two-photon microscopy is an advanced confocal laser-scanning fluorescence imaging technique that has been used in renal research for about a decade as a very powerful tool for the deep optical sectioning of the living kidney tissue (4, 6, 8, 9). Two-photon applications have changed a number of existing paradigms in renal (patho)physiology as summarized in a current review (10).
Since two-photon imaging allows the direct visualization of the glomerular filtration barrier (GFB) with submicron resolution in the intact kidney in vivo, it was used recently for the reevaluation of albumin's glomerular sieving coefficient (GSC) (12). In this interesting work, Russo et al. (12) observed mass glomerular filtration of fluorophore-conjugated albumin in normal kidneys (GSC in the 0.02–0.04 range) and its rapid endocytosis in the proximal tubule. Based on these two-photon studies, the authors (12) concluded that the GFB normally leaks albumin at nephrotic levels and that this filtered albumin load is avidly bound and retrieved by cells of the proximal tubule. In the current issue of JASN, these same authors (13) report further two-photon evidence for the tubular origin of diabetic albuminuria. Since the value of albumin GSC measured by two-photon microscopy in these studies is 50 times greater than previously measured by micropuncture (17) or calculated (7) (GSC in the 0.0006 range), the two-photon studies generated heated debate in top renal journals (1, 2, 5, 11) and at the American Society of Nephrology's 2008 Annual Meeting. One of the recurring criticisms was the low signal-to-noise fluorescence and other technical aspects of two-photon microscopy. It became clear that further work is needed in this area with better controlled two-photon experiments which would confirm or refute the high albumin GSC values. It would be even better if this came from an independent laboratory.
In the American Journal of Physiology-Renal Physiology, George Tanner (15) reports his own two-photon studies on the albumin GSC in the rat. It is important to note that although he used the same technology and imaging facility at the Indiana Center for Biological Microscopy as Russo et al. (12, 13), the brands of microscopes were different. In his independent work, Tanner (15) found that the albumin GSC was in the 0.002–0.004 range, much lower than previously reported (12, 13). He identified a number of factors that were likely responsible for the higher GSC values found by Russo et al. Several of these factors were unrelated to imaging and concerned the poor animal conditions such as hypothermia, dehydration, low blood pressure, and low glomerular filtration rate (GFR) (15). In fact, none of the Russo et al. papers measured or reported blood pressure data (12, 13) even if the perfusion pressure is the most important one of the Starling forces for filtration in the glomerular capillaries. Of the imaging related factors Tanner (15) identified the high laser outputs (high levels of illumination) and the use of external, instead of internal, photodetectors as possible further culprits which he believes resulted in the collection of significant out-of-focus fluorescence (noise and not albumin) in the Bowman's space.
The principle of two-photon microscopy is that the simultaneous absorption of two photons of equally low energy (long wavelength) can cause excitation of a fluorophore equivalent to the absorption of a single photon of double the energy (half the wavelength) (3). Two-photon images are confocal solely by excitation (excitation normally happens only in the focus) and there is no need to filter the emitted fluorescence with pinholes as with conventional confocal microscopy. For this reason, two-photon microscopy most often uses external, so-called nondescanned detectors which are very efficient, collecting close to 100% of the emitted fluorescence. In contrast, internal detection (i.e., within the scanhead) is less efficient since it uses several mirrors and the pinholes which absorb or exclude a significant portion of the emitted light. However, when imaging with two-photon in inhomogeneous, highly scattering tissues (unfortunately the kidney is a great example), the images may not be “pure” confocal when using the external detectors. This is due to the scattered exciting laser giving rise to fluorescence emission out-of-focus, usually within the first few microns in the sample (16). The out-of-focus fluorescence could be significant when imaging very superficial glomeruli in the Munich-Wistar rats (within 50 μm from the surface). Tanner (15) claims that for this reason he found better signal-to-noise ratio, lower fluorescence in the Bowman's capsule (translating to lower albumin GSC) when he used internal detectors with the classical confocal pinhole method. Of course the price one pays when using internal as opposed to external detectors is the much reduced fluorescence levels.
But there may be additional imaging-related reasons for the previously measured high GSC values by Russo et al. (12, 13), independent of the two-photon technology. Since the previous micropuncture-based albumin GSC is 0.0006 (17), performing fluorescence intensity measurements with 8-bit depth resolution (used by Russo et al. and most studies by Tanner as well) may not provide the necessary dynamic range. With 8-bit depth resolution the pixel intensities (gray scale) are in the 0–255 range, far less than what the predicted, more than 1,000-fold difference in fluorescence intensities in the two compartments would require. Most advanced imaging systems allow 12-bit depth imaging (0–4,095 gray intensity scale), which seems absolutely necessary for correct fluorescence-based GSC measurements. Consistent with the better dynamic range with 12-bit imaging, Tanner (15) found even lower albumin GSC values with an Olympus system (uses 12-bit) compared with a Zeiss system (uses 8-bit). Low average fluorescence intensities in the Bowman's capsule (values between 0 and 1) are hardly distinguishable from background under in vivo conditions. This can explain the high standard deviation in Tanner's work (15) when using 8-bit resolution (GSC was 0.004 ± 0.004) which suggests that the “real” albumin GSC is even lower.
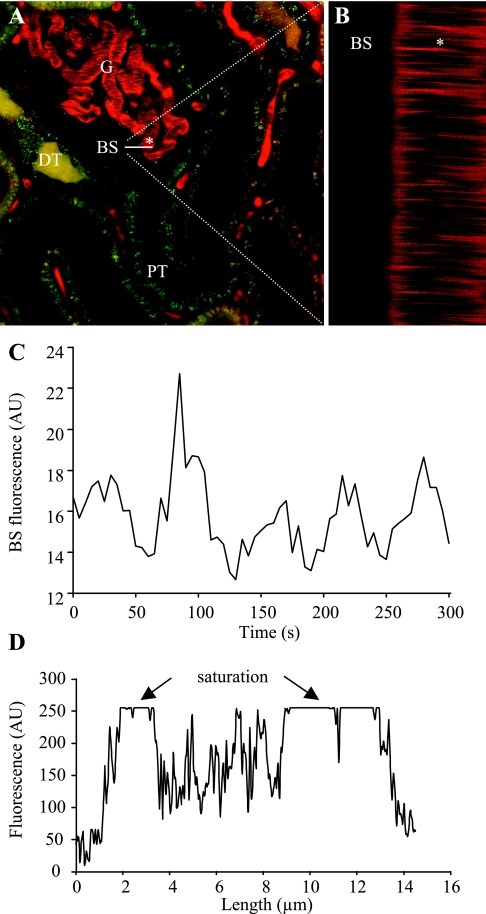
Since my laboratory at the University of Southern California routinely uses the two-photon technology for imaging the mouse and rat kidney in vivo, including function of the GFB (14), we decided to provide experimental support for the above issues. Figure 1 shows an experiment similar to those reported by Russo et al. (12, 13) and Tanner (15). A 70-kDa dextran-rhodamin B conjugate (slightly larger than albumin) was injected intravenously and fluorescence intensities in the Bowman's space and in the glomerular capillaries were detected using 8-bit depth resolution. Mean arterial blood pressure was measured during imaging as described before (6) and it was normal. The fluorescence intensity of dextran-rhodamine in the Bowman's space was low (in the 10–20 range) but distinguishable from background, and it showed regular oscillations (Fig. 1C), similar to what we reported earlier (6). These oscillations are normal and are due to two important renal physiological mechanisms that control GFR and renal hemodynamics: 1) tubuloglomerular feedback and 2) the myogenic mechanism (6). The oscillatory pattern of fluorescence actually provided assurance that we in fact measured the function of GFB. However, under the same imaging conditions and in the same glomerulus, the plasma fluorescence was saturated (Fig. 1D), meaning that the 70-kDa dextran GSC value was much lower than 0.04 (10/255). When we reduced detector sensitivity so the fluorescence intensity in the Bowman's space was around 1, a line scan of the capillary plasma (Fig. 1B) found still saturated plasma fluorescence (dextran GSC is lower than 1/255 = 0.004). The rationale for performing a line scan is that it is very fast (1,000 Hz) and it can separate the cell and plasma fractions of capillary blood much better compared with a single xy scan (6). The streaming unlabeled red blood cells can significantly scatter, absorb, and reduce dextran fluorescence in the capillaries during full-frame xy scanning, leading to the underestimation of “pure” plasma fluorescence (overestimation of albumin GSC). This was likely an additional factor in the Russo et al. studies (12, 13).
Fig. 1.
Two-photon imaging of glomerular permeability to macromolecules in the intact Munich-Wistar rat kidney in vivo. A: circulating plasma was labeled with 70-kDa dextran-rhodamine B (red), and proximal (PT) and distal (DT) renal tubules with quinacrine (green). G, glomerulus. B: line (xt) scan was performed at 1-ms intervals for 2 s along a horizontal line, ∼20 μm long as indicated on A. The left half of this line was positioned above the Bowman's space (BS) while the right half of it was over one glomerular capillary (indicated by *). Red blood cells appear as unstained dark objects (A) and leave dark bands on the line scan (B) as they move through the capillary (*). Intense plasma fluorescence appears between individual blood cells. C: recording of low levels of rhodamine B-fluorescence in the BS. Fluorescence intensity shows regular oscillations. D: linear profile of fluorescence intensities over the same glomerular capillary as selected in A, 2–14 μm indicate the outer diameter. At the edges, low-fluorescence signals are detected (capillary wall), while inside the capillary the plasma fluorescence is saturated (particularly in the relatively cell-free peripheral regions).
Even if imaging a perfectly maintained animal with 12-bit depth resolution, a number of physiological mechanisms cause normal variations in Bowman's space fluorescence, like the regular oscillations in single-nephron GFR shown in Fig. 1C and in our previous study (6). Also, the concentration of plasma (fluorescence) is higher at the efferent vs. afferent end of glomerular capillaries. Depending on the timing (Fig. 1C) and location of fluorescence GSC measurements, the results can be highly variable due to the many significant inherent errors.
In summary, two-photon microscopy is a powerful imaging tool that can detect very low levels of macromolecules (fluorescence) in the Bowman's space and at the brush-border membrane of the proximal tubule. The GSC of albumin is very low (lower than 0.004) even when fluorescence tools are used for its determination. Well-controlled systemic parameters and animal techniques (blood pressure monitoring, infusion) and adequate imaging methods (12-bit depth resolution, line scans) are absolutely required.
GRANTS
This work was supported by Grants DK-64324, DK-74754, and American Heart Association Established Investigator Award 0640056N.
REFERENCES
- 1.Christensen EI, Birn H, Rippe B, Maunsbach AB. Controversies in nephrology: renal albumin handling, facts, and artifacts. Kidney Int 72: 1192–1194, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Comper WD, Haraldsson B, Deen WM. Resolved: normal glomeruli filter nephrotic levels of albumin. J Am Soc Nephrol 19: 427–432, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Denk W, Strickler J, Webb WW. Two-photon laser scanning fluorescence microscopy. Science 248: 73–76, 1990. [DOI] [PubMed] [Google Scholar]
- 4.Dunn KW, Sandoval RM, Kelly KJ, Dagher PC, Tanner GA, Atkinson SJ, Bacallao RL, Molitoris BA. Functional studies of the kidney of living animals using multicolor two-photon microscopy. Am J Physiol Cell Physiol 283: C905–C916, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Gekle M Renal albumin handling: a look at the dark side of the filter. Kidney Int 71: 479–481, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Kang JJ, Toma I, Sipos A, McCulloch F, Peti-Peterdi J. Quantitative imaging of basic functions in renal (patho)physiology. Am J Physiol Renal Physiol 291: F495–F502, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Lazzara MJ, Deen WM. Model of albumin reabsorption in the proximal tubule. Am J Physiol Renal Physiol 292: F430–F439, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Peti-Peterdi J, Morishima S, Miyoshi T, Bell PD, Okada Y. Macula densa cell water transport assessed by two-photon laser scanning fluorescence microscopy. FASEB J 14: A134, 2000. [Google Scholar]
- 9.Peti-Peterdi J, Morishima S, Bell PD, Okada Y. Two-photon excitation fluorescence imaging of the living juxtaglomerular apparatus. Am J Physiol Renal Physiol 283: F197–F201, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Peti-Peterdi J, Toma I, Sipos A, Vargas SL. Multiphoton imaging of renal regulatory mechanisms. Physiology (Bethesda) 24: 88–96, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Remuzzi A, Sangalli F, Fassi A, Remuzzi G. Albumin concentration in the Bowman's capsule: multiphoton microscopy vs. micropuncture technique. Kidney Int 72: 1410–1411, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Russo LM, Sandoval RM, McKee M, Osicka TM, Collins AB, Brown D, Molitoris BA, Comper WD. The normal kidney filters nephrotic levels of albumin retrieved by proximal tubule cells: retrieval is disrupted in nephrotic states. Kidney Int 71: 504–513, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Russo LM, Sandoval RM, Campos SB, Molitoris BA, Comper WD, Brown D. Impaired tubular uptake explains albuminuria in early diabetic nephropathy. J Am Soc Nephrol 20: 489–494, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salmon AH, Toma I, Sipos A, Muston PR, Harper SJ, Bates DO, Neal CR, Peti-Peterdi J. Evidence for restriction of fluid and solute movement across the glomerular capillary wall by the subpodocyte space. Am J Physiol Renal Physiol 293: F1777–F1786, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Tanner GA Glomerular sieving coefficient of serum albumin in the rat: a two-photon microscopy study. Am J Physiol Renal Physiol 296: 1258–1265, 2009. [DOI] [PubMed] [Google Scholar]
- 16.Theer P, Denk W. On the fundamental imaging-depth limit in two-photon microscopy. J Opt Soc Am A Opt Image Sci Vis 23: 3139–3149, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Tojo A, Endou H. Intrarenal handling of proteins in rats using fractional micropuncture technique. Am J Physiol Renal Fluid Electrolyte Physiol 263: F601–F606, 1992. [DOI] [PubMed] [Google Scholar]