Abstract
Flow-induced cytosolic Ca2+ Cai2+ signaling in renal tubular epithelial cells is mediated in part through P2 receptor (P2R) activation by locally released ATP. The ability of P2R to regulate salt and water reabsorption has suggested a possible contribution of ATP release and paracrine P2R activation to cystogenesis and/or enlargement in autosomal dominant polycystic kidney disease (ADPKD). We and others have demonstrated in human ADPKD cyst cells the absence of flow-induced Cai2+ signaling exhibited by normal renal epithelial cells. We now extend these findings to primary and telomerase-immortalized normal and ADPKD epithelial cells of different genotype and of both proximal and distal origins. Flow-induced elevation of Cai2+ concentration ([Ca2+]i) was absent from ADPKD cyst cells, but in normal cells was mediated by flow-sensitive ATP release and paracrine P2R activation, modulated by ecto-nucleotidase activity, and abrogated by P2R inhibition or extracellular ATP hydrolysis. In contrast to the elevated ATP release from ADPKD cells in static isotonic conditions or in hypotonic conditions, flow-induced ATP release from cyst cells was lower than from normal cells. Extracellular ATP rapidly reduced thapsigargin-elevated [Ca2+]i in both ADPKD cyst and normal cells, but cyst cells lacked the subsequent, slow, oxidized ATP-sensitive [Ca2+]i recovery present in normal cells. Telomerase-immortalized cyst cells also exhibited altered CD39 and P2X7 mRNA levels. Thus the loss of flow-induced, P2R-mediated Cai2+ signaling in human ADPKD cyst epithelial cells was accompanied by reduced flow-sensitive ATP release, altered purinergic regulation of store-operated Ca2+ entry, and altered expression of gene products controlling extracellular nucleotide signaling.
Keywords: autosomal dominant polycystic kidney disease, telomerase, monocilium, shear stress, luciferase, fura 2
autosomal dominant polycystic kidney disease (ADPKD) is characterized by progressive enlargement of fluid-filled cysts originating from only 1–5% of nephrons. The disease affects between 1:400 and 1:1,000 people, and leads to end-stage renal disease in half of those affected. Between 70 and 85% of cases are caused by mutations in the PKD1 gene encoding the 4,302 amino acid (aa) protein polycystin-1 (PC1). PC1 includes a ∼3,000 aa NH2-terminal extracellular domain, ∼ 11 transmembrane spans, and a ∼200 aa COOH-terminal cytoplasmic tail. Most or all other cases of ADPKD are caused by mutations in the 968 aa polycystin-2 (PC2) (52), a cation channel interacting with and regulated by PC1, and forming heteromers with the TRPV4 and TRPC1 cation channels (1, 24, 53).
ATP release is a property shared by most cell types subjected to mechanical deformation (10, 41, 55). Epithelial monolayers of renal, respiratory, and gastrointestinal origin release ATP from both apical and basolateral surfaces (14, 20, 22, 28). The released nucleotides achieve concentrations sufficient to activate purinergic receptors, in turn regulating secretion and reabsorption of ions and water (26, 27, 35, 55). These effects of ATP on solute and water transport have encouraged the hypothesis that ATP release from cyst epithelial cells regulates cyst enlargement and possibly also cystogenesis in ADPKD (48, 60).
ADPKD cyst epithelial cells grown on permeable supports in primary culture released ATP at higher rates at rest and under hypotonic stress than did cells isolated from normal kidneys (60). ATP release can be detected in both apical and basolateral compartments. Cyst epithelial cells express P2X and P2Y receptors, which, upon activation, elevated cytosolic Ca2+ concentration ([Ca2+]i) and stimulated Cl− secretion by confluent cyst cell monolayers. Cysts microdissected from ADPKD kidneys also released ATP, and cyst fluid contained as much as 10 μM ATP, suggesting that ATP release from cyst epithelial cells might regulate cyst enlargement in ADPKD (48, 60). However, purinergic signaling may produce opposing effects on models of dominant and recessive cystic kidney disease (18, 54), and the role of purinergic signaling in early cystogenesis remains unclear.
Flow stimulates Cai2+ signaling in epithelial monolayers (42) by mechanisms proposed to include bending of the primary cilium (40, 43). Flow also regulates [Ca2+]i in intact renal tubules (14, 22, 27), contributing to modulation of ion and water transport in response to changes in luminal solute load and/or concentration (47, 55). The flow-induced Cai2+ signal evident in primary renal cortical epithelial cell monolayers isolated from mouse (33) and human kidney (34, 63) was absent from Pkd1−/− mouse embryonic collecting duct epithelial cells or cells isolated from human ADPKD cysts expressing lectin markers of nominal distal or proximal origin (33, 34, 63). Isolated, perfused collecting ducts from the orpk/orpk mouse model of recessive polycystic kidney disease exhibited attenuated flow-induced [Ca2+]i signaling apparent by postnatal day 14 (29). Reduced or absent flow-induced [Ca2+]i signaling also characterized primary embryonic orpk/orpk collecting duct epithelial monolayers, albeit accompanied by increased resting rates of apical Ca2+ entry (21, 50). In contrast, primary human ARPKD cyst epithelial cell monolayers exhibited enhanced flow-induced [Ca2+]i signaling (44).
Since flow stimulates both ATP release and elevation of [Ca2+]i, the relationship between these two events is of interest. Flow-stimulated elevation of [Ca2+]i in isolated, perfused mouse medullary thick ascending limb (MTAL) was attenuated by P2R blockade and by extracellular ATP hydrolysis (22), and was reduced in MTAL from P2y2−/− mice (22). P2R inhibition and extracellular ATP hydrolysis reduced spontaneous [Ca2+]i oscillations in resting Madin-Darby canine kidney (MDCK) monolayers and in perfused mouse MTAL. Spontaneous [Ca2+]i oscillation amplitude was also reduced in amplitude in a P2R-deficient cell line and in the perfused P2y2−/− MTAL (14). P2R inhibition and ATP hydrolysis nearly abolished flow-induced elevation of [Ca2+]i in established human and rat cholangiocyte cell lines (62).
We therefore tested the hypotheses that the normal flow-induced elevation of [Ca2+]i that is deficient in primary human ADPKD cyst epithelial cells is mediated by paracrine purinergic signaling, and that defective purinergic signaling might explain in part the loss of flow-sensitive Cai2+ elevation in ADPKD cells.
We found that flow-induced Cai2+ signaling of primary human epithelial cells is preserved in telomerase-immortalized normal kidney epithelial (NK cell lines), and its absence in ADPKD cyst cells is preserved in telomerase-immortalized cell lines of defined germline PKD1 genotype. ADPKD cyst cells exhibited enhanced resting and hypotonicity-induced ATP release. In contrast, flow-induced ATP release from ADPKD cyst cells was markedly reduced compared with that of normal renal epithelial cells, despite near-normal nucleotide-induced peak [Ca2+]i elevations in cyst cells. Purinergic control of [Ca2+]i in thapsigargin-pretreated cells was also altered in ADPKD cyst cells, and thapsigargin-sensitive stores were reduced. Variations in P2R and ecto-nucleotidase gene expression by cyst cell lines were also detected. We conclude that loss of flow-sensitive Cai2+ signaling in human ADPKD cyst epithelial cells is due in part to reduced flow-sensitive ATP release and is accompanied by altered purinergic responsiveness in the setting of nominal store depletion.
METHODS
Cell culture.
Discarded portions of human kidney cortex were harvested according to CCI/IRB protocols reviewed and approved at the Indiana University School of Medicine and Beth Israel Deaconess Medical Center. NK cortical tubular epithelial cells from individual NK57M03 and ADPKD cyst cells from an anonymized ADPKD patient undergoing nephrectomy were grown in primary culture as described (63). Aliquots of NK cells from the same individual (NK57M03) and aliquots of ADPKD cyst cells from a different, anonymized ADPKD patient kidney were similarly grown in primary culture. These pools of primary cells were referred to as NK and PKD cells (Supplemental Table 1; all supplementary material for this article are accessible on the journal web site).
Primary NK and PKD cells were subjected to fluorescence-activated cell sorting (FACS). Cells were separated according to intensity of the bound fluorophore-conjugated lectins Lotus tetragonolobus agglutinin (LTA; a marker of proximal nephron origin) and Dolichos biflorus agglutinin (DBA; a marker of distal nephron origin; Vector Laboratories, Burlingame, CA) (6, 49, 63). Marker-enriched, sorted cell pools were transformed with human telomerase (hTERT) cDNA as previously described (5, 65). The EcoRI fragment from pGRN145 encoding hTERT cDNA was subcloned into retroviral vector pBABEneo. Recombinant viruses were generated by electroporation-transfection of the ecotropic packaging line PE501 followed by selection with 400 μg/ml G418. Supernatants containing amphotropic virus released from confluent cultures were filtered through 0.45-μm filters. This virus preparation was used to infect NK and PKD cells, which were clonally selected in G418 and expanded.
Cloned cells were maintained in Clonetics REGM Renal Epithelial Cell Medium (Lonza, Portsmouth, NH) in the presence of penicillin/streptomycin and G418. After seven passages, G418 was removed from the growth medium. Cells for experiments were transferred to glass coverslips coated with Vitrogen collagen (Conhesion Technologies, Palo Alto, CA), fed every other day with REGM, and grown to confluence 5–6 days after plating, at which time they were used for experiments. Primary cells were used through passage 6 only. The hTERT-transformed cells were passaged weekly and maintained a stable growth rate and phenotype through passage 34 and beyond (Bacallao R, unpublished observations). These hTERT-immortalized cell lines were referred to as LTA+-NKTERT, DBA+-NKTERT, LTA+-PKDTERT, and DBA+-PKDTERT (Supplemental Table 1).
Genomic DNA analysis.
Genomic DNA was extracted from LTA+-PKDTERT cells by the salting-out method. All coding exons and splice junctions of the PKD1 and PKD2 genes were amplified by PCR from genomic DNA (46, 63) and directly sequenced on both strands. The duplicated region of the PKD1 gene was amplified with rTth DNA polymerase (PerkinElmer Applied Biosystems, Foster City, CA) in the supplied DMSO-containing buffer. Primers were either anchored in the single-copy region of the gene or mismatched with the homologous gene sequences to generate five, locus-specific Long Range PCR fragments of 3–9 kb in length. The DNA sequencing allowed identification of heterozygous variation in gene sequence. Any mutation found was confirmed with a second, independent amplification.
RNA preparation and RT-PCR.
Total RNA from NK and PKD cyst cells was isolated with an RNeasy kit (Qiagen, Valencia, CA). Human kidney total RNA was purchased from Ambion (Woodlands, TX). Reverse transcription was performed with a Retroscript first-strand cDNA synthesis kit (Ambion) using 1 μg of total RNA. Of the reaction volume, 5% was used for PCR with HotStart DNA polymerase (Qiagen) in a total reaction volume of 50 μl in the supplier's recommended buffer. cDNA fragments were amplified using specific primer pairs and annealing conditions for P2Y and P2X receptor products (Supplemental Table 2) and for CD39 family ecto-nucleoside triphosphate diphosphohydrolase products (NTPDases; Supplemental Table 3). Enzyme activation and initial template denaturation were at 95°C for 15 min, followed by 35–38 cycles of 45 s at 94°C, 2 min at 52–60°C, and 2 min at 72°C, with a 7-min final extension step at 72°C. PCR products were separated on 1% agarose gels, visualized with ethidium bromide, purified with a Qiagen Gel Extraction kit, and validated by DNA sequence analysis. PCR product abundance was documented (GelDoc, Bio-Rad) and semiquantitated with ImageJ (National Institutes of Health). PCR cycle number was adjusted to ensure detection of low-abundance transcripts and to maintain higher abundance transcripts within the log-linear range of amplification.
Confocal immunofluorescence microscopy.
Cell monolayers grown on glass coverslips were fixed for 30 min at room temperature with 140 mM NaCl and 10 mM sodium phosphate, pH 7.4 (PBS) containing 3% (wt/vol) paraformaldehyde. Fixed cells were extensively rinsed with PBS, quenched with 50 mM lysine HCl, pH 8.0, exposed to 1% SDS in PBS for 15 min for permeabilization and epitope unmasking, and blocked for 15 min in PBS with 1% bovine serum albumin and 0.05% saponin. For lectin staining, cells were incubated for 2 h with FITC-conjugated LTA or with FITC-conjugated DBA, then washed. For PC1 and PC2 staining, after 4°C overnight incubation with 1:100 dilutions of rabbit polyclonal antibody NM005 against human PC1 COOH-terminal aa 4070–4302 or rabbit polyclonal antibody against GST fusion protein with human PC2 C-terminal aa 687–968, coverslips were incubated with Cy3-conjugated donkey anti-rabbit Ig secondary antibody (1:500, Jackson ImmunoResearch, West Grove, PA) for 2 h at room temperature as described (63). Some coverslips immunostained and fixed as above for PC1 or PC2 were subsequently stained with mouse monoclonal antibody to the ciliary marker N-acetylated α-tubulin (1:500, Sigma, St. Louis, MO). P2X7 immunostaining was performed by overnight incubation (1:100) with polyclonal antibody to rat P2X7 intracellular COOH-terminal tail aa 576–595 KIRKEFPKTQGQYSGFKYPY (Alomone, Jerusalem, Israel). Anti-CD39 immunostaining with mAb clone BU-61 (Bayport, MN) was performed by overnight incubation of fixed cells, followed by PBS wash and visualization with Cy3-conjugated donkey anti-mouse Ig secondary antibody (1:500). In some experiments as indicated, antibodies were pre- and coincubated with excess specific peptide antigen. Immunostained cells on coverslips were imaged with a Zeiss LSM 510 Meta confocal laser scanning microscope.
Measurement of [Ca2+]i.
NK and PKD cells cultured to confluence on collagen-coated 35-mm glass coverslips were loaded with 5 μM fura 2-AM (Molecular Probes, Eugene, OR) in HEPES-buffered HBSS (pH 7.4) at 37°C for 30 min. Extracellular fura 2-AM was removed by washing twice with HEPES-HBSS. Coverslips were then mounted into the base of a parallel-plate flow chamber 0.5 cm in width and 0.0254 cm in depth (GlycoTech, Gaithersburg, MD) and perfused with a Harvard Syringe pump at 37°C using a calibrated, in-line heater (WPI, Sarasota, FL), with monitoring of inflow and outflow temperatures. Perfusion medium (Hanks’ solution) composition was (in mM) 127 NaCl, 5.4 KCl, 1.27 CaCl2, 1 MgCl2, 5.6 glucose, and 11.6 HEPES, final pH 7.4. [Ca2+]i was measured by fluorescence ratio imaging with a Metafluor digital imaging system (Universal Imaging, West Chester, PA) equipped with an Olympus IMT-2 inverted microscope, and a CoolSNAP CCD camera (Photometrics, Tucson, AZ). Fura 2 emission images were recorded at 510 nm during alternating excitation at 340 and 380 nm. Fura 2 fluorescence ratio values determined by in situ calibration in immortalized epithelial cells did not differ from values determined by in vitro calibration for [Ca2+]i, and so were calibrated in vitro with the same experimental settings for the imaging system, using a Ca2+ Calibration Kit No. 2 (Molecular Probes) with concentrations between 36 nM and 4 μM (32). The minimal fluorescence ratio (Rmin) was determined at “zero Ca2+” (<10 nM) and the maximal fluorescence ratio (Rmax) at 4 μM total Ca2+. The equilibrium constant (Kb) was determined by fitting experimental fluorescence ratio R values at various free [Ca2+] with the equation [Ca2+]free = Kb (Sf2/Sb2)[(R − Rmin)/(Rmax − R)], where the factor Sf2/Sb2 corrects for fura 2 ion selectivity at 380 nm. For each coverslip, one visual field was selected as a region of interest, recorded before and during imposition of a uniform rate of fluid flow. Shear stress τω was calculated as τω = 6μQ/a2b, where μ = apparent viscosity of superfusate (1.00 for H2O at 20°C, 0.70 at 37°C), Q = volumetric flow rate (ml/s), and a and b = flow chamber depth and width, respectively.
Measurement of ATP release.
To measure flow-induced ATP release, confluent monolayers of 5 × 104 NK or PKD cells mounted in the Glycotech chamber were subjected to defined laminar flow rates at 37°C as described above. Perfusate volumes of at least 200 μl were collected at a single time for each flow rate. At 0.1 dyne/cm2 (flow of 50 μl/min), the collection period was 6 min (including 2 min to fill the 100 μl of initially empty postchamber dead space at the start of flow, followed by 4 min to collect 200 μl containing ATP released under flow, in addition to that accumulated in the 25-μl chamber volume during the 15-min preincubation period before the initiation of flow). At both 0.75 dyne/cm2 (flow of 320 μl/min) and 10 dyne/cm2 (flow of 5,000 μl/min), the collection period was 1 min.
Tonicity-regulated ATP release was measured after 30-min exposure of 5 × 105 confluent cells in 35-cm culture dishes to isotonic Hanks’ solution or to a hypotonic 4:6 vol/vol mixture of Hanks’ solution and distilled water, with a relative osmolarity of 40%. ATP concentration was measured by luciferin-luciferase assay (Sigma). Duplicate 100-μl aliquots of perfusate or incubation medium were added to glass tubes containing 100 μl ATP assay mix dilution buffer (25-fold dilution), and luminescence intensity was measured (Lumat LB 9507, Berthold, Oak Ridge, TN). Flow-induced ATP release from 5 × 104 cells was expressed as picomoles per minute. Tonicity-regulated ATP release from 5 × 105 cells during a 30-min period was expressed as picomoles. Identity of the ATP-associated luciferase signal was confirmed by its complete abolition after apyrase treatment.
ATP concentrations of samples were extrapolated from a calibration curve constructed with ATP standards in isotonic Hanks’ solution. Although chloride can inhibit luciferase (8, 19), several subsequent studies of ATP release by renal and respiratory epithelial cells exposed to low [Cl−] hypotonic solutions have shown luciferase light emission across this range of [Cl−] to not differ significantly (21, 45, 51, 60) or to differ by <10% (35).
Statistics.
Data are presented as means ± SE for n measurements. Means of the data were compared by Student's two-tailed unpaired t-test, with P < 0.05 defined as the threshold for significance.
RESULTS
Shear stress elevates [Ca2+]i in both primary and telomerase-immortalized NK epithelial cells.
We showed previously that confluent primary human NK cells expressing predominantly the proximal nephron marker LTA exhibit flow-sensitive Cai2+ signaling, whereas predominantly LTA+ primary ADPKD cyst cells harboring the germline mutation ΔL2433 lack this response (63). Previous reports have suggested that shear sensitivity of human and mouse NK cells expressing the distal marker DBA (33, 34) is higher than that of human primary LTA+-NK cells (63). We therefore generated and studied hTERT-immortalized clonal isolates of NK and ADPKD cyst cells of nominal proximal origin (LTA+) and distal origin (DBA+). The latter are shown in Supplemental Fig. 1.
LTA+-PKDTERT cells harbor the heterozygous C-to-T transition at nucleotide 12010 of PKD1 cDNA (counting A of the ATG imitator codon as +1), converting amino acid residue Q4004 of polycystin-1 (PC1) to a nonsense codon (Q4004X; Supplemental Fig. 2). The previously reported PKD1 Q4004X mutation (39) encodes a polypeptide lacking the COOH-terminal 300 aa of PC1, a region encompassing terminal transmembrane spans and the COOH-terminal cytoplasmic tail. No mutation was found in the coding region of the PKD2 gene of LTA+-PKDTERT cells.
LTA+-NKTERT cells subjected to superfusion at low (Fig. 1A, 0.75 dyne/cm2) and high (Fig. 1C, 10 dyne/cm2) levels of shear stress transiently elevated [Ca2+]i, whereas LTA+-PKDTERT cyst cells lines failed to elevate [Ca2+]i in response to superfusion at either low (Fig. 1B) or high (Fig. 1D) shear stress. Similar differences between LTA+-NKTERT and LTA+-PKDTERT cells were observed at shear stress levels of 0.32 and 2.3 dyne/cm2 (not shown). [Ca2+]i elevation in NK cells remained at a submaximal plateau value in the presence of higher shear stress (Fig. 1C). Thus hTERT immortalization and cell cloning did not change the flow-sensitivity phenotypes of either LTA+-NK or LTA+-PKD cells.
Fig. 1.
Human telomerase (hTERT)-immortalized, clonal Lotus tetragonolobus agglutinin (LTA+) autosomal dominant polycystic kidney disease (ADPKD) cyst cells (LTA+-PKDTERT) lack the flow-sensitive elevation in cytosolic Ca2+ concentration ([Ca2+]i) exhibited by LTA+-NKTERT cells, where NK refers to normal kidney. Imposition of laminar flow at t = 0 increases [Ca2+]i in LTA+ NKTERT cells at low (0.75 dyne/cm2; A) and high levels of shear stress (10 dyne/cm2; C). In contrast, laminar flow fails to increase [Ca2+]i in LTA+-PKDTERT cells in response to shear stress at low (B) or high levels (D). Values are means ± SE for (n) coverslips, with fura 2 ratio recorded from a single ×20 visual field encompassing 40–60 confluent cells on each coverslip.
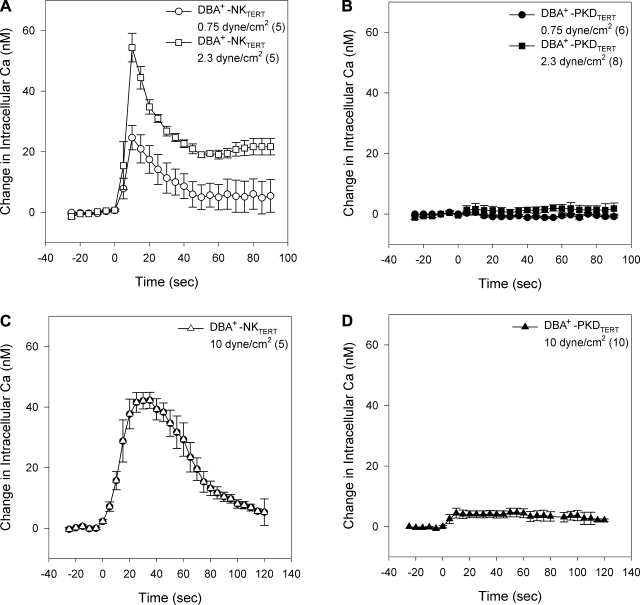
DBA+-NKTERT cells also elevated [Ca2+]i in response to low levels of shear stress (Fig. 2A), with a peak response at lower shear stress (2.3 dyne/cm2) than that exhibited by primary (63) and by LTA+-NKTERT cells at 10 dyne/cm2 (Fig. 1). In contrast, [Ca2+]i in DBA+-PKDTERT cells was unresponsive to either low (Fig. 2B) or high levels (Fig. 2D) of shear stress. As was true for primary NK and ΔL2433 PKD cells (63), resting [Ca2+]i did not differ between LTA+-NKTERT and Q4004X LTA+-PKDTERT cyst cells (not shown). Thus the loss of flow-induced [Ca2+]i elevation characterizes primary and immortalized ADPKD cyst cells of distinct nephron segment origin, and bearing distinct heterozygous germline mutations.
Fig. 2.
hTERT-immortalized, clonal Dolichos biflorus agglutinin (DBA+) ADPKD cyst cells (DBA+-PKDTERT) lack the flow-sensitive elevation in [Ca2+]i exhibited by DBA+-NKTERT cells. Imposition of laminar flow at t = 0 increases [Ca2+]i in DBA+-NKTERT cells at low levels of shear stress (0.75 or 2.3 dyne/cm2; A). Higher shear stress (10 dyne/cm2; C) slightly reduces the magnitude of peak [Ca2+]i, but prolongs peak duration. In contrast, laminar flow fails to increase [Ca2+]i in DBA+-PKDTERT cyst cells in response to shear stress at low (B) or high levels (D). Values are means ± SE for (n) coverslips, with 40–60 confluent cells/×20 visual field on each coverslip.
LTA+-PKDTERT cells form primary cilia lacking PC1.
Among their multiple sites of subcellular localization (25), PC1 and PC2 are expressed along with many other cystic kidney disease gene products in the primary cilium (33, 34, 63). Figure 3 shows PC1 (Fig. 3A, left) and PC2 (Fig. 3A, right) in the cilium of LTA+-NKTERT cells. However, PC1 was undetectable (Fig. 3B, left) and PC2 nearly so (Fig. 3B, right) in LTA+-PKDTERT cell cilia 5.0 ± 0.2 μm in length (n = 11), indistinguishable from the 4.8 ± 0.3-μm (n = 19) length of LTA+-NKTERT cilia. Ciliary PC2 localization in the x-y plane is shown in Supplemental Fig. 3. DBA+-NKTERT cell cilia of 8.7+0.4 μM in length (n = 21), and the slightly shorter DBA+-PKDTERT cilia of 7.4 ± 0.3 μM in length (n = 25; P < 0.05 compared with NK) each were significantly longer than LTA+-TERT cells (P < 0.01). Thus clonal PKDTERT cells heterozygous for the PC1 germline mutation Q4004X resemble mixed primary PKD cyst cells with the PC1 germline mutation ΔL2433 in their absence of detectable ciliary PC1, but they are more severely impaired in ciliary PC2 localization than were ΔL2433 primary cyst cells, 30% of which expressed apparently normal ciliary levels of PC2 (63). Extraciliary localization of PC1 and PC2 in NKTERT and ADPKDTERT cells did not differ (Supplemental Fig. 4).
Fig. 3.
Ciliary localization of polycystins PC1 and PC2 is reduced or absent in PKDTERT cells. Confocal x-z plane reconstructions of LTA+-PKDTERT cells show undetectable ciliary PC1 (B, left) and reduced levels of ciliary PC2 (B, right), as judged by colocalization with N-acetylated α-tubulin in contrast to the presence of PC1 (A, left) and PC2 (A, right) in cilia of LTA+-NKTERT cells. Scale bar = 10 μm.
Shear stress-induced net ATP release is selectively reduced in PKD cyst cells compared with NK cells.
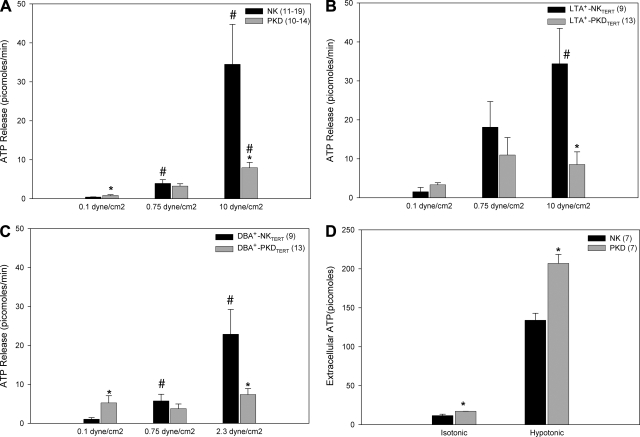
NKTERT cells released ATP in response to various uncontrolled types of mechanical stimulation (not shown), including complete replacement of medium, tilting of the monolayer, and touching of the cell monolayer surface with a glass pipette, as reported with other cell types (16, 59). Although slow superfusion at 0.1 dyne/cm2 produced little net ATP release into the medium, the net ATP release at this low flow rate from primary ΔL2433 cells (Fig. 4A) and from Q4004X PKDTERT cells (Fig. 4, B and C) exceeded that from NK (Fig. 4A) or NKTERT cells (Fig. 4, B and C). This elevated net ATP release was especially marked in DBA+-PKDTERT cells (Fig. 4C), consistent with previous reports of elevated resting net ATP release by primary ADPKD cyst cells (48). Low (0.75 dyne/cm2) and high flow (10 dyne/cm2) elicited higher magnitudes of net ATP release from primary NK and PKD cells (Fig. 4A) and from LTA+ (Fig. 4B)-or DBA+ (Fig. 4C)-NKTERT and PKDTERT cells. Primary ΔL2433 ADPKD cyst cells (Fig. 4A) and Q4004X LTA+-PKD cells (Fig. 4B) released substantially less net ATP in response to 10 dyne/cm2 shear stress than did the corresponding NK cells (P < 0.05). Q4004X DBA+-PKD cells (Fig. 4C) released less net ATP in response to 2.3 dyne/cm2 shear stress than did NK cells. In contrast, primary ΔL2433 PKD cyst cells at rest in isotonic and hypotonic conditions released significantly more net ATP than did primary NK cells (Fig. 4D; P < 0.05). Apyrase treatment of the collected perfusates abolished the luciferase signal, confirming its source as released ATP (n = 3, data not shown). The applied shear stresses and times did not detach, loosen, or morphologically alter the cells under study, and cessation of flow returned [Ca2+]i to preflow levels in all cases. The results demonstrate a selective defect in shear stress-induced net ATP release from both primary ΔL2433 primary PKD cells and Q4004X PKDTERT cells.
Fig. 4.
Shear stress-induced net ATP release from ADPKD cyst cells is greater than from NK cells. Primary NK and PKD cells (5 × 104; A), LTA+-NKTERT and LTA+-PKDTERT cells (B), or DBA+-NKTERT and DBA+-PKDTERT cells (C) were subjected to shear stress at the indicated levels. PKD cells of each type exhibited more net ATP release than did corresponding NK cells at the lowest tested flow. In contrast, PKD cells of each type exhibited far less net ATP release than did corresponding NK cells in response to the highest tested flow. D: hypotonic stress increased net ATP release from primary NK and primary PKD cells (5 × 105 cells). In both isotonic and hypotonic static (no-flow) conditions, net ATP release from primary PKD cells exceeded that from primary NK cells. As noted in the description of the luciferase assay in methods, the values for ATP release into hypotonic (low [Cl−]) medium may overestimate ATP release compared with isotonic values by as much as 10% or more. Black bars, NK cells; gray bars, ADPKD cells. *P < 0.05 for PKD or PKDTERT cells compared with NK or NKTERT cells in the same condition. #P < 0.05 for cells at intermediate or high shear stress compared with the same cell type at 0.1 dyne/cm2.
Shear stress-induced ATP release mediates flow-induced Cai2+ in NK cells.
We hypothesized that flow-induced ATP release might mediate shear stress-induced [Ca2+]i increase in NK cells. Thus primary NK cells were pretreated with P2 receptor blockers or with the ATP hydrolase apyrase for 10 min before the onset of flow at 10 dyne/cm2 shear stress in the continued presence of the drugs. As shown in Fig. 5A, flow-induced elevation in [Ca2+]i was abolished by apyrase (5 U/ml) and by the semiselective P2Y receptor antagonist suramin (300 μM). Figure 5B shows that the semiselective P2X receptor antagonist PPADS (100 μM) also abolished the flow-induced [Ca2+]i increase. Suramin also greatly reduced flow-induced [Ca2+]i elevation in LTA+-NKTERT cells (Fig. 5C) and abolished it in DBA+-NKTERT cells (Fig. 5D). Conversely, inhibition of ATP hydrolysis with the 5′-ecto-nucleotidase inhibitor ARL67156 (300 μM) prolonged the plateau-phase duration of [Ca2+]i elevation in response to both high and low shear stress (Fig. 6, A and B). This potentiation suggests that released ATP is actively degraded by cellular nucleotidase activities and that the values of net ATP released in response to hypotonic swelling (Fig. 4D) and in response to shear stress (Fig. 4, A–C) are underestimates. The data indicate that released ATP is a crucial intermediate in the transduction of a flow signal leading to elevation of [Ca2+]i in NK cells. Defective flow-sensitive autocrine/paracrine ATP release by PKD cyst cells likely contributes to their inability to elevate [Ca2+]i in response to flow.
Fig. 5.
ATP released in response to flow mediates flow-induced Cai2+ signaling by NK cells. A: primary NK cell [Ca2+]i increase in response to 10 dyne/cm2 shear stress was monitored in absence or continued presence of the nucleotidase apyrase (5 U/ml) or the P2Y receptor antagonist suramin (300 μM). B: primary NK cell [Ca2+]i increase in response to 10 dyne/cm2 shear stress was monitored in absence or continued presence of the P2X receptor antagonist PPADS (100 μM). C: LTA+-NKTERT cell [Ca2+]i increase in response to 10 dyne/cm2 shear stress was monitored in the absence or continued presence of suramin. D: DBA+-NKTERT cell [Ca2+]i increase in response to 2.3 dyne/cm2 shear stress was monitored in the absence or continued presence of suramin.
Fig. 6.
Ecto-nucleotidase inhibition prolongs the flow-induced elevation of [Ca2+]i. NK cell [Ca2+]i increase in response to 10 (A) or 0.75 dyne/cm2 (B) shear stress was monitored in absence (○) or continued presence of the 5′-ectonucleotidase inhibitor ARL67156 (300 μM, •).
PKDTERT cyst cells exhibit intact ATP-induced [Ca2+]i elevation but modestly reduced [Ca2+]i elevation in response to other nucleotide P2 receptor ligands.
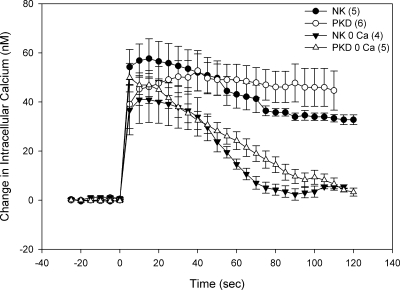
Since PKDTERT cells lack flow-responsive Cai2+ signaling, and exhibit decreased flow-responsive net ATP release, we tested the possibility that ATP responsiveness was also deficient. Exogenous extracellular ATP triggered similar [Ca2+]i responses in primary NK and ΔL2433 PKD cells (63). Exposure to extracellular ATP elevated [Ca2+]i in both LTA+-NKTERT and Q4004X LTA+-PKDTERT cells, and differed only in the more prolonged and stable plateau phase of the PKDTERT cells (Fig. 7; P = 0.03 at 90 s, 0.01 at 100 s compared with NKTERT). Nominally Ca2+-free medium did not change peak ATP-induced [Ca2+]i in PKDTERT cells but reduced the peak by ∼30% in NKTERT cells. Ca2+-free medium also accelerated the [Ca2+]i decline from peak ATP-stimulated values in both NKTERT and PKDTERT cells (Fig. 7). Similar [Ca2+]i differences between cell lines and conditions were evident at 20°C (not shown). The nucleotide-induced Cai2+ increase in primary NK cells was elicited by nucleotides in a concentration-dependent manner with the rank order of potency UTP > ATP > ADP (Supplemental Fig. 5A) and in ΔL2433 PKD cells UTP = ATP > ADP (Supplemental Fig. 5B). Similar peak [Ca2+]i elevations and rank orders of potency in response to 10 μM UTP, ADP, and ATP were measured in LTA+ (Suppl. Fig. 5C) and DBA+ (Suppl Fig. 5D) clones of NKTERT and Q4004X PKDTERT cells. These findings are consistent with the previously reported expression of multiple functional P2X and P2Y receptor subtypes in human primary cultures of normal and ADPKD renal epithelia (48).
Fig. 7.
NKTERT and PKDTERT cells exhibit similar [Ca2+]i elevation and extracellular Ca2+ dependence of that elevation in response to exogenous ATP. LTA+-NKTERT (filled symbols) and LTA+-PKDTERT cells (open symbols) were treated at t = 0 with 10 μM ATP in the presence (circles) or nominal absence of extracellular Ca2+ (triangles) as indicated.
P2 receptor mRNA expression in NKTERT and PKDTERT cyst cells.
The apparent role of purinergic signaling in renal epithelial cell flow sensitivity prompted RT-PCR survey screening of P2 receptor mRNA expression in NKTERT and PKDTERT cells. Among P2Y receptors, amplification of P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y13, and P2Y14 mRNAs yielded single bands (Fig. 8, A and B). P2Y12 mRNA was undetectable in NKTERT and PKDTERT cells (after 38 cycles), although present in the human kidney (Fig. 8B). Among P2X receptors, P2X1 and P2X2 mRNAs were undetected in NKTERT and PKDTERT cells, despite their presence in the human kidney (Fig. 8C). Consistent with the absence of P2X1 mRNA, neither LTA+-NKTERT nor LTA+-PKDTERT cells elevated [Ca2+]i in response to the P2X1 agonist β-methyl-ATP (100 μM; n = 3–4, not shown). mRNAs encoding P2X4, P2X5, P2X6, and P2X7 mRNAs were detected in NKTERT and PKDTERT cells of both lectin types (Fig. 8, C–E).
Fig. 8.
RT-PCR analysis of P2 receptor mRNA expression in NK and ADPKD cells. mRNA expression of P2Y (A and B) and P2X receptor subtype mRNAs (C–E) in human kidney (K) and in confluent 5-day cultures of LTA+-NK (NL) and LTA+-PKD (PL) was assessed by RT-PCR. D and E: P2X6 and P2X7 mRNA levels were also assessed in DBA+-NK (ND) and DBA+-PKD cells (PD). F: summary of similar experiments, measuring intensity of both amplification products. Values are means ± SE; n = 3–4. **P < 0.01.
Human kidney mRNA consistently yielded two P2X7 amplification products of similar intensity (Fig. 8E). The larger product included intron 10 and encoded a P2X7 polypeptide with a severely truncated COOH-terminal cytoplasmic tail (data not shown), encoding a polypeptide retaining partial cation channel function but unable to mediate the slower nonspecific pore formation (3) and endowed with dominant negative properties (2, 9). RT-PCR amplification product bands of each P2X7 transcript were of lower intensity in LTA+- and DBA+-PKDTERT cells than in NKTERT cells of corresponding lectin status (Fig. 8, E and F). Two previously reported transcript variants of P2X6 (7) were also detected, but neither RT-PCR amplification product band varied consistently between NKTERT and PKDTERT cells (n = 3, not shown).
P2X7 protein expression in NKTERT and PKDTERT cells.
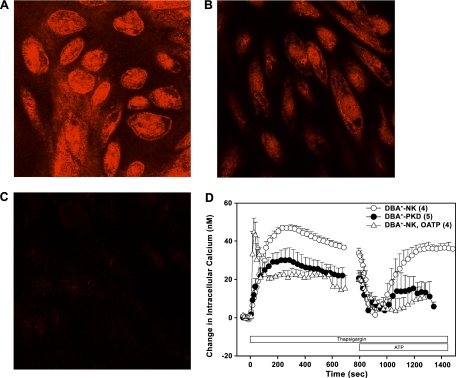
Both DBA+-NKTERT (Fig. 9A) and DBA+-PKDTERT cells (Fig. 9B) revealed a punctate distribution of P2X7 immunostaining at the cell periphery, with greater signal intensity in intracellular compartments. Preadsorption of the P2X7 antibody with its peptide antigen completely abolished these signals (Fig. 9C). P2X7 staining intensity was lower in DBA+-PKDTERT cells than in DBA+-NKTERT cells (Fig. 9, A and B). Similar differences were noted between LTA+-NK and LTA+-PKD cells (not shown). Thus PKDTERT cells express reduced levels of P2X7 mRNA and polypeptide. The [Ca2+]i response to ATP was examined in thapsigargin-treated DBA+-NKTERT and DBA+-PKDTERT cells (Fig. 9E) to isolate a P2X receptor component of Ca2+ signaling in these cells (64). The thapsigargin-induced elevation of [Ca2+]i in NK cells (○) was attenuated in PKDTERT cells (•), as observed previously in primary PKD cells (63). NKTERT cell pretreatment with the P2X7 antagonist oxidized ATP (O-ATP; 300 μM, ▵) enhanced the rate of [Ca2+]i rise elicited by thapsigargin, but attenuated the magnitude of the plateau phase. Subsequent exposure to 1 mM ATP, a concentration sufficient for near-maximal P2X7 activation, produced a biphasic effect on [Ca2+]i. In the first phase, ATP acutely reduced the thapsigargin-elevated [Ca2+]i to near resting levels. In NKTERT cells, this rapid fall in [Ca2+]i was followed within ∼30 s by a slower secondary recovery phase, during which [Ca2+]i regained its plateau value (Fig. 9E). The recovery phase was strongly inhibited to similar extents by both P2X7 antagonists O-ATP and (not shown) 1-[N,O-bis(5-isoquinoline sulphonyl)-N-methyl-l-tyrosyl]-4-phenylpiperazine (KN-62; 10 μM; n = 3). This secondary recovery phase was severely attenuated in PKDTERT cells (Fig. 9D), a phenotype consistent with reduced P2X7 expression in these cyst cells.
Fig. 9.
Reduced P2X7 protein and a reduced index of P2X7 function in ADPKD cyst cells. P2X7 immunostaining in DBA+-NK cells (A), DBA+-PKD cells (B), and DBA+-NK cells in the added presence of peptide antigen (C). Scale bar = 10 μm. D: biphasic effect of 1 mM extracellular ATP on [Ca2+]i in 500 nM thapsigargin-treated NKTERT cells (○) and PKDTERT cells (•) and in thapsigargin-treated NKTERT cells pretreated with and in the continued presence of the P2X7 antagonist oxidized ATP (O-ATP; 300 μM, ▵).
CD39 gene family mRNA expression in NKTERT and PKDTERT cells.
Since pharmacological inhibition of ecto-nucleotidase activity markedly prolonged the plateau phase of flow-induced [Ca2+]i elevation in NKTERT cells (Fig. 6), we assessed expression of CD39 gene family members encoding nucleoside triphosphate diphosphohydrolases expressed in the kidney (11, 23, 56). Comparative RT-PCR screening (Supplemental Fig. 6) revealed greatly reduced band intensities of CD39 RT-PCR amplification products in both LTA+- and DBA+-PKDTERT cells compared with lectin-matched NKTERT cells. In contrast, band intensities of RT-PCR amplification products encoding CD39L2, CD39L3, and CD39L4 did not differ consistently between NK and ADPKD cells of either lectin type. Immunoreactive CD39 was less abundant in PKDTERT than in NKTERT cells of LTA+ (Supplemental Fig. 7) and DBA+ lectin types (not shown).
DISCUSSION
This report presents several novel findings. hTERT-immortalized ADPKD cyst cells heterozygous for the germline mutation Q4004X share the loss of normal flow-sensitive [Ca2+]i signaling that characterizes primary ADPKD cyst cells heterozygous for the distinct germline mutation ΔL2433 (63) and SV40-transformed cells hetero- or hemizygous for the germline mutation Q2556X (34). hTERT-immortalized clonal NK cell lines (NKTERT) selected for expression of proximal or distal markers exhibited slightly different flow sensitivities, but hTERT-immortalized clonal ADPKD cyst cell lines (PKDTERT) expressing either marker were similarly unresponsive to flow. These immortalized cyst cells possessed cilia of nearly normal length, but lacked ciliary localization of PC1 and PC2. Despite elevated net ATP release in resting and hypotonic conditions, primary and immortalized ADPKD cyst cells of both lectin types exhibited markedly reduced flow-sensitive ATP release. Released ATP acts through P2Y and P2X receptors to mediate flow-sensitive Cai2+ signaling, and ecto-nucleotidase inhibition prolonged the plateau phase of the flow-induced Cai2+ signal. However, Cai2+ signaling amplitude in response to exogenously added ATP was only moderately reduced in PKDTERT cells. These changes in cyst cells were accompanied by reduced mRNA and protein levels of P2X7 and CD39 and by a reduction in a pharmacological index of P2X7 activity. These observations are the first to demonstrate paracrine purinergic mediation of flow-sensitive Ca2+ signaling in human NK cells and to show deficient flow-sensitive net ATP release from ADPKD cells.
Role of genotype and nephron segment in flow-sensitive Cai2+ signaling.
The present work adds to the range of defined PKD1 genotypes associated with loss of flow-sensitive Cai2+ signaling to include heterozygosity for the mutation Q4004X in addition to the previously reported heterozygosity for the mutation ΔL2433 (63) and heterozygosity and likely hemizygosity for the germline mutation Q2556X (34). These germline mutations together encompass a range of total PC1 polypeptide expression that is normal or elevated (this work and Ref. 63), or reduced, or absent (34). They also now extend across the gamut of primary cells, SV40-transformed cell lines, and hTERT-immortalized cell lines. The defective flow phenotype is present in cells of both proximal and distal origin, as judged by lectin expression. Thus loss of flow-sensitive [Ca2+]i signaling has proven to be a robust phenotype of ADPKD cyst cells in culture, thus far regardless of germline genotype or of nephron segment of apparent origin.
The emerging picture is more complicated in recessive cystic disease. Embryonic orpk/orpk collecting duct cells with stunted cilia exhibited a moderate reduction of the flow-induced [Ca2+]i elevation observed in Tg737/polaris-rescued cells. This reduction was noted in the context of increased “basal” Ca2+ permeability and elevated apical PC2 expression (50). Similar reduction in flow-triggered [Ca2+]i elevation was evident in isolated, perfused orpk/orpk collecting ducts from 2-wk-old, but not 1-wk-old mice (29). Small interference RNA knockdown (∼90%) of the ARPKD gene fibrocystin produced reductions of comparable magnitude in flow-induced [Ca2+]i elevation in mIMCD3 and murine embryonic collecting duct cells (58). In contrast, both clonal and pooled SV40-immortalized human ARPKD cyst cells responded to flow with [Ca2+]i elevations twofold higher than those of immortalized collecting duct cells from age-matched normal kidneys (44).
The reported requirements of flow-sensitive Cai2+ signaling for extracellular Ca2+ entry and for Ca2+ release from intracellular stores have also varied. In human ADPKD cyst cells and in murine Pkd1−/− embryonic kidney cells, Ca2+ entry was required, and stores were ryanodine-sensitive (33, 34, 63). MDCK cells (42) and intact or split-open rabbit collecting duct (30) required Ca2+ entry, but the stores were inositol triphosphate (IP3)-sensitive. orpk/orpk tubules also required Ca2+ entry (29), but flow-induced elevation of [Ca2+]i in cell monolayers was bath Ca2+-independent (21). The pharmacosensitivity of flow-induced Cai2+ elevation reported in the orpk/orpk tubule experiments did not define the nature of the Ca2+ stores (29, 50). Flow-induced [Ca2+]i increase in the isolated, perfused mouse MTAL did not require luminal Ca2+ but did require basolateral extracellular Ca2+ to sustain the post-peak plateau response (22). These differences likely reflect species, developmental stage, and differentiation state, with differing contributions of shear stress, mechanical stretch, and hydrostatic pressure according to experimental system and geometry.
Role of ATP release in flow-sensitive Cai2+ signaling.
The selective reduction in flow-sensitive net ATP release observed in ADPKD cyst cells (Fig. 4) suggests that it contributes to the loss of flow-sensitive Cai2+ signaling in ADPKD cells. The role of paracrine ATP signaling in flow-induced Ca2+ signaling has been investigated in several cell types. Flow-induced [Ca2+]i increase in perfused mouse MTAL was inhibited by apyrase and suramin, mediated by P2y2 receptors on both apical and basolateral membranes, and substantially reduced in P2y2−/− tubules (22). P2y2 activation was also found to underlie temperature-dependent, spontaneous [Ca2+]i oscillations in MDCK cells at rest (14). In orpk/orpk mouse collecting duct cells rescued with the Tg737/polaris transgene, flow-induced elevation of [Ca2+]i was blocked by apyrase and suramin. In contrast to the selective defect in flow-induced net ATP release of ADPKD cyst cells, the unrescued orpk/orpk cells with stubby cilia exhibited attenuated net ATP release in response to three different stimuli: hypotonic shock, harsh pipetting, and ionomycin (21).
Flow elevates [Ca2+]i in isolated rat bile duct segments and in cholangiocytes by a cilium-dependent mechanism (31). Flow also triggers cholangiocytes to release ATP, which mediates flow-induced Cai2+ elevation by paracrine P2 receptor stimulation (62). P2R activation in cholangiocytes activates anion channels in parallel with increased bicarbonate secretion bile flow. However, flow-induced ATP release and subsequent purinergic elevation of [Ca2+]i in some human cholangiocyte cell lines was not cilium dependent (62). Thus the properties of flow-stimulated net ATP release appear to differ among cell types, may reflect predominant utilization of distinct ATP release pathways, and may be differentially affected by mutations in different cystic disease genes.
Purinergic receptors and nucleotidases in flow sensing.
Intraluminal ATP concentrations in superficial proximal tubules of anesthetized rats have been estimated at 100–300 nM, several-fold higher than in Bowman's space, and several-fold higher than in superficial distal convoluted tubule (57). These concentrations likely underestimate true juxtamembrane concentrations, in view of the high activities of both secreted and apical membrane ecto-nucleotidases. Thus released luminal ATP is believed to reach concentrations sufficient for activation of apical purinergic receptors (27, 55). A subset of ADPKD cyst fluids contained ATP at the higher concentrations of 0.5–10 μM (60).
Normal and ADPKD kidneys express a wide range of purinergic receptor and ecto-nucleotidase mRNAs and immunoreactive polypeptides (27, 48, 55). The absence in LTA+-NK and PKD cyst cells of some P2R subtype mRNAs expressed in the kidney (Fig. 8) may reflect either axial heterogeneity of P2R expression or alterations in gene expression accompanying immortalization. Although ATP-responsive [Ca2+]i elevation was normal or modestly reduced in NK and ADPKD cyst cells, cyst cell responses to UTP and ADP were reduced to greater extents (Supplemental Fig. 5). PKDTERT cells displayed lower mRNA levels and immunostaining intensity for P2X7 and CD39 than detected in NKTERT cells of either lectin type (Figs. 8 and 9; Supplemental Figs. 6 and 7). ADPKD cyst cells also exhibited a delayed and attenuated [Ca2+]i recovery following the profound reduction in [Ca2+]i induced by 1 mM ATP, a recovery sensitive to the P2X7 antagonists O-ATP and KN-62 (Fig. 9). Thus reductions in flow-induced net ATP release and flow-induced, extracellular ATP-dependent Cai2+ signaling in PKDTERT cells were associated with apparent decreases in P2X7 expression and in Cai2+ signaling sensitive to P2X7 inhibitors.
The ATP-induced rapid fall in thapsigargin-elevated [Ca2+]i observed in NKTERT cells has been observed in other cell types. In thapsigargin-pretreated CFPAC-1 cells, 10 μM ATP rapidly reduced [Ca2+]i by 40% without decreasing plasmalemmal Ca2+ entry (as measured by Mn2+ quench of Cai2+ signal) and without increasing Cai2+ extrusion by either Na+/Ca2+ exchange or vanadate-sensitive Ca2+-ATPase (61). Similar inhibitory effects of ATP were noted in HT-29 cells. In rat brown adipocytes, 10 μM ATP also rapidly depressed thapsigargin-elevated [Ca2+]i by 92% (37) by a mechanism insensitive to phorbol ester but sensitive to high concentrations of suramin and of PPADS (36). The inhibitory effect of ATP on thapsigargin-elevated [Ca2+]i correlated with increased peripheral actin polymerization and was blocked by pharmacological disruption of the actin cytoskeleton (38). The mechanism of this inhibitory effect of ATP on [Ca2+]i in NKTERT and PKDTERT cells, as well as in brown adipocytes, CFPAC-1, and HT-29 cells, remains unclear. The secondary elevation of depressed [Ca2+]i might be mediated by slower P2 receptor activation (or recovery from thapsigargin-associated inactivation), with pharmacological properties suggestive of P2X7.
Reduced expression of both P2X7 and CD39 in PKDTERT cells suggests important roles for these proteins in normal flow-sensitive Cai2+ signaling, but would be predicted to exert opposing effects. Generation of adenosine by CD39 and other ecto-nucleotidases may play an additional, potentially important role in terminating or otherwise modulating the flow signal. However, suramin is a poor P2X7 antagonist (13), and preliminary experiments suggest that neither P2X7 antagonist O-ATP nor KN-62 can reproduce the inhibitory effects of suramin and of PPADS on flow-induced Cai2+ signaling in LTA+ or DBA+ NKTERT cells (Xu C and Alper SL, unpublished observations). In addition, primary ADPKD cyst cells bearing the heterozygous germline mutation ΔL2433 exhibited increased levels of mRNA encoding P2X7 (n = 4) and CD39 (n = 2; not shown), in contrast to the decreased levels in immortalized Q4004X ADPKD cyst cells (Fig. 8 and Supplemental Fig. 6). Thus levels of these mRNAs may vary as a function of either genotype or immortalization state. Taken together, the data suggest that the identity of the P2 receptor(s) mediating flow-sensitive Cai2+ signaling in primary NK cells and in NKTERT cells remains to be determined. P2X7 is a potentiator of apoptosis and inflammatory cytokine release in epithelial (4) as well as in other cells (12). Alteration of P2X7 expression in ADPKD (17) may be related to the disease stage-specific, variable increases in apoptosis detected in ADPKD cyst epithelial cells (15). This variation, in turn, may be reflected in the differentiation state-dependence of P2R and ecto-nucleotidase mRNA levels.
Additional work is needed to define the pathway(s) of flow-sensitive ATP release that are selectively impaired in ADPKD cyst cells while ATP release in basal and hypotonic swelling conditions is elevated. The central role of vectorial fluid flow-induced paracrine signaling in the embryonic node in the developmental establishment of left-right asymmetry strongly suggests that deficient flow-induced ATP release by ADPKD cyst cells likely has consequences for the control of gene expression. However, the roles of flow-sensitive, purinergically mediated Cai2+ signaling by renal epithelial cells in the generation and/or maintenance of normal axial tubular structure and in the prevention of cystogenesis remain to be established.
GRANTS
C. Xu was supported by postdoctoral fellowship F32 DK69049 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and by a postdoctoral fellowship from the Polycystic Kidney Disease Foundation. This work was additionally supported by NIDDK Grants R01-DK57662 to S. L. Alper, R01 DK58816 to P. C. Harris, R01 DK50141 to A. Wandinger-Ness, by a Polycystic Kidney Disease Foundation research grant to R. Bacallao, and by Shared Instrument Grant S10-RR017927 to Beth Israel Deaconess Medical Center.
Supplementary Material
Acknowledgments
We thank Drs. W. Junger and D. H. Friedman (Harvard) and O. Ibraghimova-Beskrovnaya (Genzyme) for antibodies and D. H. Friedman, W. Junger, and S. A. Ness (University of New Mexico) for helpful discussions.
REFERENCES
- 1.Bai CX, Giamarchi A, Rodat-Despoix L, Padilla F, Downs T, Tsiokas L, Delmas P. Formation of a new receptor-operated channel by heteromeric assembly of TRPP2 and TRPC1 subunits. EMBO Rep 9: 472–479, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becker D, Woltersdorf R, Boldt W, Schmitz S, Braam U, Schmalzing G, Markwardt F. The P2X7 carboxyl tail is a regulatory module of P2X7 receptor channel activity. J Biol Chem 2008. [DOI] [PubMed]
- 3.Cheewatrakoolpong B, Gilchrest H, Anthes JC, Greenfeder S. Identification and characterization of splice variants of the human P2X7 ATP channel. Biochem Biophys Res Commun 332: 17–27, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Chen L, Brosnan CF. Regulation of immune response by P2X7 receptor. Crit Rev Immunol 26: 499–513, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Condon J, Yin S, Mayhew B, Word RA, Wright WE, Shay JW, Rainey WE. Telomerase immortalization of human myometrial cells. Biol Reprod 67: 506–514, 2002. [DOI] [PubMed] [Google Scholar]
- 6.D'Agati V, Trudel M. Lectin characterization of cystogenesis in the SBM transgenic model of polycystic kidney disease. J Am Soc Nephrol 3: 975–983, 1992. [DOI] [PubMed] [Google Scholar]
- 7.da Silva RL, Resende RR, Ulrich H. Alternative splicing of P2X6 receptors in developing mouse brain and during in vitro neuronal differentiation. Exp Physiol 92: 139–145, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Denburg JL, McElroy WD. Anion inhibition of firefly luciferase. Arch Biochem Biophys 141: 668–675, 1970. [DOI] [PubMed] [Google Scholar]
- 9.Feng YH, Li X, Wang L, Zhou L, Gorodeski GI. A truncated P2X7 receptor variant (P2X7-j) endogenously expressed in cervical cancer cells antagonizes the full-length P2X7 receptor through hetero-oligomerization. J Biol Chem 281: 17228–17237, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fitz JG Regulation of cellular ATP release. Trans Am Clin Climatol Assoc 118: 199–208, 2007. [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman DJ, Rennke HG, Csizmadia E, Enjyoji K, Robson SC. The vascular ectonucleotidase ENTPD1 is a novel renoprotective factor in diabetic nephropathy. Diabetes 56: 2371–2379, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Marcos M, Pochet S, Marino A, Dehaye JP. P2X7 and phospholipid signalling: the search of the “missing link” in epithelial cells. Cell Signal 18: 2098–2104, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Gever JR, Cockayne DA, Dillon MP, Burnstock G, Ford AP. Pharmacology of P2X channels. Pflügers Arch 452: 513–537, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Geyti CS, Odgaard E, Overgaard MT, Jensen ME, Leipziger J, Praetorius HA. Slow spontaneous [Ca2+]i oscillations reflect nucleotide release from renal epithelia. Pflügers Arch 455: 1105–1117, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Goilav B, Satlin LM, Wilson PD. Pathways of apoptosis in human autosomal recessive and autosomal dominant polycystic kidney diseases. Pediatr Nephrol 23: 1473–1482, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Grygorczyk R, Hanrahan JW. CFTR-independent ATP release from epithelial cells triggered by mechanical stimuli. Am J Physiol Cell Physiol 272: C1058–C1066, 1997. [DOI] [PubMed] [Google Scholar]
- 17.Hillman KA, Burnstock G, Unwin RJ. The P2X7 ATP receptor in the kidney: a matter of life or death? Nephron Exp Nephrol 101: e24–30, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Hillman KA, Johnson TM, Winyard PJ, Burnstock G, Unwin RJ, Woolf AS. P2X(7) receptors are expressed during mouse nephrogenesis and in collecting duct cysts of the cpk/cpk mouse. Exp Nephrol 10: 34–42, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Holmsen H, Holmsen I, Bernhardsen A. Microdetermination of adenosine diphosphate and adenosine triphosphate in plasma with firefly luciferase system. Anal Biochem 17: 456–473, 1966. [DOI] [PubMed] [Google Scholar]
- 20.Homolya L, Steinberg TH, Boucher RC. Cell to cell communication in response to mechanical stress via bilateral release of ATP and UTP in polarized epithelia. J Cell Biol 150: 1349–1360, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hovater MB, Olteanu D, Hanson EL, Cheng NL, Siroky B, Fintha A, Komlosi P, Liu W, Satlin LM, Bell PD, Yoder BK, Schwiebert EM. Loss of apical monocilia on collecting duct principal cells impairs ATP secretion across the apical cell surface and ATP-dependent and flow-induced calcium signals. Purinergic Signal 4: 155–170, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jensen ME, Odgaard E, Christensen MH, Praetorius HA, Leipziger J. Flow-induced [Ca2+]i increase depends on nucleotide release and subsequent purinergic signaling in the intact nephron. J Am Soc Nephrol 18: 2062–2070, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Kishore BK, Isaac J, Fausther M, Tripp SR, Shi H, Gill PS, Braun N, Zimmermann H, Sevigny J, Robson SC. Expression of NTPDase1 and NTPDase2 in murine kidney: relevance to regulation of P2 receptor signaling. Am J Physiol Renal Physiol 288: F1032–F1043, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Kottgen M, Buchholz B, Garcia-Gonzalez MA, Kotsis F, Fu X, Doerken M, Boehlke C, Steffl D, Tauber R, Wegierski T, Nitschke R, Suzuki M, Kramer-Zucker A, Germino GG, Watnick T, Prenen J, Nilius B, Kuehn EW, Walz G. TRPP2 and TRPV4 form a polymodal sensory channel complex. J Cell Biol 182: 437–447, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kottgen M, Walz G. Subcellular localization and trafficking of polycystins. Pflügers Arch 451: 286–293, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Lazarowski ER, Tarran R, Grubb BR, van Heusden CA, Okada S, Boucher RC. Nucleotide release provides a mechanism for airway surface liquid homeostasis. J Biol Chem 279: 36855–36864, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leipziger J Control of epithelial transport via luminal P2 receptors. Am J Physiol Renal Physiol 284: F419–F432, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Lewis SA, Lewis JR. Kinetics of urothelial ATP release. Am J Physiol Renal Physiol 291: F332–F340, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Liu W, Murcia NS, Duan Y, Weinbaum S, Yoder BK, Schwiebert E, Satlin LM. Mechanoregulation of intracellular Ca2+ concentration is attenuated in collecting duct of monocilium-impaired orpk mice. Am J Physiol Renal Physiol 289: F978–F988, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Liu W, Xu S, Woda C, Kim P, Weinbaum S, Satlin LM. Effect of flow and stretch on the [Ca2+]i response of principal and intercalated cells in cortical collecting duct. Am J Physiol Renal Physiol 285: F998–F1012, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Masyuk AI, Masyuk TV, Splinter PL, Huang BQ, Stroope AJ, LaRusso NF. Cholangiocyte cilia detect changes in luminal fluid flow and transmit them into intracellular Ca2+ and cAMP signaling. Gastroenterology 131: 911–920, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merlin D, Jiang L, Strohmeier GR, Nusrat A, Alper SL, Lencer WI, Madara JL. Distinct Ca2+- and cAMP-dependent anion conductances in the apical membrane of polarized T84 cells. Am J Physiol Cell Physiol 275: C484–C495, 1998. [DOI] [PubMed] [Google Scholar]
- 33.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 33: 129–137, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Nauli SM, Rossetti S, Kolb RJ, Alenghat FJ, Consugar MB, Harris PC, Ingber DE, Loghman-Adham M, Zhou J. Loss of polycystin-1 in human cyst-lining epithelia leads to ciliary dysfunction. J Am Soc Nephrol 17: 1015–1025, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Okada SF, Nicholas RA, Kreda SM, Lazarowski ER, Boucher RC. Physiological regulation of ATP release at the apical surface of human airway epithelia. J Biol Chem 281: 22992–23002, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Omatsu-Kanbe M, Isono T, Matsuura H. Multiple P2 receptors contribute to a transient increase in intracellular Ca2+ concentration in ATP-stimulated rat brown adipocytes. Exp Physiol 87: 643–652, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Omatsu-Kanbe M, Matsuura H. Inhibition of store-operated Ca2+ entry by extracellular ATP in rat brown adipocytes. J Physiol 521: 601–615, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Omatsu-Kanbe M, Shibata M, Yamamoto T, Isono T, Matsuura H. Actin filaments play a permissive role in the inhibition of store-operated Ca2+ entry by extracellular ATP in rat brown adipocytes. Biochem J 381: 389–396, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peltola P, Lumiaho A, Miettinen R, Pihlajamaki J, Sandford R, Laakso M. Genetics and phenotypic characteristics of autosomal dominant polycystic kidney disease in Finns. J Mol Med 83: 638–646, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Praetorius HA, Frokiaer J, Nielsen S, Spring KR. Bending the primary cilium opens Ca2+-sensitive intermediate-conductance K+ channels in MDCK cells. J Membr Biol 191: 193–200, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Praetorius HA, Leipziger J. Fluid flow sensing and triggered nucleotide release in epithelia. J Physiol 586: 2669, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Praetorius HA, Spring KR. Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol 184: 71–79, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Praetorius HA, Spring KR. Removal of the MDCK cell primary cilium abolishes flow sensing. J Membr Biol 191: 69–76, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Rohatgi R, Battini L, Kim P, Israeli S, Wilson PD, Gusella GL, Satlin LM. Mechanoregulation of intracellular Ca2+ in human autosomal recessive polycystic kidney disease cyst-lining renal epithelial cells. Am J Physiol Renal Physiol 294: F890–F899, 2008. [DOI] [PubMed] [Google Scholar]
- 45.Roman RM, Feranchak AP, Davison AK, Schwiebert EM, Fitz JG. Evidence for Gd3+ inhibition of membrane ATP permeability and purinergic signaling. Am J Physiol Gastrointest Liver Physiol 277: G1222–G1230, 1999. [DOI] [PubMed] [Google Scholar]
- 46.Rossetti S, Consugar MB, Chapman AB, Torres VE, Guay-Woodford LM, Grantham JJ, Bennett WM, Meyers CM, Walker DL, Bae K, Zhang QJ, Thompson PA, Miller JP, Harris PC. Comprehensive molecular diagnostics in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 18: 2143–2160, 2007. [DOI] [PubMed] [Google Scholar]
- 47.Satlin LM, Carattino MD, Liu W, Kleyman TR. Regulation of cation transport in the distal nephron by mechanical forces. Am J Physiol Renal Physiol 291: F923–F931, 2006. [DOI] [PubMed] [Google Scholar]
- 48.Schwiebert EM, Wallace DP, Braunstein GM, King SR, Peti-Peterdi J, Hanaoka K, Guggino WB, Guay-Woodford LM, Bell PD, Sullivan LP, Grantham JJ, Taylor AL. Autocrine extracellular purinergic signaling in epithelial cells derived from polycystic kidneys. Am J Physiol Renal Physiol 282: F763–F775, 2002. [DOI] [PubMed] [Google Scholar]
- 49.Silva FG, Nadasdy T, Laszik Z. Immunohistochemical and lectin dissection of the human nephron in health and disease. Arch Pathol Lab Med 117: 1233–1239, 1993. [PubMed] [Google Scholar]
- 50.Siroky BJ, Ferguson WB, Fuson AL, Xie Y, Fintha A, Komlosi P, Yoder BK, Schwiebert EM, Guay-Woodford LM, Bell PD. Loss of primary cilia results in deregulated and unabated apical calcium entry in ARPKD collecting duct cells. Am J Physiol Renal Physiol 290: F1320–F1328, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Taylor AL, Kudlow BA, Marrs KL, Gruenert DC, Guggino WB, Schwiebert EM. Bioluminescence detection of ATP release mechanisms in epithelia. Am J Physiol Cell Physiol 275: C1391–C1406, 1998. [DOI] [PubMed] [Google Scholar]
- 52.Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet 369: 1287–1301, 2007. [DOI] [PubMed] [Google Scholar]
- 53.Tsiokas L, Kim S, Ong EC. Cell biology of polycystin-2. Cell Signal 19: 444–453, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Turner CM, King BF, Srai KS, Unwin RJ. Antagonism of endogenous putative P2Y receptors reduces the growth of MDCK-derived cysts cultured in vitro. Am J Physiol Renal Physiol 292: F15–F25, 2007. [DOI] [PubMed] [Google Scholar]
- 55.Vallon V P2 receptors in the regulation of renal transport mechanisms. Am J Physiol Renal Physiol 294: F10–F27, 2008. [DOI] [PubMed] [Google Scholar]
- 56.Vekaria RM, Shirley DG, Sevigny J, Unwin RJ. Immunolocalization of ectonucleotidases along the rat nephron. Am J Physiol Renal Physiol 290: F550–F560, 2006. [DOI] [PubMed] [Google Scholar]
- 57.Vekaria RM, Unwin RJ, Shirley DG. Intraluminal ATP concentrations in rat renal tubules. J Am Soc Nephrol 17: 1841–1847, 2006. [DOI] [PubMed] [Google Scholar]
- 58.Wang S, Zhang J, Nauli SM, Li X, Starremans PG, Luo Y, Roberts KA, Zhou J. Fibrocystin/polyductin, found in the same protein complex with polycystin-2, regulates calcium responses in kidney epithelia. Mol Cell Biol 27: 3241–3252, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Watt WC, Lazarowski ER, Boucher RC. Cystic fibrosis transmembrane regulator-independent release of ATP. Its implications for the regulation of P2Y2 receptors in airway epithelia. J Biol Chem 273: 14053–14058, 1998. [DOI] [PubMed] [Google Scholar]
- 60.Wilson PD, Hovater JS, Casey CC, Fortenberry JA, Schwiebert EM. ATP release mechanisms in primary cultures of epithelia derived from the cysts of polycystic kidneys. J Am Soc Nephrol 10: 218–229, 1999. [DOI] [PubMed] [Google Scholar]
- 61.Wolff T, Leipziger J, Fischer KG, Klar B, Nitschke R, Greger R. Evidence for agonist-induced export of intracellular Ca2+ in epithelial cells. Pflügers Arch 424: 423–430, 1993. [DOI] [PubMed] [Google Scholar]
- 62.Woo K, Dutta AK, Patel V, Kresge C, Feranchak AP. Fluid flow induces mechanosensitive ATP release, calcium signalling and Cl− transport in biliary epithelial cells through a PKCzeta-dependent pathway. J Physiol 586: 2779–2798, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu C, Rossetti S, Jiang L, Harris PC, Brown-Glaberman U, Wandinger-Ness A, Bacallao R, Alper SL. Human ADPKD primary cyst epithelial cells with a novel, single codon deletion in the PKD1 gene exhibit defective ciliary polycystin localization and loss of flow-induced Ca2+ signaling. Am J Physiol Renal Physiol 292: F930–F945, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yeung D, Zablocki K, Lien CF, Jiang T, Arkle S, Brutkowski W, Brown J, Lochmuller H, Simon J, Barnard EA, Gorecki DC. Increased susceptibility to ATP via alteration of P2X receptor function in dystrophic mdx mouse muscle cells. FASEB J 20: 610–620, 2006. [DOI] [PubMed] [Google Scholar]
- 65.Yi X, Tesmer VM, Savre-Train I, Shay JW, Wright WE. Both transcriptional and posttranscriptional mechanisms regulate human telomerase template RNA levels. Mol Cell Biol 19: 3989–3997, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.