Abstract
Purpose of the review
This review discusses recent progress in research that seeks to understand the regeneration of hair cells and it highlights findings that may hold importance for the eventual development of regenerative therapies for hearing and balance impairments.
Recent findings
Signaling via the Notch receptor and the bHLH transcription factors has important roles in the development and regeneration of hair cells. The cytoskeletal properties and cell-matrix interactions of supporting cells in mice of different ages may hold part of the explanation for the age-related differences in their proliferative responses to damage and the differences between mammals and non-mammals in hair cell regeneration. Progress also has been made in deriving stem cells from inner ear tissues and other sources and in the evaluation of their potential uses as source of new hair cells and tools for biomedical research.
Summary
Much has been accomplish since the discovery of postembryonic hair cell production and hair cell regeneration in non-mammals decades ago. No therapies for hair cell regeneration are under clinical trials, but research is yielding potentially important discoveries that are likely to lead to the development of therapeutic methods for inducing hair cell regeneration in the mammalian inner ear.
Keywords: ear, repair, regeneration, proliferation, hair cell
INTRODUCTION
Hair cell loss in humans and other mammals appears to be permanent and cumulative, but thousands of hair cells are added throughout life in the ears of many non-mammalian vertebrates (reviewed in [1]). When hair cells in non-mammals are killed by trauma, toxicity, or other causes they are replaced and begin to restore hearing and balance sensitivity within weeks. This article will address the possibility for therapeutic regeneration while highlighting recent important findings related to the molecules that may regulate inner ear regeneration, postnatal changes in mammalian ears that may limit regeneration, and potential roles for stem cells in the treatment of ear disorders.
Signals that control proliferation
Embryonic signals that control when, where, and which cells divide and when they will stop dividing and develop into the specialized cells of the ear may have importance for the eventual development of regenerative therapies. Nearly all the cells stop dividing in the auditory epithelia of birds and mammals before birth [2, 3]. But damage to the auditory epithelium in birds evokes an innate capacity for renewed supporting cell proliferation that leads to the regeneration of hair cells [4, 5]. Molecules such as retinoblastoma protein, pRb, an important tumor suppressor that prevents cell proliferation have recently been explored. Several years ago it was discovered that Rb deletion can lead to overproduction of mammalian hair cells via renewed cycling [6, 7, 8]. More recent investigations in postnatal ears have confirmed that hair cells that lack pRb will replicate their DNA as they reenter the cell cycle, but that is quickly followed by hair cell death [9••]. Thus, deletion of Rb by itself does not lead to lasting hair cell replacement in postnatal ears.
Cell production in the inner ear is also regulated by Cip/Kip and Ink4 cyclin-dependent kinase inhibitors, CKIs. Mice that carry a mutant form of the widely expressed CKI, p27Kip1, develop supernumerary hair cells and supporting cells in the organ of Corti [10, 11]. Another Cip/Kip family member, p21Cip1, and the Ink4 family member, p19Ink4d, also help to maintain the post-mitotic state of hair cells. In mice where p21Cip1 and p19Ink4d have been deleted auditory hair cells re-enter the cell cycle, but quickly die by apoptosis while the vestibular hair cells appeared to remain intact [12].
Forced expression of Skp2, which triggers the degradation of p27Kip1, has been found to increase proliferation in cells adjacent to the organ of Corti. Ectopic hair cells were also observed, but only when Skp2 was overexpressed together with Atoh1 [13•]. Taken together, the recent results suggest that the development of therapeutic regeneration is likely to require something more than the absence of cell cycle inhibitors. The importance of tumor suppression mechanisms has led to the evolution of multiple redundant controls, such as p53-mediated apoptosis, that act to limit cell proliferation in mammals.
Signals that influence cell fate
Considerable gains have been made in understanding the molecules that regulate cell fate during development and regeneration in the ear. Atoh1 (formerly known as Math1) is a basic helix-loop-helix (bHLH) transcription factor protein necessary for hair cell differentiation. In its absence, mice do not develop hair cells and their cochleae do not label for supporting cell markers, consistent with a vital role for Atoh1 in the establishment of the prosensory domain and cell fate determination [14, 15]. Similar roles recently have been attributed to two Atoh1 genes in Zebrafish [16••].
Atoh1 mRNA is expressed in embryonic cochlear progenitor cells in mice, but the Atoh1 protein is only found in differentiating hair cells [14, 17–20]. In chickens, Atoh1 protein is expressed in the cells of developing sensory patches [21, 22]. Atoh1 expression is found in the avian utricular sensory epithelium, where the hair cell population turns over continually, but is not found in the undamaged and quiescent auditory sensory epithelium. However, ototoxic damage leads to the expression of Atoh1 in supporting cells and regenerated hair cells in both types of sensory epithelia [23•].
The bHLH transcription factors of the Inhibitor of Differentiation (Id) family are expressed within the developing cochlea, and appear to strongly inhibit the expression of Atoh1 and the differentiation of hair cells [24]. Another bHLH transcription factor, Neurogenin1, and Atoh1 have recently been found to cross-inhibit each in the early otocyst as it transitions from neurogenesis to sensory cell formation, and Atoh1 was found to positively regulate its own expression [25••]. Fgf3 and Fgf8 act as upstream activators of Atoh1 in the Zebrafish otic vesicle and fox1, pax8, and dlx genes regulate Atoh1 in the preplacode [16••]. A recent study has provided evidence that Atoh1 activates Hes6 transcription through binding to three E-boxes in its promoter [26•].
Several years ago it was discovered that ectopic hair cells could be induced to develop in Kollicker’s organ through the forced overexpression of Atoh1, and forced expression in vitro also resulted in new hair cells in damaged postnatal utricles [27, 28]. Nearly complete in vivo recovery of hair cells and supporting cells and partial recovery of auditory function are reported to follow adenoviral delivery of the Atoh1 gene into the cochlea of guinea pigs after toxic injury [29]. More recently, adenoviral delivery of Atoh1 in vivo has been reported to lead to vestibular function recovery in mice after aminoglycoside damage [30]. Caution may be warranted, however, in interpreting these remarkable early reports. A scientific axiom is that extraordinary claims require extraordinary evidence. In the absence of evidence for intermediate stages in the recovery process the data reported thus far have not reached that level and they await replication in other laboratories.
The Notch signaling pathway
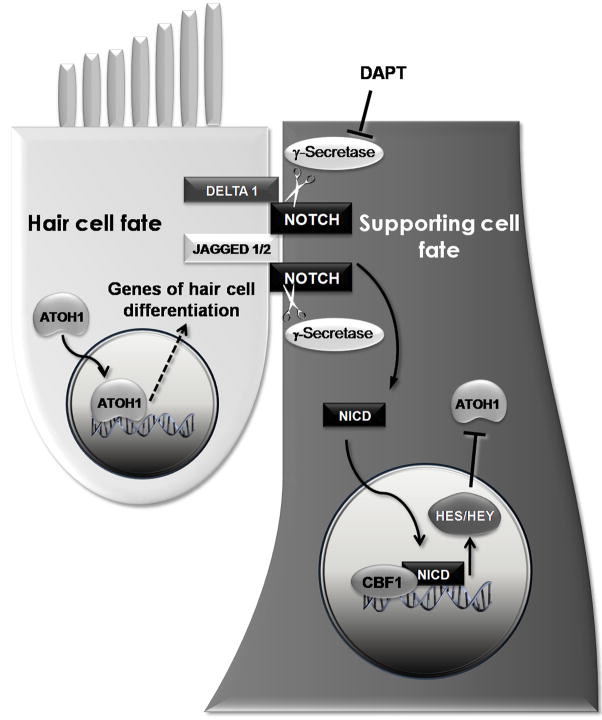
Notch signaling influences the development and specialization of cells in diverse tissues, and Notch mutations have been identified in certain forms of cancer and other conditions. When the cell-attached ligands of the DSL family bind to Notch receptors that leads to cleavage and release of the Notch intracellular domain (NICD), which translocates to the nucleus where it converts the CBF1 repressor complex (formerly known as Rbpsuh) into an activator complex. The NICD/CBF1 activator complex upregulates the basic helix-loop-helix (bHLH) transcriptional regulators Hes and Hes-related protein genes, which antagonize proneural genes like neurogenin and the “prosensory” gene Atoh1. This antagonism is central to the inhibition of hair cell differentiation that followed Notch activation (Figure 1).
Figure 1. Notch signaling in cell fate determination.
Developing hair cells express the ligands Jagged and Delta. Those ligands bind to the Notch receptors of adjacent cells, and that leads to γ-secretase mediated cleavage and generation of a Notch intracellular domain (NICD). After tranlocation to the nucleus, NICD forms a complex with CBF1 and other proteins, and upregulates the expression of Hairy and enhancer of split (HES) and related HEY genes, which block the effects of Atoh1, leading to inhibition of hair cell fate and the development of the cell as a supporting cell. Developing hair cells express Atoh1 which is necessary for hair cell differentiation. Inhibitors of γ-secretases such as DAPT appear to interrupt Notch signaling by blocking the generation of the NICD.
Notch signaling activated by binding to the ligands Jagged1, Jagged 2, and Delta1 has been implicated in early the establishment of prosensory domains [31–34], and the lateral inhibitory interactions that determine hair cell and supporting cell fate during later development of the mammalian ear [31, 32, 35–40]. In the developing inner ear, genetic deletions of Notch ligands [31, 36, 37] or the CBF-1/Rbpsuh gene [41] result in the development of supernumerary hair cells. This effect has also been produced pharmacologically using γ-secretase inhibitors [41•, 42•] as well by oligonucleotide knockdowns directed at Jagged1 and Notch1 [40].
Notch signaling is reactivated during regeneration in the avian ear [43] and the zebrafish lateral line where it appears to limit the hair cell production by acting as a brake on supporting cell proliferation [44••]. Recently, in epithelia of adults guinea pigs that had been damaged by ototoxic treatments, Notch signaling components were found to be upregulated, and when γ-secretase inhibitors were administered that led to the formation of ectopic auditory hair [45•]. An increasing number of receptors have been discovered to undergoγ-secretase mediated cleavage so specific disruption of Notch signaling will be required to establish the mechanism of the γ-secretase inhibitors in the inner ear. γ-secretase inhibitors and other pharmacological agents that influence intermediates in the Notch-bHLH transcription factor pathway may provide important potential candidates for regenerative therapies, but much remain to be discovered in these future studies.
Other signaling pathways
The canonical Wnt, sonic hedgehog (Shh), bone morphogenetic protein (BMP) and fibroblast growth factor (FGF) signaling pathways have important roles in regionalization and early embryonic specification of the developing ear (reviewed in [46–48]). Those pathways are likely to have important roles in hair cell regeneration, but much remains to be investigated.
Gene expression analysis
The development of microarray technology for assessing gene expression can provide comprehensive and relatively unbiased information about molecular regulation at the transcriptional level. These approaches are being used to investigate inner ear development, hair cell death and regeneration. Investigations of avian sensory epithelia determined that the expression of 605 genes in the cochlea and 188 genes in the utricle changed hours after they were damaged by laser ablation or ototoxicity [49••]. Another study of the developing mouse inner ear identified genes whose expression patterns corresponded with the organogenesis of the cochlea, utricle, and saccule and provided novel evidence of roles for β-adrenergic, amyloid, estrogen receptor, circadian rhythm, and immune pathways in inner ear development [50••].
MicroRNAs
Short noncoding, RNAs (20-22 nucleotides) termed microRNAs (miRNAs) have recently emerged as important regulators of protein expression [51, 52]. miRNAs play a role in fin regeneration in zebrafish [53, 54] and other regenerative processes (review in [55]). Recently, Tsonis et al. reported that members of the let-7 family of miRNAs are downregulated during hair cell regeneration in newts, and suggested that they may have a role in phenotypic conversion of supporting cells into hair cells [56]. These findings may presage an important, but still largely unexplored role for miRNAs in hair cell regeneration.
Gene therapy
The inner ear may provide a privileged site for the therapeutic delivery of genes in an isolated compartment. As a first approach to that potential goal, an in vitro system for study gene therapy in the human inner ear has been developed and was successfully used to demonstrate expression of exogenous genes delivered into hair cells and supporting cells via second-generation adenovirus transfection [57••]. Other approaches have been recently reviewed [58].
Potential limits to regeneration in mammals
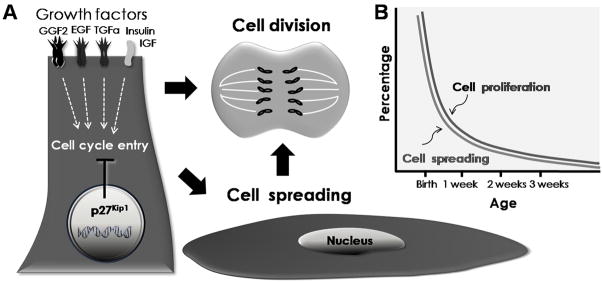
When balance epithelia from newborn rodents are isolated and cultured with appropriate mitogens the majority of the supporting cells replicate their DNA and begin to proliferate (Figure 2) [59–65]. But a sharp, decline in cell production capacity progresses during the first postnatal weeks and appears to leave mammalian ears uniquely vulnerable to permanent hair cell loss (Figure 2) [60, 61••]. That decline was recently correlated with a progressive loss of the capacity for supporting cells to change from their normal, columnar shapes to thin and widely spread, squamous cells that have a high incidence of proliferation (Figure 2) [66••]. Wounding assays in cultures of whole utricles, assessed spreading of supporting cells on their natural substrate and demonstrated that the number of cells that begin to proliferate is correlated with number of cells spreading to cover a wound [67••].
Figure 2. Cell proliferation in mammalian supporting cells (SC) correlates with SC spreading and is influenced by growth factors and cyclin-dependent kinase inhibitors.
A. Glial Growth factor (GGF2), Epidermal growth factor (EGF), Transforming growth factor (TGFα), Insulin, Insulin-like Growth Factor and other extracellular signals can promote robust cell production in sensory epithelia from neonatal mammals. On the other hand, the cyclin-dependent kinase inhibitor p27Kip1 helps to maintain supporting cell in a mitotically quiescent state. When supporting cells change from their normal elongated columnar shape to spread and more flattened shapes their capacity for proliferation is enhanced. B. In mammalian vestibular epithelia, the capacity for cellular spreading and cell proliferation decline during the first week after birth and that appears to contribute to the limited regeneration that is characteristic of mammals.
Cell shape may also influence differentiation in the inner ear. As will be discussed below, when widely spread proliferating cells derived from passaged cultures of avian hair cell progenitors were lifted off a 2D culture substrate and aggregated in suspension the cells took on columnar shapes and differentiated into hair cells and supporting cells [68••]. Also, after the cochlea has been damaged by high concentrations of ototoxic drugs, a flattened epithelium consisting of thinly spread and highly proliferative nonsensory cells can replace the organ of Corti [69]. When transfected with Atoh1 those cells do not differentiate into hair cells [70], which may indicate that a flattened shape prevents them from differentiating, as it does for human epidermal keratinocytes [71].
The cytoskeleton and matrix interactions
Recent preliminary investigations in utricles from mice revealed substantial differences in the amount of filamentous actin that bracket the apical junctions between supporting cells in the utricles of young and old mice, suggesting that increases in cytoskeletal stiffness may contribute to the loss in their capacity to spread and proliferate [67]. The cytoskeleton also appears to have a role in determining how hair cells are lost. A recent study revealed that cultured bullfrog saccules which were treated with myosin-contraction inhibitors lost nearly twice as many hair cells as controls [72•]. The substantial loss of hair cells after these treatments points to myosin-mediated tension as a potential mechanism for retaining hair cells within the epithelium. Disruption of microtubule polymerization also increased the loss of hair cell, and treatments with actin polymerization inhibitors appeared to slow the closure of voids left by extruded hair cells [72•].
Age-related changes in integrin expression and activation also may contribute to restrictions in shape change and proliferation in mammalian hair cell epithelia. In sensory epithelia isolated from the utricles of embryonic and postnatal day 6 mice cellular spreading and proliferation were dependent onα6 integrin, which disappeared from lateral cell membranes by P6 and co-localized with β4 integrin near the basement membrane at both ages [66]. α6β1 promotes migration in multiple cell types while α6β4 promotes firm adhesion and structural stability, these developmental changes could contribute to stronger substrate adhesions and the decreased spreading and proliferation that develops postnatally in mammalian ears. Given the intimate relations between shape change, the cytoskeleton, and the cell’s connections to its substrate and other cells, further work to characterize potential differences between species that differ in their capacity for innate regeneration will be of interest.
Potential roles of inner ear stem cells
Stem cells are defined by their capacity for self-renewal and differentiation into more than one cell type. Supporting cells are important stem cells in the inner ear, but there is the potential for the existence of other tissue resident stem cells that might be held in reserve and could contribute to the regenerative replacement of hair cells. If such cells exist, they remain to be identified, but recent studies have shown that the mammalian ear contains cells that have the ability to self-renew and differentiate into a variety of cell types both in vitro and after xenograft transplantation into the ears of chick embryos developing in ovo [73]. Cells that have been isolated from the greater epithelial ridge of the neonatal rat cochlea [74] and from the organ of Corti of the newborn mouse cochlea [75, 76] also appear to be able to proliferate and generate cells that exhibit some properties of hair cells.
FACS sorting has been used to produce samples enriched for viable supporting cells from the cochlea of p27(kip1)-GFP expressing mice. These supporting cells retain the ability to divide and appear to be able to adopt the phenotype of hair cells in culture [77••]. Embryonic stem cells (ES cells) [78••], bone marrow mesenchymal stem cells [79], and adult mouse olfactory precursor cells [80] also can differentiate into cells that express proteins and other characteristics normally found in hair cells. Transplantation of ES-cell-derived cells into the early otocysts of chicken embryos resulted in the differentiation of more normal appearing hair cells, indicating that the inner ear microenvironment may facilitate hair cell differentiation [78]. Cells isolated from the inner ears of the mouse embryos were shown to aggregate in vitro and develop into the normal alternating patterns of hair cells surrounded by supporting cells, indicating that the cells are likely to express signals that normally direct pattern formation in vivo [77••, 81]. Elements of the Notch signaling pathway and the bHLH transcription factors are likely to contribute to the development of these patterns, and these in vitro systems hold the potential to reveal other signals as yet unidentified that may be important for regeneration.
Supporting cells derived from dissociated sheets of utricular sensory epithelium from embryonic chickens were found to undergo an epithelia-to-mesenchymal transition and proliferate for more than 20 population doublings when cultured on 2-D substrates. Those cultures can be frozen, shipped to other laboratories, thawed and greatly expanded as a source of hair cells. When dissociated and cultured in suspension, these cells aggregate and pass through a mesenchymal-to-epithelial transition that gives rise spherical epithelia which develop hair cells that are crowned by hair bundles, comprised of a single kinocilium and an asymmetric array of stereocilia [68]. Vials of these frozen cells provide the capacity to produce bona fide hair cells completely in vitro and may be useful for increasing the pace of various research approaches to treatments for hearing loss and other inner ear disorders.
CONCLUSION
Recent research has identified molecules that regulate the production and differentiation of hair cells during development. Such knowledge is likely to be important for the development of methods for stimulating hair cell regeneration in mammalian ears. Questions remain as to what limits hair cell regeneration in mammals, but recent cellular studies have revealed candidate mechanisms. Stem cells have been isolated from the inner ear and derived from other sources. These cells are likely to become important tools for inner ear research and they hold the potential for therapeutic uses if major challenges can be overcome.
References
- 1.Meyers JR, Corwin JT. Morphological Correlates of Regeneration and Repair. In: Salvi RJ, Fay RR, Popper AN, editors. Hair Cell Regeneration, Repair, and Protection. Springer; New York City: 2008. pp. 39–75. [Google Scholar]
- 2.Katayama A, Corwin JT. Cell production in the chicken cochlea. J Comp Neurol. 1989;281(1):129–35. doi: 10.1002/cne.902810110. [DOI] [PubMed] [Google Scholar]
- 3.Ruben RJ. Development of the inner ear of the mouse: a radioautographic study of terminal mitoses. Acta Otolaryngol. 1967;(Suppl 220):1–44. [PubMed] [Google Scholar]
- 4.Corwin JT, Cotanche DA. Regeneration of sensory hair cells after acoustic trauma. Science. 1988;240(4860):1772–4. doi: 10.1126/science.3381100. [DOI] [PubMed] [Google Scholar]
- 5.Ryals BM, Rubel EW. Hair cell regeneration after acoustic trauma in adult Coturnix quail. Science. 1988;240(4860):1774–6. doi: 10.1126/science.3381101. [DOI] [PubMed] [Google Scholar]
- 6.Mantela J, Jiang Z, Ylikoski J, et al. The retinoblastoma gene pathway regulates the postmitotic state of hair cells of the mouse inner ear. Development. 2005;132(10):2377–88. doi: 10.1242/dev.01834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sage C, Huang M, Karimi K, et al. Proliferation of functional hair cells in vivo in the absence of the retinoblastoma protein. Science. 2005;307(5712):1114–8. doi: 10.1126/science.1106642. [DOI] [PubMed] [Google Scholar]
- 8.Sage C, Huang M, Vollrath MA, et al. Essential role of retinoblastoma protein in mammalian hair cell development and hearing. Proc Natl Acad Sci U S A. 2006;103(19):7345–50. doi: 10.1073/pnas.0510631103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9•.Weber T, Corbett MK, Chow LM, et al. Rapid cell-cycle reentry and cell death after acute inactivation of the retinoblastoma gene product in postnatal cochlear hair cells. Proc Natl Acad Sci U S A. 2008;105(2):781–5. doi: 10.1073/pnas.0708061105. Inactive Rb in auditory HC of neonatal mice using a hair cell specific inducible Cre mouse line results in HCs entering the cell cycle, but they fail to divide and subsequently undergo apoptosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen P, Segil N. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development. 1999;126(8):1581–90. doi: 10.1242/dev.126.8.1581. [DOI] [PubMed] [Google Scholar]
- 11.Lowenheim H, Furness DN, Kil J, et al. Gene disruption of p27(Kip1) allows cell proliferation in the postnatal and adult organ of corti. Proc Natl Acad Sci U S A. 1999;96(7):4084–8. doi: 10.1073/pnas.96.7.4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laine H, Doetzlhofer A, Mantela J, et al. p19(Ink4d) and p21(Cip1) collaborate to maintain the postmitotic state of auditory hair cells, their codeletion leading to DNA damage and p53-mediated apoptosis. J Neurosci. 2007;27(6):1434–44. doi: 10.1523/JNEUROSCI.4956-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13•.Minoda R, Izumikawa M, Kawamoto K, et al. Manipulating cell cycle regulation in the mature cochlea. Hear Res. 2007;232(1–2):44–51. doi: 10.1016/j.heares.2007.06.005. p27Kip1 was shown to be expressed in non-sensory cells around the mature guinea pig organ of Corti, and overexpression of SKP2 alone leads to their proliferation. Overexpression of SKP2 in combination with Atoh1 resulted in ectopic hair cell production. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woods C, Montcouquiol M, Kelley MW. Math1 regulates development of the sensory epithelium in the mammalian cochlea. Nat Neurosci. 2004;7(12):1310–8. doi: 10.1038/nn1349. [DOI] [PubMed] [Google Scholar]
- 15.Bermingham NA, Hassan BA, Price SD, et al. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999;284(5421):1837–41. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- 16••.Millimaki BB, Sweet EM, Dhason MS, Riley BB. Zebrafish atoh1 genes: classic proneural activity in the inner ear and regulation by Fgf and Notch. Development. 2007;134(2):295–305. doi: 10.1242/dev.02734. Two atoh1 proteins necessary for HC development were expressed in Zebrafish, and one is involved in establishment of prosensory patches while the other in HC fate determination. Notch and FGF signaling appears to regulate atoh1 genes together with other transcription factors such as foxi1, pax8 and dlx. [DOI] [PubMed] [Google Scholar]
- 17.Chen P, Johnson JE, Zoghbi HY, Segil N. The role of Math1 in inner ear development: Uncoupling the establishment of the sensory primordium from hair cell fate determination. Development. 2002;129(10):2495–505. doi: 10.1242/dev.129.10.2495. [DOI] [PubMed] [Google Scholar]
- 18.Lanford PJ, Shailam R, Norton CR, et al. Expression of Math1 and HES5 in the cochleae of wildtype and Jag2 mutant mice. J Assoc Res Otolaryngol. 2000;1(2):161–71. doi: 10.1007/s101620010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matei V, et al. Smaller inner ear sensory epithelia in Neurog 1 null mice are related to earlier hair cell cycle exit. Dev Dyn. 2005;234(3):633–50. doi: 10.1002/dvdy.20551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zine A, et al. Hes1 and Hes5 activities are required for the normal development of the hair cells in the mammalian inner ear. J Neurosci. 2001;21(13):4712–20. doi: 10.1523/JNEUROSCI.21-13-04712.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pujades C, Kamaid A, Alsina B, Giraldez F. BMP-signaling regulates the generation of hair-cells. Dev Biol. 2006;292(1):55–67. doi: 10.1016/j.ydbio.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Stone JS, Shang JL, Tomarev S. Expression of Prox1 defines regions of the avian otocyst that give rise to sensory or neural cells. J Comp Neurol. 2003;460(4):487–502. doi: 10.1002/cne.10662. [DOI] [PubMed] [Google Scholar]
- 23•.Cafaro J, Lee GS, Stone JS. Atoh1 expression defines activated progenitors and differentiating hair cells during avian hair cell regeneration. Dev Dyn. 2007;236(1):156–70. doi: 10.1002/dvdy.21023. In chicks, Atoh1 is not expressed in undamaged, quiescent basilar papilla but is expressed in some cells in the utricle. Atoh1 is upregulated after ototoxic damage in both cochlear and utricular sensory epithelia and may have potential role in determining hair cell fate. [DOI] [PubMed] [Google Scholar]
- 24.Jones JM, Montcouquiol M, Dabdoub A, et al. Inhibitors of differentiation and DNA binding (Ids) regulate Math1 and hair cell formation during the development of the organ of Corti. J Neurosci. 2006;26(2):550–8. doi: 10.1523/JNEUROSCI.3859-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25••.Raft S, Koundakjian EJ, Quinones H, et al. Cross-regulation of Ngn1 and Math1 coordinates the production of neurons and sensory hair cells during inner ear development. Development. 2007;134(24):4405–15. doi: 10.1242/dev.009118. Utricular and saccular epithelia were shown to derive from Ngn-expressing otocytic cells, and Ngn1 and Atoh1 were shown to negatively regulate each other to generate neurons or sensory cells, respectively. Moreover, Ngn1 negatively autoregulates itself through notch signaling and Math1 directly autoregulates itself during development. [DOI] [PubMed] [Google Scholar]
- 26•.Scheffer D, Sage C, Corey D, Pingault V. Gene expression profiling identifies Hes6 as a transcriptional target of ATOH1 in cochlear hair cells. FEBS Lett. 2007;581(24):4651–6. doi: 10.1016/j.febslet.2007.08.059. This paper shows that Atoh1 can bind to the Hes6 promoter in vitro and that Hes6 expression during development parallels Atoh1, suggesting that Atoh1 may regulate Hes6 expression. [DOI] [PubMed] [Google Scholar]
- 27.Zheng JL, Gao WQ. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat Neurosci. 2000;3(6):580–6. doi: 10.1038/75753. [DOI] [PubMed] [Google Scholar]
- 28.Shou J, Zheng JL, Gao WQ. Robust generation of new hair cells in the mature mammalian inner ear by adenoviral expression of Hath1. Mol Cell Neurosci. 2003;23(2):169–79. doi: 10.1016/s1044-7431(03)00066-6. [DOI] [PubMed] [Google Scholar]
- 29.Izumikawa M, Minoda R, Kawamoto K, et al. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med. 2005;11(3):271–6. doi: 10.1038/nm1193. [DOI] [PubMed] [Google Scholar]
- 30.Staecker H, Praetorius M, Baker K, Brough DE. Vestibular hair cell regeneration and restoration of balance function induced by math1 gene transfer. Otol Neurotol. 2007;28(2):223–31. doi: 10.1097/MAO.0b013e31802b3225. [DOI] [PubMed] [Google Scholar]
- 31.Brooker R, Hozumi K, Lewis J. Notch ligands with contrasting functions: Jagged1 and Delta1 in the mouse inner ear. Development. 2006;133(7):1277–86. doi: 10.1242/dev.02284. [DOI] [PubMed] [Google Scholar]
- 32.Daudet N, Lewis J. Two contrasting roles for Notch activity in chick inner ear development: specification of prosensory patches and lateral inhibition of hair-cell differentiation. Development. 2005;132(3):541–51. doi: 10.1242/dev.01589. [DOI] [PubMed] [Google Scholar]
- 33.Kiernan AE, Ahituv N, Fuchs H, et al. The Notch ligand Jagged1 is required for inner ear sensory development. Proc Natl Acad Sci U S A. 2001;98(7):3873–8. doi: 10.1073/pnas.071496998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kiernan AE, Xu J, Gridley T. The Notch ligand JAG1 is required for sensory progenitor development in the mammalian inner ear. PLoS Genet. 2006;2(1):e4. doi: 10.1371/journal.pgen.0020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haddon C, Jiang YJ, Smithers L, Lewis J. Delta-Notch signalling and the patterning of sensory cell differentiation in the zebrafish ear: evidence from the mind bomb mutant. Development. 1998;125(23):4637–44. doi: 10.1242/dev.125.23.4637. [DOI] [PubMed] [Google Scholar]
- 36.Kiernan AE, Cordes R, Kopan R, et al. The Notch ligands DLL1 and JAG2 act synergistically to regulate hair cell development in the mammalian inner ear. Development. 2005;132(19):4353–62. doi: 10.1242/dev.02002. [DOI] [PubMed] [Google Scholar]
- 37.Lanford PJ, Lan Y, Jiang R, et al. Notch signalling pathway mediates hair cell development in mammalian cochlea. Nat Genet. 1999;21(3):289–92. doi: 10.1038/6804. [DOI] [PubMed] [Google Scholar]
- 38.Lewis J. Notch signalling and the control of cell fate choices in vertebrates. Semin Cell Dev Biol. 1998;9(6):583–9. doi: 10.1006/scdb.1998.0266. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Z, Nadeau P, Song W, et al. Presenilins are required for gamma-secretase cleavage of beta-APP and transmembrane cleavage of Notch-1. Nat Cell Biol. 2000;2(7):463–5. doi: 10.1038/35017108. [DOI] [PubMed] [Google Scholar]
- 40.Zine A, Van De Water TR, de Ribaupierre F. Notch signaling regulates the pattern of auditory hair cell differentiation in mammals. Development. 2000;127(15):3373–83. doi: 10.1242/dev.127.15.3373. [DOI] [PubMed] [Google Scholar]
- 41•.Yamamoto N, Tanigaki K, Tsuji M, et al. Inhibition of Notch/RBP-J signaling induces hair cell formation in neonate mouse cochleas. J Mol Med. 2006;84(1):37–45. doi: 10.1007/s00109-005-0706-9. This article shows induction of ectopic myosin7a-positive cells in the region lateral to hair cells in the cochlea of neonatal mice by treatment with gamma-secretase inhibitors and by disruption of Rbpsuh gene to a lesser extent. [DOI] [PubMed] [Google Scholar]
- 42•.Takebayashi S, Yamamoto N, Yabe D, et al. Multiple roles of Notch signaling in cochlear development. Dev Biol. 2007;307(1):165–78. doi: 10.1016/j.ydbio.2007.04.035. γ-secretase inhibitors were shown to increase hair cell number in a proliferation-independent manner and to decreased Hes 1/5 mRNA levels and increase Atoh1 levels during cochlear development (E14.5–E17.5). γ-secretase inhibitors also produced an increase in proliferation of progenitor cells. [DOI] [PubMed] [Google Scholar]
- 43.Stone JS, Rubel EW. Delta1 expression during avian hair cell regeneration. Development. 1999;126(5):961–73. doi: 10.1242/dev.126.5.961. [DOI] [PubMed] [Google Scholar]
- 44••.Ma EY, Rubel EW, Raible DW. Notch signaling regulates the extent of hair cell regeneration in the zebrafish lateral line. J Neurosci. 2008;28(9):2261–73. doi: 10.1523/JNEUROSCI.4372-07.2008. This article shows that mRNA levels of atoh1a, notch 3 and delta A are increased during hair cell regeneration in the Zebrafish lateral line. Notch inhibition during regeneration using γ-secretase inhibitors results in increased delta A expression, and excess regenerated hair cells via increased supporting cell division, but Notch inhibition has no effect in undamaged tissue. A model where Notch signaling regulates the proliferation of hair cell progenitors is discussed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45•.Hori R, Nakagawa T, Sakamoto T, et al. Pharmacological inhibition of Notch signaling in the mature guinea pig cochlea. Neuroreport. 2007;18(18):1911–4. doi: 10.1097/WNR.0b013e3282f213e0. This article shows upregulation of notch and jagged1 in aminoglycoside-damaged auditory epithelia and generation of ectopic hair cells in damaged cochlea of adult guinea pigs treated with γ-secretase inhibitors in vivo. [DOI] [PubMed] [Google Scholar]
- 46.Whitfield TT, Hammond KL. Axial patterning in the developing vertebrate inner ear. Int J Dev Biol. 2007;51(6–7):507–20. doi: 10.1387/ijdb.072380tw. [DOI] [PubMed] [Google Scholar]
- 47.Kelley MW. Regulation of cell fate in the sensory epithelia of the inner ear. Nat Rev Neurosci. 2006;7(11):837–49. doi: 10.1038/nrn1987. [DOI] [PubMed] [Google Scholar]
- 48.Schneider-Maunoury S, Pujades C. Hindbrain signals in otic regionalization: walk on the wild side. Int J Dev Biol. 2007;51(6–7):495–506. doi: 10.1387/ijdb.072345ss. [DOI] [PubMed] [Google Scholar]
- 49••.Hawkins RD, Bashiardes S, Powder KE, et al. Large scale gene expression profiles of regenerating inner ear sensory epithelia. PLoS ONE. 2007;2(6):e525. doi: 10.1371/journal.pone.0000525. The changes in gene expression of over 1700 transcription factors in the sensory epithelium of wounded chicken cochleas and utricles were quantified with microarrays. The sensory epithelium from both of these organs was explanted and cultured, and hair cells were specifically wounded with either 1 mM neomycin or laser microbeam ablation. The cultures were then collected for microarray analysis at various timepoints after the damage regimen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50••.Sajan SA, Warchol ME, Lovett M. Toward a systems biology of mouse inner ear organogenesis: gene expression pathways, patterns and network analysis. Genetics. 2007;177(1):631–53. doi: 10.1534/genetics.107.078584. Differential expression of genes in the developing inner ear organs of mice aged E9–E15 was monitored with microarray analysis. Gene expression patterns specific to both tissue and developmental stage were observed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ambros V, Lee RC. Identification of microRNAs and other tiny noncoding RNAs by cDNA cloning. Methods Mol Biol. 2004;265:131–58. doi: 10.1385/1-59259-775-0:131. [DOI] [PubMed] [Google Scholar]
- 52.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 53.Tanaka EM, Weidinger G. Micromanaging regeneration. Genes Dev. 2008;22(6):700–5. doi: 10.1101/gad.1660508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yin VP, Thomson JM, Thummel R, et al. Fgf-dependent depletion of microRNA-133 promotes appendage regeneration in zebrafish. Genes Dev. 2008;22(6):728–33. doi: 10.1101/gad.1641808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang Z, Wu J. MicroRNAs and regenerative medicine. DNA Cell Biol. 2007;26(4):257–64. doi: 10.1089/dna.2006.0548. [DOI] [PubMed] [Google Scholar]
- 56.Tsonis PA, Call MK, Grogg MW, et al. MicroRNAs and regeneration: Let-7 members as potential regulators of dedifferentiation in lens and inner ear hair cell regeneration of the adult newt. Biochem Biophys Res Commun. 2007;362(4):940–5. doi: 10.1016/j.bbrc.2007.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57••.Kesser BW, Hashisaki GT, Fletcher K, et al. An in vitro model system to study gene therapy in the human inner ear. Gene Ther. 2007;14(15):1121–31. doi: 10.1038/sj.gt.3302980. Sensory epithelia from human vestibular organs were harvested and maintained in viably in culture for up to 5 days. Adenovirus can transfect both hair cells and supporting cells and rates of transfection depended on viral titer. Expression of deafness genes was successfully accomplished in at least 10 % of cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hildebrand MS, Newton SS, Gubbels SP, et al. Advances in molecular and cellular therapies for hearing loss. Mol Ther. 2008;16(2):224–36. doi: 10.1038/sj.mt.6300351. [DOI] [PubMed] [Google Scholar]
- 59.Gu R, Marchionni M, Corwin JT. Glial growth factor enhances supporting cell proliferation in rodent vestibular epithelia cultured in isolation. Soc Neurosci Abstr. 1996;21 [Google Scholar]
- 60.Gu R, Marchionni M, Corwin JT. Assoc Res Otolaryngol Abstr. Vol. 20 1997. Age-related decreases in proliferation within isolated mammalian vestibular epithelia cultured in control and glial growth factor 2 medium. [Google Scholar]
- 61••.Gu R, Montcouquiol M, Marchionni M, Corwin JT. Proliferative responses to growth factors decline rapidly during postnatal maturation of mammalian hair cell epithelia. Eur J Neurosci. 2007;25(5):1363–72. doi: 10.1111/j.1460-9568.2007.05414.x. >40% of supporting cell nuclei are BrdU-positive in macular explants from neonate rat utricles when cultured for 72 hr with BrdU and growth factors. This proliferative response declines postnatally so that sometime between P27 and P35, no labeling is observed in supporting cell nuclei. [DOI] [PubMed] [Google Scholar]
- 62.Montcouquiol M, Corwin JT. Brief treatments with forskolin enhance s-phase entry in balance epithelia from the ears of rats. J Neurosci. 2001;21(3):974–82. doi: 10.1523/JNEUROSCI.21-03-00974.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Montcouquiol M, Corwin JT. Intracellular signals that control cell proliferation in mammalian balance epithelia: key roles for phosphatidylinositol-3 kinase, mammalian target of rapamycin, and S6 kinases in preference to calcium, protein kinase C, and mitogen-activated protein kinase. J Neurosci. 2001;21(2):570–80. doi: 10.1523/JNEUROSCI.21-02-00570.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zheng JL, Frantz G, Lewis AK, et al. Heregulin enhances regenerative proliferation in postnatal rat utricular sensory epithelium after ototoxic damage. J Neurocytol. 1999;28(10–11):901–12. doi: 10.1023/a:1007078307638. [DOI] [PubMed] [Google Scholar]
- 65.Zheng JL, Gao WQ. Analysis of rat vestibular hair cell development and regeneration using calretinin as an early marker. J Neurosci. 1997;17(21):8270–82. doi: 10.1523/JNEUROSCI.17-21-08270.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66••.Davies D, Magnus C, Corwin JT. Developmental changes in cell-extracellular matrix interactions limit proliferation in the mammalian inner ear. Eur J Neurosci. 2007;25(4):985–98. doi: 10.1111/j.1460-9568.2007.05355.x. In macular explants taken from utricles of mice of various ages, the ability to spread over 72 hr in culture significantly declined from E18 to P6. The loss in spreading directly correlated with a decline in proliferation. These changes were paralleled by changes in integrin expression, and pharmalogical treatments disrupting integrins in the older tissue resulted in increased spreading and proliferation. [DOI] [PubMed] [Google Scholar]
- 67••.Meyers JR, Corwin JT. Shape change controls supporting cell proliferation in lesioned mammalian balance epithelium. J Neurosci. 2007;27(16):4313–25. doi: 10.1523/JNEUROSCI.5023-06.2007. Excision wounds were made in utricles from mice of different ages which were placed in whole organ culture for 72 hrs. Wounds in utricles from E18 mice closed in <24 hrs, while those in 2-week-old mice remained open at 48 hrs. The spread area of individual supporting cells covering lesions in these treated cultures directly correlated with their ability for S-phase entry, with 85% of those cells that had spread >300 μm2 entering S-phase. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68••.Hu Z, Corwin JT. Inner ear hair cells produced in vitro by a mesenchymal-to-epithelial transition. Proc Natl Acad Sci U S A. 2007;104(42):16675–80. doi: 10.1073/pnas.0704576104. Bona fide hair cells were produced in vitro when purified supporting cells from embryonic chicken utricles were continuously passaged and then allowed to form spheres. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim YH, Raphael Y. Cell division and maintenance of epithelial integrity in the deafened auditory epithelium. Cell Cycle. 2007;6(5):612–9. doi: 10.4161/cc.6.5.3929. [DOI] [PubMed] [Google Scholar]
- 70.Izumikawa M, Batts SA, Miyazawa T, et al. Response of the flat cochlear epithelium to forced expression of Atoh1. Hear Res. 2008;240(1–2):52–6. doi: 10.1016/j.heares.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Watt FM, Jordan PW, O’Neill CH. Cell shape controls terminal differentiation of human epidermal keratinocytes. Proc Natl Acad Sci U S A. 1988;85(15):5576–80. doi: 10.1073/pnas.85.15.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72•.Hordichok AJ, Steyger PS. Closure of supporting cell scar formations requires dynamic actin mechanisms. Hear Res. 2007;232(1–2):1–19. doi: 10.1016/j.heares.2007.06.011. The cytoskeleton in cells of the Bullfrog saccule treated with gentamycin was perturbed with actin, mircotubule, and myosin inhibitors. Microtubule and myosin disruption agents enhanced extrusion of hair cells while actin inhibitors had the opposite effect. Disruption of actin and myosin slowed down scar formation, but microtubule disruption had no effect. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li H, Liu H, Heller S. Pluripotent stem cells from the adult mouse inner ear. Nat Med. 2003;9(10):1293–9. doi: 10.1038/nm925. [DOI] [PubMed] [Google Scholar]
- 74.Zhang Y, Zhai SQ, Shou J, et al. Isolation, growth and differentiation of hair cell progenitors from the newborn rat cochlear greater epithelial ridge. J Neurosci Methods. 2007;164(2):271–9. doi: 10.1016/j.jneumeth.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 75.Oshima K, Grimm CM, Corrales CE, et al. Differential distribution of stem cells in the auditory and vestibular organs of the inner ear. J Assoc Res Otolaryngol. 2007;8(1):18–31. doi: 10.1007/s10162-006-0058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang Z, Jiang H, Yan Y, et al. Characterization of proliferating cells from newborn mouse cochleae. Neuroreport. 2006;17(8):767–71. doi: 10.1097/01.wnr.0000215781.22345.8b. [DOI] [PubMed] [Google Scholar]
- 77••.White PM, Doetzlhofer A, Lee YS, et al. Mammalian cochlear supporting cells can divide and trans-differentiate into hair cells. Nature. 2006;441(7096):984–7. doi: 10.1038/nature04849. Purified post-mitotic supporting cells were isolated from the postnatal mouse cochlea using FACS. These supporting cells retain the ability to divide and trans-differentiate into cells expressing hair cell specific proteins in culture. Downregulation of the cyclin-dependent kinase inhibibor, p27 kip1, is related to the age-dependent changes in supporting cell proliferation capacity. [DOI] [PubMed] [Google Scholar]
- 78••.Li H, Roblin G, Liu H, Heller S. Generation of hair cells by stepwise differentiation of embryonic stem cells. Proc Natl Acad Sci U S A. 2003;100(23):13495–500. doi: 10.1073/pnas.2334503100. Stem cells from the adult mouse utricular sensory epithelium were isolated and characterized. These stem cells are able to self-renew and differentiate into a variety of cell types, including cells representative of ectodermal, endodermal and mesodermal lineages. These sphere-forming stem cells express marker genes of the developing inner ear and differentiate into hair cell-like cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jeon SJ, Oshima K, Heller S, Edge AS. Bone marrow mesenchymal stem cells are progenitors in vitro for inner ear hair cells. Mol Cell Neurosci. 2007;34(1):59–68. doi: 10.1016/j.mcn.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Doyle KL, Kazda A, Hort Y, et al. Differentiation of adult mouse olfactory precursor cells into hair cells in vitro. Stem Cells. 2007;25(3):621–7. doi: 10.1634/stemcells.2006-0390. [DOI] [PubMed] [Google Scholar]
- 81.Bianchi LM, Person AL, Penney EB. Embryonic inner ear cells reaggregate into specific patterns in vitro. J Assoc Res Otolaryngol. 2002;3(4):418–29. doi: 10.1007/s101620020042. [DOI] [PMC free article] [PubMed] [Google Scholar]