Abstract
Endothelial progenitor cells (EPC) contribute to repair and maintenance of the vascular system, but in patients with chronic kidney disease (CKD), the number and function of EPC may be affected by kidney dysfunction. We assessed numbers and the angiogenic function of EPC from patients with CKD in relation to disease progression. In a cross-sectional, prospective study, 50 patients with varying degrees of CKD, including 20 patients undergoing dialysis and 10 healthy controls, were included. Mononuclear cells were isolated, and circulating EPC were quantified by flow cytometry based on expression of CD14 and CD34. EPC were cultured on fibronectin-coated supramolecular films of oligocaprolactone under angiogenic conditions to determine their angiogenic capacity and future use in regenerative medicine. CKD patients had normal numbers of circulating CD14+ EPC but reduced numbers of circulating CD34+ EPC. Furthermore, EPC from patients with CKD displayed functional impairments, i.e., hampered adherence, reduced endothelial outgrowth potential, and reduced antithrombogenic function. These impairments were already observed at stage 1 CKD and became more apparent when CKD progressed. Dialysis treatment only partially ameliorated EPC impairments in patients with CKD. In conclusion, EPC number and function decrease with advancing CKD, which may hamper physiological vascular repair and can add to the increased risk for cardiovascular diseases observed in CKD patients.
Keywords: cardiovascular disease, CD34, CD14, hemodialysis, peritoneal dialysis
circulating endothelial progenitor cells (EPC) play a role in the maintenance and regeneration of the cardiovascular system (reviewed in Refs. 10 and 44). EPC are a self-renewing cell population present in the bone marrow and circulation, which can differentiate into functional endothelial cells in vitro and in vivo (15). We and others have identified two distinct EPC subsets, the CD34+ EPC (1, 27) and the CD14+ EPC (19, 29), in the peripheral blood of healthy subjects. These EPC subsets have been proposed and applied as tools in, among others, cell therapy for ischemic diseases (18, 22), and the generation of bioartificial tissues, such as endothelialized antithrombogenic hemodialysis access (24) or replacement blood vessels (17, 20).
In patients with chronic kidney disease (CKD), the risk for cardiovascular diseases (CVD), as well as cardiovascular morbidity and mortality, is increased (30, 34). Therefore, in these patients, EPC may serve as a potential tool for cell therapy. However, we and others have described functional and numerical impairment of EPC in patients with various CVD (25, 40), including CKD (5, 28). The latter reports may explain why traditional CVD risk factors, such as hypertension and dislipidemia, only partly account for the increase in CVD-associated morbidity and mortality in CKD patients. Surprisingly, EPC functionality has not been investigated in the early stages of CKD, nor during the progression of CKD. This information gap must be filled to make predictions about the suitability of EPC for physiological repair or their application in regenerative medicine of the kidney. Here, we hypothesized that EPC number and function are affected by by CKD progression and asked whether dialysis treatment ameliorates EPC dysfunction.
We studied the number and endothelial outgrowth potential of circulating EPC derived from patients with CKD in relation to disease progression (CKD K/DOQI, stages 1–5) (26). Endothelial outgrowth was performed on fibronectin-coated supramolecular diureido-pyrimidione-modified oligocaprolactone (PCLdiUPy) (7, 8) biomaterial to explore the use of patient-derived EPC in regenerative medicine. Adherence of EPC to the PCLdiUPy biomaterial was assessed, as well as the capacity for endothelial differentiation. Furthermore, cell function of endothelial outgrowth cells (EOC) was assessed by thrombin generation assays.
MATERIALS AND METHODS
Subjects.
In a cross-sectional, prospective study, 50 patients with various stages of CKD and matched healthy controls (n = 10/group) were included from the outpatient renal clinic of the University Medical Center Groningen (The Netherlands) and the Dialysis Center Groningen (The Netherlands). Patients had various underlying causes of kidney disease, and we included 10 patients in each of the following CKD catagories; stages 1 and 2 CKD [estimated glomerualr filtration rate (eGFR; expressed as ml·min−1·1.73m−2 throughout) > 60], stage 3 CKD (30 < eGFR < 60), stages 4 and 5 CKD but not yet in dialysis (eGFR < 30), hemodialysis, and peritoneal dialysis. Patients with diabetes mellitus, vasculitides, acute infections, neoplasms, acute (within past 6 mo) cardiovascular events, or using immunosuppressive medication (including corticosteroids) were excluded. Patients maintained their regular medication. All participants provided informed consent, and the study was conducted according to the principles of the Declaration of Helsinki.
Isolation and quantification of EPC.
Mononuclear cells (MNC) were isolated from peripheral blood by density-gradient centrifugation of Lymphoprep (Nycomed Pharma) as previously described (19, 21). Aliquots of 1 × 106 MNC were subsequently labeled using monoclonal antibodies to the EPC markers CD34 (BD Pharmingen, San Jose, CA) and CD14 (IQ Products, Groningen, The Netherlands), and EPC were quantified by flow cytometry (BD Biosciences). Peripheral blood MNC were cultured on fibronectin (1 μg/cm2; Harbor Bio-Products, Norwood, MA)-coated diureido-pyrimidinone-polycaprolacton (PCLdiUPy) (7) in medium-199 supplemented with 20% FCS (BioWhittaker, Verviers, Belgium), 2 mM l-glutamine, 1% penicillin/streptomycin (both Sigma, St. Louis, MO), 5 U/ml heparin (Leo Pharma, Ballerup, Denmark), 10 ng/ml bFGF, 20 ng/ml HGF, 10 ng/ml IGF-1, and 10 ng/ml VEGF (all PeproTech, Rockhill, NJ) at a density of 5,000 cells/mm2.
Adherence and apoptosis of EOC.
To assess cell adhesion, nonadherent cells were removed from 5-day-old cultures by extensive washing. The remaining adherent cells were fixed with 2% paraformaldehyde (PFA; Sigma), and the nuclei were labeled using 3 μM 4′,6-diamidino-2-phenylindole (DAPI; Sigma). Nuclei were counted manually in 15 high-power fields (×40 objective magnification) using a Leica DM IL fluorescent microscope (Leica Microsystems, Wetzlar, Germany).
To determine cell apoptosis, all cells were removed from culture. Nonadherent cells were removed by pipetting, after which adherent cells were dissociated by accutase (PAA Laboratories, Pasching, Austria) treatment according to manufacturer's protocol. Cells were pooled and pelleted by centrifugation. Next, cell pellets were resuspended in annexin V binding buffer and incubated with 5 μl fluorescein-conjugated annexin V and 0.75 mM propidium iodide (all BioVision, Mountain View, CA) at room temperature for 5 min. Propidium iodide incorporation and annexin V binding was determined by flow cytormetry (FACSCalibur, BD Biosciences).
Characterization of cultured EOC.
After 3 wk in culture, adherent cells were dissociated using accutase treatment and stained for protein expression analysis of endothelial cell marker molecules CD31 (PECAM-1), CD144 (VE-Cadherin), von Willebrand factor (vWF), endothelial cell nitric oxide synthase (eNOS), and macrophage marker molecule CD163 (Scavenger Receptor M130). Cells were pelleted and resuspended in FACS buffer containing 0.5% fetal calf serum and 2 mM EDTA. Next, samples were stained with PE-conjugated mouse monoclonal antibodies to either 1) human CD31 (5 μg/ml; IQ Products), 2) human CD144 (10 μg/ml; R&D Systems, Minneapolis, MN), 3) human CD163 (10 μg/ml; BD Pharmingen), or mouse IgGs (10 μg/ml; IQ Products) at 4°C for 30 min. Excess antibodies were removed by repeated washing. Next, samples were fixed with 2% PFA at room temperature for 20 min, permeabilized with 0.1% saponin (Sigma), and stained with either rabbit polyclonal antibodies to 1) human vWF (7 μg/ml; DakoCytomation, Glostrup, Denmark), 2) human eNOS (7 μg/ml; BD Transduction Laboratories, San Jose, CA), or 3) fluorescein-conjugated rabbit IgGs (10 μg/ml; IQ Products) at 4°C for 30 min. The samples were resuspended in FACS buffer and incubated with fluorescein-conjugated donkey F(ab′)2 antibody fragments to rabbit IgG (10 μg/ml; Jackson ImmunoResearch, Suffolk, UK) at 4°C for 15 min. After removal of excess antibodies, protein expression was determined by flow cytometry on a FACSCalibur (BD Biosciences).
Proliferation and antithrombogenicity of EOC.
To quantify proliferation of cultured EOC, cells were dissociated by accutase treatment and analyzed for Ki67 expression, essentially as described above. Briefly, fixed and permeabilized cells were incubated with fluorescein-conjugated mouse monoclonal antibodies to human Ki67 (5 μg/ml; BD Pharmingen) at 4°C for 30 min. After removal of excess antibodies, cells were analyzed by flow cytometry.
Endothelial cell function of EOC was assayed by a modified thrombin generation assay (HaemoScan, Groningen, The Netherlands). EOC were dissociated using accutase and replated in fibronectin/gelatin-coated (both 10 μg/ml) 96-wells plates at a density of 50,000 cells/cm2. After 24 h, nonadherent cells were removed and adhering cells washed with PBS. Next, cells were incubated with fibrinogen-depleted plasma under normal culture conditions, activating the intrinsic coagulation cascade. After 15 min, a mixture of 30 mM CaCl2 and phospholipids was added, which results in the formation of thrombin. Samples (5 μl) were taken at regular intervals and added to ice-cold 25 mM Tris·HCl to prevent further formation of thrombin. Finally, the diluted samples were incubated with 3 mM thrombin substrate S2238 that results in a change of color, which was measured at 405 nm with 540 nm as the reference wavelength in a microtiter plate reader (Bio-Rad, Hercules, CA). A calibration curve of known thrombin concentrations was used to quantify thrombin formation in the experimental samples. Human umbilical vein endothelial cells and bare PCLdiUPy were included as negative and positive controls, respectively.
Statistical analysis.
Subject data are expressed as the mean (line) of the individual values (dots) and as means ± SE within the text. To analyze mean differences between patient groups and healthy controls, data were analyzed using a one-way Kruskall-Wallis test followed by a post hoc Dunn's multiple comparison test. To analyze mean differences between prehemodialysis data and posthemodialysis data, the Mann-Whitney U-test was performed. Univariate association studies were performed using Pearson's correlation, followed by multivariate ANCOVA. Probabilities of P < 0.05 were considered to be statistically significant.
RESULTS
Subject characteristics.
We studied 50 patients in various stages of CKD (n = 30), dialysis therapy (n = 20), and 10 healthy controls. Patients had various underlying causes of kidney disease. Some classic risk factors for cardiovascular disease were present, particularly hypertension (n = 46) and past cardiovascular events (n = 9). All patients used medication, including RAAS-blockade (n = 50), multivitamin supplements (n = 14), statins (n = 17), or erythropoietin (n = 18). Medication was unchanged during the study. Subject characteristics are summarized in Table 1.
Table 1.
Patient demography
| Healthy Controls | eGFR >60 (Stage 1–2 CKD) | 60 < eGFR <30 (Stage 3 CKD) | eGFR <30 (Stages 4–5 CKD) | ESRD (Stage 5 CKD), Hemodialysis* | ESRD (Stage 5 CKD), Peritoneal Dialysis | |
|---|---|---|---|---|---|---|
| n | 10 | 10 | 10 | 10 | 10 | 10 |
| Age, yr | 41.1±3.8 | 46.1±3.2 | 53.2±3.8 | 56.1±3.8 | 51.5±3.3 | 52.2±5.4 |
| Gender, male/female | 6/4 | 9/1 | 7/3 | 6/4 | 8/2 | 8/2 |
| BMI, kg/m2 | 23.2±1.1 | 28.1±1.6 | 26.7±0.8 | 28.5±1.6 | 23.5±1.3 | |
| eGFR, ml·min−1·1.73 m−2 | ND | 83.4±4.0 | 47.7±3.4 | 17.1±3.0 | 6.0±1.1 | 10.1±3.2 |
| Blood analyses | ||||||
| Serum creatinine, μmol/l | ND | 90.0±4.2 | 138.7±9.6 | 397.6±68.4 | 976.6±155.9 | 765.2±66.0 |
| Creatine clearance, ml/min | ND | 120.7±15.4 | 68.5±5.4 | 28.9±5.9 | 24.9±7.4 | 27.1±9.3 |
| Serum urea, mmol/ | ND | 6.7±0.6 | 13.3±2.3 | 23.7±2.4 | 26.8±2.5 | 19.8±2.6 |
| Blood pressure, mmHg | ||||||
| Diastolic pressure | ND | 74.6±2.6 | 74.4±3.3 | 72.2±2.7 | 83.7±4.6 | 77.6±1.7 |
| Systolic pressure | ND | 125.4±3.8 | 121.1±5.0 | 127.1±4.5 | 149.4±9.7 | 134.5±3.4 |
| White blood cells, ×106/ml | ND | 7.0±1.1 | 7.6±0.7 | 6.8±0.7 | 6.7±0.6 | 8.6±0.8 |
| Mononuclear cells, ×106/ml | 1.1±0.1 | 1.1±0.1 | 1.4±0.1 | 1.7±0.3 | 1.5±0.2 | 1.5±0.3 |
| Granulocytes, ×106/ml | ND | 5.9±1.0 | 6.1±0.7 | 5.1±0.7 | 5.3±0.6 | 7.0±0.7 |
| Thrombocytes, ×106/ml | ND | 214.9±23.7 | 235.8±28.5 | 208.8±20.9 | 202.5±25.1 | 265.0±49.5 |
| Hemoglobin, mmol/l | ND | 9.5±0.2 | 8.7±0.4 | 7.5±0.3 | 7.2±0.4 | 7.5±0.1 |
| Total cholesterol, mmol/l | ND | 5.0±0.3 | 5.2±0.3 | 4.6±0.4 | 4.0±0.3 | 4.1±0.2 |
| HDL cholesterol, mmol/l | ND | 1.2±0.1 | 1.3±0.1 | 1.4±0.2 | 1.3±0.1 | 1.1±0.2 |
| LDL cholesterol, mmol/l | ND | 3.4±0.3 | 3.2±0.3 | 3.0±0.3 | 1.9±0.2 | 2.3±0.1 |
| Total serum protein, g/l | ND | 74.8±1.3 | 73.1±1.5 | 75.0±1.8 | 68.6±2.1 | 63.9±7.0 |
| Serum calcium, mmol/l | ND | 2.36±0.02 | 2.33±0.03 | 2.36±0.04 | 2.20±0.05 | 2.45±0.07 |
| Serum phosphate, mmol/l | ND | 0.85±0.18 | 0.97±0.11 | 1.38±0.10 | 1.87±0.20 | 1.62±0.13 |
| Urine analyses | ||||||
| pH | ND | 6.4±0.3 | 5.8±0.2 | 6.2±0.2 | 6.5±0.3 | 6.1±0.1 |
| Urine creatinine, mmol/24 h | ND | 15.5±1.8 | 12.7±1.3 | 11.2±1.0 | 6.5±1.6 | 6.3±1.0 |
| Urine urea, mmol/24 h | ND | 374.5±29.4 | 335.0±26.5 | 319.5±30.2 | 80.4±17.2 | 99.5±17.8 |
| Urine total protein, g/24 h | ND | 0.74±0.23 | 1.55±0.74 | 1.73±0.40 | 1.14±0.44 | 0.78±0.37 |
| Urine sodium, mmol/24 h | ND | 188,9±19.9 | 157.1±24.0 | 153.0±13.4 | 53.7±21.8 | 78.8±15.8 |
| Urine potassium, mmol/24 h | ND | 85.7±8.5 | 72.17±4.9 | 63.9±9.9 | 34.8±7.1 | 32.0±6.2 |
| Dialysis | ||||||
| Time on dialysis, mo | 34.4±10.3 | 33.8±4.4 | ||||
| Dialysis efficiency, Kt/V | 1.37±0.06 | 2.22±0.10 | ||||
| Risk factors | ||||||
| History of CVD, % | 0% | 0% | 10% | 10% | 50% | 20% |
| Hypertension, % | 0% | 100% | 100% | 100% | 60% | 100% |
| Proteinuria, % | 0% | 70% | 50% | 80% | 80% | 60% |
| Vascular comorbidity, % | 0% | 0% | 0% | 30% | 60% | 20% |
| Previous renal transplant, % | 0% | 0% | 0% | 0% | 30% | 10% |
| Medication | ||||||
| Anticoagulants, % | 0% | 0% | 0% | 0% | 70% | 10% |
| Antihypertensive drugs, % | 0% | 100% | 100% | 100% | 50% | 60% |
| ACE inhibitors, % | 0% | 50% | 60% | 50% | 30% | 10% |
| ANG II-receptor antagonists, % | 0% | 40% | 70% | 10% | 0% | 20% |
| β-Blockers, % | 0% | 10% | 10% | 20% | 50% | 40% |
| Diuretics, % | 0% | 50% | 100% | 100% | 10% | 30% |
| Calcium antagonists, % | 0% | 20% | 10% | 10% | 0% | |
| Other, % | 0% | 0% | 0% | 10% | 10% | 0% |
| Anti-inflammatory agents, % | 10% | 0% | 0% | 0% | 20% | 0% |
| Erythropoietin, % | 0% | 0% | 0% | 10% | 90% | 80% |
| Iron supplements, % | 0% | 0% | 0% | 20% | 20% | 0% |
| Statins, % | 0% | 10% | 60% | 20% | 50% | 30% |
| Other, % | 20% | 0% | 50% | 60% | 100% | 100% |
| of which multivitamins, % | 10% | 0% | 0% | 0% | 40% | 100% |
Values are means ± SE. n, no. of subjects; CKD, chronic kidney disease; ESRD, end-stage renal disease; BMI, body mass index; eGFR, estimated glomerular filtration rate; CVD, cardiovascular disease; ND, not determined.
Predialysis values.
Advancing CKD (higher CKD stage or lower eGFR) was associated with higher serum creatinin levels (r = −0.95; P < 0.0001), lower creatinin clearance (r = 0.85; P < 0.0001), higher serum urea levels (r = −0.81; P < 0.0001), and higher serum phosphate levels (r = −0.73; P < 0.0001; Supplement 1; all supplemental material for this article is available on the journal web site). The number of white blood cells, mononuclear cells, granulocytes, and thrombocytes did not vary between the different stages of CKD (Supplement 1), but the hemoglobin content decreased with advancing CKD (r = 0.63; P = 0.0001). Total serum protein, total serum cholesterol, and HDL- and LDL-cholesterol and serum calcium were not different between the stages of CKD (Supplement 1).
Number of circulating EPC.
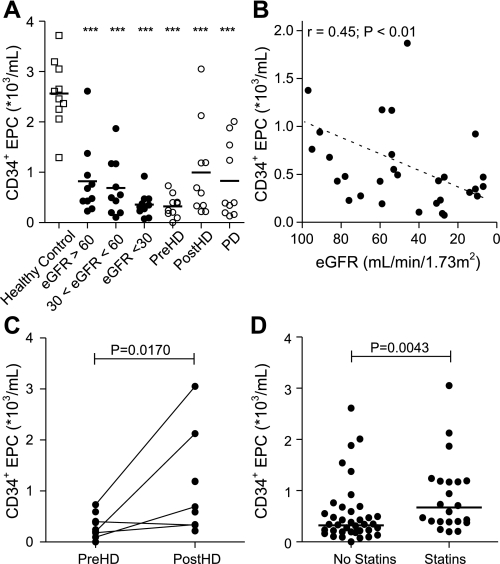
Circulating CD34+ EPC and CD14+ EPC were detectable by flow cytometry in all patient groups and healthy controls. The mean number of circulating CD34+ EPC in healthy subjects was 2.6 × 103 CD34+ EPC/ml peripheral blood (range 1.3–3.7 × 103 CD34+ EPC/ml). Already in patients with an eGFR > 60, the number of CD34+ EPC was lower (mean 0.8; range 0.2–2.6 × 103 CD34+ EPC/ml peripheral blood; 68% reduction; P < 0.001) compared with healthy controls. Patients with 30 < eGFR < 60 had even lower numbers of CD34+ EPC (mean 0.7; range 0.11–1.9 × 103 CD34+ EPC/ml peripheral blood; 73% reduction; P < 0.001). CKD patients in the eGFR < 30 group showed the highest reduction in CD34+ EPC number (mean 0.4; range 0.08–0.9 × 103 CD34+ EPC/ml peripheral blood; 89% reduction; P < 0.001; Fig. 1A).
Fig. 1.
Determinants of circulating CD34+ endothelial progenitor cell (EPC) numbers. CD34+ EPC decreased in early stages of chronic kidney disease (CKD) and decreased further during disease progression (A and B). Statin use increased the number of circulating CD34+ EPC (C). Also, hemodialysis treatment resulted in mobilization of CD34+ EPC (D). □, Healthy controls; •, CKD patients; ○, hemodialysis (HD) and peritoneal dialysis (PD) patients. ***P < 0.001 vs. healthy controls.
In univariate analysis, the reduction in CD34+ EPC numbers was associated with CKD stage (eGFR; r = 0.44; P = 0.008; Fig. 1B). Interestingly, the number of circulating CD34+ EPC increased during hemodialysis treatment (0.3 ± 0.07 vs. 1.0 ± 0.29 × 103 CD34+ EPC/ml peripheral blood; pre- vs. post-hemodialysis; 208% increase; P = 0.02; Fig. 1C), but not to the level of healthy controls (1.0 ± 0.29 vs. 2.6 ± 0.21 × 103 CD34+ EPC/ml peripheral blood; post-hemodialysis vs. healthy control; 62% decrease; P < 0.001). Similarly, patients receiving peritoneal dialysis had higher CD34+ cell numbers than patients in stage 5 CKD who did not receive renal replacement therapy (0.8 ± 0.24 vs. 0.4 ± 0.08 × 103 CD34+ EPC/ml peripheral blood; peritoneal dialysis vs. eGFR < 30, 132% increase; P = 0.04). However, the CD34+ EPC number did not reach normal levels (0.8 ± 0.24 vs. 2.6 ± 0.21 × 103 CD34+ EPC/ml peripheral blood; peritoneal dialysis vs. healthy control; 68% decrease; P < 0.001).
In untreated CKD patients, with the use of multivariate analysis, the number of CD34+ EPC no longer correlated with eGFR (r = 0.18; P = 0.34) but was correlated with serum urea levels (r = −0.28; P = 0.03), serum phosphate levels (r = −0.88; P < 0.01), and the presence of concomitant risk factors for cardiovascular disease, such as age (r = −0.50; P = 0.02), LDL-cholesterol (r = 0.28; P < 0.05) (Table 2), and a history of cardiovascular disease (P = 0.03), but not with body mass index, blood pressure, HDL-cholesterol, total cholesterol levels, or the presence of vascular comorbidity. There was no association with gender or the use of erythropoietin (Supplement 2). Statin use increased the number of circulating CD34+ EPC (P < 0.01; Fig. 1D).
Table 2.
Determinants of CD34+ cell number
|
Univariate Analysis |
Multivariate Analysis
|
|||
|---|---|---|---|---|
| r Value | Probability | Adjusted r value | Probability | |
| eGFR, ml·min−1·1.73 m−2 | 0.45 | 0.006 | 0.18 | 0.343 |
| Age, yr | −0.46 | 0.005 | −0.50 | 0.021 |
| BMI, kg/m2 | 0.05 | 0.392 | ||
| Blood pressure | ||||
| Systolic, mmHg | −0.01 | 0.494 | ||
| Diastolic, mmHg | −0.10 | 0.302 | ||
| Serum urea, mmol/l | −0.33 | 0.046 | −0.28 | 0.033 |
| Serum phosphate, mmol/l | −0.38 | 0.015 | −0.88 | 0.008 |
| Total cholesterol, mmol/l | 0.13 | 0.245 | ||
| HDL cholesterol, mmol/l | −0.24 | 0.100 | ||
| LDL cholesterol, mmol/l | 0.31 | 0.050 | 0.28 | 0.047 |
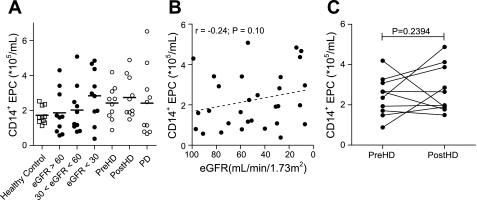
In contrast to CD34+ EPC, the numbers of CD14+ EPC were similar in patients and healthy controls (Fig. 2A) and did not associate with increasing CKD stage (Fig. 2B). No significant association was found between the number of circulating CD14+ EPC and known cardiovascular risk factors or the use of statins or erythropoietin by CKD patients (Supplement 3). Also, hemodialysis (Fig. 2C) and peritoneal dialysis did not alter the number of circulating CD14+ EPC.
Fig. 2.
Determinants of circulating CD14+ EPC numbers. CD14+ EPC numbers were similar in CKD patients and healthy controls (A), but the number of CD14+ EPC increased slightly during CKD progression (B). There was no mobilization effect caused by hemodialysis treatment (C). Symbols are as defined for Fig. 1.
Adherence and apoptosis of EOC.
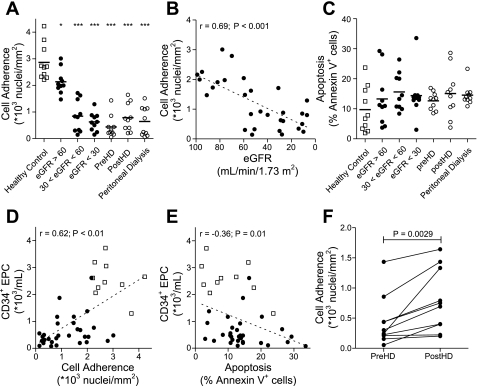
Peripheral blood MNC were plated on fibronectin-coated PCLdiUPy at a density of 5.0 × 103 cells/mm2 and cultured according to standard protocols for the culture of EOC (2, 14). Cell adhesion in patients with mild CKD (eGFR > 60) was lower than in healthy controls (2.13 ± 0.15 vs. 2.86 ± 0.21 × 103 cells/mm2, respectively; 25% reduction; P < 0.05), and declined further in advancing CKD stages [0.84 ± 0.19 × 103 cells/mm2 (30 < eGFR < 60); 71% reduction; P < 0.001]. Cell adhesion was lowest in patients with eGFR < 30 (0.63 ± 0.11 × 103 cells/mm2; 78% reduction; P < 0.001; Fig. 3, A and B). Reduced cell adhesion was not caused by apoptosis, because patients and controls had comparable percentages of apoptotic cells (14.5 ± 2.5 vs 9.7 ± 2.4% respectively; P = 0.09; Fig. 3C). Also, there was no correlation between the percentage of apoptotic cells and the number of adhered cells. Interestingly, in CKD patients, the number of circulating CD34+ EPC was associated with cell adhesion (r = 0.62; P < 0.001; Fig. 3D) and reduced apoptosis (r = −0.36; P = 0.01; Fig. 3E). The reduction in cell adhesion observed in CKD patients was not ameliorated by hemodialysis, nor by peritoneal dialysis (Fig. 3A), nor did dialysis treatment augment apoptosis of cells in culture (Fig. 3C). Notably, adhesion of MNC isolated directly before hemodialysis adhered less than cells isolated directly after hemodialysis (0.44 ± 0.13 vs. 0.79 ± 0.16 × 103 cells/mm2 respectively; 80% increase; P < 0.01; Fig. 3F).
Fig. 3.
Adherence and apoptosis of endothelial outgrowth cells. The adherence of endothelial outgrowth cells from patients with CKD was hampered as early as stage 1 (eGFR > 60; A). Dysfunctional adhesion was associated with CKD disease progression (B). Apoptosis of endothelial outgrowth cells did not contribute to reduced adhesion, for apoptosis was similar in all patient groups and healthy controls (C). Increased numbers of CD34+ EPC were associated with increased cell adherence (D) and reduced apoptosis (E). Activation of cells through hemodialysis also increased cell adherence (F). Symbols are as defined for Fig. 1. *P < 0.05 vs. health controls. ***P < 0.001 vs. healthy controls.
Endothelial phenotype of cultured EOC.
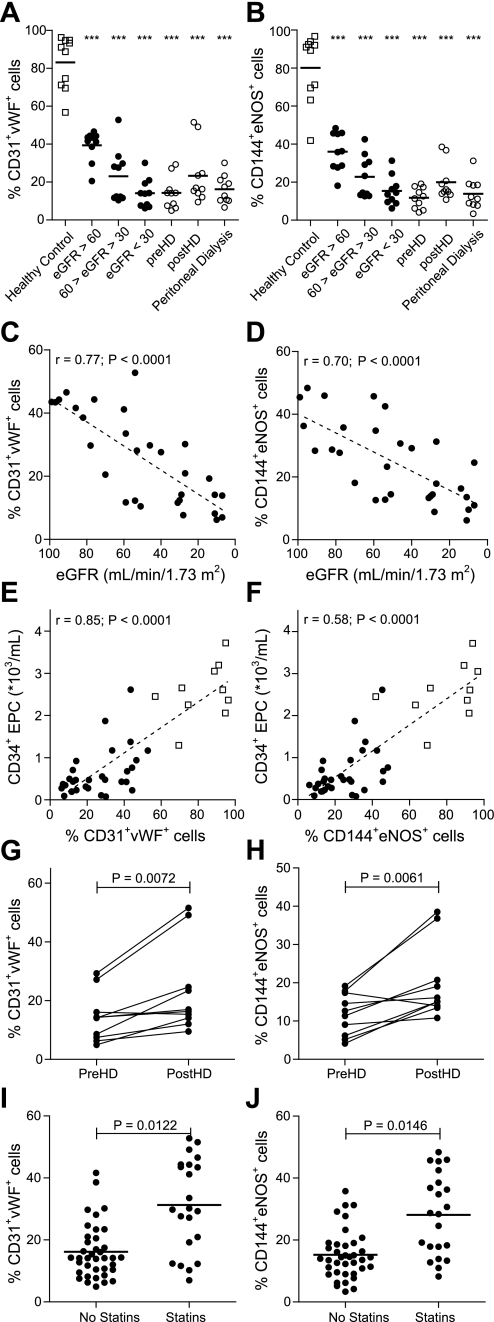
To investigate the endothelial outgrowth potential of cultured cells, cells were examined for the coexpression of endothelial cell markers CD31 and vWF or CD144 and eNOS. Differentiation of cells into macrophages was determined by assessing CD163 expression (Table 3). Endothelial cell markers were not present at day 0 of culture, but high numbers of endothelial cells had formed in cultures from healthy controls (83.12 ± 4.40% CD31+vWF+ cells and 80.20 ± 5.02% CD144+eNOS+ cells) after 21 days. Endothelial outgrowth was reduced in all patient groups (Fig. 4, A and B). This reduction was associated with a reduction in eGFR (r = 0.77; P < 0.0001 and r = 0.70; P < 0.0001, CD31+vWF+ cells and CD144+eNOS+ cells, respectively; Fig. 4, C and D), and furthermore associated with decreased numbers of circulating CD34+ EPC (both CD31+vWF+ cells and CD144+eNOS+ cells, r = 0.85; P < 0.0001; Fig. 4, E and F), but did not correlate with the number of circulating CD14+ EPC (r = −0.16; P = 0.17 and r = −0.15; P = 0.18, CD31+vWF+ cells and CD144+eNOS+ cells, respectively). Neither hemodialysis nor peritoneal dialysis resulted in full restoration of endothelial outgrowth (Fig. 4, A and B), but cells from hemodialysis patients isolated directly after hemodialysis showed higher endothelial outgrowth compared with cells isolated directly before hemodialysis (P < 0.01, both CD31+vWF+ cells and CD144+eNOS+ cells; Fig. 4, G and H). Furthermore, the use of statins increased endothelial outgrowth in patients with CKD (both CD31+vWF+ cells and CD144+eNOS+ cells P < 0.001; Fig. 4, I and J).
Table 3.
Endothelial cell marker expression by endothelial outgrowth cells
| Healthy Controls | eGFR >60 (stages 1–2 CRF) | 60 < eGFR <30 (stage 3 CRF) | eGFR <30 (stages 4–5 CRF) | Prehemodialysis | Posthemodialysis | Peritoneal Dialysis | |
|---|---|---|---|---|---|---|---|
| CD31 | 86.02±4.01† | 40.72±2.51* | 25.15±5.61*† | 15.01±2.65*† | 13.24±2.18*† | 25.26±4.08*†‡ | 16.94±2.34*† |
| vWF | 88.53±4.21† | 43.88±2.88* | 26.56±3.72*† | 15.48±2.40*† | 16.64±3.22*† | 25.57±4.51*†‡ | 17.60±2.35*† |
| CD144 | 83.20±5.02† | 36.84±3.46* | 23.13±3.36* | 18.30±2.87*† | 12.61±1.77*† | 19.84±2.62*†‡ | 14.37±2.52*† |
| eNOS | 91.76±5.27† | 37.28±4.18* | 26.92±3.89* | 16.97±2.22*† | 13.58±2.43*† | 24.73±4.80*†‡ | 15.59±2.32*† |
| CD163 | 7.83±1.08 | 4.47±0.61* | 3.90±0.77 | 6.03±0.88 | 5.12±1.09 | 3.71±0.67* | 3.87±0.73* |
Values are means ± SE expressed as the percentage of positive cells. vWF, von Willebrand factor; eNOS, endothelial nitric oxide synthase.
P < 0.05 vs. healthy controls.
P < 0.05 vs. eGFR >60.
P < 0.05 vs. prehemodialysis.
Fig. 4.
Endothelial cell differentiation by cultured endothelial outgrowth cells. Endothelial outgrowth cells from patients with CKD lost the ability to differentiate into mature endothelial cells, as indicated by reduced coexpression of CD31 and von Willebrand factor (vWF; A) and CD144 and endothelial nitric oxide synthase (eNOS; B). Although differentiation was affected at the early stages of disease, differentiation capacity decreased progressively during disease progression (C and D). The level of endothelial cell differentiation was associated with the number of CD34+ EPC (E and F) and increased after hemodialysis treatment (G and H). Furthermore, the use of prescribed statins increased endothelial cell differentiation (I and J). Symbols are as defined for Fig. 1. ***P < 0.001 vs. healthy controls.
Cell proliferation and endothelial function.
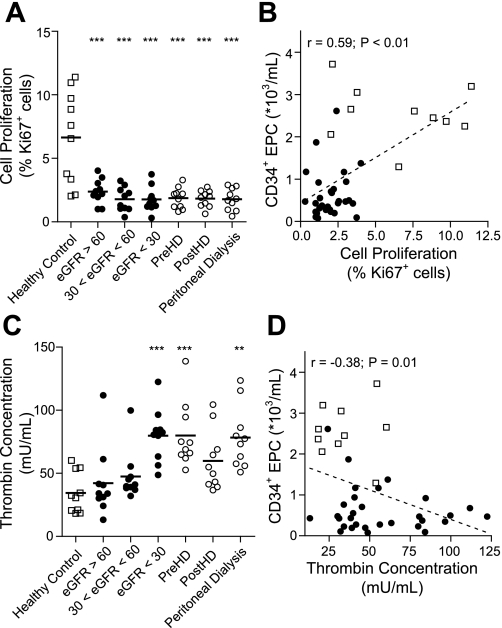
We assessed the proliferative potential of cultured endothelial cells by determining the expression of the nuclear proliferation marker Ki67 at day 21. Proliferation was reduced in EOC from CKD patients (average 70% reduction), independently of the disease stage (P < 0.01 vs. healthy controls) (Fig. 5A). In CKD patients who did not receive dialysis treatment, the percentage of proliferating cells was associated with the number of circulating CD34+ EPC (r = 0.59; P < 0.001; Fig. 5B), but this did not correlate with the number of CD14+ EPC (r = −0.22; P = 0.08). Hemodialysis and peritoneal dialysis had no effect on the number of proliferating cells, nor did the number of proliferating cells change following hemodialysis (prehemodialysis vs. posthemodialysis).
Fig. 5.
Endothelial cell proliferation and function of cultured endothelial outgrowth cells. Endothelial outgrowth cells from CKD patients showed drastic decreases in cell proliferation (A). Proliferation of endothelial outgrowth cells was associated with the presence of CD34+ EPC (B). Furthermore, endothelial outgrowth cells from end-stage renal failure patients showed reduced antithrombogenic behavior (C), indicating improper maturation. In CKD patients, functional maturation depended partly on the number of circulating CD34+ EPC numbers (D). Symbols are as defined for Fig. 1.
We asked whether EOC from CKD patients exerted antithrombogenic behavior, an endothelial cell function. Using a modified thrombin generation assay (21), we examined the ability of cells to inhibit thrombin formation in an in vitro coagulation assay. EOC from healthy controls inhibited the formation of thrombin, and maximum thrombin concentration did not exceed thrombin formation by human umbilical cord endothelial cells (data not shown). Cells from patients with an eGFR > 30 were also able to inhibit the formation of thrombin [maximum thrombin concentration 47.46 ± 6.16 mU/ml (30 < eGFR < 60) vs. 34.41 ± 5.11 mU/ml (healthy controls); P > 0.10]. Thereafter, the antithrombogenic property of EOC decreased, resulting in increased thrombin formation [79.87 ± 6.61 mU/ml (eGFR < 30); P < 0.01 vs. healthy controls; Fig. 5C]. Although no difference in thrombin formation was found between the initial stages of CKD and healthy controls, increased thrombin generation associated with disease progression (r = −0.47; P = 0.004), indicative of a reduction in EOC function during CKD progression. Also, thrombin generation was negatively correlated with the number of circulating CD34+ EPC (r = −0.38; P < 0.01; Fig. 5D) in CKD patients, indicating the importance of CD34+ EPC in the functional maturation of EOC. In contrast to peritoneal dialysis, hemodialysis restored endothelial cell function (Fig. 5C).
DISCUSSION
We have investigated the number and endothelial outgrowth capacity of circulating CD34+ EPC and CD14+ EPC in patients with CKD and found that 1) the numbers of circulating CD34+ EPC decreased with increasing kidney disease as reflected by increased serum urea and phosphate levels; 2) the numbers of circulating CD34+ EPC did not correlate with eGFR; 3) adherence and endothelial outgrowth of EPC from patients with CKD is progressively reduced during kidney disease, 4) the antithrombogenic function of EOC was hampered in patients with end-stage renal disease; and that 5) EPC dysfunction is not fully ameliorated by dialysis treatment. Taken together, EPC dysfunction in patients with progressive CKD may hamper physiological vascular repair, adding to increased risk for cardiovascular diseases observed in these patients. Furthermore, EPC dysfunction may challenge the use of patient-derived EPC for regenerative medicine therapies.
Although EPC dysfunction has been described for late-stage kidney disease (stages 4–5 CKD), we are the first to show a marked decrease in the number of circulating CD34+ EPC as early as stage 1 CKD, which worsened during CKD progression, indicated by increasing serum urea levels. Corroboratory, Sturiale et al. (38) and Choi et al. (5) have described reduced numbers of CD34+ and CD133+ EPC in patients with CKD that undergo hemodialysis treatment. Commonly, this implies that the production, or mobilization of CD34+ EPC from the bone marrow is impaired in patients with CKD.
This impairment may be explained by chronic uremia, since increases in plasma urea concentration are associated with decreased CD34+ EPC numbers. Similarly, others have described decreases in erythropoiesis (11, 13), increases in progenitor cell apoptosis (45) and impairment of EPC migration (6, 9) caused by uremic toxins present in serum from CKD patients. However, we cannot exclude interference of antihypertensive drugs as confounder in our analysis, since all CKD patients were kept on their regular medication. Interestingly, the number of CD34+ EPC increased following hemodialysis. Although, the CD34+ EPC mobilization may have increased by a (temporary) decrease in uremic toxins following hemodialysis, most patients (90%) received erythropoietin before hemodialysis. Since erythropoietin is a known attractant for CD34+ EPC (3, 12), we feel that the biological effects of erythropoietin, and not the decrease in uremic toxins, caused the observed increase in circulating CD34+ EPC. However, the true mechanism behind hemodialysis-induced CD34+ EPC mobilization remains to be elucidated.
In contrast to the reduction in CD34+ EPC numbers, there was no effect of CKD on the number of circulating CD14+ EPC. Therefore, we hypothesize that the chemoattractants for CD14+ EPC and CD34+ EPC are differentially expressed in patients with CKD. Although we did not include such analysis in our current study, there are multiple reports on the increased expression of MCP-1, a chemoattractant for CD14+ EPC during CKD, which seem to corroborate this hypothesis (16, 37). However, little mechanistic insight is known on the impaired mobilization of CD34+ EPC, and future research will have to elucidate this phenomenon.
When used in regenerative medicine, e.g., for endothelialized hemodialysis shunts, endothelial outgrowth on natural or synthetic biomaterial is key. Endothelial outgrowth potential is commonly used as a surrogate marker for the quality and function of circulating EPC. Here, we tested the ability of CKD patient-derived EPC to adhere to fibronectin-coated PCLdiUPy and found a high reduction in the number of adhering cells (up to 78%) compared with EPC from healthy controls. This reduction in adherent EPC numbers was not caused by EPC apoptosis in CKD patients (45), since apoptosis frequencies were comparable in CKD patients and healthy controls. Moreover, endothelial outgrowth of adherent EPC was also highly reduced (up to 80%) in patients with CKD compared with healthy controls.
Endothelial outgrowth and proliferation of CD14+ EPC depend on paracrine signaling by CD34+ EPC (21). We and others have previously described that endothelial outgrowth of CD14+ EPC depends on interaction between CD34+ EPC and CD14+ EPC (21). Herein, CD34+ EPC secrete various proangiogenic cytokines and growth factors by CD34+ EPC that augment the behavior of CD14+ EPC (21, 39). Here, we found that adhesion of EOC correlated positively with CD34+ EPC numbers. Secretion of proangiogenic factors by CD34+ EPC may therefore explain the relationship between the number of CD34+ EPC and endothelial outgrowth and proliferation observed here.
We therefore postulate that there is a disturbed balance between CD34+ EPC and CD14+ EPC in the circulation of CKD patients, which may cause the observed impairments. Restoration of this balance between CD34+ EPC and CD14+ EPC numbers, by mobilization of CD34+ EPC from the bone marrow, may therefore restore endothelial outgrowth potential and vascular regeneration in vivo. We and others have described increased numbers of circulating CD34+ EPC with statin (41, 42) use or erythropoietin (4) and improved endothelial outgrowth percentage. Additionally, recent clinical results show improved cardiovascular outcome of high-dose statin use in patients with end-stage renal failure (31–33), which argues for prophylactic prescription of EPC-mobilizing agents for CKD patients.
Antithrombogenic behavior is a key property of endothelial cells, which, physiologically, maintain a balance between thrombolytic and thrombogenic activities, preventing the formation of blood clots. In this study, thrombin generation was efficiently prevented by EOC from healthy controls, but this property was lost during disease progression. We are the first to describe loss of in vitro hemostatic control in EOC from patients with end-stage renal disease, and this loss may contribute to the increased occurrence of thrombosis within this patient group (23, 43). Since the EOC in the in vitro coagulation assay were expressing endothelial markers CD31, VE-Cadherin, vWF, and eNOS, the loss of antithrombogenic behavior may also reflect improper maturation of EOC.
Remarkably, EPC dysfunction persists in the presence of nonuremic fetal calf serum, as was the case in our in vitro assays. This would argue for long-lasting changes in the EPC that may be elicited by uremic toxins, but do not need these toxins to remain in effect. Such changes may be introduced in the EPC epigenome (as reviewed in Refs. 35 and 36) but remain to be elucidated.
In conclusion, patients with CKD have reduced numbers of circulating CD34+ EPC, which decrease progressively with advancing disease severity and increasing serum urea levels. EPC dysfunction results in a functional impairment in cell adherence and endothelial outgrowth formation. Although dialysis caused some improvement, functional impairments were not completely alleviated. These observations are compatible with the increased occurrence of cardiovascular disease in patients suffering from CKD. Moreover, decreased numbers and functional impairment of circulating CD34+ EPC pose a major limitation for their use in regenerative medicine. Proper mobilization and revitalization strategies, e.g., treatment with erythropoietins or statins, need to be investigated to prevent further decay of progenitor cell function. Early intervention may reduce cardiovascular morbidity in CKD patients through increased physiological vascular regeneration.
GRANTS
This work was supported by a pilot research grant from the graduate school for BioMaterial Science and Application (BMSA) of the University of Groningen, Groningen, The Netherlands.
Acknowledgments
Present addreess of P. Y. W. Dankers: Dept. of Biomedical Engineering, Molecular Science, and Technology, Eindhoven University of Technology, Eindhoven, The Netherlands.
REFERENCES
- 1.Asahara T, Kawamoto A. Endothelial progenitor cells for postnatal vasculogenesis. Am J Physiol Cell Physiol 287: C572–C579, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Asahara T, Murohara T, Sullivan A, Silver M, vanderZee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science 275: 964–967, 1997. [DOI] [PubMed] [Google Scholar]
- 3.Bahlmann FH, de Groot K, Spandau JM, Landry AL, Hertel B, Duckert T, Boehm SM, Menne J, Haller H, Fliser D. Erythropoietin regulates endothelial progenitor cells. Blood 103: 921–926, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Bahlmann FH, Degroot K, Duckert T, Niemczyk E, Bahlmann E, Boehm SM, Haller H, Fliser D. Endothelial progenitor cell proliferation and differentiation is regulated by erythropoietin. Kidney Int 64: 1648–1652, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Choi JH, Kim KL, Huh W, Kim B, Byun J, Suh W, Sung J, Jeon ES, Oh HY, Kim DK. Decreased number and impaired angiogenic function of endothelial progenitor cells in patients with chronic renal failure. Arterioscler Thromb Vasc Biol 24: 1246–1252, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Coppolino G, Bolignano D, Campo S, Loddo S, Teti D, Buemi M. Circulating progenitor cells after cold pressor test in hypertensive and uremic patients. Hypertens Res 31: 717–724, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Dankers PYW, Harmsen MC, Brouwer LA, van Luyn MJA, Meijer EW. A modular and supramolecular approach to bioactive scaffolds for tissue engineering. Nat Mater 4: 568–574, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Dankers PYW, van Leeuwen ENM, van Gemert GML, Spiering AJH, Harmsen MC, Brouwer LA, Janssen HM, Bosman AW, van Luyn MJA, Meijer EW. Chemical and biological properties of supramolecular polymer systems based on oligocaprolactones. Biomaterials 27: 5490–5501, 2006. [DOI] [PubMed] [Google Scholar]
- 9.de Groot K, Hermann Bahlmann F, Sowa J, Koenig J, Menne J, Haller H, Fliser D. Uremia causes endothelial progenitor cell deficiency. Kidney Int 66: 641–646, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Dimmeler S, Zeiher AM. Vascular repair by circulating endothelial progenitor cells: the missing link in atherosclerosis? J Mol Med 82: 671–677, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Freedman MH, Cattran DC, Saunders EF. Anemia of chronic renal failure: inhibition of erythropoiesis by uremic serum. Nephron 35: 15–19, 1983. [DOI] [PubMed] [Google Scholar]
- 12.Heeschen C, Aicher A, Lehmann R, Fichtlscherer S, Vasa M, Urbich C, Mildner-Rihm C, Martin H, Zeiher AM, Dimmeler S. Erythropoietin is a potent physiologic stimulus for endothelial progenitor cell mobilization. Blood 102: 1340–1346, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Hotta T, Maeda H, Suzuki I, Chung TG, Saito A. Selective inhibition of erythropoiesis by sera from patients with chronic renal failure. Proc Soc Exp Biol Med 186: 47–51, 1987. [DOI] [PubMed] [Google Scholar]
- 14.Hur J, Yoon C, Kim H, Choi J, Kang H, Hwang K, Oh BH, Lee M, Park Y. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol 24: 288–293, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz M, Gilley D, Yoder M. Identification of a novel hierarchy of endothelial progenitor cells utilizing human peripheral and umbilical cord blood. Blood 104: 2752–2760, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Jacobson SH, Egberg N, Hylander B, Lundahl J. Correlation between soluble markers of endothelial dysfunction in patients with renal failure. Am J Nephrol 22: 42–47, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Kaushal S, Amiel GE, Guleserian KJ, Shapira OM, Perry T, Sutherland FW, Rabkin E, Moran AM, Schoen FJ, Atala A, Soker S, Bischoff J, Mayer JE Jr. Functional small-diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nat Med 7: 1035–1040, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawamoto A, Asahara T, Losordo DW. Transplantation of endothelial progenitor cells for therapeutic neovascularization. Cardiovasc Radiat Med 3: 221–225, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Krenning G, Dankers PYW, Jovanovic D, van Luyn MJA, Harmsen MC. Efficient differentiation of CD14+ monocytic cells into endothelial cells on degradable biomaterials. Biomaterials 28: 1470–1479, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Krenning G, Moonen JR, van Luyn MJ, Harmsen MC. Generating new blood flow: integrating developmental biology and tissue engineering. Trends Cardiovasc Med 18: 312–323, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Krenning G, van der Strate B, Schipper M, Gallego y van Seijen XJ, Fernandes B, van Luyn MJA, Harmsen MC. CD34+ cells augment endothelial cell differentiation of CD14+ endothelial progenitor cells in vitro. J Cell Mol Med. In press. [DOI] [PMC free article] [PubMed]
- 22.Krenning G, van Luyn MJA, Harmsen MC. Endothelial progenitor cell-based neovascularization: implications for therapy. Trends Mol Med 15: 180–189, 2009. [DOI] [PubMed] [Google Scholar]
- 23.Mahmoodi BK, ten Kate MK, Waanders F, Veeger NJ, Brouwer JL, Vogt L, Navis G, van der MJ. High absolute risks and predictors of venous and arterial thromboembolic events in patients with nephrotic syndrome: results from a large retrospective cohort study. Circulation 117: 224–230, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Mall J, Philipp AW, Rademacher A, Paulitschke M, Buttemeyer R. Re-endothelialization of punctured ePTFE graft: an in vitro study under pulsed perfusion conditions. Nephrol Dial Transplant 19: 61–67, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Moonen JRAJ, de Leeuw K, van Seijen X, Kallenberg C, van Luyn MJA, Bijl M, Harmsen MC. Reduced number and impaired function of circulating progenitor cells in patients with systemic lupus erythematosus. Arthritis Res Ther 9: R84, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 39: S1–S266, 2002. [PubMed] [Google Scholar]
- 27.Popa ER, Harmsen MC, Tio RA, van der Strate BWA, Brouwer LA, Schipper M, Koerts J, de Jongste MJL, Hazenberg A, Hendriks M, van Luyn MJA. Circulating CD34+ progenitor cells modulate host angiogenesis and inflammation in vivo. J Mol Cell Cardiol 41: 86–96, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez-Ayala E, Yao Q, Holmen C, Lindholm B, Sumitran-Holgersson S, Stenvinkel P. Imbalance between detached circulating endothelial cells and endothelial progenitor cells in chronic kidney disease. Blood Purif 24: 196–202, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Romagnani P, Annunziato F, Liotta F, Lazzeri E, Mazzinghi B, Frosali F, Cosmi L, Maggi L, Lasagni L, Scheffold A, Kruger M, Dimmeler S, Marra F, Gensini G, Maggi E, Romagnani S. CD14+CD34(low) cells with stem cell phenotypic and functional features are the major source of circulating endothelial progenitors. Circ Res 97: 314–322, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 108: 2154–2169, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Shepherd J, Kastelein JJP, Bittner V, Deedwania P, Breazna A, Dobson S, Wilson DJ, Zuckerman A, Wenger NK, Treating to New Targets Investigators. Effect of intensive lipid lowering with atorvastatin on renal function in patients with coronary heart disease: The Treating to New Targets (TNT) Study. Clin J Am Soc Nephrol 2: 1131–1139, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Shepherd J, Barter P, Carmena R, Deedwania P, Fruchart JC, Haffner S, Hsia J, Breazna A, LaRosa J, Grundy S, Waters D, Treating to New Targets Investigators. Effect of lowering LDL cholesterol substantially below currently recommended levels in patients with coronary heart disease and diabetes: The Treating to New Targets (TNT) Study. Diabetes Care 29: 1220–1226, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Shepherd J, Kastelein JJP, Bittner V, Deedwania P, Breazna A, Dobson S, Wilson DJ, Zuckerman A, Wenger NK. Intensive lipid lowering with atorvastatin in patients with coronary heart disease and chronic kidney disease: The TNT (Treating to New Targets) Study. J Am Coll Cardiol 51: 1448–1454, 2008. [DOI] [PubMed] [Google Scholar]
- 34.Shishehbor MH, Oliveira LPJ, Lauer MS, Sprecher DL, Wolski K, Cho L, Hoogwerf BJ, Hazen SL. Emerging cardiovascular risk factors that account for a significant portion of attributable mortality risk in chronic kidney disease. Am J Cardiol 101: 1741–1746, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stenvinkel P, Ekstrom TJ. Epigenetics and the uremic phenotype: a matter of balance. Contrib Nephrol 161: 55–62, 2008. [DOI] [PubMed] [Google Scholar]
- 36.Stenvinkel P, Ekstrom TJ. Does the uremic milieu affect the epigenotype? J Ren Nutr 19: 82–85, 2009. [DOI] [PubMed] [Google Scholar]
- 37.Stinghen AE, Goncalves SM, Martines EG, Nakao LS, Riella MC, Aita CA, Pecoits-Filho R. Increased plasma and endothelial cell expression of chemokines and adhesion molecules in chronic kidney disease. Nephron Clin Pract 111: c117–c126, 2009. [DOI] [PubMed] [Google Scholar]
- 38.Sturiale A, Coppolino G, Loddo S, Criseo M, Campo S, Crasci E, Bolignano D, Nostro L, Teti D, Buemi M. Effects of haemodialysis on circulating endothelial progenitor cell count. Blood Purif 25: 242–251, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Umland O, Heine H, Miehe M, Marienfeld K, Staubach K, Ulmer A. Induction of various immune modulatory molecules in CD34+ hematopoietic cells. J Leukoc Biol 75: 671–679, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Valgimigli M, Rigolin GM, Fucili A, Porta MD, Soukhomovskaia O, Malagutti P, Bugli AM, Bragotti LZ, Francolini G, Mauro E, Castoldi G, Ferrari R. CD34+ and endothelial progenitor cells in patients with various degrees of congestive heart failure. Circulation 110: 1209–1212, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Vasa M, Fichtlscherer S, Adler K, Aicher A, Martin H, Zeiher A, Dimmeler S. Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation 103: 2885–2890, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Walter D, Rittig K, Bahlmann FH, Kirchmair R, Silver M, Murayama T, Nishimura H, Losordo DW, Asahara T, Isner JM. Statin therapy accelerates reendothelialization: a novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells. Circulation 105: 3017–3024, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Wattanakit K, Cushman M, Stehman-Breen C, Heckbert SR, Folsom AR. Chronic kidney disease increases risk for venous thromboembolism. J Am Soc Nephrol 19: 135–140, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Werner N, Nickenig G. Endothelial progenitor cells in health and atherosclerotic disease. Ann Med 39: 82–90, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Westerweel PE, Hoefer IE, Blankestijn PJ, de Bree P, Groeneveld D, van Oostrom O, Braam B, Koomans HA, Verhaar MC. End-stage renal disease causes an imbalance between endothelial and smooth muscle progenitor cells. Am J Physiol Renal Physiol 292: F1132–F1140, 2007. [DOI] [PubMed] [Google Scholar]