Abstract
Spiral ganglion neurons often degenerate in the deaf ear, compromising the function of cochlear implants. Cochlear implant function can be improved by good preservation of the spiral ganglion neurons, which are the target of electrical stimulation by the implant. Brain derived neurotrophic factor (BDNF) has previously been shown to enhance spiral ganglion survival in experimentally deafened ears. Providing enhanced levels of BDNF in human ears may be accomplished by one of several different methods. The goal of these experiments was to test a modified design of the cochlear implant electrode that includes a coating of fibroblast cells transduced by a viral vector with a BDNF gene insert. To accomplish this type of ex vivo gene transfer, we transduced guinea pig fibroblasts with an adenovirus with a BDNF gene cassette insert, and determined that these cells secreted BDNF. We then attached BDNF-secreting cells to the cochlear implant electrode via an agarose gel, and implanted the electrode in the scala tympani. We determined that the BDNF expressing electrodes were able to preserve significantly more spiral ganglion neurons in the basal turns of the cochlea after 48 days of implantation when compared to control electrodes. This protective effect decreased in the higher cochlear turns. The data demonstrate the feasibility of combining cochlear implant therapy with ex vivo gene transfer for enhancing spiral ganglion neuron survival.
Keywords: guinea pig, adenovirus vector, nerve protection, ex vivo gene therapy, BDNF
Introduction
Cochlear implantation is a well established method of rehabilitating severe to profound deafness. Most implant recipients achieve considerable benefit, but for some, however, the benefits are modest. The variability in hearing performance after implantation is likely due to preoperative variables such as onset of deafness (prelingual vs. postlingual), duration of deafness, cause of deafness (El-Hakim et al., 2002; Gantz et al., 1993; Osberger et al., 2002), and/or operative and postoperative variables such as surgical approach, the type of electrode used, and position within cochlea (Blamey et al., 1992; Cohen, 1997; Cohen et al., 1993). Additional post-operative factors include communication/rehabilitative environment and speech processing strategy (Geers et al., 2002).
One possible common link between the perioperative factors and the outcome of the cochlear implant procedure is the survival of eighth nerve structures, especially the spiral ganglion neurons (Nadol et al., 1989), which are directly stimulated by the cochlear implant electrode (Clopton et al., 1980). Both quantity and quality of surviving spiral ganglion neurons appears to be important for the success of the cochlear implant procedure (Fayad et al., 1991; Khan et al., 2005; Leake-Jones and Rebscher, 1983; Nadol et al., 2001; Sutton and Miller, 1983). Recent studies in humans revealed lower spiral ganglion neuron density in implanted vs. unimplanted patients (Khan et al., 2005), demonstrating possible detrimental effects of the implant and/or the stimulation on the spiral ganglion, at least in some cases. The possibility for adverse effects of the implant on the neurons further emphasizes the need for measures to enhance spiral ganglion survival and optimize the condition of the neurons.
A number of growth factors (including neurotrophins) influence the development and maintenance of spiral ganglion neurons in the cochlea. Brain derived neurotrophic factor (BDNF) is a neurotrophin involved in the development and maintenance of spiral ganglion cells (Ernfors et al., 1995; Fritzsch et al., 1997; Malgrange et al., 1996; Pirvola et al., 1992). Addition of BDNF to the cochlear fluids can prevent the degeneration of spiral ganglion neurons after hair cells are lost in mature ears (Miller et al., 1997; Nakaizumi et al., 2004; Staecker et al., 1998). Various techniques of introducing BDNF into the cochlea have been used, including direct infusion into the scala tympani (Miller et al., 1997) and gene transfer using recombinant viral vectors (Nakaizumi et al., 2004; Staecker et al., 1998). Direct cochlear infusion of BDNF and other neurotrophic factors using mini-osmotic pumps allows regulation of the duration and quantity of infusion, but their use increases surgical complexity. Viral vectors may be advantageous for long term production of BDNF in the cochlea, but their use involves risk of immune response and toxicity, especially when used for the second time (Ishimoto et al., 2003), limiting the potential for safe clinical application of this technology in humans. Alternative approaches for delivering growth factors in combination with the implanted electrode need to be developed and tested.
Here we report experiments to test a modified electrode capable of secreting BDNF using the ex vivo gene transfer paradigm. The electrode is coated with fibroblasts transduced with the BDNF transgene, thereby acting as a self-contained BDNF “factory”. Implantation of this device does not involve transduction of endogenous cochlear cells with viral vectors. We determined that the modified electrode enhanced spiral ganglion protection in deafened animals.
Methods
Electrode Manufacture
Platinum/Iridium alloy (Pt–Ir 90%/10%) wire (0.0055″) coated with Teflon was used to make the electrode (A-M System Inc, Carlsborg, WA). The end intended for implantation was melted into a ball (300–400um) using a propane/oxygen torch (Figure 1a). Silicone (Dow Corning Corporation, Midland, MI) was used to coat the bare part of the electrode in an hour glass configuration (Figure 1b) to help position and stabilize the agarose/fibroblast matrix near the electrode ball. Care was taken to insure the ball was left uncoated. The total length of the electrode was 4 cm.
Figure 1.
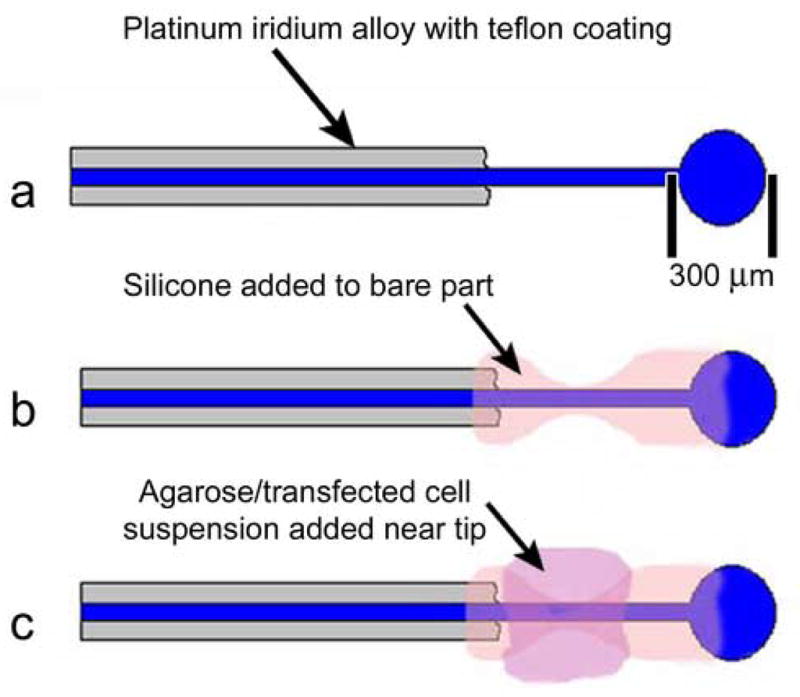
A schematic drawing showing the design and construction stages of the cochlear implant electrode: (a) Platinum iridium wire end formed in to ball (arrow points to Teflon coating of platinum iridium wire). (b) The bare end of wire coated in silicone in hour glass configuration (arrow points to silicone coating). (c) Agarose/cell matrix in place in “waist” of silicone (arrow points to agarose/cell matrix).
Coating electrodes with agarose and cells
Electrodes were coated using allogeneic fibroblasts. Guinea pig footpad fibroblast cell culture were established (see below) and expanded to eight confluent flasks (passaged 5 times). The fibroblasts were transduced with a viral vector 24 hrs prior to being seeded on the electrode.
On the day of electrode coating, a 6.25% low melt agarose solution (Boehringer-Mannheim, Mannheim, Germany) in MEM (GIBCO) was prepared. The solution was heated to 80°C and 200μl were transferred to microcentrifuge tubes, taking care to remove air bubbles. The tubes were then autoclaved. Only one tube is required for the coating. Occasionally however the agarose will “boil” due to expansion of air bubbles and therefore 2 or 3 spare tubes were prepared. Because the agarose must be kept in liquid state, the microcentrifuge tubes were transferred to a hotplate at 40°C immediately after being removed from the autoclave, ensuring that they were not allowed to cool down to room temperature.
The fibroblast culture flasks were passaged with the exception that all the fibroblasts from eight confluent flasks were resuspended in 10ml MEM. The fibroblasts were centrifuged (5min, 1000RPM, 400g) and resuspended in 50μl of MEM. This suspension was transferred to a microcentrifuge tube on the hotplate at 40°C. Once the fibroblasts warmed to 40°C, they were added to the 200μl low melt agarose and mixed.
The ball end of the previously prepared and autoclaved electrodes was dipped into the agarose/cell mixture with slow up and down movement. The ball end was held within the sterile laminar flow hood for a few seconds to allow the agarose to cool and solidify. With practice, a small quantity of agarose will fill the area in the “waist” part of the hour glass zone of silicone (Figure 1c and 2a). If the amount of agarose was too small, the electrode could be re-dipped into the agarose. It was not possible to remove excess agarose. The coated electrodes were transferred to a large Petri-dish with 20 ml of fresh culture media. The coated end was fully immersed in the media.
Figure 2.
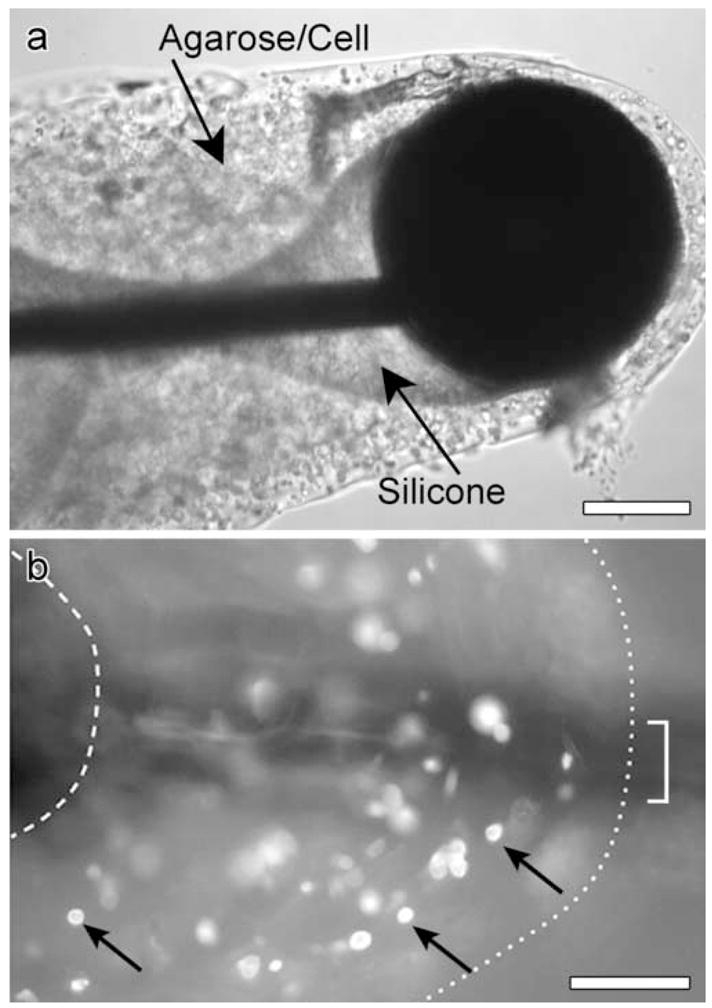
a. A light micrograph of an electrode coated with cell/agarose matrix. b. An epi-fluorescence micrograph showing reporter gene expression in cells (arrows) within agarose matrix retrieved from coated electrode after implantation for 7 days. The bracket shows the thickness of the electrode, the right dotted line shows that border of the agarose and the left dotted line shows the proximal border of the ball. Scale bars = 100 μm (in a and b).
Primary cell culture
In order to coat the electrode with fibroblasts, a primary cell culture was established. All cell culture handling was carried out under sterile conditions within a laminar flow hood. A donor guinea pig was deeply anaesthetized (xylazine 10 mg/kg, i.m., ketamine 40 mg/kg, i.m.). The footpad of the posterior left limb was prepped with 10% povidine-iodine (Triad Disposables, Brookfield, WI) and 70% isopropyl alcohol (Humco, Texarkana, TX) solution. A 4 mm by 3 mm ellipse of skin (epidermis and dermis) was removed. The wound was closed with 4/0 Ethilon (Ethicon, Somerville, New Jersey) and the foot bandaged. The ellipse of skin was cleaned three times by immersion in Earl’s Balanced Salt Solution (GIBCO-BRL, Rockville, MD) with 1% penicillin/streptomycin (10,000 units penicillin G sodium and 10,000 microgram/ml streptomycin sulphate, GIBCO) and 0.5 μg Amophotericin B (GIBCO) for 5 min. The skin was cut into six pieces and placed dermis down in a 25 cm2 culture flask (Corning Incorporate, Corning, NJ). Two ml of Dulbecco modified Eagle medium (DMEM) (GIBCO) containing 10% fetal calf serum (Summit, Fort Collins, CO) and 1% penicillin/streptomycin was added. The flask was placed in a 37° C incubator with 5% CO2. After 10 days the fibroblasts had migrated onto the flask bottom and the cells were passaged as follows. Cells were resuspended with 0.05% Trypsin, spun at 1000RPM for 5 min (800g) and the pellet resuspended in 4 ml of medium and replated in four 25 cm2 flasks. Once cells were confluent (3–7 days), further passaging was performed. Cells from the primary cultures were later used for preparation of the cell/agarose matrix, coated on to the cochlear implant electrode and implanted into guinea pig ears.
Adenoviral vectors and cell transduction
We used recombinant adenoviruses (serotype 5 human adenovirus) with E1A, E1B and E3 regions deleted. Gene inserts were driven by the CMV promoter. The BDNF vector (Ad.BDNF) was a gift from Dr. Adriana Di Polo (Montreal, Canada). The vector was amplified by the University of Michigan Viral Vector Core. Ad.empty and Ad.LacZ were purchased from the Vector Core. All vectors were at a titer of 1×1012 plaque forming units (PFU) per ml. Viral stocks were aliquoted and stored in 10% glycerol at −80°C until use. After thawing, 25 μl of the appropriate viral suspension was added to each confluent 25 cm2 flask (0.1–2 × 1010 cells). The flask was returned to the incubator. After 1 hr the supernatant was discarded and the flask rinsed twice with media. The flask was returned to the incubator and the transduced fibroblasts were used to coat electrodes after 24 hrs.
Testing Ad.BDNF activity in vitro
To assay for Ad.BDNF activity, we transduced guinea pig firbroblasts as described above. We exposed the cells to the viral vector by adding 25 μl of Ad.BDNF to 4 ml of DMEM medium for 1 hr. Four days later, the medium was sampled and prepared for testing with a BDNF ELISA kit to determine the amount of BDNF produced by the fibroblast cells. Medium from non-transfected cells served as control. BDNF levels in the medium were determined using ChemiKine™ BDNF Sandwich ELISA kit, following kit instructions. The experiment was done twice.
Hair cell elimination and spiral ganglion counts
On day 0 the animals were deafened with kanamycin (420 mg/kg s.c.) and ethacrynic acid (52.5mg/kg i.v.). After confirming successful deafening (n=14) with acoustically-evoked auditory brainstem response audiometry (a minimum threshold shift of 60 dB) the animals were divided into two groups. On day 7, 8 animals were implanted (left ear) with electrodes coated with agarose and fibroblasts transduced with Ad.BDNF (designated BDNF group) and 6 animals were implanted (left ear) with an electrode coated with agarose and fibroblasts transduced with Ad.empty (control group).
Implantation
Outbred pigmented guinea pigs (Elm Hill Laboratories, Chelmsford, MA) were used in this experiment and were 300–400g at the onset of the experiments. The University Committee for the Use and Care of Animals approved the animal experiments. The University of Michigan is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
The guinea pigs were deeply anaesthetized (xylazine 10 mg/kg, i.m., ketamine 40 mg/kg, i.m.) and given chloramphenicol succinate (30mg/kg, i.m.) as a prophylactic antibiotic. The postauricular tissues were infiltrated with 250 μl of 1% lidnocaine and 1/80000 epinephrine. The bulla was exposed by means of a postauricular approach and opened to reveal the middle ear cavity. An electric drill with 1.5 mm drill bit was used to create a cochleostomy by enlarging the round window inferiorly. This allowed a straighter insertion angle compared to that via the unmodified round window, and longer insertion length than a separate cochleostomy. The coated electrode was inserted and small pieces of muscle used to seal the cochleostomy. The electrode was secured to the bulla with carboxylate cement (Durelon ESPE America, Norristown, PA). The subcutaneous tissues and skin were closed in two layers with 3/0 Vicryl and 4/0 Ethilon. Ten milliliters of warmed saline were administered subcutaneously and the animal recovered.
Retrieval of the electrodes and inner ear tissues
Guinea pigs were deeply anaesthetized (xylazine 10 mg/kg, i.m., ketamine 40 mg/kg, i.m.) and decapitated. The temporal bones were removed, taking care not to open the bulla at this stage. The anterior bulla was then opened and the electrode traversing the middle ear cut with a microscissor. The rest of the bulla was opened, the basal turn of the cochlea revealed and the electrode removed carefully (ensuring the agarose coating remains on the electrode), and placed in 2% paraformaldehyde fixative.
Histology, spiral ganglion counts and statistics
On day 48, animals were euthanized, the inner ears harvested and prepared for spiral ganglion counts. Briefly, tissues were decalcified in 2% EDTA and 0.25% glutaraldehyde for three weeks, and the electrode was removed by pulling from the round window, leaving the agarose coating within the basal turn of the cochlea. Ears were embedded in JB4 (Electron Microscopy Scientific, Washington, PA) and sectioned at 3 μm with glass knives at the near mid-modiolar plane. Section spacing, selection and counting methods were performed by a blinded assessor as previously described (Kanzaki et al., 2002). The area of Rosenthal’s canal was measured using Metamorph (Universal Imaging Corporation, Downingtown, PA). The neuron density (number of cells/10000μm2) for each cochlea’s lower six Rosenthal’s canal sections was determined using Metamorph image analysis software.
For statistical analysis, the spiral ganglion density for all six sections was summed for each animal and the difference in the total was compared between the BDNF group and control group using the univariate F-test. The spatial distribution of cells was tested by computing the mean values for each half turn and evaluating the rank order correlation (Spearman’s rho, SPSS v. 13); linear regression was not used because turn position is not a linear function of distance. Differences between means for each half turn were also evaluated by t-test, and the sequential Bonferroni criterion for multiple comparisons was applied.
Reporter gene expression and cell survival
To test in vivo the survival of transduced fibroblasts on the implanted electrodes, we implanted four guinea pigs with electrodes coated with agarose and fibroblasts transduced with Ad.LacZ. Animals were euthanized on day 7 (N=1), 19 (N=1), or 28 (N=2) days after implantation, and the electrodes were retrieved and assessed for reporter gene expression in cells embedded in the agarose. Coated electrodes were fixed for 2 hrs, rinsed in PBS, incubated with Triton X-100 for 10 min, then incubated with a rabbit polyclonal antibody against β-galactosidase (Chemicon, Temecula, CA) diluted 1:1000 in PBS, for one hour. Following three rinses with PBS the electrodes were incubated with rhodamine-conjugated goat anti-rabbit secondary antibody (Jackson Immunoresearch Laboratories, West Grove, PA) diluted 1:100 in PBS, for 30 min. After a final rinse in PBS the electrodes were viewed with a Leica DMRB epifluorescence microscope.
In order to assess cell survival the agarose was carefully removed from the electrode after fixation, rinsed twice in PBS and placed in 30% sucrose solution for 12 hrs. The agarose was transferred to a specimen holder. To aid identification of specimen during cryosectioning the agarose bead was immersed in 2% alcian blue (Sigma-Aldrich, St. Louis, MO) for 1 min, placed in OCT (VWR International, West Chester, PA) and frozen in a mixture of dry ice and 100% ethanol. The OCT was sectioned in a Leica CM3000 cryostat. Ten μm sections were air dried and stained in hematoxylin and eosin (Sigma-Aldrich). Fibroblast survival was quantified by counting cells with nuclei as a percentage of all cells.
Results
Ad.BDNF activity
In vitro assays for BDNF production were performed twice. In the first experiment, the measured BDNF concentration in control medium was 60 pg/ml, and the two experimental samples contained 239 and 230 pg/ml. In the second experiment the control contained 104 pg/ml and the two experimental samples measured 755 and 688 pg/ml. These data represent approximately 4 and 7-fold increases in BDNF after fibroblasts are transduced with Ad.BDNF, and demonstrate the ability of these cells to express the transgene and secrete BDNF.
Reporter gene expression of coated electrode and cell survival in coating
In some cases, electrodes retrieved after 7 days in vivo showed sites that appeared to exhibit cell migration (Figure 2a, under the ball). It is not clear whether this was a defect caused by the extraction process or an area where fibroblasts were migrating from the implant to the surrounding tissue. Reporter gene expression was evident on electrodes retrieved after 7 days in vivo (Figure 2b). No reporter gene expression was evident on electrodes removed from the guinea pig ears after 28 days (data not shown). Cryosections performed on the agarose removed from the day 19 electrode revealed that approximately 50% of the fibroblasts had nuclei and appeared viable (Figure 3a) and on day 28 less than 25% of fibroblasts were viable (not shown). These data suggest that under these conditions, the life span of the fibroblasts is limited to several weeks.
Figure 3.
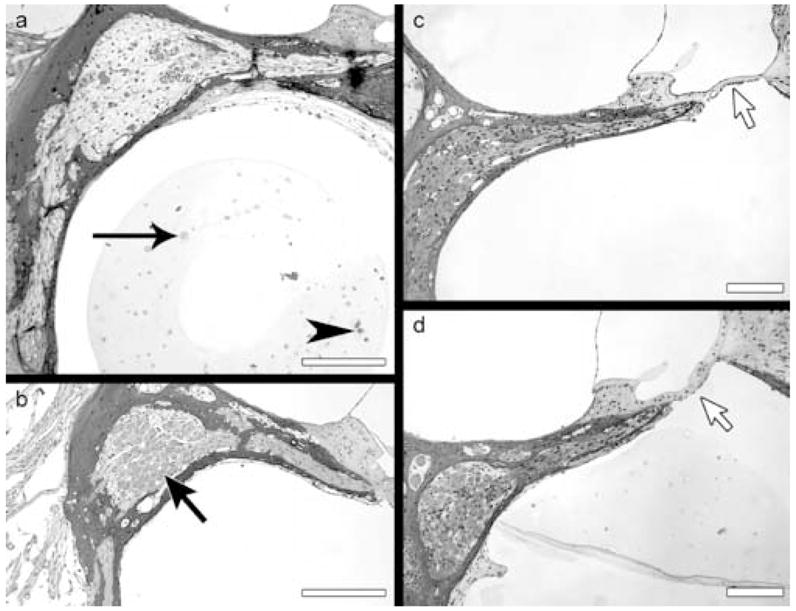
Light microscope images of toluidine blue-stained plastic sections through Rosenthal’s canal in the basal turn of the four cochleae: Ad.empty group ear is shown in (a) and Ad.BDNF group ears in (b–d). a. The agarose/cell matrix contains viable cells (arrow head) and non-viable cells appearing as ghosts (arrow). Rosenthal’s canal is nearly devoid of neurons. b. Rosenthal’s canal is evenly populated by spiral ganglion neurons (arrow). The edge contour of the neurons appears smooth. c–d. No major loss of neurons is seen in the canal, while the organ of Corti is completely devoid of hair cells (arrow). Differentiated supporting cells are absent, replaced by a simple epithelium resting on the basilar membrane. The agarose was extracted in the ears shown in b and c. Scale bars = 100 μm.
Histology and spiral ganglion density
Cochlear fluids appeared clear upon sacrificing the animals. No signs of inflammatory reaction have been noted in any of the animals. The fluid surrounding the agarose was clear and contained no cells or other evidence for inflammation (Figure 3). The mesothelial cells that line the scala tympani also appeared normal.
The population of spiral ganglion neurons in Ad.empty ears (Figure 3a) appeared to have a low density. Cells appeared denser and healthier in the BDNF group ears (Figure 3b–d). The neurons were distributed evenly throughout Rosenthal’s canal and their edges were smooth (Figure 3b) as seen in healthy spiral ganglion neurons (data not shown). Slightly lower magnification images of two other Ad.BDNF ears show the dense population of spiral ganglion neurons and the severe lesion in the organ of Corti associated with long term degeneration following the kanamycin and ethacrynic acid insult (Figure 3c–d). The data demonstrate complete loss of hair cells in all deafened ears, severe degeneration of neurons in deafened ears receiving Ad.empty and substantial survival of neurons in ears receiving the BDNF secreting implant.
Of 14 guinea pigs that had successful deafening, 8 were implanted with electrodes coated with agarose containing Ad.BDNF-transduced cells, and 6 with Ad.empty. The density of spiral ganglion neurons in each half turn of Rosenthal’s canal in the Ad.BDNF group was compared with the Ad.empty (control) group. The total number of cells in animals receiving BDNF-producing implants was significantly greater than in animals receiving control implants (p<0.001). The spatial distribution of surviving cells also differed between BDNF-treated and control groups (Figure 4). The mean number of surviving neurons was significantly and negatively correlated with distance from the base in treated animals (p < 0.001) but not in control animals (p = .072). This difference between spatial patterns is reflected in separate analyses of cell counts for each half turn, which clearly find significant differences in cell number between groups in lower sections 1 and 3 (p values = 0.007 and 0.008, respectively), but not in the upper sections (4–6) (p values = 0.221, 0.580 and 0.440). The difference between groups in section 2 also is not significant (p = 0.034, greater than the sequential Bonferroni criterion of p > 0.017 for 3 comparisons); however, the smaller difference between groups in this section reflects the higher variation in the control group, not a deviation from the general trend observed in the treated group.
Figure 4.
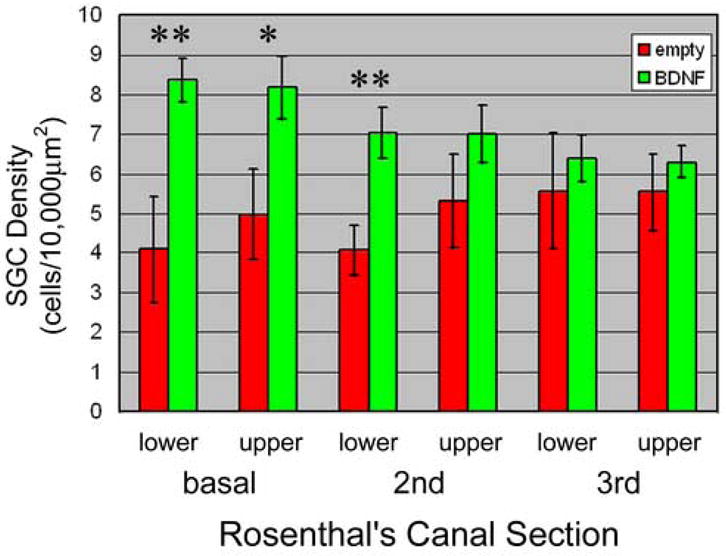
Means and standard errors (estimated confidence intervals) for numbers of spiral ganglion cells in each of 6 areas of Rosenthal’s canal (lower and upper cross sections of base, 2nd and 3rd turns) of animals receiving Ad.BDNF treated implants (n=8) compared to animal receiving untreated (Ad.empty) implants (n=6). Probability that the difference between BDNF and empty group is greater than zero: * = p<0.05, ** = p<0.01.
Discussion
In most cochlear implantation patients, hearing and speech performance improves but in some patients the results can be disappointing. One possible factor influencing the success of the cochlear implant procedure is the condition of the auditory nerve. Both qualitative and quantitative parameters in spiral ganglion survival are likely to influence the function of the electrode (Nadol et al., 1989). Spiral ganglion cell survival has been shown to be influenced by treatment with several growth factors. Specifically, neurotophic factors such as GDNF, NT-3 and BDNF can protect the eighth nerve from degeneration (Bowers et al., 2002; Lalwani et al., 2002; Miller et al., 1997; Staecker et al., 1998; Yagi et al., 2000; Ylikoski et al., 1998). Attempts to combine cochlear implantation with over-expression of a neurotrophic factor were performed using viral vectors to over-express GDNF (Kanzaki et al., 2002) or passive release from NT-3 from a polypyrrole polymer (Richardson et al., 2007). The potential advantage of the viral vector is long term production and secretion, but the risk is immune response and other side effects. The advantage of release from a pre-soaked polymer is lack of side effects and toxicity, but the disadvantage is short term release (hours or days). The approach presented here combined the electrode with ex vivo gene transfer methods and has the potential to accomplish long term growth factors secretion with minimal side effects. To extend the duration of cell survival beyond the number of weeks described here, it may be necessary to design alternative matrix in which cells can survive longer than they do in the agarose. It is also necessary to verify that transplanted cells do not proliferate, invade and obliterate the cochlea. Use of irradiated cells that are replication incompetent is one possibility for ascertaining quiescence of the coating cells. Finally, the ability of the electrode to deliver appropriate electrical stimulation should also be studied in depth. We have only performed a very preliminary test of the ability of the coated electrode to deliver electrical stimulation. One animal underwent eABR measurement 3 days after implantation and a recognizable waveform was evident at 90μA (data not shown), suggesting that coated electrodes can deliver electrical stimulation.
The data demonstrate that eighth nerve degeneration in severely deafened guinea pigs was reduced using a modified cochlear electrode coated with allogeneic fibroblasts expressing and secreting BDNF. The complete absence of inflammatory response in the ears treated with the transduced fibroblasts demonstrated the ability of the viral vector to be “hidden” from the immune system while they act as a self-enclosed “BDNF factory”. Although the lack of immune response is a clear advantage of ex vivo gene transfer, other parameters need to be optimized. The expression of the BDNF transgene in this experiment is unregulated in both quantity and duration. For better results and enhanced clinical applicability, it will be necessary to use a regulated system for transgene expression.
We selected an agarose matrix for coating cochlear implant electrodes with allogeneic fibroblasts because agarose allows for a large number of cells to be coated onto the electrode. Previous work transplanting Islet of Langerhans in agarose beads in diabetic animals has demonstrated the ability of agarose to allow diffusion of insulin, reverse the diabetic state and prevent xenotransplant rejection (Iwata et al., 1989). These animals were implanted with agarose beads intra-peritoneally. There was minimal tissue reaction surrounding the xeno-transplanted beads. In our experiment there was no noticeable inflammatory reaction surrounding the agarose. We speculate that the agarose acted as a barrier and prevented allogeneic cells from coming into contact with the host immune cells. The agarose also reduces the exchange of nutrients and waste products from the cells and may influence free circulation of perilymph. These factors are likely to have contributed to the eventual death of the fibroblasts in the agarose in our experiment. This may be a problem since withdrawal of BDNF protection resulted in accelerated loss of spiral ganglion neurons (Gillespie et al., 2003). Thus, alternative substances may need to be developed for extending cell survival and growth factor production. However, it is possible that once electrical stimulation is delivered, the neurons will stabilize and the need for BDNF will be reduced.
The protective action of the BDNF-secreting electrode is significant in the basal turns and less robust in the lower frequency regions. This pattern of protection has been previously noted in viral mediated gene therapy via round window inoculations (Lalwani et al., 2002; Yagi et al., 2000). This is likely to reflect the pattern of diffusion of released neurotrophic factors in the scala tympani. The agarose-confined transduced fibroblasts used in our experiment are likely to produce BDNF predominantly available to cells in the region of the scala tympani where the implant is present. Although protection throughout the cochlea would be desirable, the basal turn in the human cochlea, where speech frequencies are transduced, is the more important target for protection.
Trauma associated with the insertion of the electrode/agarose complex is likely to also influence the surrounding cochlear tissues, including the neurons and the epithelium. As such, the protection afforded by the Ad.BDNF group is best controlled for by the Ad.empty group, as done in our results (Figure 4). It is noted that supporting cells did not survive in a differentiated state in both Ad.BDNF and Ad.empty groups. Considering that presence of supporting cells of the organ of Corti is associated with enhanced survival of the auditory nerve (Sugawara et al., 2005), the extent of neuronal survival seen here without remaining supporting cells is another indication for the effectiveness of the BDNF secreting implant in preserving the ganglion. Future development of this method for potential clinical applicability will need to consider addition of transgenes that may also help preserve supporting cells. The choice of materials and insertion technique that will minimize tissue damage due to the surgical placement of the prosthesis should also be considered.
The extent of spiral ganglion neuron protection with our BDNF electrode is comparable to previous work using viral vectors and neurotrophic factor transgenes including GDNF and BDNF (Kanzaki et al., 2002; Lalwani et al., 2002), even after differences in experimental design are taken into account. In all these experiments, spiral ganglion cell protection was significant but incomplete. The incomplete rescue of neurons can be explained in part by early degeneration of some nerve cells, before elevated levels of growth factors become available to them. This may be less of a problem in the human ear, where degeneration of dennervated spiral ganglion neurons is usually much slower than in the guinea pig. Enhancement of survival is shown to be a feasible goal with potential therapeutic benefit, and ex vivo gene transfer a viable approach to achieving that goal. Using this approach to provide a combination of several protective molecules may yield even better results.
In conclusion, we have shown that cells seeded on a cochlear implant can survive for weeks in the cochlea in vivo and that ex vivo gene transfer of BDNF via cells in an agarose matrix placed on the cochlear implant can reduce eighth nerve degeneration in deaf guinea pigs. The presence of the transduced cells did not elicit an immune response. Once improved and optimized, the concept of combining the cochlear implant electrode with an ex vivo gene delivery system may be used to enhance spiral ganglion survival in ears that receive a cochlear implant.
Acknowledgments
We thank Diane Prieskorn for technical assistance. DR is a TWJ Foundation Scholar. Supported by the R. Jamison and Betty Williams Professorship, gifts from Berte and Alan Hirschfield and the CHD, a grant from the Royal National Institute for Deaf People (RNID) and NIH NIDCD grants DC05401, DC007634, 8-P41-EB-2030 and P30-DC05188.
List of abbreviations
- hr
hour
- BDNF
brain derived neurotrophic factor
- Ad
adenovirus
- min
minutes
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blamey PJ, Pyman BC, Gordon M, Clark GM, Brown AM, Dowell RC, Hollow RD. Factors predicting postoperative sentence scores in postlinguistically deaf adult cochlear implant patients. Ann Otol Rhinol Laryngol. 1992;101:342–348. doi: 10.1177/000348949210100410. [DOI] [PubMed] [Google Scholar]
- Bowers WJ, Chen X, Guo H, Frisina DR, Federoff HJ, Frisina RD. Neurotrophin-3 transduction attenuates Cisplatin spiral ganglion neuron ototoxicity in the cochlea. Mol Ther. 2002;6:12–18. doi: 10.1006/mthe.2002.0627. [DOI] [PubMed] [Google Scholar]
- Clopton BM, Spelman FA, Miller JM. Estimates of essential neural elements for stimulation through a cochlear prosthesis. Ann Otol Rhinol Laryngol Suppl. 1980;89:5–7. doi: 10.1177/00034894800890s202. [DOI] [PubMed] [Google Scholar]
- Cohen NL. Cochlear implant soft surgery: fact or fantasy? Otolaryngol Head Neck Surg. 1997;117:214–216. doi: 10.1016/s0194-5998(97)70176-1. [DOI] [PubMed] [Google Scholar]
- Cohen NL, Waltzman SB, Fisher SG. A prospective, randomized study of cochlear implants. The Department of Veterans Affairs Cochlear Implant Study Group. N Engl J Med. 1993;328:233–237. doi: 10.1056/NEJM199301283280403. [DOI] [PubMed] [Google Scholar]
- El-Hakim H, Abdolell M, Mount RJ, Papsin BC, Harrison RV. Influence of age at implantation and of residual hearing on speech outcome measures after cochlear implantation: binary partitioning analysis. Ann Otol Rhinol Laryngol Suppl. 2002;189:102–108. doi: 10.1177/00034894021110s521. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Van De Water T, Loring J, Jaenisch R. Complementary roles of BDNF and NT-3 in vestibular and auditory development [published erratum appears in Neuron 1995 Sep;15(3):739] Neuron. 1995;14:1153–1164. doi: 10.1016/0896-6273(95)90263-5. [DOI] [PubMed] [Google Scholar]
- Fayad J, Linthicum FH, Jr, Otto SR, Galey FR, House WF. Cochlear implants: histopathologic findings related to performance in 16 human temporal bones. Ann Otol Rhinol Laryngol. 1991;100:807–811. doi: 10.1177/000348949110001004. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Silos-Santiago I, Bianchi LM, Farinas I. The role of neurotrophic factors in regulating the development of inner ear innervation. Trends Neurosci. 1997;20:159–164. doi: 10.1016/s0166-2236(96)01007-7. [DOI] [PubMed] [Google Scholar]
- Gantz BJ, Woodworth GG, Knutson JF, Abbas PJ, Tyler RS. Multivariate predictors of audiological success with multichannel cochlear implants. Ann Otol Rhinol Laryngol. 1993;102:909–916. doi: 10.1177/000348949310201201. [DOI] [PubMed] [Google Scholar]
- Geers A, Brenner C, Nicholas J, Uchanski R, Tye-Murray N, Tobey E. Rehabilitation factors contributing to implant benefit in children. Ann Otol Rhinol Laryngol Suppl. 2002;189:127–130. doi: 10.1177/00034894021110s525. [DOI] [PubMed] [Google Scholar]
- Gillespie LN, Clark GM, Bartlett PF, Marzella PL. BDNF-induced survival of auditory neurons in vivo: Cessation of treatment leads to accelerated loss of survival effects. J Neurosci Res. 2003;71:785–790. doi: 10.1002/jnr.10542. [DOI] [PubMed] [Google Scholar]
- Ishimoto S, Kawamoto K, Stover T, Kanzaki S, Yamasoba T, Raphael Y. A glucocorticoid reduces adverse effects of adenovirus vectors in the cochlea. Audiol Neurootol. 2003;8:70–79. doi: 10.1159/000069000. [DOI] [PubMed] [Google Scholar]
- Iwata H, Amemiya H, Matsuda T, Takano H, Hayashi R, Akutsu T. Evaluation of microencapsulated islets in agarose gel as bioartificial pancreas by studies of hormone secretion in culture and by xenotransplantation. Diabetes. 1989;38(Suppl 1):224–225. doi: 10.2337/diab.38.1.s224. [DOI] [PubMed] [Google Scholar]
- Kanzaki S, Stover T, Kawamoto K, Prieskorn DM, Altschuler RA, Miller JM, Raphael Y. Glial cell line-derived neurotrophic factor and chronic electrical stimulation prevent VIII cranial nerve degeneration following denervation. J Comp Neurol. 2002;454:350–360. doi: 10.1002/cne.10480. [DOI] [PubMed] [Google Scholar]
- Khan AM, Handzel O, Damian D, Eddington DK, Nadol JB., Jr Effect of cochlear implantation on residual spiral ganglion cell count as determined by comparison with the contralateral nonimplanted inner ear in humans. Ann Otol Rhinol Laryngol. 2005;114:381–385. doi: 10.1177/000348940511400508. [DOI] [PubMed] [Google Scholar]
- Lalwani AK, Han JJ, Castelein CM, Carvalho GJ, Mhatre AN. In vitro and in vivo assessment of the ability of adeno-associated virus-brain-derived neurotrophic factor to enhance spiral ganglion cell survival following ototoxic insult. Laryngoscope. 2002;112:1325–1334. doi: 10.1097/00005537-200208000-00001. [DOI] [PubMed] [Google Scholar]
- Leake-Jones PA, Rebscher SJ. Cochlear pathology with chronically implanted scala tympani electrodes. Ann N Y Acad Sci. 1983;405:203–223. doi: 10.1111/j.1749-6632.1983.tb31634.x. [DOI] [PubMed] [Google Scholar]
- Malgrange B, Lefebvre P, Van de Water TR, Staecker H, Moonen G. Effects of neurotrophins on early auditory neurones in cell culture. Neuroreport. 1996;7:913–917. doi: 10.1097/00001756-199603220-00016. [DOI] [PubMed] [Google Scholar]
- Miller JM, Chi DH, O’Keeffe LJ, Kruszka P, Raphael Y, Altschuler RA. Neurotrophins can enhance spiral ganglion cell survival after inner hair cell loss. Int J Dev Neurosci. 1997;15:631–643. doi: 10.1016/s0736-5748(96)00117-7. [DOI] [PubMed] [Google Scholar]
- Nadol JB, Jr, Young YS, Glynn RJ. Survival of spiral ganglion cells in profound sensorineural hearing loss: implications for cochlear implantation. Ann Otol Rhinol Laryngol. 1989;98:411–416. doi: 10.1177/000348948909800602. [DOI] [PubMed] [Google Scholar]
- Nadol JB, Jr, Shiao JY, Burgess BJ, Ketten DR, Eddington DK, Gantz BJ, Kos I, Montandon P, Coker NJ, Roland JT, Jr, Shallop JK. Histopathology of cochlear implants in humans. Ann Otol Rhinol Laryngol. 2001;110:883–891. doi: 10.1177/000348940111000914. [DOI] [PubMed] [Google Scholar]
- Nakaizumi T, Kawamoto K, Minoda R, Raphael Y. Adenovirus-mediated expression of brain-derived neurotrophic factor protects spiral ganglion neurons from ototoxic damage. Audiol Neurootol. 2004;9:135–143. doi: 10.1159/000077264. [DOI] [PubMed] [Google Scholar]
- Osberger MJ, Zimmerman-Phillips S, Koch DB. Cochlear implant candidacy and performance trends in children. Ann Otol Rhinol Laryngol Suppl. 2002;189:62–65. doi: 10.1177/00034894021110s513. [DOI] [PubMed] [Google Scholar]
- Pirvola U, Ylikoski J, Palgi J, Lehtonen E, Arumae U, Saarma M. Brain-derived neurotrophic factor and neurotrophin 3 mRNAs in the peripheral target fields of developing inner ear ganglia. Proc Natl Acad Sci U S A. 1992;89:9915–9919. doi: 10.1073/pnas.89.20.9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson RT, Thompson B, Moulton S, Newbold C, Lum MG, Cameron A, Wallace G, Kapsa R, Clark G, O’Leary S. The effect of polypyrrole with incorporated neurotrophin-3 on the promotion of neurite outgrowth from auditory neurons. Biomaterials. 2007;28:513–523. doi: 10.1016/j.biomaterials.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Staecker H, Gabaizadeh R, Federoff H, Van De Water TR. Brain-derived neurotrophic factor gene therapy prevents spiral ganglion degeneration after hair cell loss. Otolaryngol Head Neck Surg. 1998;119:7–13. doi: 10.1016/S0194-5998(98)70194-9. [DOI] [PubMed] [Google Scholar]
- Sugawara M, Corfas G, Liberman MC. Influence of supporting cells on neuronal degeneration after hair cell loss. J Assoc Res Otolaryngol. 2005;6:136–147. doi: 10.1007/s10162-004-5050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton D, Miller JM. Cochlear implant effects on the spiral ganglion. Ann Otol Rhinol Laryngol. 1983;92:53–58. doi: 10.1177/000348948309200113. [DOI] [PubMed] [Google Scholar]
- Yagi M, Kanzaki S, Kawamoto K, Shin B, Shah PP, Magal E, Sheng J, Raphael Y. Spiral ganglion neurons are protected from degeneration by GDNF gene therapy. J Assoc Res Otolaryngol. 2000;1:315–325. doi: 10.1007/s101620010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylikoski J, Pirvola U, Virkkala J, Suvanto P, Liang XQ, Magal E, Altschuler R, Miller JM, Saarma M. Guinea pig auditory neurons are protected by glial cell line-derived growth factor from degeneration after noise trauma. Hear Res. 1998;124:17–26. doi: 10.1016/s0378-5955(98)00095-1. [DOI] [PubMed] [Google Scholar]


