Abstract
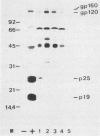
A gelatin particle agglutination assay was compared with indirect immunofluorescence by using 663 serum samples from acquired immunodeficiency syndrome and acquired immunodeficiency syndrome-related complex patients and asymptomatic male homosexuals in the United States and from hemophiliacs and healthy adult controls in Japan. The results showed that all 104 samples which were positive by indirect immunofluorescence were also positive by particle agglutination, while 5 additional samples were positive by particle agglutination only. The coincidence rate for antibody-positive and antibody-negative specimens was 99% (658 of 663) between particle agglutination and immunofluorescence. Four of the five samples which were positive by particle agglutination only were found by radioimmunoprecipitation to contain anti-env gene products of human immunodeficiency virus. Antibody titers of samples giving a positive reaction by particular agglutination varied from low (titer, 256) to remarkably high (256 X 10(5)). All specimens having particle agglutination titers of more than 10(5) were positive by immunofluorescence. A high correlation (r = 0.66) was observed between the titers of antibodies determined by particle agglutination and those determined by immunofluorescence. After fractionation of a serum sample from an individual at high risk by using high-performance liquid chromatography, it was shown that immunoglobulin M as well as immunoglobulin G human immunodeficiency virus antibody was detected by particle agglutination. Additional serum samples with a potential risk of giving false-positive results, such as heat-treated specimens, specimens containing antibodies to HLA, specimens containing auto-antibodies, and serum samples from individuals with a history of multiple blood transfusions, were shown to be clearly negative by particle agglutination.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Bedarida G., Cambiè G., D'Agostino F., Ronsivalle M. G., Berto E., Grisi M. E. HIV IgM antibodies in risk groups who are seronegative on ELISA testing. Lancet. 1986 Sep 6;2(8506):570–571. doi: 10.1016/s0140-6736(86)90133-9. [DOI] [PubMed] [Google Scholar]
- Feorino P. M., Kalyanaraman V. S., Haverkos H. W., Cabradilla C. D., Warfield D. T., Jaffe H. W., Harrison A. K., Gottlieb M. S., Goldfinger D., Chermann J. C. Lymphadenopathy associated virus infection of a blood donor--recipient pair with acquired immunodeficiency syndrome. Science. 1984 Jul 6;225(4657):69–72. doi: 10.1126/science.6328663. [DOI] [PubMed] [Google Scholar]
- Gallo R. C., Salahuddin S. Z., Popovic M., Shearer G. M., Kaplan M., Haynes B. F., Palker T. J., Redfield R., Oleske J., Safai B. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984 May 4;224(4648):500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- Gomperts E. D., Feorino P., Evatt B. L., Warfield D., Miller R., McDougal J. S. LAV/HTLV III presence in peripheral blood lymphocytes of seropositive young hemophiliacs. Blood. 1985 Jun;65(6):1549–1552. [PubMed] [Google Scholar]
- Harada S., Purtilo D. T., Koyanagi Y., Yamamoto N. Serological differences between human T-lymphotropic virus type-I (HTLV-I) and HTLV-III/LAV. Microbiol Immunol. 1986;30(6):533–544. doi: 10.1111/j.1348-0421.1986.tb02979.x. [DOI] [PubMed] [Google Scholar]
- Hinuma Y., Komoda H., Chosa T., Kondo T., Kohakura M., Takenaka T., Kikuchi M., Ichimaru M., Yunoki K., Sato I. Antibodies to adult T-cell leukemia-virus-associated antigen (ATLA) in sera from patients with ATL and controls in Japan: a nation-wide sero-epidemiologic study. Int J Cancer. 1982 Jun 15;29(6):631–635. doi: 10.1002/ijc.2910290606. [DOI] [PubMed] [Google Scholar]
- Hinuma Y., Nagata K., Hanaoka M., Nakai M., Matsumoto T., Kinoshita K. I., Shirakawa S., Miyoshi I. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6476–6480. doi: 10.1073/pnas.78.10.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M., Fujino R., Matsui T., Yoshida T., Komoda H., Imai J. A new agglutination test for serum antibodies to adult T-cell leukemia virus. Gan. 1984 Oct;75(10):845–848. [PubMed] [Google Scholar]
- Kalyanaraman V. S., Cabradilla C. D., Getchell J. P., Narayanan R., Braff E. H., Chermann J. C., Barré-Sinoussi F., Montagnier L., Spira T. J., Kaplan J. Antibodies to the core protein of lymphadenopathy-associated virus (LAV) in patients with AIDS. Science. 1984 Jul 20;225(4659):321–323. doi: 10.1126/science.6330889. [DOI] [PubMed] [Google Scholar]
- Koyanagi Y., Harada S., Yamamoto N. Establishment of a high production system for AIDS retroviruses with a human T-leukemic cell line Molt-4. Cancer Lett. 1986 Mar;30(3):299–310. doi: 10.1016/0304-3835(86)90054-6. [DOI] [PubMed] [Google Scholar]
- Levy J. A., Hoffman A. D., Kramer S. M., Landis J. A., Shimabukuro J. M., Oshiro L. S. Isolation of lymphocytopathic retroviruses from San Francisco patients with AIDS. Science. 1984 Aug 24;225(4664):840–842. doi: 10.1126/science.6206563. [DOI] [PubMed] [Google Scholar]
- Maeda Y., Furukawa M., Takehara Y., Yoshimura K., Miyamoto K., Matsuura T., Morishima Y., Tajima K., Okochi K., Hinuma Y. Prevalence of possible adult T-cell leukemia virus-carriers among volunteer blood donors in Japan: a nation-wide study. Int J Cancer. 1984 Jun 15;33(6):717–720. doi: 10.1002/ijc.2910330602. [DOI] [PubMed] [Google Scholar]
- Parry J. V., Mortimer P. P. Place of IgM antibody testing in HIV serology. Lancet. 1986 Oct 25;2(8513):979–980. doi: 10.1016/s0140-6736(86)90632-x. [DOI] [PubMed] [Google Scholar]
- Popovic M., Sarngadharan M. G., Read E., Gallo R. C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984 May 4;224(4648):497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- Sarngadharan M. G., Popovic M., Bruch L., Schüpbach J., Gallo R. C. Antibodies reactive with human T-lymphotropic retroviruses (HTLV-III) in the serum of patients with AIDS. Science. 1984 May 4;224(4648):506–508. doi: 10.1126/science.6324345. [DOI] [PubMed] [Google Scholar]
- Yamamoto N., Okada M., Koyanagi Y., Kannagi M., Hinuma Y. Transformation of human leukocytes by cocultivation with an adult T cell leukemia virus producer cell line. Science. 1982 Aug 20;217(4561):737–739. doi: 10.1126/science.6980467. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Miyoshi I., Hinuma Y. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2031–2035. doi: 10.1073/pnas.79.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T., Matsui T., Kobayashi S., Harada S., Kurimura T., Hinuma Y., Yamamoto N. A novel agglutination test for the human immunodeficiency virus antibody: a comparative study with enzyme-linked immunosorbent assay and immunofluorescence. Jpn J Cancer Res. 1986 Dec;77(12):1211–1213. [PubMed] [Google Scholar]