Abstract
Chronic kidney disease (CKD) is associated with decreased renal nitric oxide (NO) production and increased plasma levels of methylarginines. The naturally occurring guanidino-methylated arginines N-monomethyl-l-arginine (l-NMMA) and asymmetric dimethyl-l-arginine (ADMA) inhibit NO synthase activity. We hypothesized that ADMA and l-NMMA compromise the integrity of the glomerular filtration barrier via NO depletion. We studied the effect of ADMA on albumin permeability (Palb) in isolated glomeruli and examined whether this effect involves NO- and superoxide (O2•−)-dependent mechanisms. ADMA at concentrations found in circulation of patients with CKD decreased cGMP and increased Palb in a dose-dependent manner. A similar increase in Palb was caused by l-NMMA but at a concentration two orders of magnitude higher than that of ADMA. NO donor DETA-NONOate or cGMP analog abrogated the effect of ADMA on Palb. The SOD mimetic tempol or the NAD(P)H oxidase inhibitor apocynin also prevented the ADMA-induced increase in Palb. The NO-independent soluble guanylyl cyclase (sGC) activator BAY 41–2272, at concentrations that increased glomerular cGMP production, attenuated the ADMA-induced increase in Palb. Furthermore, sGC incapacitation by the heme site-selective inhibitor ODQ increased Palb. We conclude that ADMA compromises the integrity of the filtration barrier by altering the bioavailability of NO and O2•− and that NO-independent activation of sGC preserves the integrity of this barrier under conditions of NO depletion. NO-independent activation of sGS may be a useful pharmacotherapeutic approach for preservation of glomerular function in CKD thereby reducing the risk for cardiovascular events.
Keywords: chronic kidney disease, cardiorenal disease
methylation of eukaryotic protein arginines at the guanidinium group ([H2N CH NH2 NH2+]) occurs during or immediately after translation and results in the formation of NG-monomethyl l-arginine (l-NMMA) and symmetric (NG N′G) or asymmetric (NG NG) dimethylarginines, SDMA and ADMA, respectively. Proteins containing methylated arginine residues are important regulators of cellular processes including signaling, transcriptional activation, and mRNA processing. Degradation of methylated proteins releases free methylarginines. Of the naturally occurring free methylarginines, l-NMMA and ADMA inhibit all three isoforms of nitric oxide synthase (NOS) while SDMA does not. Renal nitric oxide (NO) depletion accompanies chronic kidney disease (CKD) (2, 25, 26, 52) and this could, in part, be due to inhibition of NOS by increased levels of ADMA.
Plasma methylarginines including ADMA and SDMA increase up to 10-fold in patients with end-stage renal disease (ESRD) (26, 52) and plasma levels of ADMA are positively related with indicators of vascular dysfunction such as intima-media thickness of the carotid artery (34). Therefore, increased plasma ADMA is considered a risk factor for cardiovascular disease occurring in patients with CKD and may be a target molecule for therapeutic intervention. The exact cause of increase in ADMA levels in CKD or ESRD is not well-established. Reduced ADMA excretion by the failing kidney as well as its decreased degradation to dimethylamine and l-citrulline by the enzymes dimethylaminohydrolase I and II (DDAH I and II) have been implicated (28). Furthermore, DDAH may regulate NOS activity by controlling degradation of ADMA, which inhibits NOS (51).
NO modulates a large repertoire of cellular processes mediated by cGMP or cGMP-independent mechanisms. The majority of the biological effects of NO are mediated by activation of the heme-containing soluble guanylyl cyclase (sGC) that synthesizes cGMP, a second messenger for a large number of intracellular signaling pathways. Non-cGMP-mediated effects of NO include ion channel activation and cellular transport and may result from protein nitration by NO, its metabolites, or products of interaction of these metabolites with reactive oxygen species (1, 15, 17, 42).
In the kidney, NO has both hemodynamic and nonhemodynamic effects that modulate tubular and glomerular function (43). The latter includes filtration of plasma with a tight regulation of the passage of albumin and other macromolecules from blood into the glomerular filtrate. Injury to the glomerular protein filtration barrier function with the consequent increase in permeability to macromolecules (albumin) is an early event in the development of proteinuria (44). We recently reported that constitutive glomerular NO production preserves the integrity of the glomerular capillary protein permeability barrier through antagonism of the reactive oxygen species superoxide (O2•−). Depletion of NO through inhibition of NOS resulted in increased glomerular albumin permeability via an O2•−-dependent mechanism (45). Microvascular NO generation depends on NOS activity, which in turn, is regulated by its cell-specific expression, availability of substrate (arginine) or several cofactors (e.g., tetrahydrobiopterin, NADPH) and endogenous inhibitors such as methylarginines (13). Increased levels of ADMA may alter the balance between basal O2•− and NO levels through inhibition of NOS and depletion of NO, which can compromise the activity of the NO-sGC-cGMP axis. Under such conditions, sGC-targeted pharmacological interventions may be useful in maintaining cGMP production to preserve the function of the glomerular filtration barrier.
The present studies examined and compared the effect of the naturally occurring methylarginines (ADMA, SDMA, and l-NMMA) on glomerular permeability to albumin (Palb) using an in vitro assay which offers the advantage of a direct and rapid measurement of changes in protein permeability in a manner independent of hemodynamic factors. Evidence presented here supports our hypothesis that ADMA, an inhibitor of NOS activity, compromises the integrity of glomerular filtration barrier by causing an imbalance in the bioavailability of NO and O2•−. The potential role of sGC in preserving the glomerular filtration barrier is addressed and evidence is provided to show that NO modulates the glomerular filtration barrier function through several mechanisms.
MATERIALS AND METHODS
Glomeruli and Reagents
Male Sprague-Dawley rats (200 to 250 g) were obtained through the Biomolecular Research Center, Medical College of Wisconsin (Milwaukee, WI) or the Institute Pasteur Hellenique of Athens, Greece. Rats were maintained with free access to water and rat chow. Animal care was in accordance with the National Institutes of Health guidelines and experimental protocols were approved by the Institutional Animal Care and Use Committee.
Kidneys were harvested via abdominal incision after the animals were anesthetized using isoflurane (Phoenix Pharmaceuticals, St. Joseph, MO). Glomeruli were isolated using an established sieving technique. After the kidney capsule was removed, fine fragments of the outer 1- to 2-mm renal cortex were prepared and passed through consecutive screens of 80, 120, and 200 mesh size. Glomeruli were recovered from atop the 200 mesh screen. Isolation of glomeruli was carried out at room temperature in a medium which contained (in mmol/l) 115 sodium chloride, 5.0 potassium chloride, 10 sodium acetate, 1.2 dibasic sodium phosphate, 25 sodium bicarbonate, 1.2 magnesium sulfate, 1.0 calcium chloride, 5.5 glucose, 6.0 l-alanine, 1.0 sodium citrate, and 4.0 sodium lactate. BSA (5.0 g/dl) was included in the medium (isolation/incubation medium) as an oncotic agent. The pH of the medium was adjusted to 7.4. The oncotic pressure was measured using a membrane colloid osmometer (model 4100; Wescor, Logan, UT).
Stock solutions of agonists or other reagents were prepared and diluted to final concentrations in isolation/incubation medium containing 5% BSA. In each of the studies described, control glomeruli were incubated with equivalent volumes of the isolation/incubation medium, and Palb was determined as described below. All incubations were performed at 37°C for the indicated time periods. Conditions for each experiment such as the duration of incubation and concentrations of agonists used were based on preliminary data obtained during the current or earlier studies.
Effect of the Naturally Occurring Methylarginines, ADMA, SDMA, and l-NMMA, on Glomerular Palb
Isolated glomeruli were incubated with defined concentrations of freshly prepared ADMA (1–10 μM), SDMA (5–20 μM), or l-NMMA (2 mM; Cayman Chemical, Ann Arbor, MI) for 15 min at 37°C, and Palb was determined. ADMA and l-NMMA inhibit all NOS isoforms, the inhibition being reversible and competitive with respect to l-arginine while SDMA does not inhibit NOS (12). We anticipated decreased levels of glomerular NO following incubation with l-NMMA or ADMA. We demonstrated the dynamic changes in glomerular NO using fluorescence-based microscopy of glomeruli described below.
It is possible that changes in Palb caused by ADMA are not due to NOS inhibition, but rather to other effects of this methylarginine. To provide evidence that the effect of ADMA on Palb is mechanistically linked to NOS inhibition and NO depletion, we incubated glomeruli with ADMA (5 or 10 μM) in the presence and absence of the prototypic NOS inhibitor l-NMMA (2 mM), to determine whether an additive effect on Palb occurs. To further characterize this effect, submaximal concentrations of ADMA (1 μM) and l-NMMA (0.5 mM) were also used.
The NO donor diethylenetriamine NONOate (DETA-NONOate; Cayman Chemical) was included in some experiments to determine whether the combined effect of ADMA and l-NMMA on Palb is reversible. DETA-NONOate belongs to the class of 1-substituted diazen-1-ium-1, 2-diolate compounds containing the [N(O)NO] functional group with half-lives ranging from 1 min to 1 day in physiologic buffers making them suitable for a variety of applications in which controlled generation of NO is required (20). The 20-h half-life of DETA-NONOate provides a relatively constant flux of NO required in these experiments, making this compound an ideal NO donor. Isolated glomeruli were coincubated with ADMA (5 μM) and DETA-NONOate (50, 100, or 500 μM) at 37°C for 15 min. Palb in each group was then determined.
Role of sGC Activity in Preserving the Glomerular Palb
The inhibitory effect of ADMA on NOS would decrease NO production. Since NO is a major activator of sGC, activity of this enzyme to convert GTP to cGMP would be expected to decrease in the setting of increased ADMA levels. This could be a mechanism underlying changes in glomerular Palb following exposure to ADMA. To test this possibility, we 1) assessed the effect of ADMA on glomerular cGMP production, 2) explored whether sGC activation in glomeruli exposed to ADMA preserves Palb, and 3) examined whether cGMP mimics the effect of sGC activation on Palb. Two sGC activators were employed: the NO donor DETA-NONOate (see above) and the NO-independent sGC activator BAY 41-2272 (5-cyclopropyl-2-{1-(2-fluoro-benzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl}-pyrimidin-4-ylamine). BAY 41-2272 increases sGC activity by binding to a regulatory site in the cysteine 238 and cysteine 248 region of the α1 sGC subunit (47). It acts synergistically with NO in activating sGC and does not inhibit phosphodiesterase activity (39). Glomeruli were incubated with ADMA (5 μM) alone, BAY 41-2272 alone, or coincubated with ADMA (5 μM) and various concentrations of BAY 41-2272 (1, 5, and 10 μg/ml).
In separate experiments, glomeruli were incubated with ADMA in the presence or absence of the cGMP analog 8-bromo-cGMP (Sigma, St. Louis, MO) and changes in Palb were determined. All incubations were performed at 37°C for 15 min followed by determination of glomerular cGMP production or changes in Palb.
Effect of sGC Redox Status on Glomerular Palb
sGS is a heme protein where NO binds to heme Fe2+. Oxidation of iron to Fe3+ prevents the NO-mediated activation of sGC. ODQ {1H-[1,2,4] oxadiazolo-[4,3-a]quinoxalin-1-one} causes oxidation of heme iron (Fe2+ to Fe3+) thereby inhibiting sGC activation by NO without altering its catalytic activity. ODQ is widely used to test the participation of sGS in cellular signaling (56). To determine the role of sGC in preserving the glomerular filtration barrier, we incubated glomeruli with ODQ (1–10 μM) alone or 10 μM ODQ with cGMP (100 nM) at 37°C for 15 min.
Role of the Interaction Between NO and O2•− in Mediating the Change in Glomerular Palb
NO is an efficient scavenger of O2•− and inhibition of NOS might result in an increase in O2•− bioavailability, which could account for the observed changes in Palb following exposure of glomeruli to ADMA. To examine this possibility, isolated glomeruli were incubated for 15 min with ADMA (5 μM) in the presence or absence of the membrane-permeable SOD mimetic tempol (4-hydroxy-2, 2, 6, 6-tetramethyl piperidinoxyl; 5 mM; Sigma), and Palb was measured. This low molecular weight SOD mimetic has been used as a spin trap for O2•− and attenuates the superoxide-mediated injury in models of ischemia-reperfusion injury and inflammation (10).
Role of NAD(P)H Oxidase-Derived Superoxide in Mediating Changes in Palb
Highly vascular tissues, including glomeruli, contain significant levels of membrane-bound flavin-containing oxidases that use NADH or NAD(P)H as cofactors to generate O2•−. To examine whether NAD(P)H-driven O2•− production accounts for changes in Palb following exposure to ADMA, isolated glomeruli were incubated for 15 min with ADMA (5 μM) in the presence or absence of the NAD(P)H oxidase inhibitor apocynin (4-hydroxy-3-methoxy acetophenone) at concentrations 5, 20, and 40 μM. This ortho-methoxy-substituted catechol is believed to inhibit the oxidase by preventing the assembly of p47phox and p67phox subunits of the NAD(P)H oxidase complex within the cell membrane (48). Changes in Palb were measured at the completion of each incubation period. Recent studies in cultured cells suggest that apocynin can function as an inhibitor of NADPH oxidase in leukocytes as well as an anti-oxidant in vascular endothelial cells (14).
Determination of Glomerular cGMP Production
Glomerular cGMP levels were measured using an ELISA after incubating isolated glomeruli with ADMA and/or BAY 41-2272 at concentrations employed to determine changes in Palb. A typical cGMP production experiment in glomeruli incubated with ADMA is described. An aliquot of glomerular suspension (120 μl) was placed in a microcentrifuge tube. Vehicle (5 μl) or ADMA (5 μl of 140 μM stock) was added to achieve a final ADMA concentration of 5 μM and incubated in a 37°C water bath for 15 min. The phosphodiesterase inhibitor IBMX (3-isobutyl-1-methylxanthine) was then added (15 μl) to a final concentration of 0.5 mM (pH 7.4) and incubations were allowed to proceed for another 5 min. Intracellular cGMP was extracted by adding 1.5 μl of concentrated hydrochloric acid to the reaction mixture and incubating at room temperature for 30 min. Glomeruli were obtained as a pellet by centrifugation at 500 g for 5 min and the supernatant was collected for cGMP assay. The cGMP concentration was determined using the direct cGMP kit (Assay Designs, Ann Arbor, MI) following the manufacturer's instruction. Results were expressed as femtomoles per microgram of protein.
Determination of Glomerular Palb by Measuring Volume Response to an Oncotic Gradient
The volume response (ΔV) of glomerular capillaries to an oncotic gradient generated by defined concentrations of albumin was measured as previously described. This in vitro assay of Palb is a very sensitive test of the initial subtle injury to the glomerular filtration barrier (40). Glomeruli were incubated in control or media containing the aforementioned reagents, affixed to glass coverslips coated with poly-l-lysine (1 mg/ml), and observed using video microscopy before and 1 min after the initial incubation medium containing 5 g/dl BSA was replaced by medium containing 1 g/dl BSA. This replacement of medium produces an oncotic gradient across the glomerular capillary wall and results in a net fluid influx and an increase in glomerular volume. Glomerular volume was calculated from the average of four diameters of the video image, and the increase in volume (ΔV) of each glomerulus in response to the oncotic gradient was expressed as: ΔV = (Vfinal − Vinitial)/Vinitial × 100% (32).
Reflection coefficient of albumin.
There is a direct relationship between the increase in ΔV and the oncotic gradient (Δπ) applied across the capillary wall. We used this principle to calculate reflection coefficient of albumin (σalb), using the ratio of ΔV of experimental to ΔV of control glomeruli in response to identical oncotic gradients: σalb = ΔVexperimental/ΔVcontrol (40).
Convectional Palb.
Convectional Palb was defined as (1 − σalb) to describe the movement of albumin consequent to water flow. When σalb is zero, albumin moves at the same rate as water and Palb is 1.0. When σalb is 1.0, albumin cannot cross the membrane with water and Palb is zero (40).
Determination of NO Production in Glomeruli
Isolated rat glomeruli were pretreated with ADMA (5 μM), l-NMMA (2 mM), or vehicle in HEPES buffer solution consisting of (mmol/l) 138.0 NaCl, 4 KCl, 1.2 MgSO4, 1.6 CaCl2, 1.2 KH2PO4, 0.026 EDTA, 6.0 glucose, and 10 HEPES acid. DAF-FM (10 μM; Invitrogen, Carlsbad, CA) was added to the buffer and loaded. DAF-FM, like DAF-2 diacetate, is membrane permeant and forms a fluorescent product with the nitrosonium cation produced by spontaneous oxidation of NO. According to the manufacturer, DAF-FM adduct with NO is sensitive, stable at physiological pH, and resists photolysis. Fluorescence detection of NO production using DAF-FM was performed by krypton-argon laser excitation at 488 nm while recording emission at 523 nm. Images were obtained using a laser-scanning imaging system mounted on an inverted microscope (Olympus) with a ×20 objective lens. Images were obtained and analyzed using MetaMorph software suite (Universal Imaging, Molecular Dynamics, Sunnyvale, CA).
Statistical Analysis
Each Palb value is an average of 15 measurements obtained in 5 individual glomeruli isolated from one rat. Three rats were used in each group. Results were expressed as means ± SE. Unpaired t-test was used to compare the control group with experimental groups. Effect of DETA-NONOate, tempol, or apocynin on Palb was determined by comparing each with ADMA alone group. Significance of difference between groups was expressed as P values.
RESULTS
Effect of Methylarginines on Palb: Role of NO
Asymmetric methylarginines increase Palb.
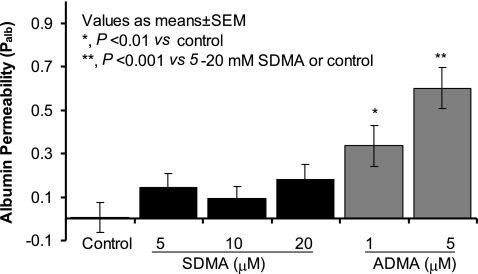
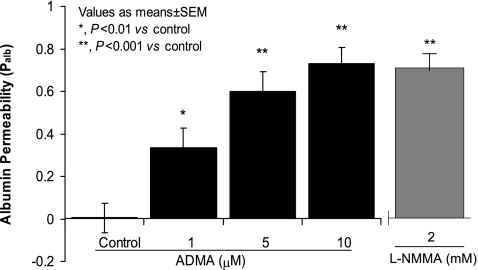
The asymmetric methylarginine ADMA significantly increased Palb (1 μM ADMA Palb: 0.34 ± 0.09 vs. control Palb: 0.005 ± 0.07, P < 0.01; 5 μM ADMA Palb: 0.6 ± 0.09 vs. control Palb: 0.005 ± 0.07, P < 0.001), while 5–20 μM symmetric methylarginine SDMA did not affect Palb (Fig. 1). The effect of ADMA on Palb was dose dependent with a statistically significant increase at 1 μM ADMA (P < 0.01 vs. control), 5 μM ADMA (P < 0.001 vs. control), or 10 μM ADMA (10 μM ADMA Palb: 0.73 ± 0.076 vs. control, P < 0.001; Fig. 2). Although 1 μM ADMA caused a significant increase in Palb, 5 μM was found to result in maximal increase under the experimental conditions used. Ten micromolar ADMA caused a greater increase in Palb (0.73 ± 0.076) compared with 5 μM ADMA (0.6 ± 0.09) but this difference was not significant (P < 0.2). Therefore, we used 5 μM ADMA in subsequent experiments.
Fig. 1.
Effect of asymmetric dimethyl-l-arginine (ADMA) and SDMA on albumin permeability (Palb). Isolated rat glomeruli were incubated with 5, 10, or 20 μM SDMA or 1 and 5 μM ADMA at 37°C for 15 min. Control glomeruli were incubated with equivalent volume of the vehicle (incubation medium containing 5% BSA). Change in glomerular Palb was determined by an ex vivo assay (see materials and methods). Palb values are presented as means ± SE derived from measurements made in 15 glomeruli isolated from 3 rats (5 glomeruli from each rat). ADMA significantly increased Palb in a dose-dependent fashion (1 μM, *P < 0.01 vs. control; 5 μM, **P < 0.001 vs. control), whereas SDMA did not increase Palb at any of the concentrations tested.
Fig. 2.
Dose-dependent effect of ADMA and effect of N-monomethyl-l-arginine (l-NMMA) on Palb. Isolated rat glomeruli were incubated with ADMA (1, 5, or 10 μM) or with l-NMMA (2 mM) at 37°C for 15 min. Control glomeruli were incubated with equivalent volume of the incubation medium containing 5% BSA. Palb values are presented as means ± SE derived from measurements made in 15 glomeruli isolated from 3 rats (5 glomeruli from each rat). ADMA caused a dose-dependent increase in Palb which was significant at 1 μM (*P < 0.01 vs. control) and peaked at 5 μM (**P < 0.001 vs. control). l-NMMA (2 mM) had an effect on Palb comparable to that of ADMA (10 μM).
l-NMMA also caused an increase in Palb comparable to that induced by ADMA (Fig. 2). However, a maximal increment in Palb was achieved at a l-NMMA concentration (2 mM) that was two orders of magnitude higher than that of ADMA (2.0 mM l-NMMA Palb: 0.75 ± 0.08 vs. control Palb: 0 ± 0.13, n = 15, P < 0.001).
ADMA and l-NMMA decrease glomerular NO.
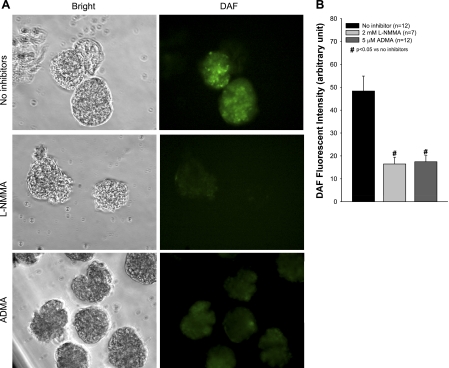
ADMA and l-NMMA inhibit NOS activity resulting in decreased NO levels. We demonstrated decreased NO levels in glomeruli incubated with ADMA or l-NMMA followed by DAF-FM loading. Photomicrographs (Fig. 3A) of glomeruli identified in bright field (left) showed DAF fluorescence (right). Vehicle-treated control glomeruli (top) showed more fluorescence compared with l-NMMA-treated (middle) and ADMA-treated (bottom) glomeruli. Image intensities were compared using MetaMorph software and presented as a bar graph (Fig. 3B). Both ADMA and l-NMMA significantly reduced DAF-FM fluorescence (P < 0.05 vs. control), indicating the decreased glomerular NO production.
Fig. 3.
Effect of ADMA and l-NMMA on glomerular nitric oxide (NO). Isolated rat glomeruli were pretreated with ADMA (5 μM), l-NMMA (2 mM), or vehicle in HEPES buffer. DAF-FM (10 μM) was added to the buffer and loaded. Fluorescence detection was performed by krypton-argon laser excitation at 488 nm while recording emission at 523 nm. Images were obtained with a laser-scanning imaging system mounted on an inverted microscope (Olympus) using a ×20 objective lens. Images were analyzed using MetaMorph (Universal Imaging). Results presented in the micrograph and bar graph demonstrate that both ADMA and l-NMMA significantly depleted glomerular NO (#P < 0.05 vs. control).
NO donor abrogates the ADMA-induced increase in Palb.
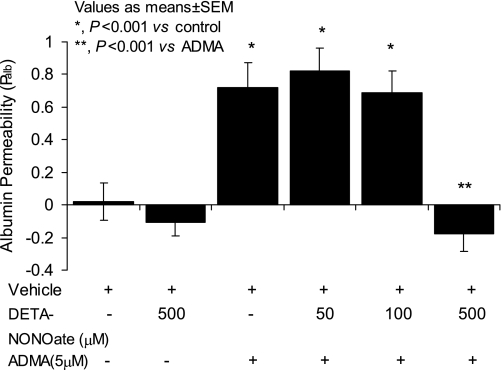
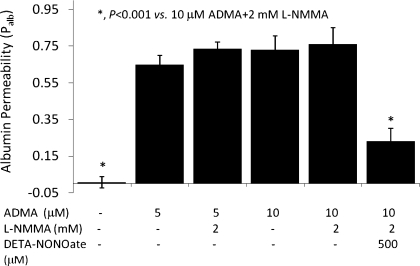
It is possible that inhibition of NOS by ADMA caused the increased Palb seen (Figs. 1 and 2) via a mechanism independent of NO depletion. To examine this possibility, glomeruli were incubated with ADMA in the absence and presence of the NO donor DETA-NONOate. DETA-NONOate at 500 μM abrogated the ADMA-induced increase in Palb (ADMA 5 μM Palb: 0.72 ± 0.15 vs. ADMA 5 μM + DETA-NONOate 500 μM Palb: −0.18 ± 0.105, P < 0.001; Fig. 4). Lower concentrations of DETA-NONOate (50 or 100 μM) failed to prevent the ADMA-induced increase in Palb.
Fig. 4.
Effect of diethylenetriamine NONOate (DETA-NONOate) on ADMA-induced increase in Palb. Isolated rat glomeruli were incubated with ADMA (5 μM) alone or in the presence of various concentrations of DETA-NONOate (50, 100, or 500 μM) at 37°C for 15 min. Control glomeruli were incubated with equivalent volume of incubation medium containing 5% BSA. Palb values are presented as means ± SE derived from measurements made in 15 glomeruli isolated from 3 rats (5 glomeruli from each rat). ADMA alone increased Palb (*P < 0.001 vs. control), whereas DETA-NONOate alone (500 μM) had no effect. Lower concentrations of DETA-NONOate (50 and 100 μM) did not alter the increase in Palb caused by ADMA but 500 μM abrogated this increase (**P < 0.001 vs. ADMA alone).
The combined effect of l-NMMA (2 mM) and 5 or 10 μM ADMA (Palb: 0.74 ± 0.03 and 0.76 ± 0.04, respectively) was not different from that of 5 μM ADMA alone (Palb: 0.6 ± 0.09; Fig. 5). DETA-NONOate abrogated the combined effect of l-NMMA + ADMA on Palb (Fig. 5). Submaximal concentrations of l-NMMA (0.5 mM, Palb: 0.22 ± 0.03) combined with submaximal concentration of ADMA (1 μM, Palb: 0.27 ± 0.04) had additive effect on Palb (0.46 ± 0.05, P < 0.01 vs. 1 μM ADMA or 0.5 mM l-NMMA).
Fig. 5.
Combined effect of ADMA and l-NMMA on Palb. Isolated glomeruli were incubated with ADMA (5 or 10 μM) alone or with l-NMMA (2 mM) at 37°C for 15 min. An aliquot of glomeruli was incubated with ADMA (10 μM), l-NMMA (2 mM), and DETA-NONOate (500 μM). Control glomeruli were incubated with equivalent volume of the incubation medium containing 5% BSA. Palb values are presented as means ± SE derived from measurements made in 15 glomeruli isolated from 3 rats (5 glomeruli from each rat). ADMA at 5 or 10 μM concentrations increased Palb (*P < 0.001 vs. control). Combined effect of ADMA (5 or 10 μM) and l-NMMA (2 mM) did not result in additional increase in Palb over ADMA alone. Inclusion of DETA-NONOate to ADMA (10 μM) + l-NMMA (2 mM) significantly blocked the combined effect of ADMA and l-NMMA (*P < 0.001 vs. ADMA + l-NMMA).
Effect of Methylarginines on Palb: Role of sGC
ADMA-induced increase in Palb is attenuated by activation of sGC.
NO reacts with multiple targets, chief among these being the enzyme sGC, which is known to be present in rat podocytes (33, 38). It is possible that in our experiments NO derived from DETA-NONOate reversed the effect of ADMA on Palb by acting on targets other than sGC (15). To evaluate the extent to which sGC activity is important in preserving the permeability barrier in glomeruli exposed to ADMA, we 1) assessed the effect of ADMA on glomerular cGMP production, 2) employed the NO-independent sGC activator compound BAY 41-2272, and 3) employed the sGC inhibitor ODQ.
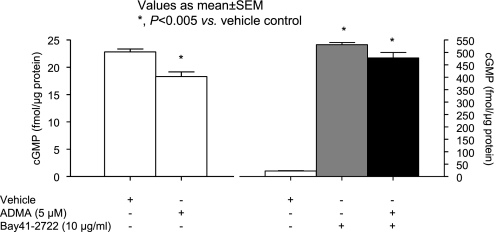
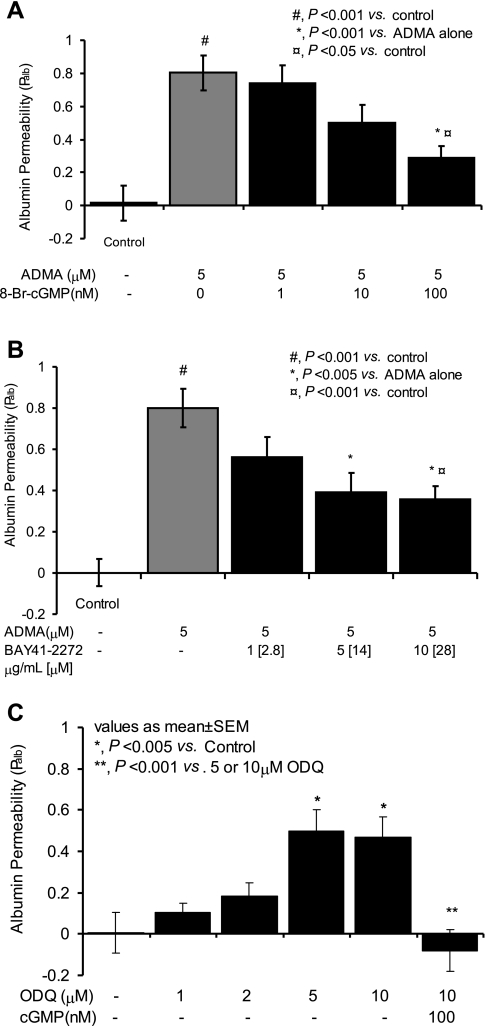
ADMA alone significantly reduced glomerular cGMP levels, whereas BAY 41-2272 alone markedly increased cGMP levels (Fig. 6). In glomeruli coincubated with ADMA and BAY 41-2272, cGMP production was no different than that observed with BAY 41-2272 alone (Fig. 6). BAY 41-2272 (5 and 10 μg/ml) partially reversed the ADMA-induced increase in Palb [Fig. 6; ADMA alone (5 μM) Palb: 0.80 ± 0.10 vs. control Palb: 0.001 ± 0.06, P < 0.001; ADMA alone vs. ADMA (5 μM) + BAY 41-2272 (1 μg/ml) Palb: 0.56 ± 0.1, NS; ADMA alone vs. ADMA (5 μM) + BAY 41-2272 (5 μg/ml) Palb: 0.39 ± 0.09, P < 0.005; ADMA alone vs. ADMA (5 μM) + BAY 41-2272 (10 μg/ml) Palb: 0.36 ± 0.06, P < 0.005].
Fig. 6.
Effect of ADMA and BAY 41-2272 on glomerular cGMP production. Isolated rat glomeruli were incubated with vehicle alone or vehicle containing ADMA (5 μM) at 37°C for 15 min (left). Glomerular cGMP content was determined using a cGMP ELISA kit as described in materials and methods. ADMA significantly decreased glomerular cGMP (*P < 0.01 vs. vehicle control). Isolated glomeruli were incubated with vehicle, BAY 41-2272 (10 μg/ml) alone, or ADMA (5 μM) and BAY 41-2272 (10 μg/ml) at 37°C for 15 min (right). Glomerular cGMP content was determined. BAY 41-2272 significantly increased cGMP content (gray bar) and reversed the ADMA-induced decrease in cGMP (black bar).
cGMP blocks the effect of ADMA on Palb.
The sGC activators NO and BAY 41-2272 were used to increase cGMP synthesis. To examine whether cGMP mediates the salutary effect of sGC activation on Palb, glomeruli were incubated with ADMA in the presence or absence of the cGMP analog 8-bromo cGMP. As shown in Fig. 7A, 8-bromo-cGMP significantly attenuated the effect of ADMA on Palb at concentrations as low as 100 nM (0.29 ± 0.07 vs. ADMA alone (5 μM) 0.74 ± 0.11, P < 0.001).
Fig. 7.
cGMP synthesis by soluble guanylyl cyclase (sGC) protects the glomerular filtration barrier. Isolated glomeruli were incubated with reagents as described for 15 min at 37°C. Control glomeruli were incubated with equivalent volume of the vehicle (incubation medium containing 5% BSA). Palb values are presented as means ± SE derived from measurements made in 15 glomeruli isolated from 3 rats (5 glomeruli from each rat). A: effect of cGMP analog on ADMA-induced increase in Palb. Isolated rat glomeruli were incubated with ADMA (5 μM) alone or ADMA (5 μM) and 8-Br-cGMP (1, 10, or 100 nM). ADMA increased Palb. 8-bromo-cGMP at 100 nM concentration significantly abrogated this increase (*P < 0.001 vs. ADMA alone). B: effect of BAY 41-2272 on ADMA-induced increase in Palb. Isolated rat glomeruli were incubated with ADMA (5 μM) alone or ADMA (5 μM) in the presence of various concentrations of BAY 41-2272 (1, 5, or 10 μg/ml). ADMA alone increased Palb. BAY 41-2272 (5 or 10 μg/ml) significantly prevented the increase in Palb (*P < 0.005 vs. control). C: effect of sGC inhibitor ODQ on Palb. Isolated rat glomeruli were incubated with ODQ (1, 2, 5, or 10 μM) alone. Additional aliquot of glomeruli was incubated with 10 μM ODQ and 100 μM 8-Br-cGMP. ODQ alone (5 and 10 μM) increased Palb (*P < 0.005 vs. control). Inclusion of 8-Br-cGMP blocked the effect of ODQ (**P < 0.001).
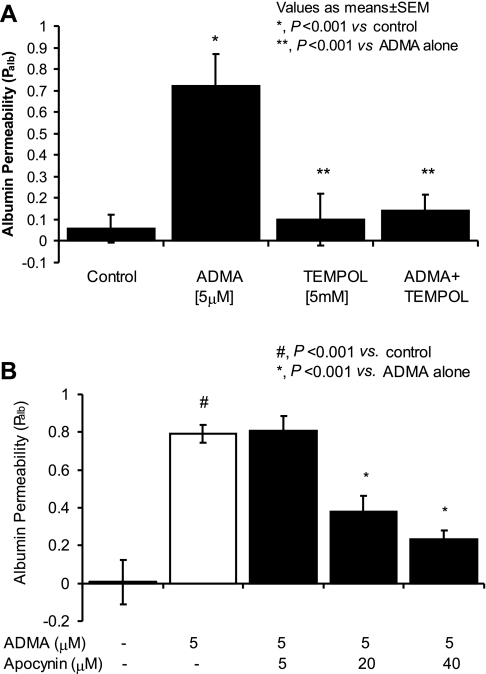
Fig. 8.
Superoxide scavenger and NADPH oxidase inhibitor block the ADMA-induced increase in Palb. Isolated glomeruli were incubated with reagents as described for 15 min at 37°C. Control glomeruli were incubated with equivalent volume of the vehicle (incubation medium containing 5% BSA). Palb values are presented as means ± SE derived from measurements made in 15 glomeruli isolated from 3 rats (5 glomeruli from each rat). A: effect of tempol on ADMA-induced increase in Palb. Isolated rat glomeruli were incubated with ADMA (5 μM) alone, the SOD mimetic tempol (5 mM) alone, or ADMA in the presence of tempol. ADMA alone increased Palb (*P < 0.001 vs. control) and tempol abrogated the effect of ADMA on Palb (**P < 0.001 vs. ADMA alone) while tempol alone did not alter Palb. B: effect of apocynin on ADMA-induced increase in Palb. Isolated rat glomeruli were incubated with ADMA (5 μM) only or ADMA and apocynin (5, 20, and 40 μM). Apocynin at 20 or 40 μM abrogated the increased Palb caused by ADMA (*P < 0.001 vs. ADMA alone).
NO-independent activation of sGC by BAY 41-2272 blocks the effect of ADMA on Palb.
The partial but statistically significant reversal of the increase in Palb by the sGC activator BAY 41-2272 (Fig. 7B) contrasts with the complete reversal achieved when 500 μM of the NO donor DETA-NONOate was used (Fig. 4).
sGC inhibition by ODQ increases Palb.
ODQ renders sGC unresponsive to NO by oxidizing its heme iron from Fe2+ to Fe3+. Thus, inhibition sGC activity through oxidation of heme iron would be expected to cause an effect on Palb similar to that of NO depletion. To confirm this, glomeruli were incubated with ODQ (1–10 μM) for 15 min and Palb was determined. We observed (Fig. 7C) a dose-dependent effect of ODQ on Palb with significant increase at 5 μM (Palb: 0.5 ± 0.1; control: 0.006 ± 0.1, P < 0.005) or 10 μM (Palb: 0.46 ± 0.09; control: 0.006 ± 0.1, P < 0.005). The increased Palb by 10 μM ODQ was attenuated by 100 nM cGMP (Palb: −0.08 ± 0.1; control: 0.006 ± 0.1, NS).
Scavenging of O2•− Prevents the ADMA-Induced Increase in Palb
Glomeruli generate O2•− both constitutively and under pathologic conditions (43) while NO is an efficient scavenger of O2•− (16). Furthermore, Palb is increased in glomeruli overproducing O2•− or exposed to exogenous O2•− (5, 27). We tested the hypothesis that the ADMA-induced increase in Palb occurred as a result of NO depletion resulting in unopposed effects of O2•−. Isolated glomeruli were incubated with ADMA (5 μM) in the presence or absence of the SOD mimetic tempol (5 mM). The increase in Palb in response to ADMA was prevented by tempol (ADMA + tempol Palb: 0.14 ± 0.07 vs. ADMA alone 0.70 ± 0.15, P < 0.001), while tempol alone had no effect on Palb (0.1 ± 0.12 vs. control, NS; Fig. 8A).
NAD(P)H Oxidase-Derived O2•− Mediates Changes in Palb in Response to ADMA
Having shown that the ADMA-induced increase in Palb was mechanistically linked to NO depletion and to an unopposed effect of O2•−, we next explored potential sources of O2•−. Several enzyme systems, including NAD(P)H oxidase, are involved in the constitutive production of O2•− in normal glomeruli. Components of the NAD(P)H oxidase complex are found in glomerular epithelial and mesangial cells (11, 19).
To examine the extent to which NAD(P)H oxidase activity contributed to the increase in Palb caused by ADMA, we used the NAD(P)H oxidase inhibitor apocynin. Glomeruli were incubated with ADMA (5 μM) in the absence and presence of apocynin at concentrations (5–40 μM) shown to inhibit formation of the NAD(P)H oxidase complex and O2•− production in a variety of cell types including endothelial cells and monocytes and to lower blood pressure in animal models (54). Apocynin significantly attenuated the increase in Palb induced by 5 μM ADMA (ADMA alone Palb: 0.80 ± 0.12 vs. ADMA + 20 μM apocynin Palb: 0.38 ± 0.08, P < 0.001; ADMA alone Palb: 0.80 ± 0.12 vs. ADMA + apocynin 40 μM Palb: 0.23 ± 0.046, P < 0.001; Fig. 8B).
DISCUSSION
Methylarginines are released into plasma through degradation of cellular proteins. Circulating levels of methylarginines are elevated in several disease states, including those characterized by endothelial dysfunction such as hypertension, preeclampsia, atherosclerosis, hypercholesterolemia, diabetes mellitus, and CKD (51). Whether increased plasma ADMA contributes to or results from endothelial dysfunction is currently being debated (28). The effect of increased ADMA on the glomerular filtration barrier is unknown. The present studies examined the effect of the naturally occurring methylarginines on glomerular permeability to albumin. We selected l-NMMA, SDMA, and ADMA as representative monomethyl-, symmetrical dimethyl-, and asymmetrical dimethylarginine, respectively. Systemic administration of ADMA or l-NMMA is known to cause hemodynamic changes (7, 21), making it difficult to determine whether the effect of methylarginines on the glomerular filtration barrier is solely due to their effect on glomerular NO production. Therefore, we used an in vitro assay that permits studies on changes in the glomerular filtration barrier without the influence of hemodynamic factors. Indeed, inhibition of NO following ADMA or l-NMMA administration to rats was shown to reduce glomerular plasma flow rate and glomerular capillary ultrafiltration coefficient. Moreover, NOS inhibition increases afferent and efferent arteriolar resistance and intracapillary hydraulic pressure (7). We used this assay extensively to determine early effects of injury on the glomerular filtration barrier as well as the protective effect of several agents (40, 44).
We demonstrated that ADMA induces a significant increase in glomerular Palb at concentrations that are within the range of plasma levels reported in human renal failure (2, 4, 26, 28) while the symmetrical stereoisomer SDMA had no effect (Fig. 1). Since ADMA but not SDMA inhibits NOS (52), we hypothesized that the effect of ADMA on Palb was linked to NO depletion and subsequent impairment of the protein permeability barrier function. This hypothesis is supported by our finding that the prototypic pharmacologic NOS inhibitor, l-NMMA, mimicked the effect of ADMA on Palb (Fig. 2). Of note, compared with 5–10 μM ADMA, a markedly higher concentration of l-NMMA (2 mM) was required to achieve a comparable increase in Palb (Fig. 2). Although ADMA and l-NMMA have been found to be approximately equipotent in inhibiting NOS in other models (9, 25, 52), the substantially lower concentrations of ADMA required for maximal increase in Palb suggest a greater sensitivity of the filtration barrier to ADMA (dimethyl arginine) compared with l-NMMA (monomethyl arginine). Furthermore, ADMA may act on non-NOS targets as well (15, 46) resulting in unrelated effects on glomerular cells, although presently we limited our investigations only to its effect on the glomerular filtration barrier function.
Both ADMA and l-NMMA are potent inhibitors of NOS activity (24, 50). We used cell-permeant DAF-FM to demonstrate the change in glomerular NO as a result of brief incubation with ADMA or l-NMMA. DAF compounds are fluorescein-derived dyes (22) that permit convenient and specific detection of low amounts of cellular NO. Results showed that both ADMA and l-NMMA caused a decrease in the intensity of fluorescence indicating decreased levels of fluorescent benzotriazole, the NO-DAF-FM adduct (Fig. 3, A and B).
Our hypothesis that the ADMA-induced increase in Palb is due to NO depletion was further supported by our findings that this increase was prevented by the NO donor DETA-NONOate (Fig. 4). The fact that a relatively high concentration of DETA-NONOate (500 μM) was required suggests that there may be a critical number of targets to which exogenous NO must bind to reverse the effect of ADMA on Palb. To verify that the effect of ADMA on Palb was mechanistically related to NOS inhibition, glomeruli were coincubated with ADMA in the presence of the prototypic pharmacologic NOS inhibitor, l-NMMA, to examine whether an additive effect on Palb could occur. Compared with the effect of ADMA alone, ADMA + l-NMMA at their optimal concentrations had no additive effect on Palb indicating that the ADMA-induced increase in Palb can be attribute to NOS inhibition. Submaximal concentrations of these inhibitors, on the other hand, showed an additive effect suggesting a common target. Furthermore, exogenous NO abrogated the effect of ADMA + l-NMMA on Palb (Fig. 5).
A key target of NO is sGC, which functions as an NO sensor in mammalian cells (38). Binding of NO to the heme moiety of sGC markedly increases cyclase activity and generation of cGMP. cGMP is a crucial second messenger in a number of signaling cascades involving protein phosphorylation and ion channel regulation. We hypothesized that exogenous NO reversed the ADMA-induced increase in Palb by activating sGC, leading to increased production of cGMP. To this end, the NO-independent sGC activator BAY 41-2272 prevented the ADMA-induced decrease in glomerular cGMP levels (Fig. 6) and attenuated the increase in Palb caused by ADMA, and did so at concentrations capable of stimulating glomerular cGMP production (Fig. 7B). Moreover, the cGMP analog 8-bromo-cGMP also attenuated the ADMA-mediated increase in Palb (Fig. 7A). The findings that the ADMA-induced increase in Palb was 1) prevented by a relatively high concentration of DETA-NONOate and 2) was only partially reversed by BAY 41-2272 or by 8-Br-cGMP lead us to propose that NO protects the permeability barrier through reactions that do not solely involve stimulation of sGC and generation of cGMP. That sGC activity is important in preserving the integrity of this barrier is supported by the observation that oxidation of sGC heme iron using ODQ increased Palb (Fig. 7C). As ODQ acts by oxidizing sGC heme iron, this observation indicates the redox state of this enzyme is important in preserving the filtration barrier.
In addition to its effect on sGC, NO reacts with O2•− at a near diffusion-controlled rate constant, a reaction that is three to four times faster than O2•− dismutation by SOD. Moreover, the half-life of NO (normally 4–50 s) is doubled in the presence of SOD (16), indicating that O2•− is an efficient NO scavenger. We hypothesized that the ADMA-induced increase in Palb could be mediated in part by increased bioavailability of O2•− occurring due to NO depletion. In this regard, we previously demonstrated that O2•− directly increases Palb (5). Tempol completely reversed the ADMA-induced increase in Palb (Fig. 8A), a finding that supports the hypothesis that O2•− mediated the effect of ADMA on Palb. These observations support the role of NO as an important antagonist of the effects of O2•− on the glomerular filtration barrier. By inhibiting NOS, ADMA could change the balance between these two reactive species and result in a net increase in the bioavailability of O2•−.
We next explored the potential sources of O2•−. Glomerular O2•− is generated by various oxidases including NAD(P)H oxidase complex found in mesangial (19) and epithelial (11) cells. We found that the NAD(P)H oxidase inhibitor apocynin significantly attenuated the increase in Palb caused by ADMA (Fig. 8B), which indicates that NAD(P)H oxidase activity generated O2•− in amounts sufficient to increase Palb in glomeruli exposed to ADMA. Further studies using other inhibitors of NADPH oxidase will be needed to address the extent to which this oxidase contributes to superoxide production since a recent report suggests that in addition to its role as an NADPH oxidase inhibitor apocynin also has an antioxidant effect (14, 48).
Under certain conditions, including methylarginine excess, constitutive NOS can generate O2•− rather than NO (35, 37), a phenomenon referred to as NOS uncoupling. Arginine is a semi-essential amino acid derived from the diet as well as cellular synthesis. Arginine, a precursor of NO, urea, certain amino acids, and polyamines, also plays a regulatory role in gene expression and cell cycle progression. Endothelial cells deprived of arginine maintain release of the endothelium-derived relaxation factor for 2 h. Thus, intracellular levels of arginine are maintained for considerable time (29, 31). The brief incubation (15 min) employed in our studies was unlikely to result in arginine depletion and NOS uncoupling as evident from unchanged Palb of the control group. Furthermore, glomeruli used in these studies were isolated from arginine-replete rats. Thus, O2•− generation in the present studies appears to be catalyzed by the NADPH oxidase system.
NOS uncoupling may also result from insufficient levels of (6R)-5,6,7,8-tetrahydro-l-biopterin (BH4) due to decreased synthesis or increased oxidation to BH2 (8, 30). Increased circulating levels of BH2 and ADMA are found in patients with acute myocardial infarction (55). Electron paramagnetic resonance studies show that BH4-depleted eNOS generates superoxide that is increased by ADMA, l-NMMA, or l-arginine (6). Thus, under pathological conditions (e.g., diabetes, hypertension) associated with CKD, increased levels of ADMA may potentiate reactive oxygen species generation and oxidative stress.
Recent studies show that ADMA causes vascular and end-organ injury through the renin-angiotensin system (49). Angiotensin II receptor AT1R antagonists restore ADMA-induced flow reduction (53) and upregulate ADMA hydrolysis (36). ADMA also participates in endothelial cell senescence (41), renal fibrosis (18, 36), and monocyte adhesion via chemokine receptor activation (3). Therefore, ADMA may exacerbate the effects of angiotensin II on the vasculature and the target tissues.
The molecular and structural elements of the glomerular filtration barrier that are altered by methylarginines and result in an increase in Palb remain to be characterized. The principal structural components of the glomerular filtration barrier are the podocytes, capillary endothelial cells, and the basement membrane. As the increase in Palb occurs within 15 min of glomerular incubation with ADMA, it is unlikely that changes in expression (mRNA or protein) of key molecular elements (i.e., nephrin, podocin) of this barrier are an underlying mechanism. Rather, signaling events or posttranslational modifications of these or other proteins that are important in preserving the integrity of the barrier are likely mechanism of increased Palb.
In summary, these studies provide evidence that methylarginines should be viewed not only as predictors of cardiovascular events (23) but also as effectors of glomerular capillary injury. ADMA may qualify as a uremic toxin as it is a product of protein metabolism, it accumulates in the course of renal failure, it is a guanidine compound, and it can cause organ damage through vascular dysfunction. These studies also suggest that the redox state of sGC is important in preserving the integrity of the glomerular filtration barrier and that NO-independent sGC activators can preserve integrity of this barrier. Strategies to decrease plasma levels of methylarginines and/or to maintain NO-independent cGMP generation will contribute to successful management of CKD. Additionally, our results suggest that regulation of the glomerular filtration barrier by NO involves antagonism of superoxide, regulation of cGMP levels via activation of sGC, and interaction with proteins that regulate the glomerular filtration barrier. We speculate that direct interactions between such proteins and NO may result in protein amino acid nitration and nitrosylation and modulate the glomerular barrier function. Thus, NO participates in maintaining the physiological integrity of this barrier function at several planes and increased levels of ADMA in CKD may disrupt the fine balance between NO and O2•−.
GRANTS
This work was supported in part by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases/National Institutes of Health (NIH R01-061588) (E. T. McCarthy), Research Development Funds from the Department of Medicine, Medical College of Wisconsin (M. Sharma), by the George P. Livanos Research Support Fund, University of Athens, Athens, Greece (E. A. Lianos), and by NIH R01-HL-080173 (H. Miura). Some of the results were presented at the Annual Meeting of the American Society of Nephrology (2007 and 2008).
Acknowledgments
We thank Dr. R. S. Reddy and R. Singhal for excellent technical support and L. Brauer for expert administrative support. We thank Dr. A. Knorr (Bayer Health Care, Wuppertal, Germany) for providing the BAY 41-2272 compound.
REFERENCES
- 1.Araujo M, Welch WJ. Oxidative stress and nitric oxide in kidney function. Curr Opin Nephrol Hypertens 15: 72–77, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Baylis C Nitric oxide deficiency; both consequence and cause of chronic renal disease (CRD). Hypertens Nephrol 5: 193–201, 2001. [Google Scholar]
- 3.Chen M, Li Y, Yang T, Wang Y, Bai Y, Xie X. ADMA induces monocyte adhesion via activation of chemokine receptors in cultured THP-1 cells. Cytokine 43: 149–159, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Cross JM, Donald A, Vallance PJ, Deanfield JE, Woolfson RG, MacAllister RJ. Dialysis improves endothelial function in humans. Nephrol Dial Transplant 16: 1823–1829, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Dileepan KN, Ram S, Stechschulte DJ, Savin VJ. Effect of superoxide on albumin permeability of isolated rat glomeruli. J Lab Clin Med 121: 797–804, 1993. [PubMed] [Google Scholar]
- 6.Druhan LJ, Forbes SP, Pope AJ, Chen CA, Zweier JL, Cardounel AJ. Regulation of eNOS-derived superoxide by endogenous methylarginines. Biochemistry 47: 7256–7263, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Ferrario R, Takahashi K, Fogo A, Badr KF, Munger KA. Consequences of acute nitric oxide synthesis inhibition in experimental glomerulonephritis. J Am Soc Nephrol 4: 1847–1854, 1994. [DOI] [PubMed] [Google Scholar]
- 8.Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation 113: 1708–1714, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Gardiner SM, Kemp PA, Bennett T, Palmer RM, Moncada S. Regional and cardiac haemodynamic effects of NG, NG, dimethyl-l-arginine and their reersibility by vasodilators in conscious rats. Br J Pharmacol 110: 1457–1464, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giugliano D, Ceriello A, Paolisso G. Diabetes mellitus, hypertension and cardiovascular disease: which role for oxidative stress? Metabolism 44: 363–368, 1995. [DOI] [PubMed] [Google Scholar]
- 11.Greiber S, Münzel T, Kästner S, Müller B, Schollmeyer P, Pavenstädt H. NAD(P)H oxidase activity in cultured human podocytes: effects of adenosine triphosphate. Kidney Int 53: 654–663, 1998. [DOI] [PubMed] [Google Scholar]
- 12.Griffith OW, Stuehr DJ. Nitric oxide syntheses: properties and catalytic mechanism. Annu Rev Physiol 57: 707–736, 1995. [DOI] [PubMed] [Google Scholar]
- 13.Herrera M, Garvin JL. Recent advances in the regulation of nitric oxide in the kidney. Hypertension 45: 1062–1067, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 51: 211–217, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Homer KL, Wanstall JC. Cyclic GMP-independent relaxation of rat pulmonary artery by spermine NONOate, a diazeniumdiolate nitric oxide donor. Br J Pharmacol 131: 673–682, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huie RE, Padmaja S. The reaction of NO with superoxide. Free Radic Res Commun 18: 195–199, 1993. [DOI] [PubMed] [Google Scholar]
- 17.Ischiropoulos H Biological selectivity and functional aspects of protein tyrosine nitration. Biochem Biophys Res Commun 305: 776–783, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Jacobi J, Maas R, Cordasic N, Koch K, Schmieder RE, Böger RH, Hilgers KF. Role of asymmetric dimethylarginine for angiotensin II-induced target organ damage in mice. Am J Physiol Heart Circ Physiol 294: H1058–H1066, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Jones SA, Hancock JT, Jones OT, Neubauer A, Topley N. The expression of NADPH oxidase components in human glomerular mesangial cells: detection of protein and mRNA for p47phox, p67phox, and p22phox. J Am Soc Nephrol 5: 1483–1491, 1995. [DOI] [PubMed] [Google Scholar]
- 20.Keefer LK, Flippen-Anderson JL, George C, Shanklin AP, Dunams TM, Christodoulou D, Saavedra JE, Sagan ES, Bohle DS. Chemistry of the diazeniumdiolates. I. Structural and spectral characteristics of the N(O)NO functional group. Nitric Oxide 5: 377–394, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Kielstein JT, Impraim B, Simmel S, Bode-Böger SM, Tsikas D, Frölich JC, Hoeper MM, Haller H, Fliser D. Cardiovascular effects of systemic nitric oxide synthase inhibition with asymmetrical dimethylarginine in humans. Circulation 109: 172–177, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Kojima H, Nakatsubo N, Kikuchi K, Kawahara S, Kirino Y, Nagoshi H, Hirata Y, Nagano T. Detection and imaging of nitric oxide with novel fluorescent indicators: diaminofluoresceins. Anal Chem 70: 2446–2453, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Krzyzanowska K, Mittermayer F, Wolzt M, Schernthaner G. Asymmetric dimethylarginine predicts cardiovascular events in patients with type 2 diabetes. Diabetes Care 30: 1834–1839, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Leiper J, Vallance P. Biological significance of endogenous methylarginines that inhibit nitric oxide synthases. Cardiovasc Res 43: 542–548, 1999. [DOI] [PubMed] [Google Scholar]
- 25.MacAllister R, Whitley GS, Vallance P. Effects of guanidino and uremic compounds on nitric oxide pathways. Kidney Int 45: 737–742, 1994. [DOI] [PubMed] [Google Scholar]
- 26.McAllister RJ, Rambausek MH, Vallance P, Williams D, Hoffmann KH, Ritz E. Concentration of dimethyl-l-arginine in the plasma of patients with end-stage renal failure. Nephrol Dial Transplant 11: 2449–2452, 1996. [DOI] [PubMed] [Google Scholar]
- 27.McCarthy ET, Sharma R, Sharma M, Li JZ, Ge XL, Dileepan KN, Savin VJ. TNF-alpha increases albumin permeability of isolated rat glomeruli through the generation of superoxide. J Am Soc Nephrol 9: 433–438, 1998. [DOI] [PubMed] [Google Scholar]
- 28.McCarty MF Vascular endothelium is the organ chiefly responsible for the catabolism of plasma asymmetric dimethylarginine–an explanation for the elevation of plasma ADMA in disorders characterized by endothelial dysfunction. Med Hypotheses 63: 699–708, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell JA, Hecker M, Anggård EE, Vane JR. Cultured endothelial cells maintain their l-arginine level despite the continuous release of EDRF. Eur J Pharmacol 182: 573–576, 1990. [DOI] [PubMed] [Google Scholar]
- 30.Moens AL, Kass DA. Therapeutic potential of tetrahydrobiopterin for treating vascular and cardiac disease. J Cardiovasc Pharmacol 50: 238–246, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Morris SM Jr. Arginine: beyond protein. Am J Clin Nutr 83: 508S–512S, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Mount PF, Power DA. Nitric oxide in the kidney: functions and regulation of synthesis. Acta Physiol (Oxf) 187: 433–446, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Mundel P, Gambaryan S, Bachmann S, Koesling D, Kriz W. Immunolocalization of soluble guanylyl cyclase subunits in rat kidney. Histochem Cell Biol 103: 75–79, 1995. [DOI] [PubMed] [Google Scholar]
- 34.Nanayakkara PWB, Teerlink T, Stehouwer CDA, Allajar D, Spijkerman A, Schalkwijk C, Ter Wee PM, Van Guldener C. Plasma asymmetric dimethylarginine (ADMA) concentration is independently associated with carotid intima-media thickness and plasma soluble vascular cell adhesion molecule-1 (sVCAM-1) concentration in patients with mild-to-moderate renal failure. Kidney Int 68: 2230–2236, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Olken NM, Marletta MA. NG-methyl-l-arginine functions as an alternate substrate and mechanism-based inhibitor of nitric oxide synthase. Biochemistry 32: 9677–9685, 1993. [DOI] [PubMed] [Google Scholar]
- 36.Onozato ML, Tojo A, Leiper J, Fujita T, Palm F, Wilcox CS. Expression of NG,NG-dimethylarginine dimethylaminohydrolase and protein arginine N-methyltransferase isoforms in diabetic rat kidney: effects of angiotensin II receptor blockers. Diabetes 57: 172–180, 2008. [DOI] [PubMed] [Google Scholar]
- 37.Pou S, Keaton L, Surichamorn W, Rosen GM. Mechanism of superoxide generation by neuronal nitric oxide synthase. J Biol Chem 274: 9573–9580, 1999. [DOI] [PubMed] [Google Scholar]
- 38.Poulos TL Soluble guanylate cyclase. Curr Opin Struct Biol 16: 736–743, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Priviero FB, Baracat JS, Teixeira CE, Claudino MA, De Nucci G, Antunes E. Mechanisms underlying relaxation of rabbit aorta by BAY 41-2272, a nitric oxide-independent soluble guanylate cyclase activator. Clin Exp Pharmacol Physiol 32: 728–734, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Savin VJ, Sharma R, Lovell HB, Welling DJ. Measurement of albumin reflection coefficient with isolated rat glomeruli. J Am Soc Nephrol 3: 1260–1269, 1992. [DOI] [PubMed] [Google Scholar]
- 41.Scalera F, Martens-Lobenhoffer J, Bukowska A, Lendeckel U, Täger M, Bode-Böger SM. Effect of telmisartan on nitric oxide–asymmetrical dimethylarginine system: role of angiotensin II type 1 receptor gamma and peroxisome proliferator activated receptor gamma signaling during endothelial aging. Hypertension 51: 696–703, 2008. [DOI] [PubMed] [Google Scholar]
- 42.Schopfer FJ, Baker PR, Freeman BA. NO-dependent protein nitration: a cell signaling event or an oxidative inflammatory response? Trends Biochem Sci 28: 646–654, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Shah SV The role of reactive oxygen metabolites in glomerular disease. Annu Rev Physiol 57: 245–262, 1995. [DOI] [PubMed] [Google Scholar]
- 44.Sharma M, Sharma R, McCarthy ET, Savin VJ. The focal segmental glomerulosclerosis permeability factor: biochemical characteristics and biological effects. Exp Biol Med 229: 85–98, 2004. [DOI] [PubMed] [Google Scholar]
- 45.Sharma M, McCarthy ET, Savin VJ, Lianos EA. Nitric oxide preserves the glomerular protein permeability barrier by antagonizing superoxide. Kidney Int 68: 2735–2744, 2005. [DOI] [PubMed] [Google Scholar]
- 46.Smith CL, Anthony S, Hubank M, Leiper JM, Vallance P. Effects of ADMA upon gene expression: an insight into the pathophysiological significance of raised plasma ADMA. PLoS Med 2: e264, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stasch JP, Becker EM, Alonso-Alija C, Apeler H, Dembowsky K, Feurer A, Gerzer R, Minuth T, Perzborn E, Pleiss U, Schröder H, Schroeder W, Stahl E, Steinke W, Straub A, Schramm M. NO-independent regulatory site on soluble guanylate cyclase. Nature 410: 212–215, 2001. [DOI] [PubMed] [Google Scholar]
- 48.Stolk J, Hiltermann TJ, Dijkman JH, Verhoeven AJ. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am J Respir Cell Mol Biol 11: 95–102, 1994. [DOI] [PubMed] [Google Scholar]
- 49.Suda O, Tsutsui M, Morishita T, Tasaki H, Ueno S, Nakata S, Tsujimoto T, Toyohira Y, Hayashida Y, Sasaguri Y, Ueta Y, Nakashima Y, Yanagihara N. Asymmetric dimethylarginine produces vascular lesions in endothelial nitric oxide synthase-deficient mice: involvement of renin-angiotensin system and oxidative stress. Arterioscler Thromb Vasc Biol 24: 1682–1688, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Tojo A, Welch WJ, Bremer V, Kimoto M, Kimura K, Omata M, Ogawa T, Vallance P, Wilcox CS. Colocalization of demethylating enzymes and NOS and functional effects of methylarginines in rat kidney. Kidney Int 52: 1593–1601, 1997. [DOI] [PubMed] [Google Scholar]
- 51.Tran CTL, Leiper JM, Vallance P. The DDAH/ADMA/NOS pathway. Atherosclerosis Suppl 4: 3–40, 2003. [DOI] [PubMed] [Google Scholar]
- 52.Vallance P, Leone A, Calver A, Collier J, Moncada S. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet 339: 572–575, 1992. [DOI] [PubMed] [Google Scholar]
- 53.Veresh Z, Racz A, Lotz G, Koller A. ADMA impairs nitric oxide-mediated arteriolar function due to increased superoxide production by angiotensin II-NAD(P)H oxidase pathway. Hypertension 52: 960–966, 2008. [DOI] [PubMed] [Google Scholar]
- 54.Williams HC, Griendling KK. NADPH oxidase inhibitors: new antihypertensive agents? J Cardiovasc Pharmacol 50: 9–16, 2007. [DOI] [PubMed] [Google Scholar]
- 55.Yada T, Kaji S, Akasaka T, Mochizuki S, Ogasawara Y, Tanemoto K, Yoshida K, Kajiya F. Changes of asymmetric dimethylarginine, nitric oxide, tetrahydrobiopterin, and oxidative stress in patients with acute myocardial infarction by medical treatments. Clin Hemorheol Microcirc 37: 269–276, 2007. [PubMed] [Google Scholar]
- 56.Zhao Y, Brandish PE, Schelvis JPM, DiValentin M, Babcock GT, Marletta MA. Inhibition of soluble guanylate cyclase by ODQ. Biochemistry 39: 10848–10854, 2000. [DOI] [PubMed] [Google Scholar]