Abstract
Chronic mountain sickness (CMS) is characterized by excessive erythrocytosis (EE) secondary to hypoventilation. Erythropoietin (Epo) and testosterone regulate erythrocyte production. Low thyroid hormone levels are also associated to hypoventilation. Hence, these hormones can play a role in etiopathogeny of EE. The purpose of this study was to elucidate the effect of sexual and thyroid hormones and Epo in residents from Lima (150 m) and Cerro de Pasco (4,340 m), Peru, and the response to human chorionic gonadotrophin stimulation (hCG). Three groups, one at low altitude and two at high altitude [1 with hemoglobin values >16–21 g/dl and the second with Hb ≥21 g/dl (EE)], were studied. hCG was administered intramuscularly in a single dose (1,000 IU), and blood samples were obtained at 0, 6, 12, 24, 48, and 72 h after injection. High-altitude natives present similar levels of gonadotropins and thyroid hormones but lower dehydroepiandrosterone sulphate (DHEAS) levels (P < 0.01) and greater Epo (P < 0.01), 17α-hydroxyprogesterone (P < 0.01), and testosterone levels (P < 0.01) than those at 150 m. Serum testosterone levels (524.13 ± 55.91 μg/dl vs. 328.14 ± 53.23 ng/dl, means ± SE; P < 0.05) and testosterone/DHEAS ratios are higher (7.98 ± 1.1 vs. 3.65 ± 1.1; P < 0.01) and DHEAS levels lower in the EE group (83.85 ± 14.60 μg/dl vs. 148.95 ± 19.11 ug/dl; P < 0.05), whereas Epo was not further affected. Testosterone levels were highest and DHEAS levels lowest in the EE group at all times after hCG stimulation. In conclusion, high androgen activity could be involved in the etiopathogeny of CMS. This evidence provides an opportunity to develop new therapeutic strategies.
Keywords: sex hormones, altitude, estradiol, dehydroepiandrosterone sulfate
more than 140 million people live permanently at high altitude (>2,500 m) in North America, Central America, South America, East Africa, and Asia (31). Approximately 30% of the Peruvian population (about 9 million people) lives at high altitude (13). One of the health problems associated with the life at high altitude has been chronic mountain sickness (CMS), also called Monge's disease, since first described by Carlos Monge Medrano in Peru in 1925 (30). This disease can eventually be fatal. Symptoms are headache, dizziness, shortness of breath, fatigue, loss of memory, and insomnia (24).
CMS, a lack of adaptation to altitude (53), is characterized by excessive erythrocytosis (EE) secondary to hypoventilation (36, 38). Hypoventilation is accentuated mainly during sleep at high altitude (48). The prevalence of CMS is increased with altitude and age, and it is more frequent in men (36). Therefore, it is important to determine mechanisms of regulation of EE at high altitude to develop strategies for treatment or prevention.
Erythropoiesis is a process hormonally regulated. At least two hormones have been described with properties to induce erythrocyte productions, namely erythropoietin (Epo) (42) and testosterone (55).
Epo is a 30.4-kDa glycoprotein hormone produced mainly by the kidney and is a key regulator of red blood cell production (42). Epo stimulates the proliferation and differentiation of bone marrow erythroid precursors (9, 28). In Andean natives, there is a high variability in Epo and erythrocytic response to high altitude; however, there is no correlation between serum Epo levels in high-altitude natives with EE and those living at high altitudes without this pathological condition (25).
Testosterone also plays an erythropoietic role (21, 27, 34). Testosterone probably acts directly on bone marrow at the level of polychromatophylic erythroblasts and enhances the synthesis of ribosomal RNA or its precursors and stimulates a nuclear ribonuclease (55). Moreover, testosterone stimulates red cell production in males in a dose-dependent manner, especially in older men (7).
Testosterone decreases ventilation (10) and increases erythropoiesis, whereas estradiol has opposed actions (8, 19, 50). For instance, higher estrogen levels have been related to higher arterial oxygen saturation (46). This may explain the differences in ventilatory responses between sexes (11, 44). In addition, a reduction in total sleep time, longer hypoxic episodes, and increases in the respiratory disturbances index (the number of apneas and hypopneas per hour) have been observed in older men exposed to high doses of testosterone (26). Therefore, the hypothesis is that testosterone could be related to EE and in turn to CMS etiopathogeny.
Other hormones as thyroid hormones could also play a role in CMS etiopathogeny since hypothyroidism is associated with hypoventilation (29, 44). Although hypothyroidism is unlikely to cause sleep apnea (44), it would be important to determine whether thyroid hormones are different in subjects with EE in relation to those without EE at high altitude.
Pulmonary hypertension is another condition associated with CMS as a result of hypoxemia (36). Dehydroepiandrosterone sulfate (DHEAS) has been shown to activate the calcium-gated potassium channels and reduce pulmonary vascular wall remodeling and right ventricle hypertrophy as well as pulmonary hypertension induced by hypoxia (17). Consequently, it is possible that low-serum DHEAS levels may be implicated in CMS etiopathogeny associated with pulmonary hypertension at high altitudes.
For these reasons, the present study aimed to determine serum levels of testosterone under basal and human chorionic gonadotropin (hCG)-stimulated conditions and basal serum levels of thyroid hormones, DHEAS, and Epo in natives of Cerro de Pasco, Peru (4,340 m), with or without erythrocytosis (Hb >21 g/dl). Additionally, measurements of the gonadotropins (LH and FSH), the serum levels of 17α-hydroxyprogesterone, a testosterone precursor, and the serum estradiol level, a testosterone metabolite, were taken. Estradiol is a major female hormone; however, recent studies have suggested a physiological role in men (2, 23, 32, 35).
Currently, therapeutic strategies for CMS are scarce and include displacement of residence to lower altitudes, repetitive blood letting, and administration of drugs such as medroxyprogesterone, enalapril, almitrine, or acetozolamide (43). However, no pharmacological or medical treatment is completely effective (41). Therefore, it is important to study other factors that could play a role in CMS etiopathogeny.
MATERIALS AND METHODS
Subjects.
Seventy adult men aged 35–65 yr volunteered to participate in this study. Forty-two men were born at high altitude (>2,000 m) and had spent ≥30 yr living in Cerro de Pasco (4,340 m), and 28 were born in and live in Lima, Peru, at 150 m.
The study was approved by the Institutional Review Board at the Universidad Peruana Cayetano Heredia. Informed consent was obtained from each participant in the study. Men were not receiving any medication for ≥3 mo before the study, nor had they had phlebotomy during the year prior to the study. They represent a prospective cohort.
Subjects were excluded from the study if they had pathologies that could aggravate the erythrocytosis, such as smoking habit, chronic obstructive pulmonary disease, cardiovascular and renal diseases, or BMI >25, or if they had received medication in the last 3 mo.
Sample size.
Sample size was calculated with an α-error of 0.05 and a power of 80%.
Experimental protocol.
A basal venous blood sample was drawn from an antecubital vein of each subject between 0800 and 1000 by a trained professional. Immediately thereafter, 1,000 IU of hCG (Pregnyl; Organon) was intramuscularly injected into each participant. Then, venous blood samples were drawn at 6, 12, 24, 48, and 72 h after a single hCG injection.
Survey.
The sociodemographic variables were recorded through a questionnaire that included alcohol, coffee and/or tobacco consumption, and time of residence at high altitude.
BMI.
Height and weight were obtained from each subject, and BMI was calculated as kg/m2.
Serum hormone measurements.
Venous blood samples were allowed to clot and then centrifuged at 1,000 g for 10 min at room temperature to obtain serum that was immediately stored at −20°C until assayed for hormonal analysis. Basal serum values of FSH, LH, thyroid hormones, DHEAS, Epo, 17α-hydroxyprogesterone, total testosterone, and estradiol in Lima and Cerro de Pasco were evaluated. Testosterone, estradiol, and DHEAS were also measured after a stimulus with an intramuscular injection of 1,000 IU hCG (Pregnyl) at 0, 6, 12, 24, 48, and 72 h. DHEAS and DHEA are readily interconvertible in the human body. Moreover, serum dehydroepiandrosterone (DHEA) and DHEAS levels were significantly elevated after the DHEA administration in men (54).
Triiodothyronine (T3), thyroxine (T4), total testosterone, estradiol, 17α-hydroxyprogesterone, and DHEAS were measured by radioimmunoassay using commercial kits (Diagnostic Products, Los Angeles, CA). LH and FSH were measured by immunoradiometric assay using commercial kits from Diagnostic Products. The hormone labeled with 125iodine was used as radioactive marker. Erythropoietin was measured by chemiluminescence using Immunolite kits. The results are expressed as mIU/ml.
Characteristics of the assays as within-assay variation, between-assay variation, and sensitivity for each hormone measured are shown in Table 1.
Table 1.
Characteristics of the assays to serum hormone measurements
| Serum Hormone | Within-Assay Variation, % | Between-Assay Variation, % | Sensitivity |
|---|---|---|---|
| 17-OHP | 6.7 | 11 | 0.07 ng/ml |
| Erythropoietin | 5.8 | 6.3 | 1.0 mIU/ml |
| Estradiol | 7.0 | 8.1 | 8 pg/ml |
| Total T4 | 2.8 | 4.2 | 0.25 μg/dl |
| Total T3 | 5.9 | 6.6 | 7 ng/dl |
| DHEAS | 4.4 | 6.3 | 1.1 μg/dl |
| Total testosterone | 5.0 | 6.7 | 4 ng/dl |
| FSH | 2.2 | 5.7 | 0.06 mIU/ml |
| LH | 1.6 | 3.3 | 0.15 mIU/ml |
17-OHP, 17α-hydroxyprogesterone; T4, thyroxine; T3, triiodothyronine; DHEAS, dehydroepiandrosterone sulfate.
Hb measurement.
Hb concentration was measured on site with a HemoCue (Anglholm, Sweden) system. The hematocrit was measured in each sample with the microhematocrit method. The coefficient of determination between Hb measurement and hematocrit was 0.99. Data presented in this paper were based only on Hb measurements. Subjects from Cerro de Pasco were grouped according to their Hb levels: Hb >16–21 g/dl (n = 20) or Hb >21 g/dl (EE; n = 22).
Pulse oxygen saturation.
The arterial oxygen saturation was measured in the second left finger by pulse oximetry using a Nellcor N-20 oximeter (Pleasanton, CA). This equipment also provides heart rate value.
Blood lead levels.
The blood sample was obtained by vein puncture (vacutainer) and collected in tubes containing EDTA. Disposable materials were used for each participant, and they were lead free. The anodic voltimetry technique (Lead Care, Chelmstord, MA) was used to determine blood lead levels. Values were expressed as μg/dl.
Statistical analysis.
Results were recorded in a database in Excel and analyzed using the statistical package Stata (version 8.0; Stata, College Station, TX). Data are expressed as means ± SE. The homogeneity of variances has been determined with the Bartlett test. If homogenous, analysis of variance (ANOVA) test was used to determine differences among groups. If there were differences, the mean comparisons between each one of the groups were determined using the Scheffé test. The variables with no homogenous distribution were analyzed using the Kruskal-Wallis nonparametric test. The comparisons between pairs of medians were determined using the Mann-Whitney U-test.
Multiple regression analyses have also been performed. Response to hCG stimulation was assessed by two-way ANOVA. Then, differences between pairs of groups or pairs of periods after injections were assessed. A P value of <0.05 is considered to be statistically significant.
RESULTS
Basal studies.
Comparisons of basal characteristics between men at low altitude and at high altitude are in Table 2. Statistical analysis showed that men at high altitude were characterized by higher age (P < 0.01), lower BMI (P < 0.01), high Hb levels (P < 0.01), high blood lead levels (P < 0.05), low pulse oxygen saturation (Spo2; P < 0.01), high serum levels of 17α-hydroxyprogesterone (P < 0.01), testosterone (P < 0.01), estradiol (P < 0.05), and Epo (P < 0.01), and low serum levels of DHEAS (P < 0.01). Serum LH, FSH, T3, and T4 were similar at low and at high altitudes (P > 0.05).
Table 2.
Age and some physiological and hormone characteristics of adult men evaluated in Lima (150 m) and Cerro de Pasco, Peru (4,340 m)
| Variables | Low Altitude (n = 28) | High Altitude (n = 41) |
|---|---|---|
| Age, yr | 42.43±1.24 | 47.34±1.17* |
| Body mass index, kg/m2 | 28.19±0.73 | 24.33±0.44* |
| Hemoglobin, μg/dl | 14.24±0.16 | 21.14±0.39* |
| %Pulse oxygen saturation | 98.5±0.2 | 86.4±0.6* |
| Heart rate, beats/min | 73.5±1.6 | 71.3±1.5 |
| Blood lead level, μg/dl | 4.1±0.3 | 6.1±0.4** |
| LH, mIU/ml | 5.05±0.67 (4.02) | 4.05±0.39 (3.91) |
| FSH, mIU/ml | 4.63±0.46 (3.91) | 5.32±0.34 (4.99) |
| Total testosterone, ng/dl | 224.10±42.99 (103.00) | 428.52±41.2* (369.5)** |
| Estradiol, pg/ml | 27.90±3.64 (25.58) | 52.16±6.60* (34.34)** |
| 17-OHP, ng/ml | 0.27±0.07 (0.12) | 0.88±0.08* (0.75)** |
| DHEAS, μg/dl | 213.95±18.18 (201.65) | 113.92±29.6* (80.08)** |
| T3, ng/dl | 109.03±2.65 (112.10) | 100.71±3.25 (102.10) |
| T4, μg/dl | 10.12±0.33 (10.44) | 9.56±0.37 (9.96) |
| Erythropoietin, mIU/ml | 12.6±1.0 (11.8) | 31.6±3.6* (25.9) |
Data are means ± SE. DHEAS, DHEA sulphate. Low altitude: men from Lima (150 m). High altitude: men from Cerro de Pasco (4,340 m); n = no. of subjects. Medians are in parentheses.
Student's t-test, P < 0.01;
rank-sum, P < 0.05 with respect to men at low altitude.
In Table 3 are presented data of men at high altitude grouped according to Hb levels. Blood lead levels (P < 0.01) and serum testosterone levels (P < 0.05) showed a significant increase for the high-Hb group.
Table 3.
Characteristics of the variables studied in men at high altitude grouped according hemoglobin levels
| Variables | CP-1 (n = 20) | CP-2 (n = 21) |
|---|---|---|
| Age, yr | 45.10±1.68 | 49.48±1.52 |
| Body mass index, kg/m2 | 24.78±0.64 | 23.91±0.61 |
| %Pulse oxygen saturation | 87.8±0.7 | 85.2±0.9 |
| Heart rate, beats/min | 69.4±2.0 | 73.2±2.4 |
| Blood lead level, μg/dl | 5.1±0.3 | 7.1±0.5* |
| LH, mIU/ml | 3.85±0.38 (3.91) | 4.34±0.74 (3.89) |
| FSH, mIU/ml | 5.53±0.53 (5.62) | 5.11±0.43 (4.91) |
| Total testosterone, ng/dl | 328.14±53.23 (242.68) | 524.13±55.91b (467.62)** |
| Estradiol, pg/ml | 50.22±9.19 (32.95) | 53.92±9.71 (35.88) |
| 17-OHP, ng/ml | 0.77±0.10 (0.58) | 0.96±0.12 (0.77) |
| DHEAS, μg/dl | 148.95±19.11 (140.80) | 83.85±14.60b (80.08)** |
| T3, ng/dl | 98.09±3.29 (100.64) | 103.06±5.50 (104.70) |
| T4, μg/dl | 10.22±0.45 (9.99) | 8.83±0.56 (8.90) |
| Erythropoietin, mIU/ml | 27.8±3.1 (25.9) | 35.0±5.1 (26.9) |
Data are means ± SE. CP-1, men from Cerro de Pasco with hemoglobin values >16–21 g/dl (without excessive erythrocytosis); CP-2, men from Cerro de Pasco with hemoglobin >21 g/dl (excessive erythrocytosis). Medians are between parentheses.
P < 0.01;
P < 0.05 with respect to high altitude.
At HA, DHEAS levels showed a significant decrease for the high Hb group (P < 0.05). Age, BMI, Spo2, heart rate, serum estradiol, and serum 17α-hydroxyprogesterone levels were similar between the groups with EE and without EE (Table 3). The median Epo levels were similar in the groups with different Hb levels at high altitude (Table 3).
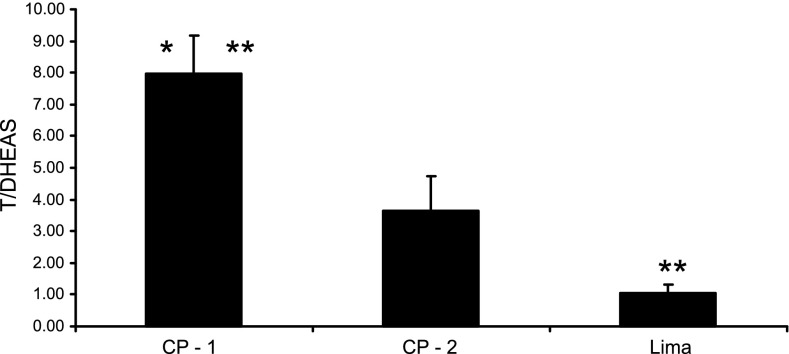
Serum testosterone/DHEAS ratio was significantly higher in Cerro de Pasco than in Lima. The ratio showed a significant increase according to Hb grouping in Cerro de Pasco (Fig. 1).
Fig. 1.
Serum testosterone/dehydroepiandrosterone sulfate (T/DHEAS) ratio in men from Lima and Cerro de Pasco, Peru, with Hb values >16–21 g/dl (CP-1) and >21 g/dl (CP-2). Data are means ± SE. *P < 0.01 with respect to Lima; **P < 0.05 with respect to values in CP-2 (men from Cerro de Pasco with Hb values >16–21 g/dl).
Sexual hormones and Epo association:.
At HA, Epo levels were not associated with serum testosterone (r2 = 0.04), estradiol (r2 = 0.002, P > 0.05), 17α-hydroxyprogesterone (r2 = 0.09, P > 0.05), or DHEAS (r2 = 0.07, P > 0.05) levels (Table 4).
Table 4.
Linear regression analysis between serum erythropoietin levels and sex hormones in adult men at high altitude
| Serum Erythropoietin Levels Vs. | Linear Regression Equation | r2 | P |
|---|---|---|---|
| 17-OHP | 20.24X + 16.83 | 0.09 | >0.05 |
| DHEAS | −0.14X + 50.95 | 0.07 | >0.05 |
| Testosterone | 0.02X + 24.21 | 0.04 | >0.05 |
| Estradiol | 0.03X + 33.09 | 0.002 | >0.05 |
r2 = Coefficient of determination.
Table 5 summarizes the regression models adjusted for age, BMI, Spo2, heart rate, and blood lead levels. Serum testosterone (P = 0.032) was directly related to increasing Hb levels at HAs, whereas DHEAS levels (P = 0.04) were inversely associated with increasing Hb levels at HAs. Epo levels in serum were not related to Hb values in men at high altitude (P = 0.57).
Table 5.
Models of multiple regression analysis for each serum hormone to assess the hemoglobin values in adult men at high altitude (4,340 m)
| Hemoglobin | Coefficient of Regression | SE | P | Confidence Interval (at 95%) |
|---|---|---|---|---|
| Serum testosterone | 0.003 | 0.001 | 0.03 | 0.0002 0.005 |
| Serum estradiol | −0.67 | 0.51 | 0.20 | −1.72 0.37 |
| Serum 17-OHP | 1.14 | 0.69 | 0.11 | −0.26 2.54 |
| DHEAS | −0.28 | 0.13 | 0.04 | −0.55 −0.01 |
| Eryhtropoietin | −0.39 | 0.68 | 0.57 | −1.79 1.00 |
| T/DHEAS ratio | 1.26 | 0.36 | 0.001 | 0.54 2.00 |
T/DHEAS, testosterone/dehydroepiandrosterone sulfate. Each line represents a model in which age, pulse oxygen saturation, body mass index, heart rate, and blood lead levels were adjusted.
hCG stimulation.
For the assessment of hormone response to hCG stimulation, serum estradiol and serum DHEAS were submitted to logarithmic transformation before analysis.
Figure 2 shows the response of men at low altitude and high altitude to 1,000 IU hCG.
Fig. 2.
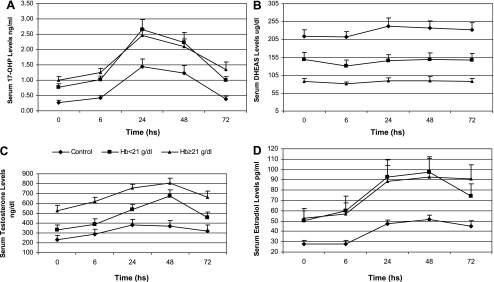
Response of men at low altitude (150 m) and at high altitude (4,340 m) to 1,000 IU of a single intramuscular injection of human chronic gonadotropin. Men from high altitudes have been grouped according to Hb levels. Men from low altitudes have been considered as control, and men from high altitudes have been grouped according to Hb levels in Hb >16–21 g/dl and Hb >21 g/dl. A: serum response of 17α-hydroxyprogesterone (17-OHP) levels. B: response of serum DHEAS. C: response of serum testosterone. D: response of serum estradiol levels. For analysis, estradiol levels and DHEAS levels were logarithmically transformed. Data are means ± SE.
Data have been assessed by two-way ANOVA to determining differences between groups and differences between times of hCG stimulus. All hormones showed differences between groups and time.
Post hoc analysis showed that serum 17α-hydroxyprogesterone values were similar at all times after hCG in both groups at high altitude. However, values were higher than at sea level at all times after hCG (P < 0.05). The three curves for DHEAS were different (P < 0.01 between men at low altitude and men with Hb >21 g/dl; P < 0.05 between men at low altitude and men with Hb >16–21 g/dl; P < 0.05 between men with Hb >16–21 g/dl and men with Hb >21 g/dl).
Testosterone response to hCG at low altitude was similar to the curve in the group at high altitude with Hb >16–21 g/dl except at 48 h (P < 0.01). Values at low altitudes were lowest at all times with respect to values in men with Hb >21 g/dl (P < 0.01). Testosterone values were higher in the group with Hb >21 g/dl than in the group with Hb >16–21 g/dl at all times except at 48 h (P > 0.05). Serum estradiol values were similar at all times after hCG in both groups at high altitude. However, values were higher than at sea level at all times after hCG (P < 0.05).
DISCUSSION
In the present study, the Spo2 value was lower at high altitude, but, as previously reported (48), it is not further decreased in the group with EE. This may indicate the existence of an additional factor to the oxygen desaturation to continue with erythropoietic stimuli to produce EE at high altitude. However, it is also possible that no significant decrease in Spo2 might be caused by too-large scattering or too-small sample.
Men with EE at high altitude have higher blood lead levels and higher testosterone levels than men at high altitude without EE. On the contrary, DHEAS levels are lower, whereas the Epo, LH, FSH, and thyroid hormones levels do not differ between groups with different hemoglobin levels at high altitude. Although hypothyroidism was associated with hypoventilation (29), the present data do not support a role for thyroid hormones in men with EE. In fact, serum thyroid hormones were similar in men with and without EE at high altitude.
The population studied at high altitude was residing in Cerro de Pasco, Peru, located at 4,340 m altitude. Cerro de Pasco is a mining city, and for that reason it is not rare to find higher blood lead levels in its population. Surprisingly, the group with highest hemoglobin levels has a higher lead blood level. However, mean blood lead levels were within normal concentrations (<25 μg/dl). Studies in lead-contaminated regions have not observed an association between blood lead and hemoglobin levels in adult women (37). The γ-aminolevulinic acid dehydratase (ALAD) seems to regulate the lead action in the organism. The carriers of the ALAD2 allele have higher blood lead levels and higher hemoglobin levels than the carriers of the ALAD1 allele (22, 47). It is possible that ALAD2 allele is more frequent in the group with EE, which would explain the presence of the highest lead and hemoglobin levels in this group compared with those without EE; however, this must be experimentally demonstrated.
Epo is known to be increased after acute exposure to high altitude (16), and it is reduced when native men at high altitude descend to sea level (40). Although the Epo is known as a hormone that regulates erythropoiesis, the studies oriented to demonstrate their participation in EE in natives at high altitude are not conclusive (25, 49, 52). According to our results, adult male residents at high altitudes greater than 4,000 m have higher Epo levels than men at low altitudes. This confirms the role of Epo in stimulating erythropoiesis in hypoxia conditions. However, when Epo is studied in subjects with different hemoglobin levels at high altitude, there is no further increase in Epo levels. This may indicate that Epo is not responsible of the EE observed at high altitude. In addition, neither systemic studies nor molecular studies of Epo and Epo receptor expression find an association between the polymorphisms related to these genes and EE (28).
Instead, our results demonstrate that basal serum testosterone, estradiol, and 17α-hydroxyprogesterone levels are higher in Cerro de Pasco than in Lima. Furthermore, in natives at high altitudes, testosterone levels are higher in men with EE than in those without EE. These results were also associated with lower DHEAS levels. In women at higher altitudes, an increase in hemoglobin levels was associated with an increase in serum testosterone (14). This may indicate that a higher bioandrogenic activity at high altitude could explain the EE observed in the Peruvian Andean population. An inverse relationship between serum testosterone levels and oxygen saturation has also been observed in obese men with obstructive sleep apnea at sea level (12). Sleep apnea is a condition associated with EE and CMS at high altitudes (36). Therefore, it is possible to expect an association between elevated serum testosterone levels with high erythropoiesis.
Previously, it has been suggested that high testosterone concentrations such as those in the upper ranges of sea level values could compromise adaptation to high altitude, particularly among older men (3). Our study demonstrates for the first time that serum testosterone levels have higher values (basal and hCG stimulated) in EE compared with men without EE at high altitudes.
Testosterone, after acting, is converted to estradiol by the aromatase enzyme. Estradiol and progesterone have been used as respiratory stimulators to reduce hypoventilation and improve erythropoiesis (44). However, testosterone may reduce their effect by downregulating estradiol and progesterone receptors (45). In that circumstance, high testosterone levels at high altitudes may blunt the estradiol and progesterone actions on ventilation favoring hypoventilation and high stimulus for erythropoiesis. Serum estradiol levels in older men are almost similar to those observed in postmenopausal women of the same age (32, 51). However, values in premenopausal women are four to five times higher than in men and postmenopausal women (32, 51).
Testosterone is produced in the Leydig cells from one of its precursors, the 17α-hydroxyprogesterone (1). The process of androgenesis indicates that 17α-hydroxyprogesterone is converted in several steps to dehydropeiandrosterone sulfate, and then after one step more it is converted to testosterone. Because 17α-hydroxyprogesterone is increased at high altitude with respect to sea level, this elevation indicates that the process of producing testosterone at high altitude is elevated since the precursor, 17α-hydroxyprogesterone, was elevated. Because serum LH level was not different between groups with increasing hemoglobin levels at high altitudes, it suggests a higher response to gonadotropin stimulus in men with EE at high altitude, as confirmed after the stimulus with 1,000 IU of hCG. In vitro studies showed that proliferation of Leydig cells and testosterone release was enhanced significantly by hypoxia. During hypoxia, administration of hCG also enhanced proliferation of Leydig cells and testosterone release (20).
DHEAS is readily convertible to DHEA (39). DHEA by action of the 3β-hydroxysteroid dehydrogenase (15) is converted to androstenedione, and this by the 17β-hydroxysteroid dehydrogenase (4) is metabolized to testosterone. Differences in testosterone/DHEAS ratio between EE and the non-EE group suggest a higher conversion rate from DHEAS to androstenedione and then to testosterone. A higher conversion from androstenedione to testosterone has been observed in women at high altitudes compared with women at low altitudes (14). It is probable that natives not adapted to live at high altitude (CMS) have higher activity of 17β-hydroxysteroid and probably 3β-hydroxysteroid, promoting a higher conversion to testosterone under basal and hCG-stimulated conditions.
At sea level, it is known that gonadotropin stimulation increases serum levels of both DHEAS and testosterone (39). Then, at high altitudes, a situation in which serum levels of DHEAS are low and serum testosterone values are high suggests a higher conversion rate from DHEAS to testosterone in this particular group.
CMS has also been associated with pulmonary hypertension (36). DHEA and DHEAS are pulmonary vasodilators, and they inhibit hypoxic-induced pulmonary hypertension (6, 17). Low serum DHEAS levels could be associated with the pulmonary hypertension observed in patients with CMS at high altitudes (36).
High DHEAS levels have been associated with coronary disease (18). The low DHEAS levels at high altitude compared with sea level could explain the low prevalence of coronary diseases at high altitude (33) and in part be protective for this pathology at high altitude.
This evidence suggests the possibility for new therapeutic strategies, including administration of DHEAS/DHEA or reducing the activity of the 17β-hydroxysteroid enzymes.
In summary, EE is associated with high serum testosterone and testosterone/DHEAS ratio. It is confirmed that Epo is responsible for increasing hemoglobin production at high altitude, but an effect on excessive erythrocytosis is discarded.
GRANTS
This study was supported by a grant from the Fogarty Program of The National Institutes of Health (NIH Research Grant No. 5-D43-TW-005746-04, funded by the Fogarty International Center, the National Institutes on Environmental Health Services, the National Institute for Occupational Safety and Health, and the Agency for Toxic Substances and Disease Registry).
Acknowledgments
The technical support from Julio Rubio is acknowledged. We also thank the volunteers who participated in the studies for their time and effort.
REFERENCES
- 1.Amory JK, Coviello AD, Page ST, Anawalt BD, Matsumoto AM, Bremner WJ. Serum 17α-hydroxyprogesterone strongly correlates with intratesticular testosterone in gonadotropin-suppressed normal men receiving various dosages of human chorionic gonadotropin. Fertil Steril 89: 380–386, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Araujo AB, Travison TG, Bhasin S, Esche GR, Williams RE, Clark RV, McKinlay JB. Association between testosterone and estradiol and age-related decline in physical function in a diverse sample of men. J Am Geriatr Soc 56: 2000–2008, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beall CM, Worthman CM, Stallings J, Strohl KP, Brittenham GM, Barragan M. Salivary testosterone concentration of Aymara men native to 3600 m. Ann Hum Biol 19: 67–78, 1992. [DOI] [PubMed] [Google Scholar]
- 4.Benghuzzi H, Mohamed A. The effects of androstenedione on renal tubule epithelial cells. Biomed Sci Instrum 43: 63–68, 2007. [PubMed] [Google Scholar]
- 5.Bernardi L, Roach RC, Keyl C, Spicuzza L, Passino C, Bonfichi M, Gamboa A, Gamboa J, Malcovati L, Schneider A, Casiraghi N, Mori A, Leon-Velarde F. Ventilation, autonomic function, sleep and erythropoietin. Chronic mountain sickness of Andean natives. Adv Exp Med Biol 543: 161–175, 2003. [PubMed] [Google Scholar]
- 6.Bonnet S, Dumas-de-La-Roque E, Bégueret H, Marthan R, Fayon M, Dos Santos P, Savineau JP, Baulieu EE. Dehydroepiandrosterone (DHEA) prevents and reverses chronic hypoxic pulmonary hypertension. Proc Natl Acad Sci USA 100: 9488–9493, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coviello AD, Kaplan B, Lakshman KM, Chen T, Singh AB, Bhasin S. Effects of graded doses of testosterone on erythropoiesis in healthy young and older men. J Clin Endocrinol Metab 93: 914–919, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cristancho E, Reyes O, Serrato M, Mora MM, Rojas JA, Robinson Y, Boning D. Arterial oxygen saturation and hemoglobin mass in postmenopausal untrained and trained altitude residents. High Alt Med Biol 8: 296–306, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Delanghe JR, Bollen M, Beullens M. Testing for recombinant erythropoietin. Am J Hematol 83: 237–241, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Favier R, Spielvogel H, Caceres E, Rodriguez A, Sempore B, Pequignot J. Differential effects of ventilatory stimulation by sex hormones and almitrine on hypoxic erythrocytosis. Pflugers Arch 434: 97–103, 1996. [DOI] [PubMed] [Google Scholar]
- 11.Fregosi RF Castrating the respiratory controller. J Physiol 561: 353, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gambineri A, Pelusi C, Pasquali R. Testosterone levels in obese male patients with obstructive sleep apnea syndrome: relation to oxygen desaturation, body weight, fat distribution and the metabolic parameters. J Endocrinol Invest 26: 493–498, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Gonzales GF Peruvian contributions to the study on human reproduction at high altitude: from the chronicles of the Spanish conquest to the present. Respir Physiol Neurobiol 158: 172–179, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Gonzales GF, Villena A. Low pulse oxygen saturation in post-menopausal women at high altitude is related to a high serum testosterone/estradiol ratio. Int J Gynaecol Obstet 71: 147–154, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Gonzales GF, Góñez C, Villena A. Adrenopause or decline of serum adrenal androgens with age in women living at sea level or at high altitude. J Endocrinol 173: 95–101, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Gunga HC, Kirsch KA, Roecker L, Kohlberg E, Tiedemann J, Steinach M, Schobersberger W. Erythropoietin regulations in humans under different environmental and experimental conditions. Respir Physiol Neurobiol 158: 287–297, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Hampl V, Bíbova J, Povýsilová V, Herget J. Dehydroepiandrosterone sulphate reduces chronic hypoxic pulmonary hypertension in rats. Eur Respir J 21: 862–865, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Hautanen A, Mänttäri M, Manninen V, Tenkanen L, Huttunen JK, Frick MH, Adlercreutz H. Adrenal androgens and testosterone as coronary risk factors in the Helsinki Heart Study. Atherosclerosis 105: 191–200, 1994. [DOI] [PubMed] [Google Scholar]
- 19.Horiguchi H, Oguma E, Kayama F. The effects of iron deficiency on estradiol-induced suppression of erythropoietin induction in rats: implications of pregnancy-related anemia. Blood 106: 67–74, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Hwang GS, Wang SW, Tseng WM, Yu CH, Wang PS. Effect of hypoxia on the release of vascular endothelial growth factor and testosterone in mouse TM3 Leydig cells. Am J Physiol Endocrinol Metab 292: E1763–E1769, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Jockenhovel F, Vogel E, Reinhardt W, Reinwein D. Effects of various modes of androgen substitution therapy on erythropoiesis. Eur J Med Res 2: 293–298, 1997. [PubMed] [Google Scholar]
- 22.Kim HS, Lee SS, Lee GS, Hwangbo Y, Ahn KD, Lee BK. The protective effect of delta-aminolevulinic acid dehydratase 1-2 and 2-2 isozymes against blood lead with higher hematologic parameters. Environ Health Perspect 112: 538–541, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lai J, Zhou D, Xia S, Shang Y, Want L, Zheng L, Zhu J. Reduced testosterone levels in males with lone atrial fibrillation. Clin Cardiol 32: 43–46, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.León-Velarde F, Ramos MA, Hernández JA, De Idiáquez D, Muñoz LS, Gaffo A, Córdova S, Durand D, Monge C. The role of menopause in the development of chronic mountain sickness. Am J Physiol Regul Integr Comp Physiol 272: R90–R94, 1997. [DOI] [PubMed] [Google Scholar]
- 25.León-Velarde F, Monge C, Vidal A, Carcagno M, Criscuolo M, Bozzini CE. Serum immunoreactive erythropoietin in high altitude natives with and without excessive erythrocytosis. Exp Hematol 19: 257–260, 1991. [PubMed] [Google Scholar]
- 26.Liu PY, Yee B, Wishart SM, Jimenez M, Jung DG, Grunstein RR, Handelsman DJ. The short-term effects of high-dose testosterone on sleep, breathing, and function in older men. J Clin Endocrinol Metab 88: 3605–3613, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Mazer NA Testosterone deficiency in women: etiologies, diagnosis, and emerging treatments. Int J Fertil Womens Med 47: 77–86, 2002. [PubMed] [Google Scholar]
- 28.Mejía O, Prchal J, León-Velarde F, Hurtado A, Stockton D. Genetic association analysis of chronic mountain sickness in an Andean high-altitude population. Haematologica 90: 13–18, 2005. [PubMed] [Google Scholar]
- 29.Misiolek M, Marek B, Namyslowski G, Scierski W, Zwirska-Korczala K, Kazmierczak-Zagorska Z, Kajdaniuk D, Misiolek H. Sleep apnea syndrome and snoring in patients with hypothyroidism with relation to overweight. J Physiol Pharmacol 58, Suppl 1: 77–85, 2007. [PubMed] [Google Scholar]
- 30.Monge MC Chronic mountain sickness. Physiol Rev 23: 166–184, 1943. [Google Scholar]
- 31.Moore LG Human genetic adaptation to high altitude. High Alt Med Biol 2: 257–279, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Ostadal B, Kolar F. Cardiac adaptation to chronic high-altitude hypoxia: beneficial and adverse effects. Respir Physiol Neurobiol 158: 224–236, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Orwoll E, Lambert LC, Marshall LM, Phipps K, Blank J, Barrett-Connor E, Cauley J, Ensrud K, Cummings S. Testosterone and estradiol among older men. J Clin Endocrinol Metab 91: 1336–1344, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Parr JH, Seed M, Godsland I, Wynn V. The effects of reverse sequential anti-androgen therapy (cyproterone acetate and ethinyl estradiol) on hematological parameters. J Endocrinol Invest 10: 237–239, 1987. [DOI] [PubMed] [Google Scholar]
- 35.Pasquier G, Rives N, Bouzouita A, Caremel R, Sibert L. [Comparison of oestradiol and testosterone levels in peripheral blood and spermatic cord blood in patients with secretory azoospermia]. Prog Urol 18: 663–668, 2008. [DOI] [PubMed] [Google Scholar]
- 36.Peñaloza D, Arias-Stella J. The heart and pulmonary circulation at high altitudes: healthy highlanders and chronic mountain sickness. Circulation 115: 1132–1146, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Rabstein S, Unfried K, Ranft U, Illig T, Kolz M, Mambetova C, Vlad M, Roman C, Weiss T, Becker D, Brüning T, Pesch B. Lack of association of delta-aminolevulinate dehydratase polymorphisms with blood lead levels and hemoglobin in Romanian women from a lead-contaminated region. J Toxicol Environ Health A 71: 716–724, 2008. [DOI] [PubMed] [Google Scholar]
- 38.Reeves JT, Leon-Velarde F. Chronic mountain sickness: recent studies of the relationship between hemoglobin concentration and oxygen transport. High Alt Med Biol 5: 147–155, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Remer T, Manz F, Pietrzik K. Re-examination of the effect of hCG on plasma levels and renal excretion of dehydroepiandrosterone sulfate in healthy males. Steroids 60: 204–209, 1995. [DOI] [PubMed] [Google Scholar]
- 40.Rice L, Ruiz W, Driscoll T, Whitley CE, Tapia R, Hachey DL, Gonzales GF, Alfrey CP. Neocytolysis on descent from altitude: a newly recognized mechanism for the control of red cell mass. Ann Intern Med 134: 652–656, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Richalet JP, Rivera-Ch M, Maignan M, Privat C, Pham I, Macarlupu JL, Petitjean O, León-Velarde F. Acetazolamide for Monge's disease: efficiency and tolerance of 6-month treatment. Am J Respir Crit Care Med 177: 1370–1376, 2008. [DOI] [PubMed] [Google Scholar]
- 42.Richmond TD, Chohan M, Barber DL. Turning cells red: signal transduction mediated by erythropoietin. Trends Cell Biol 15: 146–155, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Rivera-Ch M, León-Velarde F, Huicho L. Treatment of chronic mountain sickness: critical reappraisal of an old problem. Respir Physiol Neurobiol 158: 251–265, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Saaresranta T, Polo O. Hormones and breathing. Chest 122: 2165–2182, 2002. [DOI] [PubMed] [Google Scholar]
- 45.Saaresranta T, Polo O. Sleep-disordered breathing and hormones. Eur Respir J 22: 161–172, 2003. [DOI] [PubMed] [Google Scholar]
- 46.Saaresranta T, Polo-Kantola P, Virtanen I, Vahlberg T, Irjala K, Polo O. Menopausal estrogen therapy predicts better nocturnal oxyhemoglobin saturation. Maturitas 55: 255–263, 2006. [DOI] [PubMed] [Google Scholar]
- 47.Scinicariello F, Murray HE, Moffett DB, Abadin HG, Sexton MJ, Fowler BA. Lead and delta-aminolevulinic acid dehydratase polymorphism: where does it lead? A meta-analysis. Environ Health Perspect 115: 35–41, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spicuzza L, Casiraghi N, Gamboa A, Keyl C, Schneider A, Mori A, Leon-Velarde F, Di Maria GU, Bernardi L. Sleep-related hypoxaemia and excessive erythrocytosis in Andean high-altitude natives. Eur Respir J 23: 41–46, 2004. [DOI] [PubMed] [Google Scholar]
- 49.Schmidt W, Spielvogel H, Eckardt KU, Quintela A, Peñaloza R. Effects of chronic hypoxia and exercise on plasma erythropoietin in high-altitude residents. J Appl Physiol 74: 1874–1878, 1993. [DOI] [PubMed] [Google Scholar]
- 50.Tatsumi K, Pickett CK, Jacoby CR, Weil JV, Moore LG. Role of endogenous female hormones in hypoxic chemosensitivity. J Appl Physiol 83: 1706–1710, 1997. [DOI] [PubMed] [Google Scholar]
- 51.Waaseth M, Bakken K, Dumeaux V, Olsen KS, Rylander C, Figenschau Y, Lund E. Hormone replacement therapy use and plasma levels of sex hormones in the Norwegian Women and Cancer postgenome cohort—a cross-sectional analysis. BMC Womens Health 8: 1, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu TY Chronic mountain sickness on the Qinghai-Tibetan plateau. Chin Med J (Engl) 118: 161–168, 2005. [PubMed] [Google Scholar]
- 53.Xing G, Qualls C, Huicho L, Rivera-Ch M, Stobdan T, Slessarev M, Prisman E, Ito S, Wu H, Norboo A, Dolma D, Kunzang M, Norboo T, Gamboa JL, Claydon VE, Fisher J, Zenebe G, Gebremedhin A, Hainsworth R, Verma A, Appenzeller O. Adaptation and mal-adaptation to ambient hypoxia; Andean, Ethiopian and Himalayan patterns. PLoS ONE 3: e2342, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamada Y, Sekihara H, Omura M, Yanase T, Takayanagi R, Mune T, Yasuda K, Ishizuka T, Ueshiba H, Miyachi Y, Iwasaki T, Nakajima A, Nawata H. Changes in serum sex hormone profiles after short-term low-dose administration of dehydroepiandrosterone (DHEA) to young and elderly persons. Endocr J 54: 153–162, 2007. [DOI] [PubMed] [Google Scholar]
- 55.Zitsmann M Effects of testosterone replacement and its pharmacogenetics on physical performance and metabolism. Asian J Androl 10: 364–372, 2008. [DOI] [PubMed] [Google Scholar]