Abstract
Transplantation of neural tissue has been attempted as a treatment method for neurodegenerative disorders. Grafted neurons survive to a lesser extent into middle-aged or aged hosts, and survival rates of < 10% of grafted neurons is common. Antioxidant diets, such as blueberry, can exert powerful effects on developing neurons and blood vessels in vitro, but studies are lacking that examine the effects of these diets on transplanted tissues. In this study, we examined the effects of a blueberry diet on survival, growth, and vascularization of fetal hippocampal tissue to the anterior chamber of the eye of young or middle-aged female rats. Previous work from our group showed significant increase in neuronal survival and development with blueberry diet in grafts. However, the effects of antioxidant diet on vascular development in grafts have not been explored previously. The age of the host affected individual vessel morphology in that aged hosts contained grafts with thick, undeveloped walls, and wider lumen. The blood–brain barrier also appeared to be affected by the age of the host. The blueberry diet did not affect vessel morphology or density of vessel-associated protein markers but gave rise to significantly increased growth capacity, cytoarchitecture, and the final size of hippocampal grafts.
Keywords: aging, angiogenesis, development, hippocampal formation, transplantation
Introduction
Anecdotal evidence that dietary intake of fruits and vegetables improves overall health in humans has recently gained support in dietary epidemiologic studies of antioxidants such as green tea, red wine, and the so-called Mediterranean diet (Hardy et al, 2003). Recent animal studies of dietary supplements have corroborated these observations and have shown that nutritional compounds can have profound effects on physiologic processes such as cardiovascular health (Stoclet et al, 2004), cancer prevention (Dulak, 2005), and neurologic performance (Stoclet et al, 2004; Ramassamy, 2006). One such dietary substance that has been shown to have direct effects on the central nervous system is blueberry extract (Joseph et al, 2005). Dietary supplementation with blueberry extract has been shown to prevent and reverse memory loss in aged rats and mice (Joseph et al, 1999, 2005; Andres-Lacueva et al, 2005), to improve functional recovery after experimentally induced Parkinson’s disease (Stromberg et al, 2005), to prevent behavioral deficits in an animal model of Alzheimer’s disease (Joseph et al, 2003), and to improve behavioral recovery and cellular survival after stroke (Sweeney et al, 2002; Wang et al, 2005).
The effects of dietary blueberry supplementation on neuronal survival after stroke have been attributed to the antioxidant or anti-inflammatory actions of blueberry compounds. However, the influence of dietary blueberry supplementation on angiogenesis or vascularization of neural tissue has not been fully explored. Other nutritional substances such as resveratrol, green tea, and the spice curcumin have been shown to have differential effects on angiogenesis, depending on the type of tissue studied (Dulak, 2005). Dietary administration of resveratrol, for example, will inhibit angiogenesis and retard the growth of ovarian cancer cells (Cao et al, 2004), but will also enhance neovascularization in the injured heart (Kaga et al, 2005). Dietary blueberry supplementation has also been shown to inhibit angiogenesis in a human skin cell line (Roy et al., 2002; Bagchi et al, 2004), but the effects of dietary blueberry supplementation on vascularization of the central nervous system is not known.
One technique that lends itself well to studies of vascularization of neural tissue is transplantation. Vascularization of neural tissue transplants is instrumental for transplant survival, and can be influenced by factors such as age of host (Eriksdotter-Nilsson and Olson, 1989), growth factor levels (Calza et al, 2001), and expression of the angiogenic factor vascular endothelial growth factor (Lee et al, 2007). This study uses an intraocular neural transplantation technique in which graft vascularization has been extensively characterized (Eriksdotter-Nilsson and Olson, 1989; Granholm et al, 1996; Tuba and Kalman, 1997). Intraocular transplantation exploits the anterior eye chamber of adult rats for the growth and maturation of discrete areas of the fetal brain. Neural grafts to the anterior eye chamber of adult host animals attach to and become vascularized by the host iris within 2 weeks after transplantation (Granholm et al, 1996), providing continual vascular support of developing tissue. Intraocular graft development and maturation parallels that of corresponding brain areas in situ (Olson et al, 1977, 1983; Granholm, 1991). Intraocular grafts will grow and survive in the anterior eye chamber for as long as the host animal is alive, and growth and vascularization of neural intraocular grafts can be readily monitored over time through the translucent cornea of host animals (Olson et al, 1983). One overwhelming advantage of intraocular transplantation over traditional intracranial methods is the preservation of tissue cytoarchitecture and organization in grafted tissue. Intraocular transplantation of the hippocampal formation, for example, maintains the intact cellular organization of this multilayered brain region (Olson et al, 1977). As neurons in the hippocampus belong to distinct populations with specific anatomic distributions, transplantation of the fetal hippocampal formation allows for a more detailed study of cell survival after transplantation.
We have previously shown that dietary supplementation with blueberry extract will improve the growth and organization of intraocular grafts of fetal hippocampal formation to aged hosts (Willis et al, 2005). Specifically, we found that the maturation of specific hippocampal cell layers was enhanced with blueberry supplementation. Although hippocampal grafts to aged control hosts exhibited an unorganized morphology, with lack of pyramidal neurons and missing distinct neuronal cell layers, the blueberry-supplemented aged hosts exhibited organotypical cell layers and developed markers characteristic of the mature hippocampus in situ (calbindin, NeuN, and Doublecortin; see Willis et al, 2005). Further, in our previous study, we found that Doublecortin, a marker for neurons undergoing neurogenesis, was abundant in grafts to aged control hosts, suggesting increased level of immaturity, whereas Doublecortin was present only in few neurons that were incorporated in the dentate cell layers in blueberry-treated grafts. Other markers for mature neurons (NeuN and calbindin) also showed a high level of organization in blueberry-treated hosts when compared with aged control grafts (Willis et al, 2005). Although some candidates for this strong enhancement of graft survival by blueberries were proposed in our previous publications, the biologic mechanisms are not known to date. As appropriate growth of neural transplants depends on tissue vascularization, this study explores the potential role of dietary blueberry supplementation in improving vascularization of fetal hippocampal intraocular grafts in young and aged hosts. Another goal of this study was to perform a pooled analysis of graft growth during different conditions (aged and young hosts) as well as different treatments (blueberry diet or isocaloric control diet) to determine whether there were reproducible effects of these two different conditions on graft growth and survival. Random effects statistical models were applied to examine transplant growth in oculo of transplants examined in five different experiments.
Materials and methods
Subjects
Young (5 to 6 months) and middle-aged (17 to 18 months) female Fisher 344 rats were used as recipients for intraocular grafts of fetal hippocampal formation. Fetal tissue donors were timed pregnant Fisher 344 dams (Harlan, Indianapolis, IN, USA) at embryonic day 18 (E18). The data used for the growth analysis (Figure 1) represent a pooled analysis of transplants in five different experiments, which were analyzed statistically to determine whether there were global effects of blueberry diet and/or age of host animals in terms of rate of growth (see Figure 1). The total number of animals for the growth analysis was 21 young animals (10 control; 11 treated) and 40 old animals (19 control; 21 treated) with bilateral intraocular transplants. A subset of these animals has been used previously for studies with neuronal markers (see Willis et al, 2005), and another subset is used in this study for vascular markers (see below). By combining data across experiments, we were able to explore global effects of blueberry treatment and age of recipient rats.
Figure 1.

Actual (A) and predicted (B) growth curves for the transplants from the four groups up to 12 weeks postgrafting. A pooled analysis was performed of transplant growth from five different experiments to determine potential interactions between growth, age of host, and treatment with blueberry diets (BB). As can be gleaned from these two curves, data showed that differences in graft growth were alleviated by blueberry treatment, suggesting that this antioxidant diet could have major effects on growth of CNS tissue in both young and aged recipients.
In this study, we used a total of 16 aged and 18 young host animals to examine vascular markers. One week before transplantation, the host animals were randomly divided into two groups receiving chow ad libitum: one that received control chow and one that received NIH-31 chow (Harlan Teklad, Madison, WI, USA) supplemented with 2% blueberry (Van Drunen Farms, Momence, IL, USA). Within both diet groups, subjects were further divided into either a short-term (1 to 2 weeks) or long-term (8 to 12 weeks) group, yielding four host animals per each treatment paradigm, except for the young control diet long-term group, which contained six host animals. The blueberry diet was prepared by homogenizing, centrifuging, and freeze-drying fresh blueberries (Vaccinium angustifolium). The blueberry was then added to the control diet in place of cornstarch, yielding a chow consisting of control diet plus blueberry at 2% (g per kg diet). The diets used in this study are isocaloric and have been used previously (Joseph et al, 1999, 2003; Willis et al, 2005); for additional information regarding dietary formulations see Joseph et al, 1999). The transplant recipients were maintained on the respective diets for the duration of the study. Weekly monitoring of animal weight and food consumption revealed no food preference or weight changes for each diet (Willis et al, 2005). Animals were kept on a standard 12:12-h light/dark cycle. All surgical procedures were performed according to the National Institutes of Health guidelines for animal use and were approved by the local Institutional Animal Care and Use Committee.
Dissection and Transplantation
Embryonic day 18 (E18) dams were euthanized with an overdose of halothane; fetuses were removed and placed on ice until dissection. The fetal brain was removed and hippocampal formation was dissected and cut into several pieces along the longitudinal axis, each piece containing CA1–CA3 and dentate gyrus, as described previously (Granholm, 1991). Recipient host animals were anesthetized with xylazine (Rompun, 12 mg/kg i.p.) and ketamine (Ketaset, 80 mg/kg i.p.). One drop of 1% atropine solution was placed on the cornea to dilate the pupil and facilitate grafting. Fetal hippocampal formation was inserted into the anterior eye chamber through a razor blade slit in the cornea using a modified syringe as described previously (Olson et al, 1983; Granholm, 1991). Each host contained one hippocampal graft in each eye, yielding eight transplants per group, except for the young control diet long-term group, which contained six hosts and 12 transplants.
Quantification of Graft Growth
Survival, vascularization, and growth of grafts in the anterior eye chamber were monitored at the time of transplantation and once a week until time of killing. Intraocular grafts are readily visible through the translucent cornea of hosts. Length and width measurements of grafts in lightly anesthetized hosts (halothane) were performed through the use of a dissecting microscope and a caliper with a millimeter scale. Graft area was calculated by multiplying length by width (Willis et al, 2005). Transplants from five individual experiments were assessed for growth and vascularization to determine whether there were global effects of age and treatment (blueberry in the diet) on hippocampal tissue growth.
Lipopolysaccharide Treatment
At 8 weeks after transplantation, two subjects from the young control diet group were subjected to lipopolysaccharide (LPS) exposure. Subjects were anesthetized with xylazine (Rompun, 12 mg/kg i.p.) and ketamine (Ketaset, 80 mg/kg i.p.). Lipopolysaccharide (2.5 mg/kg; Sigma-Aldrich, St Louis, MO, USA) was administered intravenously through the sublingual vein. Subjects were monitored continuously and killed 24 h after injection, and the tissues were harvested and processed as described below. Owing to the already compromised vascular tree in grafts in the aged host (see results), we did not carry out LPS treatment of transplants to aged hosts in this study.
Morphologic assessment of intraocular grafts
After either a short-term (1 to 2 weeks) or long-term (8 to 12 weeks; n = 8 for all groups) graft growth period, host animals were deeply anesthetized with halothane and transcardially perfused with 0.1 mol/L phosphate-buffered saline (PBS) followed by 4% paraformaldehyde. Intraocular grafts were removed and transferred to 4% paraformaldehyde for 24 h, followed by storage in 30% sucrose in PBS. Grafts were then sectioned to a thickness of 10 μm on a cryostat and then mounted directly on glass slides. Sections were mounted in such a way that each slide contained every 10th section throughout the entire graft. Hematoxylin and eosin (H&E) staining was performed on every 10th section to visualize not only general tissue morphology but also to identify the presence of infiltrating peripheral blood cells. Hematoxylin and eosin staining was performed by first incubating slides in xylene, followed by decreasing concentrations of ethanol. Slides were then incubated in Mayer’s hemalum for 4 mins, followed by washing in tap water and eosin incubation for 1 min. Finally, slides were differentiated in 95% ethanol, washed in absolute ethanol and xylene, and then coverslipped in Permount (Fisher Scientific, Pittsburgh, PA, USA).
Immunohistochemistry
Specific detection of blood vessels and peripheral immune cells was achieved through the use of fluorescent immunohistochemistry. Briefly, slides were first washed with PBS, followed by incubation with 0.5% Triton X-100 in PBS for 1 h to make membranes permeable. After another PBS wash (10 mins), the slides were incubated with blocking serum for 1 h in room temperature and finally incubated with primary antibody solution (laminin: 1:500, Abcam Inc., Cambridge, MA, USA; Glut1: 1:500, Abcam; ED1 (CD68): 1:1,000, Millipore, Billerica, MA, USA; CD3: 1:1,000, BD Biosciences, San Jose, CA, USA) in PBS overnight at 4°C. After three washes in PBS (3 × 5 mins), the sections were incubated with fluorescent secondary antibody (Invitrogen, Carlsbad, CA, USA). A secondary goat anti-rat IgG conjugated with rhodamine (1:30; Sigma-Aldrich) was used on separate sections to examine the functionality of the blood–brain barrier (BBB) in grafted tissues (see Figure 7). Sections were cover-slipped with polyvinyl alcohol mounting medium with Dabco (PVA/DABCO) (Sigma-Aldrich) solution and examined in a Nikon Eclipse E-600 microscope.
Figure 7.

(A–D) Immunofluorescence showing anti-rat IgG conjugated with rhodamine in a graft from an aged blueberry-treated (A), aged control (B), young LPS (C), and young control (D) host. (E) Percentage of graft area expressing Glut1 in hippocampal grafts from young and aged hosts maintained on a control or blueberry-supplemented diet at 1 to 2 weeks and 8 to 12 weeks after transplantation, and young hosts treated with LPS (n = 8 for all dietary groups; n = 4 for LPS). Note that there was a significant increase in Glut1 immunoreactive staining in aged versus young recipients, as well as in the LPS-treated grafts in young hosts. (F) Densitometry showing significant increase in IgG staining in the parenchyma from the aged control compared to young control graft (*P < 0.05). The aged blueberry group was not significantly different from the young control group.
Quantification of Cellular Markers
Quantification of peripheral blood components in H&E-stained sections was performed with the aid of the optical fractionator probe (StereoInvestigator; Microbrightfield, MBF Bioscience, Williston, VT, USA) and a computer-assisted image analysis system including a Nikon Eclipse E-600 microscope coupled to a Prior H128 motorized stage and an Olympus 750 video camera system. Infiltrating immune cells were identified on the basis of morphology and H&E staining pattern. Every 10th section throughout each graft was outlined under low magnification, and the number of cells was measured using a systematic random design of disector counting frames. A × 60 objective lens with a 1.4 numerical aperture was used to count individual cells within the counting frames. The number of cells per millimeter of graft area was obtained by dividing the total number of cells per graft by the area in millimeters in which the cells had been counted (no. of cells/(area of counting frame × no. of counting frames) = no. of cells per mm2). Glucose transporter 1 (Glut1) expression in grafts was quantified by densitometry. Briefly, background fluorescence was subtracted and the gray scale was adjusted using density slicing. This approach captures all labeled profiles above a threshold density and interactively discriminates them from density values below the threshold. Computer-assisted imaging software (Scion Corporation, Frederick, MD, USA) then automatically measures the mean optical density and the number of pixels per area of the extracted profiles in the outlined regions. Two parameters were obtained from this procedure: the area covered by the specific profile population (graft area) and the mean density of the specific profile (Glut1 expression). The percentage of graft area expressing Glut1 was obtained by dividing the area of blood vessels expressing Glut1 by the total graft area.
Statistics
The number of infiltrating peripheral immune cells and density of staining of Glut1 was analyzed with analysis of variance (Statview Version 5.0.1, SAS Institute Inc., Cary, NC, USA). To examine graft growth longitudinally, we pooled the results from five different experiments, which varied in terms of the groups that were examined as well as the timing of the follow-up period. Normally, the presence of different follow-up intervals would pose problems for traditional repeated-measures analysis of variance. In this study, we, therefore, applied different random effects models (Singer, 2003; Little et al, 2006) to the analysis of change in graft growth for the two age groups and two treatment groups to examine possible global effects of host age and blueberry diet. Although this method provides the same basic information as traditional repeated-measures analysis, in terms of longitudinal changes over time, there are a number of advantages to this method of data analysis. Chief among the advantages of this method of data analysis is the ability to adapt to variations in the follow-up interval as well as the ability to include subjects for whom complete data are not available, two features that are present in the current analysis.
Results
Dietary Blueberry Supplementation Improved Graft Growth
The observed means for the graft growth per postgrafting time (expressed in weeks) are shown in Figure 1A. These graft growth curves are summarized from five different experiments, showing that there was a reproducible increase in transplant growth with blueberry treatment, both in young and in aged graft recipients. As seen in the figure, each of the groups exhibited growth over the course of the 13-week follow-up period after grafting. However, the figure also shows that the graft growth was not purely linear. As a result, the initial random effects model estimated linear, quadratic, and cubic effects for time. The results indicated that all three of these time effects were statistically significant (linear: estimate = 1.63, s.e. = 0.13, P < 0.001; quadratic: estimate = −0.16, s.e. = 0.03, P < 0.001; cubic: estimate = 0.01, s.e. = 0.001, P < 0.001). We then took this basic model and added interactions between each of the time effects (linear, quadratic, and cubic) and age (young, old), treatment (control, blueberry), as well as a three-way interaction with age and treatment. For the linear effect of time, there was a statistically significant interaction with age (estimate = −1.06, s.e. = 0.34, P < 0.01) but there were no effects for the treatment interaction or the three-way interaction with age and treatment. For the quadratic effect of time, the interaction with age was statistically significant (estimate = 0.18, s.e. = 0.07, P < 0.05), as was the interaction with age and treatment (estimate = −0.19, s.e. = 0.10, P < 0.05). The interaction between quadratic time and treatment was not statistically significant. Finally, for the cubic time effect, the interaction with age was statistically significant (estimate = −0.01, s.e. = 0.003, P < 0.05), as was the three-way interaction (estimate = 0.01, s.e. = 0.005, P < 0.05), but the interaction with treatment was not statistically significant.
To decompose the age by treatment interactions with time, we ran time by age models that were stratified by treatment status (control, blueberry; ‘BB’). The predicted means for the four different groups (young control, young BB, old control, and old BB) are shown in Figure 1B. For the control condition, the interactions between time and age were statistically significant for linear time (estimate = −1.02, s.e. = 0.30, P < 0.001), quadratic time (estimate = 0.18, s.e. = 0.06, P < 0.01), and cubic time (estimate = −0.008, s.e. = 0.003, P < 0.05). These results indicated that there were significant age differences in the time effects for the graft growth, and Figure 1 shows that the young control group exhibited greater growth than the old-control group. For the treatment group, none of the corresponding interactions between linear time (estimate = −0.41, s.e. = 0.39, P = 0.293), quadratic time (estimate = −0.002, s.e. = 0.08, P = 0.984), or cubic time (estimate = 0.002, s.e. = 0.004, P = 0.628) were statistically significant. In this case, there were no age differences in graft growth rate across the two age groups in the treated condition. Taken together, the results of this analysis show that grafts in young recipients showed greater graft growth, but only when grafts in young versus old recipients in the control condition were compared, because blueberry-treated grafts in aged recipients grew as well or even better than grafts in young control recipients. Thus, as can be seen from the predicted growth patterns in Figure 1B, there were no age differences in graft growth rate when all groups were compared, suggesting that the blueberry treatment had alleviated effects of age on growth of intraocular grafts.
Dietary Blueberry Supplementation Improved Graft Organization
Considering that the fetal hippocampal formation is a highly organized structure with cellular layers corresponding to the dentate gyrus, CA1, and CA3 and that this cellular organization is preserved in intraocular grafts (Granholm, 1991), we assessed cellular organization of hippocampal grafts by means of H&E staining. Hematoxylin and eosin staining of hippocampal intraocular grafts harvested at 1 to 2 weeks revealed that grafts from young hosts exhibited a highly organized cytoarchitecture (Figures 2A and 2B) composed of distinct cellular layers. Dietary supplementation of aged hosts with 2% blueberry imparted a similar cellular organization as that seen in young hosts (Figure 2F). Grafts from aged hosts maintained on a control diet, however, exhibited a perturbed cellular organization (Figure 2E), with cells of distinct morphology present but not integrated into specific patterns or layers. This difference in cellular organization extended to grafts harvested at 8 to 12 weeks: grafts from young hosts of both diets (Figures 3A to 3D) and aged hosts maintained on a blueberry diet (Figures 3F and 3H) exhibited a cellular organization typical of intraocular hippocampal grafts in young recipients (Granholm, 1991), whereas grafts from aged hosts maintained on a control diet appeared more disorganized (Figures 3E and 3G), suggesting that the blueberry may have had effects on graft organization. Transplants to blueberry-treated hosts have previously been examined thoroughly for neuronal markers, and we found significant differences between the supplemented and control groups using these markers as well (Willis et al, 2005).
Figure 2.
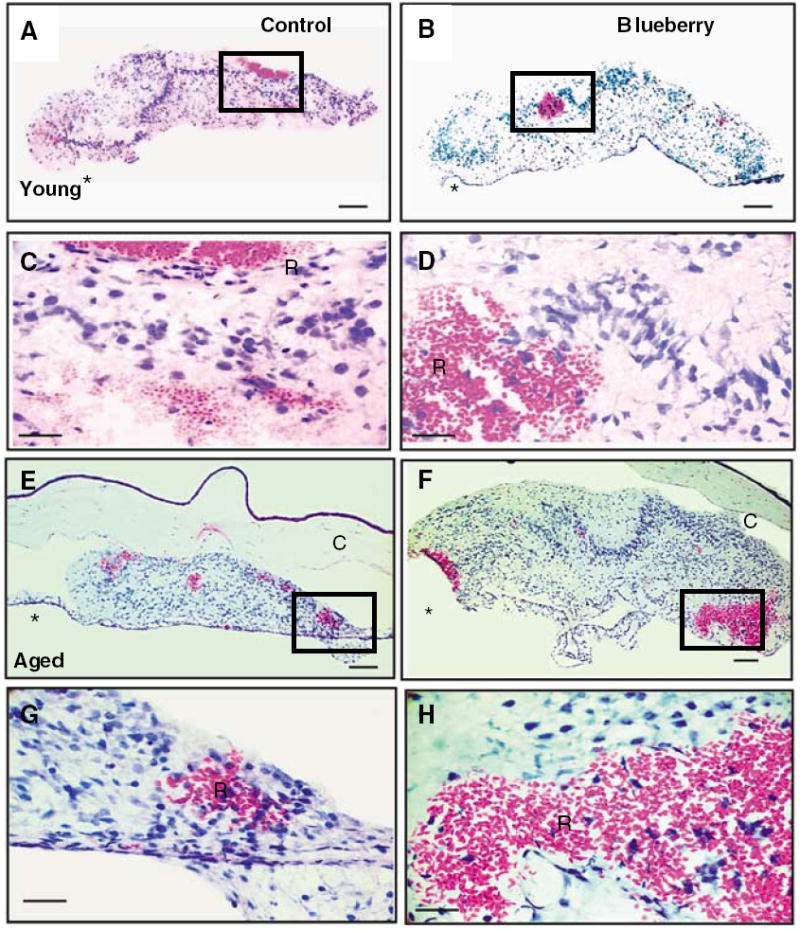
Hematoxylin and eosin staining of hippocampal grafts 1 to 2 weeks after transplantation from young (A to D) and aged (E to H) hosts maintained on a control (A, C, E, G) or blueberry supplemented (B, D, F, H) diet. Minimal numbers of peripheral immune cells were observed in control and blueberry grafts of either host age. The box outlined in A, B, E, and F outline higher magnification seen in C, D, G, and H and shows blood pools in grafted tissue filled with red blood cells and some white blood cells as well at this early stage. Note that the hippocampal grafts are well organized with specific cell layers obvious also at this early age, at least in young grafts of both groups and aged blueberry treated grafts *, host iris attachment; R, red blood cells; C, cornea; scale bar = 100 μm (A, B, E, and F) and 25 μm (C, D, G, and H).
Figure 3.

Hematoxylin and eosin staining of hippocampal grafts 8 to 12 weeks after transplantation from young (A to D) and aged (E to H) hosts maintained on a control (A, C, E, G) or blueberry-supplemented (B, D, F, H) diet and young hosts treated with LPS (I, J). The box in A, B, E, and F demarcate the higher magnification area shown below for each image. Note the difference in morphologic organization between the aged control graft (E) and all other grafts at this time point after grafting (8 to 12 weeks). Peripheral immune cells were not observed in control or blueberry grafts of either host age. Lipopolysaccharide treatment, however, induced the infiltration of peripheral immune cells, including macrophages (J, arrows) and granulocytes (J, arrowheads). *, host iris attachment; R, red blood cells; scale bar = 100 μm (A, B, E, F, and I) and 25 μm (C, D, G, H, and J).
Hematoxylin and eosin staining was used to visualize the presence of pools of eosin-positive red blood cells in both young (Figures 2A to 2D) and aged (Figures 2E to 2H) host animals. Red blood cell pools appeared at 1 to 2 weeks after transplantation in grafts from all groups, indicating that timing of angiogenesis in grafted tissues was not altered by blueberry supplementation neither was it altered by the age of the recipient animal at the time of grafting.
Peripheral Immune Cells did not Infiltrate Hippocampal Intraocular Grafts
To determine whether lymphocytes or granulocytes from the blood stream infiltrated intraocular grafts, white blood cells were quantified in H&E-stained sections, on the basis of morphologic appearance (Table 1). Basophil, eosinophil, and neutrophil granulocytes, as well as lymphocytes, monocytes, and macrophages, were identified and counted in grafted tissues according to the H&E staining pattern and morphology using a × 60 objective. Grafts harvested at 1 to 2 weeks after transplantation in all groups contained very low numbers of identifiable white blood cells (Figure 2, Table 1). However, even fewer white blood cells could be observed in grafts harvested at 8 to 12 weeks after transplantation (Figure 3, Table 1). To verify the efficacy of H&E staining in peripheral immune cell identification, a positive control was generated by subjecting two young control diet hosts (i.e., four grafts, one per eye of each recipient) to intravenous LPS treatment. Lipopolysaccharide induced infiltration of numerous granulocytes and macrophages in intraocular grafts (Figures 3I and 3J), which were readily visible by H&E staining. The presence of infiltrating immune cells in intraocular transplants was examined using fluorescent immunohistochemistry for the lymphocyte marker CD3 and the macrophage marker ED1. Lipopolysaccharide treatment gave rise to an increase in both ED1 and CD3 immunohistochemistry, whereas no positive signal was observed in grafts from young or aged control subjects or in any of the groups that were supplemented with blueberry in the diet (data not shown, as all but one group showed no immunostaining with these markers).
Table 1.
Quantification of peripheral blood components in grafted tissue
| Host age | Weeks after transplantation | Treatment | Blood pools | Macrophages (cells/mm2) | White blood cells (cells/mm2) |
|---|---|---|---|---|---|
| Young | 1–2 | Control | + | 0 | 0.001 |
| Blueberry | + | 0 | 0 | ||
| Young | 8–12 | Control | − | 0 | 0 |
| Blueberry | − | 0 | 0 | ||
| Aged | 1–2 | Control | + | 0.003 | 0.005 |
| Blueberry | + | 0 | 0.001 | ||
| Aged | 8–12 | Control | − | 0 | 0 |
| Blueberry | − | 0 | 0 | ||
| Young | 8 | LPS | − | 0.196 | 0.094 |
Abbreviations: H&E, hematoxylin and eosin; LPS, lipopolysaccharide.
H&E staining revealed the presence of red blood cells in grafts from both groups 1 to 2 weeks after transplantation, with no macrophages or granulocytes detected. Grafts harvested at 8 to 12 weeks after transplantation exhibited mature vasculature with very few or no observed peripheral blood cells. In grafts from young hosts treated with LPS at 8 weeks after transplantation, several macrophages and lymphocytes were observed throughout the tissue (n = 8 for all dietary groups, n = 4 for LPS; Data presented as number of cells/mm2 of area counted in each graft).
Dietary Blueberry Supplementation did not Impact Expression of Vascular Markers
An initial assessment of graft vascularization at 8 to 12 weeks after transplantation was performed using immunohistochemistry for the blood vessel marker laminin (Figure 4). Laminin expression was markedly different in grafts from young versus aged hosts and revealed a distinct morphologic effect of host age on graft vascularization. To confirm the morphologic differences observed with laminin expression, we also performed immunohistochemistry for the BBB marker Glut1 in intraocular grafts at 1 to 2 weeks and at 8 to 12 weeks after transplantation. Glucose transporter 1 expression corroborated the morphologic differences observed with laminin expression at 1 to 2 weeks (Figure 5) and at 8 to 12 weeks (Figure 6) after transplantation. Hippocampal grafts from young hosts exhibited many thin, compact blood vessels with fairly small lumina throughout the graft regardless of dietary manipulation. This expression pattern was seen in grafts from young hosts both at early (Figures 5A to 5D) and late (Figures 6A to 6D) time points after transplantation. In grafts from aged hosts, however, blood vessels appeared abnormal, with thick walls and an enlarged lumen at 1 to 2 weeks (Figures 5E to 5H) and at 8 to 12 weeks (Figures 6E to 6H) after transplantation. Dietary blueberry supplementation did not appear to affect blood vessel morphology regardless of the age of graft recipients. Blood vessel morphology in grafts harvested from young hosts treated with LPS (Figure 6I and 6J) was severely disrupted and resembled vessels in grafts from aged hosts rather than the grafts from young hosts. As the aged host transplants already displayed this disrupted blood vessel morphology, studies were not initiated to perform LPS injections to the aged hosts. Vessels in LPS-treated grafts appeared larger, with a disorganized structure, thick walls, larger lumen, and patchy Glut1 expression within blood vessel walls (Figure 6J), indicative of severely altered vessel morphology and/or permeability.
Figure 4.

Laminin expression in hippocampal grafts 8 to 12 weeks after transplantation from young (A, B) and aged (C, D) hosts maintained on a control (A, C) or blueberry-supplemented (B, D) diet. Laminin expression indicated that blood vessel morphology was distinctly different in grafts from young versus aged hosts, but dietary blueberry supplementation did not appear to affect morphology of laminin-positive blood vessels. (scale bar = 100 μm (A to D); *, iris).
Figure 5.

Glucose transporter 1 expression in hippocampal grafts 1 to 2 weeks after transplantation from young (A to D) and aged (E to H) hosts maintained on a control (A, C, E, G) or blueberry-supplemented (B, D, F, H) diet. Glucose transporter 1 expression revealed an extensive thin-walled blood vessel network in grafts from young hosts (A, B) with larger, thick-walled vessels present at the site of iris attachment (I; C, D). Grafts from aged hosts exhibited fewer blood vessels (E, F) that appeared thicker (G, H) and less evenly distributed throughout grafted tissue (scale bar = 25 μm (A, B, E, and F) and 100 μm (C, D, G, and H); *, iris).
Figure 6.
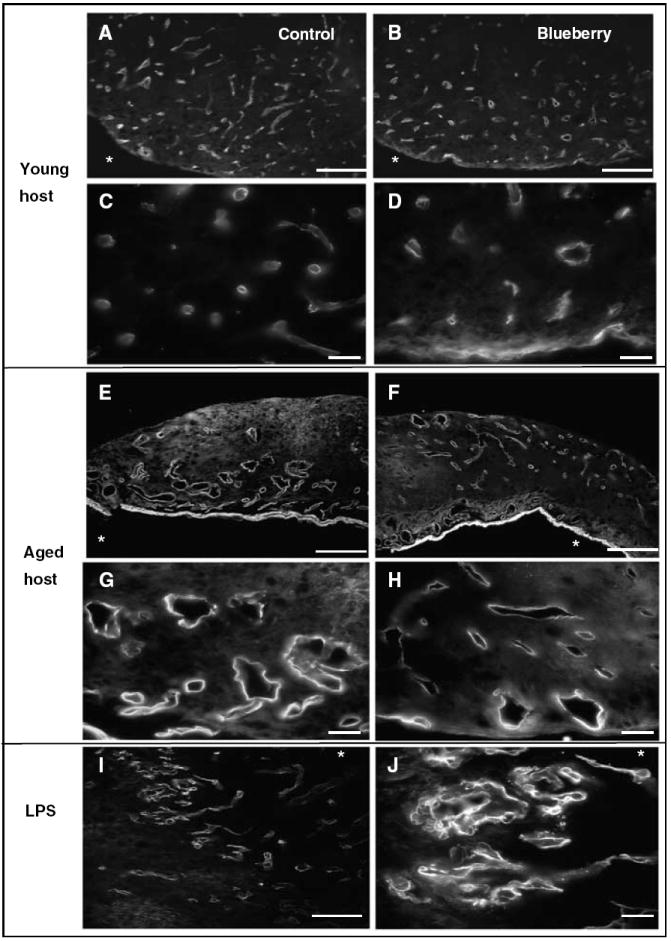
Glucose transporter 1 expression hippocampal grafts 8 to 12 weeks after transplantation from young (A to D) and aged (E to H) hosts maintained on a control (A, C, E, G) or blueberry-supplemented (B, D, F, H) diet, and grafts from young hosts treated with LPS (I, J). Grafts from young hosts contained many thin blood vessels throughout the graft (A, B) in contrast to grafts from aged hosts (E, F), which contained fewer thicker vessels with larger lumina. Higher magnification revealed that vessels in grafts from young hosts appeared small and thin-walled (C, D), whereas vessels in grafts from aged hosts (G, H) appeared enlarged with thick walls and patchy Glut1 immunoreactivity. Blood vessels in LPS-treated grafts resembled those in grafts from aged hosts (scale bar = 25 μm (A, B, E, F, and I) and 100 μm (C, D, G, H, and J)). The * in figures represents location of host iris.
The degree of Glut1 expression was quantified in hippocampal grafts from all groups through densitometry (Figure 7E). At 1 to 2 weeks after transplantation, grafts from young hosts exhibited a significantly lower area of Glut1 immunoreactivity (young control 4.92% ± 1.45%; young blueberry 3.12% ± 0.42%) as compared with grafts from aged hosts (aged control 18.92% ± 3.59%; aged blueberry 19.16% ± 2.61%), reflecting the morphologic appearance of Glut1-positive blood vessels in grafts from young versus aged hosts. This trend in Glut1 expression was extended to grafts harvested at 8 to 12 weeks after transplantation. Grafts from young hosts exhibited a lower level of Glut1 expression regardless of dietary condition (young control 5.99% ± 1.16%; young blueberry 4.93% ± 0.69%). The extent of Glut1 expression in grafts from aged hosts was higher than that seen in young hosts (aged control 17.95% ± 4.78%; aged blueberry 14.21% ± 3.95%), but there was no significant effect of blueberry supplementation on Glut1 expression in aged hosts. Interestingly, grafts from young host animals treated with LPS also exhibited an elevated level of Glut1 expression (22.42% ± 4.12%), which was not significantly different from grafts from aged hosts.
Blood–Brain Barrier in Intraocular Grafts
A common and noninvasive method for examination of the condition of BBB in neuronal rat tissues is to stain with an anti-rat IgG conjugated with a fluorescent marker, such as rhodamine. We have used this method in a previous published work to examine whether the barrier is intact during different conditions (Granholm et al, 1996). If the BBB is intact, one will only detect fluorescence within vessels, and if leakage is occurring, it is easy to detect anti-rat IgG outside vessels in the brain parenchyma. Figure 7 depicts anti-rat IgG conjugated with rhodamine in the four different groups, and Figure 7F shows densitometry assessments of this immunostaining. As can be seen in Figures 7A to 7D, hippocampal grafts to aged hosts exhibited increased IgG staining, indicative of leakage occurring into surrounding graft parenchyma. This was also obvious in young grafts treated with LPS (Figure 7C), but not in young control grafts (Figure 7D). Interestingly, the aged blueberry (BB, Figure 7A) appeared to have less overall anti-rat IgG staining compared to aged controls. The densitometry revealed a significant increase in IgG staining in aged controls compared to young hosts (P < 0.05; see Figure 7F), but there was no significant difference between aged BB and young grafts (P > 0.1). Thus, it is possible that the blueberry diet may alter the functional properties of the BBB, although this will be the focus of future, more invasive studies.
Discussion
The primary findings in this study were that blood vessels in intraocular grafts to aged hosts exhibited a perturbed organization and vascular tree and that dietary blueberry supplementation improved size and cellular organization of hippocampal intraocular grafts to aged host rats without affecting the vascular morphology or density of blood vessels in grafted tissues. Further, we found that hippocampal grafts to aged hosts exhibited a ‘leaky’ BBB, with abundant parenchymal staining of fluorescent anti-rat IgG. Although we did not observe effects of the blueberry treatment on general vascularization of the grafted tissue or infiltration of peripheral blood cells, we present here preliminary data suggesting that blueberry diets may affect BBB development or maintenance. To our knowledge, the fact that BBB is affected in hippocampal grafts to aged hosts has not been published previously. At the very least, these data have ruled out vascular development as the key factor for blueberry function in our model, pointing instead to several other interesting mechanisms for antioxidant diets in aged hosts, such as BBB function, glial development, growth factors, or cytokines.
In agreement with our previous study (Willis et al, 2005), blueberry supplementation significantly improved the size of intraocular grafts to aged hosts. In this study, we have expanded these previous studies by showing significant differences in graft growth between young and aged recipients, as well as significant effects of blueberry treatment also in grafts in young recipients. In addition to an increased size of hippocampal transplants to aged hosts, dietary blueberry supplementation also improved graft organization. The fetal hippocampal formation begins to develop around E13 with the infolding of the telencephalic wall. By E18, the fetal stage used here, the hippocampal formation, exhibits a rudimentary organization including a distinct dentate gyrus, CA1, and CA3 (Granholm, 1991). The continued maintenance of this hippocampal cytoarchitecture has been consistently reported in intraocular grafts (Olson et al, 1977, 1983; Granholm, 1991) and is disrupted when hippocampus is grafted to aged hosts, as reported by our group previously (Eriksdotter-Nilsson and Olson, 1989; Willis et al, 2005), although previous studies have not examined the effect of the age of the host on BBB development. In our study, blueberry supplementation in aged hosts prevented this age-associated disruption and maintained hippocampal grafts in an appropriately organized state.
As graft survival and growth is dependent on vascular support from the host, the present study focused on the impact of age and blueberry supplementation on vascularization and infiltration of circulatory cells. The formation of red blood cells pools is an early step in the formation of a vascular tree in intraocular grafts (Tuba and Kalman, 1997), and the formation of an intact BBB is completed at approximately 2 weeks after transplantation in intraocular grafts (Granholm et al, 1996). A delay in the formation of an intact BBB could account for the differences in growth and cell survival seen in our study, but previous work has shown that hippocampal grafts develop normally even after graft vascularization has been delayed (Eriksdotter-Nilsson and Olson, 1989). This is contrary to the earlier findings from this research group showing that intraocular grafts of cortical tissue are dependent on normal vascularization to develop organotypically (Bjorklund et al, 1983). Thus, it is possible that the hippocampal formation is guided by factors other than vascular trophic molecules in its development, contrary to other cortical regions. These findings, along with the consistent appearance of red blood cells in grafts from all groups at 1 to 2 weeks after transplantation, imply that the effects of blueberry supplementation on size and organization of grafts to aged hosts cannot be exclusively attributed to the initial rate of vascularization of tissue, even though our studies using anti-rat IgG suggest that there may be remaining effects of aging and blueberry supplementation on the BBB function even several weeks after grafting.
Once vascularization of grafts is complete, tight junctions in the BBB of the graft are fully formed and graft blood vessels express markers seen in the brain in situ (Granholm et al, 1996). We quantified the presence of leukocytes, microphages, and monocytes in grafted tissues and found very low numbers of peripheral immune cells both early (1 to 2 weeks) and several weeks (8 to 12 weeks) after grafting. Blood–brain barrier endothelial cells express low levels of adhesion molecules required in the trafficking of white blood cells across the barrier (Bart et al, 2000), and leukocyte infiltration into central nervous system tissues is a process that is tightly regulated (Hickey, 2001). Lipopolysaccharide was used as a positive control, as it will increase levels of proinflammatory cytokines in circulation and induce a breakdown of the BBB (Abbott and Revest, 1991). Although several leukocytes and macrophages were detected in LPS-treated grafts, very few infiltrating cells were observed in grafts from either young or aged hosts. However, there was significant leakage of IgG across the BBB in grafts to aged hosts as compared to tissues grafted to young hosts (Figure 7). Preliminary studies suggest that this leakage of IgG may occur to a lesser extent in blueberry-treated aged hosts, suggesting that further studies of BBB physiology in grafted tissues may be warranted.
Glucose transporter 1 immunohistochemistry revealed a dramatic difference in blood vessel morphology in grafts from young versus aged hosts, which could be attributed to a number of age-related changes in the ocular environment with aging. Aged animals exhibit increased levels of proangiogenic factors such as vascular endothelial growth factor, basic fibroblast growth factor, and transforming growth factor-β in the iris and the aqueous humor of the eye (Tong et al, 2006), which can induce the expression and translocation of Glut1 in endothelial cell membranes (Sone et al, 2000). Additionally, as levels of proangiogenic factors become elevated during aging, antiangiogenic factors decline with age (Ogata et al, 2004; Funaki et al, 2001). These angiogenic factors can initiate neovascularization, which has been associated with age-related ocular disorders (Glaser, 1990). As intraocular grafts attach to the host iris and are surrounded by ocular fluid, angiogenic factors in the aged eye could account for the alterations in blood vessels seen in hippocampal grafts to aged hosts. Interestingly, blood vessel morphology and Glut1 expression in the LPS-treated grafts from young hosts closely resembled those of grafts in aged hosts. The effects of LPS on Glut1 expression corroborate previous studies showing that proinflammatory mediators can increase Glut1 expression in equine articular chondrocytes (Phillips et al, 2005) and in various cell lines in culture (Cohen et al, 1996). The proinflammatory cytokine interleukin-6 is elevated in patients with neovascular glaucoma, a condition defined by excessive iris vascularization (Chen et al, 1999), suggesting that the systemic inflammation caused by LPS injection may also result in elevated Glut1 expression in the grafts. Inflammatory processes may also have an important function in the perturbation of blood vessels in intraocular grafts to aged hosts in this study, as inflammation has been shown to increase during aging in humans and animals (Ye and Johnson, 1999; Krabbe et al, 2004) and can disrupt neuronal survival (Kempermann and Neumann, 2003), particularly in fetal neural tissue grafts (McGuire et al, 2001). Proinflammatory cytokines increase in ocular fluid after eye surgery (Malecaze et al, 1991; King et al, 2000), and cytokine exposure during the grafting procedure might also affect graft survival and organization. Blueberry supplementation has been shown to modulate activation of microglia (Stromberg et al, 2005; Lau et al., 2007). Therefore, our future studies will include a close examination of anti-inflammatory processes as a possible mechanism for blueberry effects.
Our current findings indicate that blueberry in the diet did not affect vascular morphology of intraocular grafts in either young or aged hosts. Although other nutritional compounds have shown both antiangiogenic (Dulak, 2005) and proangiogenic (Kaga et al, 2005) activities, depending on the tissue investigated, studies on angiogenic properties of blueberry have been limited to human keratinocytes, where a blueberry-enhanced diet inhibited vascular endothelial growth factor expression and decreased angiogenesis (Roy et al., 2002). A combination of berry extracts containing blueberries was also shown to inhibit vascular endothelial growth factor expression in keratinocytes and to impair angiogenesis in a human microvascular endothelial cell line (Bagchi et al, 2004). Our results do not necessarily contradict findings of antiangiogenic properties of blueberries in other systems but instead suggest that factors other than angiogenesis may play a more major role in the effects of blueberry on the hippocampal grafts observed here.
Our previous study revealed an increase in size and organization of hippocampal grafts to aged hosts with blueberry treatment, along with an increase in the expression of neuronal markers (Willis et al, 2005). Since the present study excludes graft vascularization as a major mechanism of action of blueberries in our model, other potential actions of blueberries must be explored. Blueberries have traditionally been identified as potent antioxidants (Prior et al., 1998). However, a seminal study by Joseph and Bickford (Joseph et al, 1999) showed distinct effects of blueberries on neuronal signaling cascades when compared to other diets of the same antioxidant level. These findings indicate that blueberries could have effects on neural tissue independently of antioxidant actions. Blueberry supplementation has been found to increase extracellular signal-related kinase (ERK) activation in the hippocampus (Casadesus et al, 2004), reverse the age-associated increase in heat shock protein 70 in the hippocampus (Galli et al, 2005), and prevent loss of hippocampal CA1 pyramidal cells following lesions (Duffy et al, 2007). The improvement in size and organization of hippocampal grafts in our current study could be attributed to any of these direct effects of blueberry compounds on neuronal parameters. Although the pooled analysis of graft growth presented here also showed significant effects of blueberry on growth of hippocampal grafts in young recipients, the effects on organization of the tissue were more prominent in the aged hosts. Therefore, blueberry supplementation in our model system may be primarily affecting processes associated with aging.
In conclusion, this study revealed that vascularization of hippocampal intraocular grafts was dramatically different in young and aged graft recipients, but that dietary blueberry supplementation did not affect vascular morphology in grafts from either young or aged hosts. Further, we found that the peripheral infiltration of circulating immune cells appeared to play a minor role in the development of intraocular tissues, suggesting that factors endogenous to the grafted central nervous system tissues played a more significant role in organizational differences between the recipient ages. These findings indicate that compounds in blueberries may be acting directly on grafted neurons or on other host systems involved in graft survival.
Acknowledgments
This work was supported by a program project grant from the NIA (AG04418).
References
- Abbott NJ, Revest PA. Control of brain endothelial permeability. Cerebrovasc Brain Metab Rev. 1991;3:39–72. [PubMed] [Google Scholar]
- Andres-Lacueva C, Shukitt-Hale B, Galli R, Jauregui O, Lamuela-Raventos RM, Joseph JA. Anthocyanins in aged blueberry-fed rats are found centrally and may enhance memory. Nutr Neurosci. 2005;8:111–20. doi: 10.1080/10284150500078117. [DOI] [PubMed] [Google Scholar]
- Bagchi D, Sen CK, Bagchi M, Atalay M. Anti-angiogenic, antioxidant, and anti-carcinogenic properties of a novel anthocyanin-rich berry extract formula. Biochemistry. 2004;69:75–80. doi: 10.1023/b:biry.0000016355.19999.93. [DOI] [PubMed] [Google Scholar]
- Bart J, Groen HJ, Hendrikse NH, van der Graaf WT, Vaalburg W, de Vries EG. The blood–brain barrier and oncology: new insights into function and modulation. Cancer Treat Rev. 2000;26:449–62. doi: 10.1053/ctrv.2000.0194. [DOI] [PubMed] [Google Scholar]
- Bjorklund H, Seiger A, Hoffer B, Olson L. Trophic effects of brain areas on the developing cerebral cortex: I. Growth and histological organization of intraocular grafts. Brain Res. 1983;282:131–40. doi: 10.1016/0165-3806(83)90091-3. [DOI] [PubMed] [Google Scholar]
- Calza L, Giardino L, Giuliani A, Aloe L, Levi-Montalcini R. Nerve growth factor control of neuronal expression of angiogenetic and vasoactive factors. Proc Natl Acad Sci USA. 2001;98:4160–5. doi: 10.1073/pnas.051626998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Fang J, Xia C, Shi X, Jiang BH. Trans-3,4,5′-trihydroxystibene inhibits hypoxia-inducible factor 1alpha and vascular endothelial growth factor expression in human ovarian cancer cells. Clin Cancer Res. 2004;10:5253–63. doi: 10.1158/1078-0432.CCR-03-0588. [DOI] [PubMed] [Google Scholar]
- Casadesus G, Shukitt-Hale B, Sellwagen HM, Zhu X, Lee H-G, Smith MA, Joseph JA. Modulation of hippocampal plasticity and cognitive behavior by short-term blueberry supplementation in aged rats. Nutr Neurosci. 2004;7:309–16. doi: 10.1080/10284150400020482. [DOI] [PubMed] [Google Scholar]
- Chen KH, Wu CC, Roy S, Lee SM, Liu JH. Increased interleukin-6 in aqueous humor of neovascular glaucoma. Invest Ophthalmol Vis Sci. 1999;40:2627–32. [PubMed] [Google Scholar]
- Cohen T, Nahari D, Cerem LW, Neufeld G, Levi BZ. Interleukin 6 induces the expression of vascular endothelial growth factor. J Biol Chem. 1996;271:736–41. doi: 10.1074/jbc.271.2.736. [DOI] [PubMed] [Google Scholar]
- Duffy KB, Spangler EL, Devan BD, Guo Z, Bowker JL, Janas AM, Hagepanos A, Minor RK, Decabo R, Mouton PR, Shukitt-Hale B, Joseph JA, Ingram DK. A blueberry-enriched diet provides cellular protection against oxidative stress and reduces a kainate-induced learning impairment in rats. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.04.002. e-pub ahead of print 22 May 2007. [DOI] [PubMed] [Google Scholar]
- Dulak J. Nutraceuticals as anti-angiogenic agents: hopes and reality. J Physiol Pharmacol. 2005;56(Suppl 1):51–69. [PubMed] [Google Scholar]
- Eriksdotter-Nilsson M, Olson L. Growth of brain tissue grafts is dependent upon host age. Mech Ageing Dev. 1989;49:1–22. doi: 10.1016/0047-6374(89)90064-x. [DOI] [PubMed] [Google Scholar]
- Funaki H, Sawaguchi S, Yaoeda K, Koyama Y, Yaoita E, Funaki S, Shirakashi M, Oshima Y, Shukunami C, Hiraki Y, Abe H, Yamamoto T. Expression and localization of angiogenic inhibitory factor, chondro-modulin-I, in adult rat eye. Invest Ophthalmol Vis Sci. 2001;42:1193–200. [PubMed] [Google Scholar]
- Galli RL, Bielinski DF, Szprengiel A, Shukitt-Hale B, Joseph JA. Blueberry supplemented diet reverses age-related decline in hippocampal HSP70 neuroprotection. Neurobiol Aging. 2005;27:344–50. doi: 10.1016/j.neurobiolaging.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Glaser BM. Extracellular modulating factors and the control of intraocular neovascularization: an overview. J Diabet Complications. 1990;4:108–12. doi: 10.1016/0891-6632(90)90049-b. [DOI] [PubMed] [Google Scholar]
- Granholm AC. Hippocampal transplants in oculo. A model for establishment of isolated circuits. In: Conn M, editor. Methods in neuroscience. Vol. 7. 1991. pp. 327–48. [Google Scholar]
- Granholm AC, Curtis M, Diamond DM, Branch BJ, Heman KL, Rose GM. Development of an intact blood–brain barrier in brain tissue transplants is dependent on the site of transplantation. Cell Transplant. 1996;5:305–14. doi: 10.1177/096368979600500219. [DOI] [PubMed] [Google Scholar]
- Hardy G, Hardy I, Ball PA. Nutraceuticals—a pharmaceutical viewpoint: part II. Curr Opin Clin Nutr Metab Care. 2003;6:661–71. doi: 10.1097/00075197-200311000-00010. [DOI] [PubMed] [Google Scholar]
- Hickey WF. Basic principles of immunological surveillance of the normal central nervous system. Glia. 2001;36:118–24. doi: 10.1002/glia.1101. [DOI] [PubMed] [Google Scholar]
- Joseph JA, Arendash G, Gordon M, Diamond D, Shukitt-Hale B, Morgan D. Blueberry supplementation enhances signaling and prevents behavioral deficits in an Alzheimer disease model. Nutr Neurosci. 2003;6:153–62. doi: 10.1080/1028415031000111282. [DOI] [PubMed] [Google Scholar]
- Joseph JA, Shukitt-Hale B, Casadesus G. Reversing the deleterious effects of aging on neuronal communication and behavior: beneficial properties of fruit polyphenolic compounds. Am J Clin Nutr. 2005;81(Suppl 1):313S–6S. doi: 10.1093/ajcn/81.1.313S. [DOI] [PubMed] [Google Scholar]
- Joseph JA, Shukitt-Hale B, Denisova NA, Bielinski D, Martin A, McEwen JJ, Bickford PC. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. J Neurosci. 1999;19:8114–21. doi: 10.1523/JNEUROSCI.19-18-08114.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaga S, Zhan L, Matsumoto M, Maulik N. Resveratrol enhances neovascularization in the infarcted rat myocardium through the induction of thioredoxin-1, heme oxygenase-1 and vascular endothelial growth factor. J Mol Cell Cardiol. 2005;39:813–22. doi: 10.1016/j.yjmcc.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Neumann H. Neuroscience. Microglia: the enemy within? Science. 2003;302:1689–90. doi: 10.1126/science.1092864. [DOI] [PubMed] [Google Scholar]
- King WJ, Comer RM, Hudde T, Larkin DF, George AJ. Cytokine and chemokine expression kinetics after corneal transplantation. Transplantation. 2000;70:1225–33. doi: 10.1097/00007890-200010270-00017. [DOI] [PubMed] [Google Scholar]
- Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp Gerontol. 2004;39:687–99. doi: 10.1016/j.exger.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Lau FC, Bielinski D, Joseph JA. Inhibitory effects of blueberry extract on the production of inflammatory mediators in lipopolysaccharide-activated BV2 microglia. J Neurosci Res. 2007;85:1010–7. doi: 10.1002/jnr.21205. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Kim KS, Park IH, Kim SU. Human neural stem cells over-expressing VEGF provide neuroprotection, angiogenesis and functional recovery in mouse stroke model. PLoS ONE. 2007;2:1–14. doi: 10.1371/journal.pone.0000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little RC, M G, Stroup WW, Wolfinger RD, Schabenberger O. SAS for mixed models. 2. Cary, NC: SAS Press; 2006. [Google Scholar]
- Malecaze F, Chollet P, Cavrois R, Vita N, Arne JL, Ferrara P. Role of interleukin 6 in the inflammatory response after cataract surgery. An experimental and clinical study. Arch Ophthalmol. 1991;109:1681–3. doi: 10.1001/archopht.1991.01080120065027. [DOI] [PubMed] [Google Scholar]
- McGuire SO, Ling ZD, Lipton JW, Sortwell CE, Collier TJ, Carvey PM. Tumor necrosis factor alpha is toxic to embryonic mesencephalic dopamine neurons. Exp Neurol. 2001;169:219–30. doi: 10.1006/exnr.2001.7688. [DOI] [PubMed] [Google Scholar]
- Ogata N, Matsuoka M, Imaizumi M, Arichi M, Matsumura M. Decrease of pigment epithelium-derived factor in aqueous humor with increasing age. Am J Ophthalmol. 2004;137:935–6. doi: 10.1016/j.ajo.2003.08.058. [DOI] [PubMed] [Google Scholar]
- Olson L, Freedman R, Seiger A, Hoffer B. Electro-physiology and cytology of hippocampal formation transplants in the anterior chamber of the eye. I. Intrinsic organization. Brain Res. 1977;119:87–106. doi: 10.1016/0006-8993(77)90093-2. [DOI] [PubMed] [Google Scholar]
- Olson L, Seiger A, et al. Intraocular transplantation in rodents: a detailed account of the procedure and examples of its use in neurobiology with special reference to brain tissue grafting. In: Fedoroff S, Hertz L, editors. Advances in cellular neurobiology. Vol. 4. New York: Academic Press; 1983. pp. 407–42. [Google Scholar]
- Phillips T, Ferraz I, Bell S, Clegg PD, Carter SD, Mobasheri A. Differential regulation of the GLUT1 and GLUT3 glucose transporters by growth factors and pro-inflammatory cytokines in equine articular chondrocytes. Vet J. 2005;169:216–22. doi: 10.1016/j.tvjl.2004.01.026. [DOI] [PubMed] [Google Scholar]
- Prior RL, Cao G, Martin A, Sofic E, McEwen J, O’Brien C, Lischner N, Ehlenfeldt M, Kalt W, Krewer G, Mainland CM. Antioxidant capacity as influenced by total phenolic and anthocyanin content, maturity, and variety of Vaccinium species. J Agric Food Chem. 1998;46:2686–93. [Google Scholar]
- Ramassamy C. Emerging role of polyphenolic compounds in the treatment of neurodegenerative diseases: a review of their intracellular targets. Eur J Pharmacol. 2006;545:51–64. doi: 10.1016/j.ejphar.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Roy S, Khanna S, Alessio HM, Vider J, Bagchi D, Bagchi M, Sen C. Anti-angiogenic property of edible berries. Free Radic Res. 2002;36:1023–31. doi: 10.1080/1071576021000006662. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: modeling change and event occurrence. New York, NY: Oxford University Press; 2003. [Google Scholar]
- Sone H, Deo BK, Kumagai AK. Enhancement of glucose transport by vascular endothelial growth factor in retinal endothelial cells. Invest Ophthalmol Vis Sci. 2000;41:1876–84. [PubMed] [Google Scholar]
- Stoclet JC, Chataigneau T, Ndiaye M, Oak MH, El Bedoui J, Chataigneau M, Schini-Keith VB. Vascular protection by dietary polyphenols. Eur J Pharmacol. 2004;500:299–313. doi: 10.1016/j.ejphar.2004.07.034. [DOI] [PubMed] [Google Scholar]
- Stromberg I, Cemma C, Vila J, Bickford PC. Blueberry- and spirulina-enriched diets enhance striatal dopamine recovery and induce a rapid, transient microglia activation after injury of the rat nigrostriatal dopamine system. Exp Neurol. 2005;196:298–307. doi: 10.1016/j.expneurol.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Sweeney MI, Kalt W, MacKinnon SL, Ashby J, Gottschall-Pass KT. Feeding rats diets enriched in lowbush blueberries for six weeks decreases ischemia-induced brain damage. Nutr Neurosci. 2002;5:427–31. doi: 10.1080/1028415021000055970. [DOI] [PubMed] [Google Scholar]
- Tong JP, Chan WM, Liu DT, Lai TY, Choy KW, Pang CP, Lam DS. Aqueous humor levels of vascular endothelial growth factor and pigment epithelium-derived factor in polypoidal choroidal vasculopathy and choroidal neovascularization. Am J Ophthalmol. 2006;141:456–62. doi: 10.1016/j.ajo.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Tuba A, Kalman M. The early phase of vascularization in intraocular telencephalic transplants. J Neural Transplant Plast. 1997;6:97–103. doi: 10.1155/NP.1997.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Chang CF, Chou J, Chen HL, Deng X, Harvey BK, Cadet JL, Bickford PC. Dietary supplementation with blueberries, spinach, or spirulina reduces ischemic brain damage. Exp Neurol. 2005;193:75–84. doi: 10.1016/j.expneurol.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Willis L, Bickford PC, Zaman V, Moore AB, Granholm AC. Blueberry extract enhances survival of intraocular hippocampal transplants. Cell Transplant. 2005;14:213–23. doi: 10.3727/000000005783983142. [DOI] [PubMed] [Google Scholar]
- Ye S-M, Johnson RW. Increased interleukin-6 expression by microglia from brain of aged mice. J Neuroimmunol. 1999;93:139–48. doi: 10.1016/s0165-5728(98)00217-3. [DOI] [PubMed] [Google Scholar]


