Abstract
Background
There have been contradictory results regarding temporal changes in the antimicrobial resistance of Escherichia coli from tertiary care centres. Therefore, we performed a population-based investigation to examine in vitro antimicrobial resistance trends of E. coli bloodstream isolates.
Methods
In this retrospective population-based incidence study, we identified 461 unique patients with first episodes of E. coli bloodstream infection (BSI) from 1 January 1998 to 31 December 2007 through microbiology records at the two laboratories in Olmsted County, Minnesota. Logistic regression was used to examine temporal changes in antimicrobial resistance and Poisson regression for changes in incidence rates.
Results
The median age of patients with E. coli BSI was 69 years; 306 (66.4%) were female. The age-adjusted incidence rate of E. coli BSI per 100 000 person-years was 48.0 (95% CI: 42.5–53.4) in females and 34.0 (95% CI: 28.6–39.6) in males. The urinary tract was the most common primary source of infection (79.8%). During the study period, resistance rates of E. coli bloodstream isolates increased from 32% to 53% for ampicillin, from 23% to 45% for ampicillin/sulbactam, from 9% to 28% for trimethoprim/sulfamethoxazole and from 0% to 12% for ciprofloxacin. Resistance rates to carbapenems, cephalosporins and piperacillin/tazobactam remained low and stable.
Conclusions
To our knowledge, this is the first population-based study on antimicrobial resistance trends of E. coli bloodstream isolates in the USA. We demonstrated linear trends of increasing resistance among these isolates to three different classes of antimicrobial over the past decade.
Keywords: bacteraemia, E. coli, epidemiology, fluoroquinolones
Introduction
Escherichia coli is the most common cause of bloodstream infection (BSI) in population-based settings.1–5 There are conflicting results in the literature regarding antimicrobial resistance trends of E. coli. Although some studies have reported trends of increasing antimicrobial resistance, others have not.6–13 These studies, for the most part, have been performed at tertiary care centres where referral bias could overestimate the true antimicrobial resistance rates.14 In addition, some studies have included urinary and respiratory E. coli isolates that may not be of clinical significance, particularly in hospitalized patients.6,9–11
To our knowledge, population-based studies that examine the antimicrobial resistance trends of E. coli bloodstream isolates in the USA are lacking. Therefore, we performed a population-based study to examine antimicrobial resistance trends of E. coli bloodstream isolates in Olmsted County, Minnesota, over the past decade. We hypothesized that there was a trend of increasing resistance among these isolates to three different types of antimicrobial (ampicillin, trimethoprim/sulfamethoxazole and ciprofloxacin) from 1998 to 2007.
Materials and methods
Setting
Olmsted County is located in southeastern Minnesota with a population of 124 277 according to the 2000 census.15 With the exception of a lower prevalence of injection drug use, a higher prevalence of middle-class individuals and a higher proportion being employed in the healthcare industry, the population characteristics of Olmsted County residents are similar to those of USA non-Hispanic whites.16,17 The Rochester Epidemiology Project (REP) is a unique medical records-linkage system that encompasses care delivered to residents of Rochester and Olmsted County, Minnesota. The microbiology laboratories at the Mayo Medical Center and Olmsted Medical Center are the only two laboratories in Olmsted County. These two medical centres are geographically isolated from other urban centres as described previously;14,16,18 therefore, local residents are able to obtain healthcare within the community, rather than seeking healthcare at a distant geographic location.
Case ascertainment
We used complete enumeration of Olmsted County, Minnesota, population from 1 January 1998 to 31 December 2007. Using the microbiology databases at the Mayo Medical Center Rochester and Olmsted Medical Center, we identified 461 unique patients with first episodes of monomicrobial E. coli BSI. Medical records were reviewed by the primary investigator (M. N. A.) to confirm the diagnosis, determine patient residency status, obtain baseline clinical features and obtain in vitro antimicrobial susceptibility data. Blood cultures were identified using standard microbiology techniques according to the CLSI. Both laboratories are certified by the College of American Pathologists. CLSI methods were employed to evaluate in vitro antimicrobial susceptibility results of E. coli isolates. The study was approved by the institutional review boards of both institutions. The detailed case ascertainment and blood culture methods used were described elsewhere.5,14
Case definition
Monomicrobial E. coli BSI was defined as growth of only E. coli in a blood culture, excluding coagulase-negative staphylococci, Corynebacterium species and Propionibacterium spp. Cases were classified according to the site of acquisition into nosocomial, healthcare-associated and community-acquired, as previously defined.19 The primary source of BSI was defined using the Centers for Disease Control and Prevention criteria.20 Fluoroquinolone-resistant E. coli isolates were defined as isolates that are resistant in vitro to ciprofloxacin. All aspects of the study were prespecified in the study protocol prior to data collection.
Statistical analysis
Descriptive statistics were used to summarize the data: medians and interquartile range (IQR) for continuous variables and counts and percentages for categorical variables. The χ2 or Fisher's exact test, as appropriate, was used to evaluate associations between categorical variables.
Incidence rate (IR), expressed as the number of new cases of E. coli BSI per 100 000 person-years, was calculated assuming that the entire population of Olmsted County was at risk of E. coli BSI. Age-, gender- and calendar year-specific IRs were estimated by using the number of patients in each age, gender and calendar year group as the numerator, with corresponding denominators obtained from the 2000 Olmsted County census. A projected population growth rate of 1.9% per year after 2000 was assumed. The rates were directly adjusted to the USA 2000 white population.15 To calculate the rates, the 10 year study period was divided into five 2 year intervals (1998–99, 2000–01, 2002–03, 2004–05 and 2006–07) and age was categorized into five groups (0–18, 19–39, 40–59, 60–79 and ≥80 years). Ninety-five per cent confidence intervals (95% CIs) were derived using the Poisson distribution.
Poisson regression was used to test for a linear trend in IR for each antimicrobial separately. To examine temporal changes in antimicrobial resistance, E. coli isolates that were resistant or had intermediate susceptibility to a particular antimicrobial were classified as being resistant to that antimicrobial; otherwise, the isolate was classified as being susceptible. Logistic regression was used to test for a linear increase in the log odds of E. coli isolates that are resistant to each antimicrobial throughout the study period; the 10 year study period was divided into five 2 year intervals as described previously. Goodness of fit was evaluated using deviance and the Hosmer–Lemeshow test for each Poisson and logistic regression model, respectively. For testing goodness of fit, a significance threshold was defined as P < 0.01. The GENMOD procedure in SAS (version 8, SAS Institute Inc, Cary, NC, USA) was used to perform Poisson regression, and the LOGISTIC procedure for logistic regression. The level of significance for all statistical testing was defined as P < 0.05 (two-sided) unless otherwise noted.
Results
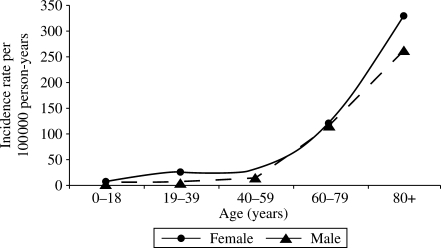
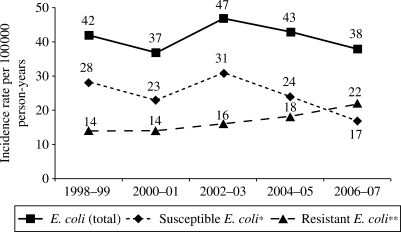
We identified 461 unique patients with E. coli BSI during the study period. The median age of patients with E. coli BSI was 69 (IQR: 50–81) years. Three hundred and six (66.4%) were female. Gender-specific IRs for each age group are illustrated in Figure 1. Age-adjusted IR per 100 000 person-years was higher in females than in males [48.0 (95% CI: 42.5–53.4) versus 34.0 (95% CI: 28.6–39.6)]. The age- and gender-adjusted IR of E. coli BSI remained relatively stable across the 10 year study period (ranging from 37 to 47 per 100 000 person-years; Figure 2) with an overall age- and gender-adjusted IR of 41.4 per 100 000 person-years (95% CI: 37.6–45.3).
Figure 1.
Incidence rate of E. coli bloodstream infection by age and gender, 1998–2007.
Figure 2.
Age- and gender-adjusted incidence rates of antimicrobial-susceptible, antimicrobial-resistant and total E. coli BSI by each 2 year interval, 1998–2007. *E. coli bloodstream isolates susceptible to all tested antimicrobials. **E. coli bloodstream isolates resistant to at least one tested antimicrobial.
Most cases were community-acquired (59.4%); the remainder were healthcare associated (31.7%) or nosocomial (8.9%). The urinary tract was the most common primary source of infection (79.8%), followed by the gastrointestinal tract (8.7%), the respiratory tract (3.0%) and other sites (1.3%). Thirty-three patients (7.2%) had primary BSI of unknown primary source. Females were more likely to have a urinary primary source of infection than males (85.6% versus 68.4%, P < 0.001).
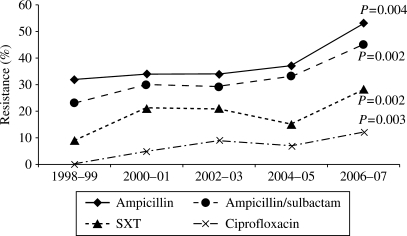
The in vitro antimicrobial resistance rates of E. coli bloodstream isolates to all tested antimicrobials are shown in Table 1. There was a linear trend of increasing resistance among E. coli bloodstream isolates to ampicillin (P = 0.004), ampicillin/sulbactam (P = 0.002), trimethoprim/sulfamethoxazole (P = 0.002) and fluoroquinolones (P = 0.004 and P = 0.003 for levofloxacin and ciprofloxacin, respectively) during the 10 year study period (Figure 3). The increase in antimicrobial resistance rates was more prominent in 2006–07. Resistance rates to gentamicin, piperacillin/tazobactam and cephalosporins remained generally low during the study period with no statistically significant linear trends of increasing resistance (Table 1). Following the introduction of the extended-spectrum β-lactamase (ESBL) screen in our microbiology laboratory in 2000, only three ESBL-producing E. coli bloodstream isolates have been identified. No carbapenem-resistant E. coli bloodstream isolates were detected in our population over the past decade.
Table 1.
In vitro antimicrobial resistance rates of E. coli bloodstream isolates to antimicrobials, 1998–2007
| Antimicrobial | 1998–99 | 2000–01 | 2002–03 | 2004–05 | 2006–07 | Overall | P value* |
|---|---|---|---|---|---|---|---|
| Ampicillin | 28/87 (32) | 27/80 (34) | 35/103 (34) | 36/98 (37) | 48/91 (53) | 174/459 (38) | 0.004 |
| Ampicillin/sulbactam | 20/87 (23) | 24/80 (30) | 30/103 (29) | 32/98 (33) | 41/91 (45) | 147/459 (32) | 0.002 |
| Cefazolin | 2/87 (2) | 1/80 (1) | 3/103 (3) | 3/98 (3) | 5/91 (5) | 14/459 (3) | 0.239 |
| Cefepime | 1/86 (1) | 0/75 (0) | 0/95 (0) | 1/89 (1) | 0/82 (0) | 2/427 (0) | 0.614 |
| Ceftazidime | 1/87 (1) | 1/80 (1) | 0/103 (0) | 1/98 (1) | 2/91 (2) | 5/459 (1) | 0.514 |
| Ciprofloxacin | 0/87 (0) | 4/80 (5) | 9/103 (9) | 7/98 (7) | 11/91 (12) | 31/459 (7) | 0.003 |
| Gentamicin | 1/87 (1) | 3/80 (4) | 4/103 (4) | 3/98 (3) | 4/91 (4) | 15/459 (3) | 0.241 |
| Imipenem | 0/86 (0) | 0/75 (0) | 0/95 (0) | 0/89 (0) | 0/82 (0) | 0/427 (0) | — |
| Levofloxacin | 0/86 (0) | 3/75 (4) | 9/95 (9) | 7/89 (8) | 9/82 (11) | 28/427 (7) | 0.004 |
| Meropenem | 0/86 (0) | 0/75 (0) | 0/95 (0) | 0/88 (0) | 0/82 (0) | 0/426 (0) | — |
| Piperacillin/tazobactam | 0/86 (0) | 0/75 (0) | 4/95 (4) | 0/89 (0) | 1/83 (1) | 5/428 (1) | 0.515 |
| Trimethoprim/sulfamethoxazole | 8/87 (9) | 17/80 (21) | 22/103 (21) | 15/98 (15) | 25/91 (27) | 87/459 (19) | 0.002 |
Data are shown as number of non-susceptible isolates/number of isolates tested (%).
In vitro antimicrobial susceptibility results were not available for two E. coli bloodstream isolates.
*P value denotes a one-degree of freedom test for linear trend using logistic regression.
Figure 3.
In vitro antimicrobial resistance rates of E. coli bloodstream isolates by each 2 year interval, 1998–2007. P value denotes a one-degree of freedom test for linear trend using logistic regression. SXT, trimethoprim/sulfamethoxazole.
Although the age- and gender-adjusted IR of E. coli BSI remained relatively stable, there was an increase in the IR of antimicrobial-resistant E. coli BSI over the study period (Figure 2). The age- and gender-adjusted IR per 100 000 person-years increased from 14 to 20 for ampicillin-resistant E. coli BSI, from 4 to 10 for trimethoprim/sulfamethoxazole-resistant E. coli BSI and from 0 to 5 for ciprofloxacin-resistant E. coli BSI during the past decade (P = 0.095, P = 0.009 and P = 0.003, respectively; test for linear trend across the 10 year study period).
Fluoroquinolone-resistant E. coli bloodstream isolates were more likely to be resistant to other antimicrobial agents than fluoroquinolone-susceptible isolates. Of 31 fluoroquinolone-resistant E. coli isolates, 23 (87.1%) were resistant to ampicillin, compared with only 147 (34.3%) of 428 fluoroquinolone-susceptible isolates (P < 0.001). Similarly, 23 (74.2%) of 31 fluoroquinolone-resistant E. coli bloodstream isolates were resistant to trimethoprim/sulfamethoxazole, compared with only 64 (15.0%) of 428 fluoroquinolone-susceptible isolates (P < 0.001).
Discussion
To our knowledge, this is the first population-based study of antimicrobial resistance trends of E. coli bloodstream isolates in the USA. We demonstrated trends of increasing resistance among these isolates to five different antimicrobials (ampicillin, ampicillin/sulbactam, trimethoprim/sulfamethoxazole, ciprofloxacin and levofloxacin) in three classes over the past decade. Resistance rates to gentamicin, piperacillin/tazobactam, cefazolin, ceftazidime and cefepime remained low and stable and there was no resistance to imipenem or meropenem among the isolates.
A previous study in the Danish population demonstrated an increase in resistance to ampicillin among E. coli bloodstream isolates from 17% to 28% between 1981 and 1997.1 Our study showed that resistance to ampicillin continued to increase (from 32% to 53% between 1998 and 2007). Although the two studies were performed in different geographic locations, it appears that resistance rates to ampicillin among E. coli bloodstream isolates have tripled in the past three decades.
More recently, similar trends of increasing resistance were reported from a hospital-based study in Spain from 1997 to 2005.12 Resistance rates among E. coli bloodstream isolates increased from 47% to 63% for ampicillin, from 24% to 34% for trimethoprim/sulfamethoxazole and from 16% to 23% for ciprofloxacin. Although the higher rates of reported resistance in the latter study compared with ours may be due to higher rates of resistance in Spain than in the USA, it remains possible that referral bias also contributed. In contrast to this study from Spain, we did not detect a trend of increasing resistance to third-generation cephalosporins.
Our results were also consistent with those of a recent population-based study from Calgary, Canada, where increasing antimicrobial resistance rates among E. coli bloodstream isolates to ampicillin, amoxicillin/clavulanate and ciprofloxacin, and stable antimicrobial resistance rates to piperacillin/tazobactam and carbapenems were observed between 2000 and 2006.21 E. coli bloodstream isolates in Calgary health region demonstrated trends of increasing resistance to cephalosporins as well.
There are several surveillance studies on E. coli antimicrobial resistance trends in the USA, but these studies, for the most part, are hospital based. In fact, many studies have exclusively examined nosocomial isolates.6,9,13 Some studies have combined bloodstream with other clinical sources of isolates (urinary, respiratory, etc.).6,9–11 Moreover, results of these studies have been conflicting. One study showed no trend of increasing resistance to ampicillin among nosocomial E. coli bloodstream isolates between 1995 and 2002.13 Another study showed a trend of increasing resistance to third-generation cephalosporins among nosocomial E. coli isolates between 1986 and 2003.9 A subsequent report demonstrated no increase in resistance to third-generation cephalosporins among intensive care unit E. coli isolates between 1992 and 2004.6 Another study reported an increase in resistance to ciprofloxacin among intensive care unit E. coli isolates from 1% to 17% between 1993 and 2004.11 To our knowledge, the only population-based study of E. coli antimicrobial resistance trends in the USA included only urinary tract isolates and, contrary to our study, showed no trends of increasing resistance to trimethoprim/sulfamethoxazole or ciprofloxacin between 1999 and 2005.22
The increase in resistance to antimicrobial agents, particularly fluoroquinolones, among E. coli bloodstream isolates in our study is disturbing. Resistance rates to fluoroquinolones have increased to 12% in the 2006–07 interval of the study. This could have a significant impact on the empirical choice of antimicrobial regimen in patients who present with possible E. coli BSI or pyelonephritis. We have previously demonstrated a similar trend of increasing resistance to fluoroquinolones among E. coli bloodstream isolates in solid organ transplant recipients between 1998 and 2007.23 An increase in resistance to fluoroquinolones in the general population carries more clinical and public health implications. Because 80% of E. coli bloodstream isolates in this study are from a urinary source, it is conceivable that the observed resistance trends in E. coli bloodstream isolates are reflective of resistance of E. coli urinary tract isolates in our population. Because fluoroquinolones, especially the newer ones, have the highest bioavailability among all available oral antimicrobials with Gram-negative activity, this trend of increasing resistance to fluoroquinolones will likely restrict availability of a reliable oral therapy for ‘switch’ therapy of serious E. coli infections. Additionally, because fluoroquinolone-resistant E. coli isolates are more likely to be resistant to ampicillin and trimethoprim/sulfamethoxazole, an increasing proportion of patients with E. coli urinary tract infections may require intravenous antimicrobial therapy for the duration of treatment, which could markedly increase the cost of therapy.24
Currently, ampicillin is rarely used for empirical therapy of E. coli BSI, and yet, ampicillin/sulbactam has remained listed as an option in some of these regimens.25 The alarmingly high resistance rate to ampicillin/sulbactam among E. coli bloodstream isolates is a notable observation in our investigation. Only 55% of E. coli bloodstream isolates were susceptible to ampicillin/sulbactam during 2006–07; therefore, empirical use of this antimicrobial for treatment of possible E. coli BSI should be discouraged, at least in our local population. Fortunately, resistance rates to other β-lactam antibiotics, including piperacillin/tazobactam, carbapenems and third- and fourth-generation cephalosporins, have remained low.
Factors operative in the increase in resistance to antimicrobials in E. coli bloodstream isolates in our population over the past decade remain to be defined. We speculate that the increasing use of antimicrobials, particularly fluoroquinolones, may be associated with an increasing rate of fluoroquinolone resistance, as previously suggested in other populations.26 Despite the availability of ciprofloxacin for human use in the USA since 1987, resistance rates to fluoroquinolones among E. coli bloodstream isolates remained very low at the beginning of our study in 1998. It is conceivable that resistance rates to fluoroquinolones have increased since the introduction of newer fluoroquinolones in the USA in 1996. A previous study of Pseudomonas aeruginosa isolates suggested that exposure to levofloxacin, but not ciprofloxacin, was associated with an increased risk of development of fluoroquinolone-resistant P. aeruginosa isolates.27 Another possible explanation for increasing fluoroquinolone resistance is use of fluoroquinolones in animals. There is a temporal association between increasing fluoroquinolone resistance in E. coli bloodstream isolates in our population and the licensing of these antimicrobials for veterinary use in 1995. A similar ecological association has been shown in other enteric Gram-negative pathogens, such as Salmonella and Campylobacter species.28–30 Clearly, additional studies that examine risk factors for the development of fluoroquinolone resistance among E. coli are warranted.
The major strength of our work is the large sample size with which to perform the proposed statistical analyses. Contrary to some previous cross-sectional studies that examined antimicrobial resistance trends over multiple interrupted points in time, we provided a more complete view of antimicrobial resistance trends by including all E. coli bloodstream isolates over the past 10 years. Additionally, the population-based design added strength and uniqueness to our work.
Our study has limitations. First, we did not perform genetic or molecular testing on E. coli bloodstream isolates to examine for specific resistance genes or enzymes. Second, our data were derived from one geographic area. Since resistance patterns may vary from one geographic location to another, studies from multiple sites may provide a more comprehensive picture of resistance trends across the country. Other limitations include the retrospective design and the reliance on one source for case ascertainment. Finally, the population of Olmsted County consists mainly of middle-class whites; therefore, our study results may be generalized only to communities with similar population characteristics.
In summary, we demonstrated a trend of increasing resistance among E. coli bloodstream isolates to three different classes of antimicrobial over the past decade. Increasing resistance, particularly to fluoroquinolones, may have an impact on choice of empirical antimicrobial therapy in patients who present with E. coli BSI or serious upper urinary tract infections.
Funding
The study received funding from the Small Grants Program and the Baddour Family Fund at the Mayo Clinic, Rochester, MN, USA. The funding source had no role in study design. This work was made possible by research grant R01-AR30582 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (National Institutes of Health, US Public Health Service).
Transparency declarations
None to declare.
M. N. A. and B. D. L. have full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Acknowledgements
This study was presented, in part, at the Forty-eighth Interscience Conference on Antimicrobial Agents and Chemotherapy/Forty-sixth Infectious Diseases Society of America Annual Meeting, Washington, DC, USA, 2008 (Abstract C2-4183).
We thank Emily Vetter and Mary Ann Butler for providing us with vital data from the microbiology laboratory databases at the Mayo Clinic, Rochester and Olmsted Medical Center. We thank Susan Schrage, Susan Stotz and all the staff at the Rochester Epidemiology Project for their administrative help and support.
References
- 1.Pedersen G, Schonheyder HC, Kristensen B, et al. Community-acquired bacteraemia and antibiotic resistance. Trends during a 17-year period in a Danish county. Dan Med Bull. 2000;47:296–300. [PubMed] [Google Scholar]
- 2.Pedersen G, Schonheyder HC, Sorensen HT. Source of infection and other factors associated with case fatality in community-acquired bacteremia—a Danish population-based cohort study from 1992 to 1997. Clin Microbiol Infect. 2003;9:793–802. doi: 10.1046/j.1469-0691.2003.00599.x. [DOI] [PubMed] [Google Scholar]
- 3.Filice GA, Van Etta LL, Darby CP, et al. Bacteremia in Charleston County, South Carolina. Am J Epidemiol. 1986;123:128–36. doi: 10.1093/oxfordjournals.aje.a114206. [DOI] [PubMed] [Google Scholar]
- 4.Gosbell IB, Newton PJ, Sullivan EA. Survey of blood cultures from five community hospitals in south-western Sydney, Australia, 1993–1994. Aust N Z J Med. 1999;29:684–92. doi: 10.1111/j.1445-5994.1999.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 5.Uslan DZ, Crane SJ, Steckelberg JM, et al. Age- and sex-associated trends in bloodstream infection: a population-based study in Olmsted County, Minnesota. Arch Intern Med. 2007;167:834–9. doi: 10.1001/archinte.167.8.834. [DOI] [PubMed] [Google Scholar]
- 6.National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004. Am J Infect Control. 2004;32:470–85. doi: 10.1016/S0196655304005425. [DOI] [PubMed] [Google Scholar]
- 7.Diekema DJ, Pfaller MA, Jones RN. Age-related trends in pathogen frequency and antimicrobial susceptibility of bloodstream isolates in North America: SENTRY Antimicrobial Surveillance Program, 1997–2000. Int J Antimicrob Agents. 2002;20:412–8. doi: 10.1016/s0924-8579(02)00204-2. [DOI] [PubMed] [Google Scholar]
- 8.Diekema DJ, Pfaller MA, Jones RN, et al. Survey of bloodstream infections due to gram-negative bacilli: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, and Latin America for the SENTRY Antimicrobial Surveillance Program, 1997. Clin Infect Dis. 1999;29:595–607. doi: 10.1086/598640. [DOI] [PubMed] [Google Scholar]
- 9.Gaynes R, Edwards JR. Overview of nosocomial infections caused by gram-negative bacilli. Clin Infect Dis. 2005;41:848–54. doi: 10.1086/432803. [DOI] [PubMed] [Google Scholar]
- 10.Jones ME, Draghi DC, Thornsberry C, et al. Emerging resistance among bacterial pathogens in the intensive care unit - a European and North American Surveillance study (2000–2002) Ann Clin Microbiol Antimicrob. 2004;3:14. doi: 10.1186/1476-0711-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lockhart SR, Abramson MA, Beekmann SE, et al. Antimicrobial resistance among Gram-negative bacilli causing infections in intensive care unit patients in the United States between 1993 and 2004. J Clin Microbiol. 2007;45:3352–9. doi: 10.1128/JCM.01284-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peralta G, Sanchez MB, Garrido JC, et al. Impact of antibiotic resistance and of adequate empirical antibiotic treatment in the prognosis of patients with Escherichia coli bacteraemia. J Antimicrob Chemother. 2007;60:855–63. doi: 10.1093/jac/dkm279. [DOI] [PubMed] [Google Scholar]
- 13.Wisplinghoff H, Bischoff T, Tallent SM, et al. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–17. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 14.Al-Hasan MN, Wilson JW, Lahr BD, et al. Incidence of Pseudomonas aeruginosa bacteremia: a population-based study. Am J Med. 2008;121:702–8. doi: 10.1016/j.amjmed.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.US Census Bureau. Olmsted County QuickFacts. http://quickfacts.census.gov. (21 April 2008, date last accessed) [Google Scholar]
- 16.Melton LJ., III History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–74. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 17.Steckelberg JM, Melton LJ, III, Ilstrup DM, et al. Influence of referral bias on the apparent clinical spectrum of infective endocarditis. Am J Med. 1990;88:582–8. doi: 10.1016/0002-9343(90)90521-e. [DOI] [PubMed] [Google Scholar]
- 18.Tleyjeh IM, Steckelberg JM, Murad HS, et al. Temporal trends in infective endocarditis: a population-based study in Olmsted County, Minnesota. JAMA. 2005;293:3022–8. doi: 10.1001/jama.293.24.3022. [DOI] [PubMed] [Google Scholar]
- 19.Friedman ND, Kaye KS, Stout JE, et al. Health care-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002;137:791–7. doi: 10.7326/0003-4819-137-10-200211190-00007. [DOI] [PubMed] [Google Scholar]
- 20.Garner JS, Jarvis WR, Emori TG, et al. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16:128–40. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 21.Laupland KB, Gregson DB, Church DL, et al. Incidence, risk factors and outcomes of Escherichia coli bloodstream infections in a large Canadian region. Clin Microbiol Infect. 2008;14:1041–7. doi: 10.1111/j.1469-0691.2008.02089.x. [DOI] [PubMed] [Google Scholar]
- 22.Smith SP, Manges AR, Riley LW. Temporal changes in the prevalence of community-acquired antimicrobial-resistant urinary tract infection affected by Escherichia coli clonal group composition. Clin Infect Dis. 2008;46:689–95. doi: 10.1086/527386. [DOI] [PubMed] [Google Scholar]
- 23.Al-Hasan MN, Razonable RR, Eckel-Passow JE, et al. Incidence rate and outcome of Gram-negative bloodstream infection in solid organ transplant recipients. Am J Transplant. 2009;9:835–43. doi: 10.1111/j.1600-6143.2009.02559.x. [DOI] [PubMed] [Google Scholar]
- 24.Jackson LA, Benson P, Neuzil KM, et al. Burden of community-onset Escherichia coli bacteremia in seniors. J Infect Dis. 2005;191:1523–9. doi: 10.1086/429344. [DOI] [PubMed] [Google Scholar]
- 25.Al-Hasan MN, Wilson JW, Lahr BD, et al. β-Lactam and fluoroquinolone combination antibiotic therapy in bacteremia caused by gram-negative bacilli. Antimicrob Agents Chemother. 2009;53:1386–94. doi: 10.1128/AAC.01231-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacDougall C, Powell JP, Johnson CK, et al. Hospital and community fluoroquinolone use and resistance in Staphylococcus aureus and Escherichia coli in 17 US hospitals. Clin Infect Dis. 2005;41:435–40. doi: 10.1086/432056. [DOI] [PubMed] [Google Scholar]
- 27.Kaye KS, Kanafani ZA, Dodds AE, et al. Differential effects of levofloxacin and ciprofloxacin on the risk for isolation of quinolone-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2006;50:2192–6. doi: 10.1128/AAC.00060-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Angulo FJ, Nargund VN, Chiller TC. Evidence of an association between use of anti-microbial agents in food animals and anti-microbial resistance among bacteria isolated from humans and the human health consequences of such resistance. J Vet Med B Infect Dis Vet Public Health. 2004;51:374–9. doi: 10.1111/j.1439-0450.2004.00789.x. [DOI] [PubMed] [Google Scholar]
- 29.Smith KE, Besser JM, Hedberg CW, et al. Quinolone-resistant Campylobacter jejuni infections in Minnesota, 1992–1998. Investigation Team. N Engl J Med. 1999;340:1525–32. doi: 10.1056/NEJM199905203402001. [DOI] [PubMed] [Google Scholar]
- 30.Wegener HC. The consequences for food safety of the use of fluoroquinolones in food animals. N Engl J Med. 1999;340:1581–2. doi: 10.1056/NEJM199905203402010. [DOI] [PubMed] [Google Scholar]