Abstract
NH3 movement across plasma membranes has traditionally been ascribed to passive, lipid-phase diffusion. However, ammonia-specific transporters, Mep/Amt proteins, are present in primitive organisms and mammals express orthologs of Mep/Amt proteins, the Rh glycoproteins. These findings suggest that the mechanisms of NH3 movement in mammalian tissues should be reexamined. Rh C glycoprotein (Rhcg) is expressed in the collecting duct, where NH3 secretion is necessary for both basal and acidosis-stimulated ammonia transport. To determine whether the collecting duct secretes NH3 via Rhcg or via lipid-phase diffusion, we generated mice with collecting duct-specific Rhcg deletion (CD-KO). CD-KO mice had loxP sites flanking exons 5 and 9 of the Rhcg gene (Rhcgfl/fl) and expressed Cre-recombinase under control of the Ksp-cadherin promoter (Ksp-Cre). Control (C) mice were Rhcgfl/fl but Ksp-Cre negative. We confirmed kidney-specific genomic recombination using PCR analysis and collecting duct-specific Rhcg deletion using immunohistochemistry. Under basal conditions, urinary ammonia excretion was less in KO vs. C mice; urine pH was unchanged. After acid-loading for 7 days, CD-KO mice developed more severe metabolic acidosis than did C mice. Urinary ammonia excretion did not increase significantly on the first day of acidosis in CD-KO mice, despite an intact ability to increase urine acidification, whereas it increased significantly in C mice. On subsequent days, urinary ammonia excretion slowly increased in CD-KO mice, but was always significantly less than in C mice. We conclude that collecting duct Rhcg expression contributes to both basal and acidosis-stimulated renal ammonia excretion, indicating that collecting duct ammonia secretion is, at least in part, mediated by Rhcg and not solely by lipid diffusion.
Keywords: NH3 movement, acid-base
ammonia1 metabolism is essential for normal renal, gastrointestinal, hepatic, central nervous system, and skeletal muscle function. Ammonia is both produced and metabolized by mammalian cells and undergoes transport across plasma membranes of a wide variety of tissues. Renal ammonia metabolism and transport are necessary to maintain systemic acid-base homeostasis (10, 36, 73). Accordingly, understanding the molecular mechanisms of ammonia transport in the kidney is central to understanding mammalian physiology.
The molecular mechanism by which ammonia crosses collecting duct plasma membranes is incompletely understood. Ammonia exists in two molecular species, NH3 and NH4+. A number of studies of the cortical collecting duct (CCD) and outer medullary collecting duct (OMCD) show that NH3 is the predominant, if not exclusive, form of ammonia transported across plasma membranes (22, 37, 38, 64, 65). Because NH3 is a small, relatively lipophilic molecule, it has been presumed that diffusive NH3 movement is the primary mechanism of collecting duct ammonia secretion.
In recent years, Rh glycoproteins, which are similar to Mep/Amt ammonia transporters in other organisms, have been identified in mammalian tissues that are involved in ammonia metabolism (41, 42). One of these, Rh C glycoprotein (Rhcg), is present in plasma membranes of epithelial cells throughout the collecting duct (11, 67). In experimental models, Rhcg expression parallels ammonia excretion (33, 40, 57, 58), suggesting that Rhcg may contribute to renal ammonia transport. However, the lack of specific Rhcg inhibitors has prevented direct testing of this model.
A recent study examined this issue by generating transgenic mice with global Rhcg deletion. These mice exhibited decreased basal and acidosis-stimulated ammonia excretion (6). Perfused collecting duct studies indicated decreased transepithelial total ammonia (NH3 plus NH4+) permeability and decreased apical NH3 permeability (6). These results fully support a critical role for Rhcg in renal ammonia transport. However, because this study used global Rhcg deletion, it cannot exclude the possibility that extrarenal Rhcg, e.g., central nervous system, regulates renal ammonia transport through indirect mechanisms not involving renal Rhcg.
For the past several years, our laboratory has been interested in the molecular mechanisms of ammonia metabolism. As part of this work, we have generated, and recently reported, a transgenic mouse with loxP sites flanking exons 5–9 of the Rhcg gene (32). This mouse, when mated appropriately with mice expressing Cre-recombinase under control of 1,329 bp of the Ksp-cadherin promoter (29, 59), yields mice with renal collecting duct-specific Rhcg deletion (32). Thus the purpose of this study was to examine the effect of genetic deletion of collecting duct Rhcg on basal ammonia and acid-base homeostasis and on the renal response to metabolic acidosis. Our results indicate that collecting duct Rhcg expression is necessary for both basal ammonia excretion and for the normal renal response to metabolic acidosis.
METHODS
Animals.
Transgenic mice with loxP sites flanking exons 5–9 of the Rhcg gene have been reported previously (32). Transgenic mice expressing Cre-recombinase under the control of 1,329 bp of the Ksp-cadherin promoter have been previously described (29, 59). Animal breeding was performed in the University of Florida College of Medicine Cancer and Genetics Transgenic Animal Core Facility by trained personnel. Mice used in this project were the result of mating mice homozygous for floxed Rhcg alleles and also expressing Ksp-cadherin-Cre (Rhcgfl/fl, Ksp-Cre+) with mice homozygous for floxed Rhcg alleles but not expressing Ksp-cadherin-Cre (Rhcgfl/fl, Ksp-Cre−). All animal studies were approved by the University of Florida College of Medicine and the North Florida/South Georgia Veterans Health System Institutional Animal Care and Use Committee.
Antibodies.
Affinity-purified antibodies to Rhcg and Rhbg generated in our laboratory have been characterized previously (44, 67). Antibodies to phosphate-dependent glutaminase (PDG) were provided by Dr. Norman Curthoys (Colorado State University). Antibodies to phosphenolpyruvate carboxykinase (PEPCK) were obtained from Cayman Chemical (Ann Arbor, MI). Antibodies to aquaporin- 2 (AQP2; AB3274) were obtained from Chemicon (Temecula, CA). Rabbit anti-thiazide-sensitive cotransporter (TSC) antibodies were supplied by Dr. David Ellison (Oregon Health Sciences University, Portland, OR).
Acid loading.
An acid diet was prepared by adding 0.4 M HCl to standard rodent chow that had been powdered. The control diet was identical, except that deionized water was substituted for HCl. Adult female animals, >8 wk of age, were placed into metabolic cages (Tecniplast diuresis metabolic cage, Fisher Scientific). Animals were allowed to acclimate for 2 days while receiving the control diet and then received the HCl diet. Daily food intake was measured. In the first set of animals, food intake, and therefore HCl ingestion, was slightly less in collecting duct-specific Rhcg deletion (CD-KO) than in control (C) mice. In a second set of animals, C mice were provided slightly less food so that food intake was similar to CD-KO mice. Measured food intake in these mice did not differ significantly between C and CD-KO mice. Results were similar for the two sets of mice and thus were combined for analysis. Daily urine excretion was collected under mineral oil and urine volume calculated. Urine samples were stored at −20°C until analyzed.
Electrolyte measurements.
Urine ammonia was measured using a commercially available kit (A7553, Pointe Scientific, Canton, MI) modified for use in 96-well plates. Serum bicarbonate was measured as total CO2 using a commercially available kit (C750-120, Pointe Scientific) modified for use with microliter quantities of serum. Serum urea nitrogen was measured using a commercially available kit (B7550-75, Pointe Scientific) according to the manufacturer's protocol. Urine urea nitrogen was measured using the same kit, with a modification to the calculations to take into account the ammonia present in the urine sample. Urine pH was measured using a micro-pH electrode (ROSS semi-micro pH, Orion 8115BN, Thermo Scientific). Plasma and urine creatinine were measured using capillary electrophoresis as described previously (77), with the exception that the injection time was 10 s, rinses used 0.1 M NaOH, LC/MS grade water, and then running buffer, with 2-min rinses between runs, and the detection wavelength was 214 nm.
Titratable acid measurement.
Urinary titratable acid was measured using the method of Chan (9) modified to use 25 or 50 μl of urine. Briefly, samples were acidified by addition of equal volume of 0.1 M HCl, boiled for 2 min, and then cooled to 37°C. The amount of 0.4 M NaOH required to titrate the sample to pH 7.40 was quantified. Samples of deionized water were analyzed in parallel, and results were subtracted from urine samples to yield net titratable acid. Net acid excretion was calculated as the sum of urinary ammonia and titratable acid excretion. In preliminary studies urine bicarbonate was less than 5 μmol/day and thus did not contribute significantly to net acid excretion.
Tissue preparation for immunolocalization.
Mice were anesthetized with inhalant isoflurane. The kidneys were preserved by in vivo cardiac perfusion with PBS (pH 7.4) followed by periodate-lysine-2% paraformaldehyde (PLP) and then cut transversely into several 2- to 4-mm-thick slices and immersed 24–48 h at 4°C in the same fixative. For light microscopy, samples of a kidney from each animal were embedded in polyester wax made using polyethylene glycol 400 distearate (Polysciences, Warrington, PA) with 10% 1-hexadecanol, and 2 or 3-μm-thick sections were cut and mounted on gelatin-coated glass slides.
Immunohistochemistry.
Immunolocalization was accomplished using standard immunoperoxidase procedures. The sections were dewaxed in ethanol, rehydrated, and then rinsed in PBS. Endogenous peroxidase activity was blocked by incubating the sections in Peroxidase Blocking Reagent (DakoCytomation, Carpinteria, CA) for 45 min. The sections were blocked for 15 min with Serum-Free Protein Block (DakoCytomation), then incubated at 4°C overnight with anti-Rhcg antibody. The sections were washed in PBS and incubated for 30 min with polymer-linked, peroxidase-conjugated goat anti-rabbit IgG (MACH2, Biocare Medical), again washed with PBS, then exposed to diaminobenzidine (DAB) for 5 min. The sections were washed in distilled water, then dehydrated with xylene, mounted, and observed by light microscopy. Comparisons of labeling were made only between sections of the same thickness from the same immunohistochemistry experiment. Sections were examined on a Nikon E600 microscope equipped with DIC optics and photographed using a DXM1200F digital camera and ACT-1 software (Nikon). Color correction was performed using Adobe Photoshop CS2 software (Adobe Systems, San Jose, CA).
Double-immunolabeling procedure.
Double immunolabeling was accomplished using sequential immunoperoxidase procedures described in detail previously (32). Briefly, tissue sections were labeled with TSC using Vector SG (Vector Laboratories) as the chromogen to produce a blue label, as described above. The above procedure was repeated with the substitution of a second primary antibody (AQP2) and the substitution of DAB for Vector SG. This label was easily distinguishable from the blue label produced by the Vector SG for detection of TSC. The sections were then washed with glass-distilled water, dehydrated with xylene, mounted with Permount, and observed by light microscopy.
Protein preparation.
Animals were anesthetized with inhalant isoflurane, and the kidneys were rinsed by in vivo cardiac perfusion with PBS (pH 7.4), rapidly removed, frozen in liquid nitrogen, and then stored frozen at −70°C until used. In some experiments, the right renal vasculature was clamped after in vivo cardiac perfusion with PBS, the right kidney was removed, and then the left kidney was perfused with PLP fixative for immunohistochemistry. Tissues were homogenized using microtube pestles (USA Scientific, Ocala, FL), and proteins were extracted using T-PER Tissue Protein Extraction Reagent (Pierce Biotechnology, Rockford, IL) according to the manufacturer's recommended procedures. An aliquot was obtained for protein determination using a BCA assay, and the remainder was stored frozen at −70°C until used.
Immunoblotting procedure.
Ten micrograms of renal protein were electrophoresed on 10% PAGE ReadyGel (Bio-Rad, Hercules, CA). Gels were then transferred electrophoretically to nitrocellulose membranes, blocked with 5 g/dl nonfat dry milk, and incubated at 4°C overnight with primary antibody diluted in Blotto buffer (50 mM Tris, 150 mM NaCl, 5 mM Na2EDTA, and 0.05% Tween 20, pH 7.65) with 5 g/dl nonfat dry milk. Loading and transfer equivalence was assessed with Ponceau S staining. After washing, membranes were exposed to secondary antibody [goat anti-rabbit IgG (Promega, Madison, WI) or goat anti-mouse IgG (Upstate, Temecula, CA) conjugated to horseradish peroxidase] at a dilution of 1:5,000. Sites of antibody-antigen reaction were visualized by using enhanced chemiluminescence (SuperSignal West Pico Substrate, Pierce) and a Kodak Image Station 440CF digital imaging system. Band density was quantified using Kodak 1D, version 3.5.4, software (Kodak Scientific Imaging, New Haven, CT). Band density was normalized such that mean density in the same region (cortex or outer medulla) in C kidneys was 100.0. Absence of saturation was confirmed by examining pixel intensity distribution in all immunoblots.
Genotyping transgenic mice.
Mice were genotyped as described previously (32). Briefly, we extracted genomic DNA from tail-clip samples obtained at ∼14 days of age. The Ksp-cadherin-Cre gene was identified using PCR amplification and the primers 5′-AGGTTCGTGCACTCATGGA-3′ and 5′-TCGACCAGTTTAGTTACCC-3′ (59). To differentiate wild-type from floxed Rhcg alleles, we used primers 5′-GTAGGGCACGGTGTCACCTGTAAAT-3′ and 5′-GGATTACAAGGTGACACTGATGCAGC-3′ flanking the 3′-loxP site. Amplification of the wild-type allele yields a product of ∼230 bp, and amplification of an allele with a loxP site yields a product of ∼400 bp.
Genomic evidence of Cre recombinase-dependent gene recombination.
Genomic evidence of cell-specific gene recombination was performed as described previously (32). Briefly, we used primers that flank both loxP sites, thereby enabling identification of genomic recombination. These primers were UF2-KOF: 5′-ATGGGAGCTGCTATGAATGGGTAAGGAC-3′, and UF2-del-3R: 5′-GATGCAGCTAGAGTTGGGGACAGAGAC-3′. PCR amplification of DNA amplifies an ∼700-bp fragment from tissue with recombination at the loxP sites and an ∼3.4-kb fragment from mice without recombination. The difference in size is due to the excised DNA.
Statistics.
Results are presented as means ± SE. Statistical analyses were performed using Student's t-test, and P < 0.05 was taken as statistically significant; n refers to the numbers of animal studied.
RESULTS
Generation of collecting duct-specific Rhcg mice.
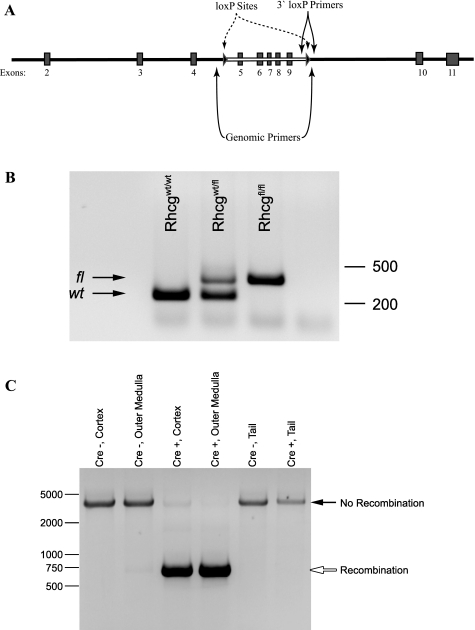
Mice used in this project were offspring from mating of an Rhcgfl/fl, Ksp-Cre+ and an Rhcgfl/fl, Ksp-Cre− parent, which gives 50% Rhcgfl/fl, Ksp-Cre+ and 50% Rhcgfl/fl, Ksp-Cre− offspring. Analysis of 14 consecutive litters showed production of male and female Rhcgfl/fl, Ksp-Cre+ and Rhcgfl/fl, Ksp-Cre− offspring in the statistically expected ratios (data not shown). All offspring were genotyped to confirm homozygous loxP expression and to determine whether they expressed Ksp-cadherin-Cre. Figure 1A shows the approximate sites of the loxP insertions relative to exons 2–11 of Rhcg. Primers flanking the 3′-loxP site were used for routine loxP genotyping (Fig. 1B).
Fig. 1.
Genomic evaluation of mice used in this study. A: location of loxP sites relative to exons 2–11 of the Rh C glycoprotein (Rhcg) gene. B: representative examination of loxP sites. Primers flanking the 3′-loxP site are used to amplify genomic DNA. Amplification of an allele without an inserted loxP site results in a product of ∼230 bp, whereas when a loxP site is present a ∼370-bp product is amplified. C: PCR amplification of genomic DNA using primers flanking both loxP sites. In the absence of genomic recombination, an ∼3.6-kb product is amplified. In the presence of recombination, genomic DNA between the loxP sites is removed and amplification using primers flanking the loxP sites results in a product of ∼750 bp. Amplification of DNA from cortex and outer medulla shows no recombination in Cre-negative mice and recombination in Cre-positive mice. No recombination is present in tail DNA from either Cre-negative or Cre-positive mice.
Confirmation of kidney-specific Rhcg recombination.
We used PCR analysis of DNA obtained from kidney and tail-clip specimens to confirm organ-specific Rhcg recombination. Figure 1C shows typical results. As reported previously, the cortex of Rhcgfl/fl, Ksp-Cre+ kidneys exhibits partial Rhcg gene recombination and the outer medulla essentially complete recombination (32). There was no recombination in tail DNA of Rhcgfl/fl, Ksp-Cre+ mice or in either kidney or tail of Rhcgfl/fl, Ksp-Cre− mice. These findings demonstrate organ-specific Rhcg gene recombination.
Immunohistochemical localization of Rhcg expression in Rhcgfl/fl, Ksp-Cre+ mice.
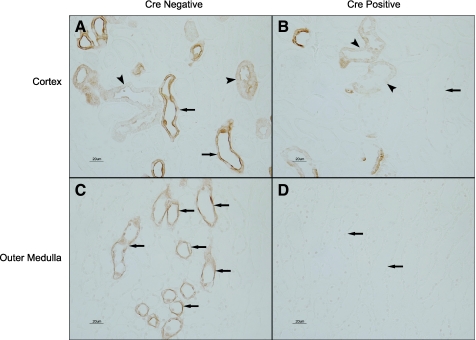
Partial Rhcg recombination suggests recombination in some, but not all renal epithelial cells. Immunohistochemistry confirmed this (Fig. 2). There was near complete loss of Rhcg immunolabel in the collecting duct of Rhcgfl/fl, Ksp-Cre+ mice with, in general, no detectable Rhcg immunolabel. Rarely, faint Rhcg immunolabel was observed in single CCD cells. Persistent Rhcg immunolabel in the connecting tubule (CNT) and distal convoluted tubule (DCT) cells was confirmed using both the morphological characteristics of these segments and serial sections labeled for Rhcg in one section and double-immunolabeled for TSC and AQP2 in the other section (Fig. 3). In the initial collecting tubule near its merger with the CCD, Rhcg immunolabel was not observed, except in occasional cells. These cells were identified as principal cells in serial sections labeled for Rhcg in one section and double-immunolabeled for AQP2 and TSC in the second and in sections double-immunolabeled for Rhcg and H+-ATPase (data not shown). Thus Rhcgfl/fl, Ksp-Cre+ results in collecting duct-specific Rhcg deletion. In the remainder of this paper, we refer to Rhcgfl/fl, Ksp-Cre+ mice as CD-KO and Rhcgfl/fl, Ksp-Cre− mice as C.
Fig. 2.
Rhcg immunolabel in Rhcgfl/fl, Ksp-cadherin-Cre negative (Cre-negative) and Rhcgfl/fl, Ksp-cadherin-Cre positive (Cre-positive) mice on a normal diet. A: Rhcg immunolabel in cortex of a kidney from an Rhcgfl/fl, Ksp-Cre− mouse. A normal distribution of Rhcg immunolabel in cortical collecting duct (CCD; arrows) and in connecting tubule (CNT) and distal convoluted tubule (DCT) segments (arrowheads) is present. B: Rhcg immunolabel in Rhcgfl/fl, Ksp-Cre+ mouse kidney. No Rhcg immunolabel is evident in CCD (arrow). Rhcg immunoreactivity is present in CNT and DCT segments (arrowheads). C: Rhcg immunolabel in the outer medulla and demonstration of normal Rhcg expression in outer medullary collecting duct (OMCD) segments (arrows). D: outer medulla from Rhcgfl/fl, Ksp-Cre+ kidney. No Rhcg immunolabel is detectable.
Fig. 3.
Serial sectioning of Rhcg immunolabel with TSC/AQP2 label. A: Rhcg immunolabel in the cortex of collecting duct-specific Rhcg deletion (CD-KO) mouse on a normal diet. B: serial section double-labeled for AQP2 (brown) and TSC (blue). Residual Rhcg immunolabel is present in a subpopulation of cells in both CNT and DCT. C and D: similar findings [Rhcg immunolabel (C), AQP2 and TSC double-immunolabel (D)] in the cortex of acid-loaded CD-KO mice. Again, residual Rhcg immunolabel is present in a subpopulation of cells in both CNT and DCT. No Rhcg immunolabel is present in cortical collecting duct (CCD) from mouse on either a normal diet or after acid loading.
Absence of nonspecific effects of Rhcg CD-KO on renal structure.
We next examined whether collecting duct-specific Rhcg deletion would have nonspecific effects on renal structure. Renal structure examined in hematoxylin- and eosin-stained tissue was not detectably different between CD-KO and C mice (Fig. 4). Serum creatinine [CD-KO, 0.082 ± 0.005 mg/dl vs. C, 0.089 ± 0.008 mg/dl; n = 8 and 5, respectively; P = not significant (NS)] and creatinine clearance (CD-KO, 191.9 ± 46.2 μl/min vs. C, 173.1 ± 40.1 μl/min; P = NS) were not significantly different between CD-KO and C mice. Similarly, blood urea nitrogen, urea clearance, and urinary urea nitrogen excretion did not differ between CD-KO and C mice (data not shown). A normal number and distribution of intercalated cells, identified as H+-ATPase-positive cells and as cells with intense basolateral Rhbg immunolabel, were present (data not shown). Finally, CD-KO and C mice appeared normal, and there were no apparent differences in birth sizes or growth rates.
Fig. 4.
Hematoxylin and eosin (H&E) staining of kidneys from CD-KO and control (C) mice. A: H&E-stained kidney from a C mouse. Normal renal structure is apparent. B: H&E-stained kidney from a CD-KO mouse. Renal structure is not detectably different from that of a C mouse.
Urinary ammonia excretion under basal conditions.
To determine Rhcg's role in basal acid-base homeostasis and renal ammonia metabolism, we examined plasma electrolytes and urinary ammonia excretion in CD-KO mice. C littermates were used as control mice. Table 1 shows these results. Collecting duct Rhcg deletion caused a slight, but significant, decrease in basal urinary ammonia excretion. Urine pH was not different between CD-KO mice and C mice on a normal diet, suggesting that differences in urinary ammonia excretion were not due to inadequate urine acidification. Plasma HCO3− did not differ significantly between CD-KO and C mice, suggesting that compensatory mechanisms maintain acid-base homeostasis in the face of this slight difference in ammonia excretion.
Table 1.
Physiological parameters under basal conditions
| Parameter | CD-KO | C | P Value |
|---|---|---|---|
| Urine ammonia, μmol/dl | 63±10 (6) | 86±5 (6) | <0.05 |
| Urine pH | 5.77±0.04 (6) | 5.74±0.05 (6) | NS |
| Urine volume, ml/day | 0.77±0.14 (6) | 0.81±0.11 (6) | NS |
| Serum HCO3−, mmol/l | 19.9±0.3 (5) | 19.4±1.1 (6) | NS |
| Body weight, g | 20.2±0.8 (12) | 19.9±0.3 (12) | NS |
Values are means ± SE. Numbers in parentheses are numbers of animals in each group. CD-KO, collecting duct-specific Rh glycoprotein (Rhcg) deletion; C, control; NS, not significant.
One possible compensatory mechanism might be differences in titratable acid excretion. However, titratable acid excretion in CD-KO mice was not increased compared with C mice (CD-KO, 49.9 ± 6.4 μmol/day vs. C, 74.6 ± 8.3 μmol/day; P = NS, n = 6/group). Increased titratable acid excretion does not compensate for decreased ammonia excretion in response to collecting duct-specific Rhcg deletion under basal conditions.
Renal response to metabolic acidosis.
Because the ability to increase urinary ammonia excretion is the primary component of the renal response to metabolic acidosis, we examined the effect of acid-loading on systemic acid-base parameters in CD-KO mice. After HCl loading for 7 days, mice with collecting duct-specific Rhcg deletion exhibited more severe metabolic acidosis, measured as serum HCO3−, than did C mice (CD-KO, 16.2 ± 1.0 mmol/l vs. C, 20.5 ± 0.8 mmol/l; P < 0.02, n = 6 and 5, respectively). Thus collecting duct Rhcg deletion results in a greater degree of metabolic acidosis in response to an exogenous acid load.
Urinary ammonia excretion in response to HCl-induced metabolic acidosis.
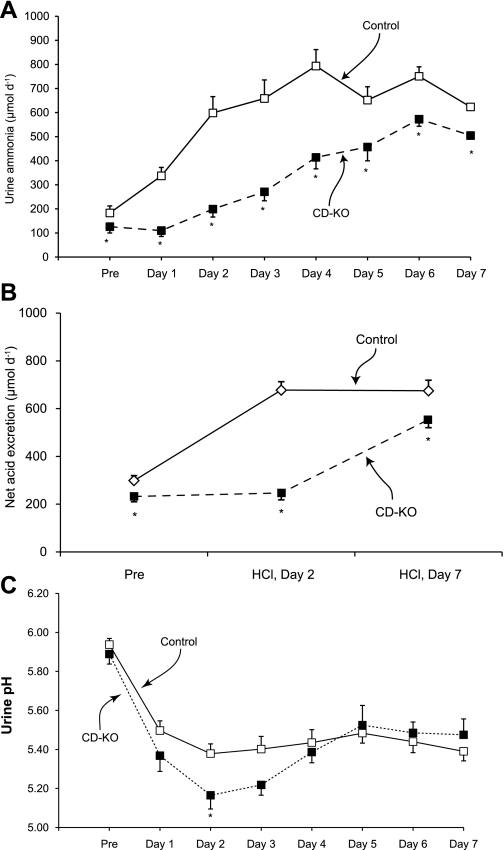
Increased renal ammonia excretion is the primary component of the increase in net acid excretion in response to metabolic acidosis, and collecting duct ammonia secretion increases dramatically to enable this. To determine whether collecting duct-specific Rhcg deletion in mice results in more severe acidosis after HCl loading due to impaired ammonia excretion, we examined urinary ammonia excretion in HCl-loaded CD-KO and C mice. Figure 5A summarizes these results. Before acid-loading, urinary ammonia excretion was significantly less in CD-KO than in C mice (P < 0.05). During the first day of acid loading, urinary ammonia excretion did not change significantly in CD-KO mice (Δ = −16 ± 19 μmol/day; P = NS, n = 6). During subsequent days, urine ammonia excretion increased, but only slowly, and did not peak until day 6 of acid loading. In contrast, in C mice urinary ammonia excretion was increased significantly on the first day of acid-loading (Δ = +154 ± 37 μmol/day; P < 0.005, n = 6) and rapidly increased to a peak on day 4. On each day of acid loading, urinary ammonia excretion was significantly less in CD-KO mice than in C mice. Thus collecting duct-specific Rhcg deletion both inhibits and delays the normal increase in renal ammonia excretion in response to HCl-induced metabolic acidosis.
Fig. 5.
Urinary ammonia excretion, net acid excretion, and urine pH in response to HCl-induced metabolic acidosis. Mice were housed in metabolic cages, and urine was collected daily under mineral oil. A: urinary ammonia excretion. While on a control diet, urinary ammonia excretion was significantly less in CD-KO than in C mice. After addition of HCl to their chow, urinary ammonia excretion increased significantly on the first day in C mice and continued to increase to a maximum on day 4. In CD-KO mice, urinary ammonia excretion did not change significantly on the first day of acid loading, did not peak until day 6, and remained less than in C mice every day. B: net acid excretion. Before acid loading and on days 2 and 7 of acid loading, net acid excretion was significantly less in CD-KO mice than in C mice. C: changes in urine pH in response to acid loading. Acid loading induced increased urine acidification in both CD-KO and C mice that was persistent throughout the course of the study, Except at day 2 of acid loading, there was no significant difference in urine pH. *P < 0.05 vs. C.
Urine pH in response to metabolic acidosis.
Because urinary acidification increases and urine alkalinization inhibits collecting duct ammonia secretion, we measured urine pH in CD-KO and C mice. Figure 5C summarizes these results. Urine pH did not differ significantly either before or during acid loading, with the exception of day 2 of acid loading when CD-KO mice exhibited more acidic urine than did C mice. Because greater degrees of urinary acidification should enhance collecting duct ammonia secretion, the impaired urinary ammonia excretion observed in acid-loaded CD-KO mice is not due to an inability to acidify urine. Instead, the more acidic urine in CD-KO mice at day 2 suggests ongoing H+ secretion in combination with impaired NH3 transport.
Titratable acid excretion in response to metabolic acidosis.
We next examined whether increased titratable acid excretion compensated for the decreased ammonia excretion in mice with collecting duct-specific Rhcg deletion exposed to metabolic acidosis. At day 2 of acid loading, titratable acid excretion was slightly less in CD-KO than C mice (CD-KO, 47.9 ± 6.1 μmol/day vs. C, 78.7 ± 7.0 μmol/day; P < 0.01), and at day 7 there was no significant difference in titratable acid excretion (CD-KO, 49.5 ± 5.7 μmol/day vs. C, 51.1 ± 5.1 μmol/day; P = NS). Figure 5B shows the comparison of net acid excretion in CD-KO and C mice. Decreased renal ammonia excretion results in decreased net acid excretion, thereby explaining the greater degree of metabolic acidosis that develops in CD-KO mice.
Rhcg expression in response to metabolic acidosis.
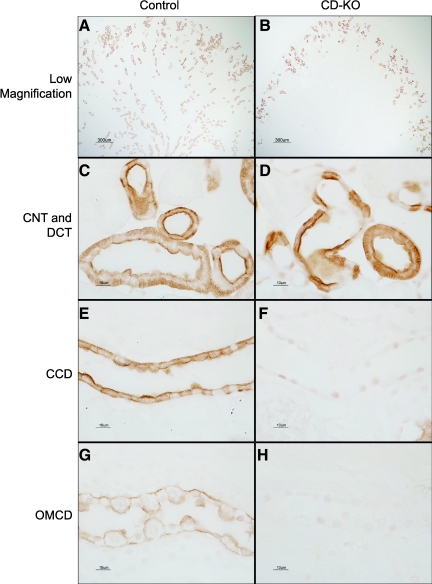
To determine whether HCl-induced metabolic acidosis induced Rhcg expression in collecting ducts of CD-KO mice, we examined Rhcg expression using immunohistochemistry. In general, Rhcg immunolabel was not evident in the CCD, OMCD, or inner medullary collecting duct (IMCD) in CD-KO mice (Fig. 6), although rare cells in the CCD expressed low levels of Rhcg immunolabel. As described above, CD-KO does not prevent Rhcg expression in the CNT and DCT. After acid loading, Rhcg immunolabel in the CNT and DCT of CD-KO mice appeared to be slightly more intense than in C mice. Increased Rhcg-mediated ammonia excretion in the DCT and CNT may contribute to the delayed increase in ammonia excretion observed in the CD-KO mice.
Fig. 6.
Rhcg expression in CD-KO and C mice after HCl-induced acid-loading. A: low-power micrograph of Rhcg expression in the cortex and outer medulla of C mouse. B: low-power micrograph of the cortex and outer medulla of a CD-KO mouse. Rhcg immunolabel is present in a substantially smaller proportion of cortical epithelial cells than in C mice, and no Rhcg immunolabel is evident in the medulla. C: CNTs and DCT in C mouse. Rhcg immunolabel in the apical and basolateral plasma membrane is present. D: CNT and DCT from a CD-KO mouse. Rhcg immunolabel is present and appears to be more intense than in acid-loaded C mice. E: CCD from acid-loaded C mouse. F: CCD from acid-loaded CD-KO mouse. No significant Rhcg immunolabel is present. G: OMCD from C mouse demonstrating the typical pattern of apical and basolateral Rhcg expression, with greater apical expression in intercalated cells than in principal cells. H: OMCD from acid-loaded CD-KO mouse showing absence of Rhcg immunolabel. All micrographs were obtained with identical microscopic settings.
Rhbg expression in response to metabolic acidosis.
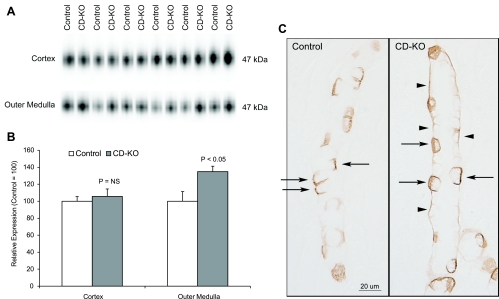
Rhbg is another member of the Rh glycoprotein family (42); it, too, transports ammonia (43, 44, 50), and it is expressed in the same epithelial cells as Rhcg (54, 67). We next examined whether Rhbg expression increased in CD-KO mice as a possible adaptive mechanism in response to Rhcg deletion. Immunoblot analysis (Fig. 7) showed a significant increase in Rhbg expression in the outer medulla (CD-KO, 134.8 ± 6.4 vs. C, 100.0 ± 11.3; P < 0.05, n = 6/group), but not in the cortex (CD-KO, 105.7 ± 8.7 vs. 100.0 ± 5.4; P = NS, n = 6/group), in acid-loaded CD-KO mice compared with acid-loaded C mice. Immunohistochemistry showed no change in the epithelial cell distribution of Rhbg immunolabel in acid-loaded CD-KO mice, but there was an increase in basolateral immunoreactivity evident in principal cells in the OMCD (Fig. 7C). These findings correlate well with the modest increase in Rhbg expression in the outer medulla by immunoblotting. In the absence of acid loading, Rhbg expression did not differ between CD-KO or C mice in either the cortex or outer medulla.
Fig. 7.
Rhbg expression in acid-loaded CD-KO and C mice. A: immunoblot analysis of Rhbg expression in the cortex and outer medulla. B: quantification of Rhbg protein expression. Cortical Rhbg expression is not significantly different between CD-KO and C mice undergoing HCl-induced metabolic acidosis. In the outer medulla, Rhbg expression is significantly greater in CD-KO mice than in C mice. C: Rhbg immunolabel in the OMCD of C and CD-KO mice. Basolateral Rhbg immunolabel in OMCD principal cells (arrowheads) is increased relative to immunolabel in acid-loaded C OMCD. Intercalated cell (arrows) basolateral Rhbg immunolabel appears unchanged.
Effect of collecting duct Rhcg deletion on ammoniagenic enzyme expression.
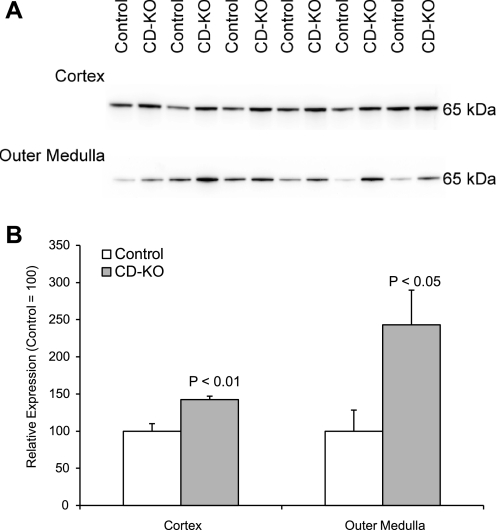
Another mechanism that might explain the delayed and blunted increase in urinary ammonia excretion in mice with collecting duct-specific Rhcg deletion is if the normal acidosis-induced increase in ammoniagenic enzymes were exaggerated in Rhcg knockout mice. To test this possibility, we examined both PEPCK and phosphate-dependent glutaminase expression. PEPCK expression was significantly greater in both the cortex and outer medulla of acid-loaded CD-KO kidneys compared with acid-loaded C kidneys (cortex: CD-KO, 142.3 ± 4.7 vs. C, 100.0 ± 10.0; P < 0.01, n = 6/group; outer medulla: CD-KO, 243.3 ± 46.4 vs. C, 100.0 ± 28.3; P < 0.05, n = 6/group). Figure 8 summarizes these findings. In contrast, phosphate-dependent glutaminase expression did not differ between acid-loaded CD-KO and C mice (cortex: CD-KO 112 ± 7.6 vs. C 100.0 ± 11.6; P = NS; outer medulla: CD-KO, 100.7 ± 7.0 vs. C, 100.0 ± 2.9; P = NS, n = 6/group) (Fig. 9). In the absence of acid loading, neither PEPCK nor phosphate-dependent glutaminase expression differed between CD-KO and C mice (data not shown).
Fig. 8.
Phosphenolpyruvate carboxykinase (PEPCK) expression in acid-loaded CD-KO and C kidneys. A: immunoblot analysis of PEPCK expression in the cortex and outer medulla of CD-KO and C mice. B: quantification of PEPCK expression. In acid-loaded mice, collecting duct Rhcg deletion results in greater PEPCK expression than in C mice in both the cortex and outer medulla.
Fig. 9.
Phosphate-dependent glutaminase (PDG) expression in acid-loaded CD-KO and C kidneys. A: immunoblot analysis of PDG expression in the cortex and outer medulla of CD-KO and C mice. B: quantification of PDG expression. There was no significant difference in PDG expression in acid-loaded C and CD-KO mice in either the cortex or outer medulla.
DISCUSSION
The current studies examine the molecular mechanisms of collecting duct ammonia secretion through the use of collecting duct-specific Rhcg gene deletion. Collecting duct Rhcg deletion decreases basal ammonia excretion and impairs the normal increase in ammonia excretion in response to acid loading. This occurs despite adaptations in other proteins involved in renal ammonia metabolism that otherwise would increase ammonia excretion. Consequently, these results indicate that renal collecting duct ammonia secretion involves Rhcg-mediated ammonia transport.
In the last decade, Rh glycoproteins have been recognized as a fundamentally distinct mammalian protein family (2, 26, 41, 42, 53, 73). They are orthologs of Mep/Amt proteins, which are ammonia transporters in more primitive organisms, such as yeast, bacteria, and plants (46–48, 52, 68). Rh glycoproteins can transport ammonia, predominantly as NH3 according to most studies (4, 5, 43, 44, 50, 55, 75, 76). Under normal conditions, they are widely expressed in ammonia-transporting and pH-responsive tissues, including kidneys, liver, gastrointestinal tract, skeletal muscle, and central nervous system (11, 25, 41, 42, 45, 54, 67, 74). However, identifying the specific physiological role of these proteins in mammalian tissues has been limited by an absence of specific inhibitors.
The current study in combination with previous studies directly demonstrates that Rhcg mediates an important role in collecting duct ammonia transport. Collecting duct ammonia secretion involves parallel NH3 and H+ secretion (12, 22, 37, 38, 64, 65). Although collecting duct NH3 transport was initially thought due to passive, lipid-phase NH3 diffusion, an increasing number of studies indicate that a carrier-mediated mechanism contributes to collecting duct NH3 secretion. Studies in cultured collecting duct cells using the ammonia analog, [14C]methylammonia, showed that a facilitated transport mechanism functionally characterized as Na+- and K+-independent electroneutral NH3 transport (or, equivalent, NH4+/H+ exchange) was the predominant mechanism of both apical and basolateral plasma membrane ammonia movement (23, 24). Notably, these are identical characteristics demonstrated for Rhcg. Metabolic acidosis, which increases renal ammonia excretion, increases Rhcg plasma membrane expression (57, 58). Finally, the current study shows that collecting duct-specific Rhcg deletion impairs both basal and acidosis-stimulated renal ammonia metabolism. Accordingly, Rhcg mediates an important role in collecting duct ammonia secretion.
Although collecting duct Rhcg deletion inhibits basal and acidosis-stimulated renal ammonia metabolism, it did not block urinary ammonia excretion completely. In part, this may be due to ammonia produced and secreted in the proximal tubule that does not undergo reabsorption in the thick ascending limb of the loop of Henle. However, only ∼20% of urinary ammonia results from this pathway (56), making it unlikely to mediate completely the urine ammonia excretion found in the CD-KO mice. Accordingly, it appears that ammonia secretion can occur, albeit less efficiently, through mechanisms that do not involve collecting duct Rhcg. Previous studies in cultured collecting duct cells support this model by showing the presence of both facilitated NH3 transport (likely mediated by Rh glycoproteins) and diffusive NH3 transport (23, 24). Although facilitated transport predominated at lower NH3 concentrations, diffusive transport increased in significance at higher concentrations. This mechanism is likely to contribute to the delayed increase in ammonia excretion in response to metabolic acidosis. A second Rhcg-independent mechanism for ammonia secretion likely involves peritubular ammonia uptake by basolateral Na+-K+-ATPase, particularly in the IMCD (70–72). Finally, residual Rhcg expression in the CNT and DCT may contribute to adaptive increases in ammonia secretion in acid-loaded CD-KO mice, which is consistent with the importance of the CNT in furosemide-induced urinary acidification (39).
Because there are at least two mechanisms of collecting duct NH3 secretion, one that is collecting duct Rhcg mediated and another that involves passive NH3 diffusion, the current studies likely underestimate collecting duct Rhcg's participation in ammonia excretion in normal mice, at least in response to metabolic acidosis. Renal ammonia metabolism also involves ammonia generation in the proximal tubule, which is enhanced by PEPCK and phosphate-dependent glutaminase. The current study, by showing that the PEPCK expression increases more in acid-loaded mice with collecting duct-specific Rhcg deletion than in acid-loaded control mice, suggests that proximal tubule ammoniagenesis is greater also. This would be expected to increase renal interstitial ammonia concentrations, which might enable greater rates of Rhcg-independent collecting duct ammonia secretion.
Shortly after initial submission of this manuscript, another report examining global Rhcg gene deletion was published (6). It, too, reported that Rhcg deletion impairs both basal and acidosis-stimulated renal ammonia excretion (6). In addition, it showed that global Rhcg deletion decreased CCD and OMCD apical NH3 permeability and decreased transepithelial ammonia permeability (6). The current manuscript adds important new information by showing that collecting duct-specific Rhcg deletion yields the same general phenotype as global deletion, in that Rhcg is necessary for normal renal ammonia excretion. Our findings indicate that altered renal ammonia metabolism under these conditions are collecting duct-specific effects and are not mediated through any of Rhcg's roles in the numerous extrarenal sites in which it is expressed. Moreover, the current study, by showing that collecting duct-specific Rhcg deletion results in impaired renal ammonia excretion, demonstrates that the CNT and DCT, despite their preserved Rhcg expression, are not the primary sites of ammonia excretion, at least in the acute response to metabolic acidosis.
Global Rhcg deletion resulted in more acidic urine compared with controls (6), whereas collecting duct-specific Rhcg deletion did not except on day 2 of acid loading (current study). These observations suggest that in the global Rhcg knockouts, H+ secretion is preserved despite impaired NH3 secretion, whereas with the collecting duct-specific Rhcg deletion impaired NH3 secretion inhibits H+ secretion, a concept that is consistent with evidence that ammonia stimulates collecting duct H+ secretion (13–15). It is possible that global Rhcg deletion affects urinary acidification in unknown ways, either by effects of Rhcg deletion in extrarenal sites or by deletion of Rhcg in the DCT and CNT, where Rhcg expression was preserved in our model. Alternatively, preserved Rhcg-mediated ammonia secretion in the DCT and CNT in mice with collecting duct-specific Rhcg deletion, but not in the mice with global Rhcg deletion, may explain this difference.
Genetic deletion of the two other mammalian Rh glycoproteins has been reported previously. Rhag is an erythroid-specific Rh glycoprotein that is one of several proteins in the Rh complex (3, 66). Its deletion decreases erythrocyte plasma membrane ammonia transport (27, 55). Rhag deletion also results in chronic hemolytic anemia characterized by stomatocytosis, reduced osmotic fragility, and abnormal autohemolysis (51). Mutations in conserved amino acids in the pore region of RhAG have recently been associated with development of overhydrated hereditary stomatocytosis, a rare autosomal dominant hemolytic anemia characterized by a profuse monovalent cation membrane leak (7).
Rhbg is an Rh glycoprotein that is closely related to Rhcg. Rhbg transports ammonia (43, 44, 50) and is expressed in essentially the same renal epithelial cells that express Rhcg (54, 57, 67). Previous observations that Rhbg expression does not change with either metabolic acidosis, reduced renal mass, or cyclosporine nephrotoxicity (33, 40, 57) and that genetic deletion of Rhbg does not alter either basal or acid-stimulated renal ammonia metabolism (8) have led some to conclude that Rhbg is not involved in renal ammonia transport. In the present study, we find a slight increase in Rhbg expression in the outer medulla in acid-loaded CD-KO mice vs. C. In mice with genetic deletion of pendrin, in which there is increased urine acidification, there is decreased Rhbg expression (34). These data raise the possibility that in specific circumstances, such the absence of collecting duct Rhcg and when renal ammonia metabolism is stimulated by metabolic acidosis, Rhbg may contribute to ammonia transport.
Another aspect of the current studies relates to the controversy regarding the possibility that mammalian Rh glycoproteins function as CO2 transporters rather than ammonia transporters. In the green algae, Chlamydomonas rheinhardtii, expression of the Rh glycoprotein Rh1 increases in response to exposure to increased media CO2 and Rh1 knockdown inhibits the normal increase in growth rates in response to changes in media CO2 concentration (62, 63). Furthermore, Rh1 knockdown does not alter extracellular ammonia uptake (62). These observations suggest that Rh1 may be involved in CO2 metabolism, not ammonia metabolism. This interpretation has been supported by evolutionary conservation and diversification studies indicating that Rh glycoproteins diverged evolutionarily from the ammonia-transporting Mep/Amt proteins (28). However, studies of genetic deletion of the nonerythroid Rh glycoproteins do not provide any evidence that they mediate CO2 transport. In particular, plasma membrane CO2 transport is necessary for collecting duct H+ secretion and urine acidification (20). Consequently, the demonstration of normal urinary acidification, both under basal conditions and in metabolic acidosis, in mice with either Rhbg (8) or Rhcg deletion (6 and current study) suggests neither Rhbg nor Rhcg is necessary for collecting duct CO2 transport.
Renal ammonia transport can now be identified as involving specific proteins in all of the major renal epithelial cells with a role in ammonia metabolism, i.e., the proximal tubule, thick ascending limb of the loop of Henle, and the collecting duct. Ammonia produced in the proximal tubule is secreted as the molecular species NH4+ by the apical Na+/H+ exchanger NHE-3 (35, 49), although some data suggest NHE-3-independent NH3 transport (60) or that multiple pathways exist, including both NHE-3 and potassium channels (61). The thick ascending limb of the loop of Henle is a major site of ammonia reabsorption, with the major mechanism involving NH4+ transport by the apical Na+-K+-2Cl− cotransporter NKCC-2 and other mechanisms including potassium channels, amiloride-sensitive NH4+ conductance, and the barium- and verapamil-sensitive K+/NH4+ antiporter (1, 16, 17, 30, 31). The net result of ammonia reabsorption in the thick ascending limb of the loop of Henle is twofold: medullary ammonia concentration (18, 19) and delivery of ammonia to the distal nephron accounting for only ∼20% of total urinary ammonia excretion (21, 56). Collecting duct ammonia secretion, which accounts for the remaining ∼80% of urinary ammonia, involves parallel H+ and NH3 secretion (10, 36). We can now add Rhcg-mediated ammonia transport in the collecting duct to this list of transporters involved in renal ammonia transport. Finally, basolateral Na+-K+-ATPase-mediated NH4+ uptake contributes to collecting duct ammonia secretion in the IMCD (69, 70, 72), but not in the CCD (37).
In summary, the current studies provide important new information regarding the molecular mechanisms of renal ammonia metabolism. By showing that collecting duct Rhcg deletion decreases both basal and acidosis-stimulated ammonia excretion, these studies clearly demonstrate that collecting duct ammonia secretion is not solely due to diffusive NH3 transport, and instead involves transport by Rhcg.
GRANTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-45788, the Medical Research Service of the North Florida/South Georgia Veterans Health System, and the Gatorade Research Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute Of Diabetes and Digestive and Kidney Diseases, the National Institutes of Health, or the Department of Veterans Affairs.
Acknowledgments
The authors thank Dr. Donald E. Kohan for numerous helpful discussions regarding generation of floxed Rhcg mice, the staff of the University of Florida College of Medicine Electron Microscopy Facility for expert tissue processing and sectioning for the light microscopic studies, the personnel of the University of Florida Cancer and Genetics Transgenic Animal Core Facility for expert care and breeding of the mice, and the University of Texas Southwestern O'Brien Kidney Research Core Center (National Institutes of Health P30-DK-079328) for performing the serum and urine creatinine measurements.
Footnotes
Ammonia consists of two molecular forms, NH3 and NH4+, that are in equilibrium with each other according to the reaction NH3 + H+ ↔ NH4+. We use the term “ammonia” to refer to the sum of these two molecular forms and refer to each molecular form as either “NH3” or “NH4+,” respectively.
REFERENCES
- 1.Amlal H, Paillard M, Bichara M. NH4+ transport pathways in cells of medullary thick ascending limb of rat kidney. NH4+ conductance and K+/NH4+ (H+) antiport. J Biol Chem 269: 21962–21971, 1994. [PubMed] [Google Scholar]
- 2.Avent ND A new chapter in Rh research: Rh proteins are ammonium transporters. Trends Mol Med 7: 94–96, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Avent ND, Reid ME. The Rh blood group system: a review. Blood 95: 375–387, 2000. [PubMed] [Google Scholar]
- 4.Bakouh N, Benjelloun F, Hulin P, Brouillard F, Edelman A, Cherif-Zahar B, Planelles G. NH3 is involved in the NH4+ transport induced by the functional expression of the human Rh C glycoprotein. J Biol Chem 279: 15975–15983, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Benjelloun F, Bakouh N, Fritsch J, Hulin P, Lipecka J, Edelman A, Planelles G, Thomas SR, Cherif-Zahar B. Expression of the human erythroid Rh glycoprotein (RhAG) enhances both NH3 and NH4+ transport in HeLa cells. Pflügers Arch 450: 155–167, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Biver S, Belge H, Bourgeois S, Van Vooren P, Nowik M, Scohy S, Houillier P, Szpirer J, Szpirer C, Wagner CA, Devuyst O, Marini AM. A role for Rhesus factor Rhcg in renal ammonium excretion and male fertility. Nature 456: 339–343, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Bruce LJ, Guizouarn H, Burton NM, Gabillat N, Poole J, Flatt JF, Brady RL, Borgese F, Delaunay J, Stewart GW. The monovalent cation leak in over-hydrated stomatocytic red blood cells results from amino acid substitutions in the Rh associated glycoprotein (RhAG). Blood 113: 1350–1357, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Chambrey R, Goossens D, Bourgeois S, Picard N, Bloch-Faure M, Leviel F, Geoffroy V, Cambillau M, Colin Y, Paillard M, Houillier P, Cartron JP, Eladari D. Genetic ablation of Rhbg in mouse does not impair renal ammonium excretion. Am J Physiol Renal Physiol 289: F1281–F1290, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Chan JC The rapid determination of urinary titratable acid and ammonium and evaluation of freezing as a method of preservation. Clin Biochem 5: 94–98, 1972. [DOI] [PubMed] [Google Scholar]
- 10.DuBose TD, Good DW, Hamm LL, Wall SM. Ammonium transport in the kidney: new physiological concepts and their clinical implications. J Am Soc Nephrol 1: 1193–1203, 1991. [DOI] [PubMed] [Google Scholar]
- 11.Eladari D, Cheval L, Quentin F, Bertrand O, Mouro I, Cherif-Zahar B, Cartron JP, Paillard M, Doucet A, Chambrey R. Expression of RhCG, a new putative NH3/NH4+ transporter, along the rat nephron. J Am Soc Nephrol 13: 1999–2008, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Flessner MF, Wall SM, Knepper MA. Permeabilities of rat collecting duct segments to NH3 and NH4+. Am J Physiol Renal Fluid Electrolyte Physiol 260: F264–F272, 1991. [DOI] [PubMed] [Google Scholar]
- 13.Frank AE, Weiner ID. Effects of ammonia on acid-base transport by the B-type intercalated cell. J Am Soc Nephrol 12: 1607–1614, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Frank AE, Wingo CS, Andrews PM, Ageloff S, Knepper MA, Weiner ID. Mechanisms through which ammonia regulates cortical collecting duct net proton secretion. Am J Physiol Renal Physiol 282: F1120–F1128, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Frank AE, Wingo CS, Weiner ID. Effects of ammonia on bicarbonate transport in the cortical collecting duct. Am J Physiol Renal Physiol 278: F219–F226, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Good DW Active absorption of NH4+ by rat medullary thick ascending limb: inhibition by potassium. Am J Physiol Renal Fluid Electrolyte Physiol 255: F78–F87, 1988. [DOI] [PubMed] [Google Scholar]
- 17.Good DW Ammonium transport by the loop of Henle. Miner Electrolyte Metab 16: 291–298, 1990. [PubMed] [Google Scholar]
- 18.Good DW, DuBose TD. Concentrations of NH3 in cortex and medulla of rat kidney. Contrib Nephrol 63: 16–20, 1988. [DOI] [PubMed] [Google Scholar]
- 19.Good DW, Knepper MA. Mechanisms of ammonium excretion: role of the renal medulla. Semin Nephrol 10: 166–173, 1990. [PubMed] [Google Scholar]
- 20.Hamm LL, Nakhoul NL. Renal acidification. In: Brenner and Rector's The Kidney, edited by Brenner BM. Philadelphia, PA: Saunders Elsevier, 2007, p. 248–279.
- 21.Hamm LL, Simon EE. Roles and mechanisms of urinary buffer excretion. Am J Physiol Renal Fluid Electrolyte Physiol 253: F595–F605, 1987. [DOI] [PubMed] [Google Scholar]
- 22.Hamm LL, Trigg D, Martin D, Gillespie C, Buerkert J. Transport of ammonia in the rabbit cortical collecting tubule. J Clin Invest 75: 478–485, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Handlogten ME, Hong SP, Westhoff CM, Weiner ID. Basolateral ammonium transport by the mouse inner medullary collecting duct cell (mIMCD-3). Am J Physiol Renal Physiol 287: F628–F638, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Handlogten ME, Hong SP, Westhoff CM, Weiner ID. Apical ammonia transport by the mouse inner medullary collecting duct cell (mIMCD-3). Am J Physiol Renal Physiol 289: F347–F358, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Handlogten ME, Hong SP, Zhang L, Vander AW, Steinbaum ML, Campbell-Thompson M, Weiner ID. Expression of the ammonia transporter proteins, Rh B glycoprotein and Rh C glycoprotein, in the intestinal tract. Am J Physiol Gastrointest Liver Physiol 288: G1036–G1047, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Heitman J, Agre P. A new face of the Rhesus antigen. Nat Genet 26: 258–259, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Hemker MB, Cheroutre G, van Zwieten R, Maaskant-van Wijk PA, Roos D, Loos JA, van der Schoot CE, dem Borne AE. The Rh complex exports ammonium from human red blood cells. Br J Haematol 122: 333–340, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Huang CH, Peng J. Evolutionary conservation and diversification of Rh family genes and proteins. Proc Natl Acad Sci USA 102: 15512–15517, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Igarashi P, Shashikant CS, Thomson RB, Whyte DA, Liu-Chen S, Ruddle FH, Aronson PS. Ksp-cadherin gene promoter. II. Kidney-specific activity in transgenic mice. Am J Physiol Renal Physiol 277: F599–F610, 1999. [DOI] [PubMed] [Google Scholar]
- 30.Kikeri D, Sun A, Zeidel ML, Hebert SC. Cell membranes impermeable to NH3. Nature 339: 478–480, 1989. [DOI] [PubMed] [Google Scholar]
- 31.Kikeri D, Sun A, Zeidel ML, Hebert SC. Cellular NH4+/K+ transport pathways in mouse medullary thick limb of Henle. Regulation by intracellular pH. J Gen Physiol 99: 435–461, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim HY, Verlander JW, Bishop JM, Cain BD, Han KH, Igarashi P, Lee HW, Handlogten ME, Weiner ID. Basolateral expression of the ammonia transporter family member, Rh C glycoprotein, in the mouse kidney. Am J Physiol Renal Physiol 296: F545–F555, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim HY, Baylis C, Verlander JW, Han KH, Reungjui S, Handlogten ME, Weiner ID. Effect of reduced renal mass on renal ammonia transporter family, Rh C glycoprotein and Rh B glycoprotein, expression. Am J Physiol Renal Physiol 293: F1238–F1247, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Kim YH, Verlander JW, Matthews SW, Kurtz I, Shin WK, Weiner ID, Everett LA, Green ED, Nielsen S, Wall SM. Intercalated cell H+/OH− transporter expression is reduced in Slc26a4 null mice. Am J Physiol Renal Physiol 289: F1262–F1272, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Kinsella JL, Aronson PS. Interaction of NH4+ and Li+ with the renal microvillus membrane Na+-H+ exchanger. Am J Physiol Cell Physiol 241: C220–C226, 1981. [DOI] [PubMed] [Google Scholar]
- 36.Knepper MA NH4+ transport in the kidney. Kidney Int 40: S95–S102, 1991. [PubMed] [Google Scholar]
- 37.Knepper MA, Good DW, Burg MB. Mechanism of ammonia secretion by cortical collecting ducts of rabbits. Am J Physiol Renal Fluid Electrolyte Physiol 247: F729–F738, 1984. [DOI] [PubMed] [Google Scholar]
- 38.Knepper MA, Good DW, Burg MB. Ammonia and bicarbonate transport by rat cortical collecting ducts perfused in vitro. Am J Physiol Renal Fluid Electrolyte Physiol 249: F870–F877, 1985. [DOI] [PubMed] [Google Scholar]
- 39.Kovacikova J, Winter C, Loffing-Cueni D, Loffing J, Finberg KE, Lifton RP, Hummler E, Rossier B, Wagner CA. The connecting tubule is the main site of the furosemide-induced urinary acidification by the vacuolar H+-ATPase. Kidney Int 70: 1706–1716, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Lim SW, Ahn KO, Kim WY, Han DH, Li C, Ghee JY, Han KH, Kim HY, Handlogten ME, Kim J, Yang CW, Weiner ID. Expression of ammonia transporters, Rhbg and Rhcg, in chronic cyclosporine nephropathy in rats. Nephron Exp Nephrol 110: e49–e58, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Z, Chen Y, Mo R, Cc Hui Cheng JF, Mohandas N, Huang CH. Characterization of human RhCG and mouse Rhcg as novel nonerythroid Rh glycoprotein homologues predominantly expressed in kidney and testis. J Biol Chem 275: 25641–25651, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Liu Z, Peng J, Mo R, Cc Hui, Huang CH. Rh type B glycoprotein is a new member of the Rh superfamily and a putative ammonia transporter in mammals. J Biol Chem 276: 1424–1433, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Ludewig U Electroneutral ammonium transport by basolateral Rhesus B glycoprotein. J Physiol 559: 751–759, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mak DO, Dang B, Weiner ID, Foskett JK, Westhoff CM. Characterization of transport by the kidney Rh glycoproteins, RhBG and RhCG. Am J Physiol Renal Physiol 290: F297–F305, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marini AM, Matassi G, Raynal V, Andre B, Cartron JP, Cherif-Zahar B. The human Rhesus-associated RhAG protein and a kidney homologue promote ammonium transport in yeast. Nat Genet 26: 341–344, 2000. [DOI] [PubMed] [Google Scholar]
- 46.Marini AM, Soussi-Boudekou S, Vissers S, Andre B. A family of ammonium transporters in Saccharomyces cerevisiae. Mol Cell Biol 17: 4282–4293, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marini AM, Vissers S, Andre B. Ammonium transport in Saccharomyces cerevisiae. Yeast 11: 425, 1995.7597846 [Google Scholar]
- 48.Marini AM, Vissers S, Urrestarazu A, Andre B. Cloning and expression of the MEP1 gene encoding an ammonium transporter in Saccharomyces cerevisiae. EMBO J 13: 3456–3463, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nagami GT Luminal secretion of ammonia in the mouse proximal tubule perfused in vitro. J Clin Invest 81: 159–164, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakhoul NL, DeJong H, Abdulnour-Nakhoul SM, Boulpaep EL, Hering-Smith K, Hamm LL. Characteristics of renal Rhbg as an NH4+ transporter. Am J Physiol Renal Physiol 288: F170–F181, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Nash R, Shojania AM. Hematological aspect of Rh deficiency syndrome: a case report and a review of the literature. Am J Hematol 24: 267–275, 1987. [DOI] [PubMed] [Google Scholar]
- 52.Ninnemann O, Jauniaux JC, Frommer WB. Identification of a high affinity NH4+ transporter from plants. EMBO J 13: 3464–3471, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Planelles G Ammonium homeostasis and human Rhesus glycoproteins. Nephron Physiol 105: 11–17, 2006. [DOI] [PubMed] [Google Scholar]
- 54.Quentin F, Eladari D, Cheval L, Lopez C, Goossens D, Colin Y, Cartron JP, Paillard M, Chambrey R. RhBG and RhCG, the putative ammonia transporters, are expressed in the same cells in the distal nephron. J Am Soc Nephrol 14: 545–554, 2003. [DOI] [PubMed] [Google Scholar]
- 55.Ripoche P, Bertrand O, Gane P, Birkenmeier C, Colin Y, Cartron JP. Human Rhesus-associated glycoprotein mediates facilitated transport of NH3 into red blood cells. Proc Natl Acad Sci USA 101: 17222–17227, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sajo IM, Goldstein MB, Sonnenberg H, Stinebaugh BJ, Wilson DR, Halperin ML. Sites of ammonia addition to tubular fluid in rats with chronic metabolic acidosis. Kidney Int 20: 353–358, 1982. [DOI] [PubMed] [Google Scholar]
- 57.Seshadri RM, Klein JD, Kozlowski S, Sands JM, Kim YH, Handlogten ME, Verlander JW, Weiner ID. Renal expression of the ammonia transporters, Rhbg and Rhcg, in response to chronic metabolic acidosis. Am J Physiol Renal Physiol 290: F397–F408, 2006. [DOI] [PubMed] [Google Scholar]
- 58.Seshadri RM, Klein JD, Smith T, Sands JM, Handlogten ME, Verlander JW, Weiner ID. Changes in the subcellular distribution of the ammonia transporter Rhcg, in response to chronic metabolic acidosis. Am J Physiol Renal Physiol 290: F1443–F1452, 2006. [DOI] [PubMed] [Google Scholar]
- 59.Shao X, Somlo S, Igarashi P. Epithelial-specific Cre/lox recombination in the developing kidney and genitourinary tract. J Am Soc Nephrol 13: 1837–1846, 2002. [DOI] [PubMed] [Google Scholar]
- 60.Simon EE, Merli C, Herndon J, Cragoe EJ Jr, Hamm LL. Determinants of ammonia entry along the rat proximal tubule during chronic metabolic acidosis. Am J Physiol Renal Fluid Electrolyte Physiol 256: F1104–F1110, 1989. [DOI] [PubMed] [Google Scholar]
- 61.Simon EE, Merli C, Herndon J, Cragoe EJ Jr, Hamm LL. Effects of barium and 5-(N-ethyl-N-isopropyl)-amiloride on proximal tubule ammonia transport. Am J Physiol Renal Fluid Electrolyte Physiol 262: F36–F39, 1992. [DOI] [PubMed] [Google Scholar]
- 62.Soupene E, Inwood W, Kustu S. Lack of the Rhesus protein Rh1 impairs growth of the green alga Chlamydomonas reinhardtii at high CO2. Proc Natl Acad Sci USA 101: 7787–7792, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Soupene E, King N, Feild E, Liu P, Niyogi KK, Huang CH, Kustu S. Rhesus expression in a green alga is regulated by CO2. Proc Natl Acad Sci USA 99: 7769–7773, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Star RA, Burg MB, Knepper MA. Luminal disequilibrium pH and ammonia transport in outer medullary collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 252: F1148–F1157, 1987. [DOI] [PubMed] [Google Scholar]
- 65.Star RA, Kurtz I, Mejia R, Burg MB, Knepper MA. Disequilibrium pH and ammonia transport in isolated perfused cortical collecting ducts. Am J Physiol Renal Fluid Electrolyte Physiol 253: F1232–F1242, 1987. [DOI] [PubMed] [Google Scholar]
- 66.Van Kim CL, Colin Y, Cartron JP. Rh proteins: Key structural and functional components of the red cell membrane. Blood Rev 20: 93–110, 2006. [DOI] [PubMed] [Google Scholar]
- 67.Verlander JW, Miller RT, Frank AE, Royaux IE, Kim YH, Weiner ID. Localization of the ammonium transporter proteins, Rh B glycoprotein and Rh C glycoprotein, in the mouse kidney. Am J Physiol Renal Physiol 284: F323–F337, 2003. [DOI] [PubMed] [Google Scholar]
- 68.von Wiren N, Gazzarrini S, Gojon A, Frommer WB. The molecular physiology of ammonium uptake and retrieval. Curr Opin Plant Biol 3: 254–261, 2000. [PubMed] [Google Scholar]
- 69.Wall SM NH4+ augments net acid secretion by a ouabain-sensitive mechanism in isolated-perfused inner medullary collecting ducts. Am J Physiol Renal Fluid Electrolyte Physiol 270: F432–F439, 1996. [DOI] [PubMed] [Google Scholar]
- 70.Wall SM Ouabain reduces net acid secretion and increases pHi by inhibiting NH4+ uptake on rat tIMCD Na+-K+-ATPase. Am J Physiol Renal Physiol 273: F857–F868, 1997. [DOI] [PubMed] [Google Scholar]
- 71.Wall SM Impact of K+ homeostasis on net acid secretion in rat terminal inner medullary collecting duct: role of the Na,K-ATPase. Am J Kidney Dis 36: 1079–1088, 2000. [DOI] [PubMed] [Google Scholar]
- 72.Wall SM, Koger LM. NH4+ transport mediated by Na+-K+-ATPase in rat inner medullary collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 267: F660–F670, 1994. [DOI] [PubMed] [Google Scholar]
- 73.Weiner ID, Hamm LL. Molecular mechanisms of renal ammonia transport. Annu Rev Physiol 69: 317–340, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weiner ID, Miller RT, Verlander JW. Localization of the ammonium transporters, Rh B Glycoprotein and Rh C Glycoprotein in the mouse liver. Gastroenterology 124: 1432–1440, 2003. [DOI] [PubMed] [Google Scholar]
- 75.Westhoff CM, Ferreri-Jacobia M, Mak DO, Foskett JK. Identification of the erythrocyte Rh-blood group glycoprotein as a mammalian ammonium transporter. J Biol Chem 277: 12499–12502, 2002. [DOI] [PubMed] [Google Scholar]
- 76.Westhoff CM, Siegel DL, Burd CG, Foskett JK. Mechanism of genetic complementation of ammonium transport in yeast by human erythrocyte Rh-associated glycoprotein (RhAG). J Biol Chem 279: 17443–17448, 2004. [DOI] [PubMed] [Google Scholar]
- 77.Zinellu A, Caria MA, Tavera C, Sotgia S, Chessa R, Deiana L, Carru C. Plasma creatinine and creatine quantification by capillary electrophoresis diode array detector. Anal Biochem 342: 186–193, 2005. [DOI] [PubMed] [Google Scholar]