Abstract
The epithelial sodium channel (ENaC) is regulated by epidermal growth factor (EGF). We investigate whether ENaC is regulated by another EGF receptor (EGFR) ligand, transforming growth factor-α (TGF-α). We show that chronic (24 h) treatment with TGF-α inhibits ENaC in Xenopus laevis kidney cells 20 times more strongly than EGF. By using single-channel measurements, we show that TGF-α significantly reduces the number of ENaC per patch. The open probability (Po) is unchanged by 24-h treatment with TGF-α. α-, β-, and γ-ENaC mRNA levels are significantly reduced by TGF-α or EGF. TGF-α or EGF reduces α- and γ-ENaC proteins in the membrane; however, β-ENaC is unchanged. TGF-α or EGF inhibits ENaC by activating EGFR since the EGFR inhibitor AG1478 blocks the effects of both. The MAPK 1/2 inhibitor U0126 also blocks the effect of TGF-α or EGF on ENaC, indicating that the MAPK1/2 pathway is involved in the TGF-α- or EGF-induced inhibition of ENaC. Interestingly, acute treatment (<1 h) with TGF-α or EGF does not inhibit ENaC current; it enhances ENaC activity by increasing Po. Pretreatment of the cells with U0126 potentiates the acute TGF-α- or EGF-induced stimulation of ENaC. This TGF-α- or EGF-induced increase in sodium current is abolished by a phosphatidylinositol 3-kinase (PI-3 kinase) inhibitor, LY294002, suggesting that PI-3 kinase is involved in the activation of sodium transport. In conclusion, chronic treatment with TGF-α or EGF inhibits ENaC by decreasing the number of channels in the membrane transcriptionally through MAPK1/2 pathways, but acute treatment with TGF-α or EGF activates ENaC by increasing Po via PI-3 kinase.
Keywords: sodium transport, EGFR, kidney
the amiloride-sensitive epithelial sodium channel (ENaC) is expressed in a variety of epithelial tissues, including kidney, urinary bladder, lung, colon, and sweat and salivary glands. ENaC expressed in the renal collecting duct plays a major role in the regulation of Na+ and fluid reabsorption, thus, in the control of blood pressure. ENaC is a heterooligomer of three homologous subunits: α-, β-, and γ- subunits. α-ENaC alone is sufficient to induce channel activity; however, all three subunits are required for the efficient synthesis, assembly, and trafficking of the channel complex (2).
ENaC is regulated by a number of hormones, such as aldosterone, vasopressin, and insulin. ENaC is also regulated by epidermal growth factor (EGF). Sodium transport was shown to be inhibited by EGF in isolated and perfused rabbit cortical collecting duct (CCD) more than a decade ago (12, 13, 22, 23). Subsequently, several groups demonstrated that ENaC is regulated by EGF in renal tubular epithelial cells (10, 16, 24), a cystic fibrosis nasal epithelial cell line (3), and Chinese hamster ovary cells overexpressing ENaC subunits (21). However, these data are not consistent regarding whether EGF stimulates ENaC or inhibits ENaC, and which signal pathways are involved in its regulation. As shown in rabbit CCD by Vehaskari et al. (22), Tong et al. (21) reported that EGF quickly decreased the activity of ENaC expressed in Chinese hamster ovary cells, and this inhibition is due to decreased open probability (Po), which is associated with depleted membrane PtdIns(4,5)P2. Their studies show that MAPK 1/2 signaling is not involved in the acute action of EGF on ENaC. Shen and Cotton (16) also reported that 24-h treatment with EGF reduced amiloride-sensitive short-circuit current in a renal CCD cell line, but this inhibition was abolished by pretreatment of the cells with PD-98059 (a MAPKK, i.e., MEK, inhibitor). Their studies indicate that the MAPK 1/2 pathway is involved in the effects of EGF on sodium transport. Contrary to the inhibitory effects of EGF observed by the above groups, Markadieu et al. (10) demonstrated that EGF provoked a rise in sodium transport in a Xenopus laevis kidney cell line by stimulation of the phosphatidylinositol 3-kinase (PI-3 kinase) pathway, and this stimulation of sodium current by EGF is increased substantially by the inhibition of the MAPK pathway.
Even though it is still controversial exactly how EGF regulates ENaC, it is thought that EGF induces its effects by binding to the epidermal growth factor receptor (EGFR/ErbB-1/HER1). EGFR is a member of the EGF receptor family of receptor tyrosine kinases (RTKs). Besides ErbB-1/EGFR, this family also includes ErbB-2 (HER2/neu), ErbB-3 (HER3), and ErbB-4 (HER4). These receptors are membrane-bound glycoproteins that comprise an extracellular ligand-binding domain, a single transmembrane domain, and an intracellular domain with ligand-activated tyrosine kinase activity. Members of the EGFR family are activated by ligand binding, which results in dimerization of the receptors, autophosphorylation, and activation of downstream intracellular signaling via several pathways: 1) the MAPK pathway, 2) PI-3 kinase/Akt pathway, 3) phospholipase C (PLC) pathway, 4) Src kinase pathways, and 5) signal transducers and activators of transcription (STAT) pathway (15).
The significance of the effects of EGF on ENaC is indicated in polycystic kidney diseases (PKDs). PKDs are genetic disorders characterized by progressive formation of fluid-filled cysts in the kidneys, which ultimately leads to renal insufficiency and end-stage renal disease (4, 16). Cyst epithelial cells in PKD exhibit abnormalities in cell polarity. EGFR is normally expressed in the basolateral membrane in the renal epithelial cells; however, EGFR is found in both apical and basolateral membranes of cyst epithelial cells (16, 26). Veizis and Cotton (24) reported that addition of EGF to the apical solution led to inhibition of sodium transport in cystic cells; they suggested that abnormal EGF-dependent regulation of ENaC function may contribute to PKD pathophysiology.
Similar to EGF, transforming growth factor (TGF)-α is another natural ligand of EGFR. TGF-α mRNA is constitutively expressed in normal adult human kidney (5), and TGF-α protein is also detected in the urine from healthy human subjects (28). Since the effects of TGF-α on ENaC have never been investigated, we examined the regulation of ENaC by TGF-α as well as EGF in our studies.
MATERIALS AND METHODS
Cell culture.
Experiments were carried out with 2F3 cells (American Type Culture Collection, Manassas, VA), a subclone of A6 cells, between passages 96 and 105. Cells were cultured as previously described (8). Briefly, cells were maintained in DMEM/nutrient mix F-12 (Invitrogen, Carlsbad, CA) and NaHCO3, modified for amphibian cells with 90 mM NaCl, 25 mM NaHCO3, 3.1 mM KCl, 0.8 mM CaCl2, 0.4 mM Na2HPO4, 0.3 mM NaH2PO4, 0.2 mM MgCl2, 0.3 mM MgSO4, and other inorganic salts, amino acids, and vitamins. Growth medium was supplemented with 5% fetal bovine serum, 1.5 μM aldosterone, 1% penicillin, and 1% streptomycin. For patch-clamp experiments, cells from the flasks were plated on permeable, glutaraldehyde-fixed collagen films attached to the bottoms of small plastic rings. For transepithelial measurements and immunoblotting experiments, cells were plated on permeable inserts (Corning, Corning, NY). Cells were grown to confluence for all experiments. Since EGFR are located in the basolateral membrane in noncystic renal cells (24), TGF-α or EGF was applied basolaterally in all experiments. In all chronic experiments, 2F3 cells were treated with TGF-α or EGF for 24 h. In acute experiments, cells were treated for the periods of time indicated in the graphs.
Patch-clamp experiments.
Patch-clamp experiments were carried out at room temperature. The pipettes were pulled from filamented borosilicate glass capillaries (TW-150, World Precision Instruments, Sarasota, FL) with a two-stage vertical puller (Narishige, Tokyo, Japan) and fire polished (Narishige). Patch pipettes contained 96 mM NaCl, 3.4 mM KCl, 0.8 mM CaCl2, 0.8 mM MgCl2, and 10 mM HEPES, pH 7.4. The resistance of these pipettes was 5–10 MΩ when they were filled with pipette solution. Cell-attached configuration was used in our studies. After formation of a high-resistance seal (∼5 GΩ) between the pipette and cell membrane, channel currents were sampled at 2 kHz with a patch-clamp amplifier (Axopatch 200B, Axon Instruments, Foster City, CA) and filtered at 100 Hz with a low-pass filter. Data were recorded by a computer with Axotape software (Axon Instruments) for 5–10 min for each patch. The channel activity, NPo, was calculated with Clampfit 9.2 data-analysis software (Molecular Devices, Sunnyvale, CA). The total number of functional channels (N) in the patch was estimated by observing the number of current levels in data recordings. Po was calculated as the ratio of NPo to N. Since the maximal number of channels that Clampfit 9.2 can analyze is eight, patches with more than eight channels are not included in the analysis of channel NPo. “Empty” patches, i.e., patches with no apparent activity, are included in the calculation.
Transepithelial measurements.
The transepithelial voltage (Vte) and resistance (Rte) across cell monolayers were measured with an epithelial volt-ohmmeter (World Precision Instruments). The transepithelial current was calculated according to Ohm's law, Ite = Vte/Rte, and normalized by the surface area of the insert. Amiloride (10 μM) was added to the apical side of the inserts at the end of each experiment, and amiloride-sensitive sodium current was calculated as the difference between total current and amiloride-insensitive current.
Total RNA preparation.
Total RNA was extracted from 2F3 cells by using an RNeasy Midi Kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. Total RNA was quantified by an ultraviolet spectrophotometer (Thermo Electron, Waltham, WI) at a wavelength of 260 nm.
cDNA synthesis.
cDNA was synthesized from total RNA with SuperScript III reverse transcription kits (Invitrogen). Five micrograms of total RNA, 1 μl of 50 μM of oligo(dT), 1 μl of 10 mM dNTP mix, and distilled H2O in a total of 10 μl were mixed and heated at 65°C for 5 min. Then, 2 μl of 10× reverse transcript buffer, 4 μl of 25 mM MgCl2, 2 μl of 0.1 M DTT, 1 μl of RNaseOUT, and 1 μl of SuperScript III reverse transcriptase were added and heated at 50°C for 60 min. Reactions were terminated at 85°C for 5 min. Total RNA was then digested by RNase H.
Real-time RT-PCR.
Real-time PCR was performed using iCycler (Bio-Rad, Hercules, CA) to quantitate the mRNA levels of α-, β-, and γ-ENaC subunits, and GAPDH was used as the endogenous RNA control. The PCR mixture contained 12.5 μl of SYBR Green Supermix (Bio-Rad), 1 μl of each primer, 5 μl of cDNA, and 5.5 μl of distilled H2O. The reaction mixtures were incubated at 95°C for 3 min, followed by 40 cycles of 95°C for 30 s, 55°C for 30 s, and 72°C for 30 s, except that 30 s at 60°C for the annealing step was used for β-ENaC. The threshold cycle is defined as the cycle number at which the fluorescence passes the calculated threshold.
Cell surface biotinylation.
2F3 cells were biotinylated as previously described (9). In brief, cells were washed twice with PBS before 0.5 mg/ml of biotin (Pierce, Rockford, IL) in borate buffer (85 mM NaCl, 4 mM KCl, 15 mM Na2B4O7, pH 7.4) was added to the apical side of the inserts. Cells were incubated with biotin solution in a cold room for 20 min; this procedure was repeated once. The biotin labeling was stopped by washing the cells twice with regular medium containing 10% serum for 15 min. Cells were then washed with PBS and lysed with RIPA buffer (PBS with 0.1% SDS, 1% Nonidet P-40, 6 mM sodium deoxycholate) containing protease inhibitors (5 μM antipain, 5 μM leupeptin, 1 mM phenylmethylsulfonyl fluoride, 100 μM 1-chloro-3-tosylamido-7-amino-2-heptanone, and 100 μM l-1-tosylamido-2-phenylethyl chloromethyl ketone, and 2 mM EGTA). Biotin-labeled proteins were isolated by incubating the cell lysate with avidin cross-linked to agarose beads (Pierce) overnight in a cold room. The beads were then washed five times with RIPA buffer, and biotinylated proteins were eluted by boiling the beads in a buffer containing 100 mM dithiothreitol and 5% SDS.
Immunoblotting.
Cell lysate or biotinylated proteins were loaded onto 7.5% SDS-PAGE and transferred to nitrocellulose membranes. Membranes were blocked in TBS buffer containing 0.1% Tween 20 and 5% nonfat milk, then probed with anti-Xenopus α-, β-, or γ-ENaC antibodies (9) overnight, and subsequently, incubated with an alkaline phophatase-conjugated secondary antibody for 1 h. Signals were detected using CDP-Star substrate (Tropix, Bedford, MA) with Kodak Image Station 200MM and Kodak 1D software (Kodak, Rochester, NY). Broad range prestained standards (Bio-Rad) were used in most of our immunoblotting experiments. Standards were loaded in different wells on the same gel as cell lysates and were exposed and captured at different settings from the experimental lanes. The different photos of the same blot were superimposed using Kodak software. PrecisionPlus protein standards (Bio-Rad) and a biotinylated protein ladder (Cell Signaling) were used in some of our later experiments for comparison. In general, PrecisionPlus and a the biotinylated protein ladder are consistent with each other; they run close to the broad-range standards at low molecular mass (∼40 kDa). However, they run 10–15 kDa lower than the broad-range standards at 60–100 kDa.
Statistical analysis.
Data are reported as means ± SE. Statistical analysis was performed using Sigmaplot software (Jandel Scientific, Chicago, IL). ANOVA was used to compare different groups, and a Student-Newman-Keuls posttest was used when appropriate. P < 0.05 was considered significant.
RESULTS
Chronic treatment with TGF-α or EGF inhibits sodium transport in 2F3 cells.
We first tested the chronic effect of TGF-α and EGF on sodium transport. Cells were treated basolaterally with TGF-α, EGF, or vehicle control for 24 h. Figure 1 shows the dose-response curves of TGFα- and EGF-induced inhibitions of amiloride-sensitive sodium current. Both TGF-α and EGF decrease sodium transport in a dose-dependent manner. The IC50 is 0.85 nM (4.7 ng/ml) for TGF-α-induced inhibition and 18 nM (110 ng/ml) for EGF-induced inhibition.
Fig. 1.
Dose-response curves for transforming growth factor (TGF)-α- and EGF-induced inhibition of amiloride-sensitive transepithelial current (Ite). 2F3 cells were treated with the indicated concentrations of TGF-α or EGF for 24 h. The values are expressed as the percentage of control amiloride-sensitive Ite in cells not treated with TGF-α and EGF.
Chronic treatment with TGF-α inhibits sodium transport by decreasing the total number of channels in the membrane but does not change ENaC Po.
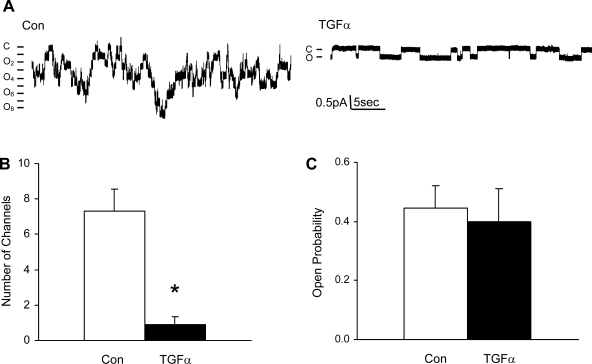
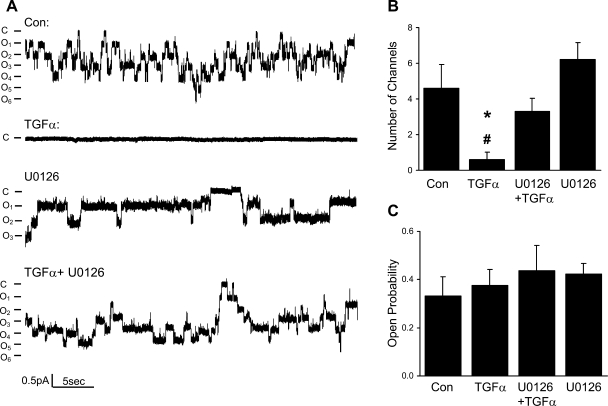
Since the activity of channels is determined by the total number of channels, Po, and unitary current (i), we performed single-channel studies to determine how TGF-α inhibits ENaC activity. Typical cell-attached single-channel recordings from control and TGF-α (20 ng/ml)-treated cells are shown in Fig. 2A; the number of current levels was reduced in TGF-α-treated cells. Figure 2B summarizes the number of active ENaC observed in each patch in control and TGF-α-treated cells. Po of ENaC was not changed by TGF-α, as shown in Fig. 2C. The ENaC unitary current also was unchanged by TGF-α.
Fig. 2.
Chronic treatment with TGF-α (20 ng/ml) or EGF (100 ng/ml) reduces the number of epithelial Na channels (ENaC) per patch, but not the open probability (Po) of the channels. A: current traces from cell-attached patches from control and TGF-α (20 ng/ml)-treated cells. The patches were held at +40 mV, with inward current downward. Closed and open states are denoted by C and O, respectively. B: summary of number of channels per patch in control and TGF-α groups. *P < 0.05 vs. Con. C: summary of ENaC Po in control and TGF-α groups.
TGF-α and EGF reduce protein levels of α- and γ-ENaC subunits in the apical membrane, but the level of β-ENaC is not changed.
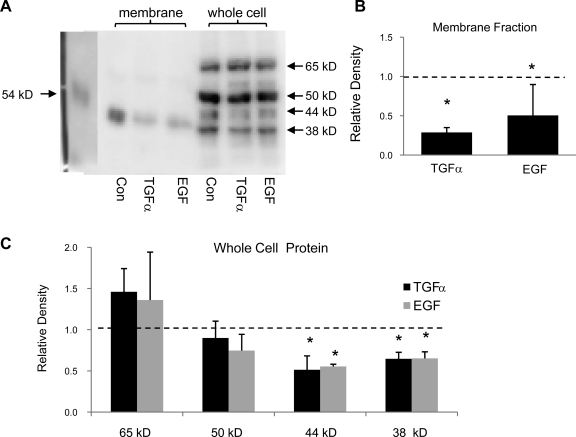
We biotinylated the apical membrane of 2F3 cells and then immunoblotted the isolated biotinylated proteins with ENaC antibodies. Under these conditions, our α-ENaC antibody detects one membrane protein at 40–50 kDa. This molecular mass is substantially less than that expected for full-length α-ENaC, but this is the only band we detected and it competed with antigenic peptide preincubation. It is likely to be a cleaved form of α-ENaC (6). Chronic treatment with either TGF-α or EGF reduced α-ENaC in the apical membrane (Fig. 3). Our α-ENaC antibody consistently detects proteins at four different molecular weights in whole cell lysates (shown in Fig. 3A). This is likely due to the cleavage and glycosylation of α-ENaC. Two of the four bands were reduced in intensity by chronic treatment with TGF-α or EGF.
Fig. 3.
Chronic treatment with TGF-α (20 ng/ml) or EGF (100 ng/ml) reduces α ENaC subunit protein in the apical membrane in 2F3 cells. A: representative immunoblot of α-ENaC protein in the apical membrane and in whole cell lysates. B: summary graph showing the change in band densities of α-ENaC in the apical membrane after treatment with TGF-α and EGF. Dashed line represents control level, which is set at 100%. C: summary graph showing the change in band densities of α-ENaC on the whole cell level after treatment with TGF-α and EGF. Dashed line represents control level, which is set at 100%. *P < 0.05 vs. Con.
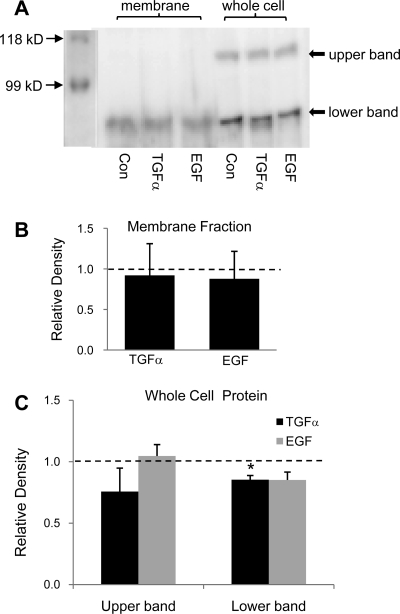
Our β-ENaC antibody detects one band in the apical membrane which is unchanged by the treatment with TGF-α or EGF (Fig. 4). Two bands were detected in whole cell lysates; the top band is likely to be the glycosylated β-ENaC. Only the bottom band is reduced by the treatment with TGF-α.
Fig. 4.
Chronic treatment with TGF-α (20 ng/ml) or EGF (100 ng/ml) does not change the level of β-ENaC in the apical membrane in 2F3 cells. A: representative immunoblot of β-ENaC protein in the apical membrane and in whole cell lysates. B: summary graph showing the change in band densities of β-ENaC in the apical membrane after treatment with TGF-α and EGF. Dashed line represents control level, which is set at 100%. C: summary graph showing the change in band densities of β-ENaC in whole cell lysates after treatment with TGF-α and EGF. Dashed line represents control level, which is set at 100%. *P < 0.05 vs. Con.
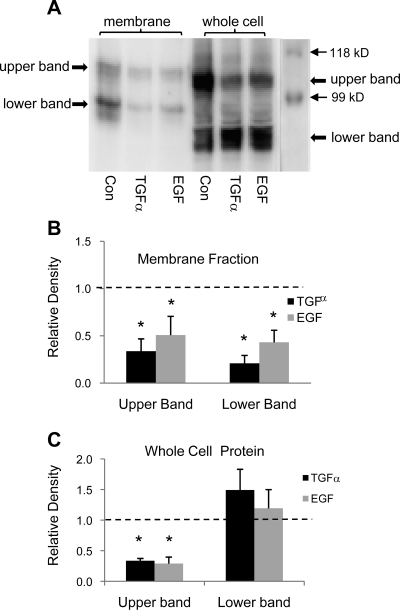
Our γ-ENaC antibody detects two membrane proteins at ∼90 and ∼70 kDa, which are likely to be the full-length and cleaved γ-ENaC, respectively (6). As illustrated in Fig. 5, chronic treatment with either TGF-α or EGF reduced γ-ENaC (both bands) in the apical membrane. Two whole cell proteins are consistently detected by our γ-ENaC antibody; only the top band is reduced by the chronic treatment with TGF-α or EGF.
Fig. 5.
Chronic treatment with TGF-α (20 ng/ml) or EGF (100 ng/ml) reduces γ-ENaC subunit protein in the apical membrane in 2F3 cells. A: representative immunoblot of γ-ENaC protein in the apical membrane and in whole cell lysates. B: summary graph showing the change in band densities of γ-ENaC in the apical membrane after treatment with TGF-α and EGF. Dashed line represents control level, which is set at 100%. C: summary graph showing the change in band densities of γ-ENaC on the whole cell level after treatment with TGF-α and EGF. Dashed line represents control level, which is set at 100%. *P < 0.05 vs. Con.
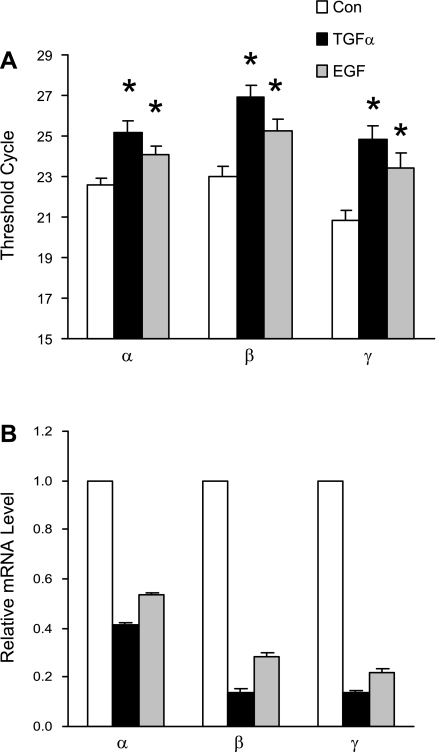
TGF-α and EGF reduce the steady-state mRNA levels of all ENaC subunits.
Since TGF-α reduces the number of active ENaC channels in the plasma membrane, we wondered whether this regulation is on a transcriptional level. We used real-time RT-PCR to quantify mRNA levels of all three ENaC subunits and GAPDH. As shown in Fig. 6, chronic treatment with TGF-α (20 ng/ml) for 24 h reduced mRNA levels of α-, β-, and γ-ENaC to 21, 8, and 7% of control, respectively, and treatment with EGF (100 ng/ml) for 24 h reduced mRNA levels of α-, β-, and γ-ENaC to 41, 23, and 18% of control, respectively.
Fig. 6.
Chronic treatment with TGF-α (20 ng/ml) or EGF (100 ng/ml) reduces the transcription of ENaC subunits. A: quantitative real-time RT-PCR of ENaC subunit mRNAs, normalized to GAPDH. *P < 0.05 vs. Con. B: steady-state mRNA levels of ENaC subunits normalized to control.
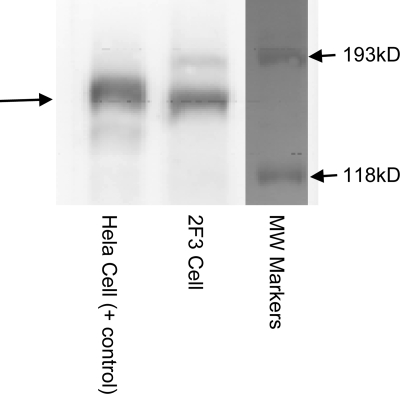
TGF-α and EGF inhibit ENaC by activating EGFR, and ErbB2 is not involved.
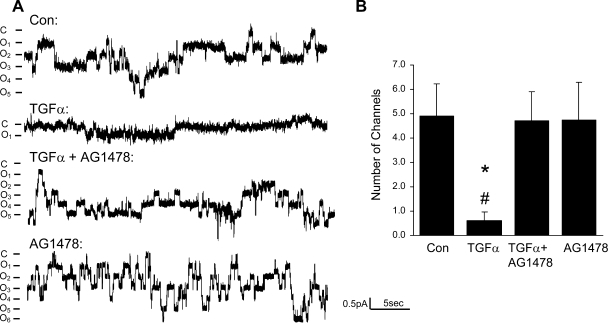
Since TGF-α and EGF are ligands of EGFR, we tested the hypothesis that TGF-α and EGF regulate ENaC by activating EGFR. The EGFR protein was detected in 2F3 cells by immunoblotting, as shown in Fig. 7. Functionally, our patch-clamp data (Fig. 8) and transepithelial measurements (Fig. 9) both demonstrated that the effect of TGF-α or EGF on ENaC was totally blocked by pretreatment of the cells with an EGFR inhibitor, AG1478 (5 μM). Figure 9 also shows that with AG1478, sodium current is higher than that in control condition, which suggests that there may be endogenous EGFR ligands produced by 2F3 cells. We also tested whether ErbB2 is involved in the inhibition of ENaC by TGF-α or EGF since ErbB2 can form heterodimers with EGFR. AG825, an ErbB2 inhibitor, at concentrations up to 10 μM did not block the TGF-α- or EGF-induced inhibition of ENaC (data not shown).
Fig. 7.
EGFR is expressed in 2F3 cells. Western blot showing that EGFR is expressed in 2F3 cells.
Fig. 8.
Chronic effect of TGF-α is blocked by the EGFR inhibitor AG1478. A: current traces from cell-attached patches from untreated cells and cells treated with TGF-α (20 ng/ml), TGF-α+AG1478 (5 μM), and AG1478 alone. The patches were held at +40 mV, with inward current downward. Closed and open states are denoted by C and O, respectively. B. summary of number of ENaC per patch in 4 conditions. *P < 0.05 vs. Con. #P < 0.05 vs. TGF-α+AG1478.
Fig. 9.
Chronic effect of TGF-α or EGF is blocked by EGFR inhibitor AG1478. Transepithelial current was measured in 2F3 cells. Cells were pretreated with vehicle control (DMSO) or AG1478 (5 μM in DMSO) both apically and basolaterally for 30 min. The basolateral solution was then replaced with either control (PBS), TGF-α (20 ng/ml in PBS), or EGF (100 ng/ml in PBS) with or without AG1478 for 24 h. At the end of the experiment, 10 μM amiloride was added to the apical surface of the cells. The ratios of amiloride-sensitive transepithelial current after and before treatments were compared with the ratio in the control group. NS, not significant. *P < 0.05.
MAPK1/2 pathway is necessary for chronic regulation of ENaC by TGF-α and EGF.
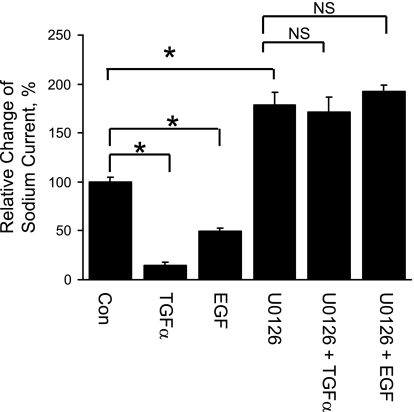
As shown in Figs. 10 and 11, both patch-clamp experiments and transepithelial measurements show that the TGF-α- or EGF-induced inhibition of ENaC is totally blocked by U0126 (MEK inhibitor). Figure 11 also shows that sodium current is higher with U0126 treatment compared with control cells, which is consistent with our hypothesis that 2F3 cells may produce endogenous EGFR ligands as suggested by Fig. 9.
Fig. 10.
Chronic effect of TGF-α on a single channel is blocked by U0126, a MEK inhibitor. A: current traces from cell-attached patches from untreated cells and cells treated with TGF-α (20 ng/ml), TGF-α+U0126 (10 μM), and U0126 alone. The patches were held at +40 mV, with inward current downward. Closed and open states are denoted by C and O, respectively. B: summary of number of ENaC per patch in all conditions. *P < 0.05 vs. Con. #P < 0.05 vs. TGF-α+U0126. C: summary of ENaC Po in all conditions.
Fig. 11.
Chronic effect of TGF-α or EGF on transepithelial current is blocked by U0126, a MEK inhibitor. Transepithelial current measurements were taken from 2F3 cells. Cells were pretreated with vehicle control (DMSO) or U0126 (10 μM in DMSO) both apically and basolaterally for 30 min. Basolateral solution was then replaced with either control (PBS), TGF-α (20 ng/ml in PBS), or EGF (100 ng/ml in PBS) with or without U0126 for 24 h. At the end of experiment, 10 μM amiloride was added to the apical surface of the cells. The ratios of amiloride-sensitive transepithelial current after and before treatments were compared with the ratio in the control group. *P < 0.05.
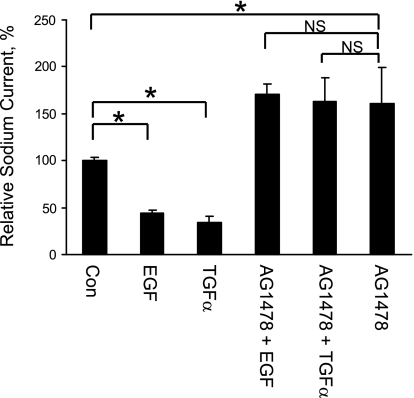
Acute treatment with TGF-α or EGF enhances sodium transport in 2F3 cells and is EGFR dependent, PI-3 kinase is involved in this activation of sodium transport, and ERK1/2 blockage potentiates this acute TGF-α- or EGF-induced stimulation of ENaC.
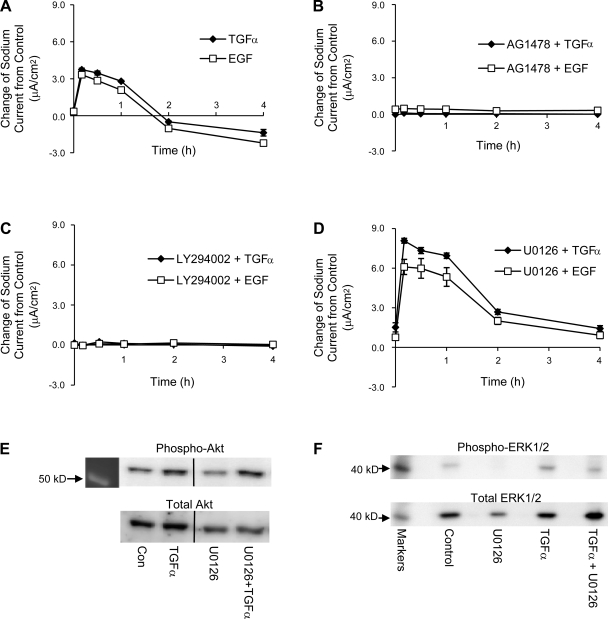
We next tested the acute effect of TGF-α or EGF on sodium transport. Cells were pretreated with vehicle control (DMSO), AG1478 (5 μM), LY294002 (50 μM), or U0126 (5 μM) both apically and basolaterally for 30 min, At time 0, the basolateral medium was changed to either control, TGF-α (20 ng/ml)- or EGF (100 ng/ml). TGF-α or EGF is referred to as “treatment” in our studies in contrast to “pretreatment” (e.g., AG 1478, etc.). Pretreatment reagents were present throughout our experiments. Transepithelial measurements were performed before the pretreatments, at time 0 and 10 min, 30 min, 1 h, 2 h, and 4 h after the treatments. The differences in the currents between treatment groups and their corresponding control groups were calculated and plotted at each time point from time 0 to 4 h. For easy viewing, data from cells with different pretreatments are shown in separately in Fig. 12.
Fig. 12.
Acute treatment with TGF-α or EGF. Transepithelial current measurements were taken from 2F3 cells. A: acute treatment (<1 h) with TGF-α or EGF enhances sodium transport. B: acute, stimulatory effects of TGF-α and EGF are blocked by AG1478, an EGFR inhibitor. C: acute, stimulatory effects of TGF-α and EGF are blocked by LY294002, a PI-3 kinase inhibitor. D: acute, stimulatory effects of TGF-α and EGF are potentiated by U0126, a MEK inhibitor. E: representative immunoblots of phospho-Akt and total Akt. F: representative immunoblots of phospho-ERK1/2 and total ERK1/2. If error bars are not visible, they are smaller than the size of the symbols.
Both TGF-α and EGF augmented sodium current within 30 min, but the sodium current returned to control level within 2 h (Fig. 12A). This acute stimulatory effect on sodium transport by TGF-α or EGF is blocked by pretreatment with AG1478, therefore it is EGFR dependent (Fig. 12B).
The TGF-α- or EGF-induced increase in sodium current is also abolished by a PI-3 kinase inhibitor, LY294002 (Fig. 12C), which suggests that PI-3 kinase is involved in the activation of sodium transport by acute treatment with TGF-α and EGF. Contrary to the chronic effect of TGF-α or EGF, inhibition of the ERK1/2 pathway by U0126 did not prevent the acute effect of TGF-α or EGF; rather, pretreatment with U0126 doubled the peak acute TGF-α- or EGF-induced stimulation of sodium transport, as shown in Fig. 12D.
Since our functional data suggest that PI-3 kinase is involved in the activation of sodium transport by acute treatment with TGF-α and the phosphorylation of Akt is a good indicator of the activation of PI-3 kinase, we detected the phosphorylation of Akt using antibodies that recognize phosphor-Akt and total Akt. As shown in Fig. 12E, the relative phosphorylation of Akt (phospho-Akt/total Akt ratio) is increased with 30 min of TGF-α treatment, and this increase is augmented further with U0126 pretreatment. The effectiveness of U0126 as a MAPK inhibitor is assessed by the detection of phosphorylation of ERK1/2. As shown in Fig. 12F, the phosphorylation of ERK1/2 is abolished by U0126, and U0126 also reduces the phosphorylation of ERK1/2 that is stimulated by TGF-α.
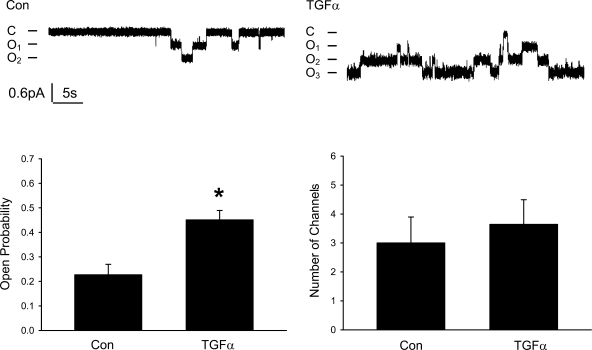
Acute treatment with TGF-α stimulates sodium transport by increasing ENaC Po, and the total number of channels in the membrane does not change.
Patch-clamp experiments were performed to investigate how ENaC is activated by the acute treatment with TGF-α. As shown in Fig. 13, P o of ENaC is significantly increased by TGF-α, but the total number of channels remained unchanged.
Fig. 13.
Acute treatment with TGF-α (20 ng/ml) or EGF (100 ng/ml) increases the Po of the channel and does not change the number of ENaC per patch. A: current traces from cell-attached patches from control and TGF-α (20 ng/ml)-treated cells (30 min to 2 h). The patches were held at +40 mV with inward current downward. Closed and open states are denoted by C and O, respectively. B: summary of ENaC Po in control and TGF-α groups. C: summary of the number of ENaC per patch in control and TGF-α groups. *P < 0.05 vs. Con.
DISCUSSION
EGF has been known for almost two decades to regulate ENaC. Our studies, for the first time, show that TGF-α, another ligand of EGFR, is not only able to regulate ENaC like EGF, but is much more potent than EGF as a regulator of sodium transport in kidney (IC50 of 0.85 nM vs. 18 nM for TGF-α and EGF, respectively). Our studies demonstrate that TGF-α and EGF regulate ENaC in 2F3 cells in two phases: an acute phase and a chronic phase.
In the acute phase (<1 h), TGF-α and EGF produce a rapid, but moderate increase in sodium transport in 2F3 cells in an EGFR-dependent manner. One of the pathways that is stimulated by activation of EGFR is the PI-3 kinase pathway, and our studies show that PI-3 kinase is required for the acute phase since LY294002 prevents the stimulation of sodium transport by TGF-α or EGF. Our immunoblotting experiments also show that the phosphorylation of Akt, an indicator of the activation of PI-3 kinase, is increased by TGF-α. PI(3,4,5)P3 (PIP3), the product of PI-3 kinase, can activate ENaC (20) and is likely the mediator of TGF-α and EGF in the acute phase. It has been shown that PIP3 activates ENaC by increasing the Po of the channel (20), and, indeed, our patch-clamp experiments show that the Po of ENaC is increased by the acute treatment with TGF-α. Therefore, it is very likely that TGF-α and EGF activate sodium transport by increasing the Po of ENaC through increased production of PIP3.
PI-3 kinase consists of a regulatory p85 subunit and a catalytic p110 subunit. The cytoplasmic domain of EGFR does not contain any typical p85 binding motif; therefore, it is thought that EGF activates PI-3 kinase indirectly. The docking protein Grb2-associated binder-1 (Gab1) has been identified as a mediator in EGF-stimulated PI-3 kinase signaling (18, 29). Following activation of EGFR, Gab1 is phosphorylated on specific tyrosine residues, which enables recruitment and activation of SH2 domain-containing proteins such as the p85 subunit of PI-3 kinase. EGF also activates the MAPK pathway by activating EGFR; interestingly, Yu and colleagues (29) suggested that EGF-stimulated MAPK activation negatively regulates the EGF-mediated interaction of Gab1 and PI-3 kinase. Their studies show that inhibiting MAPK activation with U0126 increased the EGF-induced association of Gab1 with the p85 subunit of PI-3 kinase, which is correlated with an increase in PI-3 kinase activity and greater phosphorylation of Akt. Their results explain our finding that U0126 pretreatment augments the activation of sodium current induced by acute treatment of TGF-α or EGF. Another explanation for the augmented sodium current stimulation after U0126 is that some of the stimulatory effect of TGF-α and EGF on ENaC is masked by the inhibitory effect of TGF-α and EGF on ENaC, and U0126 blocks the inhibitory effect of TGF-α and EGF on ENaC; thus the pure stimulatory effect of TGF-α and EGF on ENaC is revealed. However, since the inhibitory effect on ENaC induced by TGF-α and EGF is not significant until hours after the treatments, such an explanation probably does not explain the U0126-potentiated ENaC activation we observed within 30 min of exposure to TGF-α or EGF.
Contrary to the relatively modest increase in ENaC activity in the acute phase, TGF-α and EGF inhibit ENaC substantially in the chronic phase (>2 h). Our single-channel patch-clamp data show that chronic treatment with TGF-α reduces ENaC activity by reducing N in the membrane while Po of the channels remains unchanged. There are two possibilities for the reduced number of channels that we observed: one is that the total number of ENaC proteins that are physically present in the membrane is unchanged, but somehow the active channels are reduced; the other possibility is that the total number of channels in the membrane is reduced. Our real-time RT-PCR and immunoblotting experiments show the latter possibility to be the case: chronic TGF-α or EGF treatment reduces the mRNA levels of all ENaC subunits and reduces the protein levels of α- and γ-ENaC subunits in the membrane.
The chronic phase of TGF-α and EGF is EGFR dependent since the EGFR inhibitor AG1478 totally blocks the effects of TGF-α and EGF. On the other hand, an ErbB2 inhibitor, AG825, at concentrations up to 10 μM does not alter the effect of TGF-α or EGF on ENaC. We did observe a partial blockade of TGF-α-induced ENaC inhibition with 50 μM of AG825 (data not shown); this is probably due to the cross-inhibition of EGFR by AG825 since the IC50 of AG825 on EGFR is 19 μM (14). It is therefore unlikely that the EGFR/ErbB2 heterodimer is responsible for the TGF-α- or EGF-mediated inhibition of ENaC.
In contrast to the acute phase, the MAPK1/2 pathway is critical for the chronic phase since inhibition of the MAPK pathway with U0126 totally blocks the effects of chronic TGF-α and EGF treatments on ENaC. Besides TGF-α and EGF, progesterone (11), H2O2 (25), glucocorticoid-induced leucine zipper protein (GILZ) (19), and cAMP (27) have also been reported to regulate ENaC by either activating or suppressing the MAPK1/2 pathway. Shi et al. (17) demonstrated that MAPK1/2 phosphorylates β- and γ-ENaC and downregulates the channel by facilitating its interaction with Nedd4, thus increasing retrieval of ENaC. However, similar to the findings of other groups (7, 24), our current studies clearly show that mRNA levels of ENaC subunits are suppressed by TGF-α and EGF at steady state, even though this does not preclude the possibility that ENaC is redistributed at an earlier stage before the transcription of ENaC is reduced. How the MAPK1/2 pathway, activated by TGF-α and EGF, inhibits the transcription of ENaC remains to be investigated.
Besides blocking the TGF-α- and EGF-induced ENaC inhibition, U0126, by itself, also increases the sodium current (Fig. 9), which is consistent with our hypothesis that 2F3 cells produce endogenous EGFR ligands, as suggested by the fact that blockage of EGFR by AG1478 also increases the sodium current (Fig. 7). At least some of the EGFR ligands are either anchored to the basolateral membrane or secreted into the basolateral solution, where they can activate EGFR. The concentration of TGF-α or EGF in the interstitial fluid in the kidney has never been reported to our knowledge. If renal tubular cells can secret EGFR ligands into the basolateral side of the cells, like our 2F3 cells, the concentration of EGFR ligands in the interstitial fluid could be high enough to regulate sodium transport under physiological conditions.
Urinary TGF-α concentration has been reported to be 4.9 ± 2.8 μg/g of creatinine and to be 46.2 ± 16.6 μg/g of creatinine for EGF in healthy human subjects (28). Assuming the urinary creatinine level is 150 mg/dl (1), the concentration of TGF-α and EGF in urine from healthy adults will be 7 and 69 ng/ml, respectively. Under chronic pathological conditions where tight junctions in the collecting duct are damaged, urinary TGF-α and EGF could diffuse to the basolateral side of the tubules, activate EGFR in the membrane, and inhibit sodium transport substantially. In other conditions when EGFR is mislocalized in the apical membrane, e.g., polycystic kidney diseases, the high concentration of TGF-α and EGF in the urine could substantially reduce sodium transport.
In summary, our studies show that TGF-α is a more potent regulator of ENaC than EGF, at least in some kidney cells. Both TGF-α and EGF regulate ENaC in a biphasic pattern: an acute stimulatory phase and a chronic inhibitory phase. Both phases are mediated by activating EGFR, but through different intracellular pathways, and the two phases may interact with each other. Renal tubular cells may secrete EGFR ligands themselves, which could regulate sodium transport in the kidney under physiological and pathological conditions.
GRANTS
The work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-037963 and DK-064399.
REFERENCES
- 1.Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. Urinary creatinine concentrations in the US population: implications for urinary biologic monitoring measurements. Environ Health Perspect 113: 192–200, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature 367: 463–477, 1994. [DOI] [PubMed] [Google Scholar]
- 3.Cao LS, Owsianik G, Becq F, Nilius B. Chronic exposure to EGF affects trafficking and function of ENaC channel in cystic fibrosis cells. Biochem Biophys Res Commun 331: 503–511, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Gabow PA Autosomal dominant polycystic kidney disease. N Engl J Med 329: 332–342, 1993. [DOI] [PubMed] [Google Scholar]
- 5.Gomella LG, Sargent ER, Wade TP, Anglard P, Linehan WM, Kasid A. Expression of transforming growth factor α in normal human adult kidney and enhanced expression of transforming growth factors α and β1 in renal cell carcinoma. Cancer Res 49: 6972–6975, 1989. [PubMed] [Google Scholar]
- 6.Hughey RP, Mueller GM, Bruns JB, Kinlough CL, Poland PA, Harkleroad KL, Carattino MD, Kleyman TR. Maturation of the epithelial Na+ channel involves proteolytic processing of the α and γ-subunits. J Biol Chem 278: 37073–37082, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Kemp PJ, Borok Z, Kim KJ, Lubman RL, Danto SI, Crandall ED. Epidermal growth factor regulation in adult rat alveolar type II cells of amiloride-sensitive cation channels. Am J Physiol Cell Physiol 277: C1058–C1065, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Ling BN, Eaton DC. Effects of luminal Na+ on single Na+ channels in A6 cells, a regulatory role for protein kinase C. Am J Physiol Renal Fluid Electrolyte Physiol 256: F1094–F1103, 1989. [DOI] [PubMed] [Google Scholar]
- 9.Malik B, Schlanger L, Al Khalili O, Bao HF, Yue G, Price SR, Mitch WE, Eaton DC. ENaC degradation in A6 Cells by the ubiquitin-proteosome proteolytic pathway. J Biol Chem 276: 12903–12910, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Markadieu N, Crutzen R, Blero D, Erneux C, Beauwens R. Hydrogen peroxide and epidermal growth factor activate phosphatidylinositol 3-kinase and increase sodium transport in A6 cell monolayers. Am J Physiol Renal Physiol 288: F1201–F1212, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Michlig S, Harris M, Loffing J, Rossier BC, Firsov D. Progesterone down-regulates the open probability of the amiloride-sensitive epithelial sodium channel via a Nedd4–2-dependent mechanism. J Biol Chem 280: 38264–38270, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Muto S, Furuya H, Tabei K, Asano Y. Site and mechanism of action of epidermal growth factor in rabbit cortical collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 260: F163–F169, 1991. [DOI] [PubMed] [Google Scholar]
- 13.Ookawara S, Tabei K, Furuya H, Asano Y. The effect of EGF on electrolyte transport is mediated by tyrosine kinases in the rabbit cortical collecting duct. Miner Electrolyte Metab 25: 191–198, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Osherov N, Gazit A, Gilon C, Levitzki A. Selective inhibition of the epidermal growth factor and HER2/neu receptors by tyrphostins. J Biol Chem 268: 11134–11142, 1993. [PubMed] [Google Scholar]
- 15.Scaltriti M, Baselga J. The epidermal growth factor receptor pathway: a model for targeted therapy. Clin Cancer Res 12: 5268–5272, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Shen JP, Cotton CU. Epidermal growth factor inhibits amiloride-sensitive sodium absorption in renal collecting duct cells. Am J Physiol Renal Physiol 284: F57–F64, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Shi H, Asher C, Chigaev A, Yung Y, Reuveny E, Seger R, Garty H. Interactions of beta and gamma ENaC with Nedd4 can be facilitated by an ERK-mediated phosphorylation. J Biol Chem 277: 13539–13547, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Sithanandam G, Smith GT, Fields JR, Fornwald LW, Anderson LM. Alternate paths from epidermal growth factor receptor to Akt in malignant versus nontransformed lung epithelial Cells: ErbB3 versus Gab1. Am J Respir Cell Mol Biol 33: 490–499, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soundararajan R, Zhang TT, Wang J, Vandewalle A, Pearce D. A novel role for glucocorticoid-induced leucine zipper protein in epithelial sodium channel-mediated sodium transport. J Biol Chem 280: 39970–39981, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Tong Q, Gamper N, Medina JL, Shapiro MS, Stockand JD. Direct activation of the epithelial Na+ channel by phosphatidylinositol 3,4,5-trisphosphate and phosphatidylinositol 3,4-bisphosphate produced by phosphoinositide 3-OH kinase. J Biol Chem 279: 22654–22663, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Tong Q, Stockand JD. Receptor tyrosine kinases mediate epithelial Na+ channel inhibition by epidermal growth factor. Am J Physiol Renal Physiol 288: F150–F161, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Vehaskari VM, Heringsmith KS, Moskowitz DW, Weiner ID, Hamm LL. Effect of epidermal growth factor on sodium transport in the cortical collecting tubule. Am J Physiol Renal Fluid Electrolyte Physiol 256: F803–F809, 1989. [DOI] [PubMed] [Google Scholar]
- 23.Vehaskari VM, Herndon J, Hamm LL. Mechanism of sodium-transport inhibition by epidermal growth factor in cortical collecting ducts. Am J Physiol Renal Fluid Electrolyte Physiol 261: F896–F903, 1991. [DOI] [PubMed] [Google Scholar]
- 24.Veizis IE, Cotton CU. Abnormal EGF-dependent regulation of sodium absorption in ARPKD collecting duct cells. Am J Physiol Renal Physiol 288: F474–F482, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Wang HC, Zentner MD, Deng HT, Kim KJ, Wu R, Yang PC, Ann DK. Oxidative stress disrupts glucocorticoid hormone-dependent transcription of the amiloride-sensitive epithelial sodium channel alpha-subunit in lung epithelial cells through ERK-dependent and thioredoxin-sensitive pathways. J Biol Chem 275: 8600–8609, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Wilson PD Epithelial cell polarity and disease. Am J Physiol Renal Physiol 272: F434–F442, 1997. [DOI] [PubMed] [Google Scholar]
- 27.Yang LM, Rinke R, Korbmacher C. Stimulation of the epithelial sodium channel (ENaC) by cAMP involves putative ERK phosphorylation sites in the C termini of the channel's β- and γ-subunit. J Biol Chem 281: 9859–9868, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Yeh YC, Tsai JF, Chuang LY, Yeh HW, Tsai JH, Florine DL, Tam JP. Elevation of transforming growth factor α and its relationship to the epidermal growth factor and α-fetoprotein levels in patients with hepatocellular carcinoma. Cancer Res 47: 896–901, 1987. [PubMed] [Google Scholar]
- 29.Yu CF, Liu ZX, Cantley LG. ERK negatively regulates the epidermal growth factor-mediated interaction of Gab1 and the phosphatidylinositol 3-kinase. J Biol Chem 277: 19382–19388, 2002. [DOI] [PubMed] [Google Scholar]