Abstract
The nasal epithelium of the cystic fibrosis (CF) mouse has been used extensively in CF research because it exhibits ion transport defects similar to those of human CF airways. This tissue is composed of ∼50% olfactory (OE) and ∼50% ciliated epithelium (CE), and on the basis of previous observations, we hypothesized that a significant fraction of the bioelectric signals from murine nasal tissue may arise from OE rather than CE, while CE is the target tissue for CF gene therapy. We compared the bioelectric properties of isolated OE from the nasal cavity and CE from the nasopharynx in Ussing chamber studies. Hyperabsorption of Na+ [amiloride response; CF vs. wild type (WT)] was ∼7.5-fold greater in the OE compared with the CE. The forskolin response in native tissues did not reliably distinguish genotypes, likely due to a cyclic nucleotide-gated cation conductance in OE and a calcium-mediated Cl− conductance in CE. By potential difference assay, hyperabsorption of Na+ (CF vs. WT) and the difference in response to apical 0 Cl− buffer (CF vs. WT) were ∼2-fold greater in the nasal cavity compared with the nasopharynx. Our studies demonstrate that in the CF mouse, both the hyperabsorption of Na+ and the Cl− transport defect are of larger magnitude in the OE than in the CE. Thus, while the murine CF nasal epithelium is a valuable model for CF studies, the bioelectrics are likely dominated by the signals from the OE, and assays of the nasopharynx may be more specific for studying the ciliated epithelium.
Keywords: chloride transport, nasal potential difference, sodium transport, cystic fibrosis
the nasal epithelium of the cystic fibrosis (CF) mouse has been used as a model system for studies of CF therapies because it exhibits ion transport defects that are nearly identical to those exhibited by human CF airways (12). In the CF mouse, this tissue exhibits an enhanced response to amiloride and a defect in chloride secretion when studied with the in vivo nasal potential difference (PD) technique (12). However, the ratio of various cell types comprising the human nasal epithelium differs significantly from that of the mouse nasal epithelium. In the mouse, ∼50% of the nasal epithelium is composed of olfactory epithelium (OE), the remainder being largely ciliated (respiratory) epithelium (CE) (7, 16). In contrast, in the human, the olfactory epithelium constitutes only ∼3% of the cells in the nasal cavity, the remainder being primarily ciliated epithelium (16).
Because olfactory and ciliated epithelium have markedly different functions, it is likely that the ion transport properties differ between these two types of tissue. However, it has not been possible thus far to distinguish the contribution of the olfactory and respiratory epithelium to the murine nasal bioelectric measurements, and many studies treat the “nasal epithelia” as a homogenous tissue. Bioelectric discrimination between olfactory and respiratory epithelium is especially important to studies of candidate gene transfer agents, because the respiratory, not the olfactory, epithelium of the murine nasal cavity resembles the relevant target tissue in CF patients.
To explore the differences in ion transport function of murine nasal respiratory (ciliated) versus olfactory epithelium, we have isolated intact olfactory and ciliated epithelium from wild-type (WT) and CF mice and studied their bioelectrics, both as freshly excised and as cultured preparations. In addition, we compared the electrical PD in vivo in the nasal cavity (containing mixed olfactory and ciliated epithelium) with the nasopharynx (NP), containing ciliated epithelium with no olfactory cells present. Interestingly, different bioelectric results were obtained depending on whether tissues were studied in vitro on Ussing chambers, or in vivo, using the transepithelial PD method. In vivo, CF nasal and nasopharyngeal PDs can be discriminated from wild type by an enhanced response to amiloride and absence of a Cl− diffusion potential. In Ussing chamber studies, native tissue (OE or CE) from CF mice exhibited an enhanced response to amiloride. However, the response to forskolin (which stimulates CFTR-mediated Cl secretion in normal human airways) in native tissue (OE and CE) did not reliably distinguish between CF and WT tissue. In OE from young mice (30 days old), we observed a response to forskolin that did not differ between genotypes. In both genotypes, this response appeared to arise in the olfactory receptor neurons (ORNs) of the OE and was likely due, at least in part, to stimulation of a cAMP-mediated cation conductance, part of the signaling cascade in odorant detection. In nasopharyngeal CE, both CF and WT tissue exhibited a prominent response to forskolin that was most likely due to activation of a calcium-activated Cl− conductance we have previously described for the CF trachea (11). Our results indicate that the ion transport properties of the murine nasal cavity are complex and differ between olfactory and respiratory epithelium.
MATERIALS AND METHODS
Animals.
Mice studied were of both sexes, of a mixed strain (BALB/C, C57BL/6, DBS/2, 129/SvEv), and of various ages, as indicated in results. The WT mice were heterozygous for CFTR, and littermates to the CF-null mice (Cftrtm1Unc) when possible. Because we have been unable to distinguish a difference in airway bioelectrics between heterozygotes and homozygous normal mice, in the present study, we refer to the heterozygous mice as wild type. All mice were fed ad libitum, and the CF mice received Colyte rather than water to prevent gut obstructions. Animals were maintained and studied under protocols approved by the University of North Carolina Institutional Animal Care and Use Committee. All mice were euthanized by CO2 inhalation.
Bioelectric preparations-native tissue.
The OE was removed from the dorsal meatus as far posterior in the nasal cavity as possible. This dissection necessitated removing not only the nasal bones, as described previously (15), but in addition, several millimeters of skull were removed to aid in obtaining olfactory epithelium from the posterior aspect of the nasal cavity, uncontaminated with ciliated epithelium. The OE was easy to identify, especially in the WT mice, by its thicker, somewhat yellow appearance. All tissue was mounted, as previously described (8), on Ussing chambers having an aperture surface area of 0.014 cm2. This aperture, which was smaller than we had used previously with the nasal tissue, helped to ensure that we obtained no contaminating ciliated respiratory epithelium. At the end of each Ussing chamber study, the tissue was fixed for histological study in 10% neutral buffered formalin and examined histologically to verify that only olfactory epithelium was studied.
The ciliated respiratory epithelium was obtained from the nasopharynx after the olfactory epithelium was removed from the nasal cavity. To isolate this epithelium, the lower jaw was removed, exposing the hard and soft palate, and an incision was made through the skin and fascia over the most caudal palatine fold. The skin and fascia were then carefully removed from the soft palate by peeling the tissue caudally using fine forceps. Once the skin and fascia were stripped from the soft palate, the very thin ventral wall (composed of ciliated respiratory epithelium) was visible and the beating cilia on the apical side of the membrane could be seen. Next, the most caudal palatine bones were chipped away, exposing the epithelium lying beneath the hard palate. The exposed epithelium was cut longitudinally along the ventral aspect, and the tissue was cut through completely at the most caudal (near the glottis) border and also at the border exposed by removing the hard palate. Then, starting at the glottal pole, the tissue was carefully dissected free from the skull and rolled forward. When the tissue was completely detached at the hard palatal pole, it was removed from the mouse and was ready to mount on the Ussing chamber. Occasionally, it was difficult to determine which side of the epithelium was apical, but by careful observation, beating cilia could be visualized. This tissue was very fragile, and at times it was difficult to get a piece large enough to mount. However, with practice, the success rate of dissecting and mounting the tissue was around 60%. The tissue was mounted as described for the nasal tissue.
The olfactory and nasopharyngeal tissues were studied under open circuit conditions, using a Physiologic Instruments voltage clamp (San Diego, CA). The electrical potential difference across the tissue was continually recorded, and a constant current pulse (2–10 μA) was applied across the tissue at 1-min intervals to calculate tissue resistance. From these measurements, the equivalent short-circuit current (Ieq) was calculated. In this study, open circuit PDs ranged from −0.36 mV (WT) to −0.8 mV (CF), with resistance being in the range of 17 Ω/cm2 for OE. The ciliated epithelia harvested from the NP exhibited PDs ranging from −0.45 mV (WT) to −0.60 mV (CF), with resistance in these tissues being lower, in the range of 10 Ω/cm2. All other details of Ussing chamber techniques have been previously published (8). All drugs were obtained from Sigma Aldrich (St. Louis, MO) except for l-cis-diltiazem (Biomol, Plymouth Meeting, PA).
Tissue culture.
Tissue for these experiments was removed from the mice as described above. All tissue culture conditions, as well as Ussing chamber studies of the cultured tissue, have been previously described (13).
Electrical transepithelial PDs.
For the in vivo transepithelial electrical potential difference studies, the mice were anesthetized with ketamine-xylazine (90 mg/kg and 13 mg/kg, respectively), and a rectal thermister was inserted to monitor body temperature, which was maintained at 37°C with a heat lamp. For the nasal PD studies, all techniques were as previously described (15), with the exception of the perfusate flow, which was reduced to 1.0 μl/min. For the nasopharyngeal (NP) PD measurements, an opening was made in the trachea ∼0.5 cm distal to the larynx to ensure a patent airway. The measuring PD electrode was inserted through a small opening made just below the larynx and advanced cephalad into the nasopharynx ∼7–8 mm. The nasal and NP PDs were obtained on different mice.
Olfactory bulbectomy.
The olfactory bulb on only one side of the brain was ablated in selected mice. The surgical protocol was similar to that of Schwartz-Levey et al. (29). Briefly, the mouse was anesthetized with ketamine/xylazine, the fur over the skull was shaved, and the skin was scrubbed with betadine. The skin was retracted from the skull overlying the olfactory bulb and a hole was made in the skull over the olfactory bulb. The olfactory bulb was removed by aspiration and a piece of Gelfoam was placed in the bulb cavity to prevent bleeding. The skin was closed with surgical cement and the animal was returned to its cage to recover. Mice were given 5 mg/kg carprofen subcutaneously 1×/day for 3 days after surgery for pain. Five days after the olfactory bulbectomy (OBX) surgery, the animals were studied.
Statistical analysis.
All data are shown as means ± SE. A Student's t-test was used to compare means between two groups, and P < 0.05 was considered statistically significant.
RESULTS
Bioelectrics of native olfactory epithelium compared with ciliated epithelium.
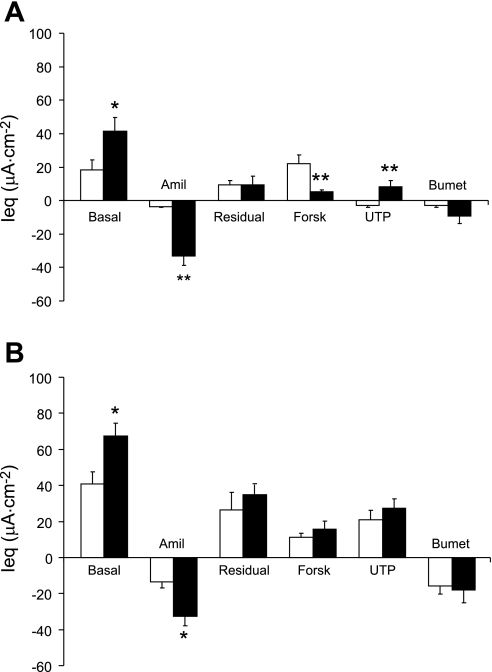
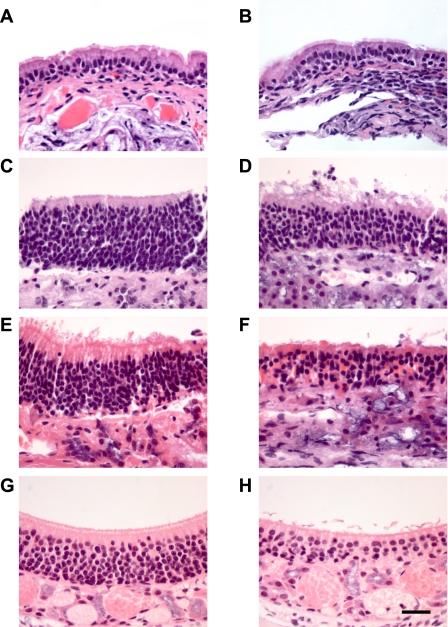
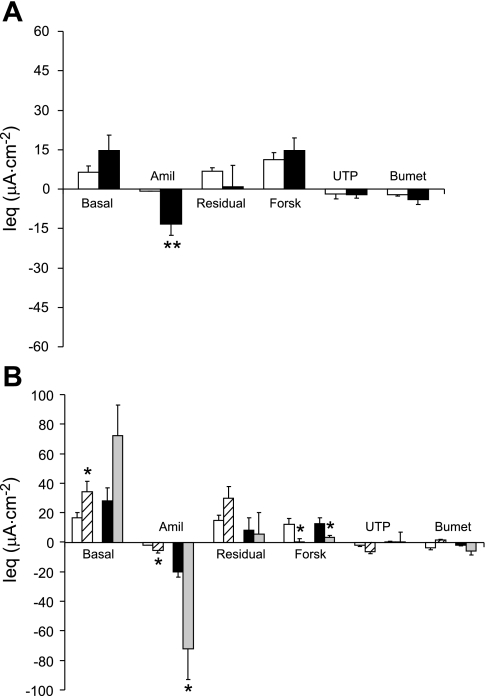
In OE isolated from CF mice (∼7 mo old), the basal Ieq was significantly elevated compared with WT, as was the amiloride-sensitive Ieq (∼10-fold vs. WT) (Fig. 1A). Moreover, the response to forskolin in the OE was significantly (P ≤ 0.01) diminished in the CF tissue compared with the response exhibited by the WT tissue. However, the forskolin response in the native WT OE does not appear to be a result of CFTR-mediated Cl− secretion (see below). Because it was difficult to obtain a large enough tissue sample of ciliated epithelium from either the nasal septum or the lateral wall of the nasal cavity without contamination with OE for Ussing chamber study, we used ciliated epithelial tissue from the NP as a model. The nasopharyngeal epithelium consists of ciliated cells and a variable number of goblet cells (Fig. 2, A and B). As in the native olfactory epithelium, the basal Ieq in the CF ciliated epithelium was significantly elevated compared with WT CE (Fig. 1B). The response to amiloride followed the same pattern, with the CF preparations exhibiting a significantly enhanced amiloride response, indicating hyperabsorption of Na+ in the CF ciliated epithelium of this tissue. However, in the nasopharyngeal ciliated cells, the amiloride-sensitive Ieq in the CF tissue was only about 2.4-fold that of the WT, compared with a ∼10-fold increase in the CF olfactory epithelium. Interestingly, we could detect no difference in the magnitude of the response to forskolin in the WT vs. CF ciliated epithelium (Fig. 1B).
Fig. 1.
A: bioelectrics of native olfactory epithelia (OE) from 7-mo-old mice. Open bars, wild-type mouse (WT); solid bars, cystic fibrosis mouse (CF) (n = 8 both groups) *P ≤ 0.05 vs. WT; **P ≤ 0.01 vs. WT. B: native nasopharyngeal ciliated epithelium. Open bars are tissue from WT mice (n = 15) and solid bars are tissue from CF mice (n = 7). *P ≤ 0.05 vs. WT. In both A and B, the basal equivalent short-circuit current (Ieq) is shown by the leftmost bar and then amiloride (Amil; 10−4 M) was added. The residual Ieq is the difference between the basal and the amiloride-sensitive Ieq. Then, changes in Ieq in response to forskolin (Forsk, 10−5 M), UTP (10−4 M), and bumetanide (Bumet, 10−4 M) are shown. All drugs were added apically except bumetanide, which was added to the basolateral side. All data are shown as means ± SE.
Fig. 2.
Histological sections of murine nasal airway. A: ciliated nasopharyngeal epithelia from WT mouse. B: CF nasopharyngeal ciliated epithelium. C: OE from 30-day-old WT mouse. D: OE from 30-day-old CF mouse. E: OE from 7-mo-old WT mouse. F: OE from 7-mo-old CF mouse. (Preparations in A–F were fixed after removal from Ussing chamber.) G: OE from 30-day-old CF mouse removed from nontreated side of mouse 5 days after undergoing olfactory bulbectomy (OBX) surgery. H: OE removed from the side receiving OBX surgery; same mouse as in G. All preparations were stained with hematoxylin and eosin. Size bar in H = 25 μM; magnification of all other sections is identical.
The response to UTP also differed between the olfactory and ciliated epithelium. In the CF olfactory epithelium, the UTP response was of a polarity consistent with anion secretion, whereas the polarity of the UTP in the OE of the WT mice was consistent with cation secretion (Fig. 1A). The response to UTP was similar to that which we previously reported for the response to ionomycin in nasal epithelia of CF and WT mice (15). In the CE, both CF and WT tissues exhibited UTP response with a polarity indicating anion secretion that did not differ between genotypes (Fig. 1B).
Bioelectrics of cultured OE and CE.
We studied the bioelectrics of cultured OE and CE from WT and CF mice to determine how closely the bioelectric phenotype of cultured cells matched that of native tissue. Olfactory and ciliated epithelia were isolated and cultured at an air/liquid interface, as previously described (13). Analysis of the OE cultures for markers of differentiation by RT-PCR demonstrated expression of cytochrome p450 Cyp2gl, a sustentacular cell marker. However, no expression of olfactory marker protein, a marker for olfactory receptor neurons, was detected. Thus, while the olfactory cells seeded were a mixture of ORNs and sustentacular cells (and possibly basal cells), our culture conditions did not promote the growth of ORNs. Also, while goblet cells were present among the native nasopharyngeal cells seeded, histologically, there was no evidence of goblet cells among the cultured cells grown from this preparation.
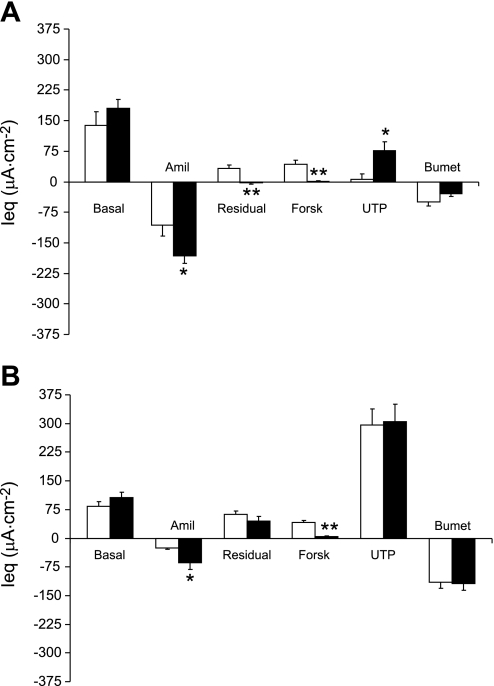
The amiloride-inhibitable Ieq was significantly elevated in the cultured CF vs. WT OE, indicating Na+ hyperabsorption (Fig. 3A). The residual (postamiloride) Ieq was significantly reduced in the CF OE, and cultured CF OE had no response to forskolin (Fig. 3A). The UTP response was significantly elevated in the CF OE, and the response to bumetanide did not differ significantly between genotypes.
Fig. 3.
A: bioelectrics of cultured murine OE. Open bars are preparations from WT mice (n = 6) and solid bars are preparations from CF mice (n = 6). B: bioelectrics of cultured murine ciliated epithelium from the nasopharynx. Open bars are tissue from WT mice (n = 9) and solid bars are tissue from CF mice (n = 8). *P ≤ 0.05 vs. WT; **P ≤ 0.01 vs. WT. All Ussing chamber conditions and abbreviations are the same as in Fig. 1.
The cultured cells derived from the ciliated nasopharynx exhibited a bioelectric phenotype that, like the cultured OE, differed somewhat from the native tissue. The basal Ieq of the cultured CE from CF mice did not differ significantly from that of the WT, but the response to amiloride was significantly increased in the CF tissue compared with the WT tissue (Fig. 3B), indicating that, like native tissue, the cultured CF ciliated epithelium exhibited hyperabsorption of Na+. Unlike the native ciliated nasopharyngeal epithelium, there was no significant response to forskolin in the cultured CF NP, whereas the cultured WT NP exhibited a significant response to forskolin. In one group of cultured ciliated cell preparations, we added the CFTR inhibitor INH 172 (21) after forskolin. In the WT preparations, this compound significantly decreased the forskolin response (preblocker Ieq 45.7 ± 2.5 μA/cm2; Δ with INH 172 −48.2 ± 3.7 μA/cm2; n = 8), whereas there was no significant response to this blocker in the CF preparations (preblocker Ieq 5.5 ± 2.5 μA/cm2, Δ with INH 172 −2.8 ± 1.8 μA/cm2; n = 6). The UTP response in the cultured ciliated epithelium did not differ between genotypes. However, the UTP response was about 4-fold greater (P ≤ 0.01) than exhibited by the cultured CF OE and about 50-fold greater compared with the UTP response in cultured WT OE.
Thus, in culture, CF cells from both the olfactory epithelium and ciliated nasopharynx exhibit an increased amiloride response and lack a forskolin response, although the magnitude of the amiloride response is greater in the OE than in the CE. The response to UTP was significantly greater in cultured ciliated nasopharynx than that exhibited by OE, suggesting a prominent calcium-activated Cl− conductance in the CE.
In vivo transepithelial electrical PD.
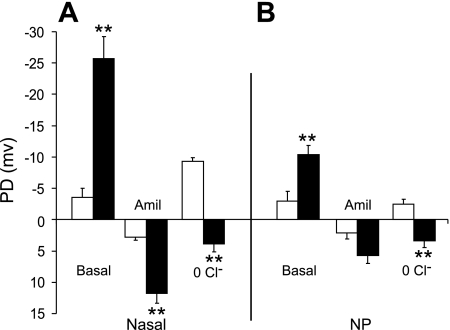
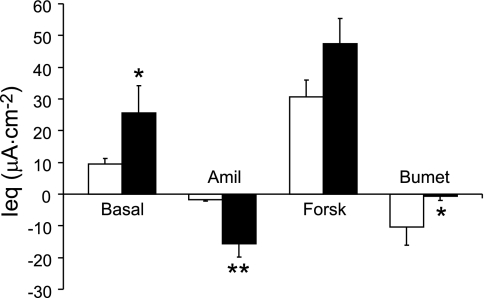
The in vivo transepithelial electrical PD studied in the nasal cavity of the CF mouse has been used extensively to indicate “correction” of the CF ion transport defects in response to various therapies. The nasal PD of the CF mouse is very distinct from that of the WT mouse, with the CF nasal PD characterized by a significantly enhanced basal PD (∼7-fold WT) and response to amiloride (∼4.5-fold WT) (Fig. 4A). In addition, the absence of PD hyperpolarization in response to low Cl− (plus amiloride) perfusion in the CF mouse nose reliably distinguishes between CF and WT epithelium (Fig. 4A).
Fig. 4.
A: in vivo potential difference (PD) data from the nasal cavity of WT (open bars n = 6) or CF (solid bars, n = 6) mice. B: in vivo PD data from the nasopharyngeal (NP) cavity from WT (open bars, n = 5) or CF (solid bars, n = 6) mice. **P ≤ 0.01 vs. WT.
In vivo electrical PD studies of the NP from the two genotypes (Fig. 4B) were performed and compared with nasal PD studies. The basal PDs were significantly elevated in the CF NP (∼3.5-fold WT), while the response to amiloride was ∼2.6-fold greater in the CF NP (as it was in the native tissue) compared with the WT (however, this difference was not significant, likely due to the small sample size) (Fig. 4B). There was a small but significant hyperpolarization of the PD in the WT NP in response to perfusion with a Cl−-free (plus amiloride) buffer. In contrast, the CF NP responded to the Cl−-free buffer with a small depolarization of the electrical PD (Fig. 4B).
Thus, although the in vivo bioelectrics are similar between the nasal cavity and the nasopharynx, the magnitude of both the amiloride response in the CF mice and the response to Cl−-free buffer in the WT mice is greater in the nasal cavity (mixed olfactory and ciliated cells) compared with the ciliated epithelium of the nasopharynx.
Olfactory epithelium effect of age and nature of the forskolin response.
We and others have previously reported a forskolin response in CF “nasal epithelium” (15, 22) that varied from not significantly different from zero (9, 22) to a forskolin response roughly half of that exhibited by the WT nasal tissue (15). In these studies, the mice ranged in age from 1 to 6 mo, and, undoubtedly, both olfactory tissue and respiratory tissue was included in the tissues studied. Because the cell type distribution in the olfactory epithelia of the CF mouse has been found to change markedly as the mice age (14, 28), we compared the bioelectrics of OE from young (∼1 mo old) CF and WT mice (Fig. 5A) with the results obtained in older (∼7 mo old) animals (see Fig. 1A). The basal Ieq from the olfactory epithelia of the young CF mice was nearly double that of the WT animals, but this difference was not significant (Fig. 5A). The CF amiloride-sensitive Ieq was significantly greater (∼18-fold) than that of the WT mice. Interestingly, in the young mice, we measured a forskolin response in both the CF and WT olfactory tissue that did not differ significantly between genotypes (Fig. 5A). The OE from the young mice responded to UTP with a small depolarization of Ieq that did not differ between genotypes. The small bumetanide response also did not differ significantly between genotypes in the OE from the young mice.
Fig. 5.
A: bioelectrics of OE from 30-day-old WT and CF mice. Open bars, WT; solid bars, CF (n = 8 both groups). B: bioelectrics of OE from 30-day-old mice having undergone unilateral OBX 5 days previously. Ussing chamber protocol is the same as in Fig. 1. Open bars, WT nontreated side; hatched bars, WT OBX side; black bars, CF nontreated side; light gray, CF OBX side. n = 8 WT nontreated; n = 7 WT OBX; n = 5 CF nontreated; n = 4 CF OBX. *P ≤ 0.05 vs. nontreated side of the respective genotype. (Note that, as in A, the response to forskolin does not differ between the two genotypes on the nontreated side.)
In the older CF animals (7 mo old; Fig. 1A), the magnitude of the response to amiloride increased (Δ with amiloride CF old vs. young CF, P ≤ 0.01) and the magnitude of the response to forskolin significantly decreased (P ≤ 0.01) (compare Figs. 1A and 5A) with age. In WT mice, a trend was present for an increase in the OE amiloride response as the mice aged, but this difference was not significant (P ≥ 0.06).
After bioelectric study on the Ussing chambers, all tissues were fixed for histological study to confirm that only olfactory tissue was studied. The OE from the 30-day-old CF mice looked histologically similar to that of the WT animals; however, as previously reported (14), even at this young age, we could detect a decrease in the number of ORNs (Fig. 2, C and D). The CF OE from the 7-mo-old animals exhibited a marked loss in the number of ORNs (Fig. 2, E and F) compared with the WT preparations.
To determine whether the loss of ORNs in the CF OE was responsible for the changes observed in bioelectrics of the OE in the older CF mice, we ablated the olfactory bulb on one side of the brain in 30-day-old WT and CF mice. In 5–6 days, this procedure causes a massive loss of the ORNs on the ablated side (24, 29), while the control side remains normal. Five days after surgery, the mice were euthanized and the OE removed for Ussing chamber study. As can be seen in Fig. 2, G and H, the number of ORNs on the OBX side (Fig. 2H) was substantially reduced compared with the number of ORNS in the untreated side (Fig. 2G) (data shown for CF only).
The basal Ieq was increased in the OBX tissue compared with the tissue from the untreated side in both the WT (2-fold increase) and CF (2.5-fold increase) preparations (Fig. 5B). However, this difference was significant only in the WT preparations. In both the WT and the CF tissue, the amiloride-sensitive Ieq was significantly elevated in the OBX tissue compared with the untreated tissue from the same animal. Because the amiloride response was actually increased in the OBX tissue from each genotype, it is unlikely that this response arose from the ORNs. It is not obvious why ablating the olfactory bulb should increase the magnitude of the amiloride response. However, there is some evidence that cytokines, likely released by apoptosis, may stimulate proliferation of sustentacular cells (24), thus increasing the surface area occupied by the sustentacular cells. Alternatively, the apoptosing ORNs may release a substance that stimulates epithelial Na+ channel (ENaC) function, or in native non-OBX tissue, the ORNs may release a substance that inhibits ENaC function.
Interestingly, ablating the olfactory bulb completely eliminated the forskolin response in the WT OBX preparations, and in the CF OBX preparations, the forskolin response was nearly completely eliminated (Fig. 5B). Note that, on the untreated side (as shown in Fig. 5A with a different group of mice), the response to forskolin in olfactory epithelium from 30-day-old mice was indistinguishable between the WT and CF preparations. Because the forskolin response was almost completely eliminated in the preparations of both genotypes by OBX, it is likely that the origin of this response resides in the olfactory receptor neurons. There were no other significant changes in the bioelectrics as a result of the OBX surgery.
To gain further insight into the origin of the forskolin response, WT olfactory epithelia were studied in bilateral Cl−-free Ringer. These tissues were incubated for 60 min in the Cl−-free Ringer and then treated with amiloride. This procedure significantly increased the magnitude of both the basal Ieq and the change in Ieq with amiloride (Fig. 6). It has previously been shown (3, 4) that by blocking apical Na+ absorption with amiloride, the apical membrane is hyperpolarized, which increases the driving force for Cl− secretion, leading to an underestimation of the amiloride-sensitive Isc if there is a significant apical Cl− conductance. In Cl−-free Ringer, the preparations should exhibit no Cl− secretion, and thus, this may be why the amiloride response was elevated. Very surprisingly, the preparations in the Cl−-free buffer exhibited an increase in Ieq in response to forskolin that did not differ significantly from preparations studied in Krebs-bicarbonate-Ringer (KBR; Fig. 6). Because the tissues were incubated in Cl−-free Ringer for ∼60 min before addition of forskolin, it is unlikely that there would be sufficient intracellular Cl− present to support the sustained response we observed (data not shown). The absence of a response to bumetanide in the Cl−-free preparations adds support to this hypothesis. Had a small pool of intracellular Cl− been responsible for the response to forskolin in Cl− free Ringer, a very transient response would have been expected. It should be noted that in the presence of KBR, the WT (as well as CF; see Fig. 1) OE exhibits a small response to bumetanide, so it appears that both the WT and CF preparations are capable of Cl− secretion.
Fig. 6.
Bioelectrics of WT nasal epithelia incubated in either Krebs-bicarbonate-Ringer (KBR; open bars) or bilateral Cl−-free Ringer for 60 min (solid bars) before study. n = 21 KBR; n = 6 Cl− free. *P ≤ 0.05, **P ≤ 0.01 vs. preparations in KBR. Mean age of mice was 44 days old.
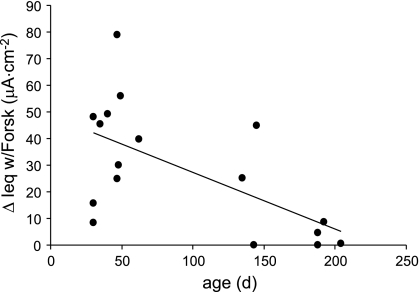
The sustained response in Cl−-free Ringer and absence of a response to bumetanide suggest that the forskolin response in Cl−-free Ringer was not a result of Cl− secretion. We have also observed a significant negative correlation between the age of the mouse and the magnitude of the response to forskolin (age range 1 mo to ∼6.5 mo) in preparations (WT) incubated in bilateral Cl−-free Ringer (Fig. 7). (No CF tissue was included in this study.) Because the number of ORNs decreases with age [the population of WT ORNs decreased ∼33% from 1 to 6 mo of age (14)], these data lend support to the hypothesis that the forskolin response in the OE originates in the ORNs.
Fig. 7.
Effect of age of mouse on the response to forskolin in WT olfactory epithelium incubated in Cl−-free buffer. R = 0.619, P ≤ 0.01, n = 17; d, days.
To determine whether the response to forskolin may be a result of HCO3− secretion, we conducted an additional study in which the response to forskolin was compared in WT OE studied in Cl−-free buffer to WT OE incubated for 1 h in Cl/HCO3−-free buffer containing 1 mM acetazolamide (to prevent endogenous HCO3− formation). After incubation, the tissues were studied in the Ussing chamber in the same buffer. (The Cl/HCO3−-free buffer contained 10 mM HEPES titrated to 7.4 and gassed with 100% oxygen.) There was no significant difference in the magnitude of the response to forskolin in the Cl−-free buffer [ΔIsc 29.2 ± 4 μA/cm2 (n = 8)] vs. those preparations incubated in the absence of both Cl− and HCO3− [ΔIsc 18 ± 5.1 μA/cm2 (n = 8)].
In the ORNs, cAMP is the first signaling molecule in the olfactory signaling cascade and activates a cyclic nucleotide-gated (CNG) cation conductance (Ca2+ and Na+) (25). Therefore, we speculated that the response to forskolin may be the result of cAMP activation of this CNG. Thus, we added l-cis-diltiazem [100 μM bilaterally; a CNG cation conductance blocker (30)] to some preparations. However, this blocker failed to diminish the magnitude of the response to forskolin in OE from 30-day-old WT mice studied in KBR [Δ with forskolin in presence of blocker, 31.1 ± 10.2 μA/cm2 (n = 5) and in the absence of blocker with forskolin 29.4 ± 8.7 (n = 5)].
DISCUSSION
The epithelium of the CF murine nasal cavity is an important model for CF research because the ion transport defects in the nasal epithelium of the CF mouse closely resemble those in human CF airways (12). However, unlike human airways, in the mouse, ∼50% of the surface area of the nasal cavity is covered by olfactory epithelium (1, 7). On the basis of previous studies, we hypothesized that a significant fraction of the bioelectric signal from the nasal epithelium may arise from the OE (15, 22, 26). Because defects in ion transport across the ciliated cell are thought to be responsible for the pulmonary phenotype in the human CF patient (18), it is important to determine ion transport characteristics of each epithelium independently.
In Ussing chamber studies of native olfactory epithelium, the rate of Na+ absorption (amiloride response) was significantly elevated in the CF OE compared with its WT counterpart in both the 30-day-old and the ∼7-mo-old mice (Table 1). In the CE (nasopharynx, native tissue), we also measured Na+ hyperabsorption, but of a much smaller magnitude than exhibited by olfactory epithelia (Table 1). The presence of Na+ hyperabsorption in both the CE and OE (likely sustentacular cells) suggests that CFTR is present in the respective WT cells.
Table 1.
Bioelectrics of murine airway epithelia
| Tissue | Method of Study |
Amiloride Response |
Response to Forskolin (Ussing chamber) and Response to 0 Cl− (PD)
|
||||
|---|---|---|---|---|---|---|---|
| WT Genotype | CF Genotype | P | WT Genotype | CF Genotype | P | ||
| Olfactory | |||||||
| Native tissue, young | Ussing chamber | + | ++++ | 0.01 | + | + | NS |
| Native tissue, old | Ussing chamber | + | +++++ | 0.01 | ++ | + | 0.01 |
| Native (young) OBX | Ussing chamber | ++ | +++++ | 0.01 | NS | ||
| Culture | Ussing chamber | + | +++ | 0.05 | + | 0.01 | |
| Respiratory-ciliated | |||||||
| Native tissue | Ussing chamber | + | ++ | 0.05 | + | + | NS |
| Culture | Ussing chamber | + | ++ | 0.05 | + | 0.01 | |
| In vivo | PD | + | ++ | NS | + | 0.01 | |
| Nasal epithelia | |||||||
| In vivo | PD | + | +++ | 0.01 | + | 0.01 | |
WT, wild-type; CF, cystic fibrosis; PD, potential difference; OBX, olfactory bulbectomy; NS, not significant.
In the OE, it is likely that the amiloride response originates in cells other than the ORNs, most likely the sustentacular cells. [The sustentacular cells surround the olfactory receptor neurons in the OE, and while these cells are in contact with the external environment, they are thought to play no role in odor detection (31).] Several lines of evidence support this hypothesis. First, Na+ hyperabsorption was present in the CF OE of both age groups, and the magnitude of the amiloride response was greater in the “old” CF mice, where significantly fewer ORNs were present. Second, in the OBX preparations, the magnitude of the amiloride response was significantly greater in both the WT and the CF preparations from the side on which the olfactory bulb had been ablated, compared with the nontreated side. Note that the OBX CF tissue still exhibited significant hyperabsorption of Na+ compared with WT OBX tissue (Table 1). Third, in support of the hypothesis that the amiloride response did not originate in the ORNs, in cultured OE (which appear to be composed of cells of sustentacular origin, because the cultured cells are positive for a sustentacular cell marker but negative for a ORN marker; see results), we observed bioelectric responses to amiloride that were qualitatively similar to the native tissue (non-OBX and OBX), i.e., a significantly enhanced response to amiloride in CF cultured OE (Table 1). The hypothesis that the amiloride response originates in the sustentacular cells is corroborated by the finding that in the OE, amiloride-sensitive ENaC have been localized to the microvilli of the sustentacular cells by immunochemistry (23).
Both CF OE (young mice) and CF CE exhibited a response to forksolin that was indistinguishable from that of WT preparations. It is clear that this response cannot be due to cAMP activation of CFTR because the CF mice used in this study have no functional CFTR. It is likely that the origin of the response to forskolin in the two tissue types is completely different. Previous studies have demonstrated a forskolin response in the nasal epithelium of CF mice (15, 22). Interestingly, in the present study, in native OE preparations from young mice, the magnitude of the forskolin response was indistinguishable between the WT and CF OE, whereas there was a significantly diminished forskolin response in the OE from the old CF mice compared with the WT counterparts (Table 1). Several lines of evidence suggest that the forskolin response arises in the olfactory receptor neurons: First, in the CF mouse, as these mice aged, both the number of ORNs (14) and the magnitude of the response to forskolin were reduced in parallel with increasing age (compare Figs. 1A and 5A). Second, the response to forskolin in the OE of the WT mice was negatively correlated with age (Fig. 7), and the number of ORNs in WT mice decreased with age (28), but not as drastically as in CF mice. Third, the response to forskolin was lost (WT), or markedly diminished (CF), in OE from mice that had been olfactory bulbectomized. Fourth, no forskolin response was obtained in our CF cultured OE, which contained no ORNs.
The current studies support the hypothesis that the response to forskolin is not calcium-activated Cl− secretion [as we had previously speculated (15)], because OE from WT mice studied in Cl−-free Ringer (equilibrated for 60 min, thus depleting intracellular Cl−) exhibited a sustained and undiminished response to forskolin. Furthermore, there was also no response to bumetanide, adding support to the hypothesis that the forskolin response observed in the Cl−-free Ringer was not a Cl− secretory response. Interestingly, Lowe et al. (20) exposed isolated OE to forskolin and recorded an electro-olfactogram (records electrical activity from the ORNs) that was sustained for many minutes. The hyperpolarization of this electro-olfactogram looked very similar to our OE forskolin response in bilateral Cl−-free Ringer, i.e., sustained and of negative polarity. Because in the ORNs, cAMP is the first signaling molecule in the olfactory signaling cascade, activating a CNG cation conductance (Ca2+ and Na+) (25), it seemed plausible that the forskolin response we recorded in the OE may at least in part be a result of cAMP activation of the CNG cation conductance in the ORNs rather than CFTR Cl− secretion. Following cAMP activation of a cation conductance, a Ca2+-activated Cl− efflux is induced, which amplifies the CNG signal (19). While the Ca2+-activated Cl− conductance is an important component of the olfactory signaling cascade, this component of the signaling cascade would not be functional in the Cl−-free buffer in the present experiments. While we were unable to block the forskolin response in OE by the CNG inhibitor l-cis-diltiazem, we have found certain other blockers ineffective in native tissue (INH 172), most likely due to ineffective cell entry, and thus, the blocker studies do not rule out the speculation that the forskolin response (at least in part) is due to a CNG cation conductance in the OE.
In the native CE of the CF mouse, the forskolin response likely reflects “cross talk” between cAMP and the calcium activated Cl− conductance, as we have previously reported for the CF murine trachea (11). The cross talk between cAMP and a calcium-activated Cl− conductance seems to have been eliminated in the cultured cells from the ciliated nasopharynx, because under these culture conditions, we can discriminate between the genotypes on the basis of the response to forskolin, i.e., WT cultures exhibited a substantial response to forskolin, whereas the CF cultures had none (Table 1). Note that culturing tracheal epithelia from WT and CF mice also eliminated most of this cross talk, allowing us to distinguish the genotype on the basis of the magnitude of the forskolin response (5). Interestingly, in the cultured cells from the NP (CF and WT), the response to UTP was much greater than we observed in any of the other tissues studied (either native or cultured). This suggests that a prominent calcium-activated Ca2+ conductance is present, which is upregulated by culture conditions, in the ciliated epithelia.
Because the murine nasal PD measurements are used as an assay to determine whether gene transfer has corrected the bioelectric defect in CF tissue, and the ciliated cell is the target tissue for correction (18), it is very important to know whether the bioelectric signal is being recorded from ciliated epithelium. However, with the murine nasal PD assay, it is difficult to determine with certainty from which tissue type the measurements are obtained (27). This is not a problem with PD measurements in the human nose, because only ∼3% of the nasal epithelium is olfactory, and the olfactory epithelium is located very distally from the measuring electrode.
We measured transepithelial PD in vivo across both the nasal (combination of OE and ciliated epithelium) and the NP (ciliated epithelium). As we and others have previously published, the WT and CF mice were easily distinguished on the basis of their nasal PDs, with the CF mouse having a significantly enhanced basal PD and response to amiloride compared with the WT mouse (Fig. 4A and Table 1). When low-Cl− buffer was perfused on the nasal epithelium of the normal mouse, a significant hyperpolarization of PD resulted, whereas in the CF animals, the low-Cl− buffer resulted in a depolarization of PD. We and others have found that forskolin or other compounds that increase cAMP levels are not useful in discriminating between the WT and CF genotypes in the murine PD assay (6, 10).
The PD responses in the ciliated nasopharynx were qualitatively similar to those in the nasal cavity (Table 1). While the basal PD was significantly elevated in the CF NP compared with the WT mouse, the magnitudes of the basal PD and the amiloride response in the ciliated epithelium of the CF mouse were much less than in the CF nasal epithelia. The Cl− diffusion potential in the NP of WT mice was small, and in some instances, difficult to distinguish from noise. However, statistically in the NP there was a significant difference in the Cl− diffusion potential between genotypes, with the WT PDs exhibiting a small hyperpolarization and the PD of CF preparations exhibiting a slight depolarization (Fig. 4B). On the basis of these data, discrimination between WT and CF mice based on the NP PD is more difficult than PD measurements in the nasal cavity.
The nasal PD assay (likely recording from both OE and ciliated cells) is useful in studies aimed at correcting the CFTR defect with various pharmacological correctors/potentiators if specific cell types are not critical in “proof of concept” studies. However, for gene transfer studies aimed at correcting ciliated cells, the nasal PD assay may be less useful in ascertaining correction due to the robust contribution of the olfactory cells to the bioelectrics of the nasal PD. In fact, in a previous study, we have reported that expression of CFTR specifically in ciliated cells of CF mice did not alter nasal PD values (26). Because some vectors more readily transduced OE compared with CE (2, 17, 32) it is critical to know from which cell type a “correction” is being measured. While the CF NP gives a less robust signal with regard to both Na+ absorption and Cl− transport defects, the nasopharyngeal PD assay may be better for ascertaining gene transfer to the ciliated epithelium of the CF mouse.
GRANTS
The following grants partially funded this study: National Institutes of Health (NIH) PPG HL-34322, MTCC NIH P30 DK-065988 (to R. C. Boucher), NIH HL-70199 (to L. E. Ostrowski), and Cystic Fibrosis Foundation Ostrow04GO (to L. E. Ostrowski).
REFERENCES
- 1.Adams DR Olfactory and non-olfactory epithelia in the nasal cavity of the mouse, Peromyscus. Am J Anat 133: 37–49, 1972. [DOI] [PubMed] [Google Scholar]
- 2.Arimoto Y, Nagata H, Isegawa N, Kumahara K, Isoyama K, Konno A, Shirasawa H. In vivo expression of adenovirus-mediated lacZ gene in murine nasal mucosa. Acta Otolaryngol 122: 627–633, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Boucher RC, Cotton CU, Gatzy JT, Knowles MR, Yankaskas JR. Evidence for reduced Cl− and increased Na+ permeability in cystic fibrosis human primary cell cultures. J Physiol (Lond) 405: 77–103, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boucher RC, Stutts MJ, Knowles MR, Cantley L, Gatzy JT. Na+ transport in cystic fibrosis respiratory epithelia. Abnormal basal rate and response to adenylate cyclase activation. J Clin Invest 78: 1245–1252, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clarke LL, Grubb BR, Gabriel SE, Smithies O, Koller BH, Boucher RC. Defective epithelial chloride transport in a gene targeted mouse model of cystic fibrosis. Science 257: 1125–1128, 1992. [DOI] [PubMed] [Google Scholar]
- 6.Griesenbach U, Smith SN, Farley R, Singh C, Alton EW. Validation of nasal potential difference measurements in gut-corrected CF knockout mice. Am J Respir Cell Mol Biol 39: 490–496, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Gross EA, Swenberg JA, Fields S, Popp JA. Comparative morphometry of the nasal cavity in rats and mice. J Anat 135: 83–88, 1982. [PMC free article] [PubMed] [Google Scholar]
- 8.Grubb BR Bioelectric measurement of CFTR function in mice. Methods Mol Med 70: 525–535, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Grubb BR, Boucher RC. Pathophysiology of gene-targeted mouse models for cystic fibrosis. Physiol Rev 79: S193–S214, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Grubb BR, Gabriel SE, Mengos A, Gentzsch M, Randell SH, van Heeckeren AM, Knowles MR, Drumm ML, Riordan JR, Boucher RC. SERCA pump inhibitors do not correct biosynthetic arrest of deltaF508 CFTR in cystic fibrosis. Am J Respir Cell Mol Biol 34: 355–363, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grubb BR, Paradiso AM, Boucher RC. Anomalies in ion transport in CF mouse tracheal epithelium. Am J Physiol Cell Physiol 267: C293–C300, 1994. [DOI] [PubMed] [Google Scholar]
- 12.Grubb BR, Pickles RJ, Ye H, Yankaskas JR, Vick RN, Engelhardt JF, Wilson JM, Johnson LG, Boucher RC. Inefficient gene transfer by adenovirus vector to cystic fibrosis airway epithelia of mice and humans. Nature 371: 802–806, 1994. [DOI] [PubMed] [Google Scholar]
- 13.Grubb BR, Rogers TD, Diggs PC, Boucher RC, Ostrowski LE. Culture of murine nasal epithelia: model for cystic fibrosis. Am J Physiol Lung Cell Mol Physiol 290: L270–L277, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Grubb BR, Rogers TD, Kulaga HM, Burns KA, Wonsetler RL, Reed RR, Ostrowski LE. Olfactory epithelia exhibit progressive functional and morphological defects in CF mice. Am J Physiol Cell Physiol 293: C574–C583, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Grubb BR, Vick RN, Boucher RC. Hyperabsorption of Na+ and raised Ca2+-mediated Cl− secretion in nasal epithelia of CF mice. Am J Physiol Cell Physiol 266: C1478–C1483, 1994. [DOI] [PubMed] [Google Scholar]
- 16.Harkema J, Carey SA, Wagner JG. The nose revisited: a brief review of the comparative structure, function, and toxicologic pathology of the nasal epithelium. Toxicol Pathol 34: 252–269, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Holtmaat AJ, Hermens WT, Oestreicher AB, Gispen WH, Kaplitt MG, Verhaagen J. Efficient adenoviral vector-directed expression of a foreign gene to neurons and sustentacular cells in the mouse olfactory neuroepithelium. Brain Res Mol Brain Res 41: 148–156, 1996. [DOI] [PubMed] [Google Scholar]
- 18.Kreda SM, Mall M, Mengos A, Rochelle L, Yankaskas J, Riordan JR, Boucher RC. Characterization of wild-type and deltaF508 cystic fibrosis transmembrane regulator in human respiratory epithelia. Mol Biol Cell 16: 2154–2167, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lowe G, Gold GH. Nonlinear amplification by calcium-dependent chloride channels in olfactory receptor cells. Nature 366: 283–286, 1993. [DOI] [PubMed] [Google Scholar]
- 20.Lowe G, Nakamura T, Gold GH. Adenylate cyclase mediates olfactory transduction for a wide variety of odorants. Proc Natl Acad Sci USA 86: 5641–5645, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma T, Thiagarajah JR, Yang H, Sonawane ND, Folli C, Galietta LJV, Verkman AS. Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion. J Clin Invest 110: 1651–1658, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacVinish LJ, Goddard C, Colledge WH, Higgins CF, Evans MJ, Cuthbert AW. Normalization of ion transport in murine cystic fibrosis nasal epithelium using gene transfer. Am J Physiol Cell Physiol 273: C734–C740, 1997. [DOI] [PubMed] [Google Scholar]
- 23.Menco BP, Birrell GB, Fuller CM, Ezeh PI, Keeton DA, Benos DJ. Ultrastructural localization of amiloride-sensitive sodium channels and Na+, K+-ATPase in the rat's olfactory epithelial surface. Chem Senses 23: 137–149, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Michel D, Moyse E, Trembleau A, Jourdan F, Brun G. Clusterin/ApoJ expression is associated with neuronal apoptosis in the olfactory mucosa of the adult mouse. J Cell Sci 110: 1635–1645, 1997. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura T, Gold GH. A cyclic nucleotide-gated conductance in olfactory receptor cilia. Nature 325: 442–444, 1987. [DOI] [PubMed] [Google Scholar]
- 26.Ostrowski LE, Yin W, Diggs PS, Rogers TD, O'Neal WK, Grubb BR. Expression of CFTR from a ciliated cell-specific promoter is ineffective at correcting nasal potential difference in CF mice. Gene Ther 14: 1492–1501, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Parsons DW, Hopkins PJ, Bourne AJ, Boucher RC, Martin AJ. Airway gene transfer in mouse nasal-airways: importance of identification of epithelial type for assessment of gene transfer. Gene Ther 7: 1810–1815, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Rosli Y, Breckenridge LJ, Smith RA. An ultrastructural study of age-related changes in mouse olfactory epithelium. J Electron Microsc (Tokyo) 48: 77–84, 1999. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz-Levey M, Chikaraishi DM, Kauer JS. Characterization of potential precursor populations in the mouse olfactory epithelium using immunocytochemistry and autoradiography. J Neurosci 11: 3556–3564, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwiebert EM, Potter ED, Hwang TH, Woo JS, Ding C, Qiu W, Guggino WB, Levine MA, Guggino SE. cGMP stimulates sodium and chloride currents in rat tracheal airway epithelia. Am J Physiol Cell Physiol 272: C911–C1022, 1997. [DOI] [PubMed] [Google Scholar]
- 31.Vogalis F, Hegg CC, Lucero MT. Ionic conductances in sustentacular cells of the mouse olfactory epithelium. J Physiol (Lond) 562: 785–799, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao H, Otaki JM, Firestein S. Adenovirus-mediated gene transfer in olfactory neurons in vivo. J Neurobiol 30: 521–530, 1996. [DOI] [PubMed] [Google Scholar]