Abstract
Trauma to the central nervous system (CNS) triggers intraparenchymal inflammation and activation of systemic immunity with the capacity to exacerbate neuropathology and stimulate mechanisms of tissue repair. Despite our incomplete understanding of the mechanisms that control these divergent functions, immune-based therapies are becoming a therapeutic focus. This review will address the complexities and controversies of post-traumatic neuroinflammation, particularly in spinal cord. In addition, current therapies designed to target neuroinflammatory cascades will be discussed.
Keywords: macrophages, lymphocytes, neuroinflammation, spinal cord injury, traumatic brain injury, blood-brain barrier
Introduction
Central nervous system (CNS) trauma, either in the form of traumatic brain injury (TBI) or spinal cord injury (SCI), causes marked neuropathology and limited functional recovery. While mechanical trauma rapidly kills neurons and glia, an insidious and delayed secondary pathology follows. The latter may be amenable to therapy and is characterized by neuronal and glial apoptosis, increased blood-CNS barrier permeability and a complex and poorly understood neuroinflammatory response that can persist for months or years after the initial trauma (44;122;140).
The role of neuroinflammation is controversial. Both beneficial and detrimental effects have been ascribed to microglia/macrophages (CNS macrophages), lymphocytes, antibodies and cytokines. The goal of this review is to address the complexities and controversies of this response with an emphasis on SCI. In addition, we will discuss pre-clinical and clinical therapies that target neuroinflammation, addressing those that suppress or enhance the immune response.
Traumatically-injured brain and spinal cord elicit distinct neuroinflammatory reactions
Although inflammation is a ubiquitous consequence of CNS trauma, the temporal sequence, composition and magnitude of this response in brain are distinct from spinal cord. Schnell and colleagues proved this point by comparing the inflammatory responses elicited by identical injuries delivered to mouse brain and spinal cord (147). Following a parasagittal incision to the cortex or a similar incision to the dorsal spinal cord, marked differences in cellular inflammation were observed. In the brain, neutrophil infiltration was minimal and was restricted to the lesion site. In contrast, twice as many neutrophils infiltrated the spinal cord lesion within 24 hours with large numbers of cells infiltrating into the surrounding parenchyma. Similarly, activation and recruitment of CNS macrophages was attenuated and restricted in distribution after brain injury relative to SCI. Lymphocyte numbers also were 2–3 times greater in the spinal cord with increased infiltration into surrounding tissue.
Similar changes were noted when neuroinflammation was elicited by non-traumatic microinjection of IL-1β or TNFα (148). In response to these cytokines, the recruitment of neutrophils and CNS macrophages was always greater in spinal cord. Following IL-1β microinjection, lymphocytes infiltrated the spinal cord but never the brain. TNFα microinjections into brain elicited a response comprised only of CNS macrophages while identical injections into spinal cord elicited neutrophils and macrophages. Molecular and anatomical differences between brain and spinal cord may explain the regional differences in leukocyte recruitment (170;171). Microvascular injury and serum extravasation in the inflamed spinal cord is increased in magnitude and duration relative to the brain (54;147) and is more susceptible to the permeabilizing effects of cytokines (148). Unique patterns of chemokine expression may also explain differential leukocyte recruitment. Specifically, neutrophil-attracting chemokines (e.g., CINC) are up-regulated to a greater extent in the injured spinal cord than in the brain (27).
There is a tendency for researchers to categorically lump mechanisms of brain and spinal cord neuroinflammation together; however, it is becoming clear that the spinal cord should not be considered simply an extension of the brain. Given the pivotal role played by immune cells in orchestrating cellular and molecular cascades of tissue injury and repair, future studies should explore the extent to which brain and spinal cord inflammation differ and define the mechanisms responsible for these differences. By doing so, novel site-specific therapies should be possible.
Species and strain-dependent differences in the neuroinflammatory response to spinal cord injury
Neuroinflammatory responses to SCI vary between species and strains of a given species. These differences are unlikely to be due to variable degrees of primary trauma between small and large animals. Spinal contusion and compression injury cause acute central hemorrhagic necrosis in all mammals and are accompanied by prominent glial activation and leukocyte infiltration (see Fig. 1B) (44;63;136;159). However, the onset, duration and composition of infiltrating leukocytes is distinct between humans and rodents, between rodent species (rat vs. mouse) and between different rat and mouse strains (44;81;136;159).
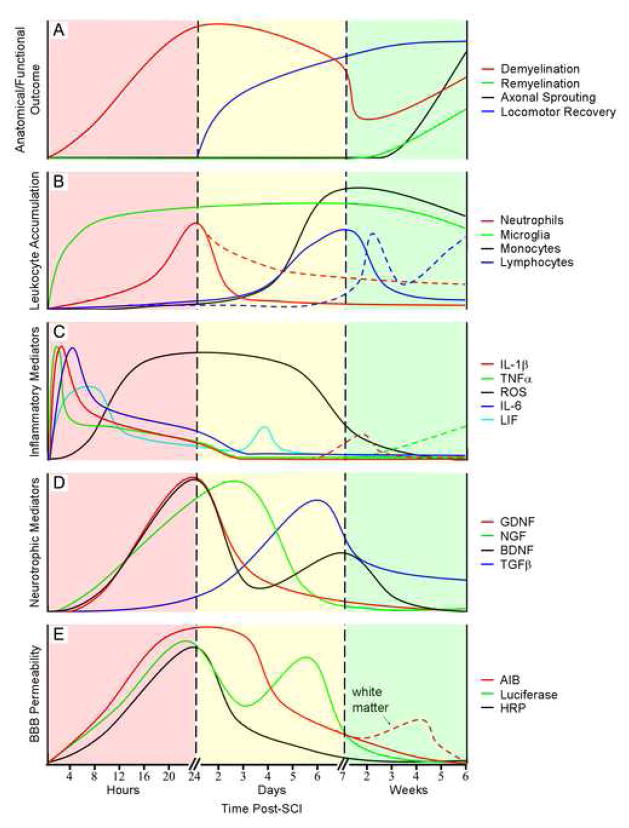
Figure 1.
Temporal correlation between inflammatory cascades, secondary neurodegenerative events and functional recovery in SCI rodents. A) Anatomical and functional outcomes, including de- and remyelination, axonal sprouting/plasticity and locomotor recovery. B) Activation of resident microglia and intraspinal accumulation of circulating leukocytes. Dashed lines departing from solid curves depict data from SCI mice whereas continuing solid curves indicate data from SCI rats. Solid curves before these break points are from both species. C) Expression of proinflammatory cytokines and reactive oxygen species (ROS). D) Expression of neurotrophic cytokines. E) Blood-brain barrier permeability to α-aminoisobutyric acid (AIB; 104 Da), horseradish peroxidase (HRP; 44000 Da), and luciferase (61000 Da). All AIB and HRP data were obtained from rat SCI models while luciferase data was from mice. Dashed curve departing from AIB solid curve indicates secondary rise in AIB permeability in white matter whereas continuing solid curve indicates permeability in gray matter. Solid curve before this break point represents permeability in both white and gray matter. Values on the vertical axis represent relative changes and are not to scale. Curves were generated using data from the following references: A) (11;67;169); B) (81;132;136;159;188): C) (129;163;180); D) (24;59;72;108); E) (120;133;179).
In all species, neutrophils accumulate within the lesion over the course of hours to days then, in most species, are rapidly cleared during the first week post-injury. In mice, elevated numbers of neutrophils persist in the lesion for months (81). In rats, lymphocytes infiltrate the lesion with monocytes 3–7 days post-injury. In contrast, lymphocyte entry is delayed in humans and mice with peak numbers evident after a delay of months post-injury (81;159). Unique to mice is the formation of a dense connective tissue matrix in the lesion in parallel with lymphocyte accumulation (44;81;136;159).
It is clear that genetics are an important determinant of post-traumatic neuroinflammation. After SCI, the MRL and 129X1/SvJ mouse strains mount a diminutive neuroinflammatory response that is associated with enhanced tissue repair and endogenous axonal plasticity (34;99). In contrast, dense fibrosis accompanies a robust inflammatory response in C57BL/6 mice but without significant axonal growth (81;99). A detailed comparative analysis in four common strains of mice after SCI failed to reveal a strict correlation between neuroinflammation, functional recovery and lesion pathology (81). Strain differences also extend to SCI rats, with increased numbers of leukocytes found in Lewis rats compared to Sprague-Dawley rats (136). The extended time course of macrophage accumulation in Lewis rats has also been described after optic nerve crush injury (154). These strain differences underscore the fact that within a genetically heterogeneous human population, attempts to manipulate inflammation to promote repair or minimize secondary injury will undoubtedly yield variable results. As diverse strains and species will continue to be used to extrapolate the human condition, future studies need to define how leukocyte populations vary between strains/species in the context of outcomes that are relevant to CNS repair. For example, do macrophages from C57BL/6 and MRL mouse strains release similar quantities of axon growth promoting proteins? Should the inflammatory contributions to remyelination be studied in BALB/c mice where post-injury inflammation is minimal relative to most other strains?
Changes in microvascular permeability after CNS injury: relationship to intraparenchymal inflammation
A prelude to the inflammatory response elicited by CNS trauma, and perhaps a consequence of this response at later times post-injury, is an increase in blood-brain barrier permeability (see Fig. 1E) (54;106;120;133;136;147;179). Using a rat model of spinal contusion injury, Noble and Wrathall initially described injury-induced changes in permeability to horseradish peroxidase (HRP) (120). They found that HRP extravasation correlated with injury severity; mild injury resulted in focal extravasation in spinal gray matter while severe injury involved gray and white matter. HRP extravasation was maximal within 1 day, with closure of the barrier by 14 days. Whetstone et al. described similar changes in acute permeability in SCI mice with a secondary rise in permeability 3–7 days post-injury (179). Interestingly, this secondary change parallels a time when blood monocytes infiltrate the injured spinal cord. A correlation between microglia activation and changes in microvascular permeability has been described in spinal cord white matter in SCI rats (133). While mechanical forces contribute to initial disruption of the blood-brain barrier, inflammatory mediators undoubtedly influence later changes in endothelial function, including maintenance of blood-to-tissue transfer.
The proinflammatory cytokines TNFα and IL-1β, which are up-regulated immediately after injury (see below and Fig. 1C), can enhance vascular permeability (148). A number of other vasoactive substances released by glia and leukocytes, including reactive oxygen species, kinins, histamines, nitric oxide and elastase, may also play a role (4;25;28;40;113;146;172). Furthermore, matrix metalloproteinase (MMP)-9, which is produced by neutrophils and endothelia, facilitates leukocyte diapedesis and may be a vascular permeabilizing factor (106). These data indicate that pathological alterations in blood-brain barrier function may be regulated by manipulating inflammatory cells and their release products. Alternatively, if properly controlled, these vasoactive properties of neuroinflammation could be harnessed to facilitate delivery of drugs to the chronically injured brain or spinal cord.
Immune-mediated injury in the traumatically injured CNS
Neutrophils and macrophages
Via the release of cytokines, free radicals, eicosanoids and proteases, activated neutrophils and macrophages can cause neuronal and glial toxicity (see Fig. 1A) (9;22;29;31;97;109;117;155). This toxic potential has been demonstrated repeatedly in various models of SCI. Protocols to deplete or neutralize neutrophils and macrophages or inhibit their functions, have provided consistent neuroprotection and improved neurological recovery (15;19;41;49;50;52;119;131;166).
Neurons and glia synthesize pro-inflammatory cytokines (e.g., TNFα and IL-1β) as part of normal intercellular communication (69;139). However, sustained elevations of TNFα and IL-1β evoke inflammation and dysregulate cytokine release causing neuron and oligodendrocyte death (see Fig. 1C) (26;66;91;98;155). Blocking TNFα or IL-1β confers neuroprotection in models of SCI, TBI and stroke (48;116;145;156). IL-6 and LIF also have been implicated in secondary neurodegeneration after CNS injury (see Fig. 1C) (77;89;100;124). Over-expression of IL-6 or LIF in spinal cord enhances leukocyte infiltration, decreases axonal growth and impairs locomotor recovery (89). Clearly, cytokines are important for maintaining homeostasis in the CNS but after injury they can become pathological.
Oxidative stress, caused by ischemia-reperfusion and inflammatory byproducts, contributes to cell death cascades after traumatic and ischemic CNS injury (5;57;94;157;180;183). Neutrophils, microglia and macrophages produce superoxide anion and nitric oxide which combine to form the highly reactive and toxic compound peroxynitrite (32;36;96;102;177;180). Free radicals produced during these processes induce apoptosis in neurons and glia via the irreversible oxidation of proteins, lipids and nucleic acids (42;92;95;109;180).
Glutamate is the chief excitatory neurotransmitter in the CNS; however, excess glutamate causes excitotoxicity in gray and white matter (33;93;107;123). Normally, glutamate is cleared from the extracellular space by astrocytes and to a limited degree by microglia (105;144;174). After injury, glutamate metabolism by astrocytes is impaired and clearance is inhibited further by TNFα and IL-1β (30;130;164), reactive oxygen species (128;175) and arachidonic acid (187). Activated microglia and macrophages are also likely to increase glutamate levels in the extracellular cleft (121;126;127). These latter changes in glutamate may be undetectable via conventional detection systems (e.g., microdialysis) but could still sensitize neurons to the effects of other substances in the microenvironment. Indeed, a feed-forward mechanism of glutamate excitotoxicity is feasible given that pro-inflammatory cytokines (e.g., TNFα) modulate the expression of synaptic AMPA and GABA receptors rendering neurons more susceptible to excitotoxicity (13;160).
Activated neutrophils and macrophages also produce neurotoxic enzymes. Phospholipase A2, a key enzyme in eicosanoid synthesis, is up-regulated in microglia, neurons and oligodendrocytes (97). Arachidonic acid and eicosanoids can be neurotoxic due to their ability to promote cyclooxygenase and free radical synthesis and by enhancing vascular permeability and leukocyte influx (3;38;76;88;103;173). Extracellular matrix-degrading enzymes (e.g., MMPs) produced by neutrophils, macrophages and endothelia also have been implicated in secondary injury (44;119;178;186).
Lymphocytes
Like microglia and macrophages, activated lymphocytes have conflicting effects on the injured CNS. Decades of experimental and clinical research in multiple sclerosis have shown the pathological potential of neuroantigen-reactive T and B lymphocytes, mostly those that recognize and mount reactions against myelin proteins (e.g., myelin basic protein; MBP) (47;55;65;68;161;176). These autoimmune responses amplify CNS macrophage effector functions resulting in blood-brain barrier injury and toxicity to oligodendrocytes and neurons (14;149;158). The result is widespread edema, axonal injury and loss of function (see Fig. 1A). The notion that traumatic or ischemic CNS injury can trigger pathological autoimmunity is a relatively new concept (1;135). Still, a growing body of evidence in animal models and human SCI has confirmed this potential (43;51;74;82). Using transgenic mice and rats vaccinated to expand MBP-reactive T cells, we have shown that autoimmune reactions exacerbate demyelination and axonal pathology, effectively increasing the size of the contusion lesion and causing loss of supraspinal neurons (73;74). This destructive potential is not restricted to MBP-reactive cells or SCI as T cells reactive with myelin oligodendrocyte glycoprotein (MOG) or ovalbumin (OVA; a non-CNS protein) exacerbate neuron loss in a model of peripheral nerve injury (2). The fact that OVA immunizations increased neuropathology indicates that T cells need not be myelin-reactive to contribute to secondary neurodegeneration. Indeed, mice and rats without T-lymphocytes (RAG knockout and athymic nude rats) have attenuated neuropathology after TBI and SCI (43;137). Also, antibody-mediated blockade of lymphocyte chemokines inhibits T cell infiltration and attenuates secondary injury after SCI (51).
Implicit to most T cell reactions is parallel activation of B cells and antibody secretion. Evidence that B cells are activated after SCI is implied from clinical data showing elevated levels of CNS autoantibodies in the serum of individuals with chronic SCI (64). We recently confirmed this potential in a mouse model of spinal contusion injury showing that SCI induces the production of autoantibodies directed against CNS proteins and systemic antigens including DNA (1). Interestingly, anti-DNA antibodies can cross-react with glutamate receptors (37). If these cross-reactive anti-DNA antibodies are pathological, then B cell-mediated pathology may transcend the spinal cord. Indeed, in systemic lupus erythematosus, anti-DNA antibodies cause cognitive deficits and widespread organ pathology. These latter parameters are not usually considered in paraplegic or quadriplegic individuals; however, SCI autoantibodies injected into intact hippocampi induced neuroinflammation and neuronal apoptosis (1).
Immune cell-mediated neuroprotection and regeneration
Neutrophils and macrophages
Given their primary function as bactericidal cells, it is doubtful that neutrophils exert neuroprotection in the CNS. This is not true for CNS macrophages. Despite being adept killers of neurons and glia, microglia may be intrinsically neuroprotective; they regularly survey the CNS and provide trophic support to neurons and glia (6;87;118). Indeed, it makes little sense to have evolved a homogeneously distributed network of cells capable of destroying the CNS from within. Instead, both microglia and macrophages derived from infiltrating monocytes produce neuroprotective cytokines and growth factors (see Fig. 1D). For example, TGFβ1 produced by macrophages after injury (90;108) has beneficial effects on neurons (79) and limits oligodendrocyte toxicity (110). Classical neurotrophic factors including CNTF, IGF, HGF, PDGF, NGF, BDNF, GDNF and NT-3 also are synthesized and released by activated CNS macrophages (39;60;78;83;114;115).
CNS macrophages may protect and repair the injured CNS by modulating glutamate excitotoxicity and by promoting the growth of injured axons (see Fig. 1A). Better known for their ability to release glutamate, microglia and macrophages increase transporters that are able to take up extracellular glutamate (144;174). Several lines of evidence suggest that CNS macrophages can promote axon growth and perhaps long-distance regeneration. Arguably the most convincing of the recent data illustrating this potential was described in a model of optic nerve injury. In that study, a novel protein called oncomodulin (OM), released by activated macrophages, was shown to be responsible for promoting regeneration of injured retinal ganglion cells (185). Interestingly, the same mode of macrophage activation that produces OM, causes the release of neurotoxic molecules (184). Thus, even though macrophages can promote axon regeneration, the potential for causing simultaneous injury exists. This will make it difficult to exploit CNS macrophage functions as a therapy in any form of CNS injury. Still, the rapid and enduring turnover of CNS macrophages from bone marrow makes it hard to ignore the possibility that these cells could be genetically-modified ex vivo and act as vehicles for drug delivery (17).
Lymphocytes
Although there is overwhelming evidence that lymphocytes can initiate and exacerbate injury to neurons and glia, recent data show that B and T cells may be an important and perhaps necessary component of CNS repair. Indeed, B and T cells can secrete a bioactive form of the neurotrophin BDNF (78). Moreover, Schwartz and colleagues have championed the idea of “protective autoimmunity” stating that autoreactive T cells, specifically those responding to myelin proteins, are an advantageous but inefficient response to CNS injury (151;152). As a result, they propose therapeutic vaccines to treat neurological disorders including SCI, TBI, glaucoma, and amyotrophic lateral sclerosis (150). Although this notion is in conflict with the prevailing dogma that autoreactive T cells are neurodestructive, the Schwartz laboratory has shown that passive or active MBP immunization limits secondary neurodegeneration in injured spinal cord and optic nerve (45;61;111). This neuroprotection is attributed to the expression of neurotrophins and antithrombin III by MBP-specific T cells (see Fig. 1D) (45). Because these protective effects are not evident in all rat or mouse strains, the application of therapeutic vaccines in humans will require a better understanding of how genetics influences autoimmunity (62;85). B cells also can exert beneficial effects in the traumatized CNS. In addition to providing neurotrophic factors, autoantibodies specific for myelin protein can promote axon regeneration and improve locomotor recovery after SCI (70).
Protective autoimmunity, as defined by Schwartz et al., requires proinflammatory myelin-reactive T cells (84). However, other investigators have suggested that neuroprotection is conferred by T cells that are not CNS-reactive after central and peripheral nerve injury (58;75;153). Importantly, these latter cells are activated along with T cells specific for MBP (112). Clearly, our understanding of lymphocyte functions in the injured nervous system is incomplete.
Immunomodulatory and cell-specific therapies for SCI
Methylprednisolone (MP), a potent immunosuppressive glucocorticoid, can successfully suppress various indices of neuroinflammation in experimental SCI models (10;46;181;182). Although MP is the current standard of care for human SCI, the effectiveness and safety of this drug have recently been questioned (35;71;141). Because immune responses in the CNS can have dual effects, global immune suppression is unlikely to yield long-term benefits. Instead, optimal treatments should be tailored to augment the beneficial functions of neuroinflammation while simultaneously minimizing those that cause injury. Currently, an immunomodulatory therapy of this type does not exist. However, a number of promising pre-clinical studies and clinical trials have been completed illustrating the therapeutic potential of cell-specific therapies after SCI.
Several groups have confirmed the therapeutic potential of activated microglia and monocyte-derived macrophages in the injured spinal cord (21;138;142;143). Two studies revealed that microglial transplants placed into lesioned spinal cord promoted neurite growth (138;142). Although functional recovery was not documented in these latter reports, partial recovery was provided by transplanting activated monocytes into the caudal stump of transected rat spinal cord (143). The success of these pre-clinical models prompted a Phase I clinical trial. This trial was completed without any adverse effects associated with macrophage transplantation (86). For more information about this trial and its implications, readers are directed to a recent review (80).
Other studies have illustrated the neuroprotective capacity of acute macrophage depletion. Indeed, studies in various species and models of SCI have independently verified that secondary loss of neurons (axons) and myelin is reduced after inhibition of monocyte, and in some cases, neutrophil, infiltration. This has been accomplished using macrophage-specific toxins (19;131), antibody-mediated blockade of integrins (7;8;52;101), chemokine antagonists (41) and pharmacological agents that inhibit microglia and/or monocyte migration and secretion (20;49). More importantly, these anatomical indices of recovery were paralleled by significant but variable improvements in motor, sensory and autonomic function.
Despite the pre-clinical success of therapeutic CNS vaccines, the safety of intentionally expanding autoreactive lymphocytes to repair the injured spinal cord remains questionable (134). Although, this type of therapy has been applied in humans with Alzheimer’s disease with some evidence of efficacy (12;53;104;168), Phase II trials were suspended due to the onset of autoimmune meningoencephalitis in a small cohort of patients (18;125).
In addition to cell-specific therapies, a number of pharmacotherapies that target the immune-CNS axis have been investigated. Systemic treatment with the anti-inflammatory cytokine IL-10 limits secondary neurodegeneration and improves locomotor recovery in some but not all SCI rodents (16;23;165). Similarly, the antibiotic minocycline, known for its ability to inhibit microglia and macrophages, has been shown to be neuroprotective and reduce neuropathic pain in rat and mouse models of SCI (56;162;167).
Conclusions
Despite extensive experimental data implicating inflammation as a pathogenic component of SCI, inflammation also appears to be pivotal for tissue repair. A challenge for researchers is to learn how to control cross-talk between the nervous and immune systems to minimize delayed neurodegeneration while promoting axonal plasticity and regeneration. Moreover, a greater appreciation for how SCI influences leukocyte development, activation and mobilization within and from peripheral lymphoid tissues is needed. Armed with this new knowledge, more effective and safer immune-based strategies will become available to treat spinal cord trauma and other CNS injuries.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.ANKENY DP, LUCIN KM, SANDERS VM, MCGAUGHY VM, POPOVICH PG. Spinal cord injury triggers systemic autoimmunity: evidence for chronic B lymphocyte activation and lupus-like autoantibody synthesis. J Neurochem. 2006;99:1073–1087. doi: 10.1111/j.1471-4159.2006.04147.x. [DOI] [PubMed] [Google Scholar]
- 2.ANKENY DP, POPOVICH PG. Central nervous system and non-central nervous system antigen vaccines exacerbate neuropathology caused by nerve injury. Eur J Neurosci. 2007;25:2053–2064. doi: 10.1111/j.1460-9568.2007.05458.x. [DOI] [PubMed] [Google Scholar]
- 3.ARAKI E, FORSTER C, DUBINSKY JM, ROSS ME, IADECOLA C. Cyclooxygenase-2 inhibitor ns-398 protects neuronal cultures from lipopolysaccharide-induced neurotoxicity. Stroke. 2001;32:2370–2375. doi: 10.1161/hs1001.096057. [DOI] [PubMed] [Google Scholar]
- 4.ARMAO D, KORNFELD M, ESTRADA EY, GROSSETETE M, ROSENBERG GA. Neutral proteases and disruption of the blood-brain barrier in rat. Brain Res. 1997;767:259–264. doi: 10.1016/s0006-8993(97)00567-2. [DOI] [PubMed] [Google Scholar]
- 5.AZBILL RD, MU X, BRUCE-KELLER AJ, MATTSON MP, SPRINGER JE. Impaired mitochondrial function, oxidative stress and altered antioxidant enzyme activities following traumatic spinal cord injury. Brain Res. 1997;765:283–290. doi: 10.1016/s0006-8993(97)00573-8. [DOI] [PubMed] [Google Scholar]
- 6.BANATI RB, GRAEBER MB. Surveillance, intervention and cytotoxicity: is there a protective role of microglia? Dev Neurosci. 1994;16:114–127. doi: 10.1159/000112098. [DOI] [PubMed] [Google Scholar]
- 7.BAO F, CHEN Y, DEKABAN GA, WEAVER LC. An anti-CD11d integrin antibody reduces cyclooxygenase-2 expression and protein and DNA oxidation after spinal cord injury in rats. J Neurochem. 2004;90:1194–1204. doi: 10.1111/j.1471-4159.2004.02580.x. [DOI] [PubMed] [Google Scholar]
- 8.BAO F, DEKABAN GA, WEAVER LC. Anti-CD11d antibody treatment reduces free radical formation and cell death in the injured spinal cord of rats. J Neurochem. 2005;94:1361–1373. doi: 10.1111/j.1471-4159.2005.03280.x. [DOI] [PubMed] [Google Scholar]
- 9.BAO F, LIU D. Peroxynitrite generated in the rat spinal cord induces neuron death and neurological deficits. Neuroscience. 2002;115:839–849. doi: 10.1016/s0306-4522(02)00506-7. [DOI] [PubMed] [Google Scholar]
- 10.BARTHOLDI D, SCHWAB ME. Methylprednisolone inhibits early inflammatory processes but not ischemic cell death after experimental spinal cord lesion in the rat. Brain Res. 1995;672:177–186. doi: 10.1016/0006-8993(94)01410-j. [DOI] [PubMed] [Google Scholar]
- 11.BASSO DM, BEATTIE MS, BRESNAHAN JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- 12.BAYER AJ, BULLOCK R, JONES RW, WILKINSON D, PATERSON KR, JENKINS L, MILLAIS SB, DONOGHUE S. Evaluation of the safety and immunogenicity of synthetic Abeta42 (AN1792) in patients with AD. Neurology. 2005;64:94–101. doi: 10.1212/01.WNL.0000148604.77591.67. [DOI] [PubMed] [Google Scholar]
- 13.BEATTIE EC, STELLWAGEN D, MORISHITA W, BRESNAHAN JC, HA BK, VON ZASTROW M, BEATTIE MS, MALENKA RC. Control of synaptic strength by glial TNFalpha. Science. 2002;295:2282–2285. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- 14.BENVENISTE EN. Role of macrophages/microglia in multiple sclerosis and experimental allergic encephalomyelitis. J Mol Med. 1997;75:165–173. doi: 10.1007/s001090050101. [DOI] [PubMed] [Google Scholar]
- 15.BERIL GH, SOLAROGLU I, OKUTAN O, CIMEN B, KAPTANOGLU E, PALAOGLU S. Metoprolol treatment decreases tissue myeloperoxidase activity after spinal cord injury in rats. J Clin Neurosci. 2007;14:138–142. doi: 10.1016/j.jocn.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 16.BETHEA JR, NAGASHIMA H, ACOSTA MC, BRICENO C, GOMEZ F, MARCILLO AE, LOOR K, GREEN J, DIETRICH WD. Systemically administered interleukin-10 reduces tumor necrosis factor-alpha production and significantly improves functional recovery following traumatic spinal cord injury in rats. J Neurotrauma. 1999;16:851–863. doi: 10.1089/neu.1999.16.851. [DOI] [PubMed] [Google Scholar]
- 17.BIFFI A, DE PALMA M, QUATTRINI A, DEL CARRO U, AMADIO S, VISIGALLI I, SESSA M, FASANO S, BRAMBILLA R, MARCHESINI S, BORDIGNON C, NALDINI L. Correction of metachromatic leukodystrophy in the mouse model by transplantation of genetically modified hematopoietic stem cells. J Clin Inves. 2004;113:1118–1129. doi: 10.1172/JCI19205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.BIRMINGHAM K, FRANTZ S. Set back to Alzheimer vaccine studies. Nat Med. 2002;8:199–200. doi: 10.1038/nm0302-199b. [DOI] [PubMed] [Google Scholar]
- 19.BLIGHT AR. Effects of silica on the outcome from experimental spinal cord injury: implication of macrophages in secondary tissue damage. euroscience. 1994;60:263–273. doi: 10.1016/0306-4522(94)90220-8. [DOI] [PubMed] [Google Scholar]
- 20.BLIGHT AR, COHEN TI, SAITO K, HEYES MP. Quinolinic acid accumulation and functional deficits following experimental spinal cord injury. Brain. 1995;118:735–752. doi: 10.1093/brain/118.3.735. [DOI] [PubMed] [Google Scholar]
- 21.BOMSTEIN Y, MARDER JB, VITNER K, SMIRNOV I, LISAEY G, BUTOVSKY O, FULGA V, YOLES E. Features of skin-coincubated macrophages that promote recovery from spinal cord injury. J Neuroimmunol. 2003;142:10–16. doi: 10.1016/s0165-5728(03)00260-1. [DOI] [PubMed] [Google Scholar]
- 22.BRADY KM, TEXEL SJ, KISHIMOTO K, KOEHLER RC, SAPIRSTEIN A. Cytosolic phospholipase A alpha modulates NMDA neurotoxicity in mouse hippocampal cultures. Eur J Neurosci. 2006;24:3381–3386. doi: 10.1111/j.1460-9568.2006.05237.x. [DOI] [PubMed] [Google Scholar]
- 23.BREWER KL, BETHEA JR, YEZIERSKI RP. Neuroprotective effects of interleukin-10 following excitotoxic spinal cord injury. Exp Neurol. 1999;159:484–493. doi: 10.1006/exnr.1999.7173. [DOI] [PubMed] [Google Scholar]
- 24.BROWN A, RICCI MJ, WEAVER LC. NGF message and protein distribution in the injured rat spinal cord. Exp Neurol. 2004;188:115–127. doi: 10.1016/j.expneurol.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 25.BUTT AM. Effect of inflammatory agents on electrical resistance across the blood-brain barrier in pial microvessels of anaesthetized rats. Brain Res. 1995;696:145–150. doi: 10.1016/0006-8993(95)00811-4. [DOI] [PubMed] [Google Scholar]
- 26.CAI Z, PANG Y, LIN S, RHODES PG. Differential roles of tumor necrosis factor-alpha and interleukin-1 beta in lipopolysaccharide-induced brain injury in the neonatal rat. Brain Res. 2003;975:37–47. doi: 10.1016/s0006-8993(03)02545-9. [DOI] [PubMed] [Google Scholar]
- 27.CAMPBELL SJ, WILCOCKSON DC, BUTCHART AG, PERRY VH, ANTHONY DC. Altered chemokine expression in the spinal cord and brain contributes to differential interleukin-1beta-induced neutrophil recruitment. J Neurochem. 2002;83:432–441. doi: 10.1046/j.1471-4159.2002.01166.x. [DOI] [PubMed] [Google Scholar]
- 28.CARLSON SL, PARRISH ME, SPRINGER JE, DOTY K, DOSSETT L. Acute inflammatory response in spinal cord following impact injury. Exp Neurol. 1998;151:77–88. doi: 10.1006/exnr.1998.6785. [DOI] [PubMed] [Google Scholar]
- 29.CHANDLER S, COATES R, GEARING A, LURY J, WELLS G, BONE E. Matrix metalloproteinases degrade myelin basic protein. Neurosci Lett. 1995;201:223–226. doi: 10.1016/0304-3940(95)12173-0. [DOI] [PubMed] [Google Scholar]
- 30.CHAO CC, HU S, EHRLICH L, PETERSON PK. Interleukin-1 and tumor necrosis factor-alpha synergistically mediate neurotoxicity: involvement of nitric oxide and of N-methyl-D-aspartate receptors. Brain Behav Immun. 1995;9:355–365. doi: 10.1006/brbi.1995.1033. [DOI] [PubMed] [Google Scholar]
- 31.CHAO CC, HU S, MOLITOR TW, SHASKAN EG, PETERSON PK. Activated microglia mediate neuronal cell injury via a nitric oxide mechanism. J Neuroimmunol. 1992;149:2736–2741. [PubMed] [Google Scholar]
- 32.CHATZIPANTELI K, GARCIA R, MARCILLO AE, LOOR KE, KRAYDIEH S, DIETRICH WD. Temporal and segmental distribution of constitutive and inducible nitric oxide synthases after traumatic spinal cord injury: effect of aminoguanidine treatment. J Neurotrauma. 2002;19:639–651. doi: 10.1089/089771502753754109. [DOI] [PubMed] [Google Scholar]
- 33.CHOI DW, KOH JY, PETERS S. Pharmacology of glutamate neurotoxicity in cortical cell culture: attenuation by NMDA antagonists. J Neurosci. 1988;8:185–196. doi: 10.1523/JNEUROSCI.08-01-00185.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.CLARK LD, CLARK RK, HEBER-KATZ E. A new murine model for mammalian wound repair and regeneration. Clin Immunol Immunopathol. 1998;88:35–45. doi: 10.1006/clin.1998.4519. [DOI] [PubMed] [Google Scholar]
- 35.COLEMAN WP, BENZEL D, CAHILL DW, DUCKER T, GEISLER F, GREEN B, GROPPER MR, GOFFIN J, MADSEN PW, III, MAIMAN DJ, ONDRA SL, ROSNER M, SASSO RC, TROST GR, ZEIDMAN S. A critical appraisal of the reporting of the National Acute Spinal Cord Injury Studies (II and III) of methylprednisolone in acute spinal cord injury. J Spinal Disord. 2000;13:185–199. doi: 10.1097/00002517-200006000-00001. [DOI] [PubMed] [Google Scholar]
- 36.COLTON CA, GILBERT DL. Production of superoxide anions by a CNS macrophage, the microglia. FEBS Letters. 1987;223(2):284–288. doi: 10.1016/0014-5793(87)80305-8. [DOI] [PubMed] [Google Scholar]
- 37.DEGIORGIO LA, KONSTANTINOV KN, LEE SC, HARDIN JA, VOLPE BT, DIAMOND B. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat Med. 2001;7:1189–1193. doi: 10.1038/nm1101-1189. [DOI] [PubMed] [Google Scholar]
- 38.EASTON AS, FRASER PA. Arachidonic acid increases cerebral microvascular permeability by free radicals in single pial microvessels of the anaesthetized rat. J Physiol. 1998;507(Pt 2):541–547. doi: 10.1111/j.1469-7793.1998.541bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.ELKABES S, DICICCO-BLOOM EM, BLACK IB. Brain microglia/macrophages express neurotrophins that selectively regulate microglial proliferation and function. J Neurosci. 1996;16:2508–2521. doi: 10.1523/JNEUROSCI.16-08-02508.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.ELLIS W. Pulsed subcutaneous electrical stimulation in spinal cord injury: preliminary results. Bioelectromagnetics. 1987;8:159–164. doi: 10.1002/bem.2250080206. [DOI] [PubMed] [Google Scholar]
- 41.ENG LF, LEE YL. Response of chemokine antagonists to inflammation in injured spinal cord. Neurochem Res. 2003;28:95–100. doi: 10.1023/a:1021652229667. [DOI] [PubMed] [Google Scholar]
- 42.EVANS TJ, COHEN J. Mediators: nitric oxide and other toxic oxygen species. Curr Top Microbiol Immunol. 1996;216:189–207. doi: 10.1007/978-3-642-80186-0_9. [DOI] [PubMed] [Google Scholar]
- 43.FEE D, CRUMBAUGH A, JACQUES T, HERDRICH B, SEWELL D, AUERBACH D, PIASKOWSKI S, HART MN, SANDOR M, FABRY Z. Activated/effector CD4+ T cells exacerbate acute damage in the central nervous system following traumatic injury. J Neuroimmunol. 2003;136:54–66. doi: 10.1016/s0165-5728(03)00008-0. [DOI] [PubMed] [Google Scholar]
- 44.FLEMING JC, NORENBERG MD, RAMSAY DA, DEKABAN GA, MARCILLO AE, SAENZ AD, PASQUALE-STYLES M, DIETRICH WD, WEAVER LC. The cellular inflammatory response in human spinal cords after injury. Brain. 2006;129:3249–3269. doi: 10.1093/brain/awl296. [DOI] [PubMed] [Google Scholar]
- 45.FRIEDMANN I, HAUBEN E, YOLES E, KARDASH L, SCHWARTZ M. T cell-mediated neuroprotection involves antithrombin activity. J Neuroimmunol. 2001;121:12–21. doi: 10.1016/s0165-5728(01)00397-6. [DOI] [PubMed] [Google Scholar]
- 46.FU ES, SAPORTA S. Methylprednisolone inhibits production of interleukin-1beta and interleukin-6 in the spinal cord following compression injury in rats. J Neurosurg Anesthesiol. 2005;17:82–85. doi: 10.1097/01.ana.0000163199.10365.38. [DOI] [PubMed] [Google Scholar]
- 47.GENAIN CP, CANNELLA B, HAUSER SL, RAINE CS. Identification of autoantibodies associated with myelin damage in multiple sclerosis. Nat Med. 1999;5:170–175. doi: 10.1038/5532. [DOI] [PubMed] [Google Scholar]
- 48.GENOVESE T, MAZZON E, CRISAFULLI C, DI PAOLA R, MUIA C, BRAMANTI P, CUZZOCREA S. Immunomodulatory effects of etanercept in an experimental model of spinal cord injury. J Pharmacol Exp Ther. 2006;316:1006–1016. doi: 10.1124/jpet.105.097188. [DOI] [PubMed] [Google Scholar]
- 49.GIULIAN D, ROBERTSON C. Inhibition of mononuclear phagocytes reduces ischemic injury in the spinal cord. Ann Neurol. 1990;27:33–42. doi: 10.1002/ana.410270107. [DOI] [PubMed] [Google Scholar]
- 50.GOK B, OKUTAN O, BESKONAKLI E, PALAOGLU S, ERDAMAR H, SARGON MF. Effect of immunomodulation with human interferon-beta on early functional recovery from experimental spinal cord injury. Spine. 2007;32:873–880. doi: 10.1097/01.brs.0000259841.40358.8f. [DOI] [PubMed] [Google Scholar]
- 51.GONZALEZ R, GLASER J, LIU MT, LANE TE, KEIRSTEAD HS. Reducing inflammation decreases secondary degeneration and functional deficit after spinal cord injury. Exp Neurol. 2003;184:456–463. doi: 10.1016/s0014-4886(03)00257-7. [DOI] [PubMed] [Google Scholar]
- 52.GRIS D, MARSH DR, OATWAY MA, CHEN Y, HAMILTON EF, DEKABAN GA, WEAVER LC. Transient blockade of the CD11d/CD18 integrin reduces secondary damage after spinal cord injury, improving sensory, autonomic, and motor function. J Neurosci. 2004;24:4043–4051. doi: 10.1523/JNEUROSCI.5343-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.HAASS C. New hope for Alzheimer disease vaccine. Nat Med. 2002;8:1195–1196. doi: 10.1038/nm1102-1195. [DOI] [PubMed] [Google Scholar]
- 54.HABGOOD MD, BYE N, DZIEGIELEWSKA KM, EK CJ, LANE MA, POTTER A, MORGANTI-KOSSMANN C, SAUNDERS NR. Changes in blood-brain barrier permeability to large and small molecules following traumatic brain injury in mice. Eur J Neurosci. 2007;25:231–238. doi: 10.1111/j.1460-9568.2006.05275.x. [DOI] [PubMed] [Google Scholar]
- 55.HAFLER DA, SLAVIK JM, ANDERSON DE, O’CONNOR KC, DE JAGER P, BAECHER-ALLAN C. Multiple sclerosis. Immunol Rev. 2005;204:208–231. doi: 10.1111/j.0105-2896.2005.00240.x. [DOI] [PubMed] [Google Scholar]
- 56.HAINS BC, WAXMAN SG. Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. J Neurosci. 2006;26:4308–4317. doi: 10.1523/JNEUROSCI.0003-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.HALL ED, BRAUGHLER JM. Central nervous system trauma and stroke: II. Pysiological and pharmacological evidence for the involvement of oxygen radicals and lipid peroxidation. Free Radical Biol Med. 1989;6:303–313. doi: 10.1016/0891-5849(89)90057-9. [DOI] [PubMed] [Google Scholar]
- 58.HAMMARBERG H, LIDMAN O, LUNDBERG C, ELTAYEB SY, GIELEN AW, MUHALLAB S, SVENNINGSSON A, LINDA H, DER MEIDE PH, CULLHEIM S, OLSSON T, PIEHL F. Neuroprotection by Encephalomyelitis: Rescue of Mechanically Injured Neurons and Neurotrophin Production by CNS-Infiltrating T and Natural Killer Cells. J Neurosci. 2000;20:5283–5291. doi: 10.1523/JNEUROSCI.20-14-05283.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.HASHIMOTO M, NITTA A, FUKUMITSU H, NOMOTO H, SHEN L, FURUKAWA S. Inflammation-induced GDNF improves locomotor function after spinal cord injury. Neuroreport. 2005;16:99–102. doi: 10.1097/00001756-200502080-00004. [DOI] [PubMed] [Google Scholar]
- 60.HASHIMOTO M, NITTA A, FUKUMITSU H, NOMOTO H, SHEN L, FURUKAWA S. Involvement of glial cell line-derived neurotrophic factor in activation processes of rodent macrophages. J Neurosci Res. 2005;79:476–487. doi: 10.1002/jnr.20368. [DOI] [PubMed] [Google Scholar]
- 61.HAUBEN E, BUTOVSKY O, NEVO U, YOLES E, MOALEM G, AGRANOV E, MOR F, LEIBOWITZ-AMIT R, PEVSNER E, AKSELROD S, NEEMAN M, COHEN IR, SCHWARTZ M. Passive or active immunization with myelin basic protein promotes recovery from spinal cord contusion. J Neurosci. 2000;20:6421–6430. doi: 10.1523/JNEUROSCI.20-17-06421.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.HAUBEN E, SCHWARTZ M. Therapeutic vaccination for spinal cord injury: helping the body to cure itself. Trends Pharmacol Sci. 2003;24:7–12. doi: 10.1016/s0165-6147(02)00013-5. [DOI] [PubMed] [Google Scholar]
- 63.HAUSMANN ON. Post-traumatic inflammation following spinal cord injury. Spinal Cord. 2003;41:369–378. doi: 10.1038/sj.sc.3101483. [DOI] [PubMed] [Google Scholar]
- 64.HAYES KC, HULL TC, DELANEY GA, POTTER PJ, SEQUEIRA KA, CAMPBELL K, POPOVICH PG. Elevated serum titers of proinflammatory cytokines and CNS autoantibodies in patients with chronic spinal cord injury. J Neurotrauma. 2002;19:753–761. doi: 10.1089/08977150260139129. [DOI] [PubMed] [Google Scholar]
- 65.HELLINGS N, RAUS J, STINISSEN P. Insights into the immunopathogenesis of multiple sclerosis. Immunol Res. 2002;25:27–51. doi: 10.1385/IR:25:1:27. [DOI] [PubMed] [Google Scholar]
- 66.HERMANN GE, ROGERS RC, BRESNAHAN JC, BEATTIE MS. Tumor necrosis factor-alpha induces cFOS and strongly potentiates glutamate-mediated cell death in the rat spinal cord. Neurobiol Dis. 2001;8:590–599. doi: 10.1006/nbdi.2001.0414. [DOI] [PubMed] [Google Scholar]
- 67.HILL CE, BEATTIE MS, BRESNAHAN JC. Degeneration and sprouting of identified descending supraspinal axons after contusive spinal cord injury in the rat. Exp Neurol. 2001;171:153–169. doi: 10.1006/exnr.2001.7734. [DOI] [PubMed] [Google Scholar]
- 68.HOHLFELD R, MEINL E, WEBER F, ZIPP F, SCHMIDT S, SOTGIU S, GOEBELS N, VOLTZ R, SPULER S, IGLESIAS A. The role of autoimmune T lymphocytes in the pathogenesis of multiple sclerosis. Neurology. 1995;45:S33–S38. doi: 10.1212/wnl.45.6_suppl_6.s33. [DOI] [PubMed] [Google Scholar]
- 69.HOPKINS SJ, ROTHWELL NJ. Cytokines and the nervous system I: expression and recognition. Trends Neurosci. 1995;18:83–88. [PubMed] [Google Scholar]
- 70.HUANG DW, MCKERRACHER L, BRAUN PE, DAVID S. A therapeutic vaccine approach to stimulate axon regeneration in the adult mammalian spinal cord. Neuron. 1999;24:639–647. doi: 10.1016/s0896-6273(00)81118-6. [DOI] [PubMed] [Google Scholar]
- 71.HURLBERT RJ. Methylprednisolone for acute spinal cord injury: an inappropriate standard of care. J Neurosurg. 2000;93:1–7. doi: 10.3171/spi.2000.93.1.0001. [DOI] [PubMed] [Google Scholar]
- 72.IKEDA O, MURAKAMI M, INO H, YAMAZAKI M, NEMOTO T, KODA M, NAKAYAMA C, MORIYA H. Acute up-regulation of brain-derived neurotrophic factor expression resulting from experimentally induced injury in the rat spinal cord. Acta Neuropathol(Berl) 2001;102:239–245. doi: 10.1007/s004010000357. [DOI] [PubMed] [Google Scholar]
- 73.JONES TB, ANKENY DP, GUAN Z, MCGAUGHY V, FISHER LC, BASSO DM, POPOVICH PG. Passive or active immunization with myelin basic protein impairs neurological function and exacerbates neuropathology after spinal cord injury in rats. J Neurosci. 2004;24:3752–3761. doi: 10.1523/JNEUROSCI.0406-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.JONES TB, BASSO DM, SODHI A, PAN JZ, HART RP, MACCALLUM RC, LEE S, WHITACRE CC, POPOVICH PG. Pathological CNS autoimmune disease triggered by traumatic spinal cord injury: implications for autoimmune vaccine therapy. J Neurosci. 2002;22:2690–2700. doi: 10.1523/JNEUROSCI.22-07-02690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.JONES TB, HART RP, POPOVICH PG. Molecular control of physiological and pathological T-cell recruitment after mouse spinal cord injury. J Neurosci. 2005;25:6576–6583. doi: 10.1523/JNEUROSCI.0305-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.KAWANO T, ANRATHER J, ZHOU P, PARK L, WANG G, FRYS KA, KUNZ A, CHO S, ORIO M, IADECOLA C. Prostaglandin E2 EP1 receptors: downstream effectors of COX-2 neurotoxicity. Nat Med. 2006;12:225–229. doi: 10.1038/nm1362. [DOI] [PubMed] [Google Scholar]
- 77.KERR BJ, PATTERSON PH. Potent pro-inflammatory actions of leukemia inhibitory factor in the spinal cord of the adult mouse. Exp Neurol. 2004;188:391–407. doi: 10.1016/j.expneurol.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 78.KERSCHENSTEINER M, GALLMEIER E, BEHRENS L, LEAL VV, MISGELD T, KLINKERT WEF, KOLBECK R, HOPPE E, OROPEZA-WEKERLE R-L, BARTKE I, STADELMANN C, LASSMAN H, WEKERLE H, HOHLFELD R. Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: A neuroprotective role of inflammation? J Exp Med. 1999;189:865–870. doi: 10.1084/jem.189.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.KIEFER R, LINDHOLM D, KREUTZBERG GW. Interleukin-6 and transforming growth factor-B1 mRNAs are induced in rat facial nucleus following motoneuron axotomy. Eur J Neurosci. 1993;5:775–781. doi: 10.1111/j.1460-9568.1993.tb00929.x. [DOI] [PubMed] [Google Scholar]
- 80.KIGERL K, POPOVICH P. Drug evaluation: ProCord - a potential cell-based therapy for spinal cord injury. IDrugs. 2006;9:354–360. [PubMed] [Google Scholar]
- 81.KIGERL KA, MCGAUGHY VM, POPOVICH PG. Comparative analysis of lesion development and intraspinal inflammation in four strains of mice following spinal contusion injury. J Comp Neurol. 2006;494:578–594. doi: 10.1002/cne.20827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.KIL K, ZANG YCQ, YANG D, MARKOWSKI J, FUOCO GS, VENDETTI GC, RIVERA VM, ZHANG JZ. T-cell responses to myelin basic protein in patients with spinal cord injury and multiple sclerosis. J Neuroimmunol. 1999;98:201–207. doi: 10.1016/s0165-5728(99)00057-0. [DOI] [PubMed] [Google Scholar]
- 83.KIM SU, DE VELLIS J. Microglia in health and disease. J Neurosci Res. 2005;81:302–313. doi: 10.1002/jnr.20562. [DOI] [PubMed] [Google Scholar]
- 84.KIPNIS J, MIZRAHI T, YOLES E, BEN NUN A, SCHWARTZ M, BEN NUR A. Myelin specific Th1 cells are necessary for post-traumatic protective autoimmunity. J Neuroimmunol. 2002;130:78–85. doi: 10.1016/s0165-5728(02)00219-9. [DOI] [PubMed] [Google Scholar]
- 85.KIPNIS J, YOLES E, SCHORI H, HAUBEN E, SHAKED I, SCHWARTZ M. Neuronal survival after CNS insult is determined by a genetically encoded autoimmune response. J Neurosci. 2001;21:4564–4571. doi: 10.1523/JNEUROSCI.21-13-04564.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.KNOLLER N, AUERBACH G, FULGA V, ZELIG G, ATTIAS J, BAKIMER R, MARDER JB, YOLES E, BELKIN M, SCHWARTZ M, HADANI M. Clinical experience using incubated autologous macrophages as a treatment for complete spinal cord injury: phase I study results. J Neurosurg Spine. 2005;3:173–181. doi: 10.3171/spi.2005.3.3.0173. [DOI] [PubMed] [Google Scholar]
- 87.KREUTZBERG GW. Microglia: A sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 88.KWON KJ, JUNG YS, LEE SH, MOON CH, BAIK EJ. Arachidonic acid induces neuronal death through lipoxygenase and cytochrome P450 rather than cyclooxygenase. J Neurosci Res. 2005;81:73–84. doi: 10.1002/jnr.20520. [DOI] [PubMed] [Google Scholar]
- 89.LACROIX S, CHANG L, ROSE-JOHN S, TUSZYNSKI MH. Delivery of hyper-interleukin-6 to the injured spinal cord increases neutrophil and macrophage infiltration and inhibits axonal growth. J Comp Neurol. 2002;454:213–228. doi: 10.1002/cne.10407. [DOI] [PubMed] [Google Scholar]
- 90.LAGORD C, BERRY M, LOGAN A. Expression of TGFbeta2 but not TGFbeta1 correlates with the deposition of scar tissue in the lesioned spinal cord. Mol Cell Neurosci. 2002;20:69–92. doi: 10.1006/mcne.2002.1121. [DOI] [PubMed] [Google Scholar]
- 91.LEE YB, YUNE TY, BAIK SY, SHIN YH, DU S, RHIM H, LEE EB, KIM YC, SHIN ML, MARKELONIS GJ, OH TH. Role of tumor necrosis factor-alpha in neuronal and glial apoptosis after spinal cord injury. Exp Neurol. 2000;166:190–195. doi: 10.1006/exnr.2000.7494. [DOI] [PubMed] [Google Scholar]
- 92.LEWEN A, MATZ P, CHAN PH. Free radical pathways in CNS injury. J Neurotrauma. 2000;17:871–890. doi: 10.1089/neu.2000.17.871. [DOI] [PubMed] [Google Scholar]
- 93.LI S, STYS PK. Mechanisms of ionotropic glutamate receptor-mediated excitotoxicity in isolated spinal cord white matter. J Neurosci. 2000;20:1190–1198. doi: 10.1523/JNEUROSCI.20-03-01190.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.LIFSHITZ J, SULLIVAN PG, HOVDA DA, WIELOCH T, MCINTOSH TK. Mitochondrial damage and dysfunction in traumatic brain injury. Mitochondrion. 2004;4:705–713. doi: 10.1016/j.mito.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 95.LIU B, GAO HM, WANG JY, JEOHN GH, COOPER CL, HONG JS. Role of nitric oxide in inflammation-mediated neurodegeneration. Ann N Y Acad Sci. 2002;962:318–331. doi: 10.1111/j.1749-6632.2002.tb04077.x. [DOI] [PubMed] [Google Scholar]
- 96.LIU D, LING X, WEN J, LIU J. The role of reactive nitrogen species in secondary spinal cord injury: formation of nitric oxide, peroxynitrite, and nitrated protein. J Neurochem. 2000;75:2144–2154. doi: 10.1046/j.1471-4159.2000.0752144.x. [DOI] [PubMed] [Google Scholar]
- 97.LIU NK, ZHANG YP, TITSWORTH WL, JIANG X, HAN S, LU PH, SHIELDS CB, XU XM. A novel role of phospholipase A2 in mediating spinal cord secondary injury. Ann Neurol. 2006;59:606–619. doi: 10.1002/ana.20798. [DOI] [PubMed] [Google Scholar]
- 98.LU KT, WANG YW, YANG JT, YANG YL, CHEN HI. Effect of interleukin-1 on traumatic brain injury-induced damage to hippocampal neurons. J Neurotrauma. 2005;22:885–895. doi: 10.1089/neu.2005.22.885. [DOI] [PubMed] [Google Scholar]
- 99.MA M, WEI P, WEI T, RANSOHOFF RM, JAKEMAN LB. Enhanced axonal growth into a spinal cord contusion injury site in a strain of mouse (129X1/SvJ) with a diminished inflammatory response. J Comp Neurol. 2004;474:469–486. doi: 10.1002/cne.20149. [DOI] [PubMed] [Google Scholar]
- 100.MA TC, ZHU XZ. Neurotoxic effects of interleukin-6 and sodium nitroprusside on cultured rat hippocampal neurons. Arzneimittelforschung. 2000;50:512–514. doi: 10.1055/s-0031-1300239. [DOI] [PubMed] [Google Scholar]
- 101.MABON PJ, WEAVER LC, DEKABAN GA. Inhibition of monocyte/macrophage migration to a spinal cord injury site by an antibody to the integrin alphaD: a potential new anti- inflammatory treatment. Exp Neurol. 2000;166:52–64. doi: 10.1006/exnr.2000.7488. [DOI] [PubMed] [Google Scholar]
- 102.MACMICKING JD, WILLENBORG DO, WEIDEMANN MJ, ROCKETT KA, COWDEN WB. Elevated secretion of reactive nitrogen and oxygen intermediates by inflammatory leukocytes in hyperacute experimental autoimmune encephalomyelitis: enhancement by the soluble products of encephalitogenic T cells. J Exp Med. 1992;176:303–307. doi: 10.1084/jem.176.1.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.MANABE Y, ANRATHER J, KAWANO T, NIWA K, ZHOU P, ROSS ME, IADECOLA C. Prostanoids, not reactive oxygen species, mediate COX-2-dependent neurotoxicity. Ann Neurol. 2004;55:668–675. doi: 10.1002/ana.20078. [DOI] [PubMed] [Google Scholar]
- 104.MARWICK C. Promising vaccine treatment for alzheimer disease found. JAMA. 2000;284:1503–1505. [PubMed] [Google Scholar]
- 105.MATUTE C, DOMERCQ M, SANCHEZ-GOMEZ MV. Glutamate-mediated glial injury: mechanisms and clinical importance. GLIA. 2006;53:212–224. doi: 10.1002/glia.20275. [DOI] [PubMed] [Google Scholar]
- 106.MAUTES AE, WEINZIERL MR, DONOVAN F, NOBLE LJ. Vascular events after spinal cord injury: contribution to secondary pathogenesis. Phys Ther. 2000;80:673–687. [PubMed] [Google Scholar]
- 107.MCDONALD JW, ALTHOMSONS SP, HYRC KL, CHOI DW, GOLDBERG MP. Oligodendrocytes from forebrain are highly vulnerable to AMPA/kainate receptor-mediated excitotoxicity. Nat Med. 1998;4:291–297. doi: 10.1038/nm0398-291. [DOI] [PubMed] [Google Scholar]
- 108.MCTIGUE DM, POPOVICH PG, MORGAN TE, STOKES BT. Localization of transforming growth factor-beta1 and receptor mRNA after experimental spinal cord injury. Exp Neurol. 2000;163:220–230. doi: 10.1006/exnr.2000.7372. [DOI] [PubMed] [Google Scholar]
- 109.MERRILL JE, IGNARRO LJ, SHERMAN MP, MELINEK J, LANE TE. Microglial cell cytotoxicity of oligodendrocytes is mediated through nitric oxide. J Immunol. 1993;151:2132–2141. [PubMed] [Google Scholar]
- 110.MERRILL JE, ZIMMERMAN RP. Natural and induced cytotoxicity of oligodendrocytes by microglia is inhibitable by TGFβ. GLIA. 1991;4:327–331. doi: 10.1002/glia.440040311. [DOI] [PubMed] [Google Scholar]
- 111.MOALEM G, LEIBOWITZ-AMIT R, YOLES E, MOR F, COHEN IR, SCHWARTZ M. Autoimmune T-cells protect neurons from secondary degeneration after central nervous system axotomy. Nat Med. 1999;5:49–55. doi: 10.1038/4734. [DOI] [PubMed] [Google Scholar]
- 112.MUHALLAB S, LUNDBERG C, GIELEN AW, LIDMAN O, SVENNINGSSON A, PIEHL F, OLSSON T. Differential expression of neurotrophic factors and inflammatory cytokines by myelin basic protein-specific and other recruited T cells infiltrating the central nervous system during experimental autoimmune encephalomyelitis. Scand J Immunol. 2002;55:264–273. doi: 10.1046/j.0300-9475.2002.01038.x. [DOI] [PubMed] [Google Scholar]
- 113.NAG S, PICARD P, STEWART DJ. Increased immunolocalization of nitric oxide synthases during blood-brain barrier breakdown and cerebral edema. Acta Neurochir Suppl(Wien) 2000;76:65–68. doi: 10.1007/978-3-7091-6346-7_13. [DOI] [PubMed] [Google Scholar]
- 114.NAKAJIMA K, HONDA S, TOHYAMA Y, IMAI Y, KOHSAKA S, KURIHARA T. Neurotrophin secretion from cultured microglia. J Neurosci Res. 2001;65:322–331. doi: 10.1002/jnr.1157. [DOI] [PubMed] [Google Scholar]
- 115.NAKAJIMA K, KOHSAKA S. Microglia: neuroprotective and neurotrophic cells in the central nervous system. Curr Drug Targets Cardiovasc Haematol Disord. 2004;4:65–84. doi: 10.2174/1568006043481284. [DOI] [PubMed] [Google Scholar]
- 116.NESIC O, XU GY, MCADOO D, HIGH KW, HULSEBOSCH C, PEREZ-POL R. IL-1 receptor antagonist prevents apoptosis and caspase-3 activation after spinal cord injury. J Neurotrauma. 2001;18:947–956. doi: 10.1089/089771501750451857. [DOI] [PubMed] [Google Scholar]
- 117.NEWMAN TA, WOOLLEY ST, HUGHES PM, SIBSON NR, ANTHONY DC, PERRY VH. T-cell- and macrophage-mediated axon damage in the absence of a CNS- specific immune response: involvement of metalloproteinases. Brain. 2001;124:2203–2214. doi: 10.1093/brain/124.11.2203. [DOI] [PubMed] [Google Scholar]
- 118.NIMMERJAHN A, KIRCHHOFF F, HELMCHEN F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 119.NOBLE LJ, DONOVAN F, IGARASHI T, GOUSSEV S, WERB Z. Matrix metalloproteinases limit functional recovery after spinal cord injury by modulation of early vascular events. J Neurosci. 2002;22:7526–7535. doi: 10.1523/JNEUROSCI.22-17-07526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.NOBLE LJ, WRATHALL JR. Distribution and time course of protein extravasation in the rat spinal cord after contusive injury. Brain Res. 1989;482:57–66. doi: 10.1016/0006-8993(89)90542-8. [DOI] [PubMed] [Google Scholar]
- 121.NODA M, NAKANISHI H, AKAIKE N. Glutamate release from microglia via glutamate transporter is enhanced by amyloid-beta peptide. Neuroscience. 1999;92:1465–1474. doi: 10.1016/s0306-4522(99)00036-6. [DOI] [PubMed] [Google Scholar]
- 122.NORENBERG MD, SMITH J, MARCILLO A. The pathology of human spinal cord injury: defining the problems. J Neurotrauma. 2004;21:429–440. doi: 10.1089/089771504323004575. [DOI] [PubMed] [Google Scholar]
- 123.OKA A, BELLIVEAU MJ, ROSENBERG PA, VOLPE JJ. Vulnerability of oligodendroglia to glutamate: pharmacology, mechanisms, and prevention. J Neurosci. 1993;13:1441–1453. doi: 10.1523/JNEUROSCI.13-04-01441.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.OKADA S, NAKAMURA M, MIKAMI Y, SHIMAZAKI T, MIHARA M, OHSUGI Y, IWAMOTO Y, YOSHIZAKI K, KISHIMOTO T, TOYAMA Y, OKANO H. Blockade of interleukin-6 receptor suppresses reactive astrogliosis and ameliorates functional recovery in experimental spinal cord injury. J Neurosci Res. 2004;76:265–276. doi: 10.1002/jnr.20044. [DOI] [PubMed] [Google Scholar]
- 125.ORGOGOZO JM, GILMAN S, DARTIGUES JF, LAURENT B, PUEL M, KIRBY LC, JOUANNY P, DUBOIS B, EISNER L, FLITMAN S, MICHEL BF, BOADA M, FRANK A, HOCK C. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology. 2003;61:46–54. doi: 10.1212/01.wnl.0000073623.84147.a8. [DOI] [PubMed] [Google Scholar]
- 126.PIANI D, FONTANA A. Involvement of the cystine transport system xc- in the macrophage-induced glutamate-dependent cytotoxicity to neurons. J Immunol. 1994;152:3578–3585. [PubMed] [Google Scholar]
- 127.PIANI D, FREI K, DO KQ, CUENOD M, FONTANA A. Murine brain macrophages induced NMDA receptor mediated neurotoxicity in vitro by secreting glutamate. Neurosci Lett. 1991;133:159–162. doi: 10.1016/0304-3940(91)90559-c. [DOI] [PubMed] [Google Scholar]
- 128.PIANI D, FREI K, PFISTER HW, FONTANA A. Glutamate uptake by astrocytes is inhibited by reactive oxygen intermediates but not by other macrophage-derived molecules including cytokines, leukotrienes or platelet-activating factor. J Neuroimmunol. 1993;48:99–104. doi: 10.1016/0165-5728(93)90063-5. [DOI] [PubMed] [Google Scholar]
- 129.PINEAU I, LACROIX S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: multiphasic expression pattern and identification of the cell types involved. J Comp Neurol. 2007;500:267–285. doi: 10.1002/cne.21149. [DOI] [PubMed] [Google Scholar]
- 130.PITT D, NAGELMEIER IE, WILSON HC, RAINE CS. Glutamate uptake by oligodendrocytes: Implications for excitotoxicity in multiple sclerosis. Neurology. 2003;61:1113–1120. doi: 10.1212/01.wnl.0000090564.88719.37. [DOI] [PubMed] [Google Scholar]
- 131.POPOVICH PG, GUAN Z, WEI P, HUITINGA I, VAN ROOIJEN N, STOKES BT. Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Exp Neurol. 1999;158:351–365. doi: 10.1006/exnr.1999.7118. [DOI] [PubMed] [Google Scholar]
- 132.POPOVICH PG, HICKEY WF. Bone marrow chimeric rats reveal the unique distribution of resident and recruited macrophages in the contused rat spinal cord. J Neuropathol Exp Neurol. 2001;60:676–685. doi: 10.1093/jnen/60.7.676. [DOI] [PubMed] [Google Scholar]
- 133.POPOVICH PG, HORNER PJ, MULLIN BB, STOKES BT. A quantitative spatial analysis of the blood-spinal cord barrier I. Permeability changes after experimental spinal contusion injury. Exp Neurol. 1996;142:258–275. doi: 10.1006/exnr.1996.0196. [DOI] [PubMed] [Google Scholar]
- 134.POPOVICH PG, JONES TB. Manipulating neuroinflammatory reactions in the injured spinal cord: back to basics. Trends Pharmacol Sci. 2003;24:13–17. doi: 10.1016/s0165-6147(02)00006-8. [DOI] [PubMed] [Google Scholar]
- 135.POPOVICH PG, STOKES BT, WHITACRE CC. Concept of autoimmunity following spinal cord injury: Possible roles for T lymphocytes in the traumatized central nervous system. J Neurosci Res. 1996;45:349–363. doi: 10.1002/(SICI)1097-4547(19960815)45:4<349::AID-JNR4>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 136.POPOVICH PG, WEI P, STOKES BT. The cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J Comp Neurol. 1997;377:443–464. doi: 10.1002/(sici)1096-9861(19970120)377:3<443::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 137.POTAS JR, ZHENG Y, MOUSSA C, VENN M, GORRIE CA, DENG C, WAITE PM. Augmented locomotor recovery after spinal cord injury in the athymic nude rat. J Neurotrauma. 2006;23:660–673. doi: 10.1089/neu.2006.23.660. [DOI] [PubMed] [Google Scholar]
- 138.PREWITT CM, NIESMAN IR, KANE CJ, HOULE JD. Activated macrophage/microglial cells can promote the regeneration of sensory axons into the injured spinal cord. Exp Neurol. 1997;148:433–443. doi: 10.1006/exnr.1997.6694. [DOI] [PubMed] [Google Scholar]
- 139.PROBERT L, SELMAJ K. TNF and related molecules: trends in neuroscience and clinical applications. J Neuroimmunol. 1997;72:113–117. doi: 10.1016/s0165-5728(96)00176-2. [DOI] [PubMed] [Google Scholar]
- 140.PROFYRIS C, CHEEMA SS, ZANG D, AZARI MF, BOYLE K, PETRATOS S. Degenerative and regenerative mechanisms governing spinal cord injury. Neurobiol Dis. 2004;15:415–436. doi: 10.1016/j.nbd.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 141.QIAN T, CAMPAGNOLO D, KIRSHBLUM S. High-dose methylprednisolone may do more harm for spinal cord injury. Med Hypotheses. 2000;55:452–453. doi: 10.1054/mehy.2000.1165. [DOI] [PubMed] [Google Scholar]
- 142.RABCHEVSKY AG, STREIT WJ. Grafting of cultured microglial cells into the lesioned spinal cord of adult rats enhances neurite outgrowth. J Neurosci Res. 1997;47:34–48. [PubMed] [Google Scholar]
- 143.RAPALINO O, LAZAROV-SPIEGLER O, AGRANOV E, VELAN GJ, YOLES E, FRAIDAKIS M, SOLOMON A, GEPSTEIN R, KATZ A, BELKIN A, HADANI M, SCHWARTZ M. Implantation of stimulated homologous macrophages results in partial recovery of paraplegic rats. Nat Med. 1998;4:814–821. doi: 10.1038/nm0798-814. [DOI] [PubMed] [Google Scholar]
- 144.RIMANIOL AC, HAIK S, MARTIN M, LE GRAND R, BOUSSIN FD, DEREUDDRE-BOSQUET N, GRAS G, DORMONT D. Na+-dependent high-affinity glutamate transport in macrophages. J Immunol. 2000;164:5430–5438. doi: 10.4049/jimmunol.164.10.5430. [DOI] [PubMed] [Google Scholar]
- 145.SANDERSON KL, RAGHUPATHI R, SAATMAN KE, MARTIN D, MILLER G, MCINTOSH TK. Interleukin-1 receptor antagonist attenuates regional neuronal cell death and cognitive dysfunction after experimental brain injury. J Cereb Blood Flow Metab. 1999;19:1118–1125. doi: 10.1097/00004647-199910000-00008. [DOI] [PubMed] [Google Scholar]
- 146.SARKER MH, EASTON AS, FRASER PA. Regulation of cerebral microvascular permeability by histamine in the anaesthetized rat. J Physiol. 1998;507(Pt 3):909–918. doi: 10.1111/j.1469-7793.1998.909bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.SCHNELL L, FEARN S, KLASSEN H, SCHWAB ME, PERRY VH. Acute inflammatory responses to mechanical lesions in the CNS: differences between brain and spinal cord. Eur J Neurosci. 1999;11:3648–3658. doi: 10.1046/j.1460-9568.1999.00792.x. [DOI] [PubMed] [Google Scholar]
- 148.SCHNELL L, FEARN S, SCHWAB ME, PERRY VH, ANTHONY DC. Cytokine-induced acute inflammation in the brain and spinal cord. J Neuropathol Exp Neurol. 1999;58:245–254. doi: 10.1097/00005072-199903000-00004. [DOI] [PubMed] [Google Scholar]
- 149.SCHROETER M, JANDER S. T-cell cytokines in injury-induced neural damage and repair. Neuromolecular Med. 2005;7:183–195. doi: 10.1385/NMM:7:3:183. [DOI] [PubMed] [Google Scholar]
- 150.SCHWARTZ M. Harnessing the immune system for neuroprotection: therapeutic vaccines for acute and chronic neurodegenerative disorders. Cell Mol Neurobiol. 2001;21:617–627. doi: 10.1023/a:1015139718466. [DOI] [PubMed] [Google Scholar]
- 151.SCHWARTZ M, KIPNIS J. Protective autoimmunity: regulation and prospects for vaccination after brain and spinal cord injuries. Trends Mol Med. 2001;7:252–258. doi: 10.1016/s1471-4914(01)01993-1. [DOI] [PubMed] [Google Scholar]
- 152.SCHWARTZ M, KIPNIS J. Multiple sclerosis as a by-product of the failure to sustain protective autoimmunity: a paradigm shift. Neuroscientist. 2002;8:405–413. doi: 10.1177/107385802236966. [DOI] [PubMed] [Google Scholar]
- 153.SERPE CJ, KOHM AP, HUPPENBAUER CB, SANDERS VM, JONES KJ. Exacerbation of facial motoneuron loss after facial nerve transection in severe combined immunodeficient (scid) mice. J Neurosci. 1999;19:RC7. doi: 10.1523/JNEUROSCI.19-11-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.SHAKED I, PORAT Z, GERSNER R, KIPNIS J, SCHWARTZ M. Early activation of microglia as antigen-presenting cells correlates with T cell-mediated protection and repair of the injured central nervous system. J Neuroimmunol. 2004;146:84–93. doi: 10.1016/j.jneuroim.2003.10.049. [DOI] [PubMed] [Google Scholar]
- 155.SHAMASH S, REICHERT F, ROTSHENKER S. The cytokine network of Wallerian degeneration: tumor necrosis factor- alpha, interleukin-1alpha, and interleukin-1beta. J Neurosci. 2002;22:3052–3060. doi: 10.1523/JNEUROSCI.22-08-03052.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.SHARMA HS, WINKLER T, STALBERG E, GORDH T, ALM P, WESTMAN J. Topical application of TNF-alpha antiserum attenuates spinal cord trauma induced edema formation, microvascular permeability disturbances and cell injury in the rat. Acta Neurochir Suppl(Wien) 2003;86:407–413. doi: 10.1007/978-3-7091-0651-8_85. [DOI] [PubMed] [Google Scholar]
- 157.SPRINGER JE, AZBILL RD, MARK RJ, BEGLEY JG, WAEG G, MATTSON MP. 4-hydroxynonenal, a lipid peroxidation product, rapidly accumulates following traumatic spinal cord injury and inhibits glutamate uptake. J Neurochem. 1997;68:2469–2476. doi: 10.1046/j.1471-4159.1997.68062469.x. [DOI] [PubMed] [Google Scholar]
- 158.SRIRAM S, RODRIGUEZ M. Indictment of the microglia as the villain in multiple sclerosis. Neurology. 1997;48:464–470. doi: 10.1212/wnl.48.2.464. [DOI] [PubMed] [Google Scholar]
- 159.SROGA JM, JONES TB, KIGERL KA, MCGAUGHY VM, POPOVICH PG. Rats and mice exhibit distinct inflammatory reactions after spinal cord injury. J Comp Neurol. 2003;462:223–240. doi: 10.1002/cne.10736. [DOI] [PubMed] [Google Scholar]
- 160.STELLWAGEN D, BEATTIE EC, SEO JY, MALENKA RC. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha. J Neurosci. 2005;25:3219–3228. doi: 10.1523/JNEUROSCI.4486-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.STINISSEN P, RAUS J, ZHANG J. Autoimmune pathogenesis of multiple sclerosis: role of autoreactive T lymphocytes and new immunotherapeutic strategies. Crit Rev Immunol. 1997;17:33–75. doi: 10.1615/critrevimmunol.v17.i1.20. [DOI] [PubMed] [Google Scholar]
- 162.STIRLING DP, KHODARAHMI K, LIU J, MCPHAIL LT, MCBRIDE CB, STEEVES JD, RAMER MS, TETZLAFF W. Minocycline treatment reduces delayed oligodendrocyte death, attenuates axonal dieback, and improves functional outcome after spinal cord injury. J Neurosci. 2004;24:2182–2190. doi: 10.1523/JNEUROSCI.5275-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.STREIT WJ, SEMPLE-ROWLAND SL, HURLEY SD, MILLER RC, POPOVICH PG, STOKES BT. Cytokine mRNA profiles in contused spinal cord and axotomized facial nucleus suggest a beneficial role for inflammation and gliosis. Exp Neurol. 1998;152:74–87. doi: 10.1006/exnr.1998.6835. [DOI] [PubMed] [Google Scholar]
- 164.TAKAHASHI JL, GIULIANI F, POWER C, IMAI Y, YONG VW. Interleukin-1beta promotes oligodendrocyte death through glutamate excitotoxicity. Ann Neurol. 2003;53:588–595. doi: 10.1002/ana.10519. [DOI] [PubMed] [Google Scholar]
- 165.TAKAMI T, OUDEGA M, BETHEA JR, WOOD PM, KLEITMAN N, BUNGE MB. Methylprednisolone and interleukin-10 reduce gray matter damage in the contused Fischer rat thoracic spinal cord but do not improve functional outcome. J Neurotrauma. 2002;19:653–666. doi: 10.1089/089771502753754118. [DOI] [PubMed] [Google Scholar]
- 166.TAOKA Y, OKAJIMA K, UCHIBA M, MURAKAMI K, KUSHIMOTO S, JOHNO M, NARUO M, OKABE H, TAKATSUKI K. Role of neutrophils in spinal cord injury in the rat. Neuroscience. 1997;79:1177–1182. doi: 10.1016/s0306-4522(97)00011-0. [DOI] [PubMed] [Google Scholar]
- 167.TENG YD, CHOI H, ONARIO RC, ZHU S, DESILETS FC, LAN S, WOODARD EJ, SNYDER EY, EICHLER ME, FRIEDLANDER RM. Minocycline inhibits contusion-triggered mitochondrial cytochrome c release and mitigates functional deficits after spinal cord injury. Proc Natl Acad Sci USA. 2004;101:3071–3076. doi: 10.1073/pnas.0306239101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.THATTE U. AN-1792 (Elan) Curr Opin Investig Drugs. 2001;2:663–667. [PubMed] [Google Scholar]
- 169.TOTOIU MO, KEIRSTEAD HS. Spinal cord injury is accompanied by chronic progressive demyelination. J Comp Neurol. 2005;486:373–383. doi: 10.1002/cne.20517. [DOI] [PubMed] [Google Scholar]
- 170.TVETEN L. Spinal cord vascularity. IV. The spinal cord arteries in the rat. Acta Radiol Diagn(Stockh) 1976;17:385–398. doi: 10.1177/028418517601700401. [DOI] [PubMed] [Google Scholar]
- 171.TVETEN L. Spinal cord vascularity. V. The venous drainage of the spinal cord in the rat. Acta Radiol Diagn(Stockh) 1976;17:653. [PubMed] [Google Scholar]
- 172.UNTERBERG A, WAHL M, BAETHMANN A. Effects of free radicals on permeability and vasomotor response of cerebral vessels. Acta Neuropathol(Berl) 1988;76:238–244. doi: 10.1007/BF00687770. [DOI] [PubMed] [Google Scholar]
- 173.UNTERBERG A, WAHL M, HAMMERSEN F, BAETHMANN A. Permeability and vasomotor response of cerebral vessels during exposure to arachidonic acid. Acta Neuropathol(Berl) 1987;73:209–219. doi: 10.1007/BF00686613. [DOI] [PubMed] [Google Scholar]
- 174.VAN LANDEGHEM FK, STOVER JF, BECHMANN I, BRUCK W, UNTERBERG A, BUHRER C, VON DEIMLING A. Early expression of glutamate transporter proteins in ramified microglia after controlled cortical impact injury in the rat. GLIA. 2001;35:167–179. doi: 10.1002/glia.1082. [DOI] [PubMed] [Google Scholar]
- 175.VOLTERRA A, TROTTI D, TROMBA C, FLORIDI S, RACAGNI G. Glutamate uptake inhibition by oxygen free radicals in rat cortical astrocytes. J Neurosci. 1994;14:2924–2932. doi: 10.1523/JNEUROSCI.14-05-02924.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.VON BUDINGEN HC, TANUMA N, VILLOSLADA P, OUALLET JC, HAUSER SL, GENAIN CP. Immune responses against the myelin/oligodendrocyte glycoprotein in experimental autoimmune demyelination. J Clin Immunol. 2001;21:155–170. doi: 10.1023/a:1011031014433. [DOI] [PubMed] [Google Scholar]
- 177.WADA K, CHATZIPANTELI K, KRAYDIEH S, BUSTO R, DIETRICH WD. Inducible nitric oxide synthase expression after traumatic brain injury and neuroprotection with aminoguanidine treatment in rats. Neurosurgery. 1998;43:1427–1436. doi: 10.1097/00006123-199812000-00096. [DOI] [PubMed] [Google Scholar]
- 178.WELLS JE, RICE TK, NUTTALL RK, EDWARDS DR, ZEKKI H, RIVEST S, YONG VW. An adverse role for matrix metalloproteinase 12 after spinal cord injury in mice. J Neurosci. 2003;23:10107–10115. doi: 10.1523/JNEUROSCI.23-31-10107.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.WHETSTONE WD, HSU JY, EISENBERG M, WERB Z, NOBLE-HAEUSSLEIN LJ. Blood-spinal cord barrier after spinal cord injury: relation to revascularization and wound healing. J Neurosci Res. 2003;74:227–239. doi: 10.1002/jnr.10759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.XIONG Y, RABCHEVSKY AG, HALL ED. Role of peroxynitrite in secondary oxidative damage after spinal cord injury. J Neurochem. 2007;100:639–649. doi: 10.1111/j.1471-4159.2006.04312.x. [DOI] [PubMed] [Google Scholar]
- 181.XU J, FAN G, CHEN S, WU Y, XU XM, HSU CY. Methylprednisolone inhibition of TNF-alpha expression and NF-kB activation after spinal cord injury in rats. Brain Res Mol Brain Res. 1998;59:135–142. doi: 10.1016/s0169-328x(98)00142-9. [DOI] [PubMed] [Google Scholar]
- 182.XU J, KIM GM, AHMED SH, XU J, YAN P, XU XM, HSU CY. Glucocorticoid receptor-mediated suppression of activator protein-1 activation and matrix metalloproteinase expression after spinal cord injury. J Neurosci. 2001;21:92–97. doi: 10.1523/JNEUROSCI.21-01-00092.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.XU W, CHI L, XU R, KE Y, LUO C, CAI J, QIU M, GOZAL D, LIU R. Increased production of reactive oxygen species contributes to motor neuron death in a compression mouse model of spinal cord injury. Spinal Cord. 2005;43:204–213. doi: 10.1038/sj.sc.3101674. [DOI] [PubMed] [Google Scholar]
- 184.YIN Y, CUI Q, LI Y, IRWIN N, FISCHER D, HARVEY AR, BENOWITZ LI. Macrophage-derived factors stimulate optic nerve regeneration. J Neurosci. 2003;23:2284–2293. doi: 10.1523/JNEUROSCI.23-06-02284.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.YIN Y, HENZL MT, LORBER B, NAKAZAWA T, THOMAS TT, JIANG F, LANGER R, BENOWITZ LI. Oncomodulin is a macrophage-derived signal for axon regeneration in retinal ganglion cells. Nat Neurosci. 2006;9:843–852. doi: 10.1038/nn1701. [DOI] [PubMed] [Google Scholar]
- 186.YONG VW, POWER C, FORSYTH P, EDWARDS DR. Metalloproteinases in biology and pathology of the nervous system. Nat Rev Neurosci. 2001;2:502–511. doi: 10.1038/35081571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.ZERANGUE N, ARRIZA JL, AMARA SG, KAVANAUGH MP. Differential modulation of human glutamate transporter subtypes by arachidonic acid. J Biol Chem. 1995;270:6433–6435. doi: 10.1074/jbc.270.12.6433. [DOI] [PubMed] [Google Scholar]
- 188.ZHANG Z, KREBS CJ, GUTH L. Experimental analysis of progressive necrosis after spinal cord trauma in the rat: etiological role of the inflammatory response. Exp Neurol. 1997;143:141–152. doi: 10.1006/exnr.1996.6355. [DOI] [PubMed] [Google Scholar]