Abstract
Developmental differences (9- to 15-year-olds) in effective connectivity in left hemisphere regions were examined using dynamic causal modeling (DCM) of functional magnetic resonance imaging (fMRI) data. Children completed spelling tasks in the visual and auditory modalities in which they were asked to determine if two words were spelled the same from the first vowel onwards. Intrinsic (anatomical) connections were strongest from primary cortical regions to unimodal association areas – from Heschl’s gyrus to superior temporal gyrus for the auditory spelling task and from calcarine to fusiform gyrus for the visual spelling task. The modulatory (experimental) effect for the visual spelling task from calcarine to superior temporal gyrus was stronger than all other effects from calcarine and this effect showed a developmental increase, suggesting automatic activation of phonology that increased with age. The modulatory effect from Heschl’s gyrus to dorsal inferior frontal gyrus also showed a developmental increase, suggesting age-related increases in phonological segmentation in verbal working memory. All together, these results suggest that there are developmental increases in automatic access into brain regions involved in phonological processing in tasks that require orthographic processing.
Keywords: Functional magnetic resonance, imaging (fMRI), Development, Children, Orthography, Phonology, Spelling, Visual, Auditory, Effective connectivity, Dynamic causal modeling, Heschl’s Gyrus, Calcarine, Superior temporal gyrus, Inferior frontal gyrus
1. Introduction
Previous studies suggest that frontal brain areas, and other regions involved in complex cognitive tasks, continue to develop throughout late childhood, while primary cortical regions involved in lower level sensory processing mature earlier (Berl et al., 2006; Brown et al., 2005). However, the processing of complex cognitive tasks may also alter primary sensory processes and their connectivity with other brain regions in late childhood. The current study examines developmental changes from childhood to adolescence in the influence of bottom–up processes emanating from primary sensory regions on other cortical regions in complex cognitive tasks, namely, spelling judgments in the visual and auditory modalities.
Neuroimaging studies with adults performing spelling tasks have shown activation in left inferior frontal gyrus, left inferior parietal cortex (supramarginal gyrus, angular gyrus, and/or inferior parietal lobule), and left inferior temporal/fusiform gyrus. These studies have used a variety of tasks including mentally writing the written word form (Sugishita et al., 1996), converting Japanese Kana words to Kanji characters and then mentally recalling their visual form (Nakamura et al., 2000), spelling judgments independent of whether words are presented in the auditory or visual modality as compared to rhyming judgments (Booth et al., 2002), physical writing of a visually presented word (Nakamura et al., 2000), physical writing of a spoken word (Menon and Desmond, 2001; Petrideset al., 1995; Tokunaga et al., 1999), physical writing of associates of a semantic category (Beeson et al., 2003), and physical writing of a word referring to a visual picture (Katanoda et al., 2001; Sugihara et al., 2006). The most consistent finding across these spelling studies in adults is activation in left inferior parietal cortex, a region that has been implicated in mapping between phonological and orthographic representations (Booth et al., 2002, 2003a). The majority of studies have also shown activation in left inferior temporal/fusiform gyrus that has been implicated in orthographic processing (Dehaene et al., 2004), and in left inferior frontal gyrus that has been implicated in modulating processes in posterior brain regions (Bitan et al., 2005) and/or phonological processing (Poldrack et al., 1999).
Although some studies have examined the neural correlates of spelling in children and adolescents (Lee et al., 1999; Richards et al., 2005), more relevant to the current investigation are studies that have directly examined skill and developmental differences on spelling judgments to auditorily and visually presented words (e.g. are grade–laid spelled the same from the first vowel onwards). In a study with adults examining brain–behavior correlations, higher accuracy on a visual spelling task was associated with greater activation in left inferior frontal gyrus (BA 46) and left fusiform gyrus (BA 37), and higher accuracy on an auditory spelling task was associated with greater activation in left supramarginal gyrus (BA 40), left angular gyrus (BA 39), and left fusiform gyrus (BA 37) (Booth et al., 2003a). These findings suggest that higher accuracy is associated with greater engagement of brain regions involved in orthographic and phonological processing. Three studies have examined differences between adults and children on spelling judgments. In one study, adults showed greater activation than children in left angular gyrus (BA 39) and left inferior frontal gyrus (BA 9) in a visual spelling task and in left inferior frontal gyrus (BA 44/45/9), left angular gyrus (BA 39) and left superior temporal gyrus (BA 22) in an auditory spelling task (Booth et al., 2004). In another study, adults showed greater modality independent activation (across auditory and visual modalities) than children in left inferior frontal gyrus (BA 9) (Booth et al., 2003b). Finally, using dynamic causal modeling (DCM) to examine effective connectivity (Friston et al., 2003; Penny et al., 2004), one study found that adults show greater top–down control than children from left inferior frontal gyrus (BA 45/46/9) to left inferior parietal lobule/precuneus (BA 40/7) in a visual spelling task (Bitan et al., 2006). Only one study has reported age-related/skill differences in activation within children (9- to 15-year-olds) during spelling tasks (Booth et al., 2007). In an auditory spelling task, developmental increases in activation were found in inferior parietal lobule/precuneus (BA 40/7) and accuracy related increases were found in left inferior frontal gyrus (BA 44/9) and left inferior parietal lobule (BA 40). Taken together, these studies converge on the importance of left inferior frontal gyrus (BA 44/9), left inferior parietal cortex (BA 40/39), and left fusiform gyrus (BA 37) for spelling tasks, showing that these regions tend to become more engaged over the course of development and with higher skill.
The current study is the first to examine developmental differences in effective connectivity between brain regions during a spelling task in the visual and auditory modalities. We employed dynamic causal modeling (DCM) to examine the directional influence (effective connectivity) that one brain region has on another. In DCM, intrinsic connections between regions are interregional influences in the absence of modulating experimental effects, whereas changes in the intrinsic connectivity between regions induced by the experimental design (in our case, the spelling tasks) are modulatory effects. In the spelling tasks, children (9- to 15-year-olds) had to determine whether two words were spelled the same from the first vowel onwards. A spelling task to auditorily presented words requires the maintenance of phonological information in memory, accessing visual orthographic information, segmentation of the onset from the rime, and a determination of whether two words have similar spelling of the rime. A spelling task presented in the visual modality could be based on visual orthographic information, however, it is likely that children would activate phonological representations because of behavioral studies showing automatic activation of phonological representations when reading (Booth et al., 1999).
Based on previous neuroimaging studies, we chose to examine connectivity with left inferior parietal cortex (BA 40), left fusiform gyrus (BA 37), and left inferior frontal gyrus. We divided the left inferior frontal gyrus into a dorsal (BA 44/9) and ventral (BA 45/47) portion because studies have suggested that the dorsal portion is involved in phonological processing, whereas the ventral portion has been implicated in semantic processing (Poldrack et al., 1999). We additionally included left superior temporal gyrus (BA 22) and left fusiform gyrus (BA 37) because studies have implicated these regions in phonological (Paulesu et al., 1996; Pugh et al., 1996) and orthographic processing (Cohen et al., 2004; Dehaene et al., 2004), respectively. Our input region for the auditory spelling task was left primary auditory cortex (Heschl’s gyrus, BA 41) and for the visual spelling task was primary visual cortex (calcarine, BA 17). The focus of this paper is on the influence of these primary cortical regions on areas involved in orthographic and phonological processing because studies have implicated primary cortical regions in reading disorders using moving visual stimuli (Demb et al., 1997) and auditory stimuli with rapid acoustic transitions (Helenius et al., 2002). Because our spelling tasks demanded access to orthographic representations and studies have shown age-related effects in fusiform gyrus when reading (Booth et al., 2003b, 2001), we may expect developmental increases in the influence from these primary cortical regions on left fusiform gyrus. However, behavioral studies suggests that there are developmental increases in the automatic activation of phonological information (Booth et al., 1999), so alternatively we may expect age-related increases in the influence from these primary regions on areas involved in phonological processing (i.e. left dorsal inferior frontal gyrus and left superior temporal gyrus) despite the orthographic requirements of the task.
2. Results
2.1. Behavioral results
A one-way ANOVA with 4 age groups (9-year-olds, 11-year-olds, 13-year-olds, and 15-year-olds) on scaled scores for the standardized measures of Verbal IQ, Performance IQ, and WRAT-III Spelling showed no significant age differences in neither the auditory nor the visual task group. A one-way ANOVA with 4 age groups (9-year-olds, 11-year-olds, 13-year-olds, and 15-year-olds) for accuracy on the spelling task showed significant difference between age groups [F(3,36)= 3.31; F(3,44)=2.72, p<0.05 for the auditory and visual tasks, respectively]. An increase of accuracy with age was confirmed by a positive correlation between accuracy and age [r(38)=0.47, p<0.005; r(46)=0.39, p<0.05 for the auditory and visual tasks, respectively]. Because age was correlated with accuracy for both tasks, all correlations involving age were partialed for accuracy and all correlations involving accuracy were partialed for age. See Table 1 for means on these measures. More detailed analyses of accuracy and reaction time in the separate spelling conditions are reported elsewhere (Bitan et al., in press; Booth et al., 2007).
Table 1.
Means (and standard deviations) for age, standardized scores and accuracy in scanner for the different age groups for the auditory spelling and visual spelling task
| 9-year-olds | 11-year-olds | 13-year-olds | 15-year-olds | |
|---|---|---|---|---|
| Auditory spelling | ||||
| N | 8 | 8 | 13 | 11 |
| Age (months) | 107 (2.5) | 128 (8.7) | 154 (2.5) | 178 (3.6) |
| WASI Verbal IQ | 121 (15.5) | 123 (12.8) | 108 (5.9) | 112 (11.2) |
| WASI Performance | 120 (10.1) | 118 (19.2) | 105 (11.3) | 99 (12.7) |
| IQ | ||||
| WRAT-III: Spelling | 115 (15.3) | 119 (12) | 113 (11.9) | 110 (7.1) |
| Accuracy in scanner | 72% (12%) | 77% (11%) | 78% (6%) | 85% (7%) |
| Visual spelling | ||||
| N | 12 | 10 | 15 | 11 |
| Age (months) | 106 (2.4) | 131 (2.8) | 155 (2.9) | 178 (3.6) |
| WASI Verbal IQ | 117 (19.1) | 118 (16.0) | 110 (10.8) | 112 (11.2) |
| WASI Performance | 117 (12.3) | 107 (19.2) | 104 (10.9) | 99 (12.7) |
| IQ | ||||
| WRAT-III: Spelling | 111 (14.2) | 116 (13.6) | 108 (14.5) | 110 (8.1) |
| Accuracy in scanner | 88% (11%) | 90% (9%) | 93% (6%) | 96% (2%) |
WASI Verbal IQ, WASI Performance IQ and WRAT-III Spelling in standard scores (M=100, SD=15). Accuracy on the spelling tasks in scanner is in percentages.
2.2. Conventional analysis
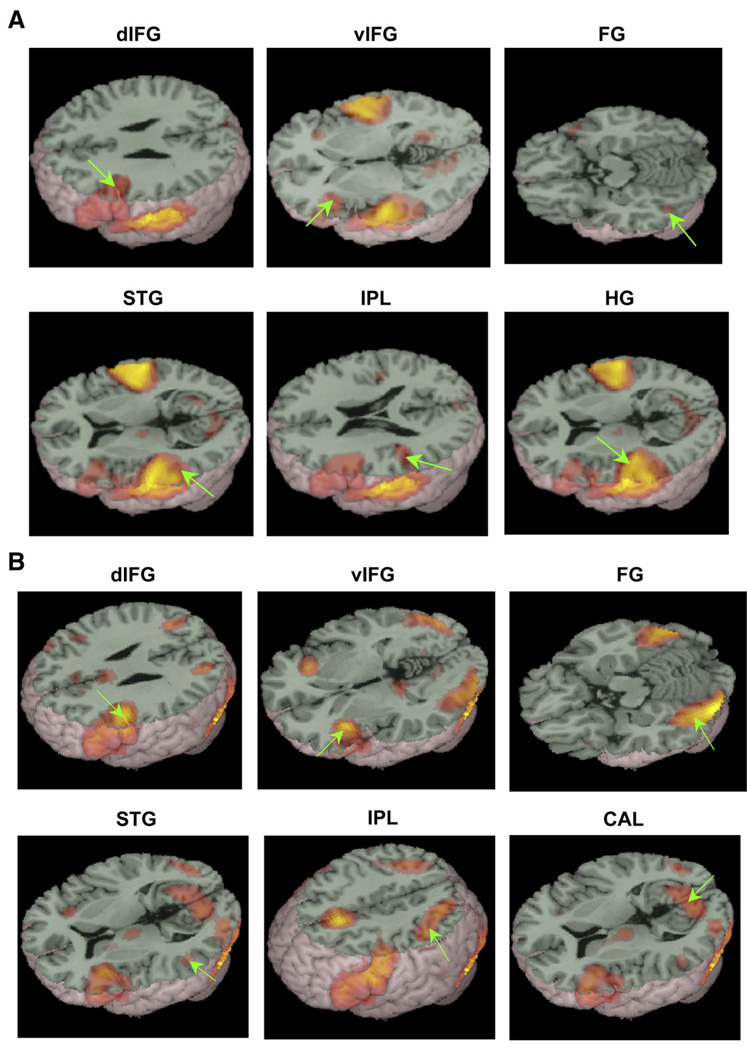
Fig. 1 and Table 2 present regions of activation for the auditory spelling task and the visual spelling task compared to null. For the auditory spelling task (Fig. 1A), there was bilateral activation in inferior frontal gyri, but it was more extensive in left hemisphere. There was also bilateral activation in Heschl’s gyri that extended bilaterally into superior/middle temporal gyri and inferior parietal lobules. Fusiform activation was confined to the left hemisphere. Additional areas of activation included bilateral lingual gyrus extending into cuneus and posterior cingulate, and bilateral medial frontal gyrus extending into anterior cingulate. For the visual spelling task (Fig. 1B), there was bilateral activation in inferior frontal gyri, but it was more extensive in left hemisphere. There was bilateral activation in superior temporal gyrus that extended into middle temporal gyrus, fusiform gyrus, inferior parietal lobule, lingual gyrus and cuneus. Additional areas of activation included bilateral medial frontal gyrus extending into anterior cingulate, left thalamus, left parahippocampal gyrus and right middle frontal gyrus.
Fig. 1.
Left hemisphere regions of interest based on the conventional analysis for the auditory spelling task (A) and visual spelling task (B). Green arrows indicate each region of interest. dIFG, dorsal inferior frontal gyrus; vIFG, ventral inferior frontal gyrus; FG, fusiform gyrus; STG, superior temporal gyrus; IPL, inferior parietal lobule; HG, Heschl’s gyrus; CAL, calcarine.
Table 2.
Main effects of words compared to null in the conventional analysis collapsed across age groups for the auditory spelling and the visual spelling task
| Task/Region | H | BA | z-value | Voxels | x | y | z |
|---|---|---|---|---|---|---|---|
| Auditory spelling | |||||||
| Heschl’s gyrus/superior temporal gyrus/middle temporal gyrus/inferior parietal lobule |
L | 41, 42, 22, 21, 40 | Inf | 996 | −57 | −15 | 3 |
| Heschl’s gyrus/superior temporal gyrus/middle temporal gyrus/inferior parietal lobule |
R | 41, 42, 22, 21, 40 | Inf | 844 | 63 | −30 | 6 |
| Medial frontal gyrus/anterior cingulate | L+R | 8, 32 | 7.17 | 292 | −6 | 15 | 48 |
| Dorsal inferior frontal gyrus | L | 44, 9 | 7.16 | 611 | −45 | 9 | 21 |
| Ventral inferior frontal gyrus | L | 45, 47 | 6.92 | 29 | −36 | 27 | −3 |
| Cerebellum (culmen) | L | 5.79 | 39 | −3 | −33 | −6 | |
| Lingual gyrus/cuneus/posterior cingulate | R | 17, 18, 19, 31 | 5.64 | 486 | 18 | −54 | −3 |
| Ventral inferior frontal gyrus | R | 45, 47 | 5.56 | 34 | 33 | 27 | −3 |
| Lingual gyrus/cuneus/posterior cingulate | L | 17, 18, 19, 31 | 5.43 | 195 | −15 | −57 | −3 |
| Fusiform gyrus | L | 37 | 5.31 | 33 | −48 | −54 | −15 |
| Visual spelling | |||||||
| Superior temporal gyrus/middle temporal gyrus/fusiform gyrus/inferior parietal lobule/lingual gyrus/cuneus |
L | 22, 21, 37, 40, 17, 18, 19 | Inf | 2489 | −42 | −72 | −15 |
| Dorsal inferior frontal gyrus | L | 44, 9 | Inf | 1875 | −45 | 6 | 24 |
| Superior temporal gyrus/middle temporal gyrus/fusiform gyrus/inferior parietal lobule/lingual gyrus/cuneus |
R | 22, 21, 37, 40, 17, 18, 19 | Inf | 1392 | 42 | −78 | −12 |
| Ventral inferior frontal gyrus | L | 45, 47 | Inf | 924 | −33 | 21 | 0 |
| Medial frontal gyrus/anterior cingulate | L+R | 8, 32 | Inf | 784 | −3 | 18 | 45 |
| Ventral inferior frontal gyrus | R | 45, 47 | 7.61 | 187 | 36 | 24 | −3 |
| Thalamus | L | 5.63 | 125 | −9 | −15 | 9 | |
| Parahippocampal gyrus | L | 27 | 5.14 | 68 | −15 | −36 | 0 |
| Middle frontal gyrus | R | 46 | 4.96 | 191 | 45 | 33 | 21 |
H: hemisphere, L: left, R: right, BA: Brodmann area. p<0.001 uncorrected, 20 or greater voxels.
2.3. Effective connectivity for auditory spelling task
Table 3 shows the significance of intrinsic connections across the whole group for the auditory spelling task. Most intrinsic connections were significant with the exception of all input to Heschl’s gyrus, output from Heschl’s gyrus to dorsal inferior frontal gyrus and to fusiform gyrus, and bidirectional connections between inferior parietal lobule and fusiform gyrus. Because our a priori connections of interest were those emanating from Heschl’s gyrus, we calculated a one-way ANOVA with 5-coupled regions from Heschl’s gyrus, with Scheffe’s contrasts between means. This analysis revealed that there were significant differences in the intrinsic connections from Heschl’s gyrus, F(38)=100.43, p<0.001. In particular, the connection from Heschl’s gyrus to superior temporal gyrus was stronger than all other connections out from Heschl’s gyrus (p<0.001; see Fig. 2A, green arrow), the connection from Heschl’s gyrus to inferior parietal lobule was stronger than all other connections out from Heschl’s gyrus except from Heschl’s to superior temporal gyrus (p<0.001), and the connection from Heschl’s gyrus to ventral inferior frontal gyrus was stronger than from Heschl’s gyrus to fusiform gyrus (p=0.006). There were no significant age or accuracy correlations with the intrinsic connections.
Table 3.
Intrinsic connections and modulatory effects for the auditory and visual spelling task: Effects go from row to column
| Auditory spelling: Intrinsic | ||||||
|---|---|---|---|---|---|---|
| →dIFG | →vIFG | →FG | →STG | →IPL | →HG | |
| dIFG→ | – | .163* | .156* | .142* | .069* | .004 |
| vIFG→ | .181* | – | .136* | .159* | .069* | .010 |
| FG→ | .160* | .125* | – | .083* | .027 | .000 |
| STG→ | .178* | .171* | .108* | – | .170* | .017 |
| IPL→ | .078* | .068* | .030 | .168* | – | .007 |
| HG→ | .036 | .085* | −.023 | .482* | .217* | – |
| Auditory spelling: Modulatory | ||||||
| →dIFG | →vIFG | →FG | →STG | →IPL | →HG | |
| dIFG→ | – | .021* | .018* | .017* | .003 | .006 |
| vIFG→ | .019* | – | .017* | .019* | .004 | .008 |
| FG→ | .016* | .016* | – | .013* | .002 | .005 |
| STG→ | .021* | .023* | .019* | – | .005 | .008 |
| IPL→ | .009* | .010 | .007* | .012* | – | .005 |
| HG→ | .121* | .117* | .112* | .139* | .016 | – |
| Visual spelling: Intrinsic | ||||||
| →dIFG | →vIFG | →FG | →STG | →IPL | →CAL | |
| dIFG→ | – | .042* | .148* | .101* | .067* | .078* |
| vIFG→ | .050* | – | .052* | .054* | .029* | .047* |
| FG→ | .168* | .051* | – | .134* | .095* | .106* |
| STG→ | .124* | .050* | .146* | – | .067* | .099* |
| IPL→ | .097* | .035* | .118* | .077* | – | .063* |
| CAL→ | .217* | .004 | .362* | .189* | .195* | – |
| Visual spelling: Modulatory | ||||||
| →dIFG | →vIFG | →FG | →STG | →IPL | →CAL | |
| dIFG→ | – | .012* | .012* | .017* | .009* | .019* |
| vIFG→ | .006* | – | .007* | .008* | .006* | .010* |
| FG→ | .013* | .015* | – | .022* | .012* | .025* |
| STG→ | .011* | .012* | .014* | – | .010* | .023* |
| IPL→ | .009* | .010* | .011* | .014* | – | .017* |
| CAL→ | .085* | .107* | .114* | .158* | .080* | – |
dIFG, dorsal inferior frontal gyrus; vIFG, ventral inferior frontal gyrus; FG, fusiform gyrus; STG, superior temporal gyrus; IPL, inferior parietal lobule; HG, Heschl’s gyrus; CAL, calcarine.
p<0.002.
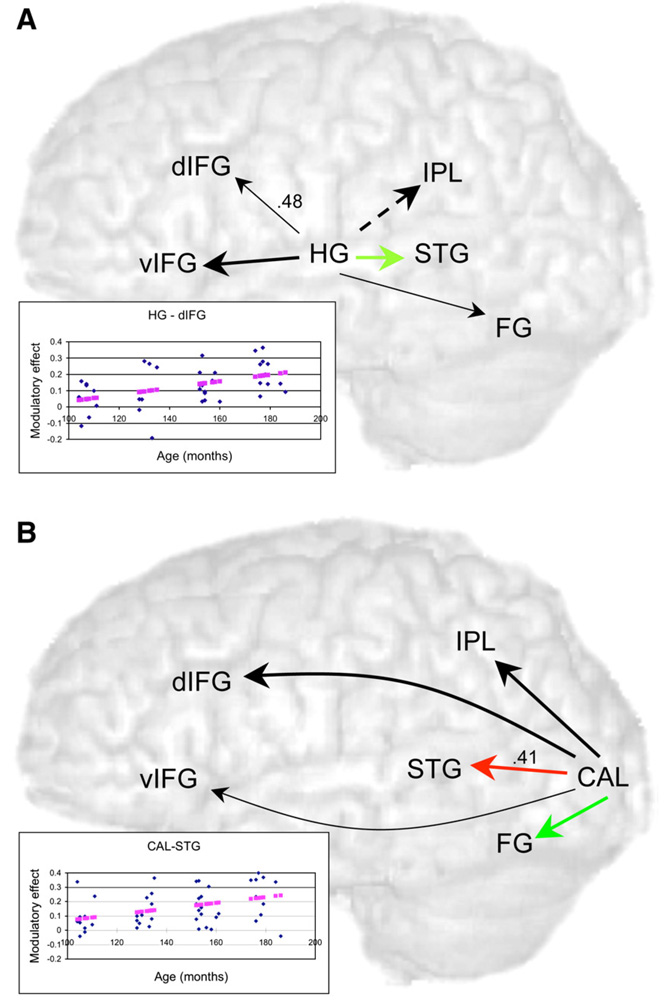
Fig. 2.
Intrinsic connections and modulatory effects from primary cortical regions for the effective connectivity analyses for the auditory spelling task (A) and the visual spelling task (B). For the auditory spelling task, the intrinsic connection from Heschl’s gyrus to superior temporal gyrus (green) was stronger than all other intrinsic connections out from Heschl’s gyrus. The modulatory effect from Heschl’s gyrus to dorsal inferior frontal gyrus was significantly correlated with age (r=.48). Scatter plot of this correlation with fitted line is in lower left corner. For the visual spelling task, the intrinsic connection from calcarine to fusiform gyrus (green) was stronger than all other intrinsic connections out from calcarine and the modulatory effect from calcarine to superior temporal gyrus (red) was stronger than other modulatory effects out from calcarine. The modulatory effect from calcarine to superior temporal gyrus was also significantly correlated with age (r=.41). Scatter plot of this correlation with fitted line is in lower left corner. dIFG, Dorsal inferior frontal gyrus; vIFG, ventral inferior frontal gyrus; FG, fusiform gyrus; STG, superior temporal gyrus; IPL, inferior parietal lobule; HG, Heschl’s gyrus; CAL, calcarine. Bold arrows indicate significant intrinsic connections (thin arrows, non-significant) and solid arrows indicate significant modulatory effects (dotted arrows, non-significant).
Table 3 shows the significance of modulatory effects across the whole group for the auditory spelling task. There were modulatory effects on all inputs to fusiform gyrus, dorsal inferior frontal gyrus, superior temporal gyrus, and ventral inferior frontal gyrus (except from inferior parietal lobule). Heschl’s gyrus and inferior parietal lobule were the only two regions with no significant modulatory input. Modulatory effects for all outputs were found for each region, except to Heschl’s gyrus. Because our a priori effects of interest were those emanating from Heschl’s gyrus, we calculated a one-way ANOVA with 5-coupled regions from Heschl’s gyrus, with Scheffe’s comparisons between means. This analysis revealed that there were significant differences in the modulatory effects from Heschl’s gyrus, F(38)=5.46, p<0.001. In particular, the modulatory effect from Heschl’s gyrus to inferior parietal lobule was weaker than all other connections out from Heschl’s gyrus (p<0.05), and there were no significant differences among the other modulatory effects. In addition, correlational analyses showed that there was a developmental increase in the modulatory effect from Heschl’s gyrus to dorsal inferior frontal gyrus [r(38)=.48, p=0.003; see Fig. 2A, scatter plot]. There were no other correlations between age and modulatory effects, and there were no correlations between accuracy and modulatory effects.
2.4. Effective connectivity for visual spelling task
Table 3 shows the significance of intrinsic connections across the whole group for the visual spelling task. All intrinsic connections were significant with the exception of the connection from calcarine to ventral inferior frontal gyrus. Because our a priori connections of interest were those emanating from calcarine, we calculated a one-way ANOVA with 5-coupled regions from calcarine, with Scheffe’s comparisons between means. This analysis revealed that there were significant differences in the intrinsic connections from calcarine, F(46)= 62.18, p<0.001. In particular, the connection from calcarine to fusiform gyrus was stronger than all other connections out from calcarine (p<0.001; see Fig. 2B, green arrow) and the connection from calcarine to ventral inferior frontal gyrus was weaker than all other connections out from calcarine (p<0.001). There were no significant age or accuracy correlations with the intrinsic connections.
Table 3 shows the significance of modulatory effects across the whole group for the visual spelling task. All modulatory effects between regions were significant. Because our a priori effects of interest were those emanating from calcarine, we calculated a one-way ANOVA with 5-coupled regions from calcarine, with Scheffe’s comparisons between means. This analysis revealed that there were significant differences in the modulatory effects from calcarine, F(46)=3.58, p=0.007. In particular, the modulatory effect from calcarine to superior temporal gyrus was stronger than from calcarine to inferior parietal lobule (p=0.025) and to dorsal inferior frontal gyrus (p=0.044; see Fig. 2B, red arrow). In addition, correlational analyses showed that there was a developmental increase in the modulatory effect from calcarine to superior temporal gyrus [r(46)=.41, p=0.004; see Fig. 2B, scatter plot]. There were no other correlations between age and modulatory effects, and there were no correlations between accuracy and modulatory effects.
3. Discussion
This study used dynamic causal modeling (DCM) to examine intrinsic connections and modulatory effects in left hemisphere regions when children (9- to 15-year-olds) performed spelling tasks in the auditory and visual modalities. Intrinsic connections are interregional influences in the absence of modulating experimental effects and modulatory effects are the changes in the intrinsic connectivity between regions induced by the spelling task. For both modalities, there were modulatory effects involving inferior frontal gyrus, inferior parietal cortex and fusiform gyrus. This is broadly consistent with studies in adults that have reported activation in these regions in a variety of spelling tasks (Beeson et al., 2003; Booth et al., 2002; Katanoda et al., 2001; Menon and Desmond, 2001; Nakamura et al., 2000; Petrides et al., 1995; Sugihara et al., 2006; Sugishita et al., 1996; Tokunaga et al., 1999). None of these studies, however, found activation in superior temporal gyrus, whereas our study revealed reliable activation in and modulatory effects on connections with superior temporal gyrus in both visual and auditory modalities. This could be explained by task differences as most of previous studies used physical writing which may have placed greater emphasis on motor processes (Beeson et al., 2003; Katanoda et al., 2001; Menon and Desmond, 2001; Nakamura et al., 2000; Petrides et al., 1995; Sugihara et al., 2006; Tokunaga et al., 1999). In contrast, our tasks involved spelling judgments to sequentially presented words, so it is more likely that phonological processes were engaged. One notable difference between the modalities in our study was that the auditory spelling task had unidirectional modulatory effects out of primary auditory cortex (Heschl’s gyrus), whereas the visual spelling task had bidirectional modulatory effects for primary visual cortex (calcarine). This presumably reflects the fact that the auditory spelling task required the mapping from phonological to orthographic forms, whereas the visual spelling task performance could be aided by lower level visual processing.
As may be expected, both tasks had strongest intrinsic connections from primary cortical regions (Heschl’s gyrus for the auditory task and calcarine for the visual task) to unimodal association regions of the respective modality (i.e. superior temporal gyrus, usually depicted in phonological processing, for the auditory task; and fusiform gyrus, usually depicted in orthographic processing, for the visual task). Interestingly, the modulatory effect of the spelling task on connections going out from the primary regions showed developmental increases for the connection going into superior temporal gyrus in the visual task, and for the connection going into dorsal inferior frontal gyrus in the auditory task. These results show that, despite the orthographic demands of the spelling tasks, there were age-related increases in the connectivity with regions involved in phonological processing. These represent the two major findings of the current study and will be discussed in turn.
3.1. Effective connectivity from primary visual cortex
Left superior temporal gyrus (BA 22) seems to be centrally involved in phonological processing. Studies have shown greater activation in this region for words with higher phonological complexity (Desai et al., 2006) and words with low phono-tactic probabilities show different effects over repeated exposure as compared to words with high phonotactic probabilities (Majerus et al., 2005). Activation of phonological representations also seems to be automatic when reading. Activation in left superior temporal gyrus has been found for briefly presented Chinese characters (Peng et al., 2004), making judgments of the stress of words (strong or weak initial stress) whether spoken or written (Aleman et al., 2005), and there is greater activation for words than for objects (Moore and Price, 1999). Languages with more regular correspondences between graphemes and phonemes seem to show more robust activation in left superior temporal gyrus (Meschyan and Hernandez, 2006), presumably because phonology is more easily accessible. Left superior temporal gyrus seems to be involved in phonological and not articulatory processes, as studies show activation in this region is not modulated by word length (Graves et al., 2007). Further, a transcranial magnetic simulation study (TMS) showed that repetition priming during pronunciation was eliminated when left inferior parietal lobule, but not when left superior temporal gyrus, was stimulated, In contrast, priming during lexical decision was eliminated when left superior temporal gyrus, but not left inferior parietal lobule, was stimulated (Nakamura et al., 2006). The authors suggested that this double dissociation reflects that left inferior parietal lobule is critical for articulation, whereas that left superior temporal cortex is centrally involved in phonological processing.
Our study found developmental increases in modulatory effects for the visual spelling task from calcarine to superior temporal gyrus. Although there were developmental increases in the modulatory effect from calcarine to superior temporal gyrus, the modulatory effect for the group as a whole from calcarine to superior temporal gyrus was stronger than other effects from calcarine, suggesting automatic activation of phonology for all children despite the orthographic requirements of the task. The developmental increase in this modulatory effect is consistent with many behavioral studies that show automatic phonological activation during reading increases with age and skill (Booth et al., 1999, 2000). Previous studies using visual spelling tasks similar to the current study have not reported developmental differences in signal intensity in superior temporal gyrus (Booth et al., 2003b, 2004). This may suggest that the nature of phonological representations in superior temporal cortex does not change substantially during late childhood and adolescence. However, automatic access to these representations from primary visual regions still increases in this age range, and may presumably account also for improvements in reading fluency (Speece and Ritchey, 2005). Although previous studies have implicated deficits in primary visual cortex in reading disorders (Demb et al., 1997), studies examining spelling tasks have not generally reported developmental or skill effects in primary cortex (Booth et al., 2003a,b, 2004, 2007) and synaptogenesis and structural MRI studies suggest early maturation of visual cortex (Huttenlocher and Dabholkar, 1997; Sowell et al., 2001). Therefore, the developmental increase in modulatory effects from calcarine to superior temporal gyrus seems to be primarily due to the maturation of these pathways and their strategic recruitment for the task, and not maturation within each brain region.
3.2. Effective connectivity from primary auditory cortex
Several studies using verbal working memory paradigms have shown activation in left dorsal inferior frontal gyrus (BA 44) during maintenance/manipulation of verbal information in memory (Barde and Thompson-Schill, 2002; Tsukiura et al., 2001) that increases with greater load demands (Woodward et al., 2006; Zarahn et al., 2005). Although these studies suggest that dorsal inferior frontal gyrus may be generally related to maintenance/manipulation in verbal memory, other studies suggest that dorsal inferior frontal gyrus is more specifically involved in phonological segmentation. One of the most consistent findings is that pseudowords produce greater activation than words in dorsal inferior frontal gyrus for both the visual and auditory modalities (Burton et al., 2005; Fiebach et al., 2002; Heim et al., 2005; Ischebeck et al., 2004). Pseudo-words place large demands on segmentation because they must be decomposed and read by grapheme to phoneme correspondences. More direct evidence for the role of dorsal inferior frontal gyrus in segmentation is that this region shows activation during initial phoneme discrimination (Heim et al., 2003), and a transcranial magnetic stimulation study (TMS) showed increased reaction time and decreased accuracy for initial sound similarity judgments and stress assignment judgments (Romero et al., 2006). Other studies suggest that dorsal inferior frontal gyrus may also be involved in segmentation during covert and overt articulatory rehearsal (Hickok and Poeppel, 2007; Horwitz et al., 2003). One study showed greater activation for high load compared to low load in articulatory rehearsal but not in verbal working memory (Chen and Desmond, 2005). Together, these studies suggest that dorsal inferior frontal gyrus is involved in verbal working memory in general and in phonological segmentation in particular.
Our study found developmental increases in modulatory effects for the auditory spelling task from Heschl’s gyrus to dorsal inferior frontal gyrus, and not to ventral inferior frontal gyrus. This suggests that brain regions implicated in phonological, and not semantic processing, were recruited for spelling (Poldrack et al., 1999). Our finding, showing the importance of dorsal inferior frontal gyrus in spelling, is consistent with several studies in adults reporting activation in BA 9/44 in spelling tasks (Booth et al., 2002; Katanoda et al., 2001; Nakamura et al., 2000; Tokunaga et al., 1999), although there is one inconsistent study showing activation in ventral inferior frontal gyrus (Beeson et al., 2003). In addition, our results of developmental increases in the modulatory effects into dorsal inferior frontal gyrus is consistent with studies using a similar auditory spelling task that have shown developmental increases in signal intensity in dorsal inferior frontal gyrus (BA 44/9) (Booth et al., 2003b, 2004). The developmental increase in effective connectivity to dorsal inferior frontal gyrus could reflect verbal working memory and phonological segmentation required by the auditory spelling task. This task requires the maintenance of phonological information in memory, accessing visual orthographic information, segmentation of the onset from the rime, and a determination of whether two words have similar spelling of the rime. Although previous studies have implicated deficits in primary auditory cortex in reading disorders (Helenius et al., 2002), studies examining spelling tasks have not generally reported developmental or skill effects in auditory cortex after age 9 (Booth et al., 2003a,b, 2004, 2007) and synaptogenesis and structural MRI studies suggest early maturation of auditory cortex (Huttenlocher and Dabholkar, 1997; Sowell et al., 2001). Therefore, the developmental increase in modulatory effects from Heschl’s gyrus to dorsal inferior frontal gyrus seems to be primarily due to the maturation of this pathway, and its strategic recruitment in the spelling task, and not maturation within auditory cortex.
3.3. Lack of developmental differences in inferior parietal lobule
Although in previous studies we found greater activation in adults compared to children and developmental increases (9-to 15-year-olds) in signal intensity in inferior parietal cortex (BA 40/39) using tasks similar to (Booth et al., 2004) or the same as (Booth et al., in press) the current study, we did not find developmental differences in effective connectivity with inferior parietal lobule in either the auditory or the visual modality. The independence of developmental differences in signal intensity and effective connectivity is consistent with a developmental study examining rhyming judgments to visually presented words (Bitan et al., 2007). The rhyming task showed developmental increases (9- to 15-year-olds) in signal intensity in inferior parietal cortex, but no developmental effects in effective connectivity with this region. They suggested that the cortical system involved in orthographic processing and in mapping of orthography to phonology may mature and become more elaborated during development, but there is no change in its interaction with other regions that reflects its contribution to the rhyming task. In both the rhyming task and in the current spelling tasks, the increase in activation may thus reflect growing connectivity within the region, rather than connectivity between regions.
3.4. Conclusion
This study showed developmental increases in the effective connectivity from Heschl’s gyrus to dorsal inferior frontal gyrus in an auditory spelling task and from calcarine to superior temporal gyrus in a visual spelling task. These findings suggest that with maturation, primary cortical regions gain direct access to regions involved in phonological representations and phonological segmentation, which are utilized automatically regardless of the requirements of the task.
4. Experimental procedures
4.1. Participants
Forty healthy children (9- to 15-year-olds, 24 females) performed the auditory spelling task. Forty-eight healthy children (9- to 15-year-olds, 24 females) performed the visual spelling task. See Table 1 for age breakdown and standardized measures (see below for statistical analyses on these measures). Parents of children were given an interview to ensure their children met the inclusionary criteria for the study. Children were reported to be right handed, native English speakers, with normal hearing and normal or corrected-to-normal vision. All children were reported to be free of neurological diseases or psychiatric disorders and were not taking medication affecting the central nervous system. Children were reported to not have a history of intelligence, reading, attention or oral-language deficits. Children were recruited from the Chicago metropolitan area.
4.2. Standardized testing
All participants were administered the Performance and Verbal portions of the Wechsler Abbreviated Scale of Intelligence (WASI) (Wechsler, 1999) and the Spelling subtest of the Wide Range Achievement Test – Third Edition (Wilkinson, 1993).
4.3. Tasks
For the auditory spelling task, two spoken words were presented in a sequential order and a black fixation-cross appeared throughout the trial. The duration of each word was between 500 and 800 ms followed by a brief period of silence, with the second word beginning 1000 ms after the onset of the first. For the visual spelling task, each word was presented visually for 800 ms followed with a second word beginning 1000 ms after the onset of the first. For both tasks, a red fixation-cross appeared on the screen after the second word, indicating the need to make a response during the subsequent 2600-ms interval. If the two words had the same spelling for all letters from the first vowel onwards, the participant was asked to press a button with the index finger; if the two words did not have the same spelling for all letters from the first vowel onwards, the participant was asked to press a different button with the middle finger.
For both tasks, twenty-four word pairs were presented in each one of four lexical conditions that independently manipulated the orthographic and phonological similarity between words. In the two non-conflicting conditions, the two words were either similar in both orthography and phonology (O+P+, e.g. gate–hate), or different in both orthography and phonology (O−P−, e.g. press–list). In the two conflicting conditions, the two words had either similar orthography but different phonology (O+P−, e.g. pint–mint), or different orthography but similar phonology (O−P+, e.g. jazz–has). Orthographic and phonological similarity was manipulated so the spelling decision could not solely be based on the sound or spelling of the word. All words were monosyllabic words, 4–7 letters long, and were matched across conditions for written word frequency in adults and children (The Educator’s Word Frequency Guide, 1996) and for written and spoken word frequency in adults (Baayen et al., 1995).
There were three kinds of control tasks. The simple perceptual control for the auditory spelling task had 24 pairs of single pure tones and for the visual spelling task had 24 pairs of single non-linguistic symbols. The complex perceptual control for the auditory spelling task had 24 pairs of three-tone stimuli and for the visual spelling task had 24 pairs of three non-linguistic symbols. For both the simple and complex perceptual controls, participants determined whether the stimuli were identical or not by pressing a yes or no button. The third control task involved 72 null events each for the auditory spelling and visual spelling tasks. The participant was instructed to press a button when a black fixation-cross at the center of the visual field turned red. Procedures for presenting the tones, symbols and fixation-crosses were the same as the word judgment tasks. Each task was administered in two 108 trial runs, in which the order of lexical, perceptual, and null trials was optimized for event-related design (Burock et al., 1998). The order of stimuli within task was fixed for all subjects.
4.4. MRI practice session
After informed consent was obtained and the standardized tests were administered, participants were invited for a practice session, in which they were trained to minimize head movement in front of a computer screen using an infrared tracking device. In addition, they performed one run of each experimental task in a simulator scanner, in order to make sure they understood the tasks and to acclimatize themselves to the scanner environment. Different stimuli were used in the practice and in the scanning sessions. Scanning took place within a week from the practice session.
4.5. MRI data acquisition
Images were acquired using a 1.5-T GE scanner, using a standard head coil. Head movement was minimized using vacuum pillow (Bionix, Toledo, OH). The stimuli were projected onto a screen, and viewed through a mirror attached to the inside of the head coil. Participants’ responses were recorded using an optical response box (Current Designs, Philadelphia, PA). The blood oxygen level dependent (BOLD) functional images were acquired using the echo planar imaging (EPI) method. The following parameters were used for scanning: time of echo (TE)=35 ms, flip angle=90°, matrix size=64×64, field of view=24 cm, slice thickness=5 mm, number of slices=24; time of repetition (TR)=2000 ms. Four runs, with 240 repetitions each, were administered for the functional images. In addition, structural T1 weighted 3D image were acquired (TR = 21 ms, TE = 8 ms, flip angle = 20°, matrix size = 256 × 256, field of view = 22 cm, slice thickness = 1 mm, number of slices = 124).
4.6. Image analysis
Data analysis was performed using SPM2 (Statistical Parametric Mapping) (http://www.fil.ion.ucl.ac.uk/spm). The images were spatially realigned to the first volume to correct for head movements. No individual runs had more than 4 mm maximum displacement. Sinc interpolation was used to minimize timing-errors between slices. The functional images were co-registered with the anatomical image, and normalized to the standard T1 Montreal Neurological Institute (MNI) template. The data was then smoothed with a 10-mm isotropic Gaussian kernel. Statistical analyses at the first level were calculated using an event-related design, with word trials, perceptual trials, null trials, auditory or visual trials (word+perceptual) as four conditions. A high pass filter with a cutoff period of 128 s was applied. Word pairs were treated as individual events for analysis and modeled using a canonical hemodynamic response function (HRF). Group results for the conventional analyses were obtained using random-effects analyses by combining subject-specific summary statistics across the group in the contrast of all words versus null.
4.7. Effective connectivity
Based on the group main effect in the conventional analysis of words versus null, five regions of interest (ROIs) were specified in lateral portions of the left hemisphere separately for the auditory spelling and visual spelling task: dorsal inferior frontal gyrus (BA 44/9), ventral inferior frontal gyrus (BA 45/47), fusiform gyrus (BA 19/37), superior temporal gyrus (BA 22), and inferior parietal lobule (BA 40/7). We chose lateral portions of the left hemisphere because the left lateralization of language and because previous studies have implicated these regions in spelling in both adults and children (Booth et al., 2002, 2004, 2007). For dorsal inferior frontal gyrus, ventral inferior frontal gyrus, fusiform gyrus, and inferior parietal lobule, subject-specific ROIs were defined as the most active voxel within 25 mm of the group maximum with the constraint that no individual had two ROIs closer than 25 mm, so for some individuals ROIs were defined based on the second or third most active local maxima in each individual. The following anatomical masks were used for the individual ROIs to insure that similar neural regions were represented across individuals: inferior frontal gyrus for dorsal and ventral inferior frontal gyrus; inferior temporal gyrus/fusiform gyrus for fusiform gyrus; superior temporal gyrus for superior temporal gyrus, and inferior/superior parietal lobule for inferior parietal lobule. Regional responses were summarized as the principal eigenvariate within a 6-mm radius sphere centered on the chosen voxel for each individual. In order to keep Heschl’s gyrus (the input region for the auditory task) distinct from superior temporal gyrus, we used a fixed ROI across individuals for superior temporal gyrus with a 6-mm sphere centered on the group maximum. In order to make the ROI selection procedure similar to the auditory task, the same ROI for superior temporal gyrus was used for the visual spelling task. In order to ensure our input region for the auditory spelling task was primary auditory area, Heschl’s gyrus (BA 41) was defined as the intersection of a 6 mm sphere centered on the group maximum and an anatomical mask of Heschl’s gyrus (based on Pick Atlas of SPM2). In order to ensure our input region for the visual spelling task was primary visual area, calcarine (BA 17) was defined as the intersection of a 6-mm sphere centered on the group maximum and an anatomical mask of calcarine (based on Pick Atlas of SPM2).
Effective connectivity analysis was performed using the dynamic causal modeling (DCM) tool in SPM2 (Friston et al., 2003; Penny et al., 2004). DCM is a nonlinear systems identification procedure that uses Bayesian estimation of parameters to make inferences about effective connectivity between neural systems and how this connectivity is affected by experimental conditions. In DCM, three sets of parameters are estimated: the direct influence of stimuli on regional activity; the intrinsic or latent connections between regions (i.e., the interregional influences in the absence of modulating experimental effects); and the changes in the intrinsic connectivity between regions induced by the experimental design (modulatory effects) (Mechelli et al., 2003). In the present experiment, modulatory effects represent the influence of the spelling task (both conflicting and non-conflicting conditions) on the connectivity between regions. Since ‘connectivity’ in DCM is measured through the coupling of changes in imaging signals, rather than anatomically, a significant unidirectional modulatory influence of one brain region upon another does not necessarily reflect the presence of a direct and unidirectional anatomical connection. Instead, the connectivity revealed by DCM reflects the inferred direction of neural influences that are specific to the imaging conditions and that may be mediated through inter-neurons or other brain regions not explicitly included in the model.
The analysis adopted a two-stage procedure that is formally identical to the summary statistic approach used in random effects analysis of neuroimaging data. The parameters from the subject-specific, first level DCM models were taken to a second, between-subject level using the random effects approach (Bitan et al., 2005). Separate DCM models were specified for the auditory and visual modalities. Subject-specific DCMs were assumed to be fully connected (resulting in 30 connections). The modulatory (bilinear) effects for each of the spelling tasks were specified on the connections among all regions. In the visual task model, direct input of the ‘visual’ condition (including words and perceptual conditions) was specified on calcarine. In the auditory task model, direct input of the ‘auditory’ condition (including words and perceptual conditions) was specified on Heschl’s gyrus.
Our a priori modulatory effects of interest were the connections going out from the input regions (Heschl’s gyrus for the auditory spelling task and calcarine for the visual spelling task). Because each of the input regions had connections with 5 other regions, we set our corrected level of significance at p<0.01 in our one-sample tests for intrinsic connections, modulatory effects, and correlations of these effects with age (partialed for accuracy) or accuracy (partialed for age). We also calculated one-way analyses of variance (ANOVAs), with Scheffe’s post-hoc tests, on the connections going out from the input regions to determine if output was significantly stronger with certain coupled regions. All other analyses reported are significant corrected for 25 connections (p<0.002).
Acknowledgments
This research was supported by grants from the National Institute of Child Health and Human Development (HD042049) and by the National Institute of Deafness and Other Communication Disorders (DC06149) to James R. Booth.
REFERENCES
- Aleman A, Formisano E, Koppenhagen H, Hagoort P, de Haan EH, Kahn RS. The functional neuroanatomy of metrical stress evaluation of perceived and imagined spoken words. Cereb. Cortex. 2005;15(2):221–228. doi: 10.1093/cercor/bhh124. [DOI] [PubMed] [Google Scholar]
- Baayen RH, Piepenbrock R, Gulikers L. The CELEX Lexical Database [CD-ROM]. Version Release 2. Philadelphia, PA: Linguistic Data Consortium, University of Pennsylvania; 1995. [Google Scholar]
- Barde LH, Thompson-Schill SL. Models of functional organization of the lateral prefrontal cortex in verbal working memory: evidence in favor of the process model. J. Cogn. Neurosci. 2002;14(7):1054–1063. doi: 10.1162/089892902320474508. [DOI] [PubMed] [Google Scholar]
- Beeson PM, Rapcsak SZ, Plante E, Chargualaf J, Chung A, Johnson SC, Trouard TP, Mail E. The neural substrates of writing: a functional magnetic resonance imaging study. Aphasiology. 2003;17(6–l7):647–665. pelagie@u.arizona.edu. [Google Scholar]
- Berl MM, Vaidya CJ, Gaillard WD. Functional neuroimaging of developmental and adaptive changes in neurocognition. NeuroImage. 2006;30:679–691. doi: 10.1016/j.neuroimage.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Bitan T, Booth JR, Choy J, Burman DD, Gitelman DR, Mesulam MM. Shifts of effective connectivity within a language network during rhyming and spelling. J. Neurosci. 2005;25:5397–5403. doi: 10.1523/JNEUROSCI.0864-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitan T, Burman DD, Lu D, Cone N, Gitelman DR, Mesulam MM, Booth JR. Weaker top–down modulation from left inferior frontal gyrus area in children. NeuroImage. 2006;33:991–998. doi: 10.1016/j.neuroimage.2006.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitan T, Burman DD, Chou TL, Dong L, Cone NE, Cao F, Bigio JD, Booth JR. The interaction of orthographic and phonological information in children: an fMRI study. Hum. Brain Mapp. doi: 10.1002/hbm.20313. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitan T, Cheon J, Lu D, Burman DD, Gitelman DR, Mesulam MM, Booth JR. Developmental changes in activation and connectivity of phonological processing. NeuroImage. 2007;38:564–575. doi: 10.1016/j.neuroimage.2007.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Perfetti CA, MacWhinney B. Quick, automatic, and general activation of orthographic and phonological representations in young readers. Dev. Psychol. 1999;35:3–19. doi: 10.1037/0012-1649.35.1.3. [DOI] [PubMed] [Google Scholar]
- Booth JR, Perfetti CA, MacWhinney B, Hunt SB. The association of rapid temporal perception with orthographic and phonological processing in reading impaired children and adults. Sci. Stud. Read. 2000;4:101–132. [Google Scholar]
- Booth JR, Burman DD, Van Santen FW, Harasaki Y, Gitelman DR, Parrish TR, Mesulam MM. The development of specialized brain systems in reading and oral-language. Child Neuropsychol. 2001;7(3):119–141. doi: 10.1076/chin.7.3.119.8740. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TR, Mesulam MM. Functional anatomy of intra- and cross-modal lexical tasks. NeuroImage. 2002;16:7–22. doi: 10.1006/nimg.2002.1081. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TR, Mesulam MM. The relation between brain activation and lexical performance. Hum. Brain Mapp. 2003a;19:155–169. doi: 10.1002/hbm.10111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Zhang L, Choy J, Gitelman DR, Parrish TR, Mesulam MM. Modality-specific and -independent developmental differences in the neural substrate for lexical processing. J. Neurolinguist. 2003b;16:383–405. [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Zhang L, Gitelman DR, Parrish TR, Mesulam MM. Development of brain mechanisms for processing orthographic and phonological representations. J. Cogn. Neurosci. 2004;16:1234–1249. doi: 10.1162/0898929041920496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Cho S, Burman DD, Bitan T. Neural correlates of mapping from phonology to orthography in children performing an auditory spelling task. Dev. Sci. 2007;10:441–451. doi: 10.1111/j.1467-7687.2007.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TT, Lugar HM, Coalson RS, Miezin FM, Petersen SE, Schlaggar BL. Developmental changes in human cerebral functional organization for word generation. Cereb. Cortex. 2005;15(3):275–290. doi: 10.1093/cercor/bhh129. [DOI] [PubMed] [Google Scholar]
- Burock MA, Buckner RL, Woldorff MG, Rosen BR, Dale AM. Randomized event-related experimental designs allow for extremely rapid presentation rates using functional MRI. NeuroReport. 1998;9:3735–3739. doi: 10.1097/00001756-199811160-00030. [DOI] [PubMed] [Google Scholar]
- Burton MW, Locasto PC, Krebs-Noble D, Gullapalli RP. A systematic investigation of the functional neuroanatomy of auditory and visual phonological processing. NeuroImage. 2005;26(3):647–661. doi: 10.1016/j.neuroimage.2005.02.024. [DOI] [PubMed] [Google Scholar]
- Chen SH, Desmond JE. Cerebrocerebellar networks during articulatory rehearsal and verbal working memory tasks. NeuroImage. 2005;24(2):332–338. doi: 10.1016/j.neuroimage.2004.08.032. [DOI] [PubMed] [Google Scholar]
- Cohen L, Jobert A, Le Bihan D, Dehaene S. Distinct unimodal and multimodal regions for word processing in the left temporal cortex. NeuroImage. 2004;23(4):1256–1270. doi: 10.1016/j.neuroimage.2004.07.052. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Jobert A, Naccache L, Ciuciu P, Poline JB, Le Bihan D, Cohen L. Letter binding and invariant recognition of masked words: behavioral and neuroimaging evidence. Psychol. Sci. 2004;15(5):307–313. doi: 10.1111/j.0956-7976.2004.00674.x. [DOI] [PubMed] [Google Scholar]
- Demb JB, Boyton GM, Heeger DJ. Brain activity in the visual cortex predicts individual differences in reading performance. Proc. Natl. Acad. Sci. U. S. A. 1997;94:13363–13366. doi: 10.1073/pnas.94.24.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai R, Conant LL, Waldron E, Binder JR. FMRI of past tense processing: the effects of phonological complexity and task difficulty. J. Cogn. Neurosci. 2006;18(2):278–297. doi: 10.1162/089892906775783633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebach C, Friederici AD, Mueller K, von Cramon DY. fMRI evidence for dual routes to the mental lexicon in visual word recognition. J. Cogn. Neurosci. 2002;14(1):11–23. doi: 10.1162/089892902317205285. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W. Dynamic causal modelling. NeuroImage. 2003;19(4):1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Graves WW, Grabowski TJ, Mehta S, Gordon JK. A neural signature of phonological access: distinguishing the effects of word frequency from familiarity and length in overt picture naming. J. Cogn. Neurosci. 2007;19(4):617–631. doi: 10.1162/jocn.2007.19.4.617. [DOI] [PubMed] [Google Scholar]
- Heim S, Opitz B, Muller K, Friederici AD. Phonological processing during language production: fMRI evidence for a shared production—comprehension network. Cogn. Brain Res. 2003;16(2):285–296. doi: 10.1016/s0926-6410(02)00284-7. [DOI] [PubMed] [Google Scholar]
- Heim S, Alter K, Ischebeck AK, Amunts K, Eickhoff SB, Mohlberg H, Zilles K, von Cramon DY, Friederici AD. The role of the left Brodmann’s areas 44 and 45 in reading words and pseudowords. Cogn. Brain Res. 2005;25(3):982–993. doi: 10.1016/j.cogbrainres.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Helenius P, Salmelin R, Richardson U, Leinonen S, Lyytinen H. Abnormal auditory cortical activation in dyslexia 100 ms after speech onset. J. Cogn. Neurosci. 2002;14(4):603–617. doi: 10.1162/08989290260045846. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech. Nat. Rev., Neurosci. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Horwitz B, Amunts K, Bhattacharyya R, Patkin D, Jeffries K, Zilles K, Braun AR. Activation of Broca’s area during the production of spoken and signed language: a combined cytoarchitectonic mapping and PET analysis. Neuropsychologia. 2003;41(14):1868–1876. doi: 10.1016/s0028-3932(03)00125-8. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J. Comp. Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Ischebeck A, Indefrey P, Usui N, Nose I, Hellwig F, Taira M. Reading in a regular orthography: an fMRI study investigating the role of visual familiarity. J. Cogn. Neurosci. 2004;16(5):727–741. doi: 10.1162/089892904970708. [DOI] [PubMed] [Google Scholar]
- Katanoda K, Yoshikawa K, Sugishita M. Hum. Brain Mapp. 1. vol. 13. US: John Wiley & Sons Inc.; 2001. A functional MRI study on the neural substrates for writing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BCP, Kuppusamy K, Grueneich R, El-Ghazzawy O, Gordon RE, Lin W, Haacke M. Hemispheric language dominance in children demonstrated by functional magnetic resonance imaging. J. Child Neurol. 1999;14(2):78–82. doi: 10.1177/088307389901400203. [DOI] [PubMed] [Google Scholar]
- Majerus S, Van der Linden M, Collette F, Laureys S, Poncelet M, Degueldre C, Delfiore G, Luxen A, Salmon E. Modulation of brain activity during phonological familiarization. Brain Lang. 2005;92(3):320–331. doi: 10.1016/j.bandl.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Price CJ, Noppeney U, Friston KJ. A dynamic causal modeling study on category effects: bottom–up or top–down mediation? J. Cogn. Neurosci. 2003;15(7):925–934. doi: 10.1162/089892903770007317. [see comment] [DOI] [PubMed] [Google Scholar]
- Menon V, Desmond JE. Left superior parietal cortex involvement in writing: integrating fMRI with lesion evidence. Cogn. Brain Res. 2001;12(2):337–340. doi: 10.1016/s0926-6410(01)00063-5. [DOI] [PubMed] [Google Scholar]
- Meschyan G, Hernandez AE. Impact of language proficiency and orthographic transparency on bilingual word reading: an fMRI investigation. NeuroImage. 2006;29(4):1135–1140. doi: 10.1016/j.neuroimage.2005.08.055. [DOI] [PubMed] [Google Scholar]
- Moore CJ, Price CJ. Three distinct ventral occipitotemporal regions for reading and object naming. NeuroImage. 1999;10(2):181–192. doi: 10.1006/nimg.1999.0450. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Honda M, Okada T, Hanakawa T, Toma K, Fukuyama H, Konishi J, Shibasaki H. Brain. 5. Vol. 123. England: Oxford Univ Press; 2000. Participation of the left posterior inferior temporal cortex in writing and mental recall of kanji orthography: A functional MRI study. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Hara N, Kouider S, Takayama Y, Hanajima R, Sakai K, Ugawa Y. Task-guided selection of the dual neural pathways for reading. Neuron. 2006;52(3):557–564. doi: 10.1016/j.neuron.2006.09.030. [see comment] [DOI] [PubMed] [Google Scholar]
- Paulesu E, Frith U, Snowling M, Gallagher A, Morton J, Frackowiak RSJ, Frith CD. Is developmental dyslexia a disconnection syndrome? Brain. 1996;119:143–157. doi: 10.1093/brain/119.1.143. [DOI] [PubMed] [Google Scholar]
- Peng D-L, Ding G-S, Perry C, Xu D, Jin Z, Luo Q, Zhang L, Deng Y, Perry CChth. fMRI evidence for the automatic phonological activation of briefly presented words. Cogn. Brain Res. 2004;20(2):156–164. doi: 10.1016/j.cogbrainres.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Penny WD, Stephan KE, Mechelli A, Ffriston KJ. Comparing dynamic causal models. NeuroImage. 2004;22(3):1157–1172. doi: 10.1016/j.neuroimage.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Petrides M, Alivisatos B, Evans AC. Functional activation of the human ventrolateral frontal cortex during mnemonic retrieval of verbal information. Proc. Natl. Acad. Sci. U. S. A. 1995;92(13):5803–5807. doi: 10.1073/pnas.92.13.5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JD. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. NeuroImage. 1999;10(1):15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Shaywitz BA, Shaywitz SE, Constable RT, Skudlarski P, Fulbright RK, Bronen RA, Shankweiler DP, Katz L, Fletcher JM, et al. Cerebral organization of component processes in reading. Brain. 1996;119(4):1221–1238. doi: 10.1093/brain/119.4.1221. [DOI] [PubMed] [Google Scholar]
- Richards T, Berninger V, Nagy W, Parsons AC, Field KM, Richards A. Brain activation during language task contrasts in children with and without dyslexia: Inferring mapping processes and assessing response to spelling instruction. Educ. Child Psychol. 2005;22:62–80. [Google Scholar]
- Romero L, Walsh V, Papagno C. The neural correlates of phonological short-term memory: a repetitive transcranial magnetic stimulation study. J. Cogn. Neurosci. 2006;18(7):1147–1155. doi: 10.1162/jocn.2006.18.7.1147. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: inverse relationships during postadolescent brain maturation. J. Neurosci. 2001;21(22):8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speece DL, Ritchey KD. A longitudinal study of the development of oral reading fluency in young children at risk for reading failure. J. Learn. Disabil. 2005;38(5):387–399. doi: 10.1177/00222194050380050201. [DOI] [PubMed] [Google Scholar]
- Sugihara G, Kaminaga T, Sugishita M. Interindividual uniformity and variety of the “writing center”: a functional MRI study. NeuroImage. 2006;32(4):1837–1849. doi: 10.1016/j.neuroimage.2006.05.035. [DOI] [PubMed] [Google Scholar]
- Sugishita M, Takayama Y, Shiono T, Yoshikawa K, Takahashi Y. Functional magnetic resonance imaging (fMRI) during mental writing with phonograms. Neuroreport: an International Journal for the Rapid Communication of Research in Neuroscience. 1996;7(12):1917–1921. doi: 10.1097/00001756-199608120-00009. [DOI] [PubMed] [Google Scholar]
- The Educator’s Word Frequency Guide. Brewster, NY: 853 Touchstone Applied Science Associates, Inc.; 1996. [Google Scholar]
- Tokunaga H, Nishikawa T, Ikejiri Y, Nakagawa Y, Yasuno F, Hashikawa K, Nishimura T, Sugita Y, Takeda M. Different neural substrates for Kanji and Kana writing: a PET study. Neuroreport: an International Journal for the Rapid Communication of Research in Neuroscience. 1999;10(16):3315–3319. doi: 10.1097/00001756-199911080-00012. [DOI] [PubMed] [Google Scholar]
- Tsukiura T, Fujii T, Takahashi T, Xiao R, Inase M, Iijima T, Yamadori A, Okuda J. Neuroanatomical discrimination between manipulating and maintaining processes involved in verbal working memory; a functional MRI study. Brain Res. Cogn. Brain Res. 2001;11(1):13–21. doi: 10.1016/s0926-6410(00)00059-8. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence: The Psychological Corporation. Harcourt Brace & Company; 1999. [Google Scholar]
- Wilkinson GS. Wide Range Achievement Test. Third Edition. Wilmington, DE: Wide Range; 1993. [Google Scholar]
- Woodward TS, Cairo TA, Ruff CC, Takane Y, Hunter MA, Ngan ET. Functional connectivity reveals load dependent neural systems underlying encoding and maintenance in verbal working memory. Neuroscience. 2006;139(1):317–325. doi: 10.1016/j.neuroscience.2005.05.043. [DOI] [PubMed] [Google Scholar]
- Zarahn E, Rakitin B, Abela D, Flynn J, Stern Y. Positive evidence against human hippocampal involvement in working memory maintenance of familiar stimuli. Cereb. Cortex. 2005;15(3):303–316. doi: 10.1093/cercor/bhh132. [DOI] [PubMed] [Google Scholar]