR reporter mRNA expression levels in our previous studies because they were not designed to address this issue, i.e., we used a reporter construct that was driven by a strong promoter (SV 40 promoter) (40).
Nutrients May Signal Alterations in Leptin Production
In addition to alterations in insulin, changes in circulating levels of glucose, amino acids, and lipids may also contribute to increases in leptin production in response to meals. Conversely, a decline in energy or nutrient availability, via a decrease in mTOR and increase in AMP-activated protein kinase (AMPK) signaling may contribute to the decreased leptin levels with fasting.
Increases in circulating branch chain amino acids (BCAAs) likely contribute to meal-induced increases in leptin. Administration of BCAAs, particularly leucine, elicits a rise in leptin after 3 h, as shown by Lynch et al. (47). This group also showed that norleucine, which does not increase serum insulin, also increases leptin, providing evidence that BCAA increase leptin translation via activation of mTOR (47). Whether amino acids and insulin effects on leptin translation are additive and which factor dominates in vivo will require further research.
Glucose also has been suggested to mediate nutritionally induced leptin levels since glucose infusion increases leptin mRNA levels in adipose tissue and plasma leptin levels (42). Furthermore, it has been suggested that the insulin effect on leptin depends on insulin-stimulated glucose uptake and metabolism in rat adipocytes (42, 55). Mueller et al. (55) concluded that insulin's effect on glucose metabolism explains its ability to stimulate leptin gene expression via a SP-1 site and thus its increased release. In their study, however, insulin stimulates leptin secretion only after 48–96 h. Thus it is unlikely that this mechanism contributes to the insulin stimulation of leptin release over a time course of hours (3, 8, 38). Using isolated rat adipocytes, Cammisotto et al. (13) showed that 5 mM compared with 0 mM glucose increased leptin release from rat adipocytes, but increasing glucose up to 25 mM did not cause a further increase. We obtained similar results using rat adipose tissue (M.-J. Lee and S. Fried, unpublished observations). Other metabolizable hexoses or lactate also stimulate leptin release (13, 41, 55), suggesting that glucose metabolism into trioses or generation of ATP, rather than glucose itself, plays a role in inducing leptin production or secretion. It therefore seems likely that the inhibition of leptin release from cultured adipocyte-treated inhibitors of glucose uptake (55) were secondary to a nonspecific effect on energy status that resulted in an activation of AMPK and subsequent inhibition of general protein synthesis. Alternatively, because leptin secretion requires energy, the decline in ATP with inhibition of glucose uptake may inhibit the process of leptin secretion per se. Studies are needed to assess the short- and long-term effect of glucose and other substrates on relative rates of leptin biosynthesis and secretion.
FA exert short- and long-term effects on leptin expression. In short-term experiments (2 h), FAs antagonize insulin-stimulated leptin secretion from rat adipocytes without affecting basal levels (12). Blocking triacylglycerol synthesis with triacsin C, which raised intracellular FA availability, also inhibits leptin release after 2 h (74). The effect of FAs on leptin release is independent of mitochondrial FA oxidation (12), suggesting that their effect over the short term is mediated by an inhibition of insulin signaling. Consistent with this idea, infusion of intralipid/heparin to humans for several hours to raise FAs does not affect baseline leptin levels (59) but blocks the stimulatory effect of insulin (22). Furthermore, decreased FA uptake secondary to CD36 deficiency is associated with elevated serum leptin despite lower adiposity (27). The higher leptin levels were attributed to the absence of FA antagonism of the stimulatory effect of a glucose load on serum leptin in the absence of CD36. On the other hand, the perilipin-null mouse exhibits high basal lipolysis, which should raise intracellular FA availability, yet it also has high leptin (78). This observation seems inconsistent with studies showing a suppressive role of FAs on leptin, pointing out the need for further analysis of the mechanisms by which endogenous and exogenous FAs affect leptin production.
FAs may also exert long-term effects on basal leptin expression in vitro (27, 74). This effect may be mediated, at least in part, via a stimulation of PPAR activity, since FAs are PPAR-γ agonists and PPAR-γ agonists are known to decrease leptin gene expression and circulating leptin in vivo (Ref. 79 and our unpublished observation in vitro in human adipose tissue). Furthermore, the higher baseline leptin mRNA levels in CD36-null mice may be secondary to lower FA uptake with consequent lower activation of PPARs (27). It will therefore be of interest to compare the effects of different types of FAs that differentially affect PPAR activation on leptin.
Signaling Pathways Involved in Insulin and Amino Acid-stimulated Leptin Translation: Integration at the Level of mTOR
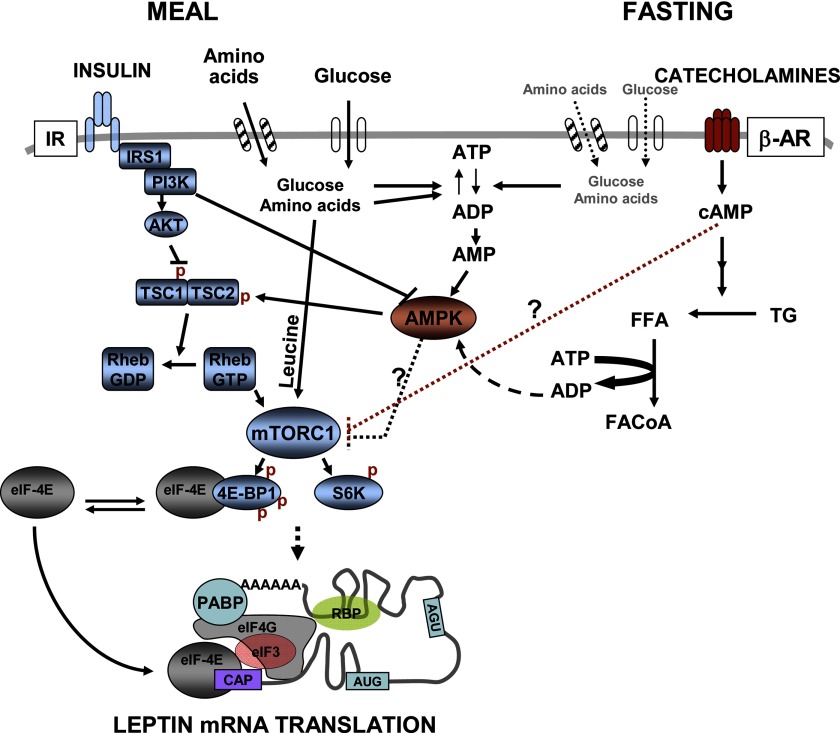
Present knowledge of the pathways that mediate the short-term control of leptin translation by hormones, nutrients, and energy status is summarized in Fig. 1. Studies using inhibitors have shown that insulin increases leptin production in adipocytes through phosphoinositide (PI) 3-kinase/PKB/mTOR. Bradley and Cheatham (8) showed that inhibitors of PI 3-kinase and MEK1/MEK2, as well as mTOR, blocked insulin-stimulated leptin release from isolated rat adipocytes without affecting leptin mRNA expression levels. Furthermore, expression of constitutively active AKT induces a more than 20-fold increase in leptin protein levels without affecting leptin mRNA levels (4). We further demonstrated that inhibitors of PI 3-kinase, AKT, or mTOR block insulin-stimulated leptin biosynthesis (40). Recent studies by Chakrabarti et al. (14) identified activation of mTOR complex 1 (mTORC1) as the critical downstream event mediating the insulin stimulation of leptin translation (14). Increasing mTORC1 activity by overexpression of Rheb or dominant negative AMPK increases leptin translation, and this does not require the presence of leptin 5′-UTR (14). In the physiological context, independent of their metabolism into glycolytic substrate or TCA cycle intermediates, amino acids can increase leptin within 2–4 h by acting as signaling molecules activating mTOR (47, 48, 66). However, the downstream events through which activation of mTOR leads to a specific increase in relative rates of leptin biosynthesis, over and above a small increase in general protein synthesis, remain to be determined.
Fig. 1.
Hormone and nutrient signaling pathways modulating leptin translation. After a meal, insulin and other nutrients stimulate leptin production by increasing leptin mRNA translation. Insulin stimulates leptin biosynthesis through a small stimulation of general protein synthesis and by specifically increasing relative rates of leptin biosynthesis. The insulin effects on leptin mRNA translation are signaled through the PI 3-kinase-mammalian target of rapamycin (mTOR) pathway. The mTOR signaling network consists of two major branches, each mediated by specific mTOR complexes (mTORC1 or mTORC2, not shown). Rapamycin-sensitive mTORC1 controls protein synthesis via phosphorylation of 4E-binding protein 1 (4E-BP1) [releasing eukaryotic initiation factor 4E (eIF-4E) and activating translation] and S6K. Amino acids, especially leucine, also increase leptin translation through mTORC1 (14). The ATP required for translation is provided by the increased supply of glucose and other metabolizable substrates. During fasting, insulin and metabolite levels decrease while sympathetic system is activated. Catecholamines activate β-adrenergic receptors (AR) signaling to decrease relative rates of leptin translation, mainly via cAMP-dependent mechanisms that decrease mTORC1 activity (P. Brauner, M.-J. Lee, S. Fried, unpublished observations). β-AR agonists also stimulate triglyceride (TG) breakdown, which may increase use of ATP for formation of fatty acyl (FA) CoA, leading to increased AMP and the activation of AMP-activated protein kinase (AMPK) (23, 87). AMPK activation is known to inhibit the mTOR pathway, decreasing general protein synthesis and possibly also relative rates of leptin mRNA translation. Thus interplay between AMPK and mTOR pathways may link low cellular energy availability during fasting to alterations in leptin synthesis. Finally, hormones and nutrients may influence the expression or binding of RNA binding proteins (RBPs) to the 5′- and 3′-untranslated regions (UTRs) of leptin mRNA level and may regulate translational efficiency and mRNA stability. IR, insulin receptor; IRS-1, insulin receptor substrate-1; PI3K, phosphoinositide 3-kinase; TSC, tuberous sclerosis complex; FFA, free fatty acid; PABP, poly(A)-binding protein.
It is notable that decreasing mTOR activity by adipose specific knockout of raptor (a component of the mTORC1) (60) results in lower leptin levels, whereas knockout of mTOR downstream translational repressor 4E-BP1 and 2 results in higher circulating leptin levels (37). However, these changes were appropriate for the concomitant changes in fat mass. The nutritional status of the mice when the leptin measurements were made was not stated, thus it is not possible to discern whether manipulating mTOR signaling specifically mediates the response to nutrient (amino acid) and hormonal (insulin) signals. In vitro studies of cultured adipocytes derived from these genetically altered mice may help define the role of the mTOR pathway in the regulation of leptin translation.
Recent studies point to the interplay between mTOR (mTORC1 complex) and AMPK signaling pathways, providing a mechanism to couple leptin synthesis with energy availability (30, 85). Kandror's group (14) demonstrated that removing the negative effect of AMPK by overexpressing dominant negative AMPK increases mTORC1 and leptin translation. This observation raises the possibility that AMPK tonically inhibits leptin translation in cultured cells and that the activation of AMPK by low cellular energy status such as fasting may inhibit leptin translation through mTOR pathway. It is not known whether this mechanism also modulates leptin synthesis in primary adipocytes. Thus the ability of insulin and/or nutrients (glucose and amino acids such as leucine) to stimulate leptin translation may result, at least in part, through relieving the inhibitory effect of AMPK. Thus cross-talk between nutrient and energy availability signaling through PI 3-kinase/PKB and AMPK may link nutritional status to the regulation of leptin production. Studies are needed to delineate the effects of AMPK activation on general protein synthesis from any possible specific effect on leptin translation.
Activation of PKA pathway may also regulate leptin release during the starved state. Increases in cAMP are likely to mediate the β-adrenergic inhibition of leptin release. Cong et al. (15) showed that insulin antagonizes the β-AR suppression of leptin release from rat primary adipocytes during 24-h cultures through PI 3-kinase-dependent activation of PDE3-decreasing cAMP. We have also found that dbcAMP can mimic the effect of β-AR to inhibit leptin translation (P. Brauner and S. Fried, unpublished observation). Thus exercise, cold stress, and fasting may decrease leptin production via cAMP signaling. Increasing intracellular cAMP has been shown to decrease mTOR kinase activity and phosphorylation of mTOR downstream targets, 4E-BP1 and S6K, in several cell types including adipocytes (26, 45, 72). Thus cAMP may decrease overall or specific rates of leptin translation through cross-talk with the mTOR pathway.
Interplay between cAMP and AMPK signaling in adipocytes may also contribute to the β-AR-mediated decrease in leptin release (23, 31, 87). Koh et al. (31) showed that β-AR agonists increase the AMP/ATP ratio and activate AMPK activity in rat adipocytes. A recent paper by Gauthier et al. (23) showed that β-AR-stimulated lipolysis increases use of ATP for FA activation and that this activates AMPK activity. Thus it seems possible that the decline in serum leptin associated with activation of the sympathetic nervous system with fasting, cold, or exercise involves the cross-talk among the PKA, AMPK, and mTOR pathways, as illustrated in Fig. 1.
In studies of rat adipocytes or adipose tissue, stimulation of β-AR did not affect basal leptin secretion but antagonized the insulin stimulation of leptin secretion after as little as 30 min of treatment (24, 65). In human adipose tissue, however, β-AR stimulation decreased basal leptin release without affecting leptin biosynthesis (65). Thus there may be significant species differences in the adrenergic regulation of leptin.
Regulation of Leptin Release at Posttranslational Steps
In addition to alterations in its synthesis, the posttranslational processing of leptin in the short term (i.e., within minutes to an hour) may also be regulated at the level of secretion per se (3, 38). Newly synthesized leptin can be stored, degraded intracellularly before being secreted, or secreted. Secreted leptin may also be subject to reuptake and secretion (38). Nutritional status (chronically or acutely) and hormonal stimuli, i.e., insulin or adrenergic agonists, could affect any of these processes to regulate leptin release.
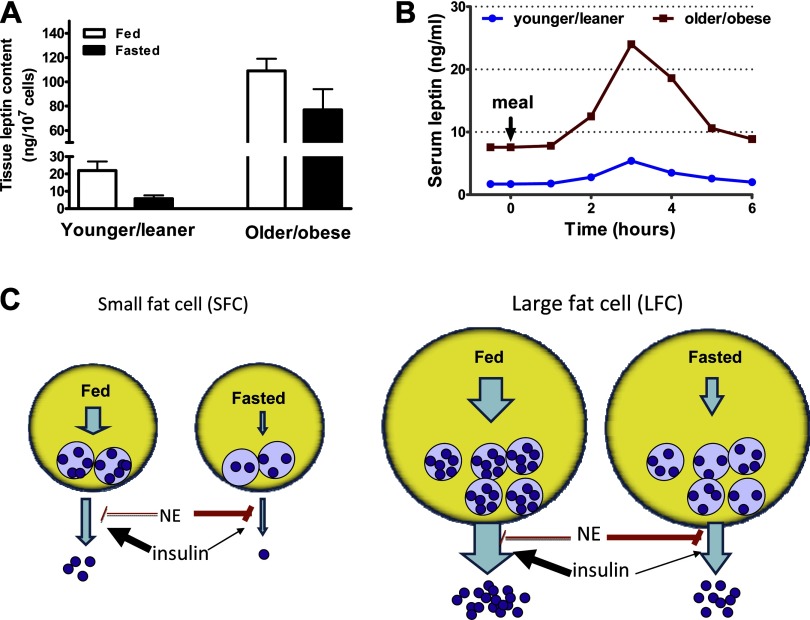
We demonstrated that a substantial quantity of leptin protein resides in a detergent-sensitive compartment of human adipose tissue, sufficient to account for leptin release from adipose tissue of humans for over 3 h (70) and rats for 1 h (38). Moreover, the amount of leptin in human adipose tissue is highly correlated with fat cell size and obesity (40, 70). The size of the preformed leptin pool also varies as a function of nutritional status and obesity. Leptin content is higher in adipose tissue from older/obese compared with younger/lean rats, and in fed compared with starved rats (Ref. 39; M.-J. Lee and S. Fried, unpublished observation; see Fig. 2). Thus we proposed that a preformed leptin pool may provide a reserve that can be rapidly released and contribute to changes in plasma leptin that occur hours after meals, as illustrated in Fig. 2. This mechanism may permit more rapid changes in circulating leptin than would be possible with alterations in de novo synthesis. For example, variations in the release of stored leptin may contribute to pulsatile variations (∼30 min) in plasma leptin levels that are documented in both humans (44) and rats (57). An increase in the rate of release of preformed leptin may also explain the bigger leptin pulse height in the obese (44) and fed conditions (2).
Fig. 2.
Influence of obesity (fat cell size, FCS) and meals on leptin storage and secretion. Large fat cells (LFC) from obese animals have larger stores of intracellular leptin compared with small fat cells (SFC) from lean controls. A: leptin content of younger/leaner (6–7 wk old, FCS = 0.05 μg lipid/cell) and older/obese (12–14 wk old, FCS = 0.17 μg lipid/cell) rat adipocytes reported in Refs. 38 and 40. (Note these were assayed at the same time so the values can be compared reliably.) B: data adapted from published papers (44, 47, 86) to illustrate that obesity is associated with higher fasting leptin levels and higher meal-induced leptin excursions. We hypothesize that this occurs, at least in part, as a result of increased secretion of preformed leptin from the LFC of the obese. C: in both SFC and LFC, the secretion of preformed leptin is modulated by insulin and catecholamines such as norepinephrine (NE) (38, 65). Under fasting conditions, in both large and small fat cells, the rate of secretion from preformed leptin stores is restrained by the increased sympathetic input to adipose tissue and higher NE and low insulin. After a meal, the rise in insulin and decrease in NE stimulates leptin secretion from preformed intracellular stores. Although drawn as one pool for simplicity, it is possible that leptin is stored in one or more as yet undefined endoplasmic reticulum and Golgi compartments and that obesity, as well as fasting and feeding, affects this distribution. Similarly, the number of vesicles, in addition to the leptin content per vesicle, may also be regulated as a function of nutritional status.
With pulse-chase experiments, we directly demonstrated that, independent of protein synthesis, insulin increases (38) while isoproterenol decreases (P. Brauner and S. Fried, unpublished results) secretion of newly synthesized leptin from rat and human adipose tissue over a time course of 30–60 min, showing that insulin or catecholamines act on the leptin secretion step. Nutritional status affects insulin induction of leptin secretion. Insulin increases leptin secretion in adipose tissue from fed but not overnight fasted rats (38). This may be due to the fact that starvation depletes a specific pool of preformed leptin that is subject to regulated secretion. Repletion of this pool in fed rats may allow insulin to promptly increase serum leptin, helping to maintain energy balance (Fig. 2).
Little is known about the molecular mechanisms controlling leptin secretion. Leptin exists in different compartments from other adipocyte secretory products, namely lipoprotein lipase, adiponectin, or glucose transporter 4 (3, 6, 67). Inhibition of vesicular trafficking with Brefeldin A (49) or monensin (42) blocks leptin release into medium and leads to its intracellular accumulation, indicating that leptin secretion is regulated by the classical secretory pathway. However, leptin does not appear to colocalize within Golgi (3), and Roh et al. (67) suggested that, at least in adipocytes from young rats, separate leptin storage vesicles may exist. Like adiponectin (84), the endoplasmic reticulum (ER) may be a critical site for regulating leptin folding (it has one disulfide bond) and secretion. Future studies in which the expression or binding activity of potential chaperones is manipulated might help to explain the cellular trafficking of leptin with variations in nutritional or physiological status.
Turnover of Newly Synthesized Leptin: Insulin Prevents Intracellular Degradation of Leptin by Increasing Secretion
Another potential site of regulatory control of leptin production is degradation. In pulse-chase experiments, only a fraction, up to 50%, of the labeled leptin that disappeared from cells could be recovered in the medium, suggesting that some of the newly synthesized leptin is degraded within adipose tissue (38). Insulin prevented this intracellular leptin degradation by moving it out of the cells, increasing the half-life of newly synthesized leptin from 50 min to 150 min (38). Although several studies (61, 88) have suggested leptin is degraded through proteasomal pathway, our results with inhibitors indicate that leptin is also degraded by another pathway. Lysosomal degradation of leptin seems likely since exogenously added 125I-leptin is degraded (38). Thus secreted leptin may be subject to reuptake and degradation, providing another level of control of net leptin secretion. Further analysis of the mechanisms regulating leptin degradation in different compartments may reveal novel mechanisms that fine tune leptin secretion as a function of nutritional state.
Conclusion
In states of chronic nutrient excess or restriction, serum leptin concentrations are regulated at the mRNA expression level through both transcriptional and posttranscriptional mechanisms. In response to meal ingestion and consequent variations in hormone levels and nutrient availability, regulation at posttranscriptional steps, including translation, storage, secretion, or degradation, adjusts the rate of leptin production and release.
Future Directions
As we understand more about how leptin is regulated at pre-and posttranscriptional levels, we will get a step closer to the development of methods to manipulating diurnal excursions and oscillations in serum leptin levels and thereby could modulate its downstream effectiveness. Additionally, it will be important to dissect how the adipocyte senses its size to adjust basal leptin production to the level of body fat and the degree of central leptin resistance. Interestingly, recent research points to the potential for cross-talk between the leptin action in the hypothalamus and leptin production in the periphery. Leptin signaling through mTOR activity in the hypothalamus regulates leptin action on food intake, and this pathway becomes resistant with high-fat-diet-induced obesity (16). The extent to which the adipocyte mTOR pathway also becomes resistant to insulin or nutrient signals may be a critical determinant of the ability of the adipocyte to upregulate leptin production and close the feedback loop. In addition, hypothalamic leptin signaling through PI 3-kinase can activate sympathetic nerves to regulate adipocyte lipogenesis and endocannabinoid production in adipose tissue (11). This mechanism may also regulate leptin transcription and translation, providing direct central control of leptin production and an additional feedback loop. Another layer of regulation involves the autocrine feedback of leptin production at the level of the adipocyte (83, 92). Although some of the nutrients (glucose, amino acids, and fatty acids) and endocrine or neural signals (insulin, GCs, catecholamines, NPY, and melanin-concentration hormone) have been identified, their relative importance and mechanism of action remain unclear. Finally, fat cells produce many other adipokines, including adiponectin, RBP 4, and serum amyloid A. Some mechanisms for regulating the production of these adipokines may be common with leptin, but others are likely to be unique. The elucidation of how the adipocyte integrates signals and functions as an endocrine cell that modulates appetite, fuel metabolism, inflammation, angiogenesis, and reproduction may point to novel therapeutic approaches to preventing obesity and its metabolic consequences.
GRANTS
This work was supported by the Geriatric Research, Education and Clinical Center, BVAMC, NIH DK52398 (S. Fried), CNRU of Maryland (P30 DK072488, S. Fried), and an AHA Post-doctoral fellowship (M.-J. Lee).
REFERENCES
- 1.Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. Role of leptin in the neuroendocrine response to fasting. Nature 382: 250–252, 1996. [DOI] [PubMed] [Google Scholar]
- 2.Bagnasco M, Kalra PS, Kalra SP. Ghrelin and leptin pulse discharge in fed and fasted rats. Endocrinology 143: 726–729, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Barr VA, Malide D, Zarnowski MJ, Taylor SI, Cushman SW. Insulin stimulates both leptin secretion and production by rat white adipose tissue. Endocrinology 138: 4463–4472, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Barthel A, Kohn AD, Luo Y, Roth RA. A constitutively active version of the Ser/Thr kinase Akt induces production of the ob gene product, leptin, in 3T3–L1 adipocytes. Endocrinology 138: 3559–3562, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Bennett BD, Solar GP, Yuan JQ, Mathias J, Thomas GR, Matthews W. A role for leptin and its cognate receptor in hematopoiesis. Curr Biol 6: 1170–1180, 1996. [DOI] [PubMed] [Google Scholar]
- 6.Bogan JS, Lodish HF. Two compartments for insulin-stimulated exocytosis in 3T3–L1 adipocytes defined by endogenous ACRP30 and GLUT4. J Cell Biol 146: 609–620, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouloumie A, Drexler HC, Lafontan M, Busse R. Leptin, the product of Ob gene, promotes angiogenesis. Circ Res 83: 1059–1066, 1998. [DOI] [PubMed] [Google Scholar]
- 8.Bradley RL, Cheatham B. Regulation of ob gene expression and leptin secretion by insulin and dexamethasone in rat adipocytes. Diabetes 48: 272–278, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Bradley RL, Kokkotou EG, Maratos-Flier E, Cheatham B. Melanin-concentrating hormone regulates leptin synthesis and secretion in rat adipocytes. Diabetes 49: 1073–1077, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Bradley RL, Mansfield JP, Maratos-Flier E, Cheatham B. Melanin-concentrating hormone activates signaling pathways in 3T3–L1 adipocytes. Am J Physiol Endocrinol Metab 283: E584–E592, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Buettner C, Muse ED, Cheng A, Chen L, Scherer T, Pocai A, Su K, Cheng B, Li X, Harvey-White J, Schwartz GJ, Kunos G, Rossetti L, Buettner C. Leptin controls adipose tissue lipogenesis via central, STAT3-independent mechanisms. Nat Med 14: 667–675, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cammisotto PG, Gelinas Y, Deshaies Y, Bukowiecki LJ. Regulation of leptin secretion from white adipocytes by free fatty acids. Am J Physiol Endocrinol Metab 285: E521–E526, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Cammisotto PG, Gelinas Y, Deshaies Y, Bukowiecki LJ. Regulation of leptin secretion from white adipocytes by insulin, glycolytic substrates, and amino acids. Am J Physiol Endocrinol Metab 289: E166–E171, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Chakrabarti P, Anno T, Manning BD, Luo Z, Kandror KV. The mTOR complex 1 regulates leptin biosynthesis in adipocytes at the level of translation. The role of the 5′-UTR in the expression of leptin mRNA. Mol Endocrinol 22: 2260–2267, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cong L, Chen K, Li J, Gao P, Li Q, Mi S, Wu X, Zhao AZ. Regulation of adiponectin and leptin secretion and expression by insulin through a PI3K-PDE3B dependent mechanism in rat primary adipocytes. Biochem J 403: 519–525, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cota D, Matter EK, Woods SC, Seeley RJ. The role of hypothalamic mammalian target of rapamycin complex 1 signaling in diet-induced obesity. J Neurosci 28: 7202–7208, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Couillard C, Mauriege P, Prud'homme D, Nadeau A, Tremblay A, Bouchard C, Despres JP. Plasma leptin response to an epinephrine infusion in lean and obese women. Obes Res 10: 6–13, 2002. [DOI] [PubMed] [Google Scholar]
- 18.De Vos P, Lefebvre AM, Miller SG, Guerre-Millo M, Wong K, Saladin R, Hamann LG, Staels B, Briggs MR, Auwerx J. Thiazolidinediones repress ob gene expression in rodents via activation of peroxisome proliferator-activated receptor gamma. J Clin Invest 98: 1004–1009, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finck BN, Johnson RW. Anti-inflammatory agents inhibit the induction of leptin by tumor necrosis factor-alpha. Am J Physiol Regul Integr Comp Physiol 282: R1429–R1435, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Fried SK, Ricci MR, Russell CD, Laferrere B. Regulation of leptin production in humans. J Nutr 130: 3127S–3131S, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Gantt KR, Cherry J, Richardson M, Karschner V, Atasoy U, Pekala PH. The regulation of glucose transporter (GLUT1) expression by the RNA binding protein HuR. J Cell Biochem 99: 565–574, 2006. [DOI] [PubMed]
- 22.Garcia-Lorda P, Nash W, Roche A, Pi-Sunyer FX, Laferrere B. Intralipid/heparin infusion suppresses serum leptin in humans. Eur J Endocrinol 148: 669–676, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gauthier MS, Miyoshi H, Souza SC, Cacicedo JM, Saha AK, Greenberg AS, Ruderman NB. AMP-activated protein kinase is activated as a consequence of lipolysis in the adipocyte: potential mechanism and physiological relevance. J Biol Chem 283: 16514–16524, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gettys TW, Harkness PJ, Watson PM. The beta 3-adrenergic receptor inhibits insulin-stimulated leptin secretion from isolated rat adipocytes. Endocrinology 137: 4054–4057, 1996. [DOI] [PubMed] [Google Scholar]
- 25.Gong DW, Bi S, Pratley RE, Weintraub BD. Genomic structure and promoter analysis of the human obese gene. J Biol Chem 271: 3971–3974, 1996. [DOI] [PubMed] [Google Scholar]
- 26.Graves LM, Bornfeldt KE, Argast GM, Krebs EG, Kong X, Lin TA, Lawrence JC Jr. cAMP- and rapamycin-sensitive regulation of the association of eukaryotic initiation factor 4E and the translational regulator PHAS-I in aortic smooth muscle cells. Proc Natl Acad Sci USA 92: 7222–7226, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hajri T, Hall AM, Jensen DR, Pietka TA, Drover VA, Tao H, Eckel R, Abumrad NA. CD36-facilitated fatty acid uptake inhibits leptin production and signaling in adipose tissue. Diabetes 56: 1872–1880, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Harris RBS, Ramsay TG, Smith SR, Bruch RC. Early and late stimulation of ob mRNA expression in meal-fed and overfed rats. J Clin Invest 97: 2020–2026, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hollenberg AN, Susulic VS, Madura JP, Zhang B, Moller DE, Tontonoz P, Sarraf P, Spiegelman BM, Lowell BB. Functional antagonism between CCAAT/Enhancer binding protein-alpha and peroxisome proliferator-activated receptor-gamma on the leptin promoter. J Biol Chem 272: 5283–5290, 1997. [DOI] [PubMed] [Google Scholar]
- 30.Kimura N, Tokunaga C, Dalal S, Richardson C, Yoshino K, Hara K, Kemp BE, Witters LA, Mimura O, Yonezawa K. A possible linkage between AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) signalling pathway. Genes Cells 8: 65–79, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Koh HJ, Hirshman MF, He H, Li Y, Manabe Y, Balschi JA, Goodyear LJ. Adrenaline is a critical mediator of acute exercise-induced AMP-activated protein kinase activation in adipocytes. Biochem J 403: 473–481, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kos K, Harte AL, James S, Snead DR, O'Hare JP, McTernan PG, Kumar S. Secretion of neuropeptide Y in human adipose tissue and its role in maintenance of adipose tissue mass. Am J Physiol Endocrinol Metab 293: E1335–E1340, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Kuo LE, Kitlinska JB, Tilan JU, Li L, Baker SB, Johnson MD, Lee EW, Burnett MS, Fricke ST, Kvetnansky R, Herzog H, Zukowska Z. Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat Med 13: 803–811, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Laferrere B, Caixas A, Fried SK, Bashore C, Kim J. Pi-Sunyer FX. A pulse of insulin and dexamethasone stimulates serum leptin in fasting human subjects. Eur J Endocrinol 146: 839–845, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laferrere B, Fried SK, Hough K, Campbell SA, Thornton J, Pi-Sunyer FX. Synergistic effects of feeding and dexamethasone on serum leptin levels. J Clin Endocrinol Metab 83: 3742–3745, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Larsson H, Ahren B. Short-term dexamethasone treatment increases plasma leptin independently of changes in insulin sensitivity in healthy women. J Clin Endocrinol Metab 81: 4428–4432, 1996. [DOI] [PubMed] [Google Scholar]
- 37.Le BO, Petroulakis E, Paglialunga S, Poulin F, Richard D, Cianflone K, Sonenberg N. Elevated sensitivity to diet-induced obesity and insulin resistance in mice lacking 4E-BP1 and 4E-BP2. J Clin Invest 117: 387–396, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee MJ, Fried SK. Multilevel regulation of leptin storage, turnover and secretion by feeding and insulin in rat adipose tissue. J Lipid Res 47: 1984–1993, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Lee MJ, Wang Y, Ricci MR, Sullivan S, Russell CD, Fried SK. Acute and chronic regulation of leptin synthesis, storage, and secretion by insulin and dexamethasone in human adipose tissue. Am J Physiol Endocrinol Metab 292: E858–E864, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Lee MJ, Yang RZ, Gong DW, Fried SK. Feeding and insulin increase leptin translation. Importance of the leptin mRNA untranslated regions. J Biol Chem 282: 72–80, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Levy JR, Gyarmati J, Lesko JM, Adler RA, Stevens W. Dual regulation of leptin secretion: intracellular energy and calcium dependence of regulated pathway. Am J Physiol Endocrinol Metab 278: E892–E901, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Levy JR, Stevens W. The effects of insulin, glucose, and pyruvate on the kinetics of leptin secretion. Endocrinology 142: 3558–3562, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Li H, Matheny M, Scarpace PJ. β3-Adrenergic-mediated suppression of leptin gene expression in rats. Am J Physiol Endocrinol Metab 272: E1031–E1036, 1997. [DOI] [PubMed] [Google Scholar]
- 44.Licinio J, Mantzoros C, Negrao AB, Cizza G, Wong ML, Bongiorno PB, Chrousos GP, Karp B, Allen C, Flier JS, Gold PW. Human leptin levels are pulsatile and inversely related to pituitary-adrenal function. Nat Med 3: 575–579, 1997. [DOI] [PubMed] [Google Scholar]
- 45.Lin TA, Lawrence JC Jr. Control of the translational regulators PHAS-I and PHAS-II by insulin and cAMP in 3T3–L1 adipocytes. J Biol Chem 271: 30199–30204, 1996. [DOI] [PubMed] [Google Scholar]
- 46.Lonnqvist F, Arner P, Nordfors L, Schalling M. Overexpression of the obese (ob) gene in adipose tissue of human obese subjects. Nat Med 1: 950–953, 1995. [DOI] [PubMed] [Google Scholar]
- 47.Lynch CJ, Gern B, Lloyd C, Hutson SM, Eicher R, Vary TC. Leucine in food mediates some of the postprandial rise in plasma leptin concentrations. Am J Physiol Endocrinol Metab 291: E621–E630, 2006. [DOI] [PubMed] [Google Scholar]
- 48.Lynch CJ, Patson BJ, Anthony J, Vaval A, Jefferson LS, Vary TC. Leucine is a direct-acting nutrient signal that regulates protein synthesis in adipose tissue. Am J Physiol Endocrinol Metab 283: E503–E513, 2002. [DOI] [PubMed] [Google Scholar]
- 49.Macdougald OA, Hwang CS, Fan HY, Lane MD. Regulated expression of the obese gene-product (leptin) in white adipose-tissue and 3T3–L1 adipocytes. Proc Natl Acad Sci USA 92: 9034–9037, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S, Kern PA, Friedman JM. Leptin levels in human and rodent—measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med 1: 1155–1161, 1995. [DOI] [PubMed] [Google Scholar]
- 51.Mandrup S, Loftus TM, Macdougald OA, Kuhajda FP, Lane MD. Obese gene expression at in vivo levels by fat pads derived from s.c. implanted 3T3–F442A preadipocytes. Proc Natl Acad Sci USA 94: 4300–4305, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matarese G, Moschos S, Mantzoros CS. Leptin in immunology. J Immunol 174: 3137–3142, 2005. [DOI] [PubMed] [Google Scholar]
- 53.Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, Sewter CP, Digby JE, Mohammed SN, Hurst JA, Cheetham CH, Earley AR, Barnett AH, Prins JB, O'Rahilly S. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature 387: 903–908, 1997. [DOI] [PubMed] [Google Scholar]
- 54.Moreno-Aliaga MJ, Stanhope KL, Havel PJ. Transcriptional regulation of the leptin promoter by insulin-stimulated glucose metabolism in 3t3-l1 adipocytes. Biochem Biophys Res Commun 283: 544–548, 2001. [DOI] [PubMed] [Google Scholar]
- 55.Mueller WM, Gregoire FM, Stanhope KL, Mobbs CV, Mizuno TM, Warden CH, Stern JS, Havel PJ. Evidence that glucose metabolism regulates leptin secretion from cultured rat adipocytes. Endocrinology 139: 551–558, 1998. [DOI] [PubMed] [Google Scholar]
- 56.Newcomer JW, Selke G, Melson AK, Gross J, Vogler GP, Dagogo-Jack S. Dose-dependent cortisol-induced increases in plasma leptin concentration in healthy humans. Arch Gen Psychiatry 55: 995–1000, 1998. [DOI] [PubMed] [Google Scholar]
- 57.Otukonyong EE, Dube MG, Torto R, Kalra PS, Kalra SP. High-fat diet-induced ultradian leptin and insulin hypersecretion are absent in obesity-resistant rats. Obes Res 13: 991–999, 2005. [DOI] [PubMed] [Google Scholar]
- 58.Papaspyrou-Rao S, Schneider SH, Petersen RN, Fried SK. Dexamethasone increases leptin expression in humans in vivo. J Clin Endocrinol Metab 82: 1635–1637, 1997. [DOI] [PubMed] [Google Scholar]
- 59.Peino R, Fernandez AJ, Penalva A, Considine RV, Rodriguez-Segade S, Rodriguez-Garcia J, Cordido F, Casanueva FF, Dieguez C. Acute changes in free-fatty acids (FFA) do not alter serum leptin levels. J Endocrinol Invest 21: 526–530, 1998. [DOI] [PubMed] [Google Scholar]
- 60.Polak P, Cybulski N, Feige JN, Auwerx J, Ruegg MA, Hall MN. Adipose-specific knockout of raptor results in lean mice with enhanced mitochondrial respiration. Cell Metab 8: 399–410, 2008. [DOI] [PubMed] [Google Scholar]
- 61.Rau H, Reaves BJ, O'Rahilly S, Whitehead JP. Truncated human leptin (delta133) associated with extreme obesity undergoes proteasomal degradation after defective intracellular transport. Endocrinology 140: 1718–1723, 1999. [DOI] [PubMed] [Google Scholar]
- 62.Rayner DV, Trayhurn P. Regulation of leptin production: sympathetic nervous system interactions. J Mol Med 79: 8–20, 2001. [DOI] [PubMed] [Google Scholar]
- 63.Rentsch J, Chiesi M. Regulation of ob gene mRNA levels in cultured adipocytes. FEBS Lett 379: 55–59, 1996. [DOI] [PubMed] [Google Scholar]
- 64.Ricci MR, Fried SK. Isoproterenol decreases leptin expression in adipose tissue of obese humans. Obes Res 7: 233–240, 1999. [DOI] [PubMed] [Google Scholar]
- 65.Ricci MR, Lee MJ, Russell CD, Wang Y, Sullivan S, Schneider SH, Brolin RE, Fried SK. Isoproterenol decreases leptin release from rat and human adipose tissue through posttranscriptional mechanisms. Am J Physiol Endocrinol Metab 288: E798–E804, 2005. [DOI] [PubMed] [Google Scholar]
- 66.Roh C, Han JR, Tzatsos A, Kandror KV. Nutrient-sensing mTOR-mediated pathway regulates leptin production in isolated rat adipocytes. Am J Physiol Endocrinol Metab 284: E322–E330, 2003. [DOI] [PubMed] [Google Scholar]
- 67.Roh C, Thoidis G, Farmer SR, Kandror KV. Identification and characterization of leptin-containing intracellular compartment in rat adipose cells. Am J Physiol Endocrinol Metab 279: E893–E899, 2000. [DOI] [PubMed] [Google Scholar]
- 68.Rosenbaum M, Goldsmith R, Bloomfield D, Magnano A, Weimer L, Heymsfield S, Gallagher D, Mayer L, Murphy E, Leibel RL. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest 115: 3579–3586, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Russell CD, Petersen RN, Rao SP, Ricci MR, Prasad A, Zhang Y, Brolin RE, Fried SK. Leptin expression in adipose tissue from obese humans: depot-specific regulation by insulin and dexamethasone. Am J Physiol Endocrinol Metab 275: E507–E515, 1998. [DOI] [PubMed] [Google Scholar]
- 70.Russell CD, Ricci MR, Brolin RE, Magill E, Fried SK. Regulation of the leptin content of obese human adipose tissue. Am J Physiol Endocrinol Metab 280: E399–E404, 2001. [DOI] [PubMed] [Google Scholar]
- 71.Saladin R, Devos P, Guerremillo M, Leturque A, Girard J, Staels B, Auwerx J. Transient increase in obese gene-expression after food-intake or insulin administration. Nature 377: 527–529, 1995. [DOI] [PubMed] [Google Scholar]
- 72.Scott PH, Lawrence JC Jr. Attenuation of mammalian target of rapamycin activity by increased cAMP in 3T3–L1 adipocytes. J Biol Chem 273: 34496–34501, 1998. [DOI] [PubMed] [Google Scholar]
- 73.Serradeil-Le Gal C, Lafontan M, Raufaste D, Marchand J, Pouzet B, Casellas P, Pascal M, Maffrand JP, Le Fur G. Characterization of NPY receptors controlling lipolysis and leptin secretion in human adipocytes. FEBS Lett 475: 150–156, 2000. [DOI] [PubMed] [Google Scholar]
- 74.Shintani M, Nishimura H, Yonemitsu S, Masuzaki H, Ogawa Y, Hosoda K, Inoue G, Yoshimasa Y, Nakao K. Downregulation of leptin by free fatty acids in rat adipocytes: effects of triacsin C, palmitate, and 2-bromopalmitate. Metabolism 49: 326–330, 2000. [DOI] [PubMed] [Google Scholar]
- 75.Sinha D, Addya S, Murer E, Boden G. 15-Deoxy-delta(12,14) prostaglandin J2: a putative endogenous promoter of adipogenesis suppresses the ob gene. Metabolism 48: 786–791, 1999. [DOI] [PubMed] [Google Scholar]
- 76.Slieker LJ, Sloop KW, Surface PL. Differentiation method-dependent expression of leptin in adipocyte cell lines. Biochem Biophys Res Commun 251: 225–229, 1998. [DOI] [PubMed] [Google Scholar]
- 77.Slieker LJ, Sloop KW, Surface PL, Kriauciunas A, LaQuier F, Manetta J, Bue-Valleskey J, Stephens TW. Regulation of expression of ob mRNA and protein by glucocorticoids and cAMP. J Biol Chem 271: 5301–5304, 1996. [DOI] [PubMed] [Google Scholar]
- 78.Tansey JT, Sztalryd C, Gruia-Gray J, Roush DL, Zee JV, Gavrilova O, Reitman ML, Deng CX, Li C, Kimmel AR, Londos C. Perilipin ablation results in a lean mouse with aberrant adipocyte lipolysis, enhanced leptin production, and resistance to diet-induced obesity. Proc Natl Acad Sci USA 98: 6494–6499, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Toruner F, Akbay E, Cakir N, Sancak B, Elbeg S, Taneri F, Akturk M, Karakoc A, Ayvaz G, Arslan M. Effects of PPARgamma and PPARalpha agonists on serum leptin levels in diet-induced obese rats. Horm Metab Res 36: 226–230, 2004. [DOI] [PubMed] [Google Scholar]
- 80.Trujillo ME, Lee MJ, Sullivan S, Feng J, Schneider SH, Greenberg AS, Fried SK. Tumor necrosis factor alpha and glucocorticoid synergistically increase leptin production in human adipose tissue: role for p38 mitogen-activated protein kinase. J Clin Endocrinol Metab 91: 1484–1490, 2006. [DOI] [PubMed] [Google Scholar]
- 81.Trujillo ME, Sullivan S, Harten I, Schneider SH, Greenberg AS, Fried SK. Interleukin-6 regulates human adipose tissue lipid metabolism and leptin production in vitro. J Clin Endocrinol Metab 89: 5577–5582, 2004. [DOI] [PubMed] [Google Scholar]
- 82.Wang B, Trayhurn P. Acute and prolonged effects of TNF-alpha on the expression and secretion of inflammation-related adipokines by human adipocytes differentiated in culture. Pflügers Arch 452: 418–427, 2006. [DOI] [PubMed] [Google Scholar]
- 83.Wang J, Liu R, Liu L, Chowdhury R, Barzilai N, Tan J, Rossetti L. The effect of leptin on Lep expression is tissue-specific and nutritionally regulated. Nat Med 5: 895–899, 1999. [DOI] [PubMed] [Google Scholar]
- 84.Wang ZV, Schraw TD, Kim JY, Khan T, Rajala MW, Follenzi A, Scherer PE. Secretion of the adipocyte-specific secretory protein adiponectin critically depends on thiol-mediated protein retention. Mol Cell Biol 27: 3716–3731, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell 124: 471–484, 2006. [DOI] [PubMed] [Google Scholar]
- 86.Yildiz BO, Suchard MA, Wong ML, McCann SM, Licinio J. Alterations in the dynamics of circulating ghrelin, adiponectin, and leptin in human obesity. Proc Natl Acad Sci USA 101: 10434–10439, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yin W, Mu J, Birnbaum MJ. Role of AMP-activated protein kinase in cyclic AMP-dependent lipolysis in 3T3–L1 adipocytes. J Biol Chem 278: 43074–43080, 2003. [DOI] [PubMed] [Google Scholar]
- 88.Yoshida A, Hirano K, Motoyashiki T, Morita T, Ueki H. Orthovanadate decreases the leptin content in isolated mouse fat pads via proteasome activation. Arch Biochem Biophys 406: 253–260, 2002. [DOI] [PubMed] [Google Scholar]
- 89.Zeigerer A, Rodeheffer MS, McGraw TE, Friedman JM. Insulin regulates leptin secretion from 3T3–L1 adipocytes by a PI 3 kinase independent mechanism. Exp Cell Res 314: 2249–2256, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang T, Kruys V, Huez G, Gueydan C. AU-rich element-mediated translational control: complexity and multiple activities of trans-activating factors. Biochem Soc Trans 30: 952–958, 2002. [DOI] [PubMed] [Google Scholar]
- 91.Zhang Y, Matheny M, Zolotukhin S, Tumer N, Scarpace PJ. Regulation of adiponectin and leptin gene expression in white and brown adipose tissues: influence of beta3-adrenergic agonists, retinoic acid, leptin and fasting. Biochim Biophys Acta 1584: 115–122, 2002. [DOI] [PubMed] [Google Scholar]
- 92.Zhang Y, Olbort M, Schwarzer K, Nuesslein-Hildesheim B, Nicolson M, Murphy E, Kowalski TJ, Schmidt I, Leibel RL. The leptin receptor mediates apparent autocrine regulation of leptin gene expression. Biochem Biophys Res Commun 240: 492–495, 1997. [DOI] [PubMed] [Google Scholar]
- 93.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature 372: 425–432, 1994. [DOI] [PubMed] [Google Scholar]
- 94.Zheng DH, Jones JP, Usala SJ, Dohm GL. Differential expression of ob mRNA in rat adipose tissues in response to insulin. Biochem Biophys Res Commun 218: 434–437, 1996. [DOI] [PubMed] [Google Scholar]