Abstract
Silent voltage-gated K+ (Kv) subunits interact with Kv2 subunits and primarily modulate the voltage dependence of inactivation of these heterotetrameric channels. Both Kv2 and silent Kv subunits are expressed in the mammalian nervous system, but little is known about their expression and function in sensory neurons. This study reports the presence of Kv2.1, Kv2.2, and silent subunit Kv6.1, Kv8.1, Kv9.1, Kv9.2, and Kv9.3 mRNA in mouse dorsal root ganglia (DRG). Immunocytochemistry confirmed the protein expression of Kv2.x and Kv9.x subunits in cultured small DRG neurons. To investigate if Kv2 and silent Kv subunits are underlying the delayed rectifier K+ current (IK) in these neurons, Kv2-mediated currents were isolated by the extracellular application of rStromatoxin-1 (ScTx) or by the intracellular application of Kv2 antibodies. Both ScTx- and anti-Kv2.1-sensitive currents displayed two components in their voltage dependence of inactivation. Together, both components accounted for approximately two-thirds of IK. A comparison with results obtained in heterologous expression systems suggests that one component reflects homotetrameric Kv2.1 channels, whereas the other component represents heterotetrameric Kv2.1/silent Kv channels. These observations support a physiological role for silent Kv subunits in small DRG neurons.
Keywords: voltage-gated K+ channels, dorsal root ganglia neurons, rStromatoxin-1, Kv2.1 antibodies
voltage-gated k+ (Kv) channels are tetramers composed of four α-subunits arranged around a central pore. Each α-subunit consists of six transmembrane segments (S1–S6) with cytoplasmic NH2- and COOH-termini. Members of the Kv1–Kv4 subfamilies generate functional K+ channels in a homotetrameric configuration. The diversity within these subfamilies is increased by the formation of heterotetramers and by interactions with auxiliary β-subunits. The Kv2 subfamily contains only two members (Kv2.1 and Kv2.2) that display very similar properties (2), but its diversity is increased by heterotetrameric interactions with α-subunits of the Kv5, Kv6, Kv8, and Kv9 subfamilies. These are designated as silent Kv subunits because they are not able to form functional homotetrameric channels despite sharing the typical topology of a Kv channel. The nonfunctionality is caused by the retention of these subunits in the endoplasmic reticulum (ER) (20, 28, 29, 31). Functionality is restored by forming heterotetrameric channels with Kv2.1 or Kv2.2 channels, thus relieving the ER retention. Therefore, these silent α-subunits act as modulating subunits for Kv2.x currents. The most profound effects consist of a reduction in amplitude, a slowing of inactivation and deactivation kinetics, and an alteration of the voltage dependence of inactivation of Kv2.1 currents (3, 11, 13, 20, 25, 28–30, 37, 42).
Kv channels play an important role in neurons by regulating the resting membrane potential, the repolarization, the frequency of firing, the shape of action potentials, and neurotransmitter release. Differences in spatial and temporal expression and the combination of several voltage-gated α- and β-subunits contribute to the heterogeneity in these neuronal electrophysiological properties. Members of the Kv2 subfamily are widely expressed in neuronal tissues, where they underlie the neuronal outward delayed rectifier K+ current (IK). Kv2 subunits have also been reported in neurons in dorsal root ganglia (DRG) (10, 12), which contain the cell bodies of visceral and somatic sensory neurons. Kv current is composed of one or more fast inactivating A-type currents and several IKs depending on the subpopulation of DRG neurons (6–8, 26, 27).
The contribution of Kv2.1 to IK in small mouse DRG neurons is unknown, and, because silent Kv subunits interact with Kv2 subunits, we also investigated the potential presence of these subunits in cultured small DRG neurons.
MATERIALS AND METHODS
Cell culture and transfection.
DRG neurons were obtained from 12- to 14-day-old mouse (FVB) embryos. Experiments were conducted in agreement with the European Communities Council Directive on the protection of animals used for experimental and other scientific purposes (86/609/EEC) and were approved by the Institutional Animal Care and Use Committee, University of Antwerp. The embryonic spinal cords with the attached DRG were mechanical dissociated. After dissociation, cells were kept in culture as previously described (23) and used for electrophysiological experiments after 4–6 wk in culture.
Mouse Ltk− cells (CCL 1.3, American Type Culture Collection) were cultured in DMEM supplemented with 10% horse serum and 1% penicillin-streptomycin under a 5% CO2 atmosphere. Ltk− cells were transiently (co-)transfected with 50 ng Kv2.1, 10 μg Kv4.2, or 0.5 μg/5 μg Kv2.1/Kv9.3 channel cDNA in combination with 0.5 μg green fluorescent protein as a transfection marker using Lipofectamine 2000 reagent according to the manufacturer's instructions (Invitrogen, San Diego, CA). Cells were trypsinized 12–24 h after transfection and used for current recordings within 5 h.
RT-PCR analysis and cDNA amplification.
After the dissection of 10 spinal cords, DRG were pinched off from the spinal cords and collected in TRIzol Reagent (Invitrogen) for RNA precipitation. Before the RT-PCR, the RNA sample was made DNA free with deoxyribonuclease I (Fermentas, Burlington, ON, Canada) to exclude genomic DNA contamination. Total RNA (1 μg) was reversed transcribed using random hexamer primers with the Thermoscripts RT-PCR System (Invitrogen) according to the manufacturer's instructions. cDNA amplification was performed for 36 or 44 cycles using gene-specific primers that spanned intron boundaries (except for intronless Kv5.1). Amplified fragments were subcloned in the TOPO-TA vector (Invitrogen) and sequenced to confirm the identity of the PCR products. Each RT-PCR analysis was performed in duplicate on two different RNA isolations.
Confocal imaging.
DRG neurons were cultured on glass-bottom dishes (MatTek, Ashland, MA) as described above. Cell fixation for confocal imaging was performed with 4% paraformaldehyde. Cells were permeabilized by 0.1 M PBS supplemented with 0.1% BSA (Aurion, Wageningen, The Netherlands), 10% horse serum, and 1% Triton X-100 for 30 min at room temperature. After permeabilization, cells were incubated overnight with primary antibodies (1:50) and dissolved in a 0.1 M PBS solution containing 10% horse serum and 0.1% Triton X-100. The following primary antibodies were used: rabbit anti-Kv2.1 (Alomone, Jerusalem, Israel), rabbit anti-Kv2.2 (Alomone), goat anti-Kv9.1 (Santa Cruz Biotechnology, Santa Cruz, CA), goat anti-Kv9.2 (Santa Cruz Biotechnology), and chicken anti-Kv9.3 (Abcam, Cambridge, UK). FITC-labeled anti-rabbit (Jackson ImmunoResearch Laboratories, West Grove, PA), FITC-labeled anti-goat (Abcam), and phycoerythrin (PE)-labeled anti-chicken (Jackson ImmunoResearch Laboratories) antibodies were used as secondary antibodies (1:100) and were dissolved in a 0.1 M PBS solution supplemented with 1% horse serum. After samples had been incubated for 1 h with secondary antibodies, confocal images were obtained on a Zeiss CLSM 510 microscope equipped with an argon laser (excitation: 488 nm) and a helium-neon laser (excitation: 543 nm) for the visualization of FITC- and PE-labeled antibodies, respectively. The immunofluorescence detection of Kv2.x and silent Kv subunits was performed on six different isolations.
Electrophysiology.
DRG neurons with diameters of ∼20 μm were chosen for the neuronal current recordings. Whole cell current recordings were obtained with an Axoclamp-2A amplifier (Axon Instruments, Union City, CA) in the two-electrode voltage-clamp mode. The amplifier was interfaced with a TL1-Labmaster (Axon Instruments). Patch pipettes with a resistance of 3–5 MΩ were pulled from 1.7-mm-diameter glass capillaries with a Brown Flaming P-87 horizontal pipette puller and heat polished. DRG neurons were superfused continuously with an extracellular solution containing (in mM) 140 N-methyl-glucamine, 5 KCl, 1 MgCl2, 1.8 CaCl2, 10 glucose, and 5 HEPES with the pH adjusted to 7.4 with HCl. The pipette solution contained (in mM) 30 KCl, 2 MgCl2, 1 CaCl2, 110 K-aspartate, 5 Na2ATP, 2 EGTA, 0.1 cAMP, and 10 HEPES with the pH adjusted to 7.4 with NaOH.
Current recordings of Ltk− cells heterologously expressing Kv subunits were made with the Axopatch-200B amplifier (Axon Instruments) in the whole cell configuration and were lowpass filtered and sampled at 1–10 kHz with a Digidata 1200A data-acquisition system (Axon Instruments). pCLAMP8 software (Axon Instruments) was used to control command voltages and data storage. Patch pipettes were pulled with laser puller P2000 (Sutter Instruments, Novato, CA) from 1.2-mm borosilicate glass (World Precision Instruments, Sarasota, FL) and heat polished. Cells were superfused with an extracellular solution containing (in mM) 145 NaCl, 4 KCl, 1.8 CaCl2, 1 MgCl2, 10 HEPES, and 10 glucose with the pH adjusted to 7.35 with NaOH. Pipettes were filled with a solution containing (in mM) 110 KCl, 5 K4BAPTA, 5 K2ATP, 1 MgCl2, and 10 HEPES with the pH adjusted to 7.2 using KOH. The differences in the solutions used for DRG or Ltk− cells were to remain comparable with previous studies using either system (and to prevent Na+ current contamination in the DRG recordings). Junction potentials were zeroed with the filled pipette in the bath solution. Experiments were excluded from analysis if the voltage errors originating from the series resistance exceeded 5 mV.
The rStromatoxin-1 (ScTx) experiments were performed by dissolving 100 nM ScTx (Alomone) in the external solution and applying it with a fast perfusion system. Alkaline phosphatase (AP)- and anti-Kv2-sensitive currents were obtained by dissolving 10 U/ml rAPID AP (Roche, Basel, Switzerland) or 10 μg/ml Kv2 antibody (Alomone) in the pipette solution, respectively. The patch pipette was dipped in the normal intracellular solution and then back filled with the AP- or anti-Kv2.1-containing solution. Anti- Kv2.1 block started 2 min after patch disruption, and steady-state block was reached after ∼8 min.
Pulse protocols and data analysis.
The pulse protocols are shown in the figures. The voltage dependence of channel activation or inactivation (activation and inactivation curves) was fitted with the following Boltzmann equation: y = 1/{1 + exp[−(E − V1/2)/k]}, where E is the applied voltage, V1/2 is the voltage at which 50% of the channels are activated or inactivated, and k is the slope factor. Results are presented as means ± SE. Statistical analysis was done using Student's t-test. P values of <0.05 were considered to be significantly different.
RESULTS
Presence of mRNA for Kv2.x and several silent Kv subunits in DRG neurons.
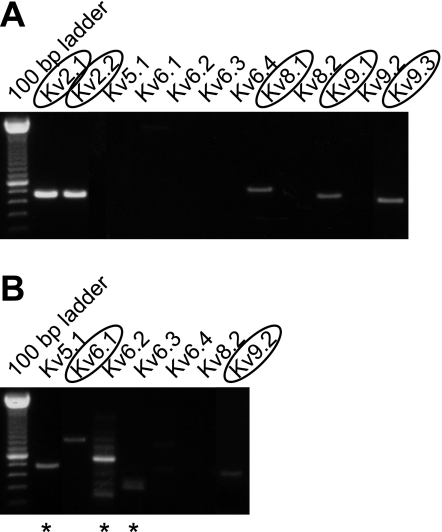
The presence of Kv2 and silent Kv subunits in mouse DRG neurons was demonstrated by RT-PCR analysis of RNA from freshly isolated DRG (as described in materials and methods). After 36 cycles of cDNA amplification with subunit-specific primers, Kv2.1 and Kv2.2 were readily observed (Fig. 1A). For each PCR analysis, two negative controls (reactions without cDNA or reverse transcriptase) and a positive control (reaction with cDNA of the Kv subunit under investigation) were performed (Supplemental Fig. 1).1 Silent subunits Kv8.1, Kv9.1, and Kv9.3 were also readily detected after 36 cycles of amplification (Fig. 1A). For the silent Kv subunits that were not detected after 36 cycles, 44 cDNA amplification cycles were performed. In the latter case, subunits Kv5.1, Kv6.1, Kv6.2, Kv6.3, and Kv9.2 were also detected, whereas Kv6.4 and Kv8.2 were not. The resulting PCR products were cloned in the TOPO-TA vector for sequencing to exclude the possibility of nonspecific amplification. This confirmed the presence of Kv2.1 and Kv2.2 subunits and silent subunits Kv6.1, Kv8.1, Kv9.1, Kv9.2, and Kv9.3. The signals obtained with Kv5.1, Kv6.2, and Kv6.3 gene-specific primers (after 44 cycles of cDNA amplification) represented nonspecific amplification (marked by astericks in Fig. 1B) of casein kinase 2, Tmem16H, and tubulin-β, respectively, which are all expressed ubiquitously in neuronal tissue.
Fig. 1.
Expression of Kv2 and silent Kv subunits in dorsal root ganglia (DRG). RT-PCR analysis was performed on freshly isolated mouse DRG. RNA was reverse transcribed with random primers, and the amplification was performed with gene-specific primers for the different Kv subunits. The silent Kv subunits that were not detected after 36 cycles of amplification (A) were subjected to an amplification reaction of 44 cycles (B). The subunits for which the expression was confirmed by sequencing are encircled. * Nonspecific amplification. The different controls are shown in Supplemental Fig. 1.
Kv2.x and Kv9.x subunits are expressed in cultured small DRG neurons.
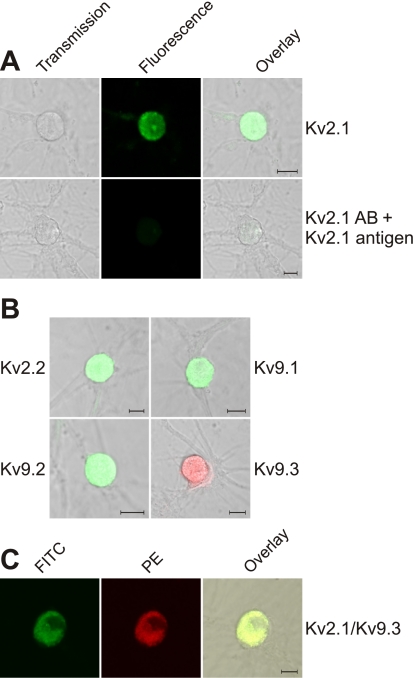
After the detection of mRNA for Kv2.x and some silent Kv subunits in freshly isolated DRG, the presence of Kv2.x and Kv9.x subunits in cultured small DRG neurons was investigated by immunofluorescence. In our DRG cultures, the DRG neurons were morphological identified and selected based on their soma shape and number of neuronal offshoots. Using subunit-specific antibodies, we detected the presence of Kv2.1 (n = 62), Kv2.2 (n = 45), Kv9.1 (n = 58), Kv9.2 (n = 55), and Kv9.3 (n = 54) subunits (Fig. 2, A and B). The specificity of each antibody was tested by pretreatment of the antibodies with an excess of the appropriate antigens (Fig. 2A and Supplemental Fig. 2, A–D). We could not confirm the expression of Kv6.1 and Kv8.1 protein due to the lack of appropriate selective antibodies. To investigate if Kv2.x and silent Kv channel subunits were coexpressed in the same DRG neurons, we incubated DRG neurons with antibodies against both Kv2.1 and Kv9.x (Fig. 2C and Supplemental Fig. 2E). Since green and red fluorescence were detected in the same neurons, at least Kv2.1 and Kv9.1, Kv9.2, or Kv9.3 subunits were present in the same neurons (n = 19, 14, and 22, respectively).
Fig. 2.
Detection of the members of the Kv2 and Kv9 subfamilies with confocal microscopy in cultured DRG neurons. A: immunofluorescent detection of Kv2.1 by incubation with a Kv2.1-specific antibody followed by a FITC-labeled secondary antibody. Left, light transmission image; middle, FITC fluorescence image; right, overlay of the left and middle images. In the top images, the presence of Kv2.1 is determined, whereas in the bottom images, the specificity of the Kv2.1 antibody is verified by pretreatment with the epitope. B: immunofluorescent detection of Kv2.2, Kv9.1, Kv9.2, and Kv9.3. Kv subunits were detected by incubation with a subunit-specific antibody followed by FITC- or phycoerythrin (PE)-labeled secondary antibodies. Images were obtained as in the overlay image in A, right. The different controls are shown in Supplemental Fig. 2. C: coexpression of Kv2.1 and Kv9.3 subunits. Kv2.1 subunits were detected by incubation with Kv2.1 antibodies followed by a FITC-labeled secondary antibody, whereas Kv9.3 subunits were detected by incubation with a Kv9.3-specific antibody followed by a PE-labeled secondary antibody. FITC and PE fluorescence are shown in the left and middle images, respectively. The right image is an overlay of the left and middle images. Scale bars = 10 μm in A–C. Similar data for Kv9.1 and Kv9.2 are shown in Supplemental Fig. 2E.
The major outward K+ current is sensitive to ScTx.
To investigate the potential contribution of Kv2.x subunits (alone and/or in combination with silent subunits) to the IK of small DRG neurons, we tested for the sensitivity of the observed total IK to ScTx. ScTx is a potent “blocker” of both Kv2.1 and Kv2.2 (Kd ≈ 13 and 21 nM, respectively) (5). It acts as a gating modifier by strongly shifting the voltage dependence of activation toward depolarized potentials, and it also blocks at least the heterotetrameric Kv2.1/ Kv9.3 channel (Kd ≈ 7 nM) (5).
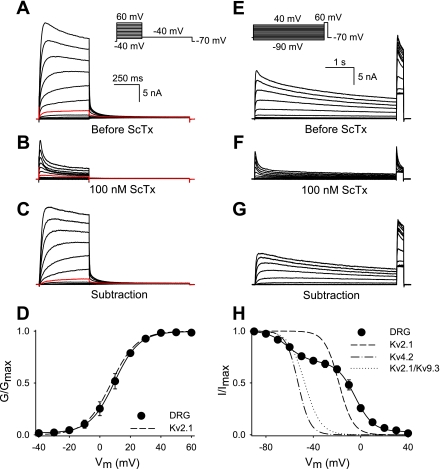
Typical current recordings of DRG neurons before and after the application of 100 nM ScTx are shown in Fig. 3. Voltage-dependent activation and inactivation of ScTx-sensitive current was examined with the voltage protocols shown in Fig. 3, A and E, respectively. The addition of 100 nM ScTx caused a large reduction of the outward K+ current (compare Fig. 3, B and F with A and E, respectively): ScTx blocked 62.0 ± 4.6% of the outward K+ current at 0 mV, a potential where 100 nM ScTx blocked Kv2.1 and Kv2.1/Kv9.3 currents almost completely (>90%, data not shown). The ScTx-sensitive current displayed a voltage dependence of activation (V1/2 = 8.5 ± 1.5 mV, k = 8.0 ± 1.2, n = 5) that was similar to that of Kv2.1 currents, as determined in heterologous expression (V1/2 = 7.4 ± 1.4 mV, k = 8.5 ± 0.3, n = 5; Fig. 3D).
Fig. 3.
Voltage-dependent activation and inactivation of the rStromatoxin-1 (ScTx)-sensitive current in DRG neurons. A: outward K+ current recordings elicited by the voltage protocol shown in the inset. The scale bar applies to A–C. B: representative current tracings elicited by the same voltage protocol as in A after the extracellular application of 100 nM ScTx. C: ScTx-sensitive current, which was obtained by subtracting the current recordings of B from those of A. The red tracings in A–C represent the current recordings at 0 mV. D: activation curve of the ScTx-sensitive current obtained from the normalized tail current amplitude at −40 mV from current recordings as in C. A single Boltzmann function (solid line) was fitted to the data. The dashed line represents the activation curve of wild-type Kv2.1 expressed in Ltk− cells. G/Gmax, conductance/maximal conductance; Vm, membrane potential. E: raw current recordings elicited by the voltage protocol shown in the inset to determine the voltage dependence of inactivation in control solution. The scale bar applies to E–G. F: representative current traces (same voltage protocol as in E) after the addition of 100 nM ScTx. G: ScTx-sensitive current. H: voltage dependence of inactivation of the ScTx-sensitive current. The inactivation curve was obtained by plotting the normalized peak currents (I/Imax) recorded during the 250-ms test pulse to 60 mV as a function of the voltage of the 5-s prepulse. Data were fitted with a sum of two Boltzmann functions (solid lines). For comparison, the inactivation curves of Kv2.1, Kv4.2, and Kv2.1/Kv9.3, obtained from heterologous expression, are also shown. The midpoints of inactivation and slope factors are shown in Table 1.
The voltage dependence of inactivation clearly displayed two components and was therefore fitted with a sum of two Boltzmann functions (Fig. 3H). The component of the voltage dependence of inactivation with a midpoint of −4.1 mV compared well with that of the homotetrameric Kv2.1 current in heterologous expression (Fig. 3H and Table 1). The second component represented 29.9 ± 3.7% of the total ScTx-sensitive current and was characterized by a midpoint of inactivation of −62.2 mV (Table 1). At least three possibilities exist for this second component: a population of A-type Kv4.2 channels, heterotetrameric Kv2/silent Kv channels, or differently phosphorylated Kv2 channels. However, the subtraction tracings, as shown in Fig. 3G, gave no indication of an early rapidly inactivating component that would correspond to Kv4.2 channels, excluding the first possibility.
Table 1.
Overview of the biophysical parameters for the voltage dependence of inactivation
| n |
First Component |
Second Component
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| V1/2, mV | k | V1/2, mV | k | |||||||
| Control | ||||||||||
| Kv2.1 | 5 | −18.4±2.8 | 6.2±0.4 | NA | NA | |||||
| Kv2.1/Kv9.3 | 6 | NA | NA | −47.2±0.7 | 6.9±0.6 | |||||
| Kv4.2 | 8 | NA | NA | −52.8±1.0 | 5.2±0.3 | |||||
| ScTx sensitive | ||||||||||
| ScTx sensitive | 10 | −4.1±2.0 | 7.1±0.8 | −62.2±1.8 | 7.8±1.0 | |||||
| AP application | ||||||||||
| Kv2.1 | 5 | −28.9±2.6 | 5.7±0.8 | NA | NA | |||||
| Kv2.1/Kv9.3 | 6 | NA | NA | −59.0±1.1 | 8.5±1.1 | |||||
| DRG | ||||||||||
| Before | 5 | −10.1±2.6 | 5.5±0.8 | −47.3±6.4 | 12.9±3.4 | |||||
| After | 5 | −22.0±3.2 | 7.3±1.4 | −65.2±4.9 | 8.3±1.5 | |||||
| Anti-Kv2.1 sensitive | ||||||||||
| Kv2.1 | 9 | −19.8±2.9 | 4.2±1.0 | NA | NA | |||||
| Kv2.1/Kv9.3 | 5 | NA | NA | −42.2±1.4 | 4.7±0.8 | |||||
| DRG | 8 | −7.0±2.1 | 6.4±0.6 | −62.3±3.1 | 5.8±1.1 | |||||
Values are means ± SE; n, number of experiments. The midpoints of inactivation (V1/2) and slope factors (k) were obtained from a single or double Boltzmann fit (see materials and methods). ScTx, rStromatoxin-1; AP, alkaline phosphatase; DRG, dorsal root ganglia; NA, not applicable. For comparison, Kv9.3 parameters are shown under “second component.”
The ScTx-sensitive current principally represents channels containing Kv2.1 subunits.
In a complementary approach, we used Kv2 antibodies to examine whether the second component of the ScTx-sensitive current originated from channels containing Kv2 subunits. Kv2 antibodies have been shown to block Kv2 currents in hippocampal and neocortical pyramidal neurons (9, 19). The Kv2 antibody-sensitive current was obtained by the subtraction of the current recordings after Kv2 antibodies had diffused into the cell (10–20 min) from those recorded immediately after patch rupture.
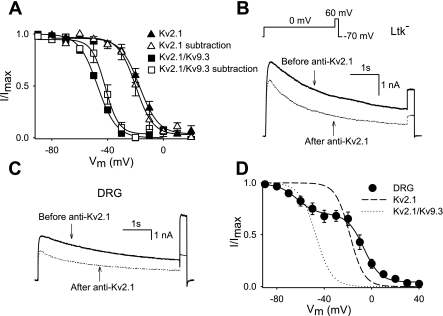
The Kv2.1 antibody blocked 30.1 ± 8.0% (during a 500-ms step to 0 mV) of the outward current in cells heterologously expressing Kv2.1, similar to previously obtained results (9, 19). The inactivation curve of the anti-Kv2.1-sensitive current was characterized by a V1/2 of −19.8 mV, which compares well with the voltage dependence of inactivation in control (Fig. 4A and Table 1). Next, we tested, in this heterologous expression system, whether these Kv2.1 antibodies also blocked Kv2.1 in a heterotetrameric configuration with silent Kv subunits. For this purpose, we used silent subunit Kv9.3 as a model. As shown in Fig. 4B, Kv2.1 antibodies blocked 29.2 ± 4.1% of the Kv2.1/Kv9.3 current. The anti-Kv2.1-sensitive current from channels in the heterotetrameric Kv2.1/Kv9.3 configuration displayed a voltage dependence of inactivation characterized by a V1/2 of −42.2 mV (Fig. 4A and Table 1). Thus, the voltage dependence of inactivation of the subtracted current was very similar to that of Kv2.1/Kv9.3 current as determined in the control. This indicates that the antibodies are not selective for homo- or heterotetrameric channels but do block heterotetrameric Kv2/silent Kv channels equally well.
Fig. 4.
Voltage dependent activation and inactivation of the anti-Kv2.1-sensitive current. A: voltage-dependent inactivation of the anti-Kv2.1-sensitive current of Kv2.1 or Kv2.1/Kv9.3 heterologous expressed. The inactivation curve was obtained as in Fig. 3H. For comparison, the inactivation curves of Kv2.1 and Kv2.1/Kv9.3 obtained from heterologous expression are also shown. B: outward K+ current traces of Kv2.1/Kv9.3 expressed in Ltk− cells before (solid line) and after (dotted lines) diffusion of Kv2.1 antibodies into the cell. The voltage protocol is shown in the inset. C: representative current tracings of the outward K+ current of DRG neurons before (solid line) and after (dotted lines) intracellular diffusion of Kv2.1 antibodies. The same voltage protocol as in B was used. D: inactivation curve of the anti-Kv2.1-sensitive current was obtained as in Fig. 3H. Data were fitted with a double Boltzmann function (solid line). The inactivation curves of wild-type Kv2.1 and wild-type Kv2.1/Kv9.3 expressed in Ltk− cells are also shown. The midpoints of inactivation and slope factors are shown in Table 1.
The addition of 10 μg/ml Kv2 antibody to small DRG neurons caused a reduction of the outward K+ current (Fig. 4C). To examine whether this reduction was due to Kv2 antibody block or due to run down, we investigated with standard pipette and bath solutions the degree of run down during a period of 20 min. After 20 min, the outward K+ current decreased 6.3 ± 0.8% (n = 4). In contrast, diffusion of Kv2.1 antibodies reduced the total outward K+ current by 24.2 ± 3.6% (n = 8). This block was comparable with the level of anti-Kv2.1 block in Kv2.1- or Kv2.1/Kv9.3-expressing cells. On the other hand, a similar application of Kv2.2 antibodies into the cells reduced the total outward K+ current by 6.5 ± 4.5% (n = 5). Since the latter was similar to run down, this argues against a significant Kv2.2-containing component in IK of the soma of cultured small DRG neurons. The voltage dependence of inactivation of the anti-Kv2.1-sensitive K+ current was again composed of two components (Fig. 4D), and the second component constituted 31.2 ± 5.2% of the total anti-Kv2.1-sensitive current. This compares well with the contribution of the second component to the ScTx-sensitive current. The first and second components of inactivation of anti-Kv2.1-sensitive current were characterized by V1/2 of −7.0 and −62.3 mV, respectively (Table 1).
Taken together, the biophysical properties of the first and second components of Kv2.1 antibody-sensitive current were comparable with those of ScTx-sensitive current. This suggests that the ScTx-sensitive current originates primarily from a population of homotetrameric Kv2.1 channels and a population of heterotetrameric Kv2/silent Kv channels, suggesting a physiological relevant function of silent Kv subunits.
The ScTx-sensitive current does not represent differentially phosphorylated Kv2.x channels.
Because phosphorylation of Kv2 channels modifies the voltage dependence of inactivation, the second component of the ScTx-sensitive current could derive from a population of channels composed of Kv2 subunits with altered phosphorylation status. Specifically, hypophosphorylation of Kv2.1 at specific residues shifts the voltage dependence of inactivation in the hyperpolarized direction (17, 21). To investigate the possibility that the second component of the ScTx-sensitive current reflected Kv2 subunits in such a hypophosphorylated state, AP was added to the intracellular solution.
As a control, the activity of AP was first tested on homotetrameric Kv2.1 channels expressed in Ltk− cells. The voltage dependence of inactivation was determined immediately after the patch was ruptured and after intracellular diffusion of AP was allowed for 10–20 min. The application of AP shifted the inactivation curve by 10 mV into the hyperpolarized direction, whereas k values did not differ significantly (Fig. 5A and Table 1). Since it is not known if hypophosphorylation might also affect the voltage dependence of inactivation of heterotetrameric Kv2/silent Kv channels, we tested the effect of AP on heterologously expressed Kv2.1/Kv9.3 channels (using Kv9.3 as a model for the group of silent Kv subunits). The results shown in Fig. 5A and Supplemental Fig. 3A demonstrate that the inactivation curve was indeed shifted (∼12 mV; Table 1) in the hyperpolarized direction after AP had diffused into the cell.
Fig. 5.
Effect of alkaline phosphatase (AP) on the voltage dependence of inactivation of the outward K+ current of Kv2.1-containing channels and DRG neurons. A: voltage dependence of inactivation of Kv2.1 and Kv2.1/Kv9.3 currents before and after the diffusion of AP into the cell. Data were fitted with a single Boltzmann function (solid lines). The application of AP shifted the voltage dependence of inactivation of both Kv2.1 as Kv2.1/Kv9.3 channels in the hyperpolarized direction to a similar extent. B: voltage dependence of inactivation of DRG outward K+ current before and after the diffusion of AP into the cell. The inactivation curve was obtained as in Fig. 3H. Data were fitted with a double Boltzmann function (solid lines). After AP diffusion, the voltage dependence of inactivation of both components was shifted in the hyperpolarized direction to a similar extent.
After the effects of AP had been characterized on heterologously expressed Kv2.1 and Kv2.1/ Kv9.3 channels, the voltage dependence of inactivation of the total outward K+ current of DRG neurons was determined before and after AP diffusion into the cell. After equilibration with AP, the outward K+ current was still composed of two components. The contribution of the second component to the total outward K+ current was not modified by the diffusion of AP into the cell (35.1 ± 3.0% before and 35.6 ± 5.6% after AP, respectively; Fig. 5B). Furthermore, AP caused a significant negative shift of the voltage dependence of inactivation for both components (Table 1 and Supplemental Fig. 3B). Before AP, the first and second component displayed midpoints of −10.1 and −47.3 mV, respectively, whereas after AP had diffused into the cell, V1/2 of the inactivation curves of the first and second component was −22.0 and −65.2 mV, respectively (Table 1). k values of both inactivation curves were not significantly different compared with those immediately after the patch had been ruptured (Table 1).
These results suggest that the second component of the ScTx-sensitive current does not represent another phosphorylation state of channels composed of Kv2 subunits. Furthermore, the AP-induced shifts in the voltage dependence of inactivation of both components were comparable with those observed for heterologous expressed Kv2.1 and Kv2.1/Kv9.3 channels, respectively. These results exclude the phosphorylation hypothesis and strengthen the idea that both current components correspond to a homotetrameric Kv2 and heterotetrameric Kv2/silent Kv channel population, respectively.
DISCUSSION
Kv2 subunits, especially Kv2.1 subunits, are highly and ubiquitously expressed throughout the mammalian brain (36), but Kv2.x subunits are also present in other parts of the central and peripheral nervous systems (1, 10, 32, 33). It has been demonstrated that Kv2 subunits interact with silent subunits and that the latter are also highly expressed in the mammalian brain (3, 29, 30, 37, 38). The heterotetramerization of Kv2 with silent subunits increases the functional diversity, which may reflect specific functions for these heterotetrameric channel complexes. For example, heterotetrameric Kv2.1 channels containing Kv9.3 subunits are involved in hypoxic pulmonary artery vasoconstriction (22), coassembly of Kv2.1 with Kv5.1 and Kv6 subunits shapes IK in smooth muscle of the mouse urinary bladder (34), and Kv8.2 subunits are involved in photoreception of the retina (41). In rat DRG neurons, both Kv2.1 and Kv2.2 have been shown to be well expressed (10, 12). However, the presence of silent Kv subunits that could modulate Kv2 properties in these sensory neurons has not been investigated to date. In this study, we confirm the presence of both Kv2 subunits, but the novel finding is that mRNA for several silent subunits (Kv6.1, Kv8.1, Kv9.1, Kv9.2, and Kv9.3) is also expressed in mouse DRG. Furthermore, we confirmed the protein expression of Kv2 and Kv9 subunits and the coexpression of Kv2.1 with Kv9.x in small DRG neurons through immunocytochemistry (Fig. 2 and Supplemental Fig. 2).
The composition of the outward Kv current of DRG neurons depends on the subpopulation. Based on the diameter of their soma, DRG neurons are divided into small (<30 μM), medium (30–50 μM), and large (>50 μM) neurons. In large cutaneous afferent DRG neurons, two transient and one sustained K+ current have been characterized (6). Based on the kinetic and pharmacological properties, two transient and one sustained K+ current have also been identified in small- and medium-sized neurons (26), whereas another report (8) has suggested the presence of four to six components. These fast transient outward K+ currents and IK probably modulate the excitability of these small-sized DRG neurons, and it has been proposed that the fast transient outward K+ current originates from Kv1.4 channels (24, 39). In addition to Kv1.4 channels, Kv3.4, Kv4.2, and Kv4.3 channels could underlie the fast transient outward K+ current in small DRG neurons (4, 7, 40). In several neurons, Kv2.1 contributes to IK, and, in some, it even represents the major outward K+ current (9, 14, 19), but the channel subunits that contribute to the IK of small DRG neurons had not yet been identified.
Using ScTx and Kv2 antibodies, we now report that Kv2.1-containing channels represent a major component of the IK in small DRG neurons. The application of 100 nM ScTx blocked ∼62% (at 0 mV) of IK in our small cultured DRG neurons, suggesting that Kv2.1-containing channels represent two-thirds of the IK in these neurons. Since ScTx is a gating modifier (voltage sensor toxin), “block” by 100 nM ScTx is diminished at voltages positive to +40 mV (5). Thus, the contribution of Kv2 and Kv2/silent Kv subunits to the IK may even slightly be underestimated.
The comparison of the properties of the anti-Kv2.1-sensitive current with those of the ScTx-sensitive current indicated that both currents consisted of similar components. The voltage dependence of activation of the ScTx-sensitive current was very similar to that of the Kv2.1 currents determined in heterologous expression (Fig. 3D and Table 1). However, based on the activation properties, it is difficult to discriminate between homo- or heterotetrameric channels composed of Kv2.1 and silent Kv subunits because of the limited modulating effects of the silent Kv subunits on the voltage dependence of activation of Kv2.1. In contrast, most silent subunits markedly affect the inactivation. Thus, heterotetrameric Kv2/silent Kv currents can be distinguished from homotetrameric Kv2 currents based on the voltage dependence of inactivation. In most cases, inactivation is slowed, and the voltage dependence is shifted into hyperpolarized direction, by up to 40 mV (3, 11, 13, 20, 25, 28, 29, 37, 42). In our case, both the ScTx- as well as anti-Kv2.1-sensitive current clearly displayed two components in their voltage dependence of inactivation (Figs. 3H and 4D). One component likely represents a population of homotetrameric Kv2.1 channels as judged by its V1/2 for the voltage dependence of inactivation, which corresponds with that of Kv2.1 in heterologous expression (Figs. 3H and 4D and Table. 1). Based on the negative position of the second component of the inactivation curve of the ScTx- and anti-Kv2.1-sensitive current, we propose that the latter is caused by channels with a heterotetrameric (Kv2/silent Kv subunit) composition. This hypothesis is supported by the fact that silent subunits were detected in DRG neurons and that the anti-Kv2.1-sensitive current still displayed two components. In the case that one of these components was not related to Kv2.1, only one component should have been blocked with Kv2.1 antibodies. To confirm that a silent subunit could be involved, we demonstrated in a heterologous expression system that Kv2.1 antibodies could indeed block heterotetrameric ion channels composed of Kv2.1/silent Kv subunits (Fig. 4, A and B), whereas the epitope (residues 841–857 of mouse Kv2.1) was only present on Kv2.1 subunits.
The phosphorylation state of Kv2.1 apparently varies between different native and heterologous expression cells (for a review, see Ref. 16), and Kv2.1 subunits are highly phosphorylated in mammalian neurons. Since the hypophosphorylation of Kv2.1 shifts the voltage-dependent inactivation in the hyperpolarized direction (17, 21), the ∼10-mV difference between the voltage dependence of inactivation of homotetrameric Kv2.1 channels recorded in heterologous cells and the voltage dependence of inactivation of the first component recorded in DRG neurons (Table 1) is probably due to a different phosphorylation state of Kv2.1 in expression cells and DRG neurons.
Since ScTx also blocks Kv2.2 channels and since the currents of homotetrameric Kv2.1 and Kv2.2 channels and heterotetrameric Kv2.1/Kv2.2 channels do not significantly differ (2), the ScTx-sensitive current could also represent Kv2.2-containing channels. However, it seems unlikely that the ScTx-sensitive current in these neurons represents homotetrameric Kv2.2 or heterotetrameric Kv2.1/Kv2.2 channels since the current reduction with Kv2.2 antibodies was small and similar to the run down current. This implies that Kv2.2 subunits play, at best, a minor role in the IK of these cultured small DRG neurons. Furthermore, Kv2.2 has a divergent, nonoverlapping distribution compared with that of Kv2.1 (for a review, see Ref. 36).
ScTx (Kd ≈ 1 nM) also blocks Kv4.2 (5), and since Kv4.2 inactivates at negative potentials, the second component of inactivation might also represent a Kv4.2 channel population. Moreover, the presence of Kv4.2 and its contribution to A-type K+ current in small rat DRG neurons have been reported (40). However, it seems unlikely that a Kv4.2 channel population reflects the second component of the ScTx-sensitive current because no clear A-type current was noticeable in the ScTx-sensitive current (Fig. 3G) and because the anti-Kv2.1-sensitive current displayed the same two components as the ScTx-sensitive current. This strengthens the idea that the ScTx-sensitive current represents only Kv2.x-containing channels in the cells that we studied.
In the case of neocortical pyramidal neurons of the rat, it has been reported that a ScTx- and anti-Kv2.1-sensitive current displayed a voltage dependence of inactivation with a midpoint of about −62 mV (9). This result is comparable with that of the second population of our ScTx- and anti-Kv2.1-sensitive current. The authors suggested that the difference compared with results of Kv2.1 in a heterologous expression system could be due to the interaction of Kv2.1 with silent Kv subunits or to a different phosphorylation state of Kv2.1 in cultured neurons compared with heterologous expression. The degree of phosphorylation influences the voltage dependent properties of Kv2.1, and different degrees of Kv2.1 phosphorylation have been reported in different cells (15, 17, 18, 21, 35). To exclude the hypothesis that differentially phosphorylated Kv2.1 channels caused the two components of the inactivation curve, we investigated the effect of dephosphorylation. While the application of AP also shifted the voltage dependence of inactivation of heterotetrameric Kv2/silent Kv currents (Fig. 5A and Table 1), it did not alter the relative size of either component in DRG neurons but shifted both equally (Fig. 5B and Table 1).
In conclusion, we propose that the two components observed in both the ScTx- and anti-Kv2.1-sensitive current reflect populations of homotetrameric Kv2.1 and heterotetrameric Kv2.1/silent Kv channels. Furthermore, since Kv4.2 does not noticeably contribute to the ScTx-sensitive current, the Kv2.1-containing channels represent the major component (∼2/3) of the IK in small mouse DRG neurons. The substantial contribution of the heterotetrameric channels supports an important physiological role of silent Kv subunits in these neurons and raises the question of how these neurons regulate the expression of homo- and heterotetrameric channels in vivo.
GRANTS
This research was funded by a PhD grant of the Institute for the Promotion of Innovation through Science and Technology in Flanders (to E. Bocksteins), by the Interuniversity Attraction Poles Program P6/31 of the Belgian Federal Science Policy Office, and by Grants G.0152.06 and G.0257.08 from the Fonds voor Wetenschappelijk Onderzoek Vlaanderen and GOA50.4426 (concerted action fund of the University of Antwerp).
Supplementary Material
Acknowledgments
We thank Jean-Pierre Timmermans for the use of the confocal microscope and Alain Labro and Natacha Ottschytsch for helpful discussions. We also thank Miguel Salinas for kindly providing the Kv9.1 and Kv9.2 channel constructs.
Footnotes
Supplemental material for this article is available online at the American Journal of Physiology-Cell Physiology website.
REFERENCES
- 1.Andrews EM, Kunze DL. Voltage-gated K+ channels in chemoreceptor sensory neurons of rat petrosal ganglion. Brain Res 897: 199–203, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Blaine JT, Ribera AB. Heteromultimeric potassium channels formed by members of the Kv2 subfamily. J Neurosci 18: 9585–9593, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castellano A, Chiara MD, Mellstrom B, Molina A, Monje F, Naranjo JR, Lopezbarneo J. Identification and functional characterization of a K+ channel alpha-subunit with regulatory properties specific to brain. J Neurosci 17: 4652–4661, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chien LY, Cheng JK, Chu D, Cheng CF, Tsaur ML. Reduced expression of A-type potassium channels in primary sensory neurons induces mechanical hypersensitivity. J Neurosci 27: 9855–9865, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Escoubas P, Diochot S, Celerier ML, Nakajima T, Lazdunski M. Novel tarantula toxins for subtypes of voltage-dependent potassium channels in the Kv2 and Kv4 subfamilies. Mol Pharmacol 62: 48–57, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Everill B, Kocsis JD. Reduction in potassium currents in identified cutaneous afferent dorsal root ganglion neurons after axotomy. J Neurophysiol 82: 700–708, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Fedulova SA, Vasilyev DV, Veselovsky NS. Voltage-operated potassium currents in the somatic membrane of rat dorsal root ganglion neurons: ontogenetic aspects. Neuroscience 85: 497–508, 1998. [DOI] [PubMed] [Google Scholar]
- 8.Gold MS, Shuster MJ, Levine JD. Characterization of six voltage-gated K+ currents in adult rat sensory neurons. J Neurophysiol 75: 2629–2646, 1996. [DOI] [PubMed] [Google Scholar]
- 9.Guan D, Tkatch T, Surmeier DJ, Armstrong WE, Foehring RC. Kv2 subunits underlie slowly inactivating potassium current in rat neocortical pyramidal neurons. J Physiol 581: 941–960, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishikawa K, Tanaka M, Black JA, Waxman SG. Changes in expression of voltage-gated potassium channels in dorsal root ganglion neurons following axotomy. Muscle Nerve 22: 502–507, 1999. [DOI] [PubMed] [Google Scholar]
- 11.Kerschensteiner D, Stocker M. Heteromeric assembly of Kv2.1 with Kv9.3: effect on the state dependence of inactivation. Biophys J 77: 248–257, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim DS, Choi JO, Rim HD, Cho HJ. Downregulation of voltage-gated potassium channel alpha gene expression in dorsal root ganglia following chronic constriction injury of the rat sciatic nerve. Brain Res Mol Brain Res 105: 146–152, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Kramer JW, Post MA, Brown AM, Kirsch GE. Modulation of potassium channel gating by coexpression of Kv2.1 with regulatory Kv5.1 or Kv6.1 α-subunits. Am J Physiol Cell Physiol 274: C1501–C1510, 1998. [DOI] [PubMed] [Google Scholar]
- 14.Malin SA, Nerbonne JM. Delayed rectifier K+ currents, IK, are encoded by Kv2 alpha-subunits and regulate tonic firing in mammalian sympathetic neurons. J Neurosci 22: 10094–10105, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Misonou H, Menegola M, Mohapatra DP, Guy LK, Park KS, Trimmer JS. Bidirectional activity-dependent regulation of neuronal ion channel phosphorylation. J Neurosci 26: 13505–13514, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohapatra DP, Park KS, Trimmer JS. Dynamic regulation of the voltage-gated Kv2.1 potassium channel by multisite phosphorylation. Biochem Soc Trans 35: 1064–1068, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Mohapatra DP, Trimmer JS. The Kv2.1 C-terminus can autonomously transfer Kv2.1-like phosphorylation-dependent localization, voltage-dependent gating, and muscarinic modulation to diverse Kv channels. J Neurosci 26: 685–695, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murakoshi H, Shi G, Scannevin RH, Trimmer JS. Phosphorylation of the Kv2.1 K+ channel alters voltage-dependent activation. Mol Pharmacol 52: 821–828, 1997. [DOI] [PubMed] [Google Scholar]
- 19.Murakoshi H, Trimmer JS. Identification of the Kv2.1 K+ channel as a major component of the delayed rectifier K+ current in rat hippocampal neurons. J Neurosci 19: 1728–1735, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ottschytsch N, Raes A, Van Hoorick D, Snyders DJ. Obligatory heterotetramerization of three previously uncharacterized Kv channel alpha-subunits identified in the human genome. Proc Natl Acad Sci USA 99: 7986–7991, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park KS, Mohapatra DP, Misonou H, Trimmer JS. Graded regulation of the Kv2.1 potassium channel by variable phosphorylation. Science 313: 976–979, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Patel AJ, Lazdunski M, Honore E. Kv2.1/Kv9.3, a novel ATP-dependent delayed-rectifier K+ channel in oxygen-sensitive pulmonary artery myocytes. EMBO J 16: 6615–6625, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ransom BR, Neale E, Henkart M, Bullock PN, Nelson PG. Mouse spinal cord in cell culture. I. Morphology and intrinsic neuronal electrophysiologic properties. J Neurophysiol 40: 1132–1150, 1977. [DOI] [PubMed] [Google Scholar]
- 24.Rasband MN, Park EW, Vanderah TW, Lai J, Porreca F, Trimmer JS. Distinct potassium channels on pain-sensing neurons. Proc Natl Acad Sci USA 98: 13373–13378, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richardson FC, Kaczmarek LK. Modification of delayed rectifier potassium currents by the Kv9.1 potassium channel subunit. Hear Res 147: 21–30, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Rola R, Witkowski G, Szulczyk PJ. Voltage-dependent K+ currents in rat cardiac dorsal root ganglion neurons. Neuroscience 119: 181–191, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Safronov BV, Bischoff U, Vogel W. Single voltage-gated K+ channels and their functions in small dorsal root ganglion neurones of rat. J Physiol 493: 393–408, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salinas M, de Weille J, Guillemare E, Lazdunski M, Hugnot JP. Modes of regulation of shab K+ channel activity by the Kv8.1 subunit. J Biol Chem 272: 8774–8780, 1997. [DOI] [PubMed] [Google Scholar]
- 29.Salinas M, Duprat F, Heurteaux C, Hugnot JP, Lazdunski M. New modulatory alpha subunits for mammalian Shab K+ channels. J Biol Chem 272: 24371–24379, 1997. [DOI] [PubMed] [Google Scholar]
- 30.Sano Y, Mochizuki S, Miyake A, Kitada C, Inamura K, Yokoi H, Nozawa K, Matsushime H, Furuichi K. Molecular cloning and characterization of Kv6.3, a novel modulatory subunit for voltage-gated K+ channel Kv2.1. FEBS Lett 512: 230–234, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Shepard AR, Rae JL. Electrically silent potassium channel subunits from the human lens epithelium. Am J Physiol Cell Physiol 277: C412–C424, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Sobko A, Peretz A, Shirihai O, Etkin S, Cherepanova V, Dagan D, Attali B. Heteromultimeric delayed-rectifier K+ channels in schwann cells: developmental expression and role in cell proliferation. J Neurosci 18: 10398–10408, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song WJ Genes responsible for native depolarization-activated K+ currents in neurons. Neurosci Res 42: 7–14, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Thorneloe KS, Nelson MT. Properties and molecular basis of the mouse urinary bladder voltage-gated K+ current. J Physiol 549: 65–74, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tiran Z, Peretz A, Attali B, Elson A. Phosphorylation-dependent regulation of Kv2.1 channel activity at tyrosine 124 by Src and by protein-tyrosine phosphatase epsilon. J Biol Chem 278: 17509–17514, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Trimmer JS, Rhodes KJ. Localization of voltage-gated ion channels in mammalian brain. Annu Rev Physiol 66: 477–519, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Vega-Saenz de Miera Modification of Kv2 EC.1. K+ currents by the silent Kv10 subunits. Brain Res Mol Brain Res 123: 91–103, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Verma-Kurvari S, Border B, Joho RH. Regional and cellular expression patterns of four K+ channel mRNAs in the adult rat brain. Brain Res Mol Brain Res 46: 54–62, 1997. [DOI] [PubMed] [Google Scholar]
- 39.Vydyanathan A, Wu ZZ, Chen SR, Pan HL. A-type voltage-gated K+ currents influence firing properties of isolectin B4-positive but not isolectin B4-negative primary sensory neurons. J Neurophysiol 93: 3401–3409, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Winkelman DL, Beck CL, Ypey DL, O'Leary ME. Inhibition of the A-type K+ channels of dorsal root ganglion neurons by the long-duration anesthetic butamben. J Pharmacol Exp Ther 314: 1177–1186, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Wu H, Cowing JA, Michaelides M, Wilkie SE, Jeffery G, Jenkins SA, Mester V, Bird AC, Robson AG, Holder GE, Moore AT, Hunt DM, Webster AR. Mutations in the gene KCNV2 encoding a voltage-gated potassium channel subunit cause “cone dystrophy with supernormal rod electroretinogram” in humans. Am J Hum Genet 79: 574–579, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu XR, Netzer R, Bohlke K, Liu Q, Pongs O. Structural and functional characterization of Kv6.2, a new gamma-subunit of voltage-gated potassium channel. Receptors Channels 6: 337–350, 1999. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.