Abstract
The molecular mechanisms by which bone cells transduce mechanical stimuli into intracellular biochemical responses have yet to be established. There is evidence that mechanical stimulation acts synergistically with parathyroid hormone PTH(1-34) in mediating bone growth. Using picosecond time-resolved fluorescence microscopy and G protein-coupled receptor conformation-sensitive fluorescence resonance energy transfer (FRET), we investigated conformational transitions in parathyroid hormone type 1 receptor (PTH1R). 1) A genetically engineered PTH1R sensor containing an intramolecular FRET pair was constructed that enabled detection of conformational activity of PTH1R in single cells. 2) The nature of ligand-dependent conformational change of PTH1R depends on the type of ligand: stimulation with the PTH(1-34) leads to conformational transitions characterized by decrease in FRET efficiency while NH2-terminal truncated ligand PTH(3-34) stimulates conformational transitions characterized by higher FRET efficiencies. 3) Stimulation of murine preosteoblastic cells (MC3T3-E1) with fluid shear stress (FSS) leads to significant changes in conformational equilibrium of the PTH1R in MC3T3-E1 cells, suggesting that mechanical perturbation of the plasma membrane leads to ligand-independent response of the PTH1R. Conformational transitions induced by mechanical stress were characterized by an increase in FRET efficiency, similar to those induced by the NH2-terminal truncated ligand PTH(3-34). The response to the FSS stimulation was inhibited in the presence of PTH(1-34) in the flow medium. These results indicate that the FSS can modulate the action of the PTH(1-34) ligand. 4) Plasma membrane fluidization using benzyl alcohol or cholesterol extraction also leads to conformational transitions characterized by increased FRET levels. We therefore suggest that PTH1R is involved in mediating primary mechanochemical signal transduction in MC3T3-E1 cells.
Keywords: mechanosensor, fluorescence resonance energy transfer, fluid shear stress, G protein-coupled receptor, mechanotransduction
mechanical loading such as physical exercise and hormones such as parathyroid hormone (PTH) play important roles in regulating bone mass (63). Depending on dosage and mechanical loading or disuse, PTH can either induce bone growth or loss. Intermittent PTH administration has been proposed as a treatment to enhance bone density for osteoporosis patients while continuous high PTH dosage induces bone loss (40, 60, 75). Increased bone mass can be a result of bone adaptation to mechanical loading while bone loss could be the consequence of mechanical disuse (65, 70, 71). Studies have also shown that mechanical loading and PTH treatment act synergistically, i.e., mechanical loading enhances the effect of intermittent PTH treatment in bone formation and PTH prevents osteoporosis caused by mechanical disuse (5, 18, 19, 30, 39, 41, 46, 47, 65, 67, 71). However, the cellular and molecular mechanisms underlining the interaction between the mechanical loading and PTH treatment is poorly understood (5, 20, 58, 69).
It has been reported that osteoblasts and osteocytes are the cells that mediate mechanotransduction in bone by responding to mechanical loading in the form of interstitial fluid shear stress (FSS) (4, 15, 36, 56, 57, 66). Type 1 PTH receptor (PTH1R) is highly expressed in bone and kidney and is vitally important for extracellular calcium homeostasis. PTH1R is a G protein-coupled receptor (GPCR) that conducts signal transduction via Gs-cAMP-PKA (6), Gq/11-PLC-PKC (50) and Gi/o pathways (49). Interaction of the PTH1R with its ligands is complex (54). Recently, multiple studies based on biophysical (14, 51), biological (79), and biochemical (76) methods suggested that certain GPCRs [e.g., bradykinin receptor in endothelial cells (14), angiotensin II type 1 receptor in cardiomyocytes (76, 79), and formyl peptide receptor in neutrophils (51)] can act as mechanosensors.
The structure and function of membrane proteins such as mechanochannels and GPCRs have been shown to be modulated by small changes in physicochemical properties of membrane bilayers (1, 2, 14, 23, 28, 31, 33, 34, 43, 53, 55). It has also been suggested that mechanical forces may initiate mechanochemical signal transduction by altering physical properties of the cell membrane (9, 14, 28, 78), which in turn leads to activation of membrane proteins.
In the present study, we test the hypothesis that PTH1R serves as a mechanosensor in osteoblasts. We show that stimulation by the PTH ligand and mechanical signaling converge and interact at the PTH1 receptor.
MATERIALS AND METHODS
Chemicals and reagents.
Human PTH(1-34) and bovine PTH(3-34) were obtained from Bachem Americas (Torrance, CA). Human Nle8,18Tyr34PTH(3-34) was from American Peptide (Sunnyvale, CA). Benzyl alcohol and methyl-β-cyclodextrin were obtained from Sigma (St. Louis, MO).
Cell culture, transfection, and shear stress experiments.
MC3T3-E1 cells (from American Type Tissue Collection, passages 2–6) were grown in α-MEM media (Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum, penicillin (100 U/ml), streptomycin (100 μg/ml), and without vitamin C. Cell cultures were maintained in a humidified 5% CO2-95% air incubator at 37°C. Transfection of MC3T3-E1 cell line was carried out using Targefect-2 and Virofect enhancer (Targeting Systems, Santee, CA) when confluency was between 70% and 90%.
Human embryonic kidney (HEK)-293 cells (American Type Tissue Collection, passages 2–10) were grown in DMEM media (Invitrogen) containing 4.5 g/l d-glucose and were transfected using Targefect-293.
The FSS experiments were performed by using a parallel plate flow microchamber that allows exposure of MC3T3-E1 cells to variable FSS in a flow channel (2 mm wide) that is optically accessible through a coverslip-based window; cells were imaged through a high numerical aperture (NA) water immersion objective (C-Apochromat ×40/1.2). The temperature of the microchamber and that of the flow media delivered into the chamber by a syringe pump were maintained at 37°C. The design of the microchamber ensured that there was no deformation of glass coverslip (axial displacement was <0.05 μm) caused by flow-induced hydrostatic pressure (14).
Construction of PTH1R FRET sensor (PTH-CC).
Primers for PCR amplifications were obtained from Sigma. The cDNA templates of Cerulean (61) and Citrine (27) were kindly provided by Dr. David W. Piston (Vanderbilt University, Nashville, TN) and Dr. Roger R. Tsien (University of California, San Diego), respectively. cDNA template for human PTH1R was obtained from the University of Missouri cDNA Resource Center (Rolla, MO). Expression vector pECFP-N1 was obtained from Clontech of Takara Bio, which was modified by replacing the sequence of enhanced cyan fluorescent protein (ECFP) with Cerulean. Cerulean was amplified by primers 5′-agaccggtgagcaagggcgaggag-3′ and 5′-tagcggccgcttacttgtacagctcgtccat-3′ such that the DNA fragment was flanked by Age1 and Not1. The digested fragment was cloned into pECFP-N1 Age1 and Not1 sites, replacing ECFP and resulting in pCerulean-N1.
The locations for insertion of Citrine into the third intracellular loop and for fusion of Cerulean to the COOH-terminal tail of PTH1R were similar to those in Ref. 72 (see Fig. 1A). DNA fragment encoding Citrine flanked by 5′-Hind3(Sac2) and 3′-EcoR1 was amplified by PCR using primers 5′-ctcggcatggacgagctgtggaattcgt-3′ and 5′-acgaattccacagctcgtccatgccgag-3′; DNA fragment encoding PTH1R from Arg396 to Ser495 flanked by 5′-EcoR1 and 3′-Age1 was amplified by PCR using primers 5′-gtggaattcgtgtgacacacggcagcag-3′ and 5′-caccggtgaatagctgctgctcccgctgcg-3′. The two DNA fragments were restriction enzyme digested and cloned into pCerulean-N1 Hind3 and Age1 sites in a one-step ligation reaction. The identified clone was then used to clone DNA fragment flanked by 5′-Hind3 and 3′-Sac2 encoding PTH1R from Met1to Gly395, which was amplified by PCR using primers 5′-gctagcgtttaaacttaagcttggt-3′ (that anneals to pcDNA3.1 sequence upstream of Kozac and start codon of PTH1R) and 5′-taccgcggcgttggtctcccgcag-3′. In the resultant construct of PTH1R FRET sensor (PTH-CC), the third intracellular loop has the sequence of ATKLRETNAA-Citrine-SCDTRQQYRKLLKST (underlined residues are altered ones to encode restriction sites in the DNA sequences), replacing PTH1R's ATKLRETNAGRCDTRQQYRKLLKST (underlined are the 2 residues where Citrine is inserted in between), and has cytoplasmic COOH terminus fused to Cerulean at residue Ser495 of PTH1R (see Fig. 1A) with insertion of two extra residues encoding Pro-Val to accommodate an Age1 restriction site. The sequences of all constructs were confirmed by sequencing service.
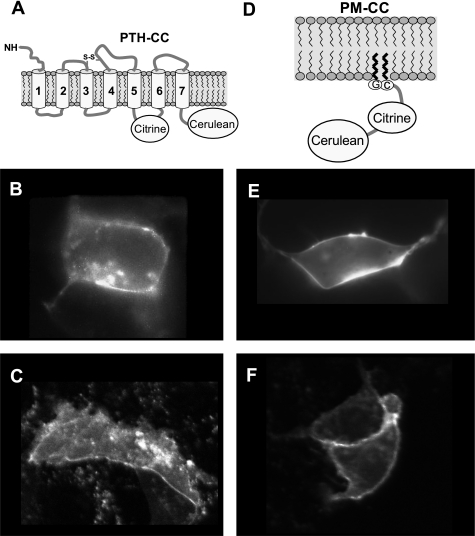
Fig. 1.
Plasma membrane (PM) localization of expressed fluorescent proteins 24 h after transfection. Expression and localization of parathyroid hormone type 1 receptor (PTH1R) fluorescence resonance energy transfer (FRET) sensor PTH-CC (A) is shown in human embryonic kidney (HEK)-293 cells (B) and MC3T3-E1 cells (C). Fluorescence images in E and F show expression and localization of FRET sensor control PM-CC (D) in HEK-293 and MC3T3-E1 cells, respectively.
Construction of plasma membrane-localized FRET sensor control (PM-CC).
We constructed a control FRET sensor that is localized to the plasma membrane of cells (see Fig. 1D). The final construct PM-CC encodes a protein that is Citrine and Cerulean linked together with a short and flexible GGGGPV (Pro-Val to encode Age1 restriction site) linker peptide to ensure Förster fluorescence energy transfer between the two fluorescent proteins, and is with a 10-residue leader peptide MGCIN SKRKD to direct its translocation to plasma membrane (77). Mammalian expression vector used to clone the molecule is pCerulean-N1. The sequences of PCR primers to amplify Citrine are 5′-gaagcttaccatgggatgtatcaacagtaaacgaaaggatgtgagcaagggcgaggagct-3′ and 5′-gaccggtccaccgcctcccttgtacagctcgtccat-3′. The PCR product was digested with Hind3 and Age1, yielding Citrine DNA fragment flanked by the 10-residue signal peptide sequence at the 5′-end and GGGGPV linker peptide sequence at the 3′-end, and cloned into pCerulean-N1 Hind3 and Age1 sites.
FRET measurements.
FRET measurement in living cells was performed as previously described (14). Briefly, fluorescence emission kinetics and spectra were measured by using a multichannel, time-correlated single photon counting spectrograph (PML-16/SPC630; Becker and Hickl, Berlin, Germany) coupled to an inverted microscope (Axiovert 200 M; Zeiss, Thornwood, NY) via fiber optic link. A femtosecond Ti:Sapphire oscillator (Spectra-Physics, Irvine, CA) was used as the excitation source. The repetition frequency of the light pulses from the oscillator was reduced to 8 MHz, and the light wavelength was doubled to 435 nm and coupled into the microscope. The excitation light (∼0.1 μW) was defocused to a spot size of 20–50 μm to enable spatially homogenous excitation of a single cell. A dichroic beamsplitter (455dclp; Chroma, Rockingham, VT) was used to separate excitation from emission light. Our experimental setup enabled detection of fluorescent decays from single cells with 16 independent wavelength channels and ≈20-ps time resolution. The photobleaching was ≈10% after 60-min illumination; it did not result in any noticeable changes of fluorescence spectra and kinetics on the timescale of our experiments. Fluorescence spectra were obtained by integrating time-resolved fluorescence data. The polarization of the detected fluorescence emission was selected by using a polarizer. Presented data were measured by detecting fluorescence emission polarized at 90° to the polarization of the excitation light at 435 nm, which enabled higher sensitivity of our measurements because Citrine emission is depolarized by the FRET process, whereas directly excited Cerulean and Citrine emissions are polarized, resulting in lower overall background signal.
Cell imaging.
Fluorescence imaging of HEK-293 cells was performed using an inverted fluorescence microscope Axiovert 200M (Zeiss) using the ×40 water immersion objective (NA 1.2), CFP (Cerulean) cube (Omega Optical, XF114-2-440AF21, 455DRLD, 480AF30), and yellow fluorescent protein (YFP; Citrine) cube (Omega Optical, XF104-2-500AF25, 525DRLP, 545AF35). MC3T3-E1 cells were imaged using confocal fluorescence microscope LSM 5 Pascal (Zeiss). Confocal images were recorded using 514 nm excitation and BP530–600 nm emission filter with ×40 oil emersion objective (NA 1.3).
RESULTS
Expression and localization of PTH-CC and PM-CC.
To monitor conformational changes of the PTH1R, we constructed a PTH1R sensor (PTH-CC) containing an intramolecular FRET pair that enables detection of conformational activity of PTH1R in single cells under mechanical stress or ligand stimulation (Fig. 1A). For control experiments we constructed a membrane-targeted FRET pair with no expected conformational activity (Fig. 1D). Expression and translocation of the two constructs, the FRET control (PM-CC), and the PTH1R activity FRET sensor (PTH-CC) were examined both in HEK-293 and in MC3T3-E1 cells. Expressed PTH-CC localizes predominantly on plasma membranes of HEK-293 cells (Fig. 1B) and MC3T3-E1 cells (Fig. 1C). Figure 1, E and F, shows that 24 h after transfection of HEK-293 and MC3T3-E1 cells, respectively, the expressed PM-CC is mostly localized on the plasma membranes. The images for both constructs appeared identical when viewed through CFP and YFP channels in the microscopes.
Response of PTH-CC to stimulation by ligands.
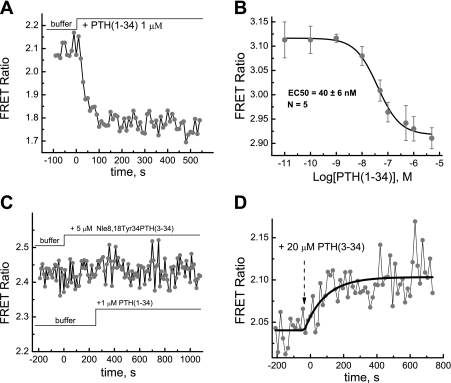
Figure 2A shows that FRET signal from PTH1R fret sensor decreases when MC3T3-E1 cells are treated with 1 μM PTH(1-34), suggesting that the agonist induces a conformational change in the receptor. We have determined that FRET signal does come back to baseline after the ligand is washed off on the timescale practically feasible in our single cell experiments (∼45 min). This suggests that the dissociation rate constant koff is very small and is also consistent with the earlier study on the green fluorescent protein-PTH1R construct where almost no ligand dissociation was observed upon washing (13) or with another recent study that showed that the majority of PTH(1-34) ligands dissociate very slowly, with half-time (t) = 4 h (17).
Fig. 2.
A: conformational response of the PTH-CC to stimulation by PTH(1-34). B: corresponding dose response. Experiments were done 24 h after transfection of MC3T3-E1 cells in chambered coverglass at 37°C. C: antagonist Nle8,18Tyr34PTH(3-34) blocks the response of the PTH-CC to stimulation by PTH(1-34). D: conformational response of the PTH-CC to stimulation by the PTH(3-34). FRET ratio was defined as ratio of Citrine emission intensity at ∼525 nm to Cerulean emission intensity at 475 nm.
Figure 2B shows the dose-response curve of PTH-CC to PTH(1-34) in HEK-293 cells. To ensure that ligand binding to the receptor at the lowest concentrations reached equilibrium, long incubation times (5–10 min) were used. The EC50 value (40 ± 6 nM) for PTH(1-34) stimulation of PTH-CC is significantly larger than the binding constant of the native receptor (∼2 nM) and is somewhat larger than the binding constant (∼15.5 nM) reported earlier for a similar PTH sensor (72). The EC50 value of the conformational change may generally be larger than the binding constant since the latter only characterizes the binding process but not the conformational change itself; the difference between the two sensors is also potentially due to slight variation (2 mutations in the flanking regions of Citrine insertion point and 2 mutations at the fusion point of Cerulean) in the sequences of the two sensors. Note that GPCR constructs of this type typically have significantly increased binding constants due to the insertion of the fluorescing proteins into the native receptor structure (14, 72).
Figure 2C shows that pretreatment of cells with a full antagonist Nle8,18Tyr34PTH(3-34) completely blocked the response to stimulation by PTH(1-34). Importantly these data also show that Nle8,18Tyr34PTH(3-34) has no effect on FRET efficiency, which is consistent with the fact that this ligand has no agonist activity (64).
In contrast to the effect of PTH(1-34), treatment of cells with the antagonist PTH(3-34) caused an opposite change (increase) in FRET signal at a concentration of 20 μM, indicating that this antagonist induced a different conformational change in PTH1R (Fig. 2D). Note that PTH(3-34) is a PTH1R antagonist only in the sense that it blocks cAMP signaling due to stimulation by PTH(1-34) (37).
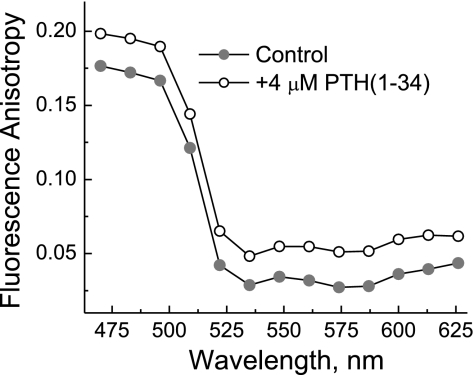
Fluorescence anisotropy data (Fig. 3) show that stimulation with PTH(1-34) leads to relatively similar increases in fluorescence anisotropy at emission wavelengths of Cerulean and Citrine; this can be explained by the fact that PTH(1-34) stimulation leads to lower FRET efficiency, resulting in stronger emission from Cerulean, which is more polarized than Citrine emission. Thus the observed FRET signal change is mainly due to relative translational rather than rotational movement between the transition dipoles of the two fluorescent proteins; this is similar to the earlier report on α2A-adrenergic receptor (73) but different from both translational and rotational movement reported for bradykinin receptor (14).
Fig. 3.
Response of fluorescence anisotropy of PTH-CC to stimulation by PTH(1-34).
FSS induces conformational changes in PTH1R.
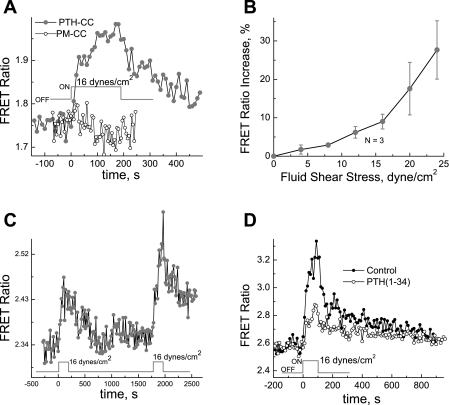
To test our hypothesis that mechanical perturbation of plasma membrane by the FSS could lead to modulation of PTH1R conformational states and possibly to activation of the PTH1R receptor, we have studied the effect of the FSS on conformational dynamics of the PTH-CC in bone cells. A monolayer of MC3T3-E1 cells transfected with the PTH1R fret sensor expression vector was subjected to the FSS of 16 dyn/cm2. As shown in Fig. 4A, the FSS leads to increase in the FRET ratio, whereas in MC3T3-E1 cells expressing the control FRET sensor (PM-CC), the FRET ratio was not affected by the FSS. The dependence of the relative FRET ratio change on the FSS magnitude is shown in Fig. 4B.
Fig. 4.
A: conformational change in PTH1R induced by fluid shear stress (FSS) (•, PTH-CC; ○, PM-CC). B: FSS induced change in FRET ratio of PTH-CC as a function of shear stress magnitude. C: reversible conformational changes in PTH1R induced by the FSS. The experiment was performed similarly as in A, except that cells were subjected to the 16 dyn/cm2 shear stress twice. D: FSS induced FRET change of PTH-CC in the presence of stimulation by the PTH(1-34). A monolayer of MC3T3-E1 cells, 24 h after being seeded and transfected either with PTH-CC or with PM-CC expression vectors on no. 1.5 coverglass, was placed on a flow chamber and equilibrated at 37°C on the microscope stage for 30 min under a flow with the FSS lower than 0.1 dyn/cm2 for the system to become stable. The cells were then subjected to shear stress at 16 dyn/cm2 for a duration of 4 min.
Figure 4C shows that the FRET ratio change of PTH1R FRET sensor can be repeatedly stimulated by the shear stress once the conformation of the PTH-CC relaxes to the initial state after the flow has been stopped.
The FSS effect can be partially inhibited by the presence of PTH(1-34) in the shearing medium; Fig. 4D shows that PTH(1-34) at 1 μM reduces the FSS response by ∼50%.
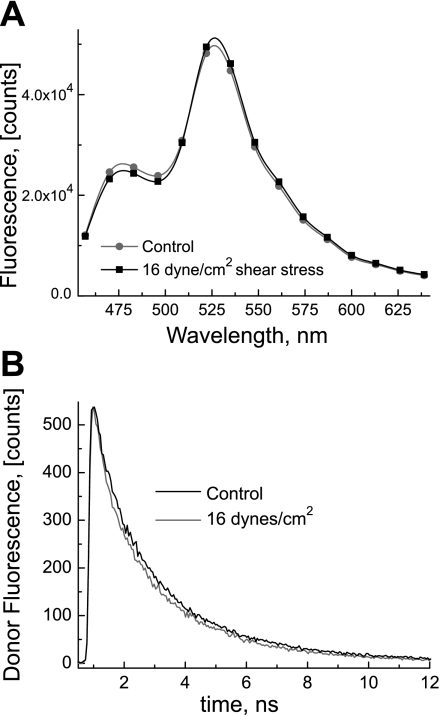
The FRET ratio increase due to the FSS was also confirmed by spectral data presented in Fig. 5A that clearly show an increase in fluorescence intensity at 530 nm and a decrease at 480 nm. Moreover, Fig. 5B shows time-resolved fluorescence decay kinetics of the fluorescence donor (Cerulean) before and during application of the FSS; a significant decrease in the fluorescence lifetime (from 2.1 ± 0.08 ns to 1.9 ± 0.08 ns) is observed, indicating that the FRET efficiency is increased by the action of the FSS.
Fig. 5.
A: response of the fluorescence spectra of the PTH-CC FRET sensor to stimulation by the FSS. The experiment was performed under the same conditions as in Fig. 4. The fluorescence spectra of the PTH1R FRET sensor were extracted from the time-resolved measurements in the presence and absence of the FSS at 16 dyn/cm2. Increased intensity at ∼525 nm and decreased intensity at 475 nm in the presence of shear stress are noticeable and indicate increase in FRET efficiency due to the FSS-induced conformational change (▪, under shear stress; •, without shear stress). B: donor (Cerulean) fluorescence lifetime decreases under stimulation by the FSS. The fluorescence decay kinetics of the fluorescence donor (Cerulean) were recorded in the presence and absence of the FSS at 16 dyn/cm2. Shortened life time in the presence of the FSS (thick line), relative to the absence of the FSS (thin line), indicates increased FRET efficiency.
A change in membrane fluidity induces conformational changes in PTH1R.
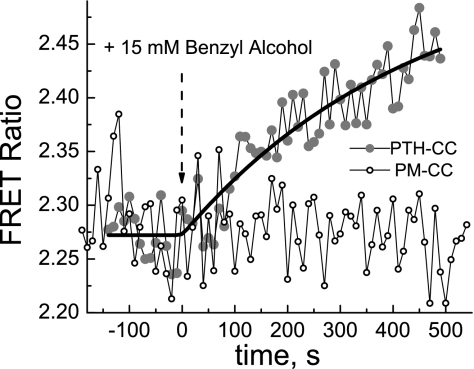
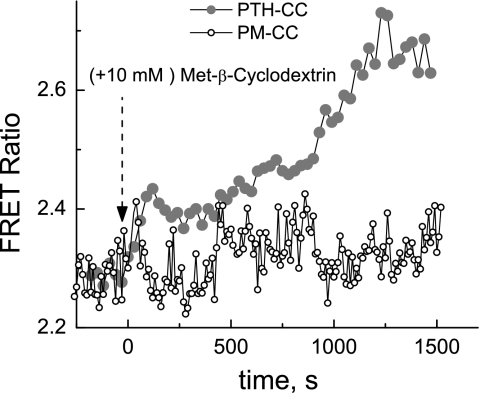
Plasma membrane fluidity in MC3T3-E1 expressing PTH1R FRET sensor was modulated by treating the cells either with 15 mM benzyl alcohol, a membrane fluidity enhancer, or with 10 mM methyl-β-cyclodextrin, which removes cholesterol from the cell membrane. The data in Figs. 6 and 7 indicate that both treatments caused FRET signal to increase, indicating conformational change that is possibly similar to that caused by the FSS.
Fig. 6.
Benzyl alcohol induces increase in the FRET efficiency of the PTH-CC FRET sensor but has no effect on the FRET ratio of the control sensor PM-CC. Experimental configuration was the same as in Fig. 2.
Fig. 7.
Methyl-β-cyclodextrin induces increase in FRET efficiency of the PTH-CC FRET sensor but has no effect on the FRET ratio of the control sensor PM-CC. Experimental configuration was the same as in Fig. 2.
DISCUSSION
PTH1R conformation is modulated by mechanical forces and membrane physical properties.
It has been suggested that certain key amino acid residues in transmembrane helices of a GPCR, interacting with each other, keep receptors preferentially locked in an inactive state. Ligand binding (or isomerization of the preloaded ligand retinal in case of rhodopsin) changes the helix-helix interactions in the GPCR and causes/enables movement of helixes 5, 6, and 7, translating into conformational change in the third cytoplasmic loop that subsequently interacts with and activates G proteins (24, 74). It has also been shown that GPCR structure is rather “plastic,” i.e., the GPCR can assume a number of different conformations depending on the specific ligand (24, 25, 59, 73); many GPCRs also exhibit agonist-independent constitutive activity, suggesting that GPCRs can adopt an active conformation spontaneously (24, 44, 45). The inherent plasticity of GPCRs (59) makes it likely that changes in membrane thickness, polarity, transmembrane lateral force profile, level of hydration, and membrane fluidity due to external mechanical force could also lead to GPCR conformational change required for activation. We have hypothesized that modulation of the physical properties of the plasma membrane by the FSS can lead to changes in conformational equilibrium of GPCRs (14). According to a simplified model, a GPCR isomerizes between two different states corresponding to inactive (R) and active conformations (R*); because of the structural constraints and in the absence of any perturbation, the equilibrium lies toward the R state (42). Our observation that the FSS leads to a change in transient FRET efficiency of the PTH-CC (see Fig. 4) can be tentatively attributed to mechanical perturbation of the plasma membrane as similarly shown earlier for the bradykinin B2 receptor (14). In contrast to the B2 receptor, the FSS-induced change in FRET efficiency in PTH-CC is of the opposite sign. However, the FSS-induced change in the PTH-CC is of the same sign as that induced by benzyl alcohol (Fig. 6) or by the extraction of cholesterol through cyclodextrin treatment (Fig. 7), both of which are known to increase membrane fluidity; thus, the FSS stimulation and changes in membrane fluidity seem to be correlated in the PTH1 and B2 receptors, although the sign of FRET change is different between the two receptors. The activity of integral membrane proteins is strongly modulated by their interactions with lipid molecules (43). Indeed, it has been recognized that mechanical perturbation of the lipid bilayer membrane may lead to changes in static and dynamic properties of the membrane such as membrane thickness (34, 52), polarity (78), structural order (8, 11, 29), and fluidity (10, 32). Specifically, it has been shown that the FSS in the lipid bilayer membrane can lead to increased membrane fluidity (10, 31).
A movement of “gating” charge was recently reported in a GPCR (7). This study suggested that the external field (due to transmembrane potential) causes a conformational change in the third intracellular loop of GPCR, which results in changed affinity of the receptor to the agonist ligand, and consequently changes the activity of the GPCR; this observation and other findings that activity of some GPCRs can be modulated by transmembrane potential (48) imply a potential sensitivity of GPCR-mediated signaling pathways to changes in electrical properties of the lipid bilayer membrane. Therefore the earlier reported sensitivity of the polarity of lipid bilayer to mechanical stress (78) provides yet another potential mechanism on how the conformational transitions of the PTH-CC could be coupled to mechanical perturbation of the membrane.
Although mechanically induced changes in physicochemical properties of the lipid bilayer membrane are not expected to be large, it is important to note that receptor-mediated signaling pathways operate as signal amplification cascades. Typically, an activated GPCR can activate hundreds of G proteins, causing a strong signal amplification (26, 35, 62), so that even a small change in the rate of one of the primary amplification steps due to the action of the FSS could cause a significant physiological response.
Insertion of fluorescent proteins into the GPCR structure strongly reduces both its ligand-binding affinity and coupling to G proteins (72) and may inhibit its interactions with other regulatory proteins; this makes it less likely that the observed effect of the FSS on the PTH-CC could be caused by interactions of the PTH-CC with some other putative mechanosensors or by intracrine or autocrine ligand activation, supporting our hypothesis that PTH1R is one of the primary mechanosensors.
PTH1R conformation is differently modulated by PTH(1-34), PTH(3-34), and shear stress.
The anabolic effect of PTH is achieved by intermittent PTH administration through activation of PKA pathway (40, 75). The activation of the Gs/ adenylyl cyclase/PKA as well as Gq/PLC/PKCβ pathways by the PTH(1-34) and its analogs is ligand and cell specific (21, 68). The PTH1R interaction with its ligands is believed to proceed through a “two-site” model where the ligand initially binds to the NH2 terminus of the receptor followed by the binding to the juxtamembrane region of the receptor (J domain), the latter binding event causing the actual receptor activation (16, 37). In contrast, the NH2-terminal truncated ligand, PTH(3-34), not only has a much lower affinity to the receptor, but also quite different specificity in activation of the signal transduction pathways (3, 22, 37, 38), indicating that PTH(3-34) can trigger a different conformation in PTH1R from that triggered by PTH(1-34). This reflects the fact that PTH1R can adopt multiple conformations and the conformations can be modulated by other proteins, e.g., Na+/H+ exchanger regulatory factor (NHERF) 1/2 (50) and G proteins in a cell-specific manner. It has been shown that natural ligand [PTH(1-34)] stimulation of similar GPCR construct containing intermolecular FRET pair leads to decrease of FRET efficiency (72), as is also observed for our PTH1R construct (Fig. 2A). However, as Fig. 2D shows, stimulation with PTH(3-34) leads to the increase of FRET efficiency, suggesting that a different type of conformational transition takes place. Such a striking difference in the nature of conformational change is not unexpected since NH2-terminal truncated ligand PTH(3-34) preferentially binds to the NH2 terminus of the receptor while binding to the J domain of the receptor is inhibited (37); this is certainly consistent with the widely accepted notion that GPCRs may adopt different conformational states (59). Our present data directly indicate that the experimentally observed different activation of the PKA and PKC pathways by PTH(1-34) and PTH(3-34) (3, 21, 68) is indeed preceded by a different conformational change in the receptor. The ability of PTH1R to selectively couple to different signal transduction pathways in response to the PTH and the PTH-related peptide is known and was recently attributed to different affinities of these two ligands to the G protein-uncoupled and coupled conformations(17). Note that, in general, an increase in FRET level of the PTH-CC could also be interpreted as shift of the conformational equilibrium from a constitutive (basal) activity level toward the inactive state. However, the basal activity of PTH1R receptors is low (12) and treatment with the antagonist Nle8,18Tyr34PTH(3-34) did not cause any FRET changes (see Fig. 2C). An increase in FRET efficiency due to stimulation by an inverse agonist was earlier observed for α2A-adrenergic receptor (73) and interpreted as being due to a distinct conformational state. The evidence that PTH(1-34) stimulates both Gq and Gs pathways while PTH(3-34) stimulates only Gq (PLC/PKC) pathway (3, 22) suggests that any PTH receptor model has to include at least one more conformational state in addition to the inactive and active [stimulated by PTH(1-34)] conformational states to explain the present results.
Our finding that an agonist can inhibit the FSS response (Fig. 4D) provides a direct link between PTH1R activation and mechanical stimulation.
The existence of the two different active conformational states of the PTH1R is consistent with the synergistic effects of the mechanical loading and anabolic effect of intermittent PTH administration. According to the prevailing theories of GPCR activation mechanisms, the agonist ligand has higher affinity to GPCR in active conformation and therefore preferentially binds to it; inverse agonists preferentially stabilize the receptor in inactive conformation. The fact that the conformational state induced by the FSS is different from the one induced by the binding of the PTH(1-34) suggests that the binding affinity and the effect of the PTH(1-34) are reduced under the effect of the FSS. Our finding that PTH(1-34) inhibits the FSS response (Fig. 4D) further confirms that interaction of the PTH(1-34) ligand with the FSS-induced state of the PTH-CC is energetically less favorable and therefore of lower affinity. This implies that the intermittent mechanical loading can lead to modulation of the PTH(1-34) stimulation even under conditions of constant ligand concentration. The action of PTH(1-34) has the anabolic effect only when its concentration varies in time, e.g., due to natural day-night cycle or due to intermittent administration. The key for the synergism is the ability of the FSS to modulate the action of the ligand; this can happen either through inhibition or through enhancement of PTH(1-34) binding to PTH1R. Such a coupling of biochemical reaction and mechanical loading constitutes a new molecular mechanism that can form the basis for explaining the synergism between the action of the PTH and mechanical loading.
Conclusions.
We have shown that stimulation of the MC3T3-E1 cells with the FSS leads to ligand-independent conformational changes in the PTH1R characterized by higher FRET efficiencies. Furthermore, we demonstrated that the nature of ligand-dependent conformational change of PTH1R GPCR depends on the type of ligand: stimulation with the natural ligand [PTH(1-34)] leads to conformational transitions characterized by decrease in FRET efficiency while stimulation with a NH2-terminal truncated ligand [PTH(3-34)] is characterized by higher FRET efficiencies. Our observation that PTH(1-34) and the FSS stimulate different conformations of the PTH1R suggests that the action of the FSS is to intermittently modulate (inhibit) the pathway stimulated by PTH(1-34), thus providing a molecular basis for the experimentally observed anabolic effect of the mechanical loading.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute Grant HL-086943 (to M. Chachisvilis).
Acknowledgments
The authors are grateful to Dr. David W. Piston ( Vanderbilt University, Nashville, TN) for the gift of template of green fluorescent protein variant Cerulean, and to Dr. Roger R. Tsien (University of California, San Diego) for the DNA template of green fluorescent protein variant Citrine. We also thank Drs. Jiunn-Chern Yeh, Ugur Ozerdem, and Ronald Y. Kwon for critical reading of the manuscript.
REFERENCES
- 1.Albert AD, Boesze-Battaglia K. The role of cholesterol in rod outer segment membranes. Prog Lipid Res 44: 99–124, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen OS, Koeppe RE. Bilayer thickness and membrane protein function: an energetic perspective. Annu Rev Biophys Biomol Struct 36: 107–130, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Azarani A, Goltzman D, Orlowski J. Structurally diverse N-terminal peptides of parathyroid hormone (PTH) and PTH-related peptide (PTHRP) inhibit the Na+/H+ exchanger NHE3 isoform by binding to the PTH/PTHRP receptor type I and activating distinct signaling pathways. J Biol Chem 271: 14931–14936, 1996. [DOI] [PubMed] [Google Scholar]
- 4.Bacabac RG, Smit TH, Mullender MG, Dijcks SJ, Van Loon JJ, Klein-Nulend J. Nitric oxide production by bone cells is fluid shear stress rate dependent. Biochem Biophys Res Commun 315: 823–829, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Bakker AD, Joldersma M, Klein-Nulend J, Burger EH. Interactive effects of PTH and mechanical stress on nitric oxide and PGE2 production by primary mouse osteoblastic cells. Am J Physiol Endocrinol Metab 285: E608–E613, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Bastepe M, Weinstein LS, Ogata N, Kawaguchi H, Juppner H, Kronenberg HM, Chung UI. Stimulatory G protein directly regulates hypertrophic differentiation of growth plate cartilage in vivo. Proc Natl Acad Sci USA 101: 14794–14799, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ben-Chaim Y, Chanda B, Dascal N, Bezanilla F, Parnas I, Parnas H. Movement of 'gating charge' is coupled to ligand binding in a G-protein-coupled receptor. Nature 444: 106–109, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Blood PD, Ayton GS, Voth GA. Probing the molecular-scale lipid bilayer response to shear flow using nonequilibrium molecular dynamics. J Phys Chem B 109: 18673–18679, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Blount P, Sukharev S, Kung C. A mechanosensitive channel protein and its gene in E. coli. Gravit Space Biol Bull 10: 43–47, 1997. [PubMed] [Google Scholar]
- 10.Butler PJ, Norwich G, Weinbaum S, Chien S. Shear stress induces a time- and position-dependent increase in endothelial cell membrane fluidity. Am J Physiol Cell Physiol 280: C962–C969, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Cantor RS Lateral pressures in cell membranes: a mechanism for modulation of protein function. J Phys Chem B 101: 1723–1725, 1997. [Google Scholar]
- 12.Carter PH, Petroni BD, Gensure RC, Schipani E, Potts JT, Gardella TJ. Selective and nonselective inverse agonists for constitutively active type-1 parathyroid hormone receptors: evidence for altered receptor conformations. Endocrinology 142: 1534–1545, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Castro M, Nikolaev VO, Palm D, Lohse MJ, Vilardaga JP. Turn-on switch in parathyroid hormone receptor by a two-step parathyroid hormone binding mechanism. Proc Natl Acad Sci USA 102: 16084–16089, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chachisvilis M, Zhang YL, Frangos JA. G protein-coupled receptors sense fluid shear stress in endothelial cells. Proc Natl Acad Sci USA 103: 15463–15468, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen NX, Geist DJ, Genetos DC, Pavalko FM, Duncan RL. Fluid shear-induced NFkappaB translocation in osteoblasts is mediated by intracellular calcium release. Bone 33: 399–410, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Dean T, Linglart A, Mahon MJ, Bastepe M, Juppner H, Potts JT Jr, Gardella TJ. Mechanisms of ligand binding to the parathyroid hormone (PTH)/PTH-related protein receptor: selectivity of a modified PTH(1–15) radioligand for GalphaS-coupled receptor conformations. Mol Endocrinol 20: 931–943, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dean T, Vilardaga JP, Potts JT Jr, Gardella TJ. Altered selectivity of parathyroid hormone (PTH) and PTH-related protein (PTHrP) for distinct conformations of the PTH/PTHrP receptor. Mol Endocrinol 22: 156–166, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donahue SW, Galley SA, Vaughan MR, Patterson-Buckendahl P, Demers LM, Vance JL, McGee ME. Parathyroid hormone may maintain bone formation in hibernating black bears (Ursus americanus) to prevent disuse osteoporosis. J Exp Biol 209: 1630–1638, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Dufour C, Holy X, Marie PJ. Transforming growth factor-β prevents osteoblast apoptosis induced by skeletal unloading via PI3K/Akt, Bcl-2, and phospho-Bad signaling. Am J Physiol Endocrinol Metab 294: E794–E801, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Duncan RL, Turner CH. Mechanotransduction and the functional response of bone to mechanical strain. Calcif Tissue Int 57: 344–358, 1995. [DOI] [PubMed] [Google Scholar]
- 21.Friedman PA, Gesek FA, Morley P, Whitfield JF, Willick GE. Cell-specific signaling and structure-activity relations of parathyroid hormone analogs in mouse kidney cells. Endocrinology 140: 301–309, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Fujimori A, Cheng SL, Avioli LV, Civitelli R. Structure-function relationship of parathyroid hormone: activation of phospholipase-C, protein kinase-A and -C in osteosarcoma cells. Endocrinology 130: 29–36, 1992. [DOI] [PubMed] [Google Scholar]
- 23.Gawrisch K, Soubias O. Structure and dynamics of polyunsaturated hydrocarbon chains in lipid bilayers-significance for GPCR function. Chem Phys Lipids 153: 64–75, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gether U Uncovering molecular mechanisms involved in activation of G protein-coupled receptors. Endocr Rev 21: 90–113, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Ghanouni P, Gryczynski Z, Steenhuis JJ, Lee TW, Farrens DL, Lakowicz JR, Kobilka BK. Functionally different agonists induce distinct conformations in the G protein coupling domain of the beta(2) adrenergic receptor. J Biol Chem 276: 24433–24436, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Gierschik P, Moghtader R, Straub C, Dieterich K, Jakobs KH. Signal amplification in HL-60 granulocytes. Evidence that the chemotactic peptide receptor catalytically activates guanine-nucleotide-binding regulatory proteins in native plasma membranes. Eur J Biochem 197: 725–732, 1991. [DOI] [PubMed] [Google Scholar]
- 27.Griesbeck O, Baird GS, Campbell RE, Zacharias DA, Tsien RY. Reducing the environmental sensitivity of yellow fluorescent protein. Mechanism and applications. J Biol Chem 276: 29188–29194, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Gudi S, Nolan JP, Frangos JA. Modulation of GTPase activity of G proteins by fluid shear stress and phospholipid composition. Proc Natl Acad Sci USA 95: 2515–2519, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gullingsrud J, Schulten K. Lipid bilayer pressure profiles and mechanosensitive channel gating. Biophys J 86: 3496–3509, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hagino H, Okano T, Akhter MP, Enokida M, Teshima R. Effect of parathyroid hormone on cortical bone response to in vivo external loading of the rat tibia. J Bone Miner Metab 19: 244–250, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Haidekker MA, L'Heureux N, Frangos JA. Fluid shear stress increases membrane fluidity in endothelial cells: a study with DCVJ fluorescence. Am J Physiol Heart Circ Physiol 278: H1401–H1406, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Haidekker MA, Ling TT, Anglo M, Stevens HY, Frangos JA, Theodorakis EA. New fluorescent probes for the measurement of cell membrane viscosity. Chem Biol 8: 123–131, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Hamill OP Twenty odd years of stretch-sensitive channels. Pflügers Arch 453: 333–351, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Hamill OP, Martinac B. Molecular basis of mechanotransduction in living cells. Physiol Rev 81: 685–740, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Heck M, Hofmann KP. Maximal rate and nucleotide dependence of rhodopsin-catalyzed transducin activation: initial rate analysis based on a double displacement mechanism. J Biol Chem 276: 10000–10009, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Hillsley MV, Frangos JA. Bone tissue engineering: the role of interstitial fluid flow. Review. Biotechnol Bioeng 43: 573–581, 1994. [DOI] [PubMed] [Google Scholar]
- 37.Hoare SR, Gardella TJ, Usdin TB. Evaluating the signal transduction mechanism of the parathyroid hormone 1 receptor. Effect of receptor-G-protein interaction on the ligand binding mechanism and receptor conformation. J Biol Chem 276: 7741–7753, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Hoare SR, Usdin TB. Quantitative cell membrane-based radioligand binding assays for parathyroid hormone receptors. J Pharmacol Toxicol Methods 41: 83–90, 1999. [DOI] [PubMed] [Google Scholar]
- 39.Jee WS, Tian XY. The benefit of combining non-mechanical agents with mechanical loading: a perspective based on the Utah Paradigm of Skeletal Physiology. J Musculoskelet Neuronal Interact 5: 110–118, 2005. [PubMed] [Google Scholar]
- 40.Jilka RL Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone 40: 1434–1446, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim CH, Takai E, Zhou H, von SD, Muller R, Dempster DW, Guo XE. Trabecular bone response to mechanical and parathyroid hormone stimulation: the role of mechanical microenvironment. J Bone Miner Res 18: 2116–2125, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Kobilka BK, Deupi X. Conformational complexity of G-protein-coupled receptors. Trends Pharmacol Sci 28: 397–406, 2007. [DOI] [PubMed] [Google Scholar]
- 43.Lee AG How lipids affect the activities of integral membrane proteins. Biochim Biophys Acta 1666: 62–87, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Lefkowitz RJ, Cotecchia S, Samama P, Costa T. Constitutive activity of receptors coupled to guanine-nucleotide regulatory proteins. Trends Pharmacol Sci 14: 303–307, 1993. [DOI] [PubMed] [Google Scholar]
- 45.Leurs R, Smit MJ, Alewijnse AE, Timmerman H. Agonist-independent regulation of constitutively active G-protein-coupled receptors. Trends Biochem Sci 23: 418–422, 1998. [DOI] [PubMed] [Google Scholar]
- 46.Li J, Duncan RL, Burr DB, Gattone VH, Turner CH. Parathyroid hormone enhances mechanically induced bone formation, possibly involving L-type voltage-sensitive calcium channels. Endocrinology 144: 1226–1233, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Ma Y, Jee WS, Yuan Z, Wei W, Chen H, Pun S, Liang H, Lin C. Parathyroid hormone and mechanical usage have a synergistic effect in rat tibial diaphyseal cortical bone. J Bone Miner Res 14: 439–448, 1999. [DOI] [PubMed] [Google Scholar]
- 48.Mahaut-Smith MP, Martinez-Pinna J, Gurung IS. A role for membrane potential in regulating GPCRs? Trends Pharmacol Sci 29: 421–429, 2008. [DOI] [PubMed] [Google Scholar]
- 49.Mahon MJ, Bonacci TM, Divieti P, Smrcka AV. A docking site for G protein betagamma subunits on the parathyroid hormone 1 receptor supports signaling through multiple pathways. Mol Endocrinol 20: 136–146, 2006. [DOI] [PubMed] [Google Scholar]
- 50.Mahon MJ, Donowitz M, Yun CC, Segre GV. Na(+)/H(+ ) exchanger regulatory factor 2 directs parathyroid hormone 1 receptor signalling. Nature 417: 858–861, 2002. [DOI] [PubMed] [Google Scholar]
- 51.Makino A, Prossnitz ER, Bunemann M, Wang JM, Yao W, Schmid-Schonbein GW. G protein-coupled receptors serve as mechanosensors for fluid shear stress in neutrophils. Am J Physiol Cell Physiol 290: C1633–C1639, 2006. [DOI] [PubMed] [Google Scholar]
- 52.Martinac B, Hamill OP. Gramicidin A channels switch between stretch activation and stretch inactivation depending on bilayer thickness. Proc Natl Acad Sci USA 99: 4308–4312, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mitchell DC, Lawrence JT, Litman BJ. Primary alcohols modulate the activation of the G protein-coupled receptor rhodopsin by a lipid-mediated mechanism. J Biol Chem 271: 19033–19036, 1996. [DOI] [PubMed] [Google Scholar]
- 54.Murray TM, Rao LG, Divieti P, Bringhurst FR. Parathyroid hormone secretion and action: evidence for discrete receptors for the carboxyl-terminal region and related biological actions of carboxyl-terminal ligands. Endocr Rev 26: 78–113, 2005. [DOI] [PubMed] [Google Scholar]
- 55.Nyholm TK, Ozdirekcan S, Killian JA. How protein transmembrane segments sense the lipid environment. Biochemistry 46: 1457–1465, 2007. [DOI] [PubMed] [Google Scholar]
- 56.Owan I, Burr DB, Turner CH, Qiu J, Tu Y, Onyia JE, Duncan RL. Mechanotransduction in bone: osteoblasts are more responsive to fluid forces than mechanical strain. Am J Physiol Cell Physiol 273: C810–C815, 1997. [DOI] [PubMed] [Google Scholar]
- 57.Pavalko FM, Gerard RL, Ponik SM, Gallagher PJ, Jin Y, Norvell SM. Fluid shear stress inhibits TNF-alpha-induced apoptosis in osteoblasts: a role for fluid shear stress-induced activation of PI3-kinase and inhibition of caspase-3. J Cell Physiol 194: 194–205, 2003. [DOI] [PubMed] [Google Scholar]
- 58.Pavalko FM, Norvell SM, Burr DB, Turner CH, Duncan RL, Bidwell JP. A model for mechanotransduction in bone cells: the load-bearing mechanosomes. J Cell Biochem 88: 104–112, 2003. [DOI] [PubMed] [Google Scholar]
- 59.Perez DM, Karnik SS. Multiple signaling states of G-protein-coupled receptors. Pharmacol Rev 57: 147–161, 2005. [DOI] [PubMed] [Google Scholar]
- 60.Potts JT, Gardella TJ. Progress, paradox, and potential: parathyroid hormone research over five decades. Ann NY Acad Sci 1117: 196–208, 2007. [DOI] [PubMed] [Google Scholar]
- 61.Rizzo MA, Springer GH, Granada B, Piston DW. An improved cyan fluorescent protein variant useful for FRET. Nat Biotechnol 22: 445–449, 2004. [DOI] [PubMed] [Google Scholar]
- 62.Roberts DJ, Waelbroeck M. G protein activation by G protein coupled receptors: ternary complex formation or catalyzed reaction? Biochem Pharmacol 68: 799–806, 2004. [DOI] [PubMed] [Google Scholar]
- 63.Robling AG, Castillo AB, Turner CH. Biomechanical and molecular regulation of bone remodeling. Annu Rev Biomed Eng 8: 455–498, 2006. [DOI] [PubMed] [Google Scholar]
- 64.Rosenblatt M, Callahan EN, Mahaffey JE, Pont A, Potts JT Jr. Parathyroid hormone inhibitors. Design, synthesis, and biologic evaluation of hormone analogues. J Biol Chem 252: 5847–5851, 1977. [PubMed] [Google Scholar]
- 65.Skerry TM The response of bone to mechanical loading and disuse: fundamental principles and influences on osteoblast/osteocyte homeostasis. Arch Biochem Biophys 473: 117–123, 2008. [DOI] [PubMed] [Google Scholar]
- 66.Stevens HY, Frangos JA. Bone cell responses to fluid flow. Methods Mol Med 80: 381–398, 2003. [DOI] [PubMed] [Google Scholar]
- 67.Sugiyama T, Saxon LK, Zaman G, Moustafa A, Sunters A, Price JS, Lanyon LE. Mechanical loading enhances the anabolic effects of intermittent parathyroid hormone (1-34) on trabecular and cortical bone in mice. Bone 43: 238–248, 2008. [DOI] [PubMed] [Google Scholar]
- 68.Takasu H, Guo J, Bringhurst FR. Dual signaling and ligand selectivity of the human PTH/PTHrP receptor. J Bone Miner Res 14: 11–20, 1999. [DOI] [PubMed] [Google Scholar]
- 69.Turner CH Bone strength: current concepts. Ann NY Acad Sci 1068: 429–446, 2006. [DOI] [PubMed] [Google Scholar]
- 70.Turner CH, Pavalko FM. Mechanotransduction and functional response of the skeleton to physical stress: the mechanisms and mechanics of bone adaptation. J Orthop Sci 3: 346–355, 1998. [DOI] [PubMed] [Google Scholar]
- 71.Ueki M, Tanaka N, Tanimoto K, Nishio C, Honda K, Lin YY, Tanne Y, Ohkuma S, Kamiya T, Tanaka E, Tanne K. The effect of mechanical loading on the metabolism of growth plate chondrocytes. Ann Biomed Eng 36: 793–800, 2008. [DOI] [PubMed] [Google Scholar]
- 72.Vilardaga JP, Bunemann M, Krasel C, Castro M, Lohse MJ. Measurement of the millisecond activation switch of G protein-coupled receptors in living cells. Nat Biotechnol 21: 807–812, 2003. [DOI] [PubMed] [Google Scholar]
- 73.Vilardaga JP, Steinmeyer R, Harms GS, Lohse MJ. Molecular basis of inverse agonism in a G protein-coupled receptor. Nat Chem Biol 1: 25–28, 2005. [DOI] [PubMed] [Google Scholar]
- 74.Warne T, Serrano-Vega MJ, Baker JG, Moukhametzianov R, Edwards PC, Henderson R, Leslie AG, Tate CG, Schertler GF. Structure of a beta1-adrenergic G-protein-coupled receptor. Nature 454: 486–491, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang D, Singh R, Divieti P, Guo J, Bouxsein ML, Bringhurst FR. Contributions of parathyroid hormone (PTH)/PTH-related peptide receptor signaling pathways to the anabolic effect of PTH on bone. Bone 40: 1453–1461, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yasuda N, Miura S, Akazawa H, Tanaka T, Qin Y, Kiya Y, Imaizumi S, Fujino M, Ito K, Zou Y, Fukuhara S, Kunimoto S, Fukuzaki K, Sato T, Ge J, Mochizuki N, Nakaya H, Saku K, Komuro I. Conformational switch of angiotensin II type 1 receptor underlying mechanical stress-induced activation. EMBO Rep 9: 179–186, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zacharias DA, Violin JD, Newton AC, Tsien RY. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science 296: 913–916, 2002. [DOI] [PubMed] [Google Scholar]
- 78.Zhang YL, Frangos JA, Chachisvilis M. Laurdan fluorescence senses mechanical strain in the lipid bilayer membrane. Biochem Biophys Res Commun 347: 838–841, 2006. [DOI] [PubMed] [Google Scholar]
- 79.Zou Y, Akazawa H, Qin Y, Sano M, Takano H, Minamino T, Makita N, Iwanaga K, Zhu W, Kudoh S, Toko H, Tamura K, Kihara M, Nagai T, Fukamizu A, Umemura S, Iiri T, Fujita T, Komuro I. Mechanical stress activates angiotensin II type 1 receptor without the involvement of angiotensin II. Nat Cell Biol 6: 499–506, 2004. [DOI] [PubMed] [Google Scholar]