Abstract
Agmatine, an endogenous metabolite of arginine, selectively suppresses growth in cells with high proliferative kinetics, such as transformed cells, through depletion of intracellular polyamine levels. In the present study, we depleted intracellular polyamine content with agmatine to determine if attrition by cell death contributes to the growth-suppressive effects. We did not observe an increase in necrosis, DNA fragmentation, or chromatin condensation in Ha-Ras-transformed NIH-3T3 cells administered agmatine. In response to Ca2+-induced oxidative stress in kidney mitochondrial preparations, agmatine demonstrated attributes of a free radical scavenger by protecting against the oxidation of sulfhydryl groups and decreasing hydrogen peroxide content. The functional outcome was a protective effect against Ca2+-induced mitochondrial swelling and mitochondrial membrane potential collapse. We also observed decreased expression of proapoptotic Bcl-2 family members and of execution caspase-3, implying antiapoptotic potential. Indeed, we found that apoptosis induced by camptothecin or 5-fluorourocil was attenuated in cells administered agmatine. Agmatine may offer an alternative to the ornithine decarboxylase inhibitor difluoromethyl ornithine for depletion of intracellular polyamine content while avoiding the complications of increasing polyamine import and reducing the intracellular free radical scavenger capacity of polyamines. Depletion of intracellular polyamine content with agmatine suppressed cell growth, yet its antioxidant capacity afforded protection from mitochondrial insult and resistance to cellular apoptosis. These results could explain the beneficial outcomes observed with agmatine in models of injury and disease.
Keywords: oxidative stress, polyamines, free radical scavenger, Bcl-2, caspase-3
agmatine, a cationic biogenic amine derived from arginine, induces a select set of physiological effects (52). The capacity to counterregulate nitric oxide (NO) as well as the proproliferative pathways of arginine places it in a pivotal position among these pathways (56). The antiproliferative effects have garnered particular interest in the treatment of neoplasms, where levels of agmatine are lower than in the surrounding normal tissue (48). Agmatine suppresses growth through the reduction of intracellular polyamine levels, which are required for growth, and inhibition of ornithine decarboxylase (ODC), the first and rate-limiting enzyme of polyamine biosynthesis, in all models studied to date (4, 16, 22, 29, 46, 59, 68). Reduction of cellular polyamine levels occurs by the induction of the polyamine autoregulatory protein antizyme (4, 29, 59), increasing activity of polyamine catabolism (16, 68), and/or decreasing ODC activity by another mechanism, possibly by reduction of ODC translation (4, 71). Furthermore, agmatine is transported into mammalian cells via the polyamine transport system (57). As polyamine transport activity correlates with proliferation rate, agmatine preferentially targets cells with high proliferative kinetics, such as transformed cells, to suppress their growth (33).
The mechanisms of growth suppression by agmatine have yet to be fully defined. As apoptotic models have implicated that polyamine depletion is a common and potentially causal factor of apoptosis (12, 15, 25, 47), we hypothesized that the induction of apoptosis by agmatine may contribute to the antigrowth effects through cellular attrition. Apoptosis occurs during normal development and aging as a compensatory mechanism to cellular proliferation in maintaining cell populations. This coordinated and energy-dependent process also occurs in disease states as tumor suppressor and host defense mechanisms against pathogens. Cellular constituents are not released to the surrounding tissue during this process, and, therefore, apoptosis is not associated with inflammation. Apoptotic cells can, however, regulate the immune response (61). Alternatively, cell death by necrosis is associated with loss of cell membrane integrity and release of cellular constituents, including chemotactic factors, resulting in an inflammatory response. The combination of cell death signal(s), cell type, and surrounding milieu all contribute to determining the mode of cell death.
Two general pathways of apoptosis are the extrinsic or death receptor pathway and the intrinsic or mitochondrial pathway. These pathways may influence one another (31) and converge at the execution caspase, caspase-3. Cleavage of caspase-3 initiates a series of apoptotic events including protein modification, DNA fragmentation, apoptotic body formation, and externalization of membrane phosphatidylserine for phagocytic recognition. Withdrawal of factors that promote the extrinsic pathway, such as growth factors, hormones, DNA damage, or cytotoxic insults, can lead to apoptosis through the mitochondrial pathway. The control and regulation of mitochondrial membrane permeability occur through members of the Bcl-2 family of proteins. Bcl-2 family members interact with one another to orchestrate either pro- or antiapoptotic activities via mitochondrial membrane regulation (73). Permeabilization of the mitochondrial membrane, in association with loss of mitochondrial transmembrane potential, ultimately results in the release of proapoptotic factors and progression to cell death.
Contrary to our expectations, we found that agmatine protects mitochondrial membrane integrity and confers resistance to apoptosis induced by camptothecin or 5-fluorourocil (5-FU). The capacity to reduce oxidative stress, protect mitochondrial function, and suppress apoptosis would contribute to the beneficial effects observed with agmatine administration in models of injury and inflammatory pathologies.
MATERIALS AND METHODS
Materials.
All chemicals were purchased from Sigma unless otherwise stated. We were unable to detect impurities by HPLC analysis in agmatine purchased from Aldrich. The Ha-Ras-transformed murine NIH-3T3 cell line [Ras/3T3; a generous gift from Dr. M. Kamps, University of California-San Diego (70)] was used for cell culture experiments. Ras/3T3 cells were maintained in DMEM (Cellgro, Herndon, VA) supplemented with 5% FBS (Atlanta Biologicals, Atlanta, GA) and antibiotics. Rat kidney mitochondria (RKM) were isolated for the evaluation of mitochondrial membrane function. [14C]agmatine was a generous gift from Dr. Maria Angelica Grillo (University of Torino, Torino, Italy).
FACS analyses of cell cycle populations.
Ras/3T3 cells were plated in the absence or presence of agmatine for the times indicated. Cells were harvested and gently fixed (62), and DNA was stained with propidium iodide and evaluated by FACS analysis. The University of California-San Diego Flow Cytometry Facility performed the staining and FACS analyses.
Hoechst staining.
Ras/3T3 cells were grown on chamber slides for 10 days with or without 1 mM agmatine, incubated with Hoechst stain (0.2 μg/ml), and viewed with an ultraviolet filter (excitation: 365 nm and emission: 480 nm).
Isolation of RKM.
RKM were isolated by conventional differential centrifugation in a standard medium containing 250 mM sucrose, 5 mM HEPES (pH 7.4), and 1 mM EGTA; EGTA was omitted from the final washing solution. Protein content was measured by the biuret method with BSA as the standard (24). These studies were performed in accordance with the guiding principles in the care and use of animals and were approved by the Italian Ministry of Health.
Standard incubation procedures for RKM.
RKM (1 mg protein/ml) were incubated in a water-jacketed cell at 20°C. The standard medium contained 200 mM sucrose, 10 mM HEPES (pH 7.4), 5 mM succinate, and 1.25 μM rotenone. Variations and/or other additions are described with the individual experiments presented.
Uptake of agmatine into RKM.
Mitochondrial incorporation of [14C]agmatine was determined by a centrifugal filtration method as previously described (66). The above-mentioned sucrose-based medium was chosen for this study to compare the obtained results with those previously determined in the liver (13) and also with those obtained for polyamine transport (for a review, see Ref. 67). The effects of higher ionic strength media were also determined. The results for RKM were similar to results previously reported in rat liver mitochondria (RLM). [14C]agmatine was prepared as previously reported (13).
Determination of mitochondrial functions.
Membrane potential (ΔΨ) was calculated on the basis of the distribution of the lipid-soluble cation tetraphenylphosphonium (TPP) through the inner membrane, measured by a TPP-positive-specific electrode prepared in our laboratory according to previously published procedures (34). Mitochondrial matrix volume was calculated from the distributions of [14C]sucrose and 3H2O according to the method of Palmieri and Klingenberg (50). Mitochondrial swelling was determined by measuring the apparent absorbance change of mitochondrial suspensions at 540 nm on a Kontron Uvikon model 922 spectrophotometer equipped with thermostatic control. The protein sulfhydryl group oxidation assay was performed as in the study by Santos et al. (55). The production of H2O2 in RLM was measured fluorometrically by the scopoletin method (45) in an Aminco-Bowman 4-8202 spectrofluorometer. A Clark electrode (Yellow Spring Instruments) in a closed vessel equipped with a thermostatic control and magnetic stirrer was used to measure respiratory parameters.
Western blot analysis.
Ras/3T3 cells were collected and lysed [lysis buffer contained 1% Triton X-100, 0.5% deoxycholic acid, 1 mM EDTA, 0.1% SDS, 4 mM NaF, complete protease cocktail (Roche Molecular Biochemicals, Mannheim, Germany), 0.7 g/ml pepstatin, and 1 mM NaVO4 in PBS]. Lysates at 50 μg/lane were resolved on 12% NuPAGE gels in MOPS buffer (Invitrogen, Carlsbad, CA). Gel proteins were transferred to nitrocellulose membranes and immunoblotted with the appropriate primary antibody as indicated. The secondary antibody was horseradish peroxidase conjugated (Santa Cruz Biotechnology, Santa Cruz, CA) for autoradiographic detection by ECL Plus (Amersham Pharmacia, Piscataway, NJ) with densitometric analysis by ImageJ software (National Institutes of Health, Bethesda, MD).
Caspase-3 activity.
Ac-DEVD-AFC (100 μM) in lysis buffer [25 mM HEPES (pH 7.5), 0.1% Triton X-100, 5 mM MgCl2, 1.3 mM EGTA, 1 mM EDTA, and 2 mM DTT] was used as a specific substrate for caspase-3 activity and detected on a Spectron Max Gemini EM Plate Reader. Incubations were for 4 days with or without 0.3 mM agmatine unless otherwise noted.
HPLC determination of polyamine content.
Ras/3T3 cells were grown in six-well plates for 4 days with or without agmatine (0.3 mM) and/or camptothecin (20 μM) (n = 3 for 6 conditions). Cells were washed and then lysed using 10% tricarboxylic acid (200 μl) as previously described (33). Cell lysates were collected and centrifuged to isolate denatured cell debris for protein analysis, and the supernatant was ether extracted and lyophilized to concentrate amino acids and polyamines. Samples and known standards were resuspended in 10 μl PBS and derivatized for fluorescence detection of primary and secondary amine groups with N-hydroxysuccinimidyl-6-aminoquinoyl carbamate as per the kit instructions (AccQ tag, Waters). The elution gradient was loosely based on the AccQ tag kit instructions. HPLC was performed using a Hewlett Packard 1100 series system with a 50 mm, 2.1-m ODS Hypersil C-18 RP column (Hewlett Packard) maintained at 45°C. Peak heights for each analyte derived from replicate runs were averaged and normalized using standards to establish the concentration of each sample. Samples were then normalized for protein content to enable the comparison of mean values among experimental conditions.
Statistical evaluations.
Variations between samples within groups were analyzed by ANOVA, with significance determined by Fisher's protected least-significant difference post hoc test. KleidaGraph software (version 4.03, Synergy Software) was used for these analyses.
RESULTS
Effects of agmatine on DNA.
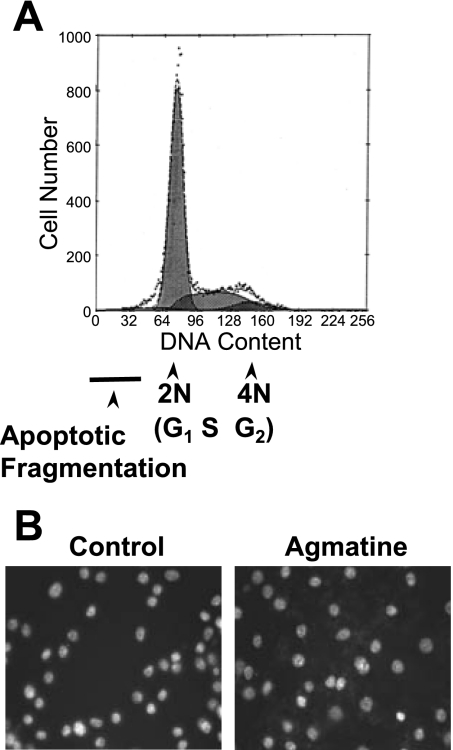
The growth-suppressive effects of agmatine may be due to a decrease in the mitotic rate, an increase in cell death, or both. We and others have shown that the administration of agmatine suppresses the mitotic rate by measuring [3H]thymidine incorporation in several cell lines (16, 53, 59) and in vivo (32). Agmatine is not toxic to any cell lines at the doses administered, as determined by trypan blue exclusion (data not shown). FACS analysis of propidium iodide in Ras/3T3 cells revealed a lack of asymmetry to the left of the 2N (G1) cell population, representing a lack of apoptotic DNA fragmentation after 10 days in culture with agmatine (Fig. 1A). Complementary to this result, Hoechst staining (Fig. 1B) did not exhibit an increase in chromatin condensation.
Fig. 1.
Apoptotic changes in DNA. A: propidium iodine/FACS analysis did not reveal an asymmetric shoulder to the left side of the 2N peak indicative of apoptotic DNA fragmentation. B: Hoechst staining of Ha-Ras-transformed NIH-3T3 (Ras/3T3) cells did not exhibit chromatin condensation typical of apoptosis. In both procedures, cells were incubated with 1 mM agmatine (AGM) for 10 days.
RKM transport of agmatine.
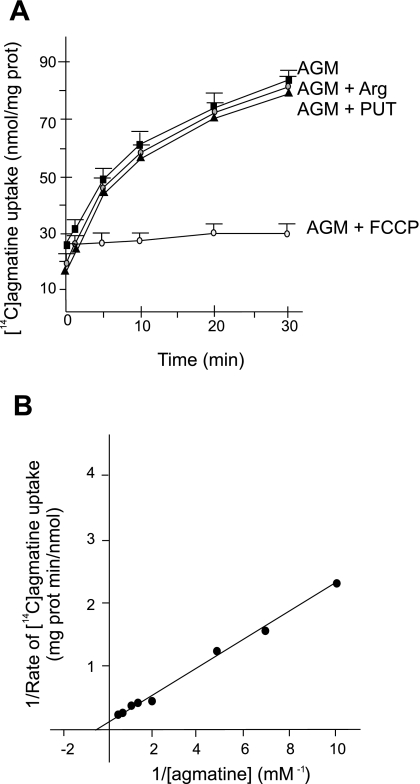
RKM took up ∼60 nmol of [14C]agmatine/mg protein in 30 min of incubation. Collapse of ΔΨ with the addition of FCCP completely inhibited transport (Fig. 2A), demonstrating an energy-dependent mode of transport. In a saline medium containing 50 mM NaCl, the net agmatine uptake was ∼6 nmol/mg protein in 30 min (Fig. 2A). In the presence of high ionic strength media, agmatine transport was also considerably inhibited in RLM (13). Taking into account that the matrix volume of 1 mg of mitochondrial protein is ∼1 μl, agmatine inner concentrations reached ∼6 mM. Matrix concentrations can also reach the millimolar level with the transport of nanomolar concentrations of exogenous agmatine. This fact allows the transport of agmatine in the presence of high ion concentrations, i.e., experimental conditions nearer to the physiological state, to generate a matrix concentration sufficient to exhibit its physiological effects. The mechanism of agmatine transport is different from that of amino acids and polyamines, as neither competed with agmatine for transport (Fig. 2A). Similar results have previously been determined in liver mitochondria (54). This specificity of transport in mitochondria is notably different than transport through the cell membrane via polyamine transporters (58). Estimation of kinetic parameters from initial rate measurements determined Km and Vmax values of 1.7 mM and 7.9 nmol·min−1·mg protein−1, respectively (Fig. 2B). These parameters are similar to those reported for agmatine transport in RLM (13), with a slightly higher rate constant and lower affinity.
Fig. 2.
AGM transport into rat kidney mitochondria (RKM). A: RKM were incubated in standard medium with 1 mM [14C]AGM (50 μCi/mmol). Labeled AGM was coincubated with 0.1 μg FCCP/mg protein, 1 mM arginine (Arg), or 1 mM putrescine (Put). The dashed line shows to AGM transport by mitochondria incubated in standard medium in which 200 mM sucrose was substituted with 50 mM NaCl plus 100 mM sucrose. B: double reciprocal plot of AGM uptake. RKM were incubated for 5 min in standard medium and [14C]AGM (50 μCi/mmol) at the indicated concentrations. The uptake of AGM was linear over the incubation period.
RKM bioenergetics in response to agmatine.
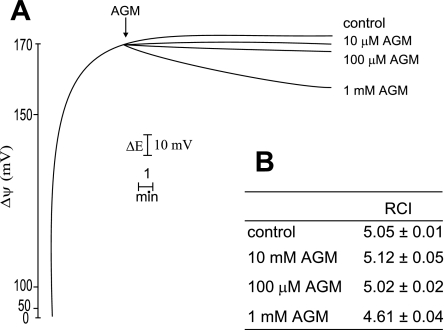
The addition of agmatine did not affect ΔΨ at lower concentrations (Fig. 3A). The gradual decrease in ΔΨ at higher concentrations is due to the transport into mitochondrial matrix. At physiological pH, agmatine is a bivalent cation, and its transport provokes a slight decrease of ΔΨ. Agmatine did not alter the respiratory control index of RKM (Fig. 3B). Hence, the amine does not result in any significant alteration of bioenergetic parameters of RKM.
Fig. 3.
Effects of AGM on mitochondrial bioenergetics. A: AGM effect on membrane potential (ΔΨ). Incubation conditions were as described in Fig. 2. AGM concentrations are shown on the right. ΔE, electrode potential. Four other experiments gave identical results. B: respiratory control index (RCI) of RKM to assess mitochondrial respiratory parameters. Values are means ± SD of 4 experiments.
Ca2+ induction in RKM of the mitochondrial permability transition.
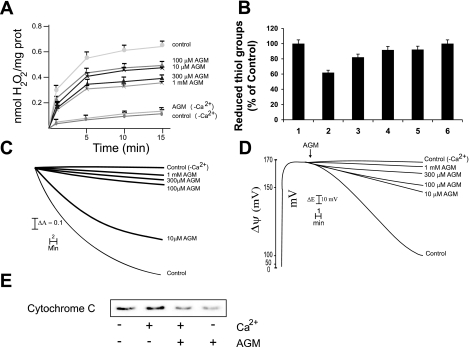
Generally, apoptotic stimuli are preceded by changes in the inner mitochondrial membrane that result in an opening of the mitochondrial permeability transition (MPT) pore. This event leads to mitochondrial swelling, loss of the mitochondrial transmembrane potential, and release of proteins from the intermembrane space into the cytosol, such as the proapoptotic protein cytochrome c. The results shown in Fig. 4 demonstrate that isolated RKM treated with supraphysiological Ca2+ concentration generate ROS, with oxidative stress and induction of the MPT ensuing, triggering the proapoptotic pathway. ROS generation by Ca2+ is due to the interaction of the cation with the cardiolipin domain of the mitochondrial inner membrane, which affects the electron flux through the respiratory chain at the level of ubiquinone pool. This effect favors an increased accumulation of the semiquinone radical, which reacts with molecular oxygen to generate superoxide anion, hydrogen peroxide, and other ROS. ROS generation induces an oxidative stress resulting in the oxidation of sulphydryl groups (Fig. 4B) and glutathione and pyridine nucleotides (results not reported). ROS generation by Ca2+ has been previously demonstrated together with its mechanism (27). Agmatine reduced hydrogen peroxide production (Fig. 4A) and oxidation of sulfhydryl groups (Fig. 4B), glutathione, and pyridine nucleotides (results not reported) in RKM in response to Ca2+ (80 μM), thereby acting as a free radical scavenger. Its protective effects were dose dependent. Generation of ROS was also confirmed using the probe Ampex red. These dose-dependent effects, in the absence of Ca2+ (control, −Ca2+) or in the presence of agmatine alone, had little effect on hydrogen peroxide production or sulfhydryl oxidation. Agmatine administration also protected, in a concentration-dependent manner, against Ca2+-induced mitochondrial swelling (Fig. 4C), reduced ΔΨ (Fig. 4D), and cytochrome c release (Fig. 4E). In this last experiment, we show only the effects of 0.3 mM agmatine. In RLM, agmatine administration increased hydrogen peroxide generation due to catabolic oxidation, decreased rat liver mitochondrial respiration, and markedly augmented Ca2+-induced MPT, especially at low concentrations (7). Thus, agmatine protects kidney mitochondria from MPT, in part through its capacity as a free radical scavenger, in contrast to the effects in RLM.
Fig. 4.
Effects of AGM on the mitochondrial permability transition (MPT) pore. RKM were incubated for 20 min in standard medium, as described in materials and methods, in the presence of 80 mM Ca2+. A: effect of AGM on H2O2 production. AGM concentrations are shown on the right. Values are means ± SD of 4 experiments. B: effect of AGM on the redox state of sulphydryl groups. AGM concentrations were as follows: control (−Ca2+ or AGM alone, 1), Ca2+ (2), Ca2+ + 10 mM AGM (3), Ca2+ + 100 mM AGM (4), Ca2+ + 300 mM AGM (5), and Ca2+ + 1 mM AGM (6). Values are means ± SD of 4 experiments. C: effect of AGM on mitochondrial swelling. AGM concentrations are shown on the right. Four other experiments gave almost identical results. Downward deflections indicate the absorbance decrease corresponding to mitochondrial swelling. D: effect of AGM on ΔΨ collapse. AGM concentrations are shown on the right. Four other experiments gave very similar results. E: protection by AGM on cytochrome c release. The AGM concentration, when present, was 300 mM. Incubations were followed by centrifugation and recovery of the supernatants, which were subjected to SDS-PAGE and immunoblot analysis to detect cytochrome c, as described in materials and methods. The data shown are typical of 3 separate experiments.
Bcl-2 family proteins.
As the mitochondrial sentinels of programmed cell death, the Bcl-2 family of proteins has either pro- or antiapoptotic activities. Incompletely understood signaling events convey intrinsic signals from an initial insult, such as DNA damage or growth factor depravation, to activate Bcl-2 proteins. Temporal analysis of protein expression revealed a significant decrease in proapoptotic Bad as well as in its phosphorylated inactivated form in response to agmatine administration (300 μM; Fig. 5). p53-upregulated modulator of apoptosis (PUMA) was also significantly decreased at day 7. Bad and PUMA are members of the BH3-only domain proteins that can activate other proapoptotic members or, in the case of PUMA, may act as a sensitizer or directly as an activator of apoptosis (40). Other Bcl-2 family members evaluated included Bcl-2, Bax, Bik, Bcl-2 modifying factor, and Bcl-xL. Total protein expression of these members was not significantly affected; however, we did not evaluate potential changes in their subcellular localization.
Fig. 5.
Expression of Bcl-2 family proteins. Western blot analysis of protein expression of Bcl-2 family member proteins is shown. Cells were incubated with 500 μM AGM for the days indicated on the x-axis. Measurements of protein expression were derived by densitometry (ImageJ software). Standard deviations for control (Con) cells not administered AGM were derived from the densitometry values of 3 independent experiments and normalized for β-actin. Standard deviations of the agmatine-treated samples were determined as percentages of the mean control value. P ≤ 0.0005, expression of both BAD and phosphorylated BAD (p-BAD) from day 2 through day 7 relative to control. P ≤ 0.05, p53-upregulated modulator of apoptosis (PUMA) expression at day 7 relative to control. Values are from 3 independent experiments.
Effects of agmatine on caspase-3.
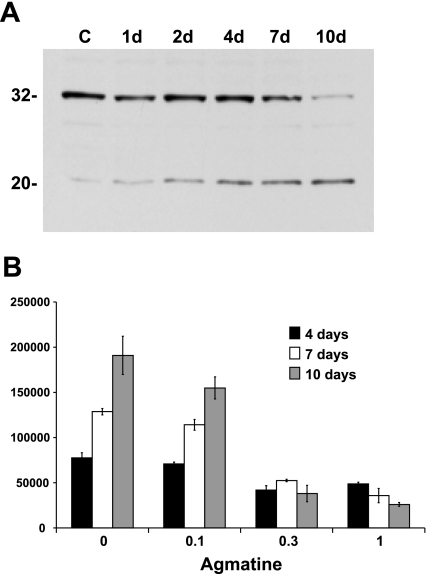
Caspase-3 is an execution caspase of the apoptotic response due, in part, to its cleavage of poly(ADP-ribose) polymerase. As shown in Fig. 6A, Ras/3T3 cells exposed to agmatine exhibited a temporal decrease in caspase-3 protein levels, with increasing caspase-3 fragments typical of proteolytic cleavage. However, caspase-3 activity in Ras/3T3 cells decreased in a time- and concentration-dependent manner in response to agmatine (Fig. 6B). The reduction of Bad expression temporally correlated with that of caspase-3.
Fig. 6.
Caspase-3 expression and activity. A: Western blot analysis of caspase-3 expression in response to 500 μM AGM over the days indicated. Caspase-3 (∼32 kDa) expression decreased over time with an increase in the potential cleavage product (∼20 kDa). C, control. B: activity of the enzyme decreased in a concentration-dependent manner over time. Activity is expressed in relative units per milligram of protein per 90 min. P ≤ 0.05, AGM relative to non-AGM samples at 0.1 mM for days 4 and 10 and at 0.3 and 1.0 mM for days 4, 7, and 10.
Agmatine suppresses apoptosis induced by 5-FU or camptothecin.
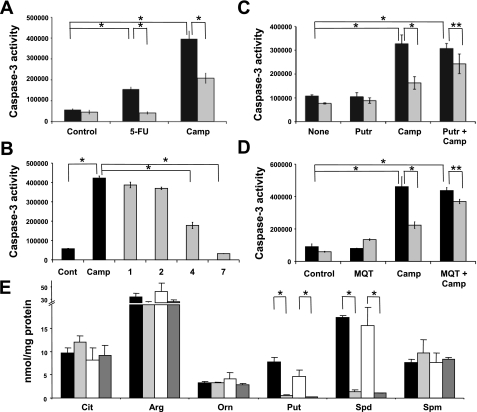
We evaluated caspase-3 activity to monitor apoptosis induced by a 24-h incubation with either 5-FU or camptothecin. Both apoptotic mediators markedly increased caspase-3 activity in Ras/3T3 cells. This induction was significantly suppressed in the presence of agmatine (300 μM; Fig. 7A). These effects were maximal by 7 days of incubation (Fig. 7B). Preloading cells with putrescine yielded no effect on basal or camptothecin-induced levels of caspase-3 activity but significantly attenuated the suppressive effects of agmatine (Fig. 7C). Inhibition of the uptake of agmatine with the polyamine transport inhibitor MQT-1483 also significantly reduced the effects of agmatine (Fig. 7D), implying that the effects are not receptor mediated. The results shown in Fig. 7E demonstrate the effects of agmatine on Ras/3T3 intracellular polyamine content. The results mirror those for the kidney proximal cell line, MCT (59), and additionally show no significant effects of camptothecin on the reduction of polyamines in response to agmatine. These results demonstrate that the antiapoptotic effects of agmatine are dependent on internalization of the amine and polyamine depletion.
Fig. 7.
AGM confers resistance to apoptosis. A: induction of apoptosis with 5-fluorouricil (5-FU; 50 mg/ml) or camptothecin (Camp; 20 μM) was suppressed by AGM (300 μM, 4 days). *P ≤ 0.0001. B: the antiapoptotic effects increased with time. AGM (300 μM) was administered for the days indicated on the x-axis. *P ≤ 0.0001. Suppression was attenuated by supplementation with the polyamine Put (1 mM; C) or blockade of uptake of AGM with administration of the polyamine transport blocker MQT-1483 (MQT; 1 μM; D). *P ≤ 0.0001; **P ≤ 0.05. P ≤ 0.001, Camp + AGM vs. Put + Camp + AGM; P ≤ 0.0001, Camp + AGM vs. MQT + Camp + AGM. E: HPLC evaluation of citrulline (Cit), arginine (Arg), ornithine (Orn), Put, spermidine (Spd), and spermine (Spm) levels in Ras/3T3 cells in the absence or presence of AGM, Camp, or AGR + Camp. There were no significant changes in Cit, Arg, Orn, or Spm levels. *P ≤ 0.01. In A–D, filled bars represent non-AGM controls and shaded bars represent AGM supplementation. In E, filled bars represent non-AGM controls, light shaded bars represent AGM supplementation, open bars represent Camp treatment, and dark shaded bars represent Camp + AGM supplementation. Caspase-3 activity is expressed in relative units per milligram of protein per 90 min.
DISCUSSION
Polyamine biosynthesis has been of interest in the study of apoptosis since the early 1990s. Initial studies on apoptosis in thymocytes observed polyamine depletion as an unsuspected finding and hypothesized that polyamine depletion contributed to the activation of apoptosis (25). Agmatine depletes intracellular polyamine levels to suppress growth (4, 16, 22, 29, 46, 59, 68). Supplementation with the polyamine putrescine attenuates this effect (22, 33, 59). Our results indicate that agmatine administration protects against indexes of apoptosis in both a transformed cell line and isolated RKM. Oxidative stress induced by Ca2+, in the presence of phosphate, leads to the opening of the MPT pore and the consequent release of cytochrome c (Fig. 4). This event triggers the intrinsic caspase-dependent apoptotic pathway, resulting in the apoptotic phenotype, in Ras/3T3 cells, as evidenced here by the increase of caspase-3 activity upon treatment with 5-FU or camptothecin (Figs. 6 and 7). Agmatine, by scavenging ROS, prevents oxidative stress, MPT induction, and cytochrome c release (Fig. 4) and decreases caspase-3 activity by blocking the intrinsic apoptotic pathway (Figs. 6 and 7). The addition of polyamines reduces this protective effect and restores camptothecin-induced apoptosis in vitro.
d,l-α-Difluoromethyl ornithine (DFMO) selectively inhibits ODC activity and has been widely used to deplete polyamine levels in vitro and in vivo. DFMO alone does not appear to induce apoptosis (49) except when used synergistically with other compounds (42, 49) or in particular cells that are predisposed to transformation (11, 51, 77). Some tumor cell lines can also undergo apoptosis when administered agmatine (71). In addition to inhibiting ODC, DFMO causes a compensatory increase in polyamine transport (10, 57) and increases oxidative stress in the cell by reducing the free radical-quenching properties inherent to polyamines (19, 28). However, several examples of polyamine depletion curbing apoptotic induction have also been described. Etoposide reduces intracellular polyamine content by increasing polyamine oxidation and thus hydrogen peroxide formation in a fibroblast cell line (43). The increased oxidative stress of polyamine metabolism is associated with apoptosis in this model. DFMO reduction of the substrate polyamines thus reduces apoptosis. DFMO also suppresses caspase and ERK activation in response to etoposide (64). A series of elegant experiments (75) delineated the transduction pathways of apoptosis in the intestinal epithelial cell line IEC-6. In this study (75), DFMO conferred protective effects against camptothecin-induced apoptosis. However, this may be cell type specific as IEC-6 cells respond to DFMO by activation of ERK1/2 (9), which is contrary to previously reported cellular responses of DFMO on ERK1/2 (6). Thus, these naturally proliferating intestinal cells may have characteristics that selectively suppress apoptosis in response to DFMO and/or may maintain sufficient free radical-scavenging capacity in the presence of reduced polyamine levels such that apoptosis is not induced.
Evidence supports a complex relationship between polyamines and apoptosis that depends on the cell type, death stimulus, extracellular milieu, and status and active pathways of intracellular polyamines (18). A compensatory upregulation of polyamine transporters by DFMO emphasizes the relevance of the extracellular milieu (63). Polyamines function as free radical scavengers and can protect against apoptosis (19, 28). The impact of DFMO on the oxidative state of cells, in combination with their varying antioxidant capacities of differing cell types, would contribute to the disparity of the reported responses of DFMO. In this regard, we show that agmatine also functions as a free radical scavenger, thereby restoring this protective function in the setting of depleted polyamine levels. The lack of other commercially accessible polyamine biosynthesis inhibitors makes DFMO a widely used and common factor among studies but also limits comparison studies by other agents. Agmatine depletes intracellular polyamine levels and, in two different model systems (ex vivo RKM and a transformed fibroblast cell line), we observed protective effects.
The results presented here can be considered of physiological relevance, since the agmatine concentrations used in the experiments and the Km calculated for transport (1.7 mM) are compatible with the concentration of agmatine normally measured in kidney cells [0.5–1 mM (Ref. 44 and A. Toninello and V. Battaglia, unpublished observations)]. It should be noted that fluctuations in these concentrations can occur in pathophysiological states (20). For example, the concentration of agmatine can increase ∼20-fold in the rat aorta after ischemic injury (14). Similarly, taking into account the calculated Vmax (7.9 nmol·min−1·mg protein−1), the accumulation of the amine in the mitochondrial matrix can reach values much higher than those obtainable with the polyamines that have Vmax values in the order of 1–1.5 nmol·min−1·mg protein−1.
Contrary to our results in RKM, low concentrations of agmatine exacerbated Ca2+- and phosphate-induced mitochondrial swelling and ΔΨ collapse in RLM preparations as well as induced apoptosis in hepatocytes in culture (7, 21). However, at high concentrations, agmatine affords protection against mitochondrial swelling and ΔΨ collapse in RLM, similar to our findings in RKM. Differences appear to be cell type specific as agmatine in kidney epithelial, pulmonary endothelial, and fibroblast cell lines induces antizyme (5, 59), whereas in hepatocytes, agmatine induces spermidine/spermine-N1-acetyltransferase (SSAT), not antizyme (68). In further support of this cell specificity in liver cells, we observed only an incremental, nonsignificant increase in SSAT expression in Ras/3T3 cells when exposed to agmatine over the course of 10 days (unpublished observations). Agmatine induction of SSAT in hepatocytes has been shown to deplete intracellular polyamines, with a correlative increase in hydrogen peroxide production in association with apoptosis (21). SSAT and amine oxidase comprise a two-step interconversion of higher-order polyamines to lower-order polyamines, i.e., spermine to spermidine and spermidine to putrescine. Hydrogen peroxide and aldehyde production from these reactions contribute to the cytotoxic effect of abnormally high intracellular polyamine levels. This pathway of increased oxidative stress and cell death has provided a new therapeutic direction in the treatment of cancer (2, 3). The biphasic response to agmatine in liver mitochondria is likely due to the balance of increased oxidative stress generated from the polyamine back conversion pathway being neutralized at higher concentrations of agmatine. At 1 mM agmatine, the redox state of mitochondrial sulfhydryl groups, glutathione and pyridine nucleotides, return to control levels in RLM preparations in the presence of Ca2+ and phosphate (7). Rat and human hepatoma cell lines also increase apoptosis in response to agmatine administration (71). Thus, the marked induction of the back conversion of polyamine pathways appears cell type selective and would explain the apoptosis in liver cells in response to agmatine in these experiments.
Glomerulonephritis is an inflammatory disease process of the kidney glomeruli. The experimental Thy-1 model of proliferative glomerulonephritis consists of an acute inflammatory phase of glomerular mesangiolysis on day 1 followed by mesangial cell proliferation and extracellular matrix deposition by day 7. Both phases negatively affect the glomerular filtration rate (GFR). In this disease model, agmatine administration results in decreased ODC activity and [3H]thymidine incorporation as well as reduced focal and segmental hypercellularity, matrix deposition, and glomerulosclerosis (32). These effects were most apparent on day 7, with glomeruli appearing normal. Interestingly, despite similar day 1 parameters of injury in the Thy-1 control and Thy-1+ agmatine-treated animals, GFRs were significantly improved on day 1 of agmatine treatment. Suppression of apoptosis in the early mesangiolytic phase of this model would account for the beneficial effect of agmatine on the GFR. Agmatine is beneficial in several other experimental models including hypoxia (17, 30), ischemia-reperfusion (26, 35, 36, 38, 65, 72), N-methyl-d-aspartic acid or glucocorticoid damage (41, 69, 76), spinal cord and nerve injury (8, 23, 37, 74), and diabetes (39). We observed selective inhibition of inducible NO synthase by the aldehyde metabolite of agmatine and beneficial effects of agmatine administration in the LPS model of endotoxic shock (60). Others have observed similar protection against LPS-induced cell death in cerebellar granule neurons (1). This selective inhibitory affect on inducible NO synthase would contribute to the antiapoptotic capacity of agmatine in models of inflammation. The capacity of agmatine to reduce oxidative stress and protect mitochondrial function likely contributes to the uniformity of these results.
In conclusion, we report that cellular attrition through apoptosis does not contribute to the antiproliferative effects of agmatine but rather that agmatine confers resistance to apoptosis. Protective effects were observed in Ras/3T3 cells in culture as well as ex vivo RKM. Aberrant apoptosis and/or proliferation were common pathological complications. A molecule that can suppress apoptosis while depleting intracellular polyamines required for proliferation without exhausting their free radical-scavenging capacity could prove a beneficial therapeutic tool. As we have previously shown in a model of glomerulonephritis (8), agmatine may be a candidate for adjunctive therapy as well as a model for drug design in this and other pathological settings.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-070123, DK-070667, DK-02920, DK-56248, and DK-28602, the University of Alabama at Birmingham-University of California-San Diego O'Brien Center for Acute Kidney Injury Research, the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, and grants-in-aid from the Ministry of Education, Science and Culture in Japan.
Acknowledgments
The authors thank Dr. F. C. White and Dr. M. Kamps (University of California-San Diego) for kindly supplying the Ras/3T3 cell line. MQT-1483 was a generous gift from MediQuest Therapeutics, Seattle, WA.
REFERENCES
- 1.Abe K, Abe Y, Saito H. Agmatine suppresses nitric oxide production in microglia. Brain Res 872: 141–148, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Agostinelli E, Tempera G, Molinari A, Salvi M, Battaglia V, Toninello A, Arancia G. The physiological role of biogenic amines redox reactions in mitochondria. New perspectives in cancer therapy. Amino Acids 33: 175–187, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Averill-Bates DA, Ke Q, Tanel A, Roy J, Fortier G, Agostinelli E. Mechanism of cell death induced by spermine and amine oxidase in mouse melanoma cells. Int J Oncol 32: 79–88, 2008. [PubMed] [Google Scholar]
- 4.Babal P, Ruchko M, Campbell CC, Gilmour SP, Mitchell JL, Olson JW, Gillespie MN. Regulation of ornithine decarboxylase activity and polyamine transport by agmatine in rat pulmonary artery endothelial cells. J Pharmacol Exp Ther 296: 372–377, 2001. [PubMed] [Google Scholar]
- 5.Babal P, Ruchko M, Olson JW, Gillespie MN. Interactions between agmatine and polyamine uptake pathways in rat pulmonary artery endothelial cells. Gen Pharmacol 34: 255–261, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Bachrach U, Wang YC, Tabib A. Polyamines: new cues in cellular signal transduction. News Physiol Sci 16: 106–109, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Battaglia V, Rossi CA, Colombatto S, Grillo MA, Toninello A. Different behavior of agmatine in liver mitochondria: Inducer of oxidative stress or scavenger of reactive oxygen species? Biochim Biophys Acta 1768: 1147–1153, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Berenholz L, Segal S, Gilad VH, Klein C, Yehezkeli E, Eviatar E, Kessler A, Gilad GM. Agmatine treatment and vein graft reconstruction enhance recovery after experimental facial nerve injury. J Peripher Nerv Syst 10: 319–328, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Bhattacharya S, Ray RM, Johnson LR. Prevention of TNF-α-induced apoptosis in polyamine-depleted IEC-6 cells is mediated through the activation of ERK1/2. Am J Physiol Gastrointest Liver Physiol 286: G479–G490, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Bogle RG, Mann GE, Pearson JD, Morgan DM. Endothelial polyamine uptake: selective stimulation by l-arginine deprivation or polyamine depletion. Am J Physiol Cell Physiol 266: C776–C783, 1994. [DOI] [PubMed] [Google Scholar]
- 11.Broaddus RR, Xie S, Hsu CJ, Wang J, Zhang S, Zou C. The chemopreventive agents 4-HPR and DFMO inhibit growth and induce apoptosis in uterine leiomyomas. Am J Obstet Gynecol 190: 686–692, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Brune B, Hartzell P, Nicotera P, Orrenius S. Spermine prevents endonuclease activation and apoptosis in thymocytes. Exp Cell Res 195: 323–329, 1991. [DOI] [PubMed] [Google Scholar]
- 13.Cabella C, Gardini G, Corpillo D, Testore G, Bedino S, Solinas SP, Cravanzola C, Vargiu C, Grillo MA, Colombatto S. Transport and metabolism of agmatine in rat hepatocyte cultures. Eur J Biochem 268: 940–947, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Delbarre G, Delbarre B, Calinon F. Determination of agmatine in abdominal aorta of rats and gerbils after ischemic reperfusion insult. Soc Neurosci Abs 21: 1495, 1995. [Google Scholar]
- 15.Desiderio MA, Grassilli E, Bellesia E, Salomoni P, Franceschi C. Involvement of ornithine decarboxylase and polyamines in glucocorticoid-induced apoptosis of rat thymocytes. Cell Growth Differ 6: 505–513, 1995. [PubMed] [Google Scholar]
- 16.Dudkowska M, Lai J, Gardini G, Stachurska A, Grzelakowska-Sztabert B, Colombatto S, Manteuffel-Cymborowska M. Agmatine modulates the in vivo biosynthesis and interconversion of polyamines and cell proliferation. Biochim Biophys Acta 1619: 159–166, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Feng Y, Piletz JE, Leblanc MH. Agmatine suppresses nitric oxide production and attenuates hypoxic-ischemic brain injury in neonatal rats. Pediatr Res 52: 606–611, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Flamigni F, Stanic I, Facchini A, Cetrullo S, Tantini B, Borzi RM, Guarnieri C, Caldarera CM. Polyamine biosynthesis as a target to inhibit apoptosis of non-tumoral cells. Amino Acids 33: 197–202, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Gaboriau F, Vaultier M, Moulinoux JP, Delcros JG. Antioxidative properties of natural polyamines and dimethylsilane analogues. Redox Rep 10: 9–18, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Galea E, Regunathan S, Eliopoulos V, Feinstein DL, Reis DJ. Inhibition of mammalian nitric oxide synthases by agmatine, an endogenous polyamine formed by decarboxylation of arginine. Biochem J 316: 247–249, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gardini G, Cabella C, Cravanzola C, Vargiu C, Belliardo S, Testore G, Solinas SP, Toninello A, Grillo MA, Colombatto S. Agmatine induces apoptosis in rat hepatocyte cultures. J Hepatol 35: 482–489, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Gardini G, Cravanzola C, Autelli R, Testore G, Cesa R, Morando L, Solinas SP, Muzio G, Grillo MA, Colombatto S. Agmatine inhibits the proliferation of rat hepatoma cells by modulation of polyamine metabolism. J Hepatol 39: 793–799, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Gilad GM, Gilad VH. Accelerated functional recovery and neuroprotection by agmatine after spinal cord ischemia in rats. Neurosci Lett 296: 97–100, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Gornall AG, Bardawill CJ, David MM. Determination of serum proteins by means of the biuret reaction. J Biol Chem 177: 751–766, 1949. [PubMed] [Google Scholar]
- 25.Grassilli E, Desiderio MA, Bellesia E, Salomoni P, Benatti F, Franceschi C. Is polyamine decrease a common feature of apoptosis? Evidence from gamma rays- and heat shock-induced cell death. Biochem Biophys Res Commun 216: 708–714, 1995. [DOI] [PubMed] [Google Scholar]
- 26.Greenberg S, George J, Wollman Y, Shapira I, Laniado S, Keren G. The effect of agmatine administration on ischemic-reperfused isolated rat heart. J Cardiovasc Pharmacol Ther 6: 37–45, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Grijalba MT, Vercesi AE, Schreier S. Ca2+-induced increased lipid packing and domain formation in submitochondrial particles. A possible early step in the mechanism of Ca2+-stimulated generation of reactive oxygen species by the respiratory chain. Biochemistry 38: 13279–13287, 1999. [DOI] [PubMed] [Google Scholar]
- 28.Ha HC, Sirisoma NS, Kuppusamy P, Zweier JL, Woster PM, Casero RA Jr. The natural polyamine spermine functions directly as a free radical scavenger. Proc Natl Acad Sci USA 95: 11140–11145, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higashi K, Yoshida K, Nishimura K, Momiyama E, Kashiwagi K, Matsufuji S, Shirahata A, Igarashi K. Structural and functional relationship among diamines in terms of inhibition of cell growth. J Biochem (Tokyo) 136: 533–539, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Hong S, Lee JE, Kim CY, Seong GJ. Agmatine protects retinal ganglion cells from hypoxia-induced apoptosis in transformed rat retinal ganglion cell line. BMC Neurosci 8: 81, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Igney FH, Krammer PH. Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer 2: 277–288, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Ishizuka S, Cunard R, Poucell-Hatton S, Wead L, Lortie M, Thomson SC, Gabbai FB, Satriano J, Blantz RC. Agmatine inhibits cell proliferation and improves renal function in anti-thy-1 glomerulonephritis. J Am Soc Nephrol 11: 2256–2264, 2000. [DOI] [PubMed] [Google Scholar]
- 33.Isome M, Lortie MJ, Murakami Y, Parisi E, Matsufuji S, Satriano J. The antiproliferative effects of agmatine correlate with the rate of cellular proliferation. Am J Physiol Cell Physiol 293: C705–C711, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Kamo N, Muratsugu M, Hongoh R, Kobatake Y. Membrane potential of mitochondria measured with an electrode sensitive to tetraphenyl phosphonium and relationship between proton electrochemical potential and phosphorylation potential in steady state. J Membr Biol 49: 105–121, 1979. [DOI] [PubMed] [Google Scholar]
- 35.Kim DJ, Kim DI, Lee SK, Suh SH, Lee YJ, Kim J, Chung TS, Lee JE. Protective effect of agmatine on a reperfusion model after transient cerebral ischemia: Temporal evolution on perfusion MR imaging and histopathologic findings. Am J Neuroradiol 27: 780–785, 2006. [PMC free article] [PubMed] [Google Scholar]
- 36.Kim JH, Yenari MA, Giffard RG, Cho SW, Park KA, Lee JE. Agmatine reduces infarct area in a mouse model of transient focal cerebral ischemia and protects cultured neurons from ischemia-like injury. Exp Neurol 189: 122–130, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Kotil K, Kuscuoglu U, Kirali M, Uzun H, Akcetin M, Bilge T. Investigation of the dose-dependent neuroprotective effects of agmatine in experimental spinal cord injury: a prospective randomized and placebo-control trial. J Neurosurg Spine 4: 392–399, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Kuo JR, Lo CJ, Chio CC, Chang CP, Lin MT. Resuscitation from experimental traumatic brain injury by agmatine therapy. Resuscitation 75: 506–514, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Lee GT, Cho YD. Regulation of fibronectin levels by agmatine and spermine in mesangial cells under high-glucose conditions. Diabetes Res Clin Pract 66: 119–128, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Letai AG Diagnosing and exploiting cancer's addiction to blocks in apoptosis. Nat Rev Cancer 8: 121–132, 2008. [DOI] [PubMed] [Google Scholar]
- 41.Lewis B, Wellmann KA, Barron S. Agmatine reduces balance deficits in a rat model of third trimester binge-like ethanol exposure. Pharmacol Biochem Behav 88: 114–121, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li L, Rao JN, Bass BL, Wang JY. NF-κB activation and susceptibility to apoptosis after polyamine depletion in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 280: G992–G1004, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Lindsay GS, Wallace HM. Changes in polyamine catabolism in HL-60 human promyelogenous leukaemic cells in response to etoposide-induced apoptosis. Biochem J 337: 83–87, 1999. [PMC free article] [PubMed] [Google Scholar]
- 44.Lortie MJ, Satriano J, Gabbai FB, Thareau S, Khang S, Deng A, Pizzo DP, Thomson SC, Blantz RC, Munger KA. Production of arginine by the kidney is impaired in a model of sepsis: early events following LPS. Am J Physiol Regul Integr Comp Physiol 287: R1434–R1440, 2004. [DOI] [PubMed] [Google Scholar]
- 45.Loschen G, Azzi A, Flohe L. Mitochondrial H2O2 formation: relationship with energy conservation. FEBS Lett 33: 84–87, 1973. [DOI] [PubMed] [Google Scholar]
- 46.Mayeur C, Veuillet G, Michaud M, Raul F, Blottiere HM, Blachier F. Effects of agmatine accumulation in human colon carcinoma cells on polyamine metabolism, DNA synthesis and the cell cycle. Biochim Biophys Acta 1745: 111–123, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Min A, Hasuma T, Yano Y, Matsui-Yuasa I, Otani S. Regulation of apoptosis of interleukin 2-dependent mouse T-cell line by protein tyrosine phosphorylation and polyamines. J Cell Physiol 165: 615–623, 1995. [DOI] [PubMed] [Google Scholar]
- 48.Molderings GJ, Kribben B, Heinen A, Schroder D, Bruss M, Gothert M. Intestinal tumor and agmatine (decarboxylated arginine): low content in colon carcinoma tissue specimens and inhibitory effect on tumor cell proliferation in vitro. Cancer 101: 858–868, 2004. [DOI] [PubMed] [Google Scholar]
- 49.Nitta T, Igarashi K, Yamamoto N. Polyamine depletion induces apoptosis through mitochondria-mediated pathway. Exp Cell Res 276: 120–128, 2002. [DOI] [PubMed] [Google Scholar]
- 50.Palmieri F, Klingenberg M. Direct methods for measuring metabolite transport and distribution in mitochondria. Methods Enzymol 56: 279–301, 1979. [DOI] [PubMed] [Google Scholar]
- 51.Ploszaj T, Motyl T, Zimowska W, Skierski J, Zwierzchowski L. Inhibition of ornithine decarboxylase by α-difluoromethylornithine induces apoptosis of HC11 mouse mammary epithelial cells. Amino Acids 19: 483–496, 2000. [DOI] [PubMed] [Google Scholar]
- 52.Raasch W, Schafer U, Chun J, Dominiak P. Biological significance of agmatine, an endogenous ligand at imidazoline binding sites. Br J Pharmacol 133: 755–780, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Regunathan S, Reis DJ. Stimulation of imidazoline receptors inhibits proliferation of human coronary artery vascular smooth muscle cells. Hypertension 30: 295–300, 1997. [DOI] [PubMed] [Google Scholar]
- 54.Salvi M, Battaglia V, Mancon M, Colombatto S, Cravanzola C, Calheiros R, Marques MP, Grillo MA, Toninello A. Agmatine is transported into liver mitochondria by a specific electrophoretic mechanism. Biochem J 396: 337–345, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Santos AC, Uyemura SA, Lopes JL, Bazon JN, Mingatto FE, Curti C. Effect of naturally occurring flavonoids on lipid peroxidation and membrane permeability transition in mitochondria. Free Radic Biol Med 24: 1455–1461, 1998. [DOI] [PubMed] [Google Scholar]
- 56.Satriano J Arginine pathways and the inflammatory response: interregulation of nitric oxide and polyamines: review article. Amino Acids 26: 321–329, 2004. [DOI] [PubMed] [Google Scholar]
- 57.Satriano J, Ishizuka S, Archer DC, Blantz RC, Kelly CJ. Regulation of intracellular polyamine biosynthesis and transport by NO and cytokines TNF-α and IFN-γ. Am J Physiol Cell Physiol 276: C892–C899, 1999. [DOI] [PubMed] [Google Scholar]
- 58.Satriano J, Isome M, Casero RA Jr, Thomson SC, Blantz RC. Polyamine transport system mediates agmatine transport in mammalian cells. Am J Physiol Cell Physiol 281: C329–C334, 2001. [DOI] [PubMed] [Google Scholar]
- 59.Satriano J, Matsufuji S, Murakami Y, Lortie MJ, Schwartz D, Kelly CJ, Hayashi S, Blantz RC. Agmatine suppresses proliferation by frameshift induction of antizyme and attenuation of cellular polyamine levels. J Biol Chem 273: 15313–15316, 1998. [DOI] [PubMed] [Google Scholar]
- 60.Satriano J, Schwartz D, Ishizuka S, Lortie MJ, Thomson SC, Gabbai F, Kelly CJ, Blantz RC. Suppression of inducible nitric oxide generation by agmatine aldehyde: beneficial effects in sepsis. J Cell Physiol 188: 313–320, 2001. [DOI] [PubMed] [Google Scholar]
- 61.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol 2: 965–975, 2002. [DOI] [PubMed] [Google Scholar]
- 62.Schmid I, Uittenbogaart CH, Giorgi JV. A gentle fixation and permeabilization method for combined cell surface and intracellular staining with improved precision in DNA quantification. Cytometry 12: 279–285, 1991. [DOI] [PubMed] [Google Scholar]
- 63.Seiler N Polyamine metabolism. Digestion 46, Suppl 2: 319–330, 1990. [DOI] [PubMed] [Google Scholar]
- 64.Stefanelli C, Tantini B, Fattori M, Stanic I, Pignatti C, Clo C, Guarnieri C, Caldarera CM, Mackintosh CA, Pegg AE, Flamigni F. Caspase activation in etoposide-treated fibroblasts is correlated to ERK phosphorylation and both events are blocked by polyamine depletion. FEBS Lett 527: 223–228, 2002. [DOI] [PubMed] [Google Scholar]
- 65.Sugiura T, Tsutsui H, Takaoka M, Kobuchi S, Hayashi K, Fujii T, Matsumura Y. Protective effect of agmatine on ischemia/reperfusion-induced renal injury in rats. J Cardiovasc Pharmacol 51: 223–230, 2008. [DOI] [PubMed] [Google Scholar]
- 66.Toninello A, Di Lisa F, Siliprandi D, Siliprandi N. Uptake of spermine by rat liver mitochondria and its influence on the transport of phosphate. Biochim Biophys Acta 815: 399–404, 1985. [DOI] [PubMed] [Google Scholar]
- 67.Toninello A, Salvi M, Mondovi B. Interaction of biologically active amines with mitochondria and their role in the mitochondrial-mediated pathway of apoptosis. Curr Med Chem 11: 2349–2374, 2004. [DOI] [PubMed] [Google Scholar]
- 68.Vargiu C, Cabella C, Belliardo S, Cravanzola C, Grillo MA, Colombatto S. Agmatine modulates polyamine content in hepatocytes by inducing spermidine/spermine acetyltransferase. Eur J Biochem 259: 933–938, 1999. [DOI] [PubMed] [Google Scholar]
- 69.Wang WP, Iyo AH, Miguel-Hidalgo J, Regunathan S, Zhu MY. Agmatine protects against cell damage induced by NMDA and glutamate in cultured hippocampal neurons. Brain Res 1084: 210–216, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.White FC, Benehacene A, Scheele JS, Kamps M. VEGF mRNA is stabilized by ras and tyrosine kinase oncogenes, as well as by UV radiation–evidence for divergent stabilization pathways. Growth Factors 14: 199–212, 1997. [DOI] [PubMed] [Google Scholar]
- 71.Wolf C, Bruss M, Hanisch B, Gothert M, von Kugelgen I, Molderings GJ. Molecular basis for the antiproliferative effect of agmatine in tumor cells of colonic, hepatic, and neuronal origin. Mol Pharmacol 71: 276–283, 2007. [DOI] [PubMed] [Google Scholar]
- 72.Yang MZ, Mun CH, Choi YJ, Baik JH, Park KA, Lee WT, Lee JE. Agmatine inhibits matrix metalloproteinase-9 via endothelial nitric oxide synthase in cerebral endothelial cells. Neurol Res 29: 749–754, 2007. [DOI] [PubMed] [Google Scholar]
- 73.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 9: 47–59, 2008. [DOI] [PubMed] [Google Scholar]
- 74.Yu CG, Marcillo AE, Fairbanks CA, Wilcox GL, Yezierski RP. Agmatine improves locomotor function and reduces tissue damage following spinal cord injury. Neuroreport 11: 3203–3207, 2000. [DOI] [PubMed] [Google Scholar]
- 75.Yuan Q, Ray RM, Johnson LR. Polyamine depletion prevents camptothecin-induced apoptosis by inhibiting the release of cytochrome c. Am J Physiol Cell Physiol 282: C1290–C1297, 2002. [DOI] [PubMed] [Google Scholar]
- 76.Zhu MY, Wang WP, Bissette G. Neuroprotective effects of agmatine against cell damage caused by glucocorticoids in cultured rat hippocampal neurons. Neuroscience 141: 2019–2027, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zou C, Vlastos AT, Yang L, Wang J, Nishioka K, Follen M. Effects of difluoromethylornithine on growth inhibition and apoptosis in human cervical epithelial and cancerous cell lines. Gynecol Oncol 85: 266–273, 2002. [DOI] [PubMed] [Google Scholar]