Abstract
Warfarin is often used as a site-specific probe for examining the binding of drugs and other solutes to Sudlow site I of human serum albumin (HSA). However, warfarin has strong binding to HSA and the two chiral forms of warfarin have slightly different binding affinities for this protein. Warfarin also undergoes a slow change in structure when present in common buffers used for binding studies. This report examined the use of four related, achiral compounds (i.e., coumarin, 7-hydroxycoumarin, 7-hydroxy-4-methylcoumarin, and 4-hydroxycoumarin) as possible alternative probes for Sudlow site I in drug binding studies. High-performance affinity chromatography and immobilized HSA columns were used to compare and evaluate the binding properties of these probe candidates. Binding for each of the tested probe candidates to HSA was found to give a good fit to a two-site model. The first group of sites had moderate-to-high affinities for the probe candidates with association equilibrium constants that ranged from 6.4 × 103 M−1 (coumarin) to 5.5 × 104 M−1 (4-hydroxycoumarin) at pH 7.4 and 37°C. The second group of weaker, and probably non-specific, binding regions, had association equilibrium constants that ranged from 3.8 × 101 M−1 (7-hydroxy-4-methylcoumarin) to 7.3 × 102 M−1 (coumarin). Competition experiments based on zonal elution indicated that all of these probe candidates competed with warfarin at their high affinity regions. Warfarin also showed competition with coumarin, 7-hydroxycoumarin and 7-hydroxy-4-methycoumarin for their weak affinity sites but appeared to not bind and or compete for all of the weak sites of 4-hydroxycoumarin. It was found from this group that 4-hydroxycoumarin was the best alternative to warfarin for examining the interactions of drugs at Sudlow site I on HSA. These results also provided information on how the major structural components of warfarin contribute to the binding of this drug at Sudlow site I.
1. INTRODUCTION
The analysis of drug binding to plasma proteins is important in the pharmaceutical industry for characterizing the pharmacokinetics and pharmacological effects of drugs [1–6]. One plasma protein that has been extensively investigated during such work is human serum albumin (HSA) [7]. HSA is the most abundant protein in plasma, with a concentration that ranges from 35–50 g/L or 0.6–0.7 mM [1,6–10]. This protein is involved in transporting and distributing many drugs within the body and also binds to a variety of endogenous and exogenous compounds to aid in their transport and to improve their solubility [8–12].
Numerous techniques have been utilized to look at HSA and drug-protein interactions, including ultrafiltration [13], ultracentrifugation [14], equilibrium dialysis [15–17], fluorescence [18,19], UV/vis absorption [19], circular dichroism [20–23], capillary electrophoresis [24–27], surface plasmon resonance [28,29], and nuclear magnetic resonance (NMR) spectroscopy [30,31]. Another technique that has been popular for some time in this type of application is high-performance affinity chromatography (HPAC) [32–36]. HPAC is a specialized form of HPLC that makes use of an immobilized biological ligand (e.g., HSA) as the stationary phase [32–35,37–39]. It has been previously shown that columns containing immobilized HSA are effective models for soluble HSA in drug binding studies, making it possible to rapidly obtain accurate and precise estimates of the association equilibrium constants and number of binding sites for drugs on HSA, while also providing a means for studying drug-drug competition for this protein [32–39]. These properties make HPAC and HSA columns appealing for the high throughput screening of drug binding to HSA.
Both the number of binding sites and affinity of a drug are important in determining the interaction of such an agent with HSA [40]. This protein is known to contain two major binding sites for drugs (i.e., Sudlow site I and II) [41,42], as well as several minor binding sites [43]. One way the binding of a drug at a particular site on HSA can be identified is by determining if this drug has direct competition with a specific probe for that site. Warfarin (i.e., 3-(α-acetonylbenzyl)-4-hydroxycoumarin) is an anti-coagulant drug that is frequently used as a probe for Sudlow site I (also often called the warfarin-azapropazone site of HSA) [44]. Warfarin has a relatively high affinity for HSA and well-characterized interactions with this protein [42]. There are, however, several disadvantages to using warfarin in binding studies. For instance, the strong binding of warfarin to HSA can lead to long retention times for this drug on HPAC columns that contain immobilized HSA [45]. In addition, although the two enantiomers of warfarin have the same binding region but slightly different affinities for HSA [12,44,45], it can be expensive to use these separate enantiomers in binding studies (see Table 1); this has lead to the frequent use of racemic warfarin as a probe in many past investigations of solute interactions with HSA [32,33,37,45]. In addition, recent studies have shown that warfarin undergoes a slow conversion in aqueous solution that can lead to measurable shifts in its binding to HSA over time [44].
Table 1.
Relative cost of warfarin and potential probe candidates for Sudlow site I of HSA
| Analyte | Relative Cost (U.S. dollars per gram)a |
|---|---|
| R-Warfarin | $72,800 |
| S-Warfarin | $74,800 |
| Racemic Warfarin | $8.38 |
| Coumarin | $0.31 |
| 7-Hydroxycoumarin | $1.32 |
| 7-Hydroxy-4-methylcoumarin | $0.35 |
| 4-Hydroxycoumarin | $0.35 |
These numbers are based on 2007/2008 list prices from Sigma-Aldrich.
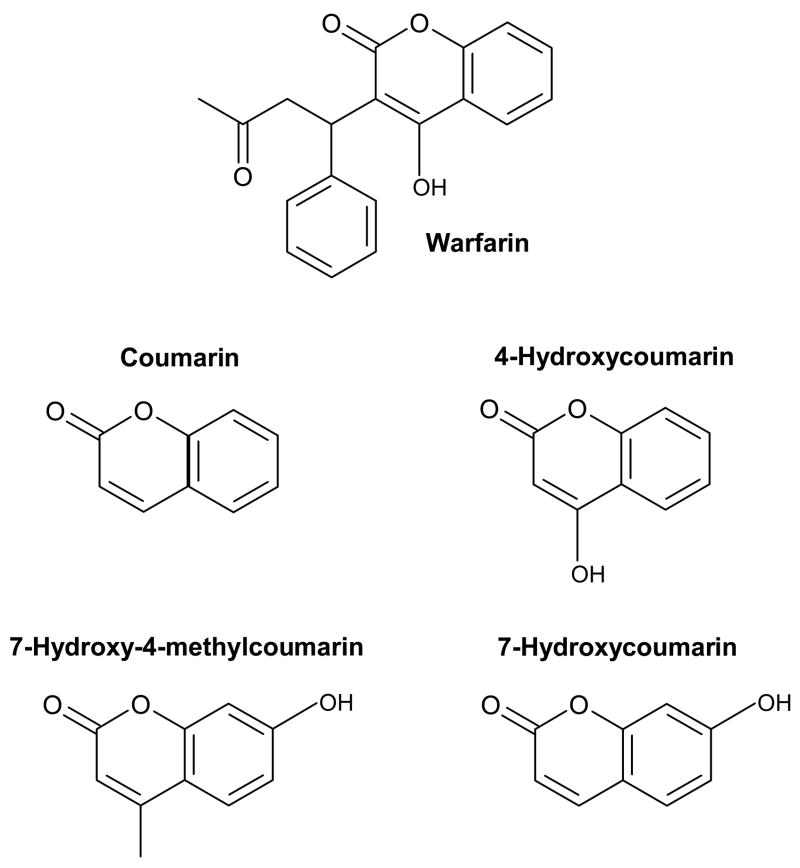
The purpose of this study is to examine several compounds that are closely-related to warfarin in structure with the goal of determining if these might be used as alternative probes for Sudlow site I in drug-protein binding studies. Ideally, a suitable warfarin replacement for high throughput studies should be specific for Sudlow site I and have few non-specific interactions with HSA or the analysis system. This probe should also have a good long-term stability in aqueous solution and be present in only a single form in solution. Figure 1 shows the various coumarin compounds that will be examined in this study as possible probes for Sudlow site I. These compounds are all achiral, which avoids the possibility of having any differences in binding by separate chiral forms; this property also makes these compounds more cost-effective to use (as illustrated in Table 1) and easier to obtain than the separate enantiomers of warfarin. In this study, the stability for each of these compounds will be examined by NMR spectroscopy3. This will be followed by an evaluation of their binding properties for HSA by using HPAC. From the results it will be possible to compare these compounds and determine which might be suitable replacements for warfarin for use in high throughput screening of drug interactions with HSA. The data obtained in this study should also provide clues as to how the various structural features of warfarin and related coumarin compounds contribute to their binding to Sudlow site I.
Figure 1.
Structures of warfarin and compounds that were examined as possible alternative probes for Sudlow site I on HSA.
2. THEORY
2.1. Frontal Analysis
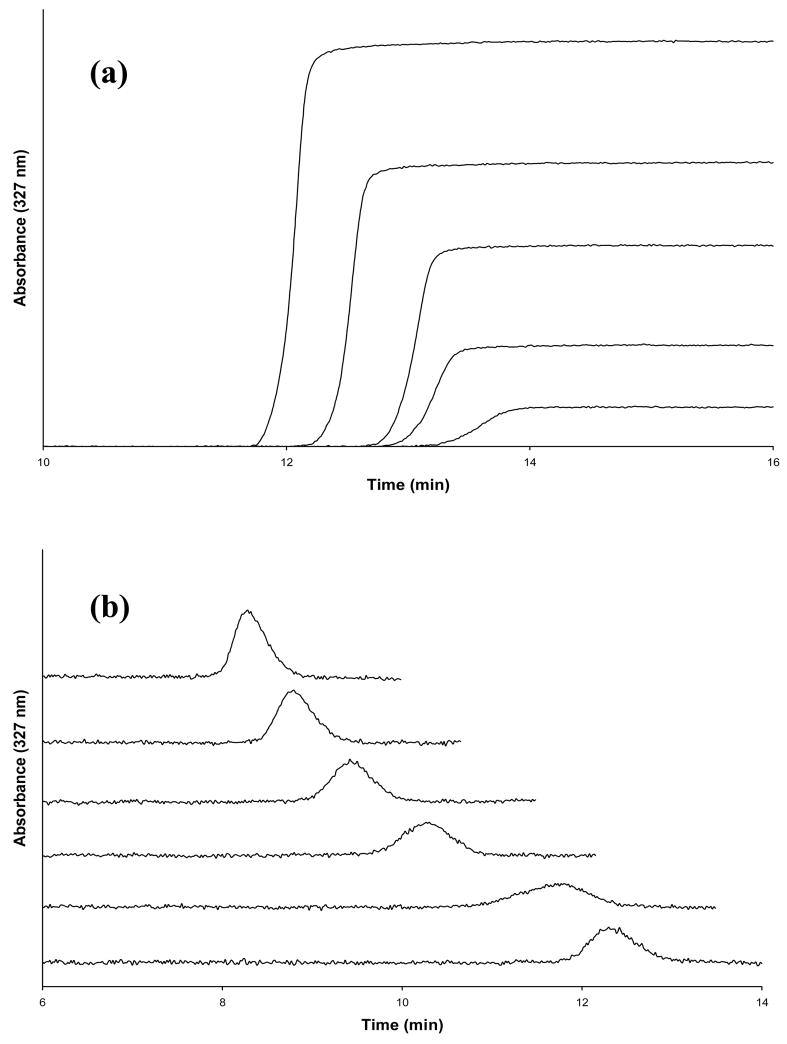
The method of frontal analysis (or frontal affinity chromatography) will be used to determine the number of binding sites and association equilibrium constants for each probe candidate examined in this study. This technique is carried out by continuously applying a solution with a known concentration of the analyte (e.g., a probe candidate) to a column that contains an immobilized ligand (e.g., HSA). As the analyte binds to the ligand, the binding sites in the column become saturated, forming a breakthrough curve like the one shown in Figure 2(a). If fast association and dissociation kinetics are present, the mean position of this breakthrough curve can be directly related to the concentration of the applied analyte [A], the total moles of active binding sites in the column for the analyte (mL), and the association equilibrium constant (Ka) for analyte-ligand binding. The following two equivalent equations can be used to relate these terms for a system where the analyte binds to a single type of site on a ligand [32,37].
Figure 2.
(a) Frontal analysis curves for 7-hydroxycoumarin at concentrations (from left-to-right) of 10, 7.5, 5.0, 2.5 or 1.0 μM. (b) Zonal elution competition studies performed with warfarin in the mobile phase while samples of 5.0 μM 7-hydroxycoumarin were injected; the concentration for warfarin in the mobile phase (from top-to-bottom) was 20, 15, 10, 5.0, 1.0 or 0 μM.
| (1) |
| (2) |
In these equations mLapp is the apparent moles of analyte that are required to reach the mean position of the breakthrough curve at any given concentration of applied analyte, [A]. According to Eq. (2), a plot of 1/mLapp versus 1/[A] for a system with 1:1 binding will make it possible to determine both the binding capacity of the column and the association equilibrium constant by finding the inverse of the intercept and the ratio of the intercept divided by the slope, respectively.
If multi-site binding occurs between the analyte and ligand, a plot prepared according to Eq. (2) will result in a non-linear relationship and produce negative deviations from a linear response at high analyte concentrations (i.e., low values for 1/[A]) [46]. To deal with this situation, Eqs. (1) and (2) can be expanded into the following forms for the case in which an analyte has two different groups of binding sites within a column [32,46].
| (3) |
| (4) |
In these expanded equations, Ka1 is the association equilibrium constant for the binding site with the highest affinity for the analyte, and Ka2 is the association equilibrium constant for the site with weaker binding, where 0 < Ka2 < Ka1. The term α1 is the fraction of all binding sites for the analyte that belong to the first group of sites (where α1 =mL1,tot/mLtot), and β2 is the ratio of the association equilibrium constants for the low affinity binding sites versus the high affinity sites (where β2=Ka2/Ka1). Similar expressions can be written for systems with more than two classes of binding sites for an analyte [46].
2.2. Zonal elution
The method of zonal elution was utilized in this study to examine the competition of warfarin with each probe candidate on HSA columns. This type of experiment is performed by continuously passing a competing agent (I) with a known concentration of [I] through a column that contains the immobilized ligand of interest (e.g., HSA). A small plug of the analyte (A) is then injected onto the column, as demonstrated in Figure 2(b). If A and I compete for a single class of binding sites on the ligand and have fast association/dissociation kinetics for their binding, the following relationship can be used to describe how the retention of A will be affected by the presence of I [32,37].
| (5) |
In this equation, k is the observed retention factor for the analyte, as given by k = (tR − tM)/tM where tR is the measured retention time for the injected analyte and tM is the column void time (i.e., the retention time for a non-retained compound). Also included in Eq. (5) are the association equilibrium constants for the competing agent and the analyte with the ligand (KaI and KaA, respectively) and the column void volume (VM). Eq. (5) is useful in studying drug-drug competition because it indicates that a plot of 1/k versus [I] should result in a linear relationship if there is direct competition between the competing agent and analyte at a single common binding site on the immobilized ligand, provided the analyte has no other separate binding sites in the column. Non-linear behavior in this plot will be seen for allosteric competition and negative deviations at low values of [I] will be noted for multi-site interactions [32].
3. EXPERIMENTAL
3.1. Reagents
The coumarin, 4-hydroxycoumarin, 7-hydroxycoumarin and 7-hydroxy-4-methylcoumarin were purchased from Aldrich (Milwaukee, WI, USA); all of these compounds were of analytical grade (>97% pure). The racemic warfarin (98%) was purchased from Sigma (St. Louis, MO, USA). The HSA (>96%, essentially fatty acid free) was also from Sigma. The Nucleosil Si-300 silica (7 μm particle diameter, 300 Å pore size) was from Macherey-Nagel (Düren, Germany). Reagents used in the bicinchoninic acid (BCA) protein assay were from Pierce (Rockford, IL, USA). All aqueous solutions were prepared using water obtained from a NANOpure system (Barnstead, Dubuque, IA, USA) and were filtered using 0.20 μm GNWP nylon membranes from Millipore (Billerica, MA, USA).
3.2. Apparatus
NMR studies were carried out on a DRX 500 MHz NMR (Bruker, Billerica, MA, USA) equipped with a cryoprobe. All 1H NMR spectra were acquired in D2O using 128 scans per sample. The chromatographic system consisted of a Waters 590 pump (Milford, MA, USA) and a Beckman 118 Solvent Module (Fullerton, CA, USA). While both of these components were used in the frontal analysis experiments, only the Waters 590 pump was required for the zonal elution experiments. The chromatographic system also contained a Jasco UV-975 UV/Vis absorbance detector (Tokyo, Japan) and a six-port Rheodyne Advantage PF valve (Cotati, CA, USA) equipped with a 20 μL sample loop during the zonal elution experiments. An Isotemp water bath from Fisher (Pittsburgh, PA, USA) was used in conjunction with a column water jacket (Alltech, IL, USA) to maintain a temperature of 37 (± 0.1) °C during all binding studies. The chromatographic data were collected and processed using LabView 5.1 or LabView 8.0 software (National Instruments, Austin, TX, USA). The BCA protein assay was carried out using a UV 160U spectrophotometer (Shimadzu, Kyoto, Japan) and the diol assay was performed on a P/ACE MDQ capillary electrophoresis system (Beckman, Fullerton, CA, USA).
3.3. Methods
1H NMR spectroscopy was used to monitor the stability of each probe candidate in a pH 7.4, 0.067 M potassium phosphate buffer. These studies were conducted using an approach identical to that described previously to examine the stability of warfarin in this same buffer [44]. The photosensitivity of each candidate probe was examined by using split samples in which one set was stored in the dark and the other set was continuously exposed to ordinary laboratory light. Both sets of samples were stored at 25°C throughout the duration of the stability studies.
The Nucleosil Si-300 silica was converted into a diol form according to the literature [47]. The diol content of the resulting material was 250 (± 20) μmol per gram silica (1 S.D.), as determined in triplicate by an iodometric capillary electrophoresis assay [48]. This diol silica was used along with the Schiff base method for the immobilization of HSA [49]. This immobilization was carried out by placing two 0.55 g portions of the diol silica into two separate 20 mL test tubes and combining each of these portions with 0.55 g sodium periodate. A 10 mL portion of a 90:10 acetic acid/water solution was then added to each test tube and mixed for 2 h at room temperature. The silica in each test tube was washed six times by centrifugation and resuspension in water. After the final washing step, 10 mL of pH 6.0, 0.10 M potassium phosphate buffer was added to each silica sample and the resulting slurries were degassed for approximately 5 min under vacuum. A 0.055 g portion of HSA was added to one of the silica slurries while the slurry in the other test tube was used as control with no HSA being added. Approximately 0.03 g of sodium cyanoborohydride was added to the slurry in each test tube, with these test tubes then being tightly covered and placed in a rocking shaker at 4°C for 6 days. The silica in each test tube was later washed three times by centrifugation and resuspension in pH 8.0, 0.10 M potassium phosphate buffer. A total of 0.1375 g sodium borohydride was slowly added in three portions to each of these test tubes over 90 min while the silica slurry was being shaken. The silica was then washed as described earlier, including three washes with pH 8.0, 0.10 M potassium phosphate buffer that contained 0.5 M sodium chloride, followed by four more washings with pH 7.4, 0.067 M potassium phosphate buffer. The final HSA silica and control support with no HSA added were then stored in pH 7.4, 0.067 M potassium phosphate buffer at 4°C until use.
The HSA silica and control silica were packed into separate 5.0 cm × 4.6 mm I.D. stainless steel columns. These columns were downward slurry packed at 3000 psi (0.21 Mbar) using pH 7.4, 0.067 M potassium phosphate buffer as the packing solution. A small amount of the remaining HSA silica was dried overnight in a vacuum oven and analyzed by using a BCA assay to determine its protein content. This assay was performed in triplicate using soluble HSA as the standard and the control support as the blank, giving a protein content of 40 (± 2) mg HSA per g silica, or 600 ( ± 30) nmol per g silica.
All samples and competing agent solutions for the chromatographic studies were prepared in pH 7.4, 0.067 M phosphate buffer. This same buffer was used as the application buffer and isocratic elution buffer during the chromatographic studies. The mobile phases were stored at 4°C and were degassed for at least 20 min prior to use. All chromatographic studies were carried out at 37°C using a flow rate of 0.5 ml/min. A six-port valve was used to change between the buffer and analyte solutions during the frontal analysis studies.
Frontal analysis studies were performed by applying to the HSA column and control column buffered solutions that consisted of the mobile phase or a known concentration of the desired probe candidate dissolved in the mobile phase. UV/vis absorbance detection was used to monitor the eluting analyte, with the detection wavelength being adjusted during the study to ensure that the signal was always within the linear response range of the detector. The concentrations of the probe candidates ranged from 1–500 μM and the detection wavelengths were as follows: 1–10 μM coumarin, 275 nm; 50–500 μM coumarin, 241 nm; 1–100 μM 7-hydroxycoumarin, 327 nm; 250–500 μM 7-hydroxycoumarin, 260 nm; 1–10 μM 7-hydroxy-4-methylcoumarin, 327 nm; 50–500 μM 7-hydroxy-4-methylcoumarin, 258 nm; 1–50 μM 4-hydroxycoumarin, 286 nm; and 65–500 μM 4-hydroxycoumarin, 325 nm. The retained analyte was eluted and the column was regenerated by changing the mobile phase to a pH 7.4, 0.067 M potassium phosphate buffer. Breakthrough times for each probe compound on the HSA column and control column were determined by using the equal area method, as outlined in Ref. [32]. The breakthrough times for the control column were subtracted from those for the HSA column to correct for non-specific binding by each probe candidate to the support and the system. The association equilibrium constants and binding capacities for each probe candidate on the HSA column were then determined by analyzing the data according to Eqs. (1)–(4).
Competition studies were performed through the use of zonal elution experiments by injecting 20 μL samples of the probe compounds onto the HSA or control column in the presence of a known concentration of warfarin in the mobile phase. Racemic warfarin was acceptable for use as a probe for Sudlow site I in this particular case because both R- and S-warfarin bind to Sudlow site I with only slightly different affinities for these interactions [12,44,45], and the primary goal of this competition study was to simply see if each achiral probe candidate could compete with warfarin for binding at this specific site. Racemic warfarin is also of general interest for such studies because it is the form of warfarin that is commonly used in therapeutic preparations. The detection wavelengths used in the competition studies were as follows: coumarin, 277 nm; 7-hydroxycoumarin, 325 nm; 7-hydroxy-4-methylcoumarin, 325 nm; and 4-hydroxycoumarin, 286 nm. A 5 μM sample of each probe candidate was injected; no significant change in the retention factors were noted by using lower concentration samples, indicating that these conditions allowed work to be performed under linear elution conditions. The concentration of warfarin that was added to the mobile phase ranged from 1–20 μM. This concentration range was determined in advance to be within the optimum range needed to observe a shift in analyte retention based on the known association equilibrium constant of warfarin with HSA [32]. The retention factors for the analyte peaks were found by using their central moments [32] using PeakFit 4.12 (Jandel Scientific Software, San Rafael, CA, USA). After correcting the data for the retention observed on the control column, the resulting retention factors were plotted according to Eq. (5) to determine the type of competition that was present for each probe candidate with warfarin.
4. RESULTS AND DISCUSSION
4.1. NMR stability studies
A previous study examined the stability of warfarin in pH 7.4, 0.067 M phosphate buffer by using 1H NMR spectroscopy [44]. It was found in this earlier report that warfarin has a slow conversion in structure from one form to another over time. It is believed that this conversion involves a change in warfarin between one cyclic epimer and another due to the presence of two chiral centers in the cyclic form of warfarin (Note: although warfarin is generally drawn in an open chain form, it is known to exist as a cyclic hemiketal in solution) [50]. This slow change in structure is temperature-dependent and follows a first-order decay process that has a rate constant of 0.0086 h−1 at 25°C [44].
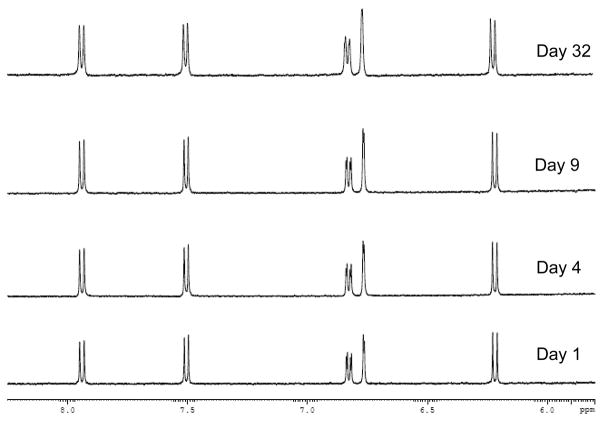
Similar experiments were conducted in this current report to examine the stability of each candidate probe. Some typical results are shown in Figure 3 for 7-hydroxycoumarin, which gave no observable change in its 1H NMR spectrum over the course of four weeks in pH 7.4, 0.067 M phosphate buffer. Similar results were obtained for all of the other candidate probes in both the presence and absence of normal laboratory lighting. These results indicated that each of these probe candidates had better long-term stability than warfarin in pH 7.4, 0.067 M phosphate buffer. This greater stability was not surprising because none of these probe candidates is capable of forming a cyclic hemiketal in solution, the feature believed to create a change in the dominant structure of warfarin over time when present in an aqueous solution [44]. These results indicated that all of these probe candidates were stable for at least one month when stored in pH 7.4, 0.067 M phosphate buffer. This feature is useful because this is the same buffer that is commonly used in drug binding studies with HSA.
Figure 3.
1H NMR spectra for 7-hydroxycoumarin when stored in pH 7.4, 0.067 M phosphate buffer for various lengths of time at 25° C. The same results were obtained for samples that were stored in the dark or in the presence of normal laboratory lighting.
4.2. Frontal analysis studies
Frontal analysis was performed using HPAC and an immobilized HSA column to determine the number of binding sites and association equilibrium constants for each probe candidate with HSA. Figure 2(a) shows some typical frontal analysis breakthrough curves that were obtained for the binding of 7-hydroxycoumarin to the HSA column. The breakthrough curves in this type of experiment shifted to the left, and to smaller breakthrough times, as the concentration of the analyte was increased. Similar results were obtained for the other probe candidates and in work performed with the control column.
Each of the probe compounds showed some non-specific binding to the support in the control column. This binding ranged from a corrected breakthrough time at 0.5 ml/min of 0.3–1.2 min (when using a void time of 1.5 min). The non-specific binding was low for most of the probe candidates and made up only 6–15% of the total binding noted on the HSA column when applying a 1.0 μM solution of the given probe candidate. The only exception was coumarin, for which non-specific binding to the support made up 48% of the capacity measured on the HSA column under the given experimental conditions. This higher level of non-specific binding may limit the usefulness of coumarin as an alternative probe to warfarin when working with columns that are based on silica supports; however, it is possible that coumarin might still be usable with HPAC columns that are prepared using other support materials.
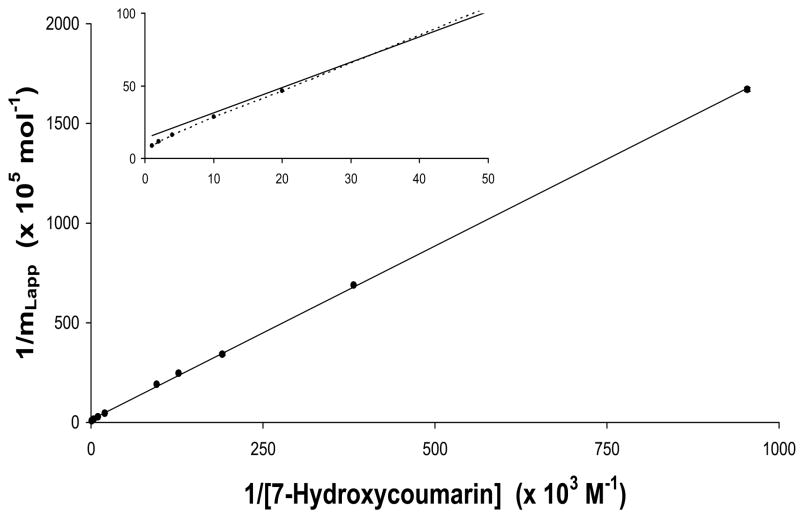
Eq. (2) was initially used to examine the frontal analysis data. A typical double reciprocal plot of 1/mLapp versus 1/[A] that was obtained is shown in Figure 4 for 7-hydroxycoumarin at applied concentrations that ranged from 1–500 μM. Similar plots were obtained for coumarin and 7-hydroxy-4-methylcoumarin. These plots had correlation coefficients that ranged from 0.9983–0.9998 (n = 10), but did show some negative deviations at high analyte concentrations (i.e., above 30–50 μM, as demonstrated by the inset in Figure 4). These results suggested that these three compounds each had a single major class of binding sites on HSA (creating the good linearity seen at low-to-moderate concentrations of these analytes), as well as a group of weaker binding sites (producing the negative deviations observed at high analyte concentrations). The same conclusion was reached for 4-hydroxycoumarin, which showed more apparent deviations from linearity at higher concentrations.
Figure 4.
Double reciprocal plot of frontal analysis data obtained for 7-hydroxycoumarin on an HSA column. The error bars represent a range of ± 1 S.D. The best-fit line was obtained by using Eq. (2).
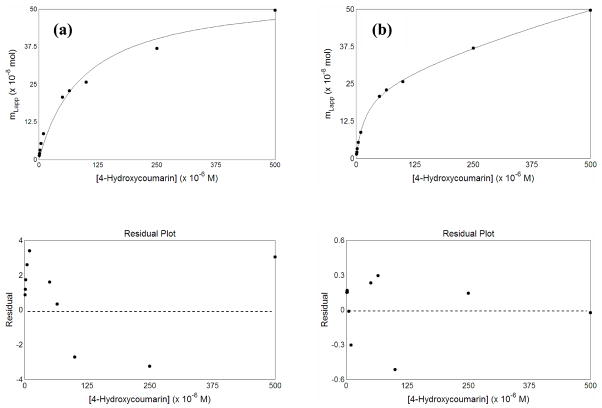
Figure 5 shows the results that were obtained when plots of mLapp versus the concentration of the applied analyte were prepared for 4-hydroxycoumarin and examined according to a one-site or two-site model. These data were found to produce the best fit to the two-site model described by Eq. (3), giving a correlation coefficient of 0.9998 (n =10) and only random variations in the corresponding residual plot, with an absolute residual sum of squares that was equal to 6.3 × 10−17 (see graph in lower part of Figure 5). When the same plot was analyzed according to a one-site model, the correlation coefficient was 0.9890 and non-random deviations were noted in the residual plot at both low and high analyte concentrations; this residual plot also showed a much larger absolute residual sum of squares (5.3 × 10−15) than that obtained with the two-site model. The other probe candidates also gave better fits to a two-site model than a one-site model for such plots, with correlation coefficients of 0.9997–0.9999, smaller absolute residual sum of squares, and only random deviations in the residual plots for the two-site model.
Figure 5.
Fit of frontal analysis data from 4-hydroxycoumarin to (a) a one-site model, as represented by Eq. (1) or (b) a two-site binding model, as represented by Eq. (3). The best-fit parameters for the graph in the two-site model are given in Table 2. The graphs at the bottom show the residual plots for the graphs immediately above each of these plots.
Table 2 summarizes the association equilibrium constants and binding capacities that were estimated from these plots based on a two-site model. The term “Site 1” in this table refers to the higher affinity binding region for each probe candidate, while “Site 2” refers to the weaker binding regions that were detected. The high affinity sites had association equilibrium constants for these probe candidates that ranged from 6.4 × 103 M−1 (for coumarin) up to 5.5 × 104 M−1 (for 4-hydroxycoumarin) at 37° C and pH 7.4. These values were approximately 4.5- to 40-fold lower than the average association equilibrium constant of 2.5 × 105 M−1 that has been reported for warfarin enantiomers with HSA under similar conditions [44,45]. It is interesting to note that the probe candidate with the closest similarity to warfarin in its structure also gave the largest association equilibrium constant for its high affinity site. This observation fits with a model in which at least some of these probe candidates were binding to Sudlow site I of HSA.
Table 2.
Association equilibrium constants (Ka) and binding capacties (mL) measured for each of the tested probe candidates on an HSA column using a two-site modela
| Analyte | Ka (M−1) | mL (mol) |
|---|---|---|
| Coumarin | Site 1: 6.4 (± 5.1) × 103 | Site 1: 1.2 (± 1.6) × 10−7 |
| Site 2: 7.3 (± 8.1) × 102 | Site 2: 7.8 (± 2.6) × 10−7 | |
| 7-Hydroxycoumarin | Site 1: 8.2 (± 0.9) × 103 | Site 1: 5.3 (± 0.7) × 10−7 |
| Site 2: 8.6 (± 1.4) × 102 | Site 2: 1.48 (± 0.02) × 10−6 | |
| 7-Hydroxy-4-methylcoumarin | Site 1: 2.2 (± 0.8) × 104 | Site 1: 2.8 (± 0.8) × 10−7 |
| Site 2: 3.8 (± 30) × 101 | Site 2: 3.7 (± 0.3) × 10−5 | |
| 4-Hydroxycoumarin | Site 1: 5.5 (± 0.5) × 104 | Site 1: 2.4 (± 0.1) × 10−7 |
| Site 2: 4.4 (± 2.5) × 102 | Site 2: 1.5 (± 0.6) × 10−6 |
The values in parenthesis represent a range of ± 1 SD. All of these measurements were made at 37°C in the presence of 7.4, 0.067 M potassium phosphate buffer.
The binding capacities obtained for the high affinity site of each probe compound were compared to the amount of HSA in the HPAC column to give the specific activities for these sites. The total moles of HSA in this column was calculated to be 224 (± 11) nmol based on the known protein content of the HSA support, the packing density of this material and the total column void volume. The resulting specific activities of the high affinity site for coumarin, 7-hydroxy-4-methylcoumarin and 4-hydroxycoumarin were in the range of 0.5–1.2 mol probe/mol HSA, as would be expected for interactions at a single binding region on HSA [44,45]. The specific activity obtained for 7-hydroxycoumarin was 2.3 mol/mol HSA, suggesting that this probe candidate might have interacted with two sites on HSA that had similar association equilibrium constants; such a feature would limit the usefulness of this particular compound if the goal is to use it as a specific probe for only Sudlow site I.
The weak affinity regions for these probe candidates had apparent association equilibrium constants in the range of only 38–860 M−1 at 37° C and pH 7.4. These interactions probably represent non-specific binding of these compounds to the structure of HSA. This conclusion is supported by the binding capacities that were estimated for these regions, which gave specific activities that ranged from 3.4–16 mol/mol HSA. These large specific activities agree with what would be expected for a group of non-selective interactions between a solute and a protein rather than binding at a specific binding site. A similar set of low affinity interactions at secondary sites has been noted between warfarin and HSA, with a reported association equilibrium constant of 1.4 × 104 M−1 at 25°C [51].
4.3 Competition studies
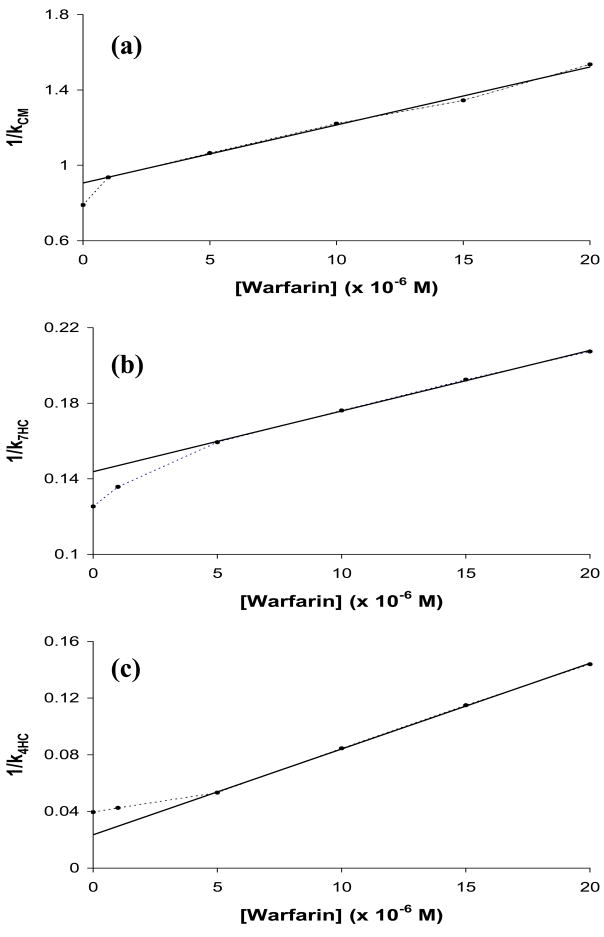
Zonal elution studies were next carried out to determine if the probe candidates could compete directly with warfarin for Sudlow site I on HSA. These studies were performed by adding various known concentrations of racemic warfarin to the mobile phase while a small and fixed amount of each candidate was injected onto the column. Figure 2(b) shows how the retention of 7-hydroxycoumarin changed with increasing concentrations of warfarin in the mobile phase. Figure 6 shows the results that were obtained when the data from such studies were analyzed according to Eq. (5). Coumarin, 7-hydroxycoumarin (see Figure 6(a)-(b)), and 7-hydroxy-4-methylcoumarin (data not shown) all gave a linear response at warfarin concentrations of at least 5 μM or higher, along with a small negative deviation from this linear behavior at lower warfarin concentrations or when no warfarin was present. The relative difference in the retention factors calculated from the best-fit intercept and actual intercept for each of these plots was in the range of 12.8 to 16.7%. 4-Hydroxycoumarin gave slightly different behavior, with a linear response being seen in Figure 6(c) at warfarin concentrations above 5 μM and a slight positive deviation being noted at lower warfarin concentrations.
Figure 6.
Zonal elution competition studies for injections of (a) coumarin (CM), (b) 7-hydroxycoumarin (7HC) and (c) 4-hydroxycoumarin (4HC) in the presence of racemic warfarin on an HSA column. The best-fit lines in (a)–(c) were found be using Eq. (5) along with data obtained at warfarin concentrations of 1–20 μM, 5–20 μM and 5–20 μM, respectively. The equations for these best-fit lines were as follows: (a) y = 3.08 (± 0.10) × 104 x + 0.906 (± 0.013); (b) y = 3.21 (± 0.06) × 103 x + 0.1437 (± 0.0009); and (c) y = 6.05 (± 0.07) × 103 x + 0.0236 (± 0.0010). A similar plot made for 7-hydroxy-4-methylcoumarin gave the following best-fit line for data obtained at warfarin concentrations of 5–20 μM: y = 4.48 (± 0.24) × 103 x + 0.135 (± 0.003). The correlation coefficients for all of these plots were in the range of 0.9971–0.9998 (n = 4–5).
For each probe candidate, the intercept of the best-fit line was consistent with the value predicted for the high affinity site by using the data in Table 2 and Eq. (5). This result indicated that competition between warfarin and the probe candidates at their high affinity sites was the dominant interaction being observed in the linear regions of these plots. In addition, the difference in the actual intercept and the best-fit intercept from the linear region for plots with negative deviations was consistent with the level of retention predicted from Table 2 for the weak affinity regions of these probe candidates. The relative size of the contributions of the weak sites to retention in the absence of any warfarin (i.e., at the y-intercept in Figure 6) was estimated from the data in Table 2 to be about 5% for 4-hydroxycoumarin, 23% for 7-hydroxycoumarin and 19% for 7-hydroxy-4-methylcoumarin. The relative contribution of the weak sites to retention was 43% for coumarin, which again suggested that this candidate would have limited usefulness as a site-specific probe for HSA.
The behavior observed for coumarin, 7-hydroxycoumarin and 7-hydroxy-4-methylcourmin for plots like those in Figure 6 is consistent with a model in which direct competition is occurring between warfarin and these probe candidates at both their high and weak affinity sites. Competition at the high affinity sites was noted to dominate at moderate-to-high concentrations of warfarin (creating the linear response seen in this region), while both the weak and high affinity sites had significant contributions to retention at lower warfarin concentrations. Similar behavior has been noted previously for other solute systems with competition at two groups of sites on HSA [32]. The results for 4-hydroxycoumarin are consistent with a slightly different model. For this probe candidate it appears that 4-hydroxycoumarin and warfarin were again competing for the high affinity site of this probe at moderate-to-low concentrations of warfarin. However, the positive deviations seen when only small concentrations of warfarin were present suggest that at least some of the weak affinity sites for 4-hydroxycoumarin were showing little or no binding for warfarin under these conditions.
The ratio of the slope and intercepts for the best-fit lines in these plots were used along with Eq. (5) to estimate the value of warfarin’s association equilibrium constant at its site of competition with each probe candidate. It was found that warfarin had an association equilibrium constant of 2.6 (± 0.1) × 105 M−1 as it underwent binding at the high affinity site for 4-hydroxycoumarin. This value is statistically identical to the average association equilibrium constant of 2.5 × 105 M−1 that has been reported for R- and S-warfarin at Sudlow site I of HSA, thus confirming that the high affinity site of 4-hydroxycoumarin was the same as this binding region [44,45]. Thus, it appeared from this result that 4-hydroxycoumarin could indeed be used as a replacement for warfarin as a probe for examining the binding of other solutes at Sudlow site I.
The association equilibrium constants determined for racemic warfarin at its site of competition with the other probe candidates were about an order of magnitude lower than the full value for warfarin at Sudlow site 1. These calculated values were as follows: competition with coumarin, 3.4 (± 0.1) × 104 M−1; competition with 7-hydroxycoumarin, 2.23 (± 0.05) × 104 M−1; and competition with 7-hydroxy-4-methylcoumarin, 3.3 (± 0.2) × 104 M−1. Because warfarin has only one major binding site on HSA, these results indicate that these probe candidates are binding to and competing with warfarin at only part of this site. A similar effect has been noted in the competition of octanoic acid with various drugs for binding to HSA [36]. This scenario is consistent with the fact that each of these three probe candidates contains only part of the structure of warfarin (i.e., as is the case for coumarin) or contain additional groups that are not present in warfarin (e.g., the 7-hydroxyl group in 7-hydroxycoumarin and 7-hydroxy-4-methylcoumarin). This would also explain why the association equilibrium constant calculated for warfarin during its competition with 4-hydroxycoumarin was essentially the same as the full value reported for warfarin at Sudlow site I because this particular probe candidate has the closest structure to that of warfarin and the best chance for fully competing with warfarin at Sudlow site I.
4.4 Effects of Coumarin Structure on Binding to Sudlow Site I
Although the main goal of this study was to identify alternatives to warfarin as probes for Sudlow site I, the results that were obtained in the frontal analysis and zonal elution studies do provide some information on the nature of the binding of warfarin and related compounds to HSA. It is known that many solutes like warfarin that bind at Sudlow site I are bulky heterocyclic compounds that also contain anionic groups near a central location of the molecule [51,52]. This general model was confirmed in this current report by the fact that the probe with the greatest similarity to warfarin in its affinity for Sudlow site I was 4-hydroxycoumarin, a compound which contains the same type of heterocyclic ring and anion-forming, acidic hydroxyl group that appears in warfarin. Removal of the hydroxyl group from this structure (leaving only the coumarin backbone) produced a decrease in Ka of 8.5-fold, as noted in Table 2 when comparing the results at Site 1 for 4-hydroxycoumarin and coumarin.
It is also clear from the data in Table 2 that the structure shared by 4-hydroxycoumarin and warfarin is only partly responsible for the high affinity of warfarin at Sudlow site I. This result is demonstrated by the 4.5-fold difference in affinity at this site that was measured for 4-hydroxycoumarin versus the average Ka value of 2.5 × 105 M−1 that has been reported for warfarin enantiomers under equivalent conditions [44,45]. This comparison indicates that the 3-(α-acetonylbenzyl) group on warfarin (see lower left portion of the warfarin structure in Figure 1) plays a significant role in contributing to the high affinity of this drug at Sudlow site I. This conclusion is consistent with recent crystal structures which show that the coumarin and 3-(α-acetonylbenzyl) groups of warfarin occupy separate sub-chambers as this drug binds to Sudlow site I (see discussion in Ref. [12]).
The positions of the hydroxyl group and other side chains about the coumarin ring were also found to affect the affinity of the tested probe compounds for HSA. Table 2 indicates that moving the hydroxyl group from the 4- to 7-position created a 6.7-fold lower affinity for 7-hydroxycoumarin versus 4-hydroxycoumarin as these compounds were bound by Sudlow site I. Placing a methyl group in the 4-position regained some of this affinity, as shown in Table 2 by the 2.7-fold increase in the association equilibrium constant at Site 1 when going from 7-hydroxycoumarin to 7-hydroxy-4-methylcoumarin.
5. CONCLUSIONS
This study examined the binding of four coumarin compounds to HSA using HPAC. It was determined by frontal analysis that all of the probe candidates had interactions with HSA that followed a two-site model, including a high affinity site and a second group of weak, non-specific binding regions. It was found in zonal elution competition studies that all of these probe candidates gave direct competition with warfarin at their high affinity sites, as well as either direct competition or no competition at their weak affinity sites (the latter behavior been noted in the case of 4-hydroxycoumarin). The results of this study not only allowed new probes for HSA to be identified, but also provided information on how the coumarin ring, hydroxyl group and 3-(α-acetonylbenzyl) group that are part of warfarin each contribute to the binding of this drug at Sudlow site I.
Of the various probe candidates that were examined, 4-hydroxycoumarin was found to be the best alternative for warfarin in its binding to Sudlow site I of HSA. Some advantages of using 4-hydroxycoumrin for this purpose include its good long term stability in a pH 7.4 phosphate buffer and its ability to be obtained in an inexpensive and single form for binding studies. 4-Hydroxycoumarin also has slightly weaker binding than warfarin to HSA, which would avoid the need for long elution times when working with such an agent in HPAC.
The other tested probes had several limitations. Coumarin had high non-specific binding to silica supports and a relatively large contribution by its weak affinity sites on HSA to its overall binding to this protein. In addition, coumarin, 7-hydroxycoumarin and 7-hydroxy-4-methylcoumarin all appeared to compete with warfarin for only part of Sudlow site I. 7-Hydroxycoumarin and 7-hydroxy-4-methylcoumarin did have small non-specific interactions with the support and with HSA, which may make them useful in some situations as probes for drug binding studies. However, binding capacity measurements did suggest that 7-hydroxycoumarin may have more than one high affinity site on HSA. Thus, 4-hydroxycoumarin was found to be the best overall alternative to warfarin as a probe for Sudlow site I of HSA.
Acknowledgments
This research was supported by the National Institutes of Health under grant R01 GM044931 and was conducted in facilities that were renovated under NIH grant RR015468-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Colmenarejo G. Med Res Rev. 2003;23:275. doi: 10.1002/med.10039. [DOI] [PubMed] [Google Scholar]
- 2.Finlay GJ, Baguley BC. Cancer Chemother Pharmacol. 2000;45:417. doi: 10.1007/s002800051011. [DOI] [PubMed] [Google Scholar]
- 3.Berezhkovskiy LM. J Pharmacokin Pharmacodyn. 2006;33:595. doi: 10.1007/s10928-006-9024-2. [DOI] [PubMed] [Google Scholar]
- 4.Otagiri M. Drug Metab Pharmacokinet. 2005;20:309. doi: 10.2133/dmpk.20.309. [DOI] [PubMed] [Google Scholar]
- 5.Liu Z, Li F, Huang Y. Biomed Chromat. 1999;13:262. doi: 10.1002/(SICI)1099-0801(199906)13:4<262::AID-BMC832>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 6.Wan H, Bergstrom F. J Liq Chrom Rel Technol. 2007;30:681. [Google Scholar]
- 7.Fasano M, Curry S, Terreno E, Galliano M, Fanali G, Narciso P, Notari S, Ascenzi P. IUBMB Life. 2005;57:787. doi: 10.1080/15216540500404093. [DOI] [PubMed] [Google Scholar]
- 8.Ascenzi P, Bocedi A, Notari S, Fanali G, Fesce R, Fasano M. Mini-Rev Med Chem. 2006;6:483. doi: 10.2174/138955706776361448. [DOI] [PubMed] [Google Scholar]
- 9.Bocedi A, Notaril S, Narciso P, Bolli A, Fasano M, Ascenzi P. IUBMB Life. 2004;56:609. doi: 10.1080/15216540400016286. [DOI] [PubMed] [Google Scholar]
- 10.Ghuman J, Zunszain PA, Petitpas I, Bhattacharya AA, Otagiri M, Curry S. J Mol Biol. 2005;353:38. doi: 10.1016/j.jmb.2005.07.075. [DOI] [PubMed] [Google Scholar]
- 11.Ascoll GA, Domenici E, Bertucci C. Chirality. 2006;18:667. doi: 10.1002/chir.20301. [DOI] [PubMed] [Google Scholar]
- 12.Petitpas I, Bhattacharya AA, Twine S, East M, Curry S. J Biol Chem. 2001;276:22804. doi: 10.1074/jbc.M100575200. [DOI] [PubMed] [Google Scholar]
- 13.Heinze A, Holzgrabe U. Int J Pharm. 2006;311:108. doi: 10.1016/j.ijpharm.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 14.Matsushita Y, Moriguchi I. Chem Pharm Bull. 1985;33:2948. doi: 10.1248/cpb.33.2948. [DOI] [PubMed] [Google Scholar]
- 15.Aarons LJ, Schary WL, Rowland M. J Pharm Pharmacol. 1979;31:322. doi: 10.1111/j.2042-7158.1979.tb13509.x. [DOI] [PubMed] [Google Scholar]
- 16.Bowmer CJ, Lindup WE. J Pharmacol Exp Ther. 1979;210:440. [PubMed] [Google Scholar]
- 17.Coulson CJ, Smith VJ. Methodological Surveys. 1981;10:210. [Google Scholar]
- 18.Seedher N, Agarwal P. Ind J Pharm Sci. 2006;68:327. doi: 10.4103/0250-474X.51958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie M, Long M, Liu Y, Qin C, Wang Y. Biochim Biophys Acta. 2006;1760:1184. doi: 10.1016/j.bbagen.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 20.Russeva V, Rakovska R, Stavreva N, Mihailova D, Berova N. Pharmazie. 1994;49:519. [PubMed] [Google Scholar]
- 21.Detrich HWI, Williams RCJ, Macdonald TL, Wilson L, Puett D. Biochemistry. 1981;20:5999. doi: 10.1021/bi00524a012. [DOI] [PubMed] [Google Scholar]
- 22.Sjoholm I, Sjodin T. Biochem Pharmacol. 1972;21:3041. doi: 10.1016/0006-2952(72)90196-7. [DOI] [PubMed] [Google Scholar]
- 23.Chignell CF. Mol Pharmacol. 1970;6:1. [PubMed] [Google Scholar]
- 24.Shiwang L, Zhang L, Zhang X. Anal Sci. 2006;22:1515. doi: 10.2116/analsci.22.1515. [DOI] [PubMed] [Google Scholar]
- 25.Zhongjiang J. Curr Pharm Anal. 2005;1:41. [Google Scholar]
- 26.Xiaocui Z, You T, Liu J, Sun X, Yan J, Yang X, Wang E. Electrophoresis. 2004;25:3422. doi: 10.1002/elps.200305930. [DOI] [PubMed] [Google Scholar]
- 27.Thormann W, Wey AB, Lurie IS, Gerber H, Byland C, Malik N, Hochmeister M, Gehrig C. Electrophoresis. 1999;20:3203. doi: 10.1002/(SICI)1522-2683(19991001)20:15/16<3203::AID-ELPS3203>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 28.Day YSN, Myszka DG. J Pharm Sci. 2003;92:333. doi: 10.1002/jps.10293. [DOI] [PubMed] [Google Scholar]
- 29.Frostell-Karlsson A, Remaeus A, Roos H, Andersson K, Borg P, Haemaelaeinen M, Karlsson R. J Med Chem. 2000;43:1986. doi: 10.1021/jm991174y. [DOI] [PubMed] [Google Scholar]
- 30.Sulkowska A, Bojko B, Rownicka J, Rezner P, Sulkowski WW. J Mol Struct. 2005:744. [Google Scholar]
- 31.Li C, Liu M. Am Biotech Lab. 2000;18:36. [Google Scholar]
- 32.Hage DS. J Chromatogr B. 2002;768:3. doi: 10.1016/s0378-4347(01)00482-0. [DOI] [PubMed] [Google Scholar]
- 33.Patel S, Wainer IW, Lough WJ. In: Handbook of Affinity Chromatography. 2. Hage DS, editor. CRC Press/Taylor & Francis; Boca Raton: 2006. p. 663. [Google Scholar]
- 34.Noctor TAG, Diaz-Perez MJ, Wainer IW. J Pharm Sci. 1993;82:675. doi: 10.1002/jps.2600820629. [DOI] [PubMed] [Google Scholar]
- 35.Domenici E, Bertucci C, Salvadori P, Felix G, Cahagne I, Motellier S, Wainer IW. Chromatographia. 1990;29:170. [Google Scholar]
- 36.Winzor DJ. J Chromatogr A. 2004;1037:351. doi: 10.1016/j.chroma.2003.11.092. [DOI] [PubMed] [Google Scholar]
- 37.Kim HS, Hage DS. J Chromatogr B. 2005;816:57. doi: 10.1016/j.jchromb.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 38.Schiel JE, Mallik R, Soman S, Joseph KS, Hage DS. J Sep Sci. 2006;29:719. doi: 10.1002/jssc.200500501. [DOI] [PubMed] [Google Scholar]
- 39.Patel S, Wainer IW, Lough WJ. In: Handbook of Affinity Chromatography. 2. Hage DS, editor. CRC Press/Taylor & Francis; Boca Raton: 2006. p. 571. [Google Scholar]
- 40.Garten S, Wosilait W. Comp Gen Pharmac. 1972;3:83. [Google Scholar]
- 41.Sudlow G, Birkett DJ, Wade DN. Mol Pharmacol. 1975;11:824. [PubMed] [Google Scholar]
- 42.Sudlow G, Birkett DJ, Wade DN. Mol Pharmacol. 1976;12:1052. [PubMed] [Google Scholar]
- 43.Sengupta A, Hage DS. Anal Chem. 1999;71:3821. doi: 10.1021/ac9903499. [DOI] [PubMed] [Google Scholar]
- 44.Moser AC, Kingsbury C, Hage DS. J Pharm Biomed Anal. 2006;41:1101. doi: 10.1016/j.jpba.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 45.Loun B, Hage DS. Anal Chem. 1994;66:3814. doi: 10.1021/ac00093a043. [DOI] [PubMed] [Google Scholar]
- 46.Tweed SA, Loun B, Hage DS. Anal Chem. 1997;69:4790. doi: 10.1021/ac970565m. [DOI] [PubMed] [Google Scholar]
- 47.Ruhn PF, Garver S, Hage DS. J Chromatogr A. 1994;669:9. doi: 10.1016/0021-9673(94)80332-3. [DOI] [PubMed] [Google Scholar]
- 48.Ruhn PF, Garver S, Hage DS. J Chromatogr A. 1994;669:9. doi: 10.1016/0021-9673(94)80332-3. [DOI] [PubMed] [Google Scholar]
- 49.Loun B, Hage DS. J Chromatogr A. 1992;579:225. [PubMed] [Google Scholar]
- 50.Giannini DD, Chan KK, Roberts JD. Proc Natl Acad Sci USA. 1974;71:4221. doi: 10.1073/pnas.71.10.4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dockal M, Chang M, Carter DC, Ruker F. Protein Sci. 2000;9:1455. doi: 10.1110/ps.9.8.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peters T., Jr . All About Albumin: Biochemistry, Genetics and Medical Applications. Academic Press; New York: 1996. [Google Scholar]