Abstract
We examined the neural representations of orthographic and phonological processing in children, while manipulating the consistency between orthographic and phonological information. Participants, aged 9–15, were scanned while performing rhyming and spelling judgments on pairs of visually presented words. The orthographic and phonological similarity between words in the pair was independently manipulated, resulting in four conditions. In the nonconflicting conditions, both orthography and phonology of the words were either (1) similar (lime‐dime) or (2) different (staff‐gain); in conflicting conditions, words had (3) similar phonology and different orthography (jazz‐has) or (4) different phonology and similar orthography (pint‐mint). The comparison between tasks resulted in greater activation for the rhyming task in bilateral inferior frontal gyri (BA 45/47), and greater activation for the spelling task in bilateral inferior/superior parietal lobules (BA 40/7), suggesting greater involvement of phonological and semantic processing in the rhyming task, and nonlinguistic spatial processing in the spelling task. Conflicting conditions were more difficult in both tasks and resulted in greater activation in the above regions. The results suggest that when children encounter inconsistency between orthographic and phonological information they show greater engagement of both orthographic and phonological processing. Hum Brain Mapp 2006. © 2006 Wiley‐Liss, Inc.
Keywords: rhyming, spelling, conflict, task‐relevance, inferior frontal gyrus
INTRODUCTION
The aim of the current study is to examine the neural correlates of orthographic and phonological representations, and the interaction between these representations in children. Since phonological and orthographic processing and the mapping between them are acquired and elaborated during development, it is important to study these processes in children. Our study attempts to differentiate between orthographic and phonological processing by using a direct comparison between an orthographic task (spelling judgment) and a phonological task (rhyming judgment). In addition, the differences and the interaction between these processes are examined by manipulating the conflict between orthographic and phonological information.
Previous studies that used the rhyming task in adults showed activation in the left superior temporal, supramarginal, and posterior inferior frontal gyri (IFG) (Booth et al., 2002a; Crosson et al., 1999; Kareken et al., 2000; Lurito et al., 2000; Paulesu et al., 1996; Pugh et al., 1996; Xu et al., 2001). The same regions were active for children in phonological tasks such as nonword reading, phoneme deletion, and rhyming judgment (Booth et al., 2004; Georgiewa, 1999; Shaywitz et al., 2002; Temple et al., 2001). However, because the baseline for comparison was usually nonlinguistic stimuli, such as line orientation judgment or passive viewing of symbol strings, it is hard to distinguish phonological from other lexical processes (i.e. orthographic and semantic) automatically invoked by the linguistic stimuli.
For the spelling judgment task, both children and adults showed activation in bilateral infero‐temporal/fusiform gyri, IFG, and superior parietal lobules (SPL), when compared with nonlinguistic stimuli (Booth et al., 2002a, 2004; Tagamets et al., 2000). A direct comparison between children and adults showed greater activation for children in the right infero‐temporal cortex (Booth et al., 2004), previously associated with visual processing of nonlinguistic stimuli such as faces and objects (Gauthier et al., 2000; Nakamura et al., 2005). This finding may suggest that children, more than adults, rely on nonlinguistic processes for making orthographic judgments in the spelling task.
In the current study, the distinction between phonological and orthographic processing is examined by directly comparing between rhyming and spelling judgments of real words. Because orthographic and phonological processes may be automatically invoked even when not required by the task, a direct comparison between tasks enables us to distinguish between automatic processes and processes, which are specifically enhanced by the requirements of the task.
The interaction between orthographic and phonological processing in the current study is further explored by independently manipulating orthographic and phonological similarity between the words. Previous studies have examined the effect of orthographic similarity of the rime (first vowel and following letters in a word) on the performance of rhyming judgment in adults (Johnston and Mcdermott, 1986; Kramer and Donchin, 1987; Levinthal and Hornung, 1992; Polich et al., 1983) and children (McPherson et al., 1997; Rack, 1985). In this paradigm, pairs of words are visually presented in one of the four conditions: words that rhyme and have a similar spelling of the rime (e.g. dime‐lime), words that rhyme but have a different spelling (e.g. jazz‐has), words that do not rhyme but have a similar spelling (e.g. pint‐mint), and words that do not match in either rhyming or spelling (e.g. staff‐gain) (not all conditions were presented in all studies). These studies found greater difficulty in pairs of words for which orthographic and phonological information did not match (i.e. jazz‐has; pint‐mint), suggesting that the conflict between orthographic and phonological information interfered with the phonological judgment. In addition, the most difficult among the conflicting pairs were pairs that were spelled the same but did not rhyme (e.g. pint‐mint) (Johnston and Mcdermott, 1986; Kramer and Donchin, 1987; Levinthal and Hornung, 1992; McPherson et al., 1997; Rack, 1985). Some of the studies used a similar approach to examine the effect of phonological information on orthographic judgments of visually presented words in adults (Kramer and Donchin, 1987; Levinthal and Hornung, 1992). In similarity to the rhyming task, conflicting pairs were more difficult than nonconflicting pairs, suggesting that the mapping from orthography to phonology is automatic in adults, and occurs even when phonological information interferes with the task.
The procedure in the current study is similar to Booth et al. (2004), with a number of important modifications. (1) We present pairs rather than triplets of words to minimize working memory load. (2) All four lexical conditions are presented (two conflicting and two nonconflicting conditions) to ensure that subjects base their judgments on the required representational system (i.e. orthography for the spelling task or phonology for the rhyming task). (3) The baseline perceptual task presented symbol strings that were more visually complex than the line judgment task. Thus, in addition to the direct comparison between tasks, each task is also compared with nonlinguistic stimuli with comparable visual complexity.
The rhyming task is predicted to differentially activate regions involved in phonological processes such as inferior frontal gyrus and temporal regions. The spelling task is predicted to differentially activate regions involved in orthographic processes such as inferior occipito‐temporal cortex. For the comparison of conflicting and nonconflicting conditions, we predict that conflicting conditions will show stronger activation in regions related to processes that are not relevant for the task, i.e. phonological processing in the spelling task, and orthographic processing in the rhyming task. This may reflect the salience of the irrelevant information and its interference with task‐relevant processing in the conflicting conditions. In addition, we predict that conflicting conditions in both tasks will engage task‐relevant processes more than the nonconflicting conditions, because this is necessary to overcome the interference from the nonrelevant information.
MATERIALS AND METHODS
Participants
Thirty‐eight healthy children (aged 9–15, mean = 11.7), 22 females, participated in the study. Children were all right handed, (mean = 78, range 50–90) according to the nine item likert‐scale questionnaire (−90 to 90, positive scores indicate right hand dominance). All children were native English speakers, with normal hearing and normal or corrected‐to‐normal vision. All children were free of neurological diseases or psychiatric disorders and were not taking medication affecting the central nervous system. Children were recruited from the Chicago metropolitan area. Parents of children were given an interview to ensure that they did not have a history of intelligence, reading, attention, or oral‐language deficits. Children were given standardized intelligence tests (Wechsler Abbreviated Scale of Intelligence (The Psychological Corporation, 1999)), which showed an average full scale IQ = 113 (range = 85–130, SD = 15.3), verbal IQ = 114 (range = 79–142, SD = 14.8), and performance IQ = 108 (range = 78–140, SD = 14.7). Reading abilities in all children were average and above, with a mean score = 111 on the Woodcock Johnson Word Identification test (Woodcock et al., 2001) (range 91–130). The Institutional Review Board at Northwestern University and Evanston Northwestern Healthcare Research Institute approved the informed consent procedures.
Tasks
Word judgment tasks
Two words were presented visually in a sequential order and the participant had to determine whether the words matched according to a predefined rule. In the spelling task, participants determined whether the words had the same rime spelling. The rime included all letters after the first consonant or consonant cluster (Bowey, 1990). The participants were instructed to “indicate whether the words were spelled the same from the first vowel on”. In the rhyming task, participants determined whether the words rhymed. Each task was performed in separate runs. Each word was presented for 800 ms followed by a 200 ms blank interval. A red fixation cross appeared on the screen after the second word, indicating the need to make a response during the subsequent 2,600 ms interval.
In both the spelling and the rhyming task, 24 word pairs were presented in each one of four lexical conditions that independently manipulated the orthographic and phonological similarity between words. In the two nonconflicting conditions, the two words were either similar in both orthography and phonology (O+P+, e.g. dime‐lime), or different in both orthography and phonology (O−P−, e.g. staff‐gain). In the two conflicting conditions, the two words had either similar orthography but different phonology (O+P−, e.g. pint‐mint), or different orthography but similar phonology (O−P+, e.g. jazz‐has). In the spelling task, the participant had to respond according to the orthographic information, whereas in the rhyming task they had to respond according to the phonological information. If there was a match according to the criterion, the participant pressed a button with the index finger; if there was no match, the participant pressed a different button with the middle finger.
Control conditions
Two perceptual control conditions were used in which two symbol strings were presented visually in sequential order and the participant had to determine whether the strings matched. In the “Simple” condition, the symbol string consisted of a single symbol, while in the “Complex” condition, the symbol string consisted of three different symbols. Timing parameters were the same as for the lexical conditions. Twenty‐four items were presented in each perceptual condition, with half of them matching. In addition to the perceptual control conditions, 72 fixation trials were included as a baseline. In the fixation condition, a black fixation cross was presented for the same duration as the stimuli in the lexical and perceptual conditions and participants were instructed to press a button when the black fixation‐cross turned red.
Stimuli Characteristics
All words were monosyllabic words, 4–7 letters long, and were matched across tasks and conditions for written word frequency in adults and children (The Educator's Word Frequency Guide, 1996) and for adult word frequency for written and spoken language (Baayen et al., 1995). A GLM analysis of the two measures for word frequency did not reveal significant differences for task or for condition or an interaction between task and condition. Two measures of word consistency were used in the experiment: phonological enemies (number of words with similar spelling but different pronunciation of the rime) and orthographic enemies (number of words with similar pronunciation but different spelling of the rime). Despite attempts to match these parameters across tasks and conditions, and due to the limited number of available words and the specific structure of the conditions, the number of phonological and orthographic enemies was matched across tasks, but not across conditions. GLM analyses of phonological and orthographic enemies as dependent variables and task and condition as independent variables showed a significant effect of condition (F(3,21) = 24.0, 13.4; P < 0.001) for phonological and orthographic enemies respectively. The largest number of phonological enemies was found in the O+P− condition in both tasks (1.2, 1.3, 5.5, and 3.3 for O+P+, O−P−, O+P− and O−P+ respectively). The largest number of orthographic enemies was found in the O−P+ condition in both tasks (5.6, 5.6, 5.3, and 13.7 for O+P+, O−P−, O+P−, and O−P+ respectively).
The symbols in the control conditions consisted of re‐arranged parts of lower‐case courier letters. In the Complex condition, a symbol did not repeat within any symbol‐string. Nonmatching pairs differed in one symbol, with the position of the nonmatching symbol equally distributed across the string. All words and symbols were presented in lower case, at the center of the screen, with a 0.5 letter offset of position between the first and second stimulus.
Experimental Procedure
After informed consent was obtained and the standardized tests were administered, participants were invited for a practice session, in which they were trained in minimizing head movement in front of a computer screen using an infrared tracking device. In addition, they preformed one run of each task (rhyming and spelling) in a simulator scanner, to make sure they understood the tasks and to acclimatize themselves to the scanner environment. Different stimuli were used in the practice and in the scanning sessions. Scanning took place within a week from the practice session. Each participant performed both the spelling and the rhyming tasks. In the scanning session, each task was administered in two 108 trial runs, in which the order of lexical, perceptual, and fixation trials and was optimized for event‐related design (Burock et al., 1998). The order of stimuli within task was fixed for all subjects, with the order of tasks counterbalanced across subjects. Prior to each run, the subjects were instructed which task they were required to perform. Altogether, four 8 min runs were administered to each participant.
MRI Data Acquisition
Images were acquired using a 1.5 T GE scanner, using a standard head coil. Head movement was minimized using vacuum pillow (Bionix, Toledo, OH). The stimuli were projected onto a screen, and viewed through a mirror attached to the inside of the head coil. Participants' responses were recorded using an optical response box (Current Designs, Philadelphia, PA). The BOLD functional images were acquired using the EPI (echo planar imaging) method. The following parameters were used for scanning: TE = 35 ms, flip angle = 90°, matrix size = 64 × 64, field of view = 24 cm, slice thickness = 5 mm, voxel size = 3.75 × 3.75 × 5 mm3, number of slices = 24; TR = 2,000 ms. Four runs, with 240 repetitions each, were administered for the functional images. In addition, structural T1 weighted 3D image were acquired (SPGR, TR = 21 ms, TE = 8 ms, flip angle = 20°, matrix size = 256 × 256, field of view = 22 cm, slice thickness = 1 mm, number of slices = 124), using an identical orientation as the functional images.
Image Analysis
Data analysis was performed using statistical parametric mapping (SPM2) (http://www.fil.ion.ucl.ac.uk/spm). The images were spatially realigned to the first volume to correct for head movements. No individual runs had more than 4 mm maximum displacement. Sinc interpolation was used to minimize timing‐errors between slices (Henson et al., 1999). The functional images were co‐registered with the anatomical image, and normalized to the standard T1 template volume (MNI), with a voxel size of 3 × 3 × 3 mm3. The data was then smoothed with a 10 mm isotropic Gaussian kernel. Four volumes were discarded from the beginning of each run. Statistical analyses at the first level were calculated using an event‐related design, separately for each task, with four lexical conditions, two perceptual conditions, and the null events as conditions of interest. A high pass filter with a cutoff period of 128 s was applied. Word pairs were treated as individual events for analysis and modeled using a canonical HRF. Group results were obtained using random‐effects analyses by combining subject‐specific summary statistics across the group as implemented in SPM2 (Penny and Holmes, 2003).
The main effect of all lexical conditions when compared with the perceptual conditions (masked by the activation in lexical conditions vs. fixation) was tested using a one‐sample t test. The comparison between tasks was done in a two‐sample t test at the group level, using the contrast of all lexical conditions when compared with fixation in each task from the individual analyses. All reported areas of activation were significant using P < 0.001 uncorrected at the voxel level and containing a cluster size greater than or equal to 10 voxels. To determine regions that are sensitive to the conflict between orthography and phonology and are common to both tasks, we used the contrast of conflicting versus nonconflicting conditions from the group analysis in one task as a mask to examine activation in the other task, and vice versa (threshold of P < 0.005 uncorrected and a cluster size greater than 10 voxels). To test the hypothesis that conflicting conditions involve greater activation of both phonological and orthographic processes, we used the task comparison maps as masks for testing the effect of conflict in each task. Regions that were more active for the rhyming task served as a mask for phonology‐related processing, while regions that were more active for the spelling task served as a mask for orthography‐related processing. Hence, the conflict effect in each task (conflicting vs. nonconflicting conditions) was separately examined using two masks: spelling > rhyming and rhyming > spelling.
To test whether the differential activation between conflicting and nonconflicting conditions is explained by the longer time on task in the conflicting conditions, we examined the correlation between reaction time and activation in the contrast of conflicting versus nonconflicting conditions. The difference in reaction time between conflicting and nonconflicting conditions for each individual was entered as a single covariate in the correlation analysis at the group level.
To test the correlation of activation with accuracy of performance on a task, the contrast of all lexical conditions vs. null in each individual was entered into a multiple regression analysis, separately for each task. Two regressors were included as covariates in the analysis: the mean accuracy across lexical conditions in the respective task and the age in months. The correlation of activation with accuracy is reported while controlling for the effect of age, with a threshold of P < 0.001 uncorrected and a cluster size greater than 10 voxels.
RESULTS
Behavioral Results
Table I shows that average accuracy of performance in the scanner was above 80% in both tasks in all conditions, with the exception of O+P− in the rhyming task (72%). A GLM analysis, with lexical conditions as a repeated measure and task as a between‐subject variable, showed a significant main effect of condition (F(3,66) = 45.1; 52.7, P < 0.001) for accuracy and RT respectively, a significant main effect of task for accuracy (F(1,66) = 8.9, P < 0.01), and a significant interaction of task and condition, for accuracy (F(3,66) = 11.9, P < 0.001) and RT (F(3,66) = 4.0, P < 0.01). The comparison within each task showed that in both tasks, performance in the conflicting conditions was less accurate and slower than in the nonconflicting conditions. Paired‐t tests of accuracy between conflicting and nonconflicting conditions showed a significant effect of conflict for the rhyming and spelling tasks (t(35) = 10.7; t(31) = 5.3 respectively, P < 0.001). Paired‐t tests of RT also showed a significant effect of conflict in the rhyming and spelling tasks (t(35) = 8.2; t(31) = 8.7 respectively, P < 0.001). A comparison between the two conflicting conditions within each task showed that O+P− was less accurate and slower than O−P+, but only in the rhyming task. A GLM analysis of two conditions by two tasks showed a significant interaction of task and condition (F(1,66) = 10.6, 8.7; P < 0.01) for accuracy and RT respectively. A paired‐t test comparing the two conflicting conditions in the rhyming task resulted in a significant difference for accuracy (t(35) = 3.8, P < 0.01) and RT (t(35) = 4.8, P < 0.001), while the same comparison in the spelling task did not show significant differences (t(31) < 1; t(31) = 1.5, for accuracy and RT respectively).
Table I.
Performance in lexical and perceptual conditions in the spelling and rhyming tasks
| Nonconflicting | Conflicting | Perceptual | ||||
|---|---|---|---|---|---|---|
| O+P+ | O−P− | O−P+ | O+P− | Simple | Complex | |
| Accuracy | ||||||
| Rhyming | ||||||
| Mean | 93 | 94 | 84 | 72 | 97 | 90 |
| SD | 1 | 1.2 | 1.4 | 3 | 0.8 | 1.4 |
| Spelling | ||||||
| Mean | 93 | 97 | 88 | 88 | 97 | 90 |
| SD | 1.7 | 1.2 | 2.1 | 2.1 | 0.8 | 1.3 |
| RT | ||||||
| Rhyming | ||||||
| Mean | 1,311 | 1,302 | 1,393 | 1,528 | 1,119 | 1,154 |
| SD | 53 | 53 | 54 | 64 | 52 | 49 |
| Spelling | ||||||
| Mean | 1,257 | 1,220 | 1,345 | 1,376 | 1,094 | 1,126 |
| SD | 65 | 60 | 62 | 70 | 56 | 46 |
Accuracy presented in percentage of correct trials, and reaction time in milliseconds.
FMRI Results
Because no significant differences were found between the analysis of correct responses alone and the analysis that includes all responses, only results from the analysis with all responses are presented, to equate the statistical power between conditions with different accuracies.
Language specific activation
Figure 1A and Table II show regions that were more active in the lexical when compared with the perceptual conditions (masked by the map of lexical conditions vs. fixation) in both tasks. These regions included left IFG (BA 45/46), superior/medial frontal gyri (BA 6/8), and extrastriate visual cortex (BA 18) in both the spelling and the rhyming tasks. A small cluster in the left inferior temporal gyrus (ITG; BA 37) (MNI coordinates: −54, −50, −12) also showed overlap of language‐specific activation between the two tasks. In addition, language‐specific activation was found in the spelling task in the left inferior parietal lobule (IPL; BA 40) and in the rhyming task in the left superior temporal gyrus (STG; BA 22).
Figure 1.
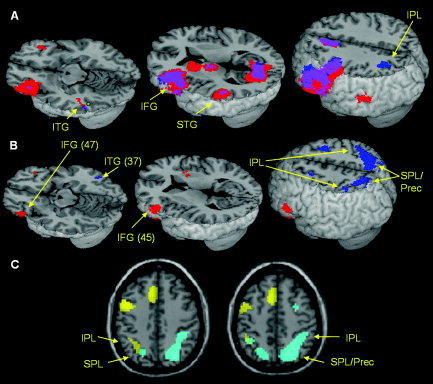
A: Language‐specific activation (words > perceptuals) in the spelling (blue) and rhyming (red) tasks, and their overlap (purple). Activation is masked by the map of words > fixation. The coordinates corresponding to the proposed Visual Word Form Area (Dehaene et al., 2004) are presented in a green mark. B: Task‐specific activation: red = rhyming > spelling, blue = spelling > rhyming. C: Overlay of language‐specific and task‐specific activation in the spelling task: yellow = words > perceptuals, cyan = spelling > rhyming, green = overlap. IFG, inferior frontal gyrus; ITG, inferior temporal gyrus; STG, superior temporal gyrus; IPL, inferior parietal lobule; SPL, superior parietal lobule; Prec, precuneus.
Table II.
Language‐specific and task‐specific activation
| Condition | Region | BA | H | Z score | Voxels | x | y | z |
|---|---|---|---|---|---|---|---|---|
| Language specific (words > symbols) | ||||||||
| Spelling | Inferior/middle frontal gyri | 45/46/47 | L | 6.2 | 1,295 | −48 | 36 | 6 |
| Superior/medial frontal gyri | 6/8 | L | 5.5 | 271 | −6 | 15 | 48 | |
| Superior/middle temporal gyri | 22 | L | 5.4 | 14 | −54 | −39 | 6 | |
| Thalamus | — | L | 4.6 | 55 | −6 | −12 | 6 | |
| Cuneus/calcarine sulcus | 30/18 | L + R | 4.3 | 164 | −6 | −75 | 6 | |
| Middle temporal gyrus | 37 | L | 4 | 10 | −57 | −51 | −12 | |
| Inferior parietal lobule | 40 | L | 3.8 | 79 | −30 | −57 | 39 | |
| Rhyming | Superior/middle temporal gyri | 22 | L | 7.4 | 134 | −60 | −42 | 12 |
| Inferior frontal gyrus | 45/46 | L | 7.4 | 2,083 | −48 | 15 | 21 | |
| Superior/medial frontal gyri | 6/8 | L | 6.2 | 382 | −6 | 9 | 60 | |
| Cuneus/calcarine sulcus | 18 | L + R | 5.7 | 559 | −9 | −87 | 12 | |
| Middle/inferior temporal gyri | 21/37 | L | 5.2 | 51 | −45 | −33 | −15 | |
| Inferior frontal gyrus | 47 | R | 4.2 | 83 | 36 | 33 | −6 | |
| Task specific | ||||||||
| Spelling > Rhyming | ||||||||
| Precuneus/superior parietal lobule/inferior parietal lobule | 7/40 | R | 5.5 | 674 | 27 | −72 | 39 | |
| Superior parietal lobule | 7 | L | 4.3 | 140 | −21 | −66 | 54 | |
| Middle frontal gyrus | 6 | R | 4 | 68 | 30 | 3 | 54 | |
| Inferior/middle temporal gyri | 37 | R | 3.8 | 13 | 54 | −48 | −12 | |
| Inferior parietal lobule | 40 | L | 3.6 | 35 | −39 | −48 | 48 | |
| Superior/middle frontal gyri | 6 | L | 3.5 | 34 | −24 | 3 | 60 | |
| Rhyming > Spelling | ||||||||
| Inferior frontal gyrus | 45 | L | 4 | 46 | −51 | 30 | 9 | |
| Inferior frontal gyrus | 47 | L | 3.7 | 23 | −45 | 30 | −12 | |
| Putamen | — | R | 3.7 | 34 | 27 | 0 | 3 | |
| Inferior frontal gyrus | 45/47 | R | 3.6 | 36 | 39 | 36 | −9 | |
| Insula/putamen | 13 | L | 3.6 | 27 | −36 | 6 | 3 | |
H, hemisphere; L, left; R, right. Activation in all tables presented with P < 0.001 uncorrected, unless specified otherwise. Clusters significant above a threshold of P < 0.05 corrected are presented in bold. Coordinates are in MNI coordinates. Task specific activation calculated from the contrasts of words versus fixation.
Task comparison
The direct comparison between the rhyming and the spelling tasks for the contrast of all lexical conditions versus fixation are presented in Figure 1B and Table II. The results show that more activation for the rhyming task was found in two clusters in the left IFG (one in BA 45 and one in BA 47), and one cluster in the right IFG (BA 45/47). On the other hand, more activation for the spelling task was found in bilateral SPL/IPL (BA 7/40). However, Figure 1C shows the overlay of the task comparison, with greater activation in the spelling when compared with the rhyming task, and the language‐specific regions in the spelling task. This shows that task‐specific activation in the spelling task (i.e. the bilateral SPL/IPL) was predominately nonoverlapping with regions that showed greater activation for the lexical when compared with the perceptual conditions in the spelling task.
Correlation with accuracy
Accuracy in the rhyming task was positively correlated with activation (in the contrast of lexical conditions vs. fixation) in the left IFG (Z = 3.8, 26 voxels, BA 47; −48, 21, −15). Accuracy in the spelling task was positively correlated with activation in the right middle occipital gyrus (BA 19; Z = 3.9, 23 voxels) and right IFG (BA 45/47; Z = 3.7, 18 voxels).
Effect of conflict
Regions that are sensitive to the conflict between orthographic and phonological information were examined in the two tasks using the comparison of conflicting and nonconflicting pairs, and are presented in Figure 2 and Table III. Regions sensitive to the conflict in both tasks, found using reciprocal masking, are presented in Figure 2A. In both the spelling and the rhyming tasks, conflicting conditions showed greater activation in anterior cingulate/medial frontal gyrus (BA 32), left IPL (BA 39/40), and left IFG (BA 44/45/9).
Figure 2.
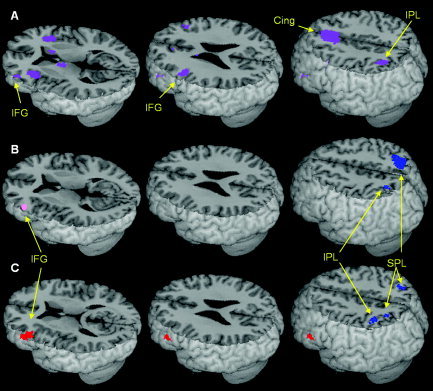
Regions sensitive to the conflict between orthography and phonology (conflicting > nonconflicting conditions). A: Conflict‐sensitive‐regions common to both the spelling and rhyming tasks (presented from the rhyming task masked by the contrast of conflicting > nonconflicting from the spelling task). B: Conflict‐sensitive‐regions in the spelling task masked by the contrast of spelling > rhyming (blue). Activation threshold of P < 0.005 uncorrected is presented. The location of subthreshold activation (P < 0.05 uncorrected) within a mask of rhyming > spelling is marked in pink. C: Conflict‐sensitive‐regions in the rhyming task masked by the contrast of spelling > rhyming (blue) and rhyming > spelling (red). Activation threshold of P < 0.005 uncorrected is presented. Cing, Cingulate gyrus (see Fig. 1 for other regions).
Table III.
Regions sensitive to the conflict between orthography and phonology
| Condition | Region | BA | H | Z score | Voxels | x | y | z |
|---|---|---|---|---|---|---|---|---|
| Common to both tasks | ||||||||
| Cingulate/superior frontal/medial frontal gyri | 32 | L + R | 7.1 | 340 | −6 | 24 | 45 | |
| Inferior frontal gyrus | 9/44/45 | L | 6.3 | 67 | −42 | 6 | 30 | |
| Insula | 13 | L | 6 | 113 | −36 | 24 | 0 | |
| Caudate nucleus | — | R | 5.9 | 35 | 12 | 15 | 3 | |
| Insula | 13 | R | 5.6 | 76 | 33 | 24 | 0 | |
| Anterior cingulate | 32 | L + R | 5 | 10 | −6 | 36 | 24 | |
| Inferior frontal gyrus | 46 | L | 4.6 | 13 | −42 | 39 | 12 | |
| Middle frontal gyrus | 46 | R | 4.5 | 16 | 45 | 36 | 21 | |
| Caudate nucleus | — | L | 4.2 | 24 | −12 | 3 | 12 | |
| Inferior parietal lobule | 39/40 | L | 4.2 | 24 | −27 | −60 | 42 | |
| Within task‐specific regions | ||||||||
| Spelling | Masked by spelling > rhyming | |||||||
| Superior parietal lobule | 7 | R | 4.4 | 203 | 33 | −63 | 51 | |
| Inferior parietal lobule | 40 | L | 3.2 | 17 | −30 | −54 | 51 | |
| Masked by rhyming > spelling | ||||||||
| No suprathershod activation (inferior frontal gyrus)a | 45/46 | L | 2.6 | 11 | −48 | 33 | 9 | |
| Rhyming | Masked by spelling > rhyming | |||||||
| Superior parietal lobule | 7 | L | 3.6 | 21 | −24 | −63 | 45 | |
| Superior parietal lobule | 7 | R | 3.4 | 49 | 30 | −69 | 45 | |
| Inferior parietal lobule | 40 | L | 3.1 | 11 | −39 | −48 | 45 | |
| Masked by rhyming > spelling | ||||||||
| Inferior frontal gyrus | 45/46 | L | 4.7 | 30 | −45 | 30 | 12 | |
| Inferior frontal gyrus | 47 | R | 3.9 | 10 | 36 | 33 | −9 | |
Coordinates for clusters common to both tasks are from conflict > no‐conflict in the rhyming task masked by conflict > no‐conflict in the spelling task. Clusters are presented with a threshold of P < 0.005 uncorrected. Bold = significant with P < 0.05 corrected.
Significant with a threshold of P < 0.05 uncorrected.
To test the prediction that conflicting conditions show greater activation associated with both task‐relevant and task‐irrelevant processes, we used the maps from the task‐comparison as masks. The effect of conflict in each task (conflicting vs. nonconflicting conditions) was separately examined in regions related to phonological processing (using the rhyming vs. spelling comparison as a mask) and in regions related to orthographic processing (using the spelling vs. rhyming comparison as a mask). The results are shown in Table III and Figure 2B,C. The effect of conflict in the rhyming task, masked by the rhyming > spelling mask (task‐relevant processing), showed activation in bilateral IFG (BA 45/46 on the left and BA 47 on the right). The effect of conflict in the rhyming task, masked by the spelling > rhyming mask (task‐irrelevant processing), resulted in activation in bilateral SPL (BA 7) and left IPL (BA 40). The effect of conflict in the spelling task, masked by spelling > rhyming mask (task‐relevant processing), showed activation in the right SPL (BA 7) and left IPL (BA 40). The effect of conflict in the spelling task masked by rhyming > spelling mask (task‐irrelevant processing) did not show any active clusters under the standard threshold, but showed activation in the left IFG (BA 45/46) under reduced threshold (P < 0.05 uncorrected at the voxel level).
To test whether the differential activation between conflicting and nonconflicting conditions is explained by the longer time on task in the conflicting conditions, we examined the correlation between this contrast and the difference in reaction time. In the spelling task, this analysis revealed activation in the right posterior medial frontal gyrus (BA 6) and right STG (BA 22). In the rhyming task, this analysis revealed activation in the left calcarine (BA 18). No activation was found in regions that were sensitive to the conflict, suggesting that the conflict effect was not explained by longer time on task.
Hard versus easy conflicting conditions in rhyming
Following the marked difference in performance between the two conflicting conditions in the rhyming task, the comparison between these conditions is presented (Table IV). Regions that were more active for the difficult conflicting condition (O+P−) when compared with the easier condition (O−P+) were medial frontal gyrus/anterior cingulate (BA 8/9/32), left IFG (BA 45), and right hemisphere regions.
Table IV.
Comparison among conflicting conditions in the rhyming task (O+P− > O−P+)
| Region | BA | H | Z score | Voxels | x | y | z |
|---|---|---|---|---|---|---|---|
| Medial frontal/anterior cingulate gyri | 8/9/32 | L + R | 4.9 | 388 | 6 | 30 | 45 |
| Middle/superior frontal gyri | 9 | R | 4.9 | 141 | 42 | 36 | 33 |
| Superior temporal gyrus | 22 | R | 4.7 | 64 | 60 | −12 | 3 |
| Precentral gyrus/insula | 44/13 | R | 4.7 | 172 | 51 | 6 | 9 |
| Middle frontal gyrus | 6 | L | 4.1 | 21 | −51 | 3 | 48 |
| Post central gyrus | 2 | L | 3.8 | 34 | −51 | −24 | 54 |
| Inferior frontal gyrus/insula | 45/13 | L | 3.7 | 25 | −33 | 21 | 6 |
DISCUSSION
Our behavioral results show that conflicting pairs of words were more difficult than nonconflicting pairs, in both the spelling and the rhyming task, suggesting that subjects were engaged in mapping of orthography to phonology in both tasks, so that conflicting information irrelevant to the task interfered with their performance. Our fMRI results show greater activation for words when compared with nonlinguistic stimuli in perisylvian language areas, and in the left infero‐temporal region, depicted as the visual word form area (Dehaene et al., 2004).
Task Comparisons
The direct comparison between tasks showed more activation for the spelling task, as compared with the rhyming task, in bilateral SPL/IPL (BA 7/40), right infero‐temporal cortex (BA 37), and bilateral middle/superior frontal cortex (Fig. 1B). This finding is consistent with previous findings showing activation in bilateral SPL for adults, and left SPL in children for the spelling when compared with a line judgment task (Booth et al., 2004). Surprisingly, regions that were differentially active in the spelling when compared with the rhyming task showed almost no overlap with language‐specific activation (words > perceptual conditions) in the same task (Fig. 1C). This finding suggests that children relied mainly on nonlinguistic information for comparing word forms, similar to the visual‐spatial processes used for comparing the symbols in the perceptual conditions. Previous studies showed that bilateral SPL is involved in nonlinguistic visual spatial tasks such as mental rotation (Alivisatos and Petrides, 1997; Cohen et al., 1996; Kosslyn et al., 1998) and shifting of spatial attention (LaBar et al., 1999). Our conclusion is consistent with our finding of greater activation in the right rather than the left infero‐temporal cortex for the spelling when compared with the rhyming task. While the left infero‐temporal cortex has been depicted in orthographic word‐specific processes (Dehaene et al., 2002; Nobre et al., 1994), its right homologue has been associated with processing of nonlinguistic visual stimuli such as faces and objects (Gauthier et al., 2000; Nakamura et al., 2005). While a previous study comparing the spelling and rhyming tasks in adults did not find any differential activation for the spelling task (Booth et al., 2002a), both tasks resulted in activation of the left fusiform gyrus, suggesting a reliance on linguistically structured representations. Furthermore, several studies show greater activation for children when compared with adults in the right infero‐temporal cortex in the spelling task (Booth et al., 2004) and in an implicit reading task (Turkeltaub et al., 2003). Altogether, these findings suggest that children rely on nonlinguistic processing in the spelling task more than adults, presumably due to their relatively limited experience with visual words. With experience, representations for linguistic stimuli presumably become more specialized, resulting in a decrease in the reliance on general visual processing.
The direct comparison between tasks showed stronger activation for the rhyming when compared with the spelling task in bilateral IFG (BA 45/47), suggesting that phonological access and manipulation were enhanced in the rhyming task. This finding is consistent with previous studies of children showing activation in left IFG (BA 44/45) in phonological tasks (Booth et al., 2004; Georgiewa, 1999; Shaywitz et al., 2002; Temple et al., 2001). However, activation in BA 47 that has previously been associated with semantic processing (Booth et al., 2002b; Devlin et al., 2003; McDermott et al., 2003; Poldrack et al., 1999; Roskies et al., 2001) suggests that the rhyming task involved greater access to semantic representations. Furthermore, activation in left BA 47 was correlated with accuracy of performance in the rhyming task, suggesting that semantic processing contributed to the children's performance and was not merely a postaccess byproduct of activating phonological representations. Activation of semantic representations may enhance phonological representations by adding to the interactive loop of orthographic, phonological, and semantic representations (Plaut et al., 1996).
A previous study that compared rhyming and spelling tasks in adults (Booth et al., 2002a) did not find differential activation in the IFG. However, the inconsistency between the current and the adults' findings is not likely to be the result of the age difference between the samples, because previous studies have actually found a developmental increase rather than a decrease in activation in IFG, in a variety of linguistic tasks (Booth et al., 2004; Gaillard et al., 2003; Shaywitz et al., 2002; Turkeltaub et al., 2003). The inclusion of the most difficult word pairs (O+P−) in the current study may have enhanced the reliance on phonological access and manipulations resulting in differential activation in the IFG for the rhyming task.
The Conflict Between Orthographic and Phonological Information
Our behavioral results, showing increased difficulty in conflicting pairs of words in both the spelling and the rhyming tasks, are consistent with studies showing this effect in both tasks in adults (Johnston and Mcdermott, 1986; Kramer and Donchin, 1987; Levinthal and Hornung, 1992; Polich et al., 1983) and in the rhyming task in children (McPherson et al., 1997; Rack, 1985). Our study further shows the conflict effect in a spelling task in children, suggesting that children automatically map orthographic to phonological representations, even when it is not required by the task. Our fMRI results further suggest what stages of processing are involved in the competition between orthographic and phonological information in conflicting pairs.
In a comparison between conflicting and nonconflicting pairs, our fMRI results show greater activation for conflicting pairs in regions that were differentially active in each respective task (Fig. 2B,C). Namely, in the rhyming task, greater activation for conflicting when compared with nonconflicting pairs was found in bilateral IFG, in the same region that showed greater activation for the rhyming when compared with the spelling task. Similarly, in the spelling task, greater activation for conflicting when compared with nonconflicting pairs was found in right SPL (BA 7), in the same region that showed greater activation for the spelling when compared with the rhyming task. These findings are consistent with our prediction that conflicting pairs will elicit greater activation of processes that are relevant for the task, i.e. phonological processing in the rhyming task and orthographic processing in the spelling task. This may reflect the need for stronger activation of task‐relevant processes to override the interference from the nonrelevant dimension.
We also predicted that conflicting pairs in each task would elicit greater activation than nonconflicting pairs in brain regions associated with processing information that is irrelevant and interfering with task performance. In other words, conflicting pairs in the rhyming task would produce activation in regions associated with orthographic processing and conflicting pairs in the spelling task would produce activation in regions associated with phonological processing. Our fMRI results confirmed this hypothesis for the rhyming task, showing greater activation in conflicting pairs in bilateral SPL, the regions that showed greater activation in the spelling when compared with the rhyming task. These findings suggest that, when making a rhyming judgment on visually presented words, orthographic processes interfere with phonological processes in the conflicting conditions. The prediction of greater activation of irrelevant (phonological) processes in conflicting conditions in the spelling task received partial support. Greater activation was found for conflicting pairs when compared with nonconflicting pairs in regions associated with phonological processes (i.e. left IFG BA 45/46), but these were only significant under a more liberal threshold.
In addition to regions that were differentially active in each task, conflicting pairs in both tasks showed greater activation than nonconflicting pairs in the dorsal aspect of left IFG (BA 9/44/45) and in left IPL (BA 40) (Fig. 2A). Activation in IPL (BA 40) has been found in tasks that involve mapping of orthography to phonology (Booth et al., 2002a; Chen et al., 2002; Demonet et al., 1992; Xu et al., 2001). Activation in BA 9/44/45 has been related to segmentation and manipulation of phonological and articulatory representations (Clark and Wagner, 2003; Demonet et al., 1992; Fiebach et al., 2002; Fiez et al., 1999; Mechelli et al., 2005). Greater activation in this region has also been found for reading inconsistent when compared with consistent words (Fiez et al., 1999; Mechelli et al., 2005). In the current study, the conflicting conditions entail a magnification of the conflict inherent in reading inconsistent words. Reading an inconsistent word (with a high proportion of phonological and orthographic enemies) is likely to require repetitive mapping of orthography to phonology, and enhanced phonological segmentation. Re‐mapping is even more likely to occur when an inconsistent word is primed by an “enemy” word. Our results therefore suggest that activation in IPL and IFG in the conflicting conditions reflect repetitive mapping between orthography and phonology, and increased phonological segmentation and covert articulation as a means for verifying the accuracy of the outcome.
We also found that the conflicting pairs produced greater activation than nonconflicting pairs for both tasks in anterior cingulate/medial frontal gyrus (BA 32/6), previously associated with monitoring responses especially when there is conflict between competing responses (Lenartowicz and McIntosh, 2005; Milham et al., 2003; Stephan et al., 2003; van de Ven et al., 2004). This finding suggests that in conflicting pairs orthographic and phonological information compete not only at early stages of generating the representations, but also at the stage of response selection. The conclusion that the conflict occurs in multiple levels of processing is consistent with previous ERP results (Kramer and Donchin, 1987).
Comparison Among the Conflicting Conditions
Our behavioral results showed significantly lower accuracy and slower reaction time for word pairs that were spelled similarly but did not rhyme (O+P−) when compared with pairs that rhymed but were not spelled the same (O−P+). This difference among the conflicting conditions, found only in the rhyming task, is consistent with previous behavioral and ERP findings (Johnston and Mcdermott, 1986; Kramer and Donchin, 1987; Levinthal et al., 1992; McPherson et al., 1997; Rack, 1985;). One study found that the O+P− condition was more vulnerable to interference by a simultaneous counting task (Johnston and Mcdermott, 1986). In a comparison between the two conflicting conditions our fMRI results show more activation for the harder condition (O+P−) in left IFG (BA 45) and anterior cingulate/medial frontal gyri. These results suggest that the O+P− condition requires enhanced phonological access and manipulation when compared with the easier conflicting condition. The activation in the anterior cingulate cluster may reflect the greater conflict presented by the more difficult word pairs.
The Left Infero‐Temporal Cortex
Only a small cluster of activation in the left ITG was more active for words when compared with nonlinguistic symbols (Fig. 1). However, the overlap between the two tasks in this language‐specific infero‐temporal activation was found in the inferior/middle temporal gyri (−54, −50, −12), very close to the previous depicted Visual Word Form Area (−48, −52, −12) (Dehaene et al., 2004). Wide‐spread activation in the left infero‐temporal cortex was previously shown for children in the comparison of words to a line judgment task (Booth et al., 2004). However, the current results show word‐specific activation in the left infero‐temporal gyrus for children even when the visual complexity in the perceptual task is similar to the linguistic task.
CONCLUSIONS
Altogether, our results show that when children are presented with visual words, they automatically engage orthographic and phonological processes, regardless of the requirements of the task. Nevertheless, in the spelling judgment task, children rely mainly on nonlinguistic processes, while in the rhyming task, children may additionally engage semantic processes. Our results further suggest that when encountering a conflict between orthographic and phonological information, such as in reading inconsistent words, the competition between the systems occurs both at early stages, of generating the representations, and at the later stage of response selection. Competition at early stages is reflected by the enhancement of both task‐relevant and task‐irrelevant processes, resulting in increased activation in left inferior frontal gyrus for phonological processing and bilateral SPL for orthographic processing in the conflicting conditions of both tasks. Competition at the stage of response selection is reflected in the activation in the anterior cingulate/medial frontal cortex.
REFERENCES
- Alivisatos B, Petrides M 1997: Functional activation of the human brain during mental rotation. Neuropsychologia 35: 111–118. [DOI] [PubMed] [Google Scholar]
- Baayen RH, Piepenbrock R, Gulikers L 1995): The CELEX Lexical Database (Version Release 2) [CD‐ROM]. Philadelphia, PA: Linguistic Data Consortium, University of Pennsylvania. [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Mesulam MM 2002a: Functional anatomy of intra‐ and cross‐modal lexical tasks. Neuroimage 16: 7–22. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Mesulam MM 2002b: Modality independence of word comprehension. Hum Brain Mapp 16: 251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Mesulam MM 2004: Development of brain mechanisms for processing orthographic and phonologic representations. J Cognit Neurosci 16: 1234–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowey JA 1990: Orthographic onsets and rimes as functional units of reading. Mem Cognit 18: 419–427. [DOI] [PubMed] [Google Scholar]
- Burock MA, Buckner RL, Woldorff MG, Rosen BR, Dale AM 1998: Randomized event‐related experimental designs allow for extremely rapid presentation rates using functional MRI. Neuroreport 9: 3735–3739. [DOI] [PubMed] [Google Scholar]
- Chen Y, Fu S, Iversen SD, Smith SM, Matthews PM 2002: Testing for dual brain processing routes in reading: A direct contrast of Chinese character and Pinyin reading using fMRI. J Cognit Neurosci 14: 1088–1098. [DOI] [PubMed] [Google Scholar]
- Clark D, Wagner AD 2003: Assembling and encoding word representations: fMRI subsequent memory effects implicate a role for phonological control. Neuropsychologia 41: 304–317. [DOI] [PubMed] [Google Scholar]
- Cohen MS, Kosslyn SM, Breiter HC, DiGirolamo GJ, Thompson WL, Anderson AK, Bookheimer SY, Rosen BR, Belliveau JW 1996: Changes in cortical activity during mental rotation—A mapping study using functional MRI. Brain 119: 89–100. [DOI] [PubMed] [Google Scholar]
- Crosson B, Rao SM, Woodley SJ, Rosen AC, Bobholz JA, Mayer A, Cunningham JM, Hammeke TA, Fuller SA, Binder JR, Cox RW, Stein EA 1999: Mapping of semantic, phonological, and orthographic verbal working memory in normal adults with functional magnetic resonance imaging. Neuropsychology 13: 171–187. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Le Clec HG, Poline JB, Le Bihan D, Cohen L 2002: The visual word form area: A prelexical representation of visual words in the fusiform gyrus. Neuroreport 13: 321–325. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Jobert A, Naccache L, Ciuciu P, Poline JB, Le Bihan D, Cohen L 2004: Letter binding and invariant recognition of masked words—Behavioral and neuroimaging evidence. Psychol Sci 15: 307–313. [DOI] [PubMed] [Google Scholar]
- Demonet JF, Chollet F, Ramsay S, Cardebat D, Nespoulous JL, Wise R, Rascol A, Frackowiak R 1992: The anatomy of phonological and semantic processing in normal subjects. Brain 115 (Part 6): 1753–1768. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Matthews PM, Rushworth MFS 2003: Semantic processing in the left inferior prefrontal cortex: A combined functional magnetic resonance imaging and transcranial magnetic stimulation study. J Cognit Neurosci 15: 71–84. [DOI] [PubMed] [Google Scholar]
- Fiebach CJ, Friederici AD, Muller K, von Cramon DY 2002: fMRI evidence for dual routes to the mental lexicon in visual word recognition. J Cognit Neurosci 14: 11–23. [DOI] [PubMed] [Google Scholar]
- Fiez J, Balota D, Raichle M, Petersen S 1999: Effects of lexicality, frequency, and spelling‐to‐sound consistency on the functional anatomy of reading. Neuron 24: 205–218. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Sachs BC, Whitnah JR, Ahmad Z, Balsamo LM, Petrella JR, Braniecki SH, McKinney CM, Hunter K, Xu B, Gradin CB 2003: Developmental aspects of language processing: fMRI of verbal fluency in children and adults. Hum Brain Mapp 18: 176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier I, Skudlarski P, Gore JC, Anderson AW 2000: Expertise for cars and birds recruits brain areas involved in face recognition. Nat Neurosci 3: 191–197. [DOI] [PubMed] [Google Scholar]
- Georgiewa P 1999: fMRI during word processing in dyslexic and normal reading children. Neuroreport 10: 3459–3465. [DOI] [PubMed] [Google Scholar]
- Henson R, Buchel C, Josephs O, Friston K 1999: The slice‐timing problem in event‐related fMRI. Neuroimage 9: S125. [DOI] [PubMed] [Google Scholar]
- Johnston RS, Mcdermott EA. 1986: Suppression effects in rhyme judgment tasks. Q J Exp Psychol A 38: 111–124. [Google Scholar]
- Kareken DA, Lowe M, Chen SHA, Lurito J, Mathews V 2000: Word rhyming as a probe of hemispheric language dominance with functional magnetic resonance imaging. Neuropsychiatry Neuropsychol Behav Neurol 13: 264–270. [PubMed] [Google Scholar]
- Kosslyn SM, Digirolamo GJ, Thompson WL, Alpert NM 1998: Mental rotation of objects versus hands: Neural mechanisms revealed by positron emission tomography. Psychophysiology 35: 151–161. [PubMed] [Google Scholar]
- Kramer AF, Donchin E 1987: Brain potentials as indexes of orthographic and phonological interaction during word matching. J Exp Psychol Learn Mem Cognit 13: 76–86. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gitelman DR, Parrish TB, Mesulam M 1999: Neuroanatomic overlap of working memory and spatial attention networks: A functional MRI comparison within subjects. Neuroimage 10: 695–704. [DOI] [PubMed] [Google Scholar]
- Lenartowicz A, McIntosh AR 2005: The role of anterior cingulate cortex in working memory is shaped by functional connectivity. J Cognit Neurosci 17: 1026–1042. [DOI] [PubMed] [Google Scholar]
- Levinthal CF, Hornung M 1992: Orthographic and phonological coding during visual word matching as related to reading and spelling abilities in college‐students. Read Writ 4: 231–243. [Google Scholar]
- Lurito JT, Kareken DA, Lowe MJ, Chen SH, Mathews VP 2000: Comparison of rhyming and word generation with FMRI. Hum Brain Mapp 10: 99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott KB, Petersen SE, Watson JM, Ojemann JG 2003: A procedure for identifying regions preferentially activated by attention to semantic and phonological relations using functional magnetic resonance imaging. Neuropsychologia 41: 293–303. [DOI] [PubMed] [Google Scholar]
- McPherson WB, Ackerman TP, Dykman RA 1997: Auditory and visual rhyme judgements reveal differences and similarities between normal and disabled adolescent readers. Dyslexia 3: 63–77. [Google Scholar]
- Mechelli A, Crinion J, Long S, Friston K, Lambon‐Ralph MA, Patterson K, McClelland JL, Price CJ 2005: Dissociating reading processes on the basis of neuronal interactions. J Cognit Neurosci 17: 1753–1765. [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Barad V 2003: Competition for priority in processing increases prefrontal cortex's involvement in top‐down control: An event‐related fMRI study of the stroop task. Brain Res Cognit Brain Res 17: 212–222. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Oga T, Okada T, Sadato N, Takayama Y, Wydell T, Yonekura Y, Fukuyama H 2005: Hemispheric asymmetry emerges at distinct parts of the occipitotemporal cortex for objects, logograms and phonograms: A functional MRI study. NeuroImage 28: 521–528. [DOI] [PubMed] [Google Scholar]
- Nobre AC, Allison T, McCarthy G 1994: Word recognition in the human inferior temporal lobe. Nature 372: 260–263. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Frith U, Snowling M, Gallagher A, Morton J, Frackowiak RSJ, Frith CD 1996: Is developmental dyslexia a disconnection syndrome? Evidence from PET scanning. Brain 119: 143–157. [DOI] [PubMed] [Google Scholar]
- Penny WD, Holmes A 2003: Random effects analysis In: Frackowiak RSJ, Friston KJ, Frith CD, editors. Human Brain Function, 2nd ed. San Diego: Academic Press; pp 843–850. [Google Scholar]
- Plaut DC, McClelland JL, Seidenberg MS, Patterson K 1996: Understanding normal and impaired word reading: Computational principles in quasi‐regular domains. Psychol Rev 103: 56–115. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JDE 1999: Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. NeuroImage 10: 15–35. [DOI] [PubMed] [Google Scholar]
- Polich J, Mccarthy G, Wang WS, Donchin E 1983: When words collide—Orthographic and phonological interference during word‐processing. Biol Psychol 16: 155–180. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Shaywitz BA, Shaywitz SE, Constable RT, Skudlarski P, Fulbright RK, Bronen RA, Shankweiler DP, Katz L, Fletcher JM, Gore JC 1996: Cerebral organization of component processes in reading. Brain 119: 1221–1238. [DOI] [PubMed] [Google Scholar]
- Rack JP 1985: Orthographic and phonetic coding in developmental dyslexia. Br J Psychol 76: 325–340. [DOI] [PubMed] [Google Scholar]
- Roskies AL, Fiez JA, Balota DA, Raichle ME, Petersen SE 2001: Task‐dependent modulation of regions in the left inferior frontal cortex during semantic processing. J Cognit Neurosci 13: 829–843. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Pugh KR, Mencl WE, Fulbright RK, Skudlarski P, Constable RT, Marchione KE, Fletcher JM, Lyon GR, Gore JC 2002: Disruption of posterior brain systems for reading in children with developmental dyslexia. Biol Psychiatry 52: 101–110. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Marshall JC, Friston KJ, Rowe JB, Ritzl A, Zilles K, Fink GR 2003: Lateralized cognitive processes and lateralized task control in the human brain. Science 301: 384–386. [DOI] [PubMed] [Google Scholar]
- Tagamets MA, Novick JM, Chalmers ML, Friedman RB 2000: A parametric approach to orthographic processing in the brain: An fMRI study. J Cognit Neurosci 12: 281–297. [DOI] [PubMed] [Google Scholar]
- Temple E, Poldrack RA, Salidis J, Deutsch GK, Tallal P, Merzenich MM, Gabrieli JDE 2001: Disrupted neural responses to phonological and orthographic processing in dyslexic children: An fMRI study. Neuroreport 12: 299–307. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Gareau L, Flowers DL, Zeffiro TA, Eden GF 2003: Development of neural mechanisms for reading. Nat Neurosci 6: 767–773. [DOI] [PubMed] [Google Scholar]
- van de Ven VG, Formisano E, Prvulovic D, Roeder CH, Linden DE 2004: Functional connectivity as revealed by spatial independent component analysis of fMRI measurements during rest. Hum Brain Mapp 22: 165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock RW, McGrew KS, Mather N 2001: Woodcock‐Johnson III. Itasca, IL: Riverside Publishing. [Google Scholar]
- Xu B, Grafman J, Gaillard WD, Ishii K, Vega‐Bermudez F, Pietrini P, Reeves‐Tyer P, DiCamillo P, Theodore W 2001: Conjoint and extended neural networks for the computation of speech codes: The neural basis of selective impairment in reading words and pseudowords. Cereb Cortex 11: 267–277. [DOI] [PubMed] [Google Scholar]


