Abstract
Myofibrillar proteins must be removed from the myofibril before they can be turned over metabolically in functioning muscle cells. It is uncertain how this removal is accomplished without disruption of the contractile function of the myofibril. It has been proposed that the calpains could remove the outer layer of filaments from myofibrils as a first step in myofibrillar protein turnover. Several studies have found that myofilaments can be removed from myofibrils by trituration in the presence of ATP. These easily releasable myofilaments (ERMs) were proposed to be intermediates in myofibrillar protein turnover. It was unclear, however, whether the ERMs were an identifiable entity in muscle or whether additional trituration would remove more myofilaments until the myofibril was gone and whether calpains could release ERMs from intact myofibrils. The present study shows that few ERMs could be obtained from the residue after the first removal of ERMs, and the yield of ERMs from well-washed myofibrils was reduced, probably because some ERMs had been removed by the washing process. Mild calpain treatment of myofibrils released filaments that had a polypeptide composition and were ultrastructurally similar to ERMs. The yield of calpain-released ERMs was two- to threefold greater than the normal yield. Hence, ERMs are an identifiable entity in myofibrils, and calpain releases filaments that are similar to ERMs. The role of ERMs in myofibrillar protein turnover is unclear, because only filaments on the surface of the myofibril would turn over, and changes in myofibrillar protein isoforms during development could not occur via the ERM mechanism.
Keywords: calpain, muscle, proteasome
similar to proteins in other cells, proteins in muscle cells turn over metabolically. The rate of this turnover varies considerably, depending on the physiological state of the animal (12, 13). During periods of muscle atrophy, the rate of muscle protein degradation exceeds the rate of muscle protein synthesis. An increased rate of muscle protein degradation is associated with the loss of muscle mass during cachexia, sepsis, prolonged bed rest, unweighting and muscle disuse, sarcopenia, and muscular dystrophy. These events have serious clinical consequences, because they result in muscle weakness and fatigue, increased recovery time, and risk of thromboembolic complications and, for some of the muscular dystrophies, lead eventually to death. Muscle protein turnover also is important to agriculture, because it directly affects the rate of muscle growth in animals, and to athletes, who wish to maximize muscle strength and/or performance. Many studies of muscle protein turnover have used inhibitors and measurements of effects of these inhibitors on the rate of loss of muscle mass. These studies have produced different results, probably because not all types of muscular atrophy involve the same biochemical pathways and also because different classes of muscle protein may be involved to different extents in loss of muscle mass. Therefore, a more detailed analysis of muscle protein turnover is warranted.
Ultimately, muscle protein degradation is the result of proteolytic enzyme activity. Skeletal muscle contains four proteolytic systems that are present in quantities that could be sufficient for metabolic turnover of muscle proteins: 1) the lysosomal system, 2) the caspase system, 3) the proteasomal system, and 4) the calpain system (18). Skeletal muscle tissue is composed of three classes of proteins based on their solubility and function: 1) the cytoplasmic or sarcoplasmic proteins, which constitute ∼30–35% by weight of total muscle protein, are soluble at low (<0.05 M) ionic strength, and contain most of the metabolic enzymes; there are likely >100–200 different proteins in this class; 2) the myofibrillar proteins, which constitute ∼55–60% of total muscle protein by weight, form the contractile structures or myofibrils in striated muscle cells, and require relatively high (>0.2 M) ionic strength for their solubilization; there are ∼20 different proteins in this class; and 3) the stroma proteins, which constitute ∼10–15% by weight of total muscle protein, are insoluble in neutral aqueous solvents, are principally extracellular, and are composed of collagen and extracellular matrix proteins in addition to some integral membrane proteins (17).
Proteins in these three classes almost certainly are not degraded by the same mechanism(s), and the rate of their degradation likely is regulated by different signaling pathways. Moreover, none of the major classes of proteolytic enzymes degrade proteins to amino acids; even the proteasome degrades proteins to small (4- to 12-amino acid) peptides, and action of a variety of peptidases in muscle cells is required to degrade muscle proteins to amino acids. Degradation of many of the stroma proteins requires extracellular proteases, whereas degradation of the sarcoplasmic and myofibrillar proteins requires intracellular proteolytic pathways. The sarcoplasmic proteins can be degraded directly by the proteasome after their ubiquitination, but the major class of muscle proteins, the myofibrillar proteins, is not degraded by the proteasome when they are assembled in the myofibrillar structure (8, 24, 36).
Consequently, measurements of muscle protein turnover using release of amino acids from muscle tissue or loss of muscle weight will measure degradation of all three of these classes of proteins, with the relative contribution from each class depending on the conditions of the experiment. Release of an amino acid such as tyrosine from muscle will measure the activity of the major class of proteolytic enzymes or the peptidase required to produce free amino acids that is rate limiting. Although the sarcoplasmic proteins turn over more rapidly than the myofibrillar or stroma proteins, the fact that the latter two classes constitute collectively 65–75% of total muscle protein indicates that, in some instances, the amino acids released or the loss of muscle weight may originate, to a considerable extent, from degradation of myofibrillar and/or stroma proteins. Hence, careful studies should distinguish between the three classes of muscle proteins in the investigation of muscle protein turnover by using measurements that identify which class is contributing or by acknowledging that the measurements may include contributions from one or more of the three classes of muscle proteins. This study describes investigations that focus on the mechanism underlying turnover of the myofibrillar proteins in skeletal muscle.
Because nearly all myofibrillar proteins in a muscle cell are assembled in the form of a myofibril and because the structural integrity of this myofibril must be maintained to preserve the contractile function of the muscle cell, the myofibrillar proteins must be disassembled from the myofibril without disrupting the contractile ability of the myofibril before they can be degraded proteolytically (18). It was proposed over 30 years ago that the calpains might be involved in this disassembly by selectively releasing filaments from the surface of the myofibril (6). At about this same time, it was discovered that skeletal muscle contains a small amount of myofilaments that can be released from myofibrils by gentle agitation in an ATP-containing solution (11, 44). These easily releasable myofilaments (ERMs) lacked α-actinin (1, 2, 44) but contained myosin and actin, as would be predicted if they had been released from the surface of the myofibril by the calpains. Incubation of muscle with leupeptin, a compound that inhibits activity of calpain and other proteases, decreased the yield of ERMs, whereas incubation with a Ca2+ ionophore, A23187, increased the yield of ERMs, suggesting that proteolytic, specifically calpain, activity was involved in their release (44). Moreover, the yield of ERMs was increased during periods of muscle atrophy induced by fasting or corticosterone treatment (5) or sepsis (46). The results suggested that the ERMs might be important intermediates in metabolic turnover of myofibrillar proteins.
It remains unclear, however, whether the ERMs are an identifiable entity in muscle cells or whether agitation in the presence of ATP simply shears the outer layer of thick and thin filaments from the surface of the myofibril and additional agitation of the myofibrils remaining after removal of the ERMs would shear off a second layer of filaments. Furthermore, it was not demonstrated that treatment of myofibrils with calpain would release ERMs from these myofibrils that were identical to those obtained by agitation. Therefore, we have attempted to prepare ERMs from the myofibril residue remaining after ERM removal and have examined the yield and nature of the filaments released from purified myofibrils during incubation with purified calpain. The results indicate that a very small amount of ERMs can be obtained from the myofibrils remaining after the initial ERM removal, showing that the ERMs are identifiable entities in muscle cells and that the filaments released from myofibrils during incubation with purified calpain are biochemically and ultrastructurally similar to the ERMs obtained by agitation.
MATERIALS AND METHODS
Materials.
Acrylamide (99.9%) was obtained from ICN Biomedicals (Aurora, OH); bisacrylamide (99.99%) from Swartz/Mann Biotech (Cleveland, OH); SDS (99%) from Bio-Rad (Hercules, CA); Tris (ultrapure, 99.8%) and 2-(N-morpholino)ethane sulfonic acid (free acid) from Mallinckrodt Baker (Phillipsburg, NJ); EDTA (free acid, 99%) from BMD Biosciences (Gibbstown, NJ); 2-mercaptoethanol, fluorescein isothiocyanate, and the protease inhibitors (except E-64) used in calpain-homogenizing buffers (40) from Sigma Chemical (St. Louis, MO); E-64 from Peptides International (Louisville, KY); and BODIPY-FL (catalog no. D-2184) from Invitrogen (Carlsbad, CA).
All other chemicals were reagent grade or purer. Unless specified otherwise, all proteins were prepared at 2–4°C with use of precooled instruments and solutions, and all experiments used doubly deionized water that had been passed through a filter to remove organic material and then through a 0.45-μm filter to remove pyrogens.
Animals and muscle samples.
Use of animals and animal tissues in these experiments was approved by the University of Arizona Institutional Animal Care and Use Committee. Myofibrils and ERMs were prepared from bovine skeletal muscle or rat skeletal muscle. Bovine muscle was removed from 400- to 800-kg animals within 30 min after exsanguination, placed on ice, and transported to the laboratory. Muscle was obtained from 9- to 12-mo-old Sprague-Dawley rats within 15 min after exsanguination and used to prepare ERMs. All procedures were carried out in a cold (2–4°C) room with use of precooled instruments.
Preparation of ERMs.
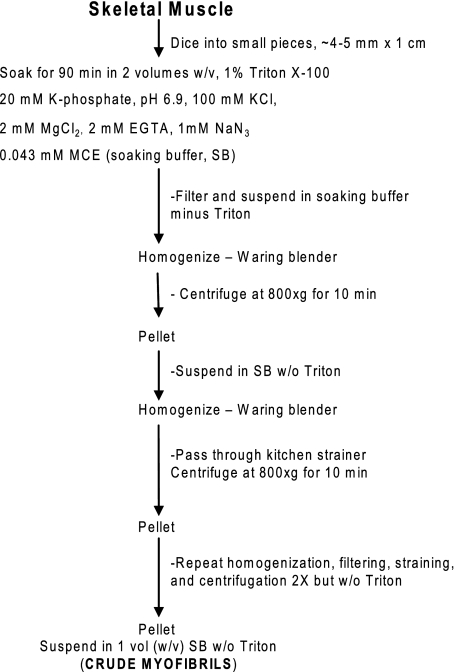
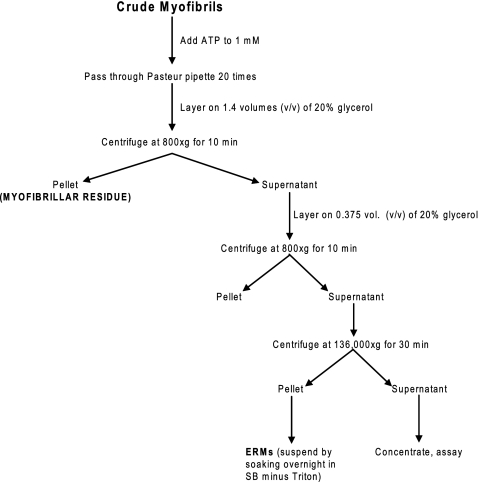
ERMs were prepared from rat hindlimb and back muscle or extensor digitorum longus (EDL) or soleus muscle or bovine diaphragm muscle according to a procedure adapted from Etlinger et al. (10, 11) and van der Westhuyzen et al. (44). The procedure is summarized in Figs. 1 and 2. Briefly, crude (i.e., not thoroughly washed) myofibrils were prepared as outlined in Fig. 1. A scalpel was used to dice muscle into small pieces, which were placed for 90 min in a “soaking buffer” containing 1% Triton X-100 to solubilize the muscle cell membranes. The buffer was changed twice during the 90-min soaking period. The diced, “soaked” muscle pieces were homogenized in soaking buffer without Triton X-100 and then washed four times with soaking buffer without Triton X-100. After the second, third, and fourth washes, the homogenate was strained through a nylon kitchen strainer. The washed suspension was called “crude myofibrils” (Fig. 1). Dicing, rather than grinding, was necessary to preserve muscle structure sufficient to yield filaments. ATP was added to the crude myofibrils to a final concentration of 1 mM, and the ATP-myofibril suspension was triturated 20 times by use of a Pasteur pipette (Fig. 2). ATP evidently dissociates the actin-myosin interaction and facilitates release of filaments that are no longer attached to the Z disk. Because the ERMs are smaller than myofibrils, they can be separated from the residual myofibrils by sedimentation of the myofibrils through a 20% glycerol solution. The myofibrillar pellet that sedimented through the 20% glycerol was called the “myofibrillar residue” (Fig. 2). The ERMs in the supernatant were purified further by sedimentation of any remaining residual myofibrils through 20% glycerol, then the ERMs were collected by sedimentation of the supernatant at 136,000 gmax for 30 min (Fig. 2).
Fig. 1.
Preparation of crude myofibrils [1st step in preparation of easily releasable myofilaments (ERMs)]. MCE, 2-mercaptoethanol.
Fig. 2.
Preparation of ERMs from crude myofibrils.
Other procedures.
Thoroughly washed myofibrils were prepared according to Goll et al. (20); this procedure involves washing the myofibrils twice with 1% Triton X-100 to remove all membranes in addition to 10 washes with dilute salt solutions, so the myofibrils are well washed. Both μ- and m-calpain were prepared from human placenta (42) or bovine skeletal muscle (40). Protein concentration was determined using the Coomassie Brilliant Blue G250 assay, as described by Bradford (4), or the biuret assay (21), as modified by Robson et al. (33); the bovine serum albumin used to prepare calibration curves was calibrated by Kjeldahl analysis. The FITC-casein and the BODIPY-FL-casein used for calpain assays were prepared as described by Wolfe et al. (47) and Thompson et al. (41), respectively. SDS-PAGE was done as described by Laemmli (26) using minigels (47). For Western blotting, the procedure described by Towbin et al. (43) and a semidry transfer procedure described by Taylor et al. (39) were used. Ratios of actin to α-actinin were obtained using a bioimaging device (Epi-Chemi II, UVP, Upland, CA). Measurements were made on the same lane of SDS gels, so differences in protein loading and staining did not affect the results.
RESULTS
Properties and yields of ERMs.
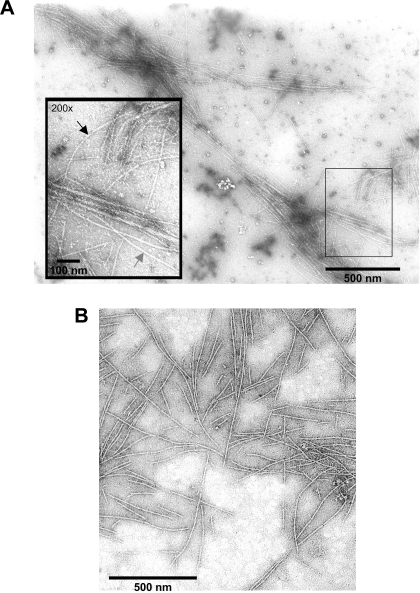
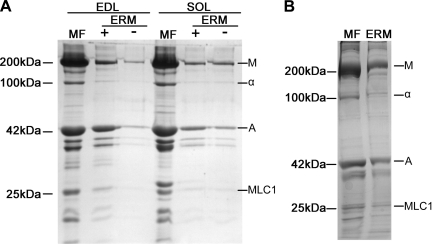
ERMs prepared from bovine diaphragm muscle (Fig. 3A) or rat back and hindlimb muscle (Fig. 3B) have the same appearance as ERMs prepared from rabbit (10, 11) or bovine (31) muscle. The diameter of thin filaments (8 nm) is slightly smaller than the diameter of thick filaments (15 nm). The ERMs would be expected to contain all the proteins that are associated with the thick or thin filament in the myofibril but should not contain the proteins that are located in the Z disk. SDS-PAGE shows that ERMs from rat (Fig. 4A) and bovine (Fig. 4B) skeletal muscle are composed primarily of myosin and actin with very little α-actinin, the principal Z disk protein. Actin constitutes ∼17–24% and α-actinin ∼2–3% of myofibrillar protein by weight (48), so a ratio of ∼0.083–0.125 would be expected for skeletal muscle myofibrils.
Fig. 3.
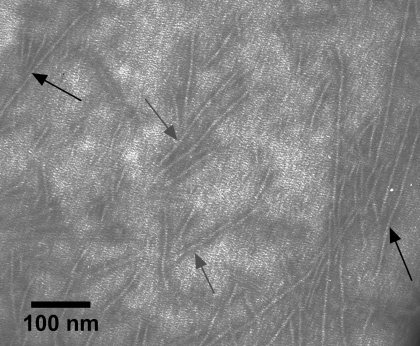
Electron micrographs of ERMs from rat skeletal muscle (A) and bovine diaphragm muscle (B). In magnified inset in A, gray arrow indicates a thick filament and black arrow indicates a thin filament.
Fig. 4.
SDS polyacrylamide gels comparing polypeptide composition of myofibrils and ERMs. A: myofibrils (MF) and ERMs prepared from rat extensor digitorum longus (EDL) and soleus muscle with (+) and without (−) use of ATP during trituration (see Fig. 2). Protein loaded from left to right: 10 μg (lane 1), 4.9 μg (lane 2), 0.33 μg (lane 3), 10 μg (lane 4), 1.07 μg (lane 5), and 1.76 μg (lane 6). Ratios of α-actinin to actin for myofibril and ERM (+) lanes are as follows: 0.0993 (lane 1), 0.0065 (lane 2), 0.0982 (lane 4), and 0.0013 (lane 5). Polypeptide composition of ERMs obtained in the presence or the absence of ATP was identical, although ∼50% as much ERM protein was obtained in the absence of ATP as in the presence of ATP. Low yield of ERMs in the absence of ATP resulted in low protein loads for ERM (−) lanes. All gels in A were 8% polyacrylamide. B: myofibrils and ERMs from bovine diaphragm muscle. Protein was loaded as follows: 8.0 μg for myofibrils and 3.0 μg for ERMs. Ratio of α-actinin to actin: 0.108 for myofibrils and 0.0046 for ERMs. Both gels in B were an 8–16% polyacrylamide gradient. Polypeptide composition of myofibrils and ERMs from rat and bovine muscle is similar, except for lack of α-actinin in ERMs, indicating that they do not have Z disk proteins. Migration rates of polypeptides having a designated molecular weight are shown at left. Major myofibril polypeptides are labeled on right: M, myosin heavy chain, α, α-actinin, A, actin; MLC1, myosin light chain 1. Analysis of different lanes showed that α-actinin-to-actin ratio in myofibrils from rat EDL and rat soleus muscle was 0.099 and 0.098, respectively, whereas α-actinin-to-actin ratio in ERMs from rat EDL and soleus muscle was 0.0065 and 0.0013, respectively.
Analysis of the α-actinin-to-actin ratios in well-washed bovine skeletal muscle myofibrils and in ERMs prepared from bovine diaphragm muscle showed that the α-actinin-to-actin ratios in these samples were 0.118 ± 0.006 (mean ± SE, n = 10) for washed myofibrils, 0.119 ± 0.002 (n = 5) for crude myofibrils, and 0.0111 ± 0.0026 (n = 5) for ERMs (Table 1). The ERMs contain tropomyosin and troponin and the myosin light chains, in addition to nebulin and titin (upper part of gels in Fig. 4); the latter two proteins would be expected to remain with the ERMs, because they are associated with the thin, actin filament (nebulin) and thick, myosin filament (titin).
Table 1.
α-Actinin-to-actin ratios in SDS-polyacrylamide gels of myofibrils and ERMs
| Tissue Source | n | Myofibrils | ERMs |
|---|---|---|---|
| Washed myofibrils | 10 | 0.118±0.006 | |
| Crude myofibrils | 5 | 0.119±0.002 | 0.0111±0.0026 |
Values are means ± SE. ERMs, easily releasable myofilaments.
The amount of ERMs obtained from two rat muscles and from bovine diaphragm muscle was <0.2% of total myofibrillar protein (Table 2). These yields are considerably lower than the ≤3% obtained from rabbit skeletal muscle by van der Westhuyzen and co-workers (44). It is unclear whether the difference is due to the different species used in the two studies (rabbit muscle in the study of van der Westhuyzen et al. and rat and bovine muscle in the present study) or to the method used to calculate yields of ERMs. We calculated yields on the basis of the protein in the original myofibrillar fraction (crude and residual myofibrils; Fig. 1). van der Westhuyzen et al. (44) evidently calculated yield as follows: ERM protein ÷ ERM protein + myofibrillar residue protein (Fig. 1). We noticed that an appreciable amount of protein was lost during trituration and centrifugation through 20% glycerol (Fig. 2), possibly because of incomplete sedimentation through 20% glycerol or because some myofibrils adhered to the Pasteur pipette or the walls of the centrifuge tube. If van der Westhuyzen et al. also lost myofibrils at this step in the process, the amount of ERM + myofibrillar protein residue would be less than the amount of myofibrillar protein that we used and would result in greater ERM yields. Rat muscle yielded almost three to four times more ERMs than bovine muscle (Table 2), so some species differences do exist. We were interested in learning whether muscles could be frozen and stored before ERM preparation, because only small amounts of muscle can be processed at one time. Freezing (−80°C) the muscle before preparation of crude myofibrils (Fig. 1), however, decreased the yield of ERMs to 15–20% of the original yield (Table 2). We did not attempt to store the crude myofibrils in 20–30% glycerol, so we do not know whether ERMs could be prepared from crude myofibrils stored in glycerol.
Table 2.
Yields of ERMs from different starting sources
|
Yield of ERMs, mg protein/100 mg starting myofibrillar protein |
||
|---|---|---|
| +ATP | −ATP | |
| Rat EDL | 0.287±0.159 (3) | 0.137±0.092 (3) |
| Rat soleus | 0.193±0.077 (3) | 0.203±0.099 (3) |
| Rat EDL after −80°C | 0.016±0.012 (3) | 0.053±0.007 (3) |
| Rat soleus after −80°C | 0.030±0.010 (3) | 0.057±0.023 (3) |
| Crude bovine myofibrils | 0.0538±0.0068 (4) | ND |
| Residual bovine myofibrils | 0.0108±0.0053 (4) | ND |
| Washed bovine myofibrils | 0.0205±0.0020 (6) | ND |
| Residual myofibrils from washed bovine myofibrils | 0.0030±0.0017 (5) | ND |
Values are means ± SE of the number of measurements indicated in parentheses. Starting material was as follows: crude myofibrils (Fig. 1) for rat extensor digitorum longus (EDL) and soleus and crude bovine myofibrils, residual bovine myofibrils, well-washed myofibrils prepared according to Goll et al. (20), and residue (Fig. 1) remaining after extraction of ERMs from well-washed myofibrils. Rat EDL and soleus after −80°C indicates storage for ∼40–45 days. ND, not determined.
As discussed in the introduction, it is important to know whether the ERMs are identifiable entities in muscle or whether they are products of shearing myofibrils so that the outer layer of filaments is removed. Therefore, we prepared ERMs from the residual myofibrils (Fig. 2) and from myofibrils that had been washed 12 times (20), a procedure that may remove some of the “easily releasable” myofilaments from the surface of the myofibril. The yield of ERMs from the residual myofibril fraction was only ∼20% of that obtained from crude myofibrils, and the yield of ERMs from washed myofibrils was only 35–40% of that obtained from crude myofibrils (Table 2). Moreover, no ERMs could be obtained from the residue remaining after a second extraction of ERMs (results not shown in Table 2) or from the residue remaining after extraction of ERMs from well-washed myofibrils (0.0030%; Table 2). The results indicate that skeletal muscle contains a small fraction of myofibrillar proteins that can be released from myofibrils by agitation in the presence of ATP and that, once removed, additional myofilaments are not removed by the same gentle agitation.
Release of ERMs by the calpains.
The calpains selectively remove Z disks from myofibrils (6, 7), and it has been proposed that the calpains might catalyze the first step in myofibrillar protein turnover by removing the outer layer of myofilaments from intact myofibrils (6). Therefore, we incubated myofibrils from rat or bovine muscle with purified μ- or purified m-calpain to determine whether these proteases would catalyze the release of ERMs. It was necessary to test several different ratios of calpain to myofibrillar protein before we obtained conditions that did not result in complete destruction of the myofibril. Finally, we incubated the myofibrils in 1:5,000–10,000 (wt/wt) calpain-myofibrillar protein for 5–30 min at 25°C. This treatment resulted in a 10- to 20-fold increase in ERM yield (Table 3). The ERMs obtained by calpain treatment appeared identical ultrastructurally in electron micrographs to the ERMs obtained by the usual procedure (Fig. 5), and their polypeptide composition was the same as evaluated by SDS-PAGE as that of the ERMs obtained in the usual manner (Fig. 6). The α-actinin-to-actin ratios of the myofibrils and calpain-released ERMs in Fig. 6 were 0.109 and 0.019, respectively.
Table 3.
Yield of ERMs after calpain treatment
|
Yield of ERMs, mg protein/100 mg starting myofibrillar protein |
||
|---|---|---|
| +Calpain | −Calpain | |
| Bovine diaphragm muscle | 1.05±0.065 (2) | 0.17 (1) |
| Rat back and hindlimb muscle | 0.99 (1) | ND |
Values are results from 1 or 2 (means ± SE) measurements, as indicated in parentheses. Starting material was crude myofibrils (Fig. 1) that were treated with purified calpain.
Fig. 5.
Electron micrograph of ERMs released by m-calpain from rat skeletal muscle myofibrils prepared from rat back and hindlimb muscle. Myofibrils were treated with m-calpain purified from bovine skeletal muscle for 30 min at 22°C and m-calpain protein-to-myofibrillar protein ratio of 1:5,000. ERMs from the incubation were prepared as described in Fig. 2. Gray arrows, thick filaments; black arrows, thin filaments.
Fig. 6.
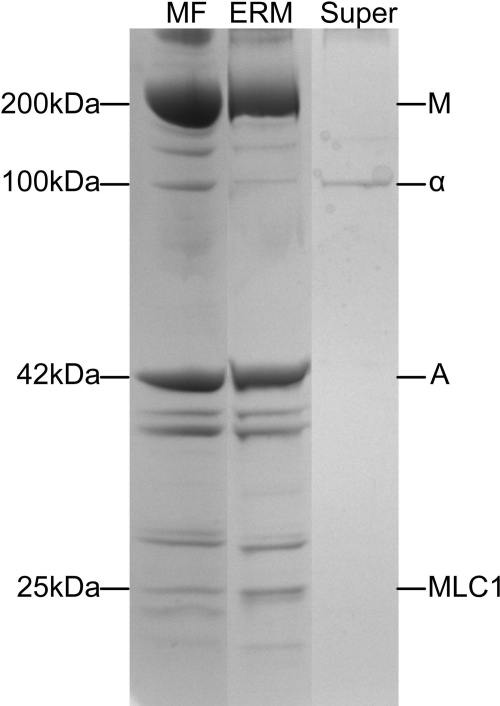
SDS polyacrylamide gels comparing polypeptide composition of rat skeletal muscle myofibrils, m-calpain-released ERMs, and the 13,600 gmax supernatant (Super) remaining after sedimentation of ERMs (see Fig 2). Myofibrils were treated with m-calpain purified from bovine skeletal muscle for 30 min at 22°C and m-calpain protein-to-myofibrillar protein of 1:5,000. ERMs from the incubation were prepared as described in Fig. 2. Ratio of α-actinin to actin is 0.109 for MF lane and 0.019 for ERM lane. ERMs released by calpain are the same ERMs shown in the electron micrographs in Fig. 5; they have the same polypeptide composition as ERMs prepared from untreated muscle (Fig. 4), indicating that calpain-released ERMs are identical to ERMs in muscle. Band at 100 kDa in Super lane is α-actinin that is released from myofibrils by the calpains (16). Gels are 8–16% polyacrylamide gradient, and each lane was loaded with 10 μg of protein. Migration rates of polypeptides with a designated molecular weight are shown at left. Major myofibril polypeptides are labeled at right: M, myosin heavy chain; α, α-actinin; A, actin; MLC1, myosin light chain 1.
DISCUSSION
The results of this study show that ERMs exist as an identifiable entity in striated muscle, at least from the two species we studied, and that ERMs can be released from skeletal muscle myofibrils by calpain. Therefore, the calpains can release ERMs from myofibrils, as first proposed over 30 years ago (6). It remains unclear, however, whether or how ERMs function in myofibrillar protein turnover. A number of studies have shown that ERMs in muscle increase during periods of rapid muscle wasting, such as in diabetes (1), starvation (5), cachexia (22), and sepsis (45, 46), and during corticosterone treatment (5, 27). It is not known whether ERMs are involved in myofibrillar protein turnover during sarcopenia (9) or during normal or exercise-induced skeletal muscle growth. If myofibrillar proteins turn over solely through an ERM pathway, the filaments on the surface of the myofibril would turn over regularly, but those in the interior of the myofibril would turn over slowly if at all. It is unclear how turnover that is restricted to or occurs primarily at the surface of the myofibril could account for the exchange of different isoforms of the contractile proteins during development. Myosin, for example, undergoes nearly a complete change in mammalian muscle from an embryonic isoform to the four isoforms that are found in myofibrils from mature animals. Moreover, myosin isoform content can change in mature muscle in response to physiological demand. It is difficult to envision how such exchange could occur if myofibrillar protein turnover were restricted solely to release and degradation of ERMs.
Several reports in the 1980s and early 1990s indicated that individual proteins in myofibrils could exchange with their counterparts in the cell cytoplasm (28, 34; see Ref. 18 for review). The significance of these results, however, is unclear, because it was not demonstrated that the molecules in the cytoplasm actually exchanged with those in the myofibril; in many instances, the results were consistent with a simple adsorption of the molecules in the solution/cytoplasm onto the surface of the myofibril. A recent report (38) found that 15% of the α-actinin in rabbit soleus myofibrils exchanged after 8 h of incubation in a solution containing labeled α-actinin. There was no net increase in the α-actinin content of the myofibrils, suggesting that an exchange had occurred, but the location of the newly incorporated α-actinin was not determined (i.e., were the added molecules all on the surface of the Z disk?). In studies of muscle contraction, skinned muscle fibers have routinely been incubated with labeled myosin light chains or troponin C, and incorporation of the label into the myofibril has been sufficient to allow spectroscopic analysis of contraction of the fibers. Hence, the labeled molecules were incorporated, and their incorporation did not alter the contractile function of the myofibril. Troponin C and myosin light chains are small molecules, however, and it is not clear whether the other larger contractile proteins can penetrate the myofibrillar lattice to exchange with molecules in the interior of the myofibril.
Spacing in the lattice structure of skeletal muscle myofibrils varies with the state of contraction: 20–50 nm between thick filaments (3), ∼10–20 nm between thick and thin filaments in the overlap area (32), and ∼20–25 nm between thin filaments as they enter the Z disk (15). The lattice spacing in the Z disk is less than that in the I band area: 17–20 nm at the edge of the Z disk to less than that in the interior of the Z disk, where α-actinin filaments exist. Its crystallographic structure shows that troponin C is an elongated molecule ∼7.5 nm long and ∼2.5 nm diameter at the two globular heads (35); in solution, this molecule has a radius of gyration of 2.3 nm (23). Hence, as the studies of muscle contraction involving labeled troponin C that had been incorporated into the myofibril demonstrate, troponin C can easily penetrate the myofibrillar lattice. It seems likely that troponin I and troponin T can also penetrate the myofibrillar lattice, at least in the I band area.
Tropomyosin is an elongated (∼42-nm-long) rod; its shape would likely slow, but not completely prevent, its access to the interior of the myofibril. Actin monomers (∼5.5 nm diameter) can also easily diffuse into and out of the myofibrillar lattice, although the effect of loss of an actin monomer on the function of the actin filament is unclear. The other major proteins in the myofibril are much larger, and it is unclear whether they could penetrate the myofibrillar lattice. The α-actinin molecule is a prolate ellipsoid with a Stokes radius of 7.69 nm and dimensions of ∼4 × 50 nm (37), so its diffusion into the Z disk would likely be slow.
Because the myosin molecule is a ∼160- to 165-nm-long rod with a calculated Stokes radius of 17.6 nm (37), its diffusion into and out of the myofibrillar lattice would be difficult. The myosin molecule contains four small (16–25 kDa) subunits, which, as indicated previously, can be exchanged with little or no effect on the contractile function of the sarcomere. The two large heavy chains in the myosin molecule, however, are each 200 kDa, and even if they were in an unordered random coil conformation, their diffusion into the interior of the myofibril would be slow. Goldfine et al. (14) found that individual myosin subunits could be incorporated into thick filaments, indicating that the separated myosin subunits are capable of independent incorporation into thick filaments. These studies, however, used myofibrils or native or synthetic thick filaments in an in vitro system, and it is not known whether the subunits were incorporated into functioning myosin molecules in the interior of the thick filaments or were simply adsorbed onto the surface. The two other major proteins in myofibrils, nebulin and titin, are very large (600–900 and 2,500–3,000 kDa, respectively) polypeptides, and it seems very unlikely that these two polypeptides could access the interior of the myofibrillar lattice.
Several studies of the rates of diffusion of proteins in living striated muscle cells have shown that the rate of diffusion of small proteins such as myoglobin (17 kDa, 3.5 nm diameter) is approximately 1/10th of the rate of diffusion of these proteins in dilute solutions (29). The rate of diffusion decreases rapidly with increasing size of the protein (30), so that diffusion of catalase (10.5 nm) in a living muscle cell is 1/20th of its diffusion in water, diffusion of ferritin (12.2 nm) is 1/50th of its diffusion in water, and there is no measurable diffusion of earthworm hemoglobin (∼30 nm). Contraction did not seem to alter the rates of diffusion of these proteins (30). It was concluded that the “molecular sieving” effect of the myofibrillar lattice progressively slows the rate of diffusion of intracellular proteins when their diameter increases to >10 nm.
It has been assumed that ERMs are released from the surface of the myofibril, and the possibility that the calpains could release myofilaments from the interior of the myofibril has not been discussed. The calpain molecule is a prolate ellipsoid with dimensions of ∼10 × 6 × 5 nm (19) and a Stokes radius of 4 nm. The calpains are concentrated in the I band and Z disk areas of the myofibril (25), and it seems likely that the calpains could penetrate the myofibrillar lattice and cleave the same sites in the interior of the myofibril as they have been proposed to cleave on the surface. It seems very unlikely, however, that any thick and thin filaments released in the interior of a myofibril could diffuse out, even if they were released by the calpains. The effect of such interior cleavages on the function of the myofibril is unclear.
In summary, the mechanism used by cells to turn over myofibrillar proteins remains unclear. It is possible that ERMs are used to turn over myofibrillar proteins during periods of rapid muscle atrophy, but it is less certain whether they are the major mechanism used to turn over myofibrillar proteins during development and perhaps also during sarcopenia. Exchange of myofibrillar proteins in the cell cytoplasm with those in the myofibrillar structure offers an attractive alternative to the ERM pathway, but the lattice spacing of the myofibril and the effects of the cell cytoplasm on rates of diffusion argue against direct exchange, at least for >15- to 20-nm proteins. Careful studies showing whether myofibrillar proteins can penetrate and are incorporated into the myofibril in the interior of the fibril are needed to show whether exchange is a route used to turn over myofibrillar proteins.
GRANTS
This work was supported by US Department of Agriculture National Research Initiative Competitive Grant 2005-35206-15268, Muscular Dystrophy Association Grant 4133, National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant AR-52108-02, and Arizona Agriculture Experiment Station Project 28, a contributing project to US Department of Agriculture Regional Research Project NC-1131.
Acknowledgments
We thank Drs. John A. Marchello and Hamdi Ahmad and their associates at the University Livestock and Meats Complex for assistance in obtaining the bovine diaphragm muscle and Dr. Robert Rhoads and Mark Morales for assistance in obtaining the rat muscle samples. The assistance of David L. Bentley was essential for obtaining electron micrographs of ERMs.
Present address of G. Neti: Department of Zoology, Dr. B. R. Ambedker Open University, Road No. 46, Jubilee Hills, Hyderabad, India 500033.
REFERENCES
- 1.Belcastro AN, Machan C, Gilchrist JS. Diabetes enhances calpain degradation of cardiac myofibrils and easily releasable myofilaments. In: The Diabetic Heart, edited by M Nagano and NS Dhalla. New York: Raven, 1991, p. 301–310.
- 2.Belcastro AN, Scrubb J, Gilchrist JS. Regulation of ATP-stimulated releasable myofilaments from cardiac and skeletal muscle myofibrils. Mol Cell Biochem 103: 113–120, 1991. [DOI] [PubMed] [Google Scholar]
- 3.Bennett P, Craig R, Starr R, Offer G. The ultrastructural location of C-protein, X-protein, and H-protein in rabbit muscle. J Muscle Res Cell Motil 7: 550–567, 1986. [DOI] [PubMed] [Google Scholar]
- 4.Bradford MM A rapid and sensitive method for the quantitation of microgram quantities of protein ultilizing the principle of dye-binding. Anal Biochem 72: 248–254, 1976. [DOI] [PubMed] [Google Scholar]
- 5.Dahlmann B, Rutschmann M, Reinauer H. Effect of starvation or treatment with corticosterone on the amount of easily releasable myofilaments in rat skeletal muscles. Biochem J 234: 659–664, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dayton WR, Goll DE, Stromer MH, Reville WJ, Zeece MG, Robson RM. Some properties of a Ca2+-activated protease that may be involved in myofibrillar protein turnover. In: Cold Spring Harbor Conferences on Cell Proliferation. Proteases and Biological Control, edited by E Reich, D Rifkin, and E Shaw. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 1975, vol. 2, p. 551–577. [Google Scholar]
- 7.Dayton WR, Reville WJ, Goll DE, Stromer MH. A Ca2+-activated protease possibly involved in myofibrillar protein turnover. Partial characterization of the purified enzyme. Biochemistry 15: 2159–2167, 1976. [DOI] [PubMed] [Google Scholar]
- 8.Du J, Wang X, Miereles C, Bailey JL, DeBigare R, Zheng B, Price R, Mitch WE. Activation of caspase-3 is an initial step triggering accelerated muscle proteolysis in catabolic conditions. J Clin Invest 113: 115–123, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dupont-Versteegden EE Apoptosis in muscle atrophy: relevance to sarcopenia. Exp Gerontol 40: 473–481, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Etlinger JD, Zak R, Fischman DA. Compositional studies of myofibrils from rabbit striated muscle. J Cell Biol 68: 123–141, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Etlinger JD, Zak R, Fischman DA, Rabinowitz M. Isolation of newly synthesized myosin filaments from skeletal muscle homogenates and myofibrils. Nature 255: 259–261, 1975. [DOI] [PubMed] [Google Scholar]
- 12.Goldberg AL Protein turnover in skeletal muscle. I. Protein catabolism during work-induced hypertrophy and growth induced with growth hormone. J Biol Chem 244: 3217–3222, 1969. [PubMed] [Google Scholar]
- 13.Goldberg AL Protein turnover in skeletal muscle. II. Effects of denervation and cortisone on protein catabolism in skeletal muscle. J Biol Chem 244: 3223–3229, 1969. [PubMed] [Google Scholar]
- 14.Goldfine SM, Einheber S, Fischman DA. Cell-free incorporation of newly synthesized myosin subunits into thick filaments. J Muscle Res Cell Motil 12: 161–170, 1991. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein MA, Schroeter JP, Michael LH. Role of the Z band in the mechanical properties of the heart. FASEB J 5: 2167–2174, 1991. [DOI] [PubMed] [Google Scholar]
- 16.Goll DE, Dayton WR, Singh I, Robson RM. Studies of the α-actinin/actin interaction in the Z-disk by using calpain. J Biol Chem 266: 8501–8510, 1991. [PubMed] [Google Scholar]
- 17.Goll DE, Kleese WC, Szpacenko A. Skeletal muscle proteases and protein turnover. In: Animal Growth Regulation, edited by DR Campion, GJ Hausman, and RJ Martin. New York: Plenum, 1989, p. 141–182.
- 18.Goll DE, Neti G, Mares SW, Thompson VF. Myofibrillar protein turnover: the proteasome and the calpains. J Anim Sci 86: 19–35, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev 83: 731–801, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Goll DE, Young RB, Stromer MH. Separation of subcellular granules by differential and density gradient centrifugation. Proc 27th Annu Recip Meat Conf, National Live Stock and Meat Board, Chicago, IL, 1974, p. 250–297.
- 21.Gornall AG, Bardawill CJ, David MM. Determination of serum proteins by means of the biuret reaction. J Biol Chem 177: 751–766, 1949. [PubMed] [Google Scholar]
- 22.Hasslegren PO, Wray C, Mammen J. Molecular regulation of muscle cachexia: it may be more than the proteasome. Biochem Biophys Res Commun 290: 1–10, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Hubbard SR, Hodgson KO, Doniach S. Small-angle X-ray scattering investigation of the solution structure of troponin C. J Biol Chem 263: 4151–4158, 1988. [PubMed] [Google Scholar]
- 24.Koohmaraie M Ovine skeletal muscle multicatalytic proteinase complex (proteasome): purification, characterization, and comparison of its effects on myofibrils with μ-calpains. J Anim Sci 70: 3697–3708, 1992. [DOI] [PubMed] [Google Scholar]
- 25.Kumamoto T, Kleece WC, Cong J, Goll DE, Pierce PR, Allen RE. Localization of the Ca2+-dependent proteinases and their inhibitor in normal, fasted, and denervated rat skeletal muscle. Anat Rec 232: 60–77, 1992. [DOI] [PubMed] [Google Scholar]
- 26.Laemmli UK Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685, 1970. [DOI] [PubMed] [Google Scholar]
- 27.Machida K, Ishibashi R, Hara T, Ohtsuka A, Hayashi K. Effects of corticosterone on Ca2+ uptake and myofibrillar disassembly in primary muscle cell cultures. Biosci Biotechnol Biochem 67: 244–249, 2003. [DOI] [PubMed] [Google Scholar]
- 28.McKenna N, Meigs JB, Wang YL. Identical distribution of fluorescently labeled brain and muscle actins in living cardiac fibroblasts and myocytes. J Cell Biol 100: 292–296, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Papadopoulos S, Endeward V, Revesz-Walker B, Jürgens KD, Gros G. Radial and longitudinal diffusion of myoglobin in single living muscle cells. Proc Natl Acad Sci USA 98: 5904–5909, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Papadopoulos S, Jürgens KD, Gros G. Protein diffusion in living skeletal muscle fibers: dependence on protein size, fiber type, and contraction. Biophys J 79: 2084–2094, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reville WJ, Murray BA, Ahern S, Zeece MG. Easily releasable myofilaments in post mortem bovine muscles. Sci Aliments 14: 431–440, 1994. [Google Scholar]
- 32.Riley DA, Bain JLW, Romatowshi G, Fitts RH. Skeletal muscle atrophy: altered thin filament density changes in slow fiber force and shortening velocity. Am J Physiol Cell Physiol 288: C360–C365, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Robson RM, Goll DE, Temple MJ. Determination of proteins in “Tris” buffer by the biuret reaction. Anal Biochem 24: 339–341, 1968. [DOI] [PubMed] [Google Scholar]
- 34.Saad AD, Dennis JE, Tan IP, Fischman DA. Visualization of myosin exchange between synthetic thick filaments. J Muscle Res Cell Motil 12: 225–234, 1991. [DOI] [PubMed] [Google Scholar]
- 35.Satyshur KA, Rao ST, Pyzalska D, Drendel W, Greaser M, Sundaralingam M. Refined structure of chicken skeletal muscle troponin C in the two-calcium state at 2-Å resolution. J Biol Chem 263: 1628–1647, 1988. [PubMed] [Google Scholar]
- 36.Solomon V, Goldberg AL. Importance of the ATP-ubiquitin-proteasome pathway in the degradation of soluble and myofibrillar proteins in rabbit muscle extracts. J Biol Chem 271: 26690–26697, 1996. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki A, Goll DE, Singh I, Allen RE, Robson RM, Stromer MH. Some properties of purified skeletal muscle α-actinin. J Biol Chem 251: 6860–6870, 1976. [PubMed] [Google Scholar]
- 38.Swartz DR Exchange of α-actinin in isolated myofibrils. J Muscle Res Cell Motil 20: 457–467, 1999. [DOI] [PubMed] [Google Scholar]
- 39.Taylor RG, Geesink GH, Thompson VF, Koohmaraie M, Goll DE. Is Z-disk degradation responsible for postmortem tenderization? J Anim Sci 73: 1351–1367, 1995. [DOI] [PubMed] [Google Scholar]
- 40.Thompson VF, Goll DE. Purification of μ-calpain, m-calpain, and calpastatin from animal tissues. In: Methods in Molecular Biology. Calpain Methods and Protocols, edited by JS Elce. Totowa, NJ: Humana, 2000, vol. 144, p. 3–16. [PubMed] [Google Scholar]
- 41.Thompson VF, Saldaña S, Cong J, Goll DE. A BODIPY fluorescent microplate assay for measuring activity of calpains and other proteases. Anal Biochem 279: 170–178, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Thompson VF, Saldaña S, Cong J, Luedke DM, Goll DE. The calpain system in human placenta. Life Sci 70: 2493–2408, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76: 4350–4354, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van der Westhuyzen DR, Matsumoto K, Etlinger JD. Easily releasable myofilaments from skeletal and cardiac muscles maintained in vitro. Role in myofibrillar assembly and turnover. J Biol Chem 256: 11791–11797, 1981. [PubMed] [Google Scholar]
- 45.Wei W, Fareed MU, Evenson A, Menconi MJ, Yang H, Petkova V, Hasselgren PO. Sepsis stimulates calpain activity in skeletal muscle by decreasing calpastatin activity but does not activate caspase-3. Am J Physiol Regul Integr Comp Physiol 288: R580–R590, 2005. [DOI] [PubMed] [Google Scholar]
- 46.Williams AB, DeCourten-Myers GM, Fisher JE, Luo G, Sun X, Hasselgren PO. Sepsis stimulates release of myofilaments in skeletal muscle by a calcium-dependent mechanism. FASEB J 13: 1435–1443, 1999. [DOI] [PubMed] [Google Scholar]
- 47.Wolfe FH, Sathe SK, Goll DE, Kleese WC, Edmunds T, Duperret SM. Chicken skeletal muscle has three Ca2+-dependent proteinases. Biochim Biophys Acta 998: 236–250, 1989. [DOI] [PubMed] [Google Scholar]
- 48.Yates LD, Greaser ML. Quantitative determination of myosin and actin in rabbit skeletal muscle. J Mol Biol 168: 123–141, 1983. [DOI] [PubMed] [Google Scholar]