Abstract
Emerging evidence indicates that mineralocorticoid receptor (MR) blockade reduces the risk of cardiovascular events beyond those predicted by its blood pressure (BP)-lowering actions; however, the underlying mechanisms remain unclear. To investigate whether protection elicited by MR blockade is through attenuation of vascular apoptosis and injury, independently of BPlowering, we administered a low dose of the MR antagonist spironolactone or vehicle for 21 days to hypertensive transgenic Ren2 rats with elevated plasma aldosterone levels. Although Ren2 rats developed higher systolic BPs compared to Sprague-Dawley (SD) littermates, low dose spironolactone treatment did not reduce systolic BP compared with untreated Ren2 rats. Ren2 rats exhibited vascular injury as evidenced by increased apoptosis, hemidesmosome-like structure loss, mitochondrial abnormalities, and lipid accumulation compared with SD, and these abnormalities were attenuated by MR antagonism. Protein kinase B (Akt) activation is critical to vascular homeostasis via regulation of cell survival and expression of apoptotic genes. Akt serine473 phosphorylation was impaired in Ren2 aortas, and restored with MR antagonism. In vivo MR antagonist treatment promoted anti-apoptotic effects by increasing phosphorylation of BAD serine136 and expression of Bcl-2 and Bcl-xL, decreasing cytochrome c release and BAD expression, and suppressing caspase-3 activation. Furthermore, MR antagonism substantially reduced the elevated NADPH oxidase activity and lipid peroxidation, expression of angiotensin II, angiotensin type 1 receptor and MR, in Ren2 vasculature. These results demonstrate that MR antagonism protects the vasculature from aldosterone-induced vascular apoptosis and structural injury via rescuing Akt activation, independent of BP effects.
Keywords: Aldosterone, Oxidative Stress, Akt Activation, Vascular Apoptosis and Injury
INTRODUCTION
Clinical studies indicate that mineralocorticoid receptor (MR) blockade exerts beneficial effects on cardiovascular events beyond those predicted by its blood pressure (BP)-lowering actions 1-4. Experimental evidence further suggests that aldosterone exerts direct adverse effects on the vasculature, contributing to vascular injury and remodeling 5-8. However, the underlying molecular mechanisms by which signaling through the MR elicit these deleterious vascular effects independently of increases in BP remain to be elucidated.
Apoptosis has been implicated in the pathogenesis of vascular injury and remodeling, leading to the development of vascular disease 9-11. Excessive apoptosis contributes to vascular cell loss, impairs endothelium-dependent vasorelaxation, promotes inflammation, and enhances procoagulant activity 12. Evidence indicates that increased hemodynamic and mechanical forces contribute to vascular cell apoptosis 13. Aldosterone further promotes apoptosis in cultured endothelial cells 14, implying a direct and/or indirect role in the pathogenesis of vascular apoptosis and injury. To what extent the vascular protective effects of MR blockade in vivo are the direct result of attenuation of apoptosis independent of reductions in BP, and the molecular mechanisms involved in this protective effect remain unclear.
Protein kinase B (Akt), a serine (Ser)/threonine (Thr) protein kinase, plays a critical role in inhibiting cardiovascular cell apoptosis including endothelial cells 15. Phosphorylation of the Akt Ser473 residue exerts anti-apoptotic actions through several mechanisms including regulation of mitochondrial membrane integrity and suppression of pro-apoptotic signaling, and/or enhancement anti-apoptotic molecule expression. BAD, a pro-apoptotic molecule, initiates apoptosis by binding to the anti-apoptotic molecules, Bcl-xL and Bcl-2 on the outer mitochondrial membrane, causing cytochorome c release into the cytosol 16-18. Akt phosphorylates BAD on Ser136, which prevents BAD from binding to Bcl-xL and Bcl-2, thus preventing the pro-apoptotic function of BAD 16. Akt also plays an important role in cell metabolism and regulation of various vascular functions. Indeed, Akt knockout mice exhibit various vascular abnormalities including increased apoptosis and lipid accumulation 19.
It was recently reported that in vitro aldosterone treatment impairs Akt activation in vascular smooth muscle cells (SMCs) 20. Accordingly, we hypothesized that aldosterone, acting through MR stimulation, suppresses vascular Akt activation, which in turn promotes vascular apoptosis and lipid accumulation and injury. We further hypothesized that the effects of aldosterone could be attenuated by in vivo treatment with the MR antagonist spironolactone. To this end, we employed the transgenic Ren2 rat model, which overexpresses the mouse renin gene in the adrenal glomerulosa resulting in elevated plasma levels of aldosterone 21, 22. Furthermore, to assess BP independent effects of MR antagonism on the vasculature protection, we utilized a dose of spironolactone, which does not reduce systolic BP in hypertensive Ren2 rats 23.
MATERIALS AND METHODS
For details of the materials and methods please see http://hyper.ahajournals.org.
Animals and Treatments
Animal procedures were followed by the University of Missouri Animal Care and Use Committee and NIH guidelines. Male transgenic heterozygous (+/-) Ren2 rats and Sprague-Dawley (SD) littermates at 6-7 wk of age were randomly assigned to SD vehicle-treated (SD-C), Ren2 vehicle-treated (Ren2-C) and Ren2 spironolactone (Ren2-Sp) groups (6-7 rats/group). Rats were implanted with a subcutaneous time-release, matrix-driven delivery pellet containing either spironolactone (5 mg; 0.24 mg/d) or vehicle for 21 day 23.
Systolic Blood Pressure (SBP)
SBP was measured prior to and after treatment 23.
Aorta Dissection and Protein Extraction
Thoracic aorta was used in this study.
NADPH Oxidase Activity Assay
NADPH oxidase activity was measured as previously described 24.
Western Blot Analysis
4-HNE, 3-nitrotyrosin, cytochrome c, BAD, BAD Ser136, total-Akt, Akt Ser473 phosphorylation, caspase-3, AT1R were analyzed by Western blot.
Immunofluorescence
Paraffin and cryostat sections were used for immunohistochemistry with the following antibodies against: BAD Ser136, Bcl-2, Bcl-xL, Akt Ser473, Akt Thr308, angiotensin II (ANG II), angiotensin type 1 receptor (AT1R), MR, Nox2, Rac1, p67phox, or 3- nitrotyrosine, respectively.
Evaluation of Apoptotic Cell Death by TUNEL
TUNEL assay was performed using In Situ Cell Death Detection Kit.
Oil Red O Staining
Oil Red O staining was performed on frozen aortic sections.
Transmission Electronic Microscopy (TEM)
The ultrastructural changes of vasculature were examined by TEM.
Statistical Analysis
All data are reported as the means ± SEM. Dunnett's test or Student's t-test were used to determine the significance among groups. A value of P<0.05 was considered to be statistically significant.
RESULTS
Spironolactone Treatment Did Not Influence Systolic Blood Pressures (SBP) in Ren2 rats
Ren2 rats at age 6-7 weeks had higher SBP than SD controls (P<0.01), further increased at age 9-10 weeks compared to 6-7 weeks of age (P<0.01). Spironolactone treatment did not reduce SBP compared with untreated Ren2 rats (P>0.05) (Fig 1A). Interestingly, despite not reducing SBP, spironolactone treatment attenuated the increases in medial wall thickness observed in untreated Ren2 rats (Fig 1B).
Figure 1.
MR blockade did not influence systolic blood pressure (SBP), but attenuated aortic tissue hypertrophy and RAAS activity in Ren2 rats. A, SBP was measured at the initial and final of experiment. B, Average aortic media thickness. C, Average gray scale intensities of immunostaining for MR. D, Representative immuostaining images for ANG II. E, Average gray scale intensities of immuostaining for ANG II. F, Representative immuostaining images for AT1R. G, Representative Western blot for AT1R (top, 50 kDa), bar graph showed the band densitometry analysis (bottom). The results are means ± SEM from 6-7 rats per group. ** P<0.01 vs. age-matched Sprague-Dawley controls (SD-C) (at the final); there was no significant difference of SBP between spironolacotne treated Ren2 (Ren2-Sp) and Ren2 controls (Ren2-C) (at the final). #P <0.05 vs. SD-C; *P<0.05 vs. Ren2-C. D and F, Magnification ×400, scale bar 50 μm.
Spironolactone Treatment Attenuated Vascular Activity of RAAS in Ren2 rats
To determine whether spironolactone treatment affects aortic tissue RAAS activity, expression of MR, ANG II, and AT1R were analyzed using immunohistochemistry and Western blot. Ren2 aortas exhibited increased expression of MR (Fig 1C), ANG II (Fig 1D & 1E) and AT1R (Fig 1F & G) compared to SD, all attenuated with spironolactone treatment.
Spironolactone Treatment Attenuated Vascular Oxidative Stress in Ren2 rats
Lipid peroxidation was determined by measuring 4-HNE and 3-nitrotyrosine by Western blot (Fig 2A-2B) and immunohistochemistry (data not shown). There was a significant increase of 4-HNE (Fig 2A) and 3-nitrotyrosine (Fig 2B) in Ren2 aortic tissues compared with SD, and spironolactone treatment decreased 4-HNE and 3-nitrotyrosine levels in Ren2 aortas (Fig 2A-2B). To determine whether attenuation of lipid peroxidation by spironolactone treatment in Ren2 aortas is due to suppression of NADPH oxidase activation, the activity and subunit expression of this enzyme were measured. Ren2 aortas exhibited greater activity (Fig 2C) and increased expression of Nox2, Rac1, p67phox subunits (Fig 2D, 2E, 2F, respectively) of NADPH oxidase compared with SD, attenuated with spironolactone treatment compared with untreated Ren2 controls (Fig 2C-2F).
Figure 2.
MR blockade attenuated vascular lipid peroxidation in Ren2 rats. A, Representative Western blot for 4-HNE (top, multiple bands), bar graph showed a quantitative densitometry analysis of 4-HNE bands (bottom). B, Representative Western blot for 3-nitrotyrosine (top, multiple bands), bar graph showed the band densitometry analysis (bottom). C, NADPH oxidase activity. D-F, Average gray scale intensities of immuostaining for Nox2, Rac1, and p67phox. The results are means ± SEM, 6-7 rats per group. **P<0.01 vs. SD-C; #P<0.05 vs. SD-C *P<0.05 vs. Ren2-C.
Spironolactone Treatment Improved Vascular Akt Activation in Ren2 rats
Akt Ser473 phosphorylation was significantly decreased in Ren2 aortas compared with SD (P<0.05), which was improved with spironolactone treatment compared with untreated Ren2 aortas (P<0.05) (Fig 3A). Immunostaining intensities for Akt Ser473 and Thr308 were decreased in Ren2 aortic sections compared with SD, similarly improved with spironolactone treatment (Fig 3B-3C).
Figure 3.

MR blockade improved Akt activation in Ren2 rats. A, Representative Western blot for Akt Ser473 (60 kDa) and total Akt (60 kDa) (top), bar graph showed a ratio of Akt Ser473/total Akt for the band densitometry analysis (bottom). B, Representative immuostaining images for Akt Ser473. C, Representative immunostaining images for Akt Thr308. The results are mean ± SEM from 6 rats for each group. #P<0.05 vs. SD-C; *P<0.05 vs. Ren2-C. M, media, Magnification ×400, scale bar 50 μm.
Spironolactone Treatment Enhanced BAD Ser136 phosphorylation and Anti-apoptotic Protein Expression in Ren2 rats
Expression of BAD Ser136 phosphorylation (Fig 4A and 4B), Bcl-2 (Fig 4C), and Bcl-xL (Fig 4D) was decreased in Ren2 aortas compared with SD, and improved with spironolactone treatment compared with untreated Ren2 aortas (Fig 4A-D). In contrast, BAD expression was significantly increased in Ren2 aortas compared with SD, and attenuated with spironolactone treatment (Fig 4E).
Figure 4.

MR blockade enhanced BAD Ser136 phosphorylation and expression of Bcl-xL and Bcl-2, and suppressed BAD expression in Ren2 rats. A, Representative immunostaining images for BAD Ser136. B, Representative Western blot for BAD Ser136 (top, 25 kDa), and bar graph showed the band densitometry analysis (bottom). C, Representative immunostaining images for Bcl-2. D, Representative immunostaining images for Bcl-xL. E, Representative Western blot for BAD (top, 25 kDa), and bar graph showed the band densitometry analysis (bottom). A, C and D, Magnification ×400, scale bar 50 μm. The results are mean ± SEM from 6 rats for each group. #P<0.05 vs. SD-C; *P<0.05 vs. Ren2-C.
Spironolactone Treatment Attenuated Vascular Mitochondrial Abnormalities and Cytochrome c Release in Ren2 Rats
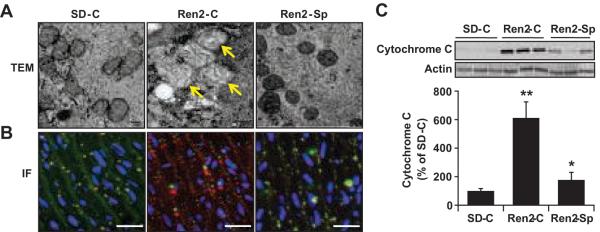
Mitochondrial damage impairs mitochondrial membrane integrity and causes cytochrome c release into cytosol, contributing to apoptosis 17, 18, 25. Mitochondria are vulnerable to damage by a variety of pathological insults including ROS 26. Ultrastructural analysis demonstrated that Ren2 vascular mitochondria displayed abnormalities including swelling, cristae disruption, and reduced matrix density compared with SD vasculature, and these abnormalities were partly corrected following spironolactone treatment (Fig 5A). Double immunostaining for mitochondrial complex IV subunit 1 and cytochrome c showed that there was a diffuse staining pattern for cytochrome c, which did not co-localize with mitochondrial complex IV subunit 1 staining in Ren2 aortas compared with SD (Fig 5B). These alterations in cytochrome c staining in Ren 2 aortic tissue were partly corrected with spironolactone treatment (Fig 5B). Western blot analysis showed that there was increased cytochrome c release into cytosol in Ren2 aortic tissues compared with SD, which was attenuated with spironolactone treatment (Fig 5C).
Figure 5.
MR blockade attenuated vascular cell mitochondria injury and cytochrome c release in the Ren2 rats. A, Representative transmission electronic microscope (TEM) images showed abnormal mitochondria with swollen and reduced matrix density in Ren2 rats (Yellow arrow), magnification ×12000, scale bar 100 nm. B, Representative double immunostaining for MTCO1 (green), cytochrome c (red), and overlap (yellow), nuclei (DAPI, blue), magnification ×400, scale bar 50 μm. C, Representative Western blot for cytochrome c in cytosolic fractions (top, 15 kDa) and bar graph shows a quantitative densitometry of cytochrome c bands (bottom). The results are means ± SEM, 6 rats per group. **P<0.01 vs. SD-C; *P<0.05 vs. Ren2-C.
Spironolactone Treatment Attenuated Vascular Apoptosis in Ren2 Rats
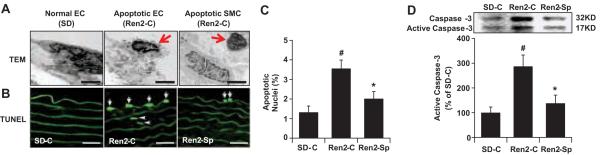
TEM ultrastructural analysis demonstrated apoptotic cells with nuclear condensation, clumping and loss of cytosolic and plasma membrane integrity in endothelial cells (ECs) and SMCs in Ren2 arteries (Fig 6A). TUNEL staining showed that there were apoptotic ECs and SMCs in Ren2 aortic sections (Fig 6B). As shown in Fig 6C, there was 2.6 fold increase of apoptotic cells in Ren2 aortic sections compared with SD (P<0.01), which was substantially reduced following spironolactone treatment (P<0.05). Immunoblot analysis further confirmed this observation that Ren2 vasculatures exhibited increased activated caspase-3, a key enzyme in apoptosis pathways, compared with SD controls (P<0.05), which were attenuated following spironolactone treatment (P<0.05) (Fig 6D).
Figure 6.
MR blockade attenuated vascular cell apoptosis in Ren2 rats. A, Representative TEM images showed apoptotic endothelial cells (EC) and smooth muscle cells (SMC) from Ren2 rats, magnification ×6000, scale bar 0.5 μm. B, Representative TUNEL staining photomicrographs showed vascular apoptotic ECs (arrow) and SMCs (arrow head), magnification ×400, scale bar 50 μm. C, Bar graph shows ratio of TUNEL-positive cell/total cells × 100%. D, Activated caspase-3 were analyzed by immunoblot, the graph indicated quantitative densitometry analysis. The results are mean ± SEM from three sections per animal and 6 rats per group. #P<0.05 compared to SD-C; *P<0.05 compared to Ren2-C.
Spironolactone Treatment Attenuated Vascular Ultrastructural Abnormalities in Ren2 Rats
Evidence suggests that activated caspase-3 cleaves integrin β4, a key component of hemidesmosomes, promoting apoptosis 27. TEM showed that Ren2 vasculatures exhibited hemidesmosome-like structure (endothelial basal adhesion plaque) loss, which was attenuated with spironolactone treatment (Fig 7A). The basal adhesion plaques that promote adhesion of the endothelium to the internal elastic matrix were structurally similar to hemidesmosomes responsible for epithelial cell-matrix attachments, and they are thus referred to as hemidesmosome-like structures. Hemidesmosome-like structure loss disrupts cell-matrix (elastin of the internal elastic lamina) interaction and adhesion, which might have contributed to increased vascular EC apoptosis observed in Ren2 vasculatures. A recent study suggests that aldosterone stimulates migration of vascular SMCs 28. Ren2 vasculatures also displayed SMC migration into intimal layer and internal elastic lamina disruption compared with SD vasculatures, attenuated with spironolactone treatment (data not shown).
Figure 7.
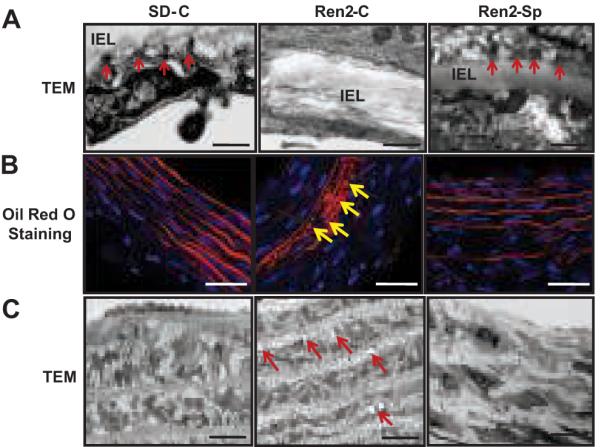
MR blockade attenuated vascular hemidesmosome-like structural loss and lipid accumulation in the Ren2 rats. A, Representative TEM images indicated hemidesmosome-like structures (arrows), IEL (internal elastic lamina), Magnification ×12000, scale bar 100 nm. B, Representative Oil Red O staining showed lipid droplets in SMC from Ren2 rats (arrow), magnification ×400, scale bar 50 μm. C, Representative TEM images showed intracellular lipid vesicles in SMC (arrow) from Ren2 rats, magnification ×3000, scale bar 10 μm.
Spironolactone Treatment Attenuated Vascular Lipid Accumulation in Ren2
Lipid accumulation in vasculature is also a characteristic of vascular injury/maladaptation 29, 30. Impaired Akt activation and damaged mitochondria have been suggested to promote vascular lipid accumulation 19. Oil red O staining indicated increased lipid accumulation in media (Fig 7B) and ECs (not shown) in Ren2 aortas compared with SD; this observation was supported by TEM (Fig 7C). Lipid accumulation in Ren2 aortas was attenuated with spironolactone treatment (Fig 7B and 7C).
DISCUSSION
Evidence suggests that MR blockade exerts a protective effects on cardiovascular outcomes 1-3. Although increased blood pressure mediated by aldosterone causes vascular injury, recent evidence suggests that aldosterone also exerts direct maladaptive effects on the vasculature 5, 7, 31. While vascular injury is a key step for initiation and progression of cardiovascular diseases, little is known regarding the molecular mechanisms underlying aldosterone-induced deleterious effects on the vasculature. The current investigation provides novel findings that MR blockade delivers a direct protection against aldosterone-induced vascular apoptosis, mitochondrial damage, lipid accumulation, and endothelial basal hemidesmosome-like structure loss via rescuing Akt activation, independent of BP/hemodynamic actions.
The transgenic Ren2 rat overexpress the mouse renin gene in the adrenal glomerulosa resulting in increased tissue ANG II and elevated plasma aldosterone levels. We have previously observed that AT1R blockade 32 and treatment with high doses of spironolactone (unpublished observation) significantly decrease blood pressure in Ren2 rats, suggesting that both ANG II and aldosterone are involved in the development of hypertension in the Ren2 rats. We report here that Ren2 aortas exhibit increased aortic tissue levels of ANG II, AT1R, and MR compared with SD. In the present investigation, we used a very low dose of spironolactone to determine whether MR blockade provides direct vasculature protection independent of blood pressure reduction. The measurement of blood pressure by the tail cuff method may be a limitation in the current study; nevertheless, low-dose spironolactone treatment reduced aortic tissue levels of ANG II, AT1R, and MR in Ren2 rats. This is consistent with a recent report that spironolactone in higher doses (50mg/kg per day) reduces the expression of angiotensin converting enzyme (ACE), MR and aldosterone synthase in streptozotocin-induced diabetic kidney 33. Our data suggest that MR blockade exerts direct protection from vascular injury, in part, through modulation of ANG II, AT1R, and MR expression in the Ren2 rat model.
Apoptosis has been implicated as an important mechanism in the pathogenesis of vascular disease 11, 34. Apoptosis promotes vascular cell loss. Apoptosis also induces vascular dysfunction, inflammatory cell infiltration, and lipid accumulation, all of which further aggravate vascular cell apoptosis 11, 12. There were significantly increased apoptotic endothelial and SMC cell deaths detected by TUNEL assay in Ren2 aortas compared with SD aortas, and these differences were substantially decreased following spironolactone treatment (Fig 4). TUNEL assay is a sensitive and standard technique for studying apoptosis in various tissues. However, TUNEL assay may also recognize cells undergoing DNA repair 35, 36. We further corroborated our TUNEL results with morphological characterization of apoptosis by light microscope and TEM as well as DNA electrophoresis. Both light microscopic (data not shown) and TEM findings (Fig 4) indicated that there were more apoptotic nuclei in Ren2 aortic sections compared with SD, and this difference was attenuated with spironolactone treatment (data not shown). Furthermore, DNA electrophoresis showed that there were increased DNA fragmentations in Ren2 aortic tissue compared with SD, attenuated with spironolactone treatment (data not shown). In addition, active caspase-3, a more specific indicator for cells going to apoptosis, was increased in Ren2 aortic tissues compared with SD, abrogated following spironolactone treatment (Fig 4). Collectively, these novel data support the notion that MR antagonism exerts a direct protective role in vascular tissue exposed to high levels of circulating aldosterone. Our results are consistent with recent reports that aldosterone induces cell apoptosis in cultured endothelial cells 14, proximal tubular cells 37, mesangial cells 38, cardiac myocytes 39. In contrast, a recently report indicates that spironolactone induces cell apoptosis in cultured mononuclear cells 40. Together, these data suggest that the effects of spironolactone on cell apoptosis may be dependent on the cell type and culture conditions. We extend these in vitro observations and report here for the first time that in vivo MR antagonism attenuates vascular cell apoptosis independent of BP-lowering action.
Our findings also suggest that the vasculature protections by MR blockade are mediated by suppression of oxidative stress and improvement of Akt activation. In this regard, Akt Ser473 phosphorylation/activation was dramatically decreased in Ren2 aortas, and was substantially improved following spironolactone treatment (Fig 3). Interestingly, immunostaining intensities of Akt Ser473 phosphorylation on ECs were comparable between SD-C, Ren2-C, and Ren2-Sp (data not shown); while immunostaining intensities of Akt Thr308 phosphorylation in ECs were dramatically decreased in Ren2 aortic sections compared with SD, increased with spironolactone treatment (data not shown). Our results are consistent with a recent report that aldosterone impairs Akt activation in cultured vascular SMCs 20. The increased levels of 4-HNE and 3-nitrotyrosine in Ren2 aortas were markedly decreased with spironolactone treatment (Fig 2). The increased activity and expression of NADPH oxidase observed in Ren2 aortas were abrogated following spironolactone treatment, suggesting that there is MR mediation of NADPH oxidase activition as a source of oxidative stress. Oxidative stress has been shown to inhibit Akt activation in various tissues including vasculature through direct or indirect actions 41.
Akt activation is crucial in cell survival 17-19, 25 through inhibiting apoptosis by suppressing pro-apoptotic and/or enhancing anti-apoptotic signal as well as regulation of mitochondrial membrane integrity and cytochrome c release. BAD is pro-apoptotic molecule and usually maintained in phosphorylated and sequestered form in the cytosol. When dephosphorylated, BAD migrates into mitochondria and binds to Bcl-2 and Bcl-xL, and release cytochrome c into the cytosol. Akt activation promotes phosphorylation and inactivation of BAD 17, 18. Akt activation increases anti-apoptotic molecule expression such as Bcl-2 17, 18. Akt phosphorylation also promotes Akt translocation to the nucleus, where it affects the transcription of target genes and exerts protective activity 42. Indeed, decreased expression of BAD Ser136 phosphorylation, Bcl-xL, and Bcl-2 was observed in Ren2 aortas compared to SD, and which was improved following spironolactone treatment. These were consistent with attenuation of mitochondrial injury and cytochrome c release into cytosol in treated Ren2 vasculatures with spironolactone. Our results are also consistent with a recent study that aldosterone treatment enhances dephosphorylation of phosphor-BAD and release of cytochrome c into cytosol in cultured mesangial cell 38. Increased cytochrome c in cytosol activates caspase-3, resulting in cell apoptosis. Active caspase-3 has been recently shown to cleave the key component integrin β4 of hemidesmosomes and impair hemidesmosome formation, promoting apoptosis 27. Ren2 arteries exhibited hemidesmosome-like structural loss, which was preserved by spironolactone treatment. These together with increased pro-apoptotic proteins contribute to increased vascular apoptosis and injury in Ren2 rats.
Akt activation also plays a vital role in regulating cell metabolism including lipid oxidation. Lipid accumulation is a prominent feature of vascular disease and also an etiological factor of vascular injury. Indeed, Akt knockout mice display increased vascular lipid accumulation and develop severe vascular injury and atherosclerosis 19. In this context, there was increased vascular lipid accumulation in Ren2 aortas, abolished by in vivo MR blockade.
In summary, the results of this investigation indicate that elevated aldosterone levels acting via the MR promote NADPH oxidase activation/ROS production, which in turn impairs Akt activation, and consequently causes activation of apoptotic signaling pathways, leading to vascular cell apoptosis, lipid accumulation and injury.
Perspectives
This investigation provides novel evidence that MR antagonism provides direct protection of vascular cells from apoptosis and injury independent of BP/hemodynamic actions. These protective effects are mediated by improvement of Akt activation and suppression of cell death pathways. These data also demonstrate that there is a potentially distinct therapeutic target mediated via rescuing Akt activation. Finally, these data help us understand some of the molecular mechanisms by which therapy with MR antagonists have provided protection against cardiovascular events in patients at high risk.
Acknowledgments
The authors would like to acknowledge Rebecca Schneider and University of Missouri Electron Microscope Core for their excellent technique and tissue preparation.
Sources of Funding This work was supported by NIH RO1-HL073101-02, VA Merit 0018 (JRS), VA VISN 15 (AWC), and the Missouri Kidney Program (AWC).
Abbreviations
- SD-C
Sprague-Dawley rat controls
- Ren2-C
Ren2 vehicle controls
- Ren2-Sp
Ren2 treated with spironolactone
- ECs
endothelial cells
- SMCs
smooth muscle cells
- RAAS
renin angiotensin aldosterone system
Footnotes
Disclosures None
References
- 1.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 2.Pitt B. Aldosterone blockade in patients with systolic left ventricular dysfunction. Circulation. 2003;108:1790–1794. doi: 10.1161/01.CIR.0000086776.15268.22. [DOI] [PubMed] [Google Scholar]
- 3.Pitt B, Reichek N, Willenbrock R, Zannad F, Phillips RA, Roniker B, Kleiman J, Krause S, Burns D, Williams GH. Effects of eplerenone, enalapril, and eplerenone/enalapril in patients with essential hypertension and left ventricular hypertrophy: the 4E-left ventricular hypertrophy study. Circulation. 2003;108:1831–1838. doi: 10.1161/01.CIR.0000091405.00772.6E. [DOI] [PubMed] [Google Scholar]
- 4.Gomez-Sanchez CE, Gomez-Sanchez EP. Role of central mineralocorticoid receptors in cardiovascular disease. Curr Hypertens Rep. 2001;3:263–269. doi: 10.1007/s11906-001-0049-z. [DOI] [PubMed] [Google Scholar]
- 5.Brown NJ. Aldosterone and vascular inflammation. Hypertension. 2008;51:161–167. doi: 10.1161/HYPERTENSIONAHA.107.095489. [DOI] [PubMed] [Google Scholar]
- 6.Rocha R, Rudolph AE, Frierdich GE, Nachowiak DA, Kekec BK, Blomme EA, McMahon EG, Delyani JA. Aldosterone induces a vascular inflammatory phenotype in the rat heart. Am J Physiol Heart Circ Physiol. 2002;283:H1802–H1810. doi: 10.1152/ajpheart.01096.2001. [DOI] [PubMed] [Google Scholar]
- 7.Schiffrin EL. Effects of aldosterone on the vasculature. Hypertension. 2006;47:312–318. doi: 10.1161/01.HYP.0000201443.63240.a7. [DOI] [PubMed] [Google Scholar]
- 8.Martinez DV, Rocha R, Matsumura M, Oestreicher E, Ochoa-Maya M, Roubsanthisuk W, Williams GH, Adler GK. Cardiac damage prevention by eplerenone: comparison with low sodium diet or potassium loading. Hypertension. 2002;39:614–618. [PubMed] [Google Scholar]
- 9.Korshunov VA, Berk BC. Smooth muscle apoptosis and vascular remodeling. Curr Opin Hematol. 2008;15:250–254. doi: 10.1097/MOH.0b013e3282f97d71. [DOI] [PubMed] [Google Scholar]
- 10.Kraemer R. Reduced apoptosis and increased lesion development in the flow-restricted carotid artery of p75(NTR)-null mutant mice. Circ Res. 2002;91:494–500. doi: 10.1161/01.res.0000035245.83233.2a. [DOI] [PubMed] [Google Scholar]
- 11.Rossig L, Dimmeler S, Zeiher AM. Apoptosis in the vascular wall and atherosclerosis. Basic Res Cardiol. 2001;96:11–22. doi: 10.1007/s003950170073. [DOI] [PubMed] [Google Scholar]
- 12.Durand E, Scoazec A, Lafont A, Boddaert J, Al HA, Addad F, Mirshahi M, Desnos M, Tedgui A, Mallat Z. In vivo induction of endothelial apoptosis leads to vessel thrombosis and endothelial denudation: a clue to the understanding of the mechanisms of thrombotic plaque erosion. Circulation. 2004;109:2503–2506. doi: 10.1161/01.CIR.0000130172.62481.90. [DOI] [PubMed] [Google Scholar]
- 13.Buus CL, Pourageaud F, Fazzi GE, Janssen G, Mulvany MJ, De Mey JG. Smooth muscle cell changes during flow-related remodeling of rat mesenteric resistance arteries. Circ Res. 2001;89:180–186. doi: 10.1161/hh1401.093575. [DOI] [PubMed] [Google Scholar]
- 14.Williams TA, Verhovez A, Milan A, Veglio F, Mulatero P. Protective effect of spironolactone on endothelial cell apoptosis. Endocrinology. 2006;147:2496–2505. doi: 10.1210/en.2005-1318. [DOI] [PubMed] [Google Scholar]
- 15.Steinberg R, Harari OA, Lidington EA, Boyle JJ, Nohadani M, Samarel AM, Ohba M, Haskard DO, Mason JC. A protein kinase Cepsilon-anti-apoptotic kinase signaling complex protects human vascular endothelial cells against apoptosis through induction of Bcl-2. J Biol Chem. 2007;282:32288–32297. doi: 10.1074/jbc.M704001200. [DOI] [PubMed] [Google Scholar]
- 16.Guo X, Chen KH, Guo Y, Liao H, Tang J, Xiao RP. Mitofusin 2 triggers vascular smooth muscle cell apoptosis via mitochondrial death pathway. Circ Res. 2007;101:1113–1122. doi: 10.1161/CIRCRESAHA.107.157644. [DOI] [PubMed] [Google Scholar]
- 17.Tsuruta F, Masuyama N, Gotoh Y. The phosphatidylinositol 3-kinase (PI3K)-Akt pathway suppresses Bax translocation to mitochondria. J Biol Chem. 2002;277:14040–14047. doi: 10.1074/jbc.M108975200. [DOI] [PubMed] [Google Scholar]
- 18.Yang L, Sun M, Sun XM, Cheng GZ, Nicosia SV, Cheng JQ. Akt attenuation of the serine protease activity of HtrA2/Omi through phosphorylation of serine 212. J Biol Chem. 2007;282:10981–10987. doi: 10.1074/jbc.M700445200. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez-Hernando C, Ackah E, Yu J, Suarez Y, Murata T, Iwakiri Y, Prendergast J, Miao RQ, Birnbaum MJ, Sessa WC. Loss of Akt1 leads to severe atherosclerosis and occlusive coronary artery disease. Cell Metab. 2007;6:446–457. doi: 10.1016/j.cmet.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hitomi H, Kiyomoto H, Nishiyama A, Hara T, Moriwaki K, Kaifu K, Ihara G, Fujita Y, Ugawa T, Kohno M. Aldosterone suppresses insulin signaling via the downregulation of insulin receptor substrate-1 in vascular smooth muscle cells. Hypertension. 2007;50:750–755. doi: 10.1161/HYPERTENSIONAHA.107.093955. [DOI] [PubMed] [Google Scholar]
- 21.Campbell DJ, Rong P, Kladis A, Rees B, Ganten D, Skinner SL. Angiotensin and bradykinin peptides in the TGR(mRen-2)27 rat. Hypertension. 1995;25:1014–1020. doi: 10.1161/01.hyp.25.5.1014. [DOI] [PubMed] [Google Scholar]
- 22.Sander M, Bader M, Djavidani B, Maser-Gluth C, Vecsei P, Mullins J, Ganten D, Peters J. The role of the adrenal gland in hypertensive transgenic rat TGR(mREN2)27. Endocrinology. 1992;131:807–814. doi: 10.1210/endo.131.2.1322284. [DOI] [PubMed] [Google Scholar]
- 23.Stas S, Whaley-Connell A, Habibi J, Appesh L, Hayden MR, Karuparthi PR, Qazi M, Morris EM, Cooper SA, Link CD, Stump C, Hay M, Ferrario C, Sowers JR. Mineralocorticoid receptor blockade attenuates chronic overexpression of the reninangiotensin-aldosterone system stimulation of reduced nicotinamide adenine dinucleotide phosphate oxidase and cardiac remodeling. Endocrinology. 2007;148:3773–3780. doi: 10.1210/en.2006-1691. [DOI] [PubMed] [Google Scholar]
- 24.Wei Y, Sowers JR, Nistala R, Gong H, Uptergrove GM, Clark SE, Morris EM, Szary N, Manrique C, Stump CS. Angiotensin II-induced NADPH oxidase activation impairs insulin signaling in skeletal muscle cells. J Biol Chem. 2006;281:35137–35146. doi: 10.1074/jbc.M601320200. [DOI] [PubMed] [Google Scholar]
- 25.Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6:513–519. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- 26.de Cavanagh EM, Inserra F, Ferder M, Ferder L. From mitochondria to disease: role of the renin-angiotensin system. Am J Nephrol. 2007;27:545–553. doi: 10.1159/000107757. [DOI] [PubMed] [Google Scholar]
- 27.Werner ME, Chen F, Moyano JV, Yehiely F, Jones JC, Cryns VL. Caspase proteolysis of the integrin beta4 subunit disrupts hemidesmosome assembly, promotes apoptosis, and inhibits cell migration. J Biol Chem. 2007;282:5560–5569. doi: 10.1074/jbc.M603669200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montezano AC, Callera GE, Yogi A, He Y, Tostes RC, He G, Schiffrin EL, Touyz RM. Aldosterone and angiotensin II synergistically stimulate migration in vascular smooth muscle cells through c-Src-regulated redox-sensitive RhoA pathways. Arterioscler Thromb Vasc Biol. 2008;28:1511–1518. doi: 10.1161/ATVBAHA.108.168021. [DOI] [PubMed] [Google Scholar]
- 29.Llorente-Cortes V, Otero-Vinas M, Camino-Lopez S, Costales P, Badimon L. Cholesteryl esters of aggregated LDL are internalized by selective uptake in human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2006;26:117–123. doi: 10.1161/01.ATV.0000193618.32611.8b. [DOI] [PubMed] [Google Scholar]
- 30.Sendra J, Llorente-Cortes V, Costales P, Huesca-Gomez C, Badimon L. Angiotensin II upregulates LDL receptor-related protein (LRP1) expression in the vascular wall: a new pro-atherogenic mechanism of hypertension. Cardiovasc Res. 2008;78:581–589. doi: 10.1093/cvr/cvn043. [DOI] [PubMed] [Google Scholar]
- 31.Zhao W, Ahokas RA, Weber KT, Sun Y. ANG II-induced cardiac molecular and cellular events: role of aldosterone. Am J Physiol Heart Circ Physiol. 2006;291:H336–H343. doi: 10.1152/ajpheart.01307.2005. [DOI] [PubMed] [Google Scholar]
- 32.Wei Y, Whaley-Connell AT, Chen K, Habibi J, Uptergrove GM, Clark SE, Stump CS, Ferrario CM, Sowers JR. NADPH oxidase contributes to vascular inflammation, insulin resistance, and remodeling in the transgenic (mRen2) rat. Hypertension. 2007;50:384–391. doi: 10.1161/HYPERTENSIONAHA.107.089284. [DOI] [PubMed] [Google Scholar]
- 33.Taira M, Toba H, Murakami M, Iga I, Serizawa R, Murata S, Kobara M, Nakata T. Spironolactone exhibits direct renoprotective effects and inhibits renal renin-angiotensinaldosterone system in diabetic rats. Eur J Pharmacol. 2008;589:264–271. doi: 10.1016/j.ejphar.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 34.Dimmeler S, Zeiher AM. Endothelial cell apoptosis in angiogenesis and vessel regression. Circ Res. 2000;87:434–439. doi: 10.1161/01.res.87.6.434. [DOI] [PubMed] [Google Scholar]
- 35.Kockx MM, Muhring J, Knaapen MW, de Meyer GR. RNA synthesis and splicing interferes with DNA in situ end labeling techniques used to detect apoptosis. Am J Pathol. 1998;152:885–888. [PMC free article] [PubMed] [Google Scholar]
- 36.Petrov VV, van Pelt JF, Vermeesch JR, Van D V, Vekemans K, Fagard RH, Lijnen PJ. TGF-beta1-induced cardiac myofibroblasts are nonproliferating functional cells carrying DNA damages. Exp Cell Res. 2008;314:1480–1494. doi: 10.1016/j.yexcr.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 37.Patni H, Mathew JT, Luan L, Franki N, Chander PN, Singhal PC. Aldosterone promotes proximal tubular cell apoptosis: role of oxidative stress. Am J Physiol Renal Physiol. 2007;293:F1065–F1071. doi: 10.1152/ajprenal.00147.2007. [DOI] [PubMed] [Google Scholar]
- 38.Mathew JT, Patni H, Chaudhary AN, Liang W, Gupta A, Chander PN, Ding G, Singhal PC. Aldosterone induces mesangial cell apoptosis both in vivo and in vitro. Am J Physiol Renal Physiol. 2008;295:F73–F81. doi: 10.1152/ajprenal.00435.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De AN, Fiordaliso F, Latini R, Calvillo L, Funicello M, Gobbi M, Mennini T, Masson S. Appraisal of the role of angiotensin II and aldosterone in ventricular myocyte apoptosis in adult normotensive rat. J Mol Cell Cardiol. 2002;34:1655–1665. doi: 10.1006/jmcc.2002.2115. [DOI] [PubMed] [Google Scholar]
- 40.Sonder SU, Woetmann A, Odum N, Bendtzen K. Spironolactone induces apoptosis and inhibits NF-kappaB independent of the mineralocorticoid receptor. Apoptosis. 2006;11:2159–2165. doi: 10.1007/s10495-006-0286-3. [DOI] [PubMed] [Google Scholar]
- 41.Cooper SA, Whaley-Connell A, Habibi J, Wei Y, Lastra G, Manrique C, Stas S, Sowers JR. Renin-angiotensin-aldosterone system and oxidative stress in cardiovascular insulin resistance. Am J Physiol Heart Circ Physiol. 2007;293:H2009–H2023. doi: 10.1152/ajpheart.00522.2007. [DOI] [PubMed] [Google Scholar]
- 42.Shiraishi I, Melendez J, Ahn Y, Skavdahl M, Murphy E, Welch S, Schaefer E, Walsh K, Rosenzweig A, Torella D, Nurzynska D, Kajstura J, Leri A, Anversa P, Sussman MA. Nuclear targeting of Akt enhances kinase activity and survival of cardiomyocytes. Circ Res. 2004;94:884–891. doi: 10.1161/01.RES.0000124394.01180.BE. [DOI] [PubMed] [Google Scholar]