Abstract
The choroid plexus epithelium (CPE) secretes the major fraction of the cerebrospinal fluid (CSF). The Na+-HCO3− transporter Ncbe/Nbcn2 in the basolateral membrane of CPE cells is important for Na+-dependent pHi increases and probably for CSF secretion. In the current study, the anion transport inhibitor DIDS had no effect on the residual pHi recovery in acidified CPE from Ncbe/Nbcn2 knockout mouse by 2′,7′-bis(2-carboxyethyl)-5(6)-carboxyfluorescein (BCECF)-fluorescence microscopy in the presence of CO2/HCO3− (Ncbe/Nbcn2-ko+DIDS 109% of control, P = 0.76, n = 5). Thus Ncbe/Nbcn2 mediates the DIDS-sensitive Na+-dependent pHi recovery in the CPE. The Na+/H+ exchanger-1 Nhe1 is proposed to mediate similar functions as Ncbe/Nbcn2 in CPE. Here, we immunolocalize the Nhe1 protein to the luminal membrane domain in mouse and human CPE. The Na+-dependent pHi recovery of Nhe1 wild-type (Nhe1-wt) mice in the absence of CO2/HCO3− was abolished in the Nhe1 knockout CPE (Nhe1-ko 0.37% of Nhe1-wt, P = 0.0007, n = 5). In Ncbe/Nbcn2-ko mice, Nhe1 was targeted to the basolateral membrane. Nevertheless, the luminal Na+-dependent pHi recovery was increased in Ncbe/Nbcn2-ko compared with wild-type littermates (Nhe1-ko 146% of Nhe1-wt, P = 0.007, n = 5). Whereas the luminal Nhe activity was inhibited by the Nhe blocker EIPA (10 μM) in the Ncbe/Nbcn2-wt, it was insensitive to the inhibitor in Ncbe/Nbcn2-ko (Ncbe/Nbcn2-ko+EIPA 100% of control, P = 0.98, n = 5). This indicates that a luminal EIPA-insensitive Nhe was induced in Ncbe/Nbcn2-ko CPE and that EIPA-sensitive Nhe activity was basolateral. The Nhe1 translocation in Ncbe/Nbcn2-ko CPE may reflect a compensatory response, which provides the cells with better means of regulating pHi or transporting Na+ after Ncbe/Nbcn2 disruption.
Keywords: membrane targeting, acid/base transporters, intracellular pH, immunohistochemistry
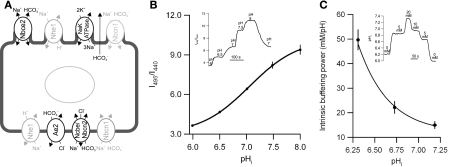
the choroid plexus epithelium (CPE) is a cerebrospinal fluid (CSF)-secreting tissue located in the brain ventricles. The secretion process in the CPE is driven by direct Na+ extrusion by the luminal Na-K-ATPase (22, 23, 31). The mechanism by which Na+ enters the CPE from the basolateral side is, however, not fully established. CSF secretion varies with basolateral pH and [HCO3−], is sensitive to the anion transport inhibitor DIDS (9, 14), and the Na+/H+ exchange inhibitor amiloride (6, 16). This indicates the involvement of Na+-dependent acid/base transporters in the secretory process. At least three Na+-dependent acid/base transporters are expressed in the CPE and may candidate as important Na+ import pathways (Fig. 1A).
Fig. 1.
A: diagram of selected transporters in the choroid plexus epithelium (CPE) cell important for intracellular pH (pHi) regulation. In the luminal membrane: Nbce2 (electrogenic Na+-HCO3− cotransporter-2); Nhe1 (Na+/H+ exchanger-1); Na-K-ATPase; Nbcn1 (electroneutral Na+-HCO3− cotransporter-1). In the basolateral membrane: Nhe1; Ae2 (anion exchanger-2); Ncbe/Nbcn2 (Na+-dependent Cl−/HCO3− exchanger or electroneutral Na+-HCO3− cotransporter-2); Nbcn1. Grey color indicates proteins with varying localization. B: calibration of intracellular 2′,7′-bis(2-carboxyethyl)-5(6)-carboxyfluorescein (BCECF) fluorescence to pHi level. Isolated clusters of CPE cells were loaded with membrane-permeable BCECF-AM, and pHi was clamped to varying known extracellular pH values by superfusion with high-K+ HEPES-buffers containing nigericin (inset). The figure shows the fluorescence excitation ratio (495 to 440 nm) as the function of pHi, and a means ± SE-weighted sigmoidal fit has been added. Data represents means ± SE of 5 experiments on wild-type, heterozygous, and Ncbe/Nbcn2-ko mice. C: intrinsic buffering power was assessed by applying NH4+ pulses in Na+-free and HCO3−/CO2-free HEPES-buffer at different values of pHi. A 5 mM NH4Cl pulse was followed by 20, 10, 5, and 0 mM NH4Cl pulses (inset). The induced pHi changes were used to calculate the mean intrinsic buffering power at the corresponding mean level of pHi. Data represents mean values ± SE of 6 pooled experiments from Ncbe/Nbcn2-ko and -wt. A means ± SE-weighted exponential fit of the data has been added.
The Slc4a10 gene product Ncbe/Nbcn2 (17, 30) is a basolateral, electroneutral, and DIDS-sensitive Na+-HCO3− importer in the CPE. Deletion of Ncbe/Nbcn2 in mice results in a 70% decreased pHi recovery rate following acidification in CPE cells (8). The brain ventricle size in the Ncbe/Nbcn2 knockout (Ncbe/Nbcn2-ko) mice was decreased by 78% compared with wild-type mice, suggesting that Ncbe/Nbcn2 may be the key blood side Na+ importer in the CPE (8). Another electroneutral Na+-HCO3− importer Nbcn1 (or NBC3, Slc4a7 gene product) is also located in the basolateral membrane of the rat and mouse CPE (20). In humans, most CPE cells also express Nbcn1 in the basolateral membrane, but some areas exhibit luminal Nbcn1 expression (21). Nbcn1 has been suggested to serve as the main mechanism for rat CPE cells to restore pHi following acidification (2). However, Nbcn1 is relatively DIDS insensitive and cannot account for the DIDS sensitivity of CSF secretion. Slc9a1 mRNA, encoding the Na+/H+ exchanger Nhe1, has also been detected in the rat choroid plexus (10). Nhe1 protein has been suggested to be expressed in the basolateral membrane of CPE cells to explain the amiloride sensitivity of CSF secretion (6, 27). The exact localization of the protein in the CPE cell has not been reported previously. The contribution of Nhe1 to pHi regulation in CPE cells is modest compared with Na+-dependent HCO3− transport in the presence of HCO3−/CO2 (2, 14). No other Na+ transporters have been described in the basolateral membrane, but Nbce2, a DIDS-sensitive electrogenic Na+-HCO3− cotransporter, is located in the luminal membrane of CPE-cells (2).
Given the pronounced effects of Ncbe/Nbcn2 disruption on CPE described above, we investigated to what extent the loss of Ncbe/Nbcn2 was compensated for by other basolateral Na+-dependent acid/base transporters. The DIDS sensitivity of residual Na+-dependent HCO3− cotransport was studied, as was protein abundances and membrane targeting of the Na+-dependent acid/base transporters expressed by the CPE. The localization and activity of the Na+/H+ exchanger Nhe1 was studied in normal mice, Nhe1 knockout mice, and Ncbe/Nbcn2 knockout mice, by semiquantitative immunofluorescence and recordings of intracellular pH regulation in CPE cells.
EXPERIMENTAL PROCEDURES
Animals and human material.
Heterozygous Ncbe/Nbcn2 null mice on mixed 129SV/C57Bl6 background (8) were bred to obtain Ncbe/Nbcn2 knockout (Ncbe/Nbcn2-ko) and Ncbe/Nbcn2 wild-type (Ncbe/Nbcn2-wt) mice. Heterozygous Nhe1 null mice on FVB background (1) were mated to produce Nhe1 knockout (Nhe1-ko) and Nhe1 wild-type (Nhe1-wt) mice. Genotypes were determined by polymerase chain reaction (PCR) of genomic DNA from tail biopsies. Tails were boiled at 95°C for 30 min in 25 mM NaOH and 0.2 mM EDTA. For the PCR reaction, a total of 20% DNA-containing solution was added 1 pmol of each primer (Table 1) and HotStarTaq Master Mix (Qiagen). After activation at 95°C for 15 min, PCR was performed for 35 cycles: denaturation at 95°C for 30 s, annealing at 58°C for 30 s (Ncbe/Nbcn2) or 57°C for 1 min (Nhe1), and elongation at 72°C for 1 min. PCR products were separated by 2% agarose gel electrophoresis with ethidium bromide and photographed under ultraviolet illumination. Littermates were compared in all experiments. All procedures were conformed to Danish animal welfare regulations. The authors are licensed to breed the mouse strains and conduct the described experiments by the Danish Ministry of Justice. Human RNA from choroid plexus and control tissues was purchased from Tebu-bio and human tissues for immunohistochemistry were obtained post mortem from donated tissue according to the Danish guidelines for use of human material.
Table 1.
Primers for genotyping and reverse transcription PCR
| Species | Primer/Direction | Sequence | |
|---|---|---|---|
| Genotyping | Mouse | NheI forward | GTGATCCCCACCATCTCAAG |
| NheI-wt reverse | CGAAGACAAGGATGTGTAGC | ||
| NheI-ko reverse | GCATGCTCCAGACTGCCTTG | ||
| Ncbe/Nbcn2 forward | CTGCAAGCAATGTGTGAGGAG | ||
| Ncbe/Nbcn2-wt reverse | GAGCAGCCCAGATGTACACCAGC | ||
| Ncbe/Nbcn2-ko reverse | CTCCCTACAGACCTCCAACAG CG | ||
| RT-PCR | Mouse | NheI forward | GGCATCGAGGACATCTGTGG |
| NheI reverse | CTGCAGACTTGGGGTGGATG | ||
| Human | NHE1 forward | TCCACCGTCTCCATGCAGAAC | |
| NHE1 reverse | GTCTCCTTGCTCCGCATCATG |
wt, Wild-type; ko, knockout. See text for more information.
Reverse transcription-PCR.
Mouse RNA was isolated using RNeasy Mini Kits (Qiagen) and was DNAse treated (RQ DNaseI, Promega). Human total RNA was DNAse treated by the provider (Tebu-bio). The RNA was reverse transcribed using 2 U/μl RT (Superscript II, Invitrogen). A total of 10% cDNA and 1 pmol of each primer (Table 1) was added HotStarTaq Master Mix. After activation at 95°C for 15 min, PCR was performed for 30 cycles: denaturation at 95°C for 30 s, annealing at 60°C for 30 s, and elongation at 72°C for 1 min. Negative controls included omission of reverse transcription or omission of cDNA. PCR for β-actin was performed to validate the template using exon spanning primers (not shown). PCR products were separated and visualized as above. Primers to mouse Nhe1 and human NHE1 yielded products of 247 bp and 502 bp, respectively.
Immunohistochemistry.
Mice from both strains were fixed via the heart with 3% paraformaldehyde in a phosphate-buffered salt solution (PBS, in mM: 167 Na+, 2.8 H2PO4−, 7.2 HPO42−; pH 7.4). The brain was removed after fixation, dehydrated, embedded in paraffin wax, and 2-μm sections were cut using a rotating microtome (Leica). The sections were dewaxed and stepwise rehydrated. Epitopes were retrieved by boiling the sections in 10 mM Tris buffer (pH 9) with 0.5 mM EGTA, and then quenched with 50 mM NH4Cl. Unspecific binding was blocked by 1% BSA in PBS with 0.2% gelatin and 0.05% saponin.
The sections were incubated overnight at 4°C with primary antibodies diluted in 0.1% BSA in PBS added 0.3% Triton X-100. Anti-Nhe1, -Nbcn1, -Ncbe/Nbcn2, and -Nbce2 antibodies were previously described and validated: Nhe1 (24, 28), Nbcn1 (5), Ncbe/Nbcn2 (8, 20), and Nbce2 (2). The interneuron marker glutamic acid decarboxylase (GAD, Alexis) was used for whole brain scanning images. The primary antibodies were visualized using Alexa Fluor 488-coupled goat anti-rabbit secondary antibodies (Invitrogen). Nuclei were visualized by Topro3 counterstaining (Invitrogen). After being washed, sections were mounted with a coverslip in Glycergel Antifade Medium (Dako) and inspected on a Leica DMIRE2 inverted microscope with TC5 SPZ confocal unit using a ×64/1.32 numerical aperture HCX Pl Apo objective. Sections from mouse kidney and duodenum were used as positive controls.
Some immunofluorescence images were merged with the corresponding differential interference contrast image to reveal the subcellular localization of the fluorescence labeling. Where necessary the luminal surface was outlined from the differential interference contrast images and background fluorescence to clarify the localization. For flat-bed laser scanning, Alexa Fluor-680 and -800 secondary antibodies were applied and the signal visualized on a LiCor Odyssey scanner. For peroxidase staining, horseradish peroxidase-conjugated goat anti-rabbit secondary antibodies (Dako) were used. Labeling was visualized by 3,3′-diaminobenzidine tetrahydrochloride dissolved in PBS with 0.1% H2O2 for 5 min in PBS. Mayer's hematoxylin was used for counterstaining, and the sections were dehydrated in graded alcohol and xylene and mounted in hydrophobic Eukitt mounting medium (O. Kindler). Brightfield microscopy was performed on a Leica DMRE microscope equipped with a Leica DM300 digital camera.
Semiquantization of immunofluorescence on brain sections.
The protein abundance of Nhe1, Nbcn1, Nbce2, and Ncbe/Nbcn2 proteins was investigated by quantifying the immunofluorescence intensities from confocal micrographs. Images were acquired in the focal plane with the highest signal intensity using fixed settings for magnification, laser power, gain, offset, and averaging for all images with a given antibody. To avoid saturation of the photomultiplier, the dynamic range (gain and offset) was adjusted to span the intensities of the most intense sample for each antibody. Linearity of the signal was verified by serial dilutions of the secondary antibody on slides mounted in antifade mounting medium (Glycergel Antifade Medium, Dako, data not shown). With the applied midlevel gain, midlevel laser power, linearity of the fluorescence signal was fully conserved as long as saturated pixels are avoided and background is kept around 1 gray value (8-bit image depth). Thus the dynamic range practically allows detection of more than 100-fold changes in fluorescence signal. The choroid plexus immunofluorescence intensities were quantified after background subtraction using Image Pro software (Media Cybernetics). For each image, the choroid plexus epithelium was outlined semiautomatically, and the total fluorescence within the region was determined and divided by the area of the outlined region. For each antibody, values were normalized to the area-corrected immunofluorescence of wild-type mouse choroid plexus.
Intracellular pH measurements.
Mice were anesthetized by isoflurane inhalation and terminated by decapitation. The brain was removed and placed in a cold HEPES-buffered salt solution (HBS in mM: 145 Na+, 0.8 Mg2+, 0.8 SO42−, 2 PO43−, 138.4 Cl−, 3.6 K+, 1.8 Ca2+, 10.0 HEPES, and 5.5 glucose; pH 7.4) and placed on ice for 20–30 min. The choroid plexus was removed from the brain ventricles by manual dissection under a stereo microscope and placed on ice in HBS. The tissue was used for experiments within 2–4 h. Two protocols were used depending on whether the investigation was focused on bilateral or luminal transport. To gain access to both luminal and basolateral transporters, the choroid plexi were digested into small clusters of cells with 4 μg/ml dispase (Invitrogen) and 4 μg/ml collagenase type IV (PAN Biotech) in Ca2+ free HBS at 37°C for 30 min. In the second protocol used for studying luminal Nhe activity, the isolated choroid plexus cells were plated directly after dissection to minimize ionic access to the basolateral transporters.
Both digested and nondigested choroid plexi were mounted on Cell-Tak-coated (BD Biosciences) coverslips for 30 min at 37°C. The tissue was loaded with the pH-sensitive fluorescent dye BCECF-AM (Invitrogen). The coverslips were mounted in a closed perfusion chamber (358 μl vol RC-21BR; Harvard Apparatus) and loaded for 10–15 min with 2 μM BCECF-AM for digested choroid plexus and 10 μM BCECF-AM for nondigested choroid plexus because of slow dye uptake. The slow dye uptake was used to ascertain the lack of basolateral access in the nondigested choroid plexus. Areas near the open end of the choroid plexus showed very rapid dye uptake. Regions of interest were chosen at least 100 μm from such areas. Leak of simple ions through the tight junctions cannot be ruled out, because the choroid plexus is a leaky epithelium. The tissue was superfused with a linear flow rate of 0.8 mm/s corresponding to 1 ml/min or 2.8 bath changes per min during recording at 37°C throughout the experiments. The dye excitation periods of 20 ms were alternating between 495 nm and 440 nm light from a monochromator (Till Photonics). The light emission at 510- to 535-nm was recorded by a 12-bit cooled monochrome CCD camera (QImaging, Retiga EXi). QED InVivo imaging software (Media Cybernetics) was used to control wavelength, light exposure time (40 ms), frequency (1 image pair each 4 s), and binning (to 348 × 260 pixel images), as well as for the data collection from user-defined regions of interest (individual cells/cytosol). Excitation ratios were calibrated from choroid plexus epithelial cells where pHi was clamped to stepwise changing extracellular pH (pHo) by 10 μM nigericin in a high-K+ buffer (Ncbe/Nbcn2 mice: Fig. 1B) (3).
Experiments were performed either in the presence or absence of HCO3−/CO2 as indicated. Cells in both protocols were first allowed to equilibrate to a baseline level in the Na+ containing buffer and were then acidified by an NH4Cl prepulse (3–5 min with equimolar substitution of Na+ by NH4+), shifted to a Na+-free buffer for 1–2 min (equimolar substitution of Na+ by N-methyl-d-glucammonium), and then shifted back to bicarbonate-buffered solution (BBS, in mM: 145 Na+, 0.8 Mg2+, 0.8 SO42−, 2 PO43−, 114.6 Cl−, 3.6 K+, 1.8 Ca2+, 10.0 HEPES, 5.5 glucose, and 24 HCO3−; pH 7.4) or HBS as indicated on figures. For inhibition of DIDS-sensitive transporters, 200 μM DIDS was added to the BBS used after acidification. For inhibition of EIPA-sensitive Nhe activity, 10 μM EIPA was added to the HBS used after the acidification protocol as indicated on figures. Each experiment was ended by a one-point calibration at pHo 7.0 in nigericin/high-K+ buffer as described by Boyarsky and co-workers (3).
The rate of pHi recovery was determined as the pHi increase over 60 s after reintroduction of Na+ (dpHi/dt). Only experiments where the washout of NH4Cl resulted in significant acidification under baseline pHi level were included in the study. The net d[H+]i/dt was calculated as the product of the intrinsic buffering power (βint) and the dpHi/dt. In studies performed in the presence of CO2/HCO3−, the total buffering power (βtot) was calculated as the sum of (βint) and the contribution of the CO2/HCO3− buffering system. Net d[H+]i/dt in the presence of CO2/HCO3− was calculated as the product of βtot and dpHi/dt (3). To avoid any involvement of transport processes, Na+ was replaced with N-methyl-d-glucamine throughout these experiments. NH4+/NH3-containing solutions were added in the order of 5, 20, 10, 5, and 0 mM to calculate the buffering power at different pHi values (Fig. 1C, inset). With the assumption that [NH3]i = [NH3]o when pHi reached a new steady state after each step and a pKa for NH4+ of 8.9 at 37°C, [NH4+]i was calculated at each steady-state pHi. The resulting change in [NH4+]i divided by the change in pHi was determined as the buffering power of the cell at the half point between the two steady-state pHi levels. The buffering power calculations were grouped into pHi intervals of 0.25 (Fig. 1C). Calibration data and buffering capacity estimates were collected from digested CPE preparations from each mouse line. The data from three individual cells per recording and one to three recordings per animal were pooled for each experiment (n). Thus each n represents one animal. The net acid extrusion or base uptake rate is reported as d[H+]i/dt values, which in effect represents the Na+-dependent pHi recovery rate, as the recovery in the absence of Na+ was negligible. An original trace for each set of experiments is shown including means ± SE values for baseline pHi levels, peak NH4Cl alkalization level, and acidification level following NH4Cl pulse for each genotype. Mean flux ± SE values are shown in bar graphs.
Statistical analysis.
Data were analyzed by two tailed t-test. For paired studies, Dunnett test was performed comparing groups to the wild-type control group. Values of P < 0.05 were considered an appropriate level of statistical significance.
RESULTS
Ncbe/Nbcn2 mediates DIDS-sensitive Na+-dependent pHi recovery in the murine choroid plexus.
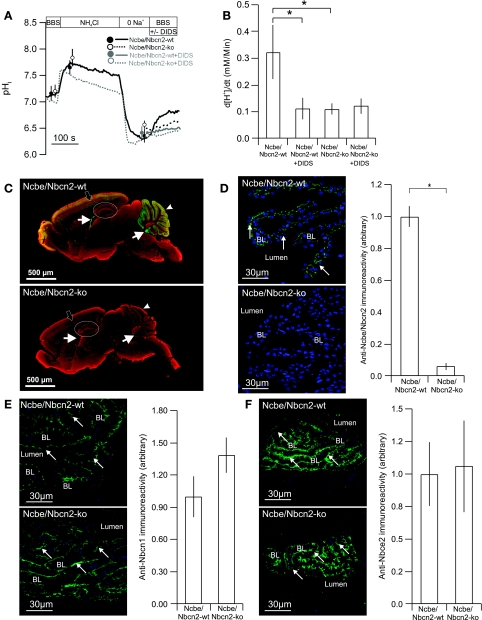
The Na+-dependent pHi recovery in the presence of CO2/HCO3− was measured on small clusters of cells from the choroid plexus from the Ncbe/Nbcn2-ko and Ncbe/Nbcn2-wt littermates. The net acid extrusion rate in the Ncbe/Nbcn2-ko was reduced by 67% compared with Ncbe/Nbcn2-wt (wt: n = 6; ko: n = 5, P < 0.01, Fig. 2, A and B). Initial pHi after acidification did not differ between Ncbe/Nbcn2-wt and Ncbe/Nbcn2-ko. Application of DIDS during pHi recovery inhibited the net acid extrusion rate in Ncbe/Nbcn2-wt, but no further inhibition was observed in Ncbe/Nbcn2-ko (n = 5, P > 0.05, Fig. 2B). Thus Ncbe/Nbcn2 seems to be the major DIDS-sensitive Na+-HCO3− transporter in the acidified choroid plexus and neither the DIDS-insensitive Na+-HCO3− transport nor the Na+/H+ exchange are increased as compensation in Ncbe/Nbcn2-ko CPE. An immunofluorescence microscopical approach was used to compare acid/base transport protein expression levels in knockout mice and wild-type littermates. Anti-Ncbe/Nbcn2 immunoreactivity was present in several brain structures including the choroid plexus (Fig. 2C, top), whereas it was absent in the Ncbe/Nbcn2-ko brain (Fig. 2C, bottom). As shown in Fig. 2D, the anti-Ncbe/Nbcn2 immunofluorescence signal was decreased by 94% in Ncbe/Nbcn2-ko compared with Ncbe/Nbcn2-wt (n = 4, P = 0.0001).
Fig. 2.
Na+-dependent pHi recovery in CPE cells from Ncbe/Nbcn2-wt and -ko mice in the presence of HCO3−/CO2. A: isolated clusters of CPE cells were acidified by a 5-min NH4Cl pulse (NH4Cl) and subsequent removal of extracellular Na+ (0 Na+). The recovery of pHi was recorded as Na+ was reintroduced to the superfusate in Ncbe/Nbcn2-wt mice (solid line) and -ko mice (dotted line), respectively. Mean values for baseline pHi, peak alkalization by NH4Cl, and pHi at maximal acidification are shown for Ncbe/Nbcn2-ko (open circles) and -wt (filled circles). BBS, Na+ containing HCO3−/CO2-buffered solution. B: mean values for net acid extrusion rate (d[H+]i/dt) in CPE cells from Ncbe/Nbcn2-wt and -ko mice in the presence and absence of DIDS, as indicated (n = 5, *statistical significance). C: immunohistochemical scanning of Ncbe/Nbcn2 expression level (green) in whole brain sections from Ncbe/Nbcn2-wt (top) and -ko mice (bottom). Filled arrows show choroid plexus, arrowheads show cerebellum, and black arrows show the cerebral cortex. Hippocampus is encircled. Red color denotes counterstaining with the interneuron marker glutamic acid decarboxylase. D: immunohistochemical detection of Ncbe/Nbcn2-expression level (green) in Ncbe/Nbcn2-wt (top left) and -ko CPE (bottom left). Blue color represents nuclear counterstaining. BL, basolateral membrane domain, arrows indicates antibody staining. Right shows semiquantization of cellular Ncbe/Nbcn2-immunoreactivity (n = 4). Immunohistochemical detection of Nbcn1- (E) and Nbce2- (F) expression levels and membrane targeting in the choroid plexus of Ncbe/Nbcn2-wt (top) and -ko mice (bottom).
Nbcn1 and NBCe2 protein abundance is not changed in Ncbe/Nbcn2-ko mice.
To support the functional lack of compensatory Nbcn1 activity (i.e., DIDS-insensitive Na+-HCO3− cotransport) in Ncbe/Nbcn2-ko choroid plexus, we stained brain sections with anti-Nbcn1 antibodies. Immunoreactivity was detected corresponding to the luminal membrane domain in both Ncbe/Nbcn2-ko and Ncbe/Nbcn2-wt CPE (Fig. 2E). The anti-Nbcn1 fluorescence intensity was unchanged in Ncbe/Nbcn2-ko compared with Ncbe/Nbcn2-wt (139% not significant, n = 4, Fig. 2E). The electrogenic Na+-HCO3− cotransporter Nbce2 is located in the luminal membrane domain of choroid plexus cells and is DIDS-sensitive like Ncbe/Nbcn2. Since we did not detect DIDS-sensitive HCO3− recovery in isolated CPE cells from Ncbe/Nbcn2-ko mice, Nbce2 seems unable to work in an inward transport mode under these circumstances. Nevertheless, anti-Nbce2 immunoreactivity was detected corresponding to the luminal CPE membranes, and the fluorescence intensities were similar in Ncbe/Nbcn2-ko and Ncbe/Nbcn2-wt CPE (106% Not significant, n = 4, Fig. 2F).
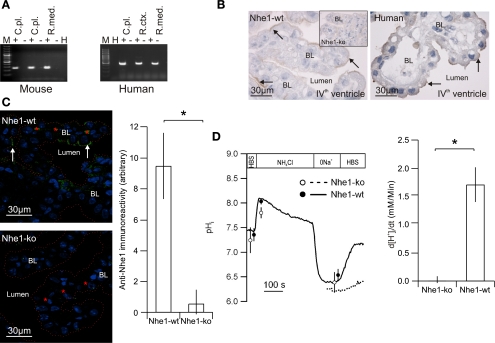
Mouse and human choroid plexus express Nhe1/NHE1 mRNA and protein.
As described above, Nhe1 has been proposed as an alternative basolateral Na+-loading mechanism in the choroid plexus. Figure 3A illustrates that RT-PCR produced a 247-bp Nhe1 product in mouse choroid plexus as well as a 502-bp NHE1 product from human choroid plexus. Anti-Nhe1 immunoreactivity was primarily found corresponding to the luminal membrane domains of choroid plexus in the Nhe1-wt mice, although weak basolateral staining cannot be ruled out (Fig. 3B, left). The inset in Fig. 3B shows the labeling pattern in Nhe1-ko mouse CPE. In human CPE, similar luminal membrane domain anti-NHE1 labeling was observed (Fig. 3B, right). The anti-Nhe1-immunoreactivity was determined in Nhe1-wt and Nhe1-ko mouse CPE to further validate the novel Nhe1 labeling pattern. Nhe-1 protein abundance was reduced by 95% in Nhe1-ko mice CPE (P = 0.0029, n = 5, Fig. 3C). A similar labeling pattern was found in mouse and human CPE with another anti-Nhe1 antibody (from J. Noël, Dept. of Physiology, University of Montreal, Canada, not shown). The Na+-dependent pHi recovery was investigated in the absence of CO2/HCO3− on small clusters of CPE to demonstrate the functional presence of Nhe1. The acid extrusion rate in the Nhe1-ko was decreased by 98.5% compared with Nhe1-wt (n = 6, P = 0.0226, Fig. 3D), indicating that Nhe1 is the only functionally active Na+/H+ exchanger in the murine choroid plexus under normal circumstances. Initial pHi after acidification did not differ between the genotypes.
Fig. 3.
Expression of Nhe1 in mouse and human choroid plexus. A: RT-PCR for Nhe1/NHE1 transcript was performed with mRNA from mouse (left) and human (right) choroid plexus (C.pl.) with renal cortex (R.ctx.) and medulla (R.med.) as positive controls and omission of reverse transcription or template as negative control (− for omitted RT and H for H2O, respectively). A 100-bp ladder was used to indicate molecular size. B: representative examples of anti-Nhe1 immunostaining in choroid plexus from Nhe1-wt mice (left, n = 5), Nhe1-ko mice (inset, n = 5), and humans (right, n = 8). C: semiquantization of cellular Nhe1-immunoreactivity in choroid plexus epithelial cells from Nhe1-wt (top) and -ko mice (bottom). Dotted line shows choroid plexus luminal surface and red stars indicate red blood cells. The bar graph show the resulting mean values for net acid extrusion rate (d[H+]i/dt, n = 5, *statistical significance). D: Na+-dependent pHi recovery in CPE cells from Nhe1-wt and -ko mice in the absence of HCO3−/CO2. Isolated clusters of CPE cells were acidified by NH4Cl pulsing (NH4Cl). The recovery of pHi was recorded as Na+ was reintroduced to the superfusate (HBS) in Nhe1-wt mice (solid line) and -ko mice (dotted line), respectively. The bar graph shows mean values for net acid extrusion ratein CPE cells from Nhe1-wt and -ko mice (n = 5). HBS, Na+-containing HEPES-buffered solution.
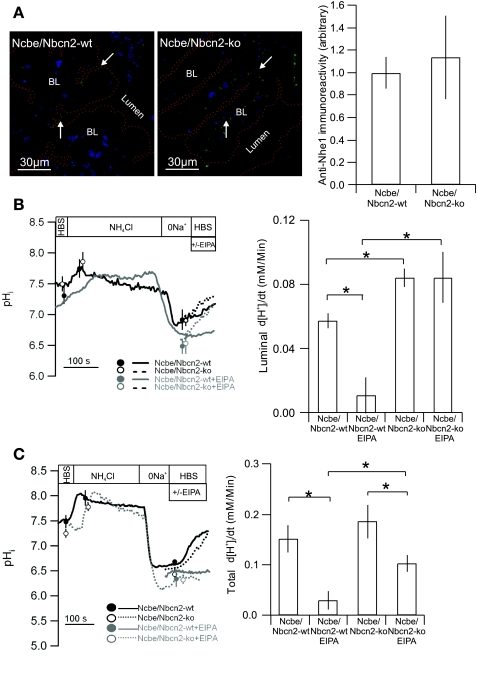
Nhe1 targeting is shifted from luminal to basolateral in Ncbe/Nbcn2-ko mice.
To investigate the possible compensatory change of Nhe1 abundance in the Ncbe/Nbcn2-ko mouse, brain sections from the Ncbe/Nbcn2-ko and Ncbe/Nbcn2-wt littermates were stained with anti-Nhe1 antibodies. In contrast to Ncbe/Nbcn2-wt mice CPE, Nhe1 was detected exclusively in the basolateral membrane domain of Ncbe/Nbcn2-ko mice (Fig. 4A). Despite this remarkable change in membrane targeting, the total Nhe1-specific fluorescence intensity was unchanged in Ncbe/Nbcn2-ko compared with Ncbe/Nbcn2-wt (113%, P = 0.6999, n = 4 for Ncbe/Nbcn2-ko and 6 for Ncbe/Nbcn2-wt, Fig. 4A).
Fig. 4.
Expression and regulation of Nhe1 in Ncbe/Nbcn2-wt and -ko mice. A: representative examples of Nhe1 immunostaining in choroid plexus from Ncbe/Nbcn21-wt (left), in Ncbe/Nbcn2-ko mice (middle), and semiquantization of cellular Nhe1-immunoreactivity in choroid plexus epithelial cells from Ncbe/Nbcn2-wt and -ko mice (right, n = 5). B: Na+-dependent pHi recovery in choroid plexus epithelial cells from Ncbe/Nbcn2-wt and -ko mice in the absence of HCO3−/CO2. Intact IVth or lateral ventricle CPE were acidified as above. Smoothed traces show NH4Cl pulses and pHi recoveries in Ncbe/Nbcn2-wt mice (solid line) and -ko mice (dotted line), respectively. Bar graph shows mean values for net acid extrusion rate (d[H+]i/dt) in intact CPE from Ncbe/Nbcn2-wt and -ko mice in the presence and absence of EIPA, as indicated (n = 5). C: isolated clusters of CPE cells were acidified by NH4Cl pulsing (NH4Cl). The recovery of pHi was recorded as Na+ was reintroduced to the superfusate (HBS) in Ncbe/Nbcn2-wt mice (solid line) and -ko mice (dotted line), respectively. Bar graph shows mean values for net acid extrusion rate (d[H+]i/dt) in CPE cells from Ncbe/Nbcn2-wt and -ko mice in the presence and absence of EIPA, as indicated (n = 5). *Statistical significance.
An EIPA-insensitive Nhe activity is induced in the choroid plexus of Ncbe/Nbcn2-ko mice.
As luminal Nhe1 immunoreactivity is absent in the Ncbe/Nbcn2-ko mice and as Nhe1 is the only Na+/H+ exchanger in the normal murine CPE, we investigated whether the Na+-dependent pHi recovery in the absence of CO2/HCO3− was abolished at the luminal side in intact choroid plexus. Surprisingly, the Ncbe/Nbcn2-ko mice showed increased luminal net acid extrusion rate compared with Ncbe/Nbcn2-wt littermates (n = 5, P < 0.05, Fig. 4B). Initial pHi after acidification did not differ between the genotypes. Whereas the luminal accessible net acid extrusion rate was reduced by 81% by EIPA in choroid plexus from Ncbe/Nbcn2-wt mice (n = 5, P < 0.05), the pHi recovery was unchanged by EIPA in the Ncbe/Nbcn2-ko mouse choroid plexus (n = 5, P > 0.05, Fig. 4B). To investigate the relative contribution of EIPA-sensitive and -insensitive transport to the total cellular Nhe activity in the Ncbe/Nbcn2-ko mice, we again measured the Na+-dependent pHi recovery rate of isolated clusters of CPE. The net acid extrusion rate did not differ between Ncbe/Nbcn2-wt and Ncbe/Nbcn2-ko (P > 0.05, n = 5, Fig. 4C). Addition of EIPA during pHi recovery resulted in an 80% inhibition of net acid extrusion rate in Ncbe/Nbcn2-wt (n = 5, P < 0.05, Fig. 4C). In Ncbe/Nbcn2-ko, addition of EIPA resulted in a 45% reduction of the net acid extrusion rate (n = 5, P < 0.05 Fig. 4C). Thus the EIPA-insensitive component of the pHi recovery was significantly larger in Ncbe/Nbcn2-ko CPE than in Ncbe/Nbcn2-wt CPE (P < 0.05) indicating an EIPA-insensitive component of Nhe in the Ncbe/Nbcn2-ko that is not present in the Ncbe/Nbcn2-wt mice. The EIPA-sensitive d[H+]i/dt, (i.e., Nhe1 activity) in the Ncbe-wt is apparently greater in isolated cell clusters than in intact tissue.
Nhe1 ablation does not change Nbcn1 or Ncbe/Nbcn2 expression levels in murine choroid plexus.
We then determined Na+-HCO3− transport protein abundances in Nhe1-ko mice to investigate whether ablation of a Na+-dependent acid/base transporter in one domain of cell in general leads to the compensatory retargeting of an alternative Na+-dependent acid/base transporter from the opposite domain. Both Ncbe/Nbcn2 and Nbcn1 were found in the basolateral membrane domain of CPE of Nhe1-ko as well as Nhe1-wt mice (Fig. 5, A and B). Neither the anti-Ncbe/Nbcn2 nor the anti-NBCn1 immunoreactivities were changed in Nhe1-ko mouse CPE compared with Nhe1-wt (Ncbe/Nbcn2: 113%, P = 0.5078, n = 5; Nbcn1: 96%, P = 0.4743, n = 5, Fig. 5, A and B).
Fig. 5.
Expression of Nbcn1 and Ncbe/Nbcn2 in choroid plexus from Nhe1-wt and -ko mice. A: immunohistochemical detection of Nbcn1-expression level and membrane targeting in the choroid plexus of Nhe1-wt (top) and -ko mice (bottom). Right shows semiquantization of cellular Nbcn1-immunoreactivity (n = 5). B: immunohistochemical detection of Ncbe/Nbcn2-expression level and membrane targeting in the choroid plexus of Nhe1-wt (top) and -ko mice (bottom). Right shows semiquantization of cellular Nbcn1-immunoreactivity (n = 5).
DISCUSSION
In the present study, we investigated the cellular implications of deleting an important Na+-dependent HCO3− importer in choroid plexus with regards to other Na+-dependent pHi regulatory mechanisms. The hypothesis was that the epithelium might compensate appropriately for the lack of Ncbe/Nbcn2 by increasing the abundance or function of other Na+-HCO3− transporters expressed in the epithelium. Alternatively, the epithelium might dedifferentiate to a less specialized form in which cell survival is the priority rather than vectorial transport of solutes and water.
In a previous study, deletion of Ncbe/Nbcn2 in the choroid plexus caused a 70% decrease in Na+-dependent pHi recovery rate following acidification (8). The present study extends this observation in that the residual acid extrusion in Ncbe/Nbcn2-ko is not DIDS sensitive. The net acid extrusion rate in Ncbe/Nbcn2-ko is similar to the DIDS-insensitive acid extrusion rate in the Ncbe/Nbcn2-wt, indicating that Ncbe/Nbcn2 mediates all DIDS-sensitive HCO3− uptake in CPE. The other DIDS-sensitive Na+-HCO3− transporter in choroid plexus Nbce2 seems not to contribute to the Na+-dependent HCO3− import, although Nbce2 is equally expressed in Ncbe/Nbcn2-wt and Ncbe/Nbcn2-ko as assessed by immunofluorescence. Originally, NBCe2 was shown to transport Na+ and HCO3− outward with a 1:3 stoichiometry (25) or inward, with a 1:2 stoichiometry (29), when expressed in Xenopus oocytes. Recently, Millar and Brown (15) evidenced an outward 1:3 transport mode for the luminal electrogenic Na+-HCO3− transport in mouse CPE. This possibly explains the lack of inward DIDS-sensitive Na+-HCO3− transport in the present study.
The DIDS-insensitive Nbcn1 was the most likely protein to compensate for Ncbe/Nbcn2 disruption, as it was previously immunolocalized to the basolateral plasma membrane (20). In the present study, we find Nbcn1 expressed in the luminal membrane domain in both the Ncbe/Nbcn2-ko and Ncbe/Nbcn2-wt mice. Luminal membrane labeling of Nbcn1 is observed in some c57bl/6 mouse strains (unpublished data); however, in other mouse strains the immunolabeling has predominantly been basolateral (20). In rats (20) and humans (21), Nbcn1 is also usually found basolaterally; however, luminal membrane labeling was also reported for parts of the human CPE. The fact that Nbcn1 is not exclusively a basolateral protein suggests that this protein is not critically involved in basolateral Na+ import. Furthermore, the DIDS-insensitive net acid extrusion, as well as Nbcn1 abundance, did not differ between Ncbe/Nbcn2-ko and -wt mice, which is consistent with Nbcn1 not being regulated significantly by Ncbe/Nbcn2 disruption.
The Na+/H+ exchangers have similar effect on pHi as electroneutral Na+-HCO3− cotransport, and Nhe1 mRNA was previously detected in rat choroid plexus (10). In this study, Nhe1/NHE1 mRNA was found in both mouse and human choroid plexus, and anti-Nhe1 immunoreactivity is demonstrated for the first time in the choroid plexus. As opposed to other epithelia, Nhe1/NHE1 is mainly located in the luminal membrane domain of CPE in Nhe1-wt mice and humans. The striking luminal localization of Nhe1 was validated by its absence in the Nhe1-ko CPE. The insignificant residual anti-Nhe1 staining seemed restricted to the cytosol as assessed by immunofluorescence. Nhe1 was thought to be a basolateral protein in CPE if it was to explain the inhibition of CSF secretion by amiloride (16, 27). However, Nhe1 is by far not the first “basolateral” protein to be expressed luminally by the CPE. The Na-K-ATPases, Nkcc1, Kcc4, and Nbcn1 are usually basolateral epithelial transporters and are luminal proteins in CPE (11, 19, 21, 22). Other transporters, as Ae2 and Kcc3, are expressed basolaterally in CPE as in other epithelia (12, 18). A Na+-dependent and HCO3−-independent pHi recovery (i.e., Nhe activity) was detected in CPE cells indicating the functional presence of Nhe protein. In the Nhe1-ko, however, the net acid extrusion was practically absent in CPE cells, revealing that Nhe1 is the only functional Nhe. We note that the net d[H+]i/dt is approximately three times larger in the presence of HCO3−/CO2 than in its absence. Therefore, the HCO3−-dependent processes seem to be more effective in restoring pHi after acidification than the Nhe proteins in the CPE.
A striking shift in Nhe1 membrane targeting was observed in Ncbe/Nbcn2-ko mouse. The luminal membrane localization of Nhe1 in Ncbe/Nbcn2-wt was shifted to basolateral membrane in Ncbe/Nbcn2-ko. However, the total Nhe1 fluorescence intensity did not differ between Ncbe/Nbcn2-ko and Ncbe/Nbcn2-wt. To demonstrate this shift functionally, the luminal Na+-dependent net acid extrusion rate in choroid plexus was compared in the Ncbe/Nbcn2-ko mice and wt littermates. The measurements were performed on undigested choroid plexus to assess the luminal membrane transport. Surprisingly, the luminal net acid extrusion rate was larger in the Ncbe/Nbcn2-ko than in Ncbe/Nbcn2-wt CPE. In addition, the Nhe inhibitor EIPA reduced net acid extrusion to a greater extend in the Ncbe/Nbcn2-wt compared with the Ncbe/Nbcn2-ko CPE. This indicated the emergence of an EIPA-insensitive luminal Na+/H+ exchanger in the Ncbe/Nbcn2-ko that was not detectable in the Ncbe/Nbcn2-wt. This perception was supported by similar measurements on cell clusters of CPE cells with access to both membrane domains. The increase in EIPA-sensitive acid extrusion rate in isolated cell clusters compared with intact Nhe1-wt tissue may reflect that the antibody does not detect all Nhe1 protein in the section. Not all cells are anti-Nhe1 immunoreactivite at the luminal surface, whereas all of the cells display EIPA-sensitive Nhe activity. The increased EIPA-sensitive Na+/H+ activity in digested preparations would then represent a basolateral pool of Nhe1 undetected by the antibodies. Alternatively, the isolation of the cells per se could somehow enhance Nhe1 activity, and finally, quantitative comparison between protocols is problematic as the calibration and buffering power estimation was made only in the digested CPE preparation.
The EIPA-insensitive Nhe form was not identified at the molecular level, because there was no obvious expression of other Nhe forms as judged by RT-PCR and immunohistochemistry. EIPA inhibits different Nhe isoforms with different potency: Nhe1>Nhe2>Nhe5>Nhe3 (13). Little is known about the inhibitor sensitivity of other plasma membrane Nhe forms that may be present in the Ncbe-ko model. It seems unfruitful to engage in functional identification of the luminal Nhe form in Ncbe/Nbcn2-ko mice, despite the emergence of more specific inhibitors for Nhe1 (HOE-694), Nhe2 (T162559R), and for Nhe3 (S-3226), for review (13). It should be noted that the net acid extrusion rates in the “Nhe1 mouse strain” are not comparable with the values from “Ncbe/Nbcn2 mouse strain.” This discrepancy most likely reflects strain-specific basal or season-dependent Nhe1 activities, because the buffering power estimations and calibration curves are similar between strains.
Thus it seems that deleting a central Na+ loader and pHi regulator in the choroid plexus causes selective changes in expression and membrane targeting of Nhe1. The translocation of Nhe1 from a predominantly luminal localization to a basolateral localization is most likely a compensation for the lack of Ncbe/Nbcn2 in the basolateral membrane. The translocation seems unique for Nhe1 among the Na+-dependent acid/base transporters in the Ncbe/Nbcn2-ko, although the basolateral targeting motifs are similar among Nhe1, Nbcn1, and Nbce2. These three proteins and Ncbe/Nbcn2 contain YXXV/YXXI as well as double lysine motifs in the intracellular NH2-terminus, which have been suggested to be basolateral signals in other proteins (7).
To our knowledge, Nhe1 has only been detected previously on the apical membrane of A6 cells early after synthesis (4). Immature/core glycosylation led to unpolarized targeting of Nhe1 in that study and mature glycosylation of Nhe1 to strict basolateral targeting. We do not find evidence for altered glycosylation of Nhe1 in the Ncbe/Nbcn2-ko or changed molecular size of the deglycosylated Nhe1 protein by immunoblotting (data not shown). Thus the mechanism behind the change in Nhe1 targeting seems not to lie in any of the usual protein features. The retinal pigment epithelium resembles the choroid plexus in the atypical cellular distribution of proteins. A single leucine residue normally targets the CD147 to the basolateral side of polarized epithelia, but this amino acid seems to be ignored by the targeting machinery in the retinal pigment epithelium, and leucine to arginine mutations lead to apical targeting as in other epithelia (7). It is feasible that the protein targeting machinery in the CPE also responds differently to usual targeting motifs and that this response can change with cellular conditions. Nhe1 may be singled out for basolateral targeting in Ncbe/Nbcn2-ko CPE as a defense against apoptosis, because this protein has been shown to improve cell survival in other stressed epithelia (26). Interestingly, Nhe1 disruption did not evoke a similar compensatory expression of other Nhe proteins. The membrane targeting and abundance of Ncbe/Nbcn2 and Nbcn1 also did not differ between Nhe1-ko and Nhe1-wt CPE. This indicates that Ncbe/Nbcn2-ko has a more profound impact on CPE cell function than Nhe deletion.
In conclusion, disruption of Ncbe/Nbcn2 had no apparent effect on the function, membrane targeting, or abundance of two other Na+-dependent transporters NBCn1 and NBCe2. In contrast, Ncbe/Nbcn2 disruption causes translocation of Nhe1 protein and activity from the luminal to the basolateral membrane domain and the appearance of an EIPA-insensitive Nhe-form in the luminal membrane of CPE. It seems that disruption of the major basolateral Na+ loader in the CPE causes less than expected compensation with regards to Na+ loading capacity.
GRANTS
Support for this study was obtained from the Danish Medical Research Council, Karen Elise Jensens Fond, Lundbeck fonden, Aarhus University Research Foundation, and the Novo Nordisk Foundation. H. H. Damkier is supported by the Faculty of Health Sciences, Aarhus University. The Water and Salt Research Centre at the University of Aarhus was established and is funded by the Danish National Research Foundation (Danmarks Grundforskningsfond). V. Prasad was supported by National Institutes of Health grant DK-50594 (to G. E. Shull).
Acknowledgments
The authors thank Inger Merete S. Paulsen and Christian V. Westberg for technical assistance. Gary Shull is deeply thanked for supplying the Nhe1-null mouse strain and Stefan Jacobs for generating the Ncbe/Nbcn2-null mice.
REFERENCES
- 1.Bell SM, Schreiner CM, Schultheis PJ, Miller ML, Evans RL, Vorhees CV, Shull GE, Scott WJ. Targeted disruption of the murine Nhe1 locus induces ataxia, growth retardation, and seizures. Am J Physiol Cell Physiol 276: C788–C795, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Bouzinova EV, Praetorius J, Virkki LV, Nielsen S, Boron WF, Aalkjaer C. Na+-dependent HCO3− uptake into the rat choroid plexus epithelium is partially DIDS sensitive. Am J Physiol Cell Physiol 289: C1448–C1456, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Boyarsky G, Ganz MB, Sterzel RB, Boron WF. pH regulation in single glomerular mesangial cells. I. Acid extrusion in absence and presence of HCO3−. Am J Physiol Cell Physiol 255: C844–C856, 1988. [DOI] [PubMed] [Google Scholar]
- 4.Coupaye-Gerard B, Bookstein C, Duncan P, Chen XY, Smith PR, Musch M, Ernst SA, Chang EB, Kleyman TR. Biosynthesis and cell surface delivery of the NHE1 isoform of Na+/H+ exchanger in A6 cells. Am J Physiol Cell Physiol 271: C1639–C1645, 1996. [DOI] [PubMed] [Google Scholar]
- 5.Damkier HH, Nielsen S, Praetorius J. An anti-NH2-terminal antibody localizes NBCn1 to heart endothelia and skeletal and vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 290: H172–H180, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Davson H, Segal MB. The effects of some inhibitors and accelerators of sodium transport on the turnover of 22Na in the cerebrospinal fluid and the brain. J Physiol 209: 131–153, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deora AA, Gravotta D, Kreitzer G, Hu J, Bok D, and Rodriguez-Boulan E. The basolateral targeting signal of CD147 (EMMPRIN) consists of a single leucine and is not recognized by retinal pigment epithelium. Mol Biol Cell 15: 4148–4165, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobs S, Ruusuvuori E, Sipila ST, Haapanen A, Damkier HH, Kurth I, Hentschke M, Schweizer M, Rudhard Y, Laatikainen LM, Tyynela J, Praetorius J, Voipio J, Hubner CA. Mice with targeted Slc4a10 gene disruption have small brain ventricles and show reduced neuronal excitability. Proc Natl Acad Sci USA 105: 311–316, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johanson CE, Parandoosh Z, Smith QR. Cl-HCO3 exchange in choroid plexus: analysis by the DMO method for cell pH. Am J Physiol Renal Fluid Electrolyte Physiol 249: F478–F484, 1985. [DOI] [PubMed] [Google Scholar]
- 10.Kalaria RN, Premkumar DR, Lin CW, Kroon SN, Bae JY, Sayre LM, LaManna JC. Identification and expression of the Na+/H+ exchanger in mammalian cerebrovascular and choroidal tissues: characterization by amiloride-sensitive [3H]MIA binding and RT-PCR analysis. Brain Res Mol Brain Res 58: 178–187, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Karadsheh MF, Byun N, Mount DB, Delpire E. Localization of the KCC4 potassium-chloride cotransporter in the nervous system. Neuroscience 123: 381–391, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Lindsey AE, Schneider K, Simmons DM, Baron R, Lee BS, Kopito RR. Functional expression and subcellular localization of an anion exchanger cloned from choroid plexus. Proc Natl Acad Sci USA 87: 5278–5282, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masereel B, Pochet L, Laeckmann D. An overview of inhibitors of Na(+)/H(+) exchanger. Eur J Med Chem 38: 547–554, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Mayer SE and Sanders-Bush E. Sodium-dependent antiporters in choroid plexus epithelial cultures from rabbit. J Neurochem 60: 1308–1316, 1993. [DOI] [PubMed] [Google Scholar]
- 15.Millar ID, Brown PD. NBCe2 exhibits a 3 HCO3−:1 Na+ stoichiometry in mouse choroid plexus epithelial cells. Biochem Biophys Res Commun 373: 550–554, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Murphy VA, Johanson CE. Alteration of sodium transport by the choroid plexus with amiloride. Biochim Biophys Acta 979: 187–192, 1989. [DOI] [PubMed] [Google Scholar]
- 17.Parker MD, Musa-Aziz R, Rojas JD, Choi I, Daly CM, Boron WF. Characterization of human SLC4A10 as an electroneutral Na/HCO3 cotransporter (NBCn2) with Cl− self-exchange activity. J Biol Chem 283: 12777–12788, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pearson MM, Lu J, Mount DB, Delpire E. Localization of the K(+)-Cl(-) cotransporter, KCC3, in the central and peripheral nervous systems: expression in the choroid plexus, large neurons and white matter tracts. Neuroscience 103: 481–491, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Plotkin MD, Kaplan MR, Peterson LN, Gullans SR, Hebert SC, Delpire E. Expression of the Na+-K+-2Cl- cotransporter BSC2 in the nervous system. Am J Physiol Cell Physiol 272: C173–C183, 1997. [DOI] [PubMed] [Google Scholar]
- 20.Praetorius J, Nejsum LN, Nielsen S. A SCL4A10 gene product maps selectively to the basolateral plasma membrane of choroid plexus epithelial cells. Am J Physiol Cell Physiol 286: C601–C610, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Praetorius J, Nielsen S. Distribution of sodium transporters and aquaporin-1 in the human choroid plexus. Am J Physiol Cell Physiol 291: C59–C67, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Quinton PM, Wright EM, Tormey JM. Localization of sodium pumps in the choroid plexus epithelium. J Cell Biol 58: 724–730, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saito Y, Wright EM. Kinetics of the sodium pump in the frog choroid plexus. J Physiol 328: 229–243, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sardet C, Counillon L, Franchi A, Pouyssegur J. Growth factors induce phosphorylation of the Na+/H+ antiporter, glycoprotein of 110 kD. Science 247: 723–726, 1990. [DOI] [PubMed] [Google Scholar]
- 25.Sassani P, Pushkin A, Gross E, Gomer A, Abuladze N, Dukkipati R, Carpenito G, Kurtz I. Functional characterization of NBC4: a new electrogenic sodium-bicarbonate cotransporter. Am J Physiol Cell Physiol 282: C408–C416, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Schelling JR and Abu Jawdeh BG. Regulation of cell survival by Na+/H+ exchanger-1. Am J Physiol Renal Physiol 295: F625–F632, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Segal MB, Burgess AM. A combined physiological and morphological study of the secretory process in the rabbit choroid plexus. J Cell Sci 14: 339–350, 1974. [DOI] [PubMed] [Google Scholar]
- 28.Tse CM, Levine SA, Yun CH, Khurana S, Donowitz M. Na+/H+ exchanger-2 is an O-linked but not an N-linked sialoglycoprotein. Biochemistry 33: 12954–12961, 1994. [DOI] [PubMed] [Google Scholar]
- 29.Virkki LV, Wilson DA, Vaughan-Jones RD, Boron WF. Functional characterization of human NBC4 as an electrogenic Na+-HCO3 cotransporter (NBCe2). Am J Physiol Cell Physiol 282: C1278–C1289, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Wang CZ, Yano H, Nagashima K, Seino The Na+-driven Cl−/HCO3− exchanger S. Cloning, tissue distribution, and functional characterization. J Biol Chem 275: 35486–35490, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Wright EM Effect of bicarbonate and other buffers on choroid plexus Na+/K+ pump. Biochim Biophys Acta 468: 486–489, 1977. [DOI] [PubMed] [Google Scholar]