Abstract
Gap-junction hemichannels are composed of six protein subunits (connexins). Undocked hemichannels contribute to physiological autocrine/paracrine cell signaling, including release of signaling molecules, cell-volume regulation, and glucose uptake. In addition, hemichannels may be pathologically activated by dephosphorylation and cell-membrane depolarization. Such hemichannel opening may induce and/or accelerate cell death. It has been suggested that connexin43 (Cx43) hemichannels are sensitive to redox potential changes and that one or more intracellular cysteines is/are important for this process. Cx46 is expressed in the lens, and its dysfunction induces cataract formation. It contains six cysteines in the extracellular loops, one in the fourth transmembrane helix, and two in the COOH-terminal domain. The latter may be susceptible to oxidation by nitric oxide (NO), which could be involved in cataract formation through cysteine S-nitrosylation. Here we report studies of the effects of the NO donor S-nitrosoglutathione (GSNO) on the electrical properties and fluorescent-dye permeability of wild-type Cx46 and mutant hemichannels expressed in Xenopus laevis oocytes. GSNO enhanced hemichannel voltage sensitivity, increased tail-current amplitude, and changed activation and closing kinetics in Cx46 and Cx46-CT43 (Cx46 mutant in which the COOH terminus was replaced with that of Cx43), but not in Cx46-C3A (Cx46 in which the intracellular and transmembrane helix 4 cysteines were mutated to alanine). We conclude that Cx46 hemichannels are sensitive to NO and that the NO effects are mediated by modification of one or more intracellular cysteines. However, it is unlikely that NO induces cataract formation due to the hemichannel activation, because at normal resting potential, NO had no major effects on Cx46 hemichannel permeability.
Keywords: connexins, gap-junctional channels, cataracts, S-nitrosylation, ion channels
gap junctions are aggregates of intercellular channels at the junctional membrane. Each channel is formed by the docking of two hemichannels or connexons, one from each adjacent cell, which are hexamers of the membrane proteins called connexins (Cxs). The presence of undocked hemichannels at the plasma membrane has been demonstrated in several cell types, using several different techniques (38). However, the roles of hemichannels in both physiological and pathophysiological processes are just being defined. A growing body of evidence supports the idea that controlled hemichannel opening allows physiological autocrine/paracrine cell signaling. Thus, it has been suggested that hemichannels participate in biological processes such as release of signaling molecules (38), cell-volume regulation (30), and glucose uptake (32). However, massive and prolonged hemichannel opening has been proposed to induce or accelerate cell death in some pathological conditions (36). Hemichannel-induced cell death can be attributed to loss of important metabolites such as ATP, amino acids and reduced glutathione, loss of ion gradients, and entry of Ca2+ (2, 37).
Recently, oxidative stress has been suggested to be an important modulator of the hemichannel properties. In metabolically inhibited astrocytes there is massive hemichannel opening that is blocked by Trolox (a free-radical scavenger) (7) or dithiothreitol [DTT; sulfhydryl (SH)-reducing agent] (31). The latter results suggest that oxidative stress affects hemichannel activity through −SH group oxidation. A good candidate to explain this phenomenon is nitric oxide (NO), because it does oxidize cysteine residues (23). In agreement with this notion, metabolic inhibition induces connexin43 (Cx43) hemichannel S-nitrosylation and exposure of astrocytes in culture to the NO donor S-nitrosoglutathione (GSNO) renders their plasma membranes permeable to hydrophilic fluorescent molecules, an effect that is blocked by hemichannel blockers (31). Additionally, it has been shown that the effect of proinflammatory molecules to open hemichannels in astrocytes is prevented by the NO synthase inhibitor nitro-l-arginine methyl ester and by DTT (32). It was suggested that COOH-terminal-domain cysteines are essential for these effects (33).
Regardless of the absence of vascular system, lens fibers survive during the entire lifespan of the individual. Nutrition and excretion from lens fibers occur through gap-junctional channels, with the intercellular fluxes of ions, metabolites, and water determined by their chemical or electrochemical gradients (14). Several connexin isoforms are expressed in the lens; cortex epithelial cells express Cx43 (3) and Cx50 (8), whereas fiber cells express Cx46 (28, 40) and Cx50 (19, 45). The importance of connexins in cataract formation is well documented. Cx43, Cx46, or Cx50 knockout mice show cataracts at early ages, suggesting that connexin function is essential to maintain lens transparency (9, 12, 46). Cataracts can also result from single mutations of Cx46 or Cx50 (13, 22).
The role of free radicals in cataract formation has been studied for a long time (20, 42), but the role of NO has become apparent only recently. Lens oxidative stress due to NO may be a risk factor for acquired cataract formation (16, 25). Inducible nitric oxide synthase (iNOS) is the enzyme involved in the generation of high levels of NO in cataracts (16). Supporting this notion, it was observed that the administration of NO scavengers or iNOS inhibitors to rats injected with selenite significantly reduced cataract formation (25). Also, the concentration of nitrite (formed only when NO is present) is higher in lenses with cataracts than in normal lenses (27). The observations above suggest that increased NO concentration is an important factor in cataract formation in humans, both in diabetes (26) and in advanced age (43). The effect of NO can be mediated by S-nitrosylation of free cysteines, because low levels of reduced glutathione (an endogenous cysteine reductor molecule) increases lens damage in response to NO exposure (43).
Because NO increases the likelihood of cataract development and Cx46 mutations induce cataracts, we tested whether the NO donor GSNO induces functional modifications in Cx46 gap-junction hemichannels. Our data demonstrate that Cx46 hemichannels are affected by GSNO, probably through S-nitrosylation of one or more cysteine residues located in the COOH-terminal domain. This work also suggests that in the lens, NO can directly affect Cx46 channels, both in terms of electrophysiological properties and permeability to charged solutes, perhaps influencing the fluxes of nutrients and toxic molecules. However, it is unlikely that NO induces cell death and/or cataract formation due to Cx46 hemichannel opening.
MATERIALS AND METHODS
Plasmid engineering.
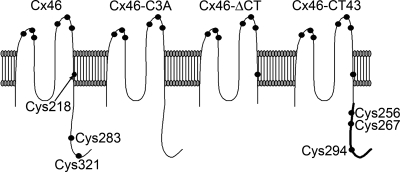
Rat Cx46 was obtained from Dr. Lisa Ebihara (University of Chicago, Chicago, IL) as plasmid pSP64T-Cx46 (28). A mutant in which Cys 218, Cys 283, and Cys 321 were substituted with Ala (Cx46-C3A) was generated by site-directed mutagenesis (Quick Change Multisite Site-Directed Mutagenesis kit, Stratagene, La Jolla, CA) using mutagenic primers where the Cys 218 and Cys 283 TGT codons were substituted with GCT, and the Cys 321 TGC codon was substituted with GCC. For the generation of the mutant with truncation of the COOH-terminal domain (Cx46ΔCT, truncated after Gly239), we first introduced two unique KpnI sites flanking the COOH-terminal domain using the following mutagenic primers (mutations underlined): 5′-GGAAGAAGCTCAAGCAGGGTACCACCAACCACTTCAACCC-3′ (proximal site) and 5′-CCTTTATAAACCTTCTCGGTACCCCTTAGTAACCAAAACCAAACC-3′ (distal site). The introduction of the proximal KpnI site resulted in the substitution of Val240 with Thr. The Cx46ΔCT mutant was then obtained by KpnI cut and self-ligation. To make the chimera in which the COOH-terminal domain of Cx43 (Lys241 to Ile382) substituted the native Cx46 COOH-terminal domain, we amplified the Cx43 sequence by PCR, with flanking, in frame, KpnI sites, and ligated the product to the Cx46ΔCT DNA cut with the same enzymes. The PCR primers were 5′-TAAGGTACCGGCGGAAGCGATCCTTACCACGCCACC-3′ (forward) and 5′-TAAGGTACCTCATTAAATCTCCAGGTCATCAGGCCG-3′ (reverse). A schematic representation of the proteins studied is presented in Fig. 1.
Fig. 1.
Schematic representation of connexin46 (Cx46) and Cx46 mutants. The diagram (extracellular-surface up) shows the location of Cys residues, denoted by circles. Cx46, wild-type rat Cx46; Cx46-C3A, Cx46 with intracellular and M4 Cys mutated to Ala; Cx46ΔCT, Cx46 with truncation of the COOH-terminal domain at position 239; Cx46-CT43, chimera in which the Cx43 COOH terminus (thicker line) was fused to Cx46ΔCT. In addition to Cys218, it has 3 additional cysteines at positions 256, 267, and 294.
cRNA preparation and injection into Xenopus laevis oocytes.
Oocytes obtained as previously described (1) were injected with 12.5 ng of antisense Cx38 oligonucleotide alone, to reduce Cx38 endogenous expression, or in combination with 25 ng of cRNA coding for one of the following: Cx46, Cx46-C3A, Cx46ΔCT, or Cx46-CT43. For SP6-directed capped cRNA synthesis (mMessage Machine, Ambion, Austin, TX), the template plasmids were linearized with Sal I. After cRNA injection, oocytes were maintained in Barth's solution (in mM: 88 NaCl, 1 KCl, 5 CaCl2, 0.8 MgCl2, and 10 HEPES/NaOH, pH 7.4) supplemented with 0.1 mg/ml gentamycin and 20 U/ml of penicillin and streptomycin for 24–48 h before experimental measurements.
Electrophysiological recordings and calculations.
Hemichannel currents were measured as previously described (6) in oocytes bathed at room temperature with ND96 solution (in mM: 96 NaCl, 2 KCl, 1.8 CaCl2, and 5 HEPES/NaOH, pH 7.4). Data acquisition and analysis were performed with pClamp 10/Digidata 1440A A/D Board (Molecular Devices, Foster City, CA). Currents were elicited by 15-s square pulses, ranging from −50 mV to +60 mV in 10-mV steps, from a holding voltage of −60 mV, with 10-s intervals between pulses. Current-voltage (I-V) relationships were calculated from the current values at the end of the pulses. In some studies, the Boltzmann equation was fit to the data:
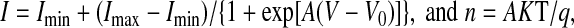 |
where A is the slope of the voltage sensitivity, K is the Boltzmann constant, T is the absolute temperature (in Kelvin), n is the gating charge, q is the valence, and Vo is the voltage at which half of the maximal current was reached.
GSNO and DTT treatment.
Oocytes were placed in plastic dishes containing ND96 plus 1 mM GSNO (a nitric oxide donor; Calbiochem, San Diego, CA). Exposure to GSNO was at 16°C for 40 to 60 min. No differences were observed in recordings from oocytes treated for 40 or 60 min. DTT (a cysteine reducing agent; Sigma, St. Louis, MO) was used at 10 mM for 15 min before the electrophysiological measurements. After treatment, oocytes were placed in the recording chamber and superfused with fresh ND96 for 3 min. Control oocytes were treated in the same manner but without the addition of GSNO or DTT to the ND96.
Dye uptake.
For dye-uptake measurements, we used 5(6)-carboxyfluorescein (CF, charge −2, Mr 376, 2 mM), ethidium bromide (EthBr, charge +1, Mr 394, 1 mM), or Lucifer yellow (LY, charge −2, Mr 457, 1 mM). The cells were incubated for 40 min at 16°C in ND96 solution (in mM: 96 NaCl, 2 KCl, 1 MgCl2, 1.8 CaCl2, and 5 HEPES-NaOH, pH 7.4) containing one of the dyes at the concentration indicated above. Uptake experiments were performed at control Ca2+ levels (1.8 mM) and in nominally Ca2+-free solution (no calcium added), and with or without GSNO treatment. After this incubation period the extracellular dye was removed by extensive washing with ice-cold ND96 solution containing 10 μM Gd3+. Gd3+, a blocker of gap-junctional hemichannels (1), was used to minimize leakage of intracellular dyes via hemichannels during washing. Individual oocytes were lysed by sonication in 2 ml of 5 mM Tris·HCl, pH 9, and the fluorescence was measured as indicated below. Each experimental point was the average of eight oocytes from the same batch, and each experiment was repeated three times in different oocyte batches. Fluorescence intensity was measured on a spectrofluorometer (Hitachi model F-7000 or SPEX Fluorolog 2) at the following excitation/emission wavelengths: CF 488/525 nm, EthBr 540/625 nm, and LY 425/520 nm. Control measurements were performed in oocytes injected with Cx38 antisense oligonucleotide alone.
Statistical analysis.
Results are expressed as means ± SE, and n refers to the number of independent experiments. For statistical analyses, each treatment was compared with its respective control, and significance was determined using a one-way ANOVA or paired Student's t-test, as appropriate. Differences were considered significant at P < 0.05.
RESULTS
Electrophysiological properties of Cx46 hemichannels under control conditions.
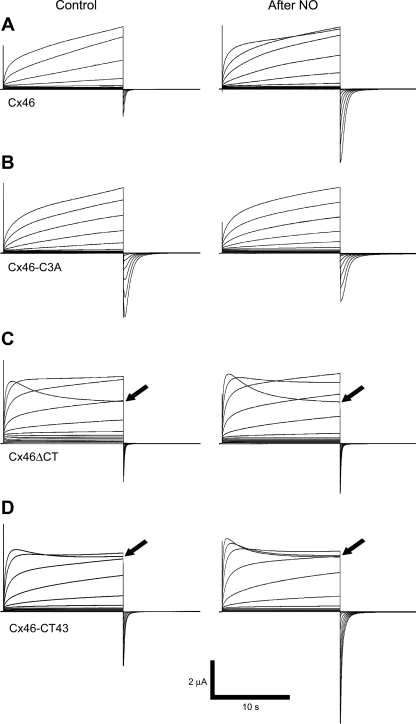
Membrane depolarization of oocytes expressing Cx46 hemichannels induced outward currents that activated slowly and did not show inactivation, even after a 15-s depolarization at +60 mV (Fig. 2A). Cx46 hemichannel currents averaged 3.77 ± 0.45 μA at +60 mV (n = 11), almost 10-fold those in oocytes injected with Cx38 antisense oligonucleotide alone (0.4 ± 0.2 μA, n = 12). Upon returning to the resting potential of −60 mV, small tail currents were observed in the Cx46-expressing oocytes, which averaged 44 ± 4% of the current at the end of the +60-mV pulse. Hemichannels formed by Cx46-C3A mutant (C218A/C283A/C321A, Fig. 1) displayed a similar response to depolarizing pulses (slow activation, without inactivation, Fig. 2B). The amplitude of the currents through hemichannels formed by Cx46-C3A were similar to those formed by Cx46 (3.48 ± 0.8 μA at +60 mV, n = 14), but the Cx46-C3A tail currents were consistently larger (101 ± 14% of the current at the end of the +60-mV pulse, Fig. 2B, n = 14 oocytes). Since the amplitude of the tail currents is expected to increase with a decreased rate of inactivation at negative voltages (upon switching the depolarizing pulses to the −60 mV holding potential), the results indicate that the presence of one or more of the mutated Cys slows down the closing of the Cx46 hemichannels at negative voltage.
Fig. 2.
Nitric oxide (NO) alters the electrophysiological properties of Cx46 hemichannels. A–D: typical whole cell current records from Xenopus oocytes expressing wild-type Cx46 and Cx46 mutants. Hemichannel current recordings were obtained under control conditions (ND96 solution plus Ca2+ and Mg2+) and after exposure to 1 mM S-nitrosoglutathione (GSNO) for 40 min (after NO). Oocytes were clamped to −60 mV, and square pulses from −60 mV to +60 mV (in 10-mV steps) were then applied for 15 s. At the end of each pulse, the membrane potential was returned to −60 mV for 10 s. Arrows point to +60 mV records (see text).
Hemichannels formed by the mutant where the COOH-terminal domain was truncated, Cx46ΔCT, exhibited a fast activation, with the peak current at +60 mV reached in ∼850 ms, followed by slow inactivation (Fig. 2C). Note that the currents at +60 mV did not reach a peak during the 15-s pulse in oocytes expressing Cx46 or Cx46-C3A hemichannels (Fig. 2, A and B). In contrast, in Cx46ΔCT-expressing oocytes, currents were activated at lower positive voltages compared with Cx46 hemichannels, and the tail currents were larger, 105 ± 15% of the currents at the end of the +60-mV pulse (Fig. 2D, black arrow). In oocytes expressing a chimera where the Cx46 COOH-terminal domain was replaced by the equivalent Cx43 domain, current activation and tail currents (103 ± 15% of the current at the end of the +60-mV pulse) were similar to those through Cx46ΔCT hemichannels (Fig. 2, C and D, black arrows). The only difference was in the degree of inactivation at +60 mV, larger in the oocytes expressing Cx46ΔCT (34 ± 4% of the peak value, n = 7) compared with those expressing Cx46-CT43 (12 ± 1%, n = 10).
In summary, wild-type Cx46 and the mutants tested form functional hemichannels when expressed in frog oocytes. The current activation by depolarizing pulses is slower in the hemichannels containing the Cx46 COOH-terminal domain (with or without the Cys residues) compared with the activation in mutants with COOH-terminal domain truncated or replaced by the corresponding Cx43 sequence. The latter two mutant hemichannels show faster activation and also inactivation at +60 mV. The tail current amplitude increased in all the mutant hemichannels, suggesting that the presence of one or more of the native Cys residues is involved in the inactivation of the currents at negative voltages.
The NO donor GSNO changes Cx46 hemichannel properties.
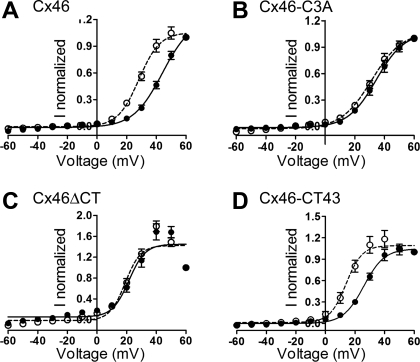
The effects of GSNO are illustrated on the right-hand side of Fig. 2. After GSNO treatment, Cx46 current activation in response to depolarizing pulses was faster (Fig. 2A). Analysis of the current activation showed two time constants, fast (τf = 540 ± 90 ms) and slow (τs 16,290 ± 390 ms), both of which were decreased by GSNO (by 40 ± 5% and 60 ± 5%, respectively). The I-V relationships measured at the end of the pulses showed that GSNO increased the voltage sensitivity of wild-type Cx46 hemichannels (Fig. 3A). Fits of the data to the Boltzmann equation indicate that GSNO displaced the voltage sensitivity (Vo) by ∼15 mV and increased the equivalent number of gating charges (Table 1). GSNO also increased the tail currents, to 2.7 ± 0.3-fold over the control value (n = 11, P < 0.05). In contrast, the activation and voltage dependence of Cx46-C3A hemichannel currents were not affected significantly by GSNO (Figs. 2B and 3B and Table 1), and the tail currents were not significantly altered (0.76 ± 0.1-fold vs. control, n = 8, P > 0.05, Figs. 2B, 3B, and 4B).
Fig. 3.
Intracellular Cys are necessary for the nitric oxide effect. A–D: current-voltage (I-V) plots of normalized currents recorded in oocytes expressing wild-type Cx46 and Cx46 mutants under control conditions (•) or after exposure to 1 mM GSNO (○). Currents were measured at the end of the 15-s pulses and were normalized to the +60-mV value. The Boltzmann equation (see text) was fitted to the data. Continuous line, control; segmented line, GSNO. See Fig. 2 for additional details.
Table 1.
Fits of Boltzmann equation to the I-V plot data in Fig. 3
|
Cx46 |
Cx46-C3A
|
Cx46ΔCT
|
Cx46-CT43
|
|||||
|---|---|---|---|---|---|---|---|---|
| Control | NO | Control | NO | Control | NO | Control | NO | |
| Imin | 0.013 | 0.005 | 0.012 | 0.024 | 0.056 | 0.014 | 0.001 | 0.013 |
| Imax | 1.2 | 1.0 | 1.1 | 1.1 | 1.5 | 1.4 | 1.0 | 1.1 |
| V0 | 44.0 | 31.1 | 34.4 | 31.3 | 21.5 | 19.0 | 25.9 | 13.9 |
| A | 0.102 | 0.141 | 0.105 | 0.098 | 0.182 | 0.192 | 0.144 | 0.184 |
| n | 2.6 | 3.6 | 2.7 | 2.5 | 4.7 | 4.9 | 3.7 | 4.7 |
I-V, current-voltage; NO, nitric oxide; V0, voltage at which half of maximal current is reached; A, slope of voltage sensitivity; n, gating charge. Currents were normalized to the value at the end of the +60-mV pulse.
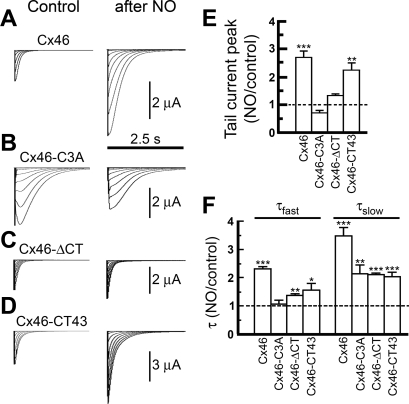
Fig. 4.
GSNO alters Cx46 and Cx46-CT43 hemichannel tail currents. A–D: typical tail-current records from Xenopus oocytes expressing wild-type Cx46 and Cx46 mutants, before and after exposure to GSNO (after NO). See Fig. 2 for details. E: effect of NO on peak tail-current amplitudes elicited upon transition from +60 mV to −60 mV. Bars are means ± SE of 11 (Cx46), 10 (Cx46-C3A), 4 (Cx46ΔCT), and 9 (Cx46-CT43) experiments. **P < 0.01 and ***P < 0.001. The dotted line denotes control values. F: effects of NO on tail-current kinetics. The data were fit to a two-exponential equation, and the resulting time constants, fast (τfast) and slow (τslow), are shown as means ± SE of the NO values relative to control. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with control.
GSNO had only marginal effects on oocytes expressing Cx46ΔCT hemichannels. The voltage dependence of the current activation at depolarizing pulses (Figs. 2C and 3C and Table 1) and the magnitude of the tail currents (1.4 ± 0.1-fold vs. control, n = 6, P > 0.05, Figs. 2C and 4C) were not significantly affected. Only a marginal change in the rate of activation was observed (no change in τf and a decrease in τs from 282 ± 36 to 213 ± 45 ms).
The changes elicited by GSNO in Cx46-CT43 hemichannels were similar to those in the wild-type hemichannels. The rate of activation by depolarizing pulses (Fig. 2D) was decreased (τf from 102 ± 24 to 51 ± 18 ms, and τs from 405 ± 81 to 162 ± 12 ms), and the voltage dependence of the currents was increased (Fig. 3D and Table 1). As was the case for wild-type Cx46 hemichannels, the magnitude of the tail currents increased to 2.8 ± 0.3-fold vs. control (n = 12, P < 0.01, Figs. 2D and 4D).
In summary, GSNO has significant effects on the activation of hemichannel currents elicited by depolarizing pulses, the voltage dependence of those currents, and their inactivation at negative voltages (tail currents), both in wild-type Cx46 hemichannels and in hemichannels in which the COOH-terminal domain was replaced with the corresponding Cx43 sequence. These results show that COOH-terminal Cys residues (absent in Cx46-C3A and Cx46ΔCT) are essential for the effect of GSNO and that the Cx43 COOH-terminal domain can restore GSNO sensitivity to COOH-terminal truncated Cx46.
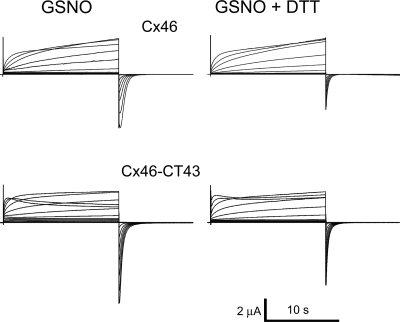
The effect of GSNO is abolished by DTT.
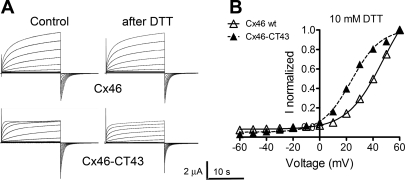
If the GSNO effects on Cx46 hemichannels are due to S-nitrosylation of cysteine residues, it is expected that the reducing agent DTT will reverse them (31). In oocytes expressing Cx46 hemichannels and treated with GSNO, addition of 10 mM DTT to the bath yielded currents close to those observed under control conditions (Fig. 5A). DTT reversed the increased voltage sensitivity of the current activation (compare Tables 1 and 2) and partially reversed the increase in the rate of current activation by depolarizing pulses and the increase in tail-current amplitude (Fig. 5). Similar results were obtained in oocytes expressing Cx46-CT43 (Fig. 5 and Table 2). The “overcorrection” of the effects of GSNO on these mutant hemichannels can be explained by a DTT effect under control conditions. While DTT had no obvious effects on wild-type Cx46 hemichannels under control conditions (Fig. 6A), it abolished the Cx46-CT43 current inactivation at +60 mV and decreased the tail-current amplitude by 35 ± 5% (Fig. 6B). These effects were opposite to those observed after exposure to GSNO (Figs. 2 and 4).
Fig. 5.
The effect of GSNO on Cx46 and Cx46-CT43 hemichannels is reversed by DTT. Shown are representative whole cell recordings of Cx46 and Cx46-CT43 hemichannel currents after 40-min exposure to 1 mM GSNO in the absence of DTT (GSNO) or after 15-min exposure to 10 mM DTT (GSNO + DTT). See Fig. 2 for details.
Table 2.
Fits of Boltzmann equation to the I-V plot data in Fig. 5
|
Cx46 |
Cx46-CT43
|
|
|---|---|---|
| DTT after NO | DTT after NO | |
| Imin | 0.001 | 0.001 |
| Imax | 1.1 | 0.9 |
| V0 | 45.5 | 31.6 |
| A | 0.112 | 0.123 |
| N | 2.8 | 3.1 |
| τ | 6.7 | 6.2 |
Currents were normalized to the value at the end of the +60-mV pulse. Tail current τ values are in ms.
Fig. 6.
DTT alters Cx46-CT43 but not Cx46 hemichannel currents. A: representative whole cell recordings of Cx46 and Cx46-CT43 hemichannel currents under control conditions and after a 15-min exposure to 10 mM DTT. B: I-V plots of the normalized currents shown in A. ▵, Cx46; ▴, Cx46-CT43. WT, wild type. The fit of the data after DTT to the Boltzmann equation is depicted by the solid (Cx46) and segmented (Cx46-CT43) lines. See Fig. 2 for details.
The data above suggest that the effect of GSNO on Cx46 hemichannels is predominantly caused by an action on intracellular cysteines, probably through nitrosylation. The effects of DTT under control conditions are in agreement with previous results showing that Cx43 hemichannels are affected by reducing agents under control conditions (33). Inasmuch as the effect of DTT is exerted only on oxidized Cys residues, the data suggest that Cx46 COOH-terminal domain cysteines are mostly reduced under control conditions, but at least one Cx46-CT43 intracellular cysteine is oxidized.
GSNO causes a small decrease in hemichannel dye permeability.
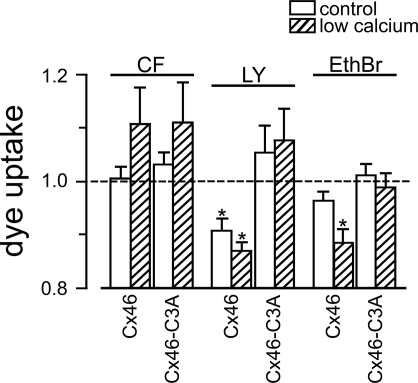
To address the effects of GSNO on the permeability of Cx46 hemichannels to larger solutes, we determined the uptakes of fluorescent dyes, both anionic (CF and LY) and cationic (EthBr) by oocytes expressing Cx46 or Cx46-C3A hemichannels. Oocytes were exposed to one of the dyes in ND96 medium plus 1.8 mM Ca2+ or in the absence of divalent cations, and in the presence or absence of 1 mM GSNO. Under control conditions (1.8 mM Ca2+, no GSNO), CF uptakes through Cx46 and Cx46-C3A hemichannels were slightly higher than those in oocytes injected with Cx38 antisense oligonucleotide alone (by 10.4 ± 0.1% and 14.2 ± 0.1%, respectively). In the absence of divalent cations, which increases hemichannel permeability, the uptake of CF through Cx46 and Cx46-C3A hemichannels increased by ∼10-fold, and those of LY and EthBr increased by 75 ± 20% and 30 ± 2%, respectively (not shown). Under control conditions, EthBr and LY uptakes were negligible and undistinguishable between the different oocyte groups. The effects of GSNO are summarized in Fig. 7. In Cx46-expressing oocytes, GSNO had no effects on CF uptake, significantly reduced LY uptake both in normal and low-Ca2+ media, and significantly reduced EthBr uptake in low-Ca2+ medium. The effects of GSNO were absent in oocytes expressing Cx46-C3A hemichannels. These results show significant, although small (10–15%), effects of GSNO on some of the tested dyes, which depend on the presence of COOH-terminal domain cysteines.
Fig. 7.
GSNO affects dye uptake through Cx46 but not Cx46-C3A hemichannels. Oocytes expressing wild-type Cx46 or Cx46-C3A were exposed to 2 mM 5(6)-carboxyfluorescein (CF), 1 mM Lucifer yellow (LY), or 1 mM ethidium bromide (EthBr), indicated at the top, for 40 min, in the absence or presence of 1 mM GSNO. After treatment, the oocytes were washed and sonicated and the dye content was measured by fluorometry. Data depicted are the normalized effects of 1 mM GSNO on dye uptake by oocytes in ND96 (1.8 mM Ca2+, open bars) or in the nominal absence of divalent cations (hatched bars). For the normalization, the dye uptake in GSNO was divided by that in the absence of GSNO at normal or low [Ca2+]. Data are from 3 independent experiments for each dye (8 oocytes per experiment). *P < 0.05, control condition vs. 1 mM GSNO.
DISCUSSION
COOH-terminal domain sequences affect Cx46 hemichannel current properties.
Our results concerning baseline properties of wild-type and mutant Cx46 hemichannels confirm and extend previous studies (28). Under control conditions, oocytes expressing wild-type Cx46 displayed currents activated by depolarizing voltages, without signs of inactivation during the 15-s pulse period. The macroscopic voltage-sensitivity parameters of Cx46 hemichannels (V0 of 44 mV and gating charge of 2.6) were similar to those estimated for Cx46 gap-junctional channels (V0 48 mV and gating charge 2.0; see Ref. 15), supporting the idea of similar properties of Cx46 gap-junctional channels and hemichannels. The properties of Cx46-C3A hemichannel currents were very similar to those of wild-type Cx46, with the exception of the tail currents, which were larger (Fig. 4). Tail currents denote hemichannel closing at negative membrane potentials, which has been associated with slow transitions from the open to the closed state (29, 41). Therefore, it seems plausible that one or more of the Cys replaced with Ala are involved in channel slow-closing kinetics. Further studies, including single-channel recordings and additional mutagenesis, will be necessary to elucidate the roles of individual Cys residues.
Hemichannels and gap junction channels are voltage sensitive, showing two molecularly different gating mechanisms (5). These are fast gating, which induces fast transitions from open to subconductive state, and loop gating, which induces slow transitions from open to closed state (5). In gap-junctional channels and hemichannels, the fast gating is activated by transjunctional voltage (Vj) changes, while slow gating is sensitive to both Vj and voltage changes at the plasma membrane (Vm) (5). Both Cx46ΔCT and Cx46-CT43 hemichannel currents showed a rapid increase followed by slow relaxation during the +60-mV pulse. Similar results were recently published for Cx46ΔCT hemichannels (44). Although single-channel studies will be needed for definitive conclusions, some of the features of Cx46ΔCT and Cx46-CT43 hemichannel currents are probably due to effects on the fast gating, because COOH-terminal truncation or enhanced green fluorescent protein fusion abolishes fast gating, but preserves slow gating, in Cx32 and Cx43 (4, 34). In agreement with this, the fast gating of truncated Cx43 is reestablished when coexpressed with the Cx43 COOH-terminal domain (24). Since the electrophysiological properties of the Cx46ΔCT and Cx46-CT43 hemichannels are very similar, we conclude that the Cx43 COOH-terminal domain cannot replace the function of the corresponding Cx46 sequence. There must be specific structural requirements of the Cx46 COOH-terminal domain essential for fast gating. In Cx43, gating appears to involve intramolecular interactions between the COOH-terminal tail and the intracellular loop, a conclusion supported by the observation that a peptide that blocks the interaction between these domains decreases fast gating (39). A similar interdomain interaction may exist in Cx46.
The NO donor GSNO alters Cx46 hemichannel properties.
After exposure to GSNO, Cx46 hemichannels became more sensitive to depolarizing pulses and showed larger tail currents. These effects of GSNO were observed in wild-type Cx46 and Cx46-CT43 hemichannels but were absent in Cx46-C3A and Cx46ΔCT hemichannels. These observations indicate that COOH-terminal domain Cys are the main sensors for redox-potential changes, similar to the case of Cx43 hemichannels (31, 33). Interestingly, although the Cx43 COOH-terminal domain cannot replace the equivalent Cx46 sequence with respect to the gating under control conditions (see above), it does reestablish sensitivity to GSNO. This suggests differences in the putative interactions of the COOH-terminal domain with other Cx46 domains during gating under control conditions vs. under the effects of GSNO.
The reducing agent DTT partially reversed the effect of GSNO on Cx46 hemichannels. Since NO can modify Cys and Tyr residues, but only Cys residues are reduced by DTT (21), a possible explanation for the partial reversal of the NO effect with DTT is that NO causes both Cys and Tyr nitration. Under control conditions, DTT by itself had no effect on Cx46 hemichannels, but it did change the Cx46-CT43 hemichannel properties. Wild-type Cx43 hemichannels expressed in HeLa cells are affected by DTT under control conditions: DTT decreases the time activation and increases the number of hemichannels sensitive to depolarization. This effect was due to reducing intracellular Cx43 Cys, because GSH (reduced gluthatione, impermeable reducing agent) mimicked the DTT effect only when applied through the recording pipette (33). These results, taken together with those reported here, suggest that Cx46 Cys are less susceptible to oxidation than the Cx43 COOH-terminal Cys, and therefore Cx43 hemichannels are more susceptible to oxidation than Cx46 hemichannels, in vivo.
It is likely that the GSNO effect is mainly mediated by direct S-nitrosylation of intracellular Cys. This is based on the following arguments: 1) GSNO has no effect on Cx46-C3A, which lacks intracellular cysteines but has the extracellular cysteines. 2) GSNO is taken up by cells (35). 3) The predominant effect of GSNO is to provide a NO radical directly to a reduced Cys, more than releasing NO to the media (10, 35). Therefore, in our system, the effect of NO products such as NO2 or N2O3 is probably less relevant to induce S-nitrosylation than transnitrosylation. Because it has been reported that, depending on the sequence of a particular protein, GSNO can induce the S-nitrosylation, S-glutathionylated or both (11), we cannot rule out the possibility of S-glutathionylation of Cx46 hemichannels in our system; additional analytical studies will be necessary to discriminate between the posttranscriptional modifications above.
Pathophysiological significance.
Gap-junctional channels between lens fibers are essential for uptake and excretion of molecules (14). Because of the role of NO as trigger and/or accelerator of cataract formation (17, 18), the role of hemichannels in cell damage (36), and the finding of direct effects of NO on Cx46 hemichannels in this study, it is relevant to address the possibility of a role of hemichannels in the pathology of cataracts. The small decreases in Cx46 hemichannel uptake of Lucifer yellow and ethidium bromide by NO could, if present in gap-junctional channels, translate in decreases in the permeability to metabolites or toxic excretion products. The development of cataracts is a slow process, and therefore the small magnitude of the NO effects observed cannot rule out a pathophysiological role. Nevertheless, measurements of long-term NO effects on ion and solute permeabilities in the lens will be needed to answer this question. Cx46 hemichannel activation by NO does not seem to play a role in cataract formation because NO does not have significant effect on Cx46 hemichannel properties at negative resting membrane potential.
In conclusion, NO enhances voltage sensitivity and increases tail-current amplitude of Cx46 hemichannels by changing their activation and closing kinetics, and slightly reduces permeabilities to some “large” solutes. These effects depend on the presence of the COOH-terminal domain Cys residues.
GRANTS
This work was supported by National Institutes of Health Grants GM-068586 and GM-79629, Texas Advanced Research Program Grant 010674-0046-2007, and Fondecyt Grant 11080061.
Acknowledgments
We thank Catherine F. Hamilton for technical support and Carolina Larraín for assistance with manuscript preparation. We also thank Dr. Lisa Ebihara for providing the Cx46 DNA.
REFERENCES
- 1.Bao X, Altenberg GA, Reuss L. Mechanism of regulation of the gap junction protein connexin 43 by protein kinase C-mediated phosphorylation. Am J Physiol Cell Physiol 286: C647–C654, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Besancon E, Guo S, Lok J, Tymianski M, Lo EH. Beyond NMDA and AMPA glutamate receptors: emerging mechanisms for ionic imbalance and cell death in stroke. Trends Pharmacol Sci 29: 268–275, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Beyer EC, Kistler J, Paul DL, Goodenough DA. Antisera directed against connexin43 peptides react with a 43-kD protein localized to gap junctions in myocardium and other tissues. J Cell Biol 108: 595–605, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bukauskas FF, Bukauskiene A, Bennett MVL, Verselis VK. Gating properties of gap junction channels assembled from connexin43 and connexin43 fused with green fluorescent protein. Biophys J 67: 137–152, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bukauskas FF, Verselis VK. Gap junction channel gating. Biochim Biophys Acta 1662: 42–60, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y, Deng Y, Bao X, Reuss J, Altenberg GA. Mechanism of the defect in gap-junctional communication by expression of a connexin 26 mutant associated with dominant deafness. FASEB J 19: 1516–1518, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Contreras JE, Sánchez HA, Véliz LP, Bukauskas FF, Bennett MV, Sáez JC. Role of connexin-based gap junction channels and hemichannels in ischemia-induced cell death in nervous tissue. Brain Res Rev 47: 290–303, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dahm R, Van Marle J, Prescott AR, Quinlan RA. Gap junctions containing alpha8-connexin (MP70) in the adult mammalian lens epithelium suggests a re-evaluation of its role in the lens. Exp Eye Res 69: 45–56, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Gao Y, Spray DC. Structural changes in lenses of mice lacking the gap junction protein connexin43. Invest Ophthalmol Vis Sci 39: 1198–1209, 1998. [PubMed] [Google Scholar]
- 10.Gaston BM, Carver J, Doctor A, Palmer LA. S-nitrosylation signalling in cell biology. Mol Interv 3: 253–263, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Giustarini D, Milzani A, Aldini G, Carini M, Rossi R, Dalle-Donne I. S-nitrosation versus S-glutathionylation of proteins sulfhydryl groups by S-nitrosoglutathione. Antioxid Redox Signal 7: 930–939, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Gong X, Li E, Klier G, Huang Q, Wu Y, Lei H, Kumar NM, Horwitz J, Gilula NB. Disruption of alpha3 connexin gene leads to proteolysis and cataractogenesis in mice. Cell 91: 833–843, 1997. [DOI] [PubMed] [Google Scholar]
- 13.Gong X, Cheng C, Xia CH. Connexins in lens development and cataractogenesis. J Membr Biol 218: 9–12, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Goodenough DA Lens gap junctions: a structural hypothesis for nonregulated low-resistance intercellular pathways. Invest Ophthalmol Vis Sci 18: 1104–1122, 1979. [PubMed] [Google Scholar]
- 15.Hopperstad MG, Srinivas M, Spray DC. Properties of gap junction channels formed by Cx46 alone and in combination with Cx50. Biophys J 79: 1954–1966, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inomata M, Hayashi M, Shumiya S, Kawashima S, Ito Y. Involvement of inducible nitric oxide synthase in cataract formation in Shumiya cataract rat (SCR). Curr Eye Res 23: 307–311, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Ito Y, Nabekura T, Takeda M, Nakao M, Terao M, Hori R, Tomohiro M. Nitric oxide participates in cataract development in selenite-treated rats. Curr Eye Res 22: 215–220, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Jurowski P, Gos R, Piasecka G. Nitric oxide levels in aqueous humor after lens extraction and poly(methylmethacrylate) and foldable acrylic intraocular lens implantation in rabbit eyes. J Cataract Refract Surg 28: 2188–2192, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Kistler J, Kirkland B, Bullivant S. Identification of a 70,000-D protein in lens membrane junctional domains. J Cell Biol 101: 28–35, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kojima K, Okochi Y, Yagi K. The opacity of the rat lens caused by fatty acid peroxide. Nippon Ganka Gakkai Zasshi 72: 1733–1739, 1968. [PubMed] [Google Scholar]
- 21.Leon L, Jeannin JF, Bettaieb A. Post-translational modifications induced by nitric oxide (NO): implication in cancer cells apoptosis. Nitric Oxide 19: 77–83, 2008. [DOI] [PubMed] [Google Scholar]
- 22.Mackay D, Ionides A, Berry V, Moore A, Bhattacharya S, Shiels A. A new locus for dominant “zonular pulverulent” cataract, on chromosome 13. Am J Hum Genet 60: 1474–1478, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martínez-Ruiz A, Lamas S. S-nitrosylation: a new paradigm in signal transduction. Cardiovasc Res 62: 43–52, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Moreno AP, Chanson M, Anumonwo J, Scerri I, Gu H, Taffet SM, Delmar M. Role of the carboxyl terminal of connexin43 in transjunctional fast voltage gating. Circ Res 90: 450–457, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Nabekura T, Koizumi Y, Nakao M, Tomohiro M, Inomata M, Ito Y. Delay of cataract development in hereditary cataract UPL rats by disulfiram and aminoguanidine. Exp Eye Res 76: 169–174, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Olofsson EM, Marklund SL, Behndig A. Glucose-induced cataract in CuZn-SOD null lenses: an effect of nitric oxide? Free Radic Biol Med 42: 1098–1105, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Ornek K, Karel F, Büyükbingöl Z. May nitric oxide molecule have a role in the pathogenesis of human cataract? Exp Eye Res 76: 23–27, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Paul DL, Ebihara L, Takemoto LJ, Swenson KI, Goodenough DA. Connexin46, a novel lens gap junction protein, induces voltage-gated currents in nonjunctional plasma membrane of Xenopus oocytes. J Cell Biol 115: 1077–1089, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pfahnl A, Dahl G. Gating of Cx46 gap junction hemichannels by calcium and voltage. Pflügers Arch 437: 343–353, 1999. [DOI] [PubMed] [Google Scholar]
- 30.Quist AP, Rhee SK, Lin H, Lal R. Physiological role of gap-junctional hemichannels. Extracellular calcium-dependent isosmotic volume regulation. J Cell Biol 148: 1063–1074, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Retamal MA, Cortés CJ, Reuss L, Bennett MV, Sáez JC. S-nitrosylation and permeation through connexin 43 hemichannels in astrocytes: induction by oxidant stress and reversal by reducing agents. Proc Natl Acad Sci USA 103: 4475–4480, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Retamal MA, Froger N, Palacios-Prado N, Ezan P, Sáez PJ, Sáez JC, Giaume C. Cx43 hemichannels and gap junction channels in astrocytes are regulated oppositely by proinflammatory cytokines released from activated microglia. J Neurosci 27: 13781–13792, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Retamal MA, Schalper KA, Shoji KF, Bennett MV, Sáez JC. Opening of connexin 43 hemichannels is increased by lowering intracellular redox potential. Proc Natl Acad Sci USA 104: 8322–8327, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Revilla A, Castro C, Barrio LC. Molecular dissection of transjunctional voltage dependence in the connexin-32 and connexin-43 junctions. Biophys J 77: 1374–1383, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romero JM, Bizzozero OA. Extracellular S-nitrosoglutathione, but not S-nitrosocysteine or N(2)O(3), mediates protein S-nitrosation in rat spinal cord slices. J Neurochem 99: 1299–1310, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Sáez JC, Contreras JE, Bukauskas FF, Retamal MA, Bennett MVL. Gap junction hemichannels in astrocytes of the CNS. Acta Physiol Scand 179: 9–22, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schalper KA, Palacios-Prado N, Retamal MA, Shoji KF, Martínez AD, Sáez JC. Connexin hemichannel composition determines the FGF-1-induced membrane permeability and free [Ca2+]i responses. Mol Biol Cell 19: 3501–3513, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schalper KA, Palacios-Prado N, Orellana JA, Sáez JC. Current used methods for identification and characterization of hemichannels. Cell Commun Adhes 15: 207–218, 2008. [DOI] [PubMed] [Google Scholar]
- 39.Seki A, Duffy HS, Coombs W, Spray DC, Taffet SM, Delmar M. Modifications in the biophysical properties of connexin43 channels by a peptide of the cytoplasmic loop region. Circ Res 95: e22–e28, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Tenbroek E, Arneson M, Jarvis L, Louis C. The distribution of the fiber cell intrinsic membrane proteins MP20 and connexin46 in the bovine lens. J Cell Sci 103: 245–257, 1992. [DOI] [PubMed] [Google Scholar]
- 41.Trexler E, Bennett MVL, Bargiello T, Verselis V. Voltage gating and permeation in a gap junction hemichannel. Proc Natl Acad Sci USA 93: 5836–5841, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Varma SD, Chand D, Sharma YR, Kuck JF, Richards RD. Oxidative stress on lens and cataract formation: role of light and oxygen. Curr Eye Res 3: 35–57, 1984. [DOI] [PubMed] [Google Scholar]
- 43.Varma SD, Hegde KR. Susceptibility of the ocular lens to nitric oxide: implications in cataractogenesis. J Ocul Pharmacol Ther 23: 188–195, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Walter WJ, Zeilinger C, Bintig W, Kolb HA, Ngezahayo A. Phosphorylation in the C-terminus of the rat connexin46 (rCx46) and regulation of the conducting activity of the formed connexons. J Bioenerg Biomembr 40: 397–405, 2008. [DOI] [PubMed] [Google Scholar]
- 45.White TW, Bruzzone R, Goodenough DA, Paul DL. Mouse Cx50, a functional member of the connexin family of gap junction proteins, is the lens fiber protein MP70. Mol Biol Cell 3: 711–720, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.White TW, Goodenough DA, Paul DL. Targeted ablation of connexin50 in mice results in microphthalmia and zonular pulverulent cataracts. J Cell Biol 143: 815–825, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]