Summary
Members of the Forkhead box O (Foxo) family of transcription factors are key regulators of cellular responses, but their function in the immune system remains incompletely understood. Here we show that T cell-specific deletion of Foxo1 gene in mice led to spontaneous T cell activation, effector T cell differentiation, autoantibody production, and the induction of inflammatory bowel disease in a transfer model. In addition, Foxo1 was critical for the maintenance of naïve T cells in the peripheral lymphoid organs. Transcriptome analyses of T cells identified Foxo1-regulated genes encoding, among others, cell surface molecules, signaling proteins, and nuclear factors that control gene expression. Functional studies validated interleukin 7 receptor-α as a Foxo1 target gene essential for Foxo1 maintenance of naïve T cells. These findings reveal crucial functions of Foxo1-dependent transcription in control of T cell homeostasis and tolerance.
Introduction
T lymphocytes are well maintained in the peripheral lymphoid organs under the immune system steady state (Jameson, 2002; Surh and Sprent, 2005). Both cell-intrinsic and cell-extrinsic mechanisms operate to ensure the homeostasis and tolerance of T cells. Survival and homeostatic proliferation of T cells is primarily mediated by the cytokine interleukin 7 (IL-7) (Ma et al., 2006; Schluns and Lefrancois, 2003), in part via IL-7 control of expression of target genes including the pro-survival factor Bcl-2 (Akashi et al., 1997; Maraskovsky et al., 1997), and through IL-7 regulation of protein stability of the cyclin-dependent kinase inhibitor p27Kip1 (Barata et al., 2001; Li et al., 2006b). IL-7 exerts its biological effects by binding to the IL-7 receptor complex composed of IL-7 receptor-α chain (IL-7R) and the common γ-chain, resulting in the activation of the JAK kinases and the Stat5 transcription factor (Jiang et al., 2005). Neutralization of IL-7 results in compromised survival of mature T cells (Schluns et al., 2000; Tan et al., 2001), whereas transgenic expression of IL-7 expands T cells (Kieper et al., 2002). Memory T cells also fail to flourish in IL-7-deficient mice (Kondrack et al., 2003; Li et al., 2003; Schluns et al., 2000; Seddon et al., 2003), emphasizing a prerequisite for IL-7 in control of T cells at various differentiation states. IL-7 is constitutively produced by stromal cells, and the control of IL-7 signaling is largely through the regulation of IL-7R expression on T cells (Mazzucchelli and Durum, 2007). Indeed, IL-7R is not detectable on CD4+CD8+ immature T cells, but is expressed on positively selected mature CD4+ and CD8+ T cells. IL-7R is down-regulated upon T cell activation, but is re-expressed on memory T cells. Several transcription factors including GABP and Gfi-1 have been shown to regulate IL-7R expression in T cells (Chandele et al., 2008; Park et al., 2004; Xue et al., 2004; Yucel et al., 2003). However, the mechanisms that control IL-7R expression at the various stages of T cell differentiation remain incompletely understood.
The stochastic process by which the T cell receptors with different antigen-binding specificities are generated creates the inherent problem that some receptors have a high affinity for self-antigens or for innocuous environmental antigens such as those from commensal organisms. Multiple mechanisms have evolved to control T cell-mediated immunopathology, including deletion of self-reactive T cell clones in the thymus and active immune suppression by cytokine TGF-β1 or CD4+CD25+Foxp3+ regulatory T cells in the periphery (Li and Flavell, 2008; Mathis and Benoist, 2004; Sakaguchi et al., 2008). In addition to these cell-extrinsic mechanisms, it has been postulated that peripheral T cell tolerance might be regulated by T cell-intrinsic factors including the Forkhead box O (Foxo) family of transcription factors (Yusuf and Fruman, 2003).
Foxo proteins (Foxo1, Foxo3a, Foxo4, and Foxo6) are mammalian homologues of the Caenorhabditis elegans transcription factor DAF-16, which have critical functions in control of cell metabolism, survival, proliferation, and differentiation (Burgering, 2008). Foxo activity is down-regulated by protein kinase B (PKB)-mediated phosphorylation at three conserved sites that triggers nuclear export of Foxo proteins in complex with the 14-3-3 protein (Huang and Tindall, 2007). In resting T cells, Foxo proteins reside in the nucleus. PKB activation via the stimulation of the T cell receptor, CD28, and cytokine signaling pathways inactivates Foxo proteins, which is associated with the induction of T cell proliferation (Peng, 2008). Indeed, ectopic expression of a PKB-insensitive Foxo1 mutant suppresses T cell proliferation (Fabre et al., 2005; Medema et al., 2000), suggesting that inactivation of Foxo1 is an obligatory step for T cells to enter the cell cycle. In another study, over-expression of a constitutively active form of Foxo3a results in T cell apoptosis (Brunet et al., 1999). Foxo control of T cell proliferation and apoptosis has been associated with Foxo induction of expression of the cell cycle inhibitor p27Kip1 and the pro-apoptotic factor Bim respectively (Medema et al., 2000; Stahl et al., 2002). These gain-of-function studies imply that different Foxo family members might regulate different target gene expression resulting in differential T cell responses.
The functions of the individual Foxo family proteins in control of T cell tolerance, and of T cell responses in general remain largely undefined in vivo. Expression of a dominant-negative mutant of Foxo1 in T cells leads to the reduced number of thymocytes in mice; yet the mechanisms underlying this defect was not defined (Leenders et al., 2000). Deletion of Foxo3a gene in mice results in a mild lymphoproliferative syndrome, and the development of inflammatory lesions in multiple organs (Lin et al., 2004). These autoimmune phenotypes are associated with spontaneous T cell activation and CD4+ T cell differentiation into T helper 1 (Th1) and Th2 cells. However, in disagreement with the afore-mentioned over-expression study, Foxo3a-deficient T cells exhibit uncompromised apoptosis in assays of activation-induced cell death. Instead, Foxo3a-deficient T cells display enhanced activation of the transcription factor NF-κB as a possible consequence of the reduced expression of IκB proteins (Lin et al., 2004). Therefore, Foxo3a appears to be a critical regulator of T cell tolerance in mice. The functions of the other Foxo family proteins in control of T cell responses in vivo remain to be determined.
To investigate the definitive function of Foxo1 in T cells, we generated mice with T cell-specific deletion of the Foxo1 gene. Thymic T cell differentiation did not appear to be compromised in the absence of Foxo1. However, in the periphery, increased numbers of CD4+ and CD8+ Foxo1-deficient T cells exhibited an activated phenotype and differentiated into effector T cells, concomitant with the induction of autoantibody. In addition, the naïve T cell number was reduced in Foxo1-deficient mice. Gene expression profiling of naïve T cells revealed novel Foxo1 target genes including Il7r. Indeed, expression of IL-7R protein was markedly diminished in Foxo1-deficient naïve T cells which was associated with compromised IL-7 signaling, and reduced Bcl-2 expression. Foxo1-deficient naïve T cells were refractory to IL-7-induced survival in vitro, and exhibited compromised homeostatic proliferation in a lymphopenic environment. Bone marrow chimera experiments revealed that diminished IL-7R expression was a consequence of intrinsic defects of Foxo1-deficient T cells, which was in line with the observation that Foxo1 bound to the evolutionarily conserved transcription regulatory sequences of Il7r gene in wild-type T cells. Foxo1-deficient OT-II T cells exhibited a naïve T cell phenotype, expressed undetectable levels of IL-7R and reduced Bcl-2, and were depleted from the peripheral lymphoid organs. Re-expression of IL-7R on these cells restored Bcl-2 gene expression, and rescued OT-II T cell number in the periphery. These findings demonstrated a critical role for Foxo1 in control of T cell tolerance, and of naïve T cell homeostasis through the induction of IL-7R expression.
Results
Generation of Mice with T Cell-specific Deletion of Foxo1 Gene
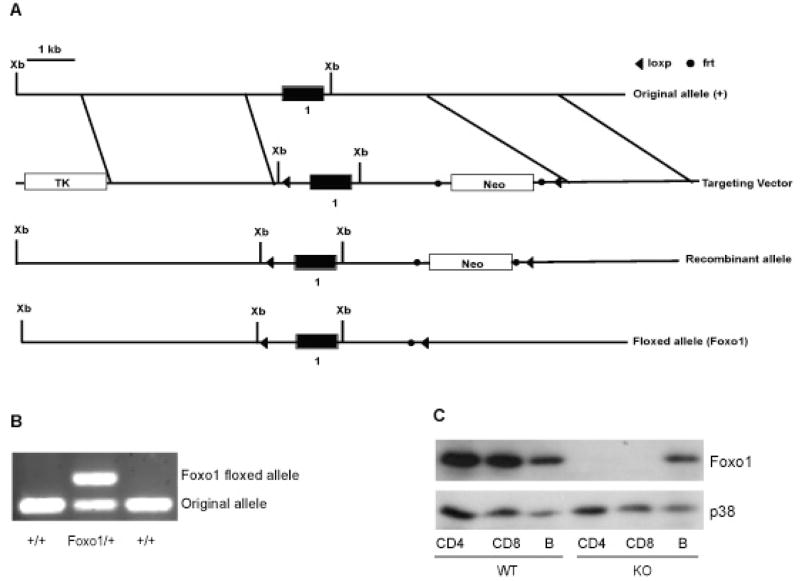
The embryonic lethal phenotype of Foxo1-deficient mice prohibited their usage in the study of T cells (Furuyama et al., 2004; Hosaka et al., 2004). To investigate the cell-type-specific function of Foxo1 in vivo, we generated mice with a mutated Foxo1 allele by the insertion of two loxP sites flanking its promoter region and the first exon (Figure 1A). The translation start codon of Foxo1 protein resides in exon 1. Deletion of exon 1 was therefore expected to create a null mutation of Foxo1 gene. loxP sites were introduced into the Foxo1 locus by homologous recombination in mouse embryonic stem (ES) cells. ES cell clones carrying the recombinant Foxo1 locus were used for generating chimeric mice that produced heterozygous mice after germline transmission. These heterozygous mice were bred with a strain of FLP1 recombinase transgenic mice, which led to the excision of the neomycin resistant gene (Neo) flanked by the frt sites, and the creation of a floxed Foxo1 allele (Figure 1B).
Figure 1. Generation of Mice with T Cell-specific Deletion of the Foxo1 Gene.
(A) Schematic presentation of original allele (+), targeting vector, the recombinant allele, and the floxed allele (Foxo1). Filled boxes represent the first exon of Foxo1 gene. The locations of restriction-enzyme sites (Xb, XbaI) are indicated. Loxp and Frt sites are showed as arrowheads and filled circles respectively.
(B) PCR genotyping of the Floxed Foxo1 allele and the original allele.
(C) CD4+, CD8+ T cells and B cells were purified from WT (Foxo1/Foxo1) and KO (4Cre-Foxo1/Foxo1) mice by FACS sorting. The amounts of Foxo1 and p38 were determined by immunoblotting. p38 was used as a loading control.
Mice with two floxed Foxo1 alleles developed normally and did not show any sign of disease. These mice, designated as WT (wild-type), were used as the control group in our analysis. To study the function of Foxo1 in T cells, we crossed mice carrying the two floxed Foxo1 alleles with CD4-Cre transgenic mice, in which Cre is specifically expressed in T cells (Lee et al., 2001). These mice are designated here as KO (knockout). Foxo1 protein was not detectable in either CD4+ or CD8+ T cells isolated from the KO mice (Figure 1C), whereas B cells from KO mice expressed comparable amounts of Foxo1 to those from WT mice (Figure 1C). These observations reveal efficient and specific ablation of Foxo1 protein in T cells from KO mice.
T Cell Development in the Absence of T cell Foxo1
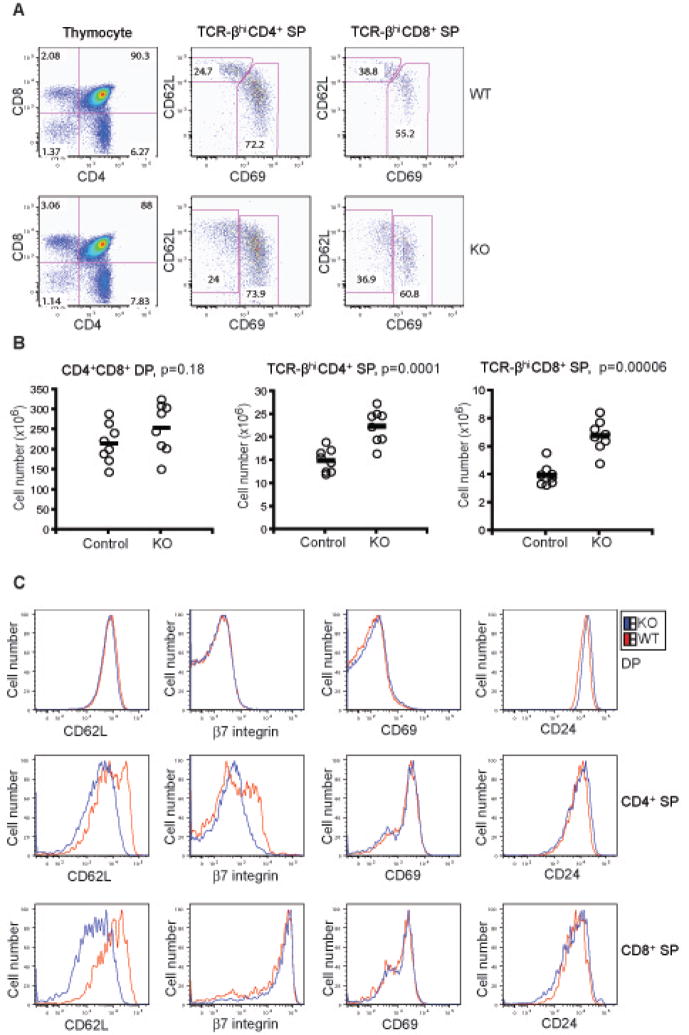
To investigate the consequences of loss of Foxo1 in T cells, we first evaluated thymic T cell development in Foxo1 KO mice aged between 6 to 8 weeks. The CD4 and CD8 profile of KO thymocytes was not drastically different from that of WT thymocytes, although a slight increase of TCR-βhiCD4+ and TCR-βhiCD8+ mature T cells was observed (Figure 2A and 2B). We further examined CD69 and CD62L expression in these T cells, and found that up-regulation of CD62L was compromised in the CD69− T cell population from the KO mice (Figure 2A and 2C). These findings are in line with a recent study showing that the expression of a constitutively active form of Foxo1 in human T cells induces CD62L expression, which has been associated with Foxo1 induction of the transcription factor Kruppel-like factor 2 (KLF2) (Fabre et al., 2008). KLF2 is an important regulator of T cell migration (Carlson et al., 2006; Sebzda et al., 2008), and additionally controls the expression of multiple T cell maturation marker proteins including β7 integrin, CD69, and CD24 (Carlson et al., 2006). However, unlike KLF2-deficient T cells, expression of these cell surface molecules appeared uncompromised in Foxo1 KO T cells (Figure 2C). Taken together, these observations reveal a specific role for Foxo1 in promoting CD62L expression in mature CD4+ and CD8+ thymocytes in mice.
Figure 2. T Cell Development in Foxo1-deficient Mice.
(A) Thymic CD4 and CD8 profile of WT and KO mice at 6 weeks old. CD69 and CD62L were used as maturation makers for TCR-βhiCD4+ and TCR-βhiCD8+ single positive (SP) thymocytes. These are representative results of eight mice per group analyzed.
(B) Number of thymic CD4+CD8+ (double positive, DP), TCR-βhiCD4+ SP and TCR-βhiCD8+ SP cells in control (Foxo1/Foxo1) and KO (4Cre-Foxo1/Foxo1) mice (n=8) at 6–8 weeks old. The p values between two groups of T cell number are shown.
(C) Expression of CD62L, β7 integrin, CD69 and CD24 in thymocytes from WT and KO mice at 6 weeks old. These are representative results of four mice per group analyzed.
T cell Activation and Autoimmunity in T Cell Foxo1-deficient Mice
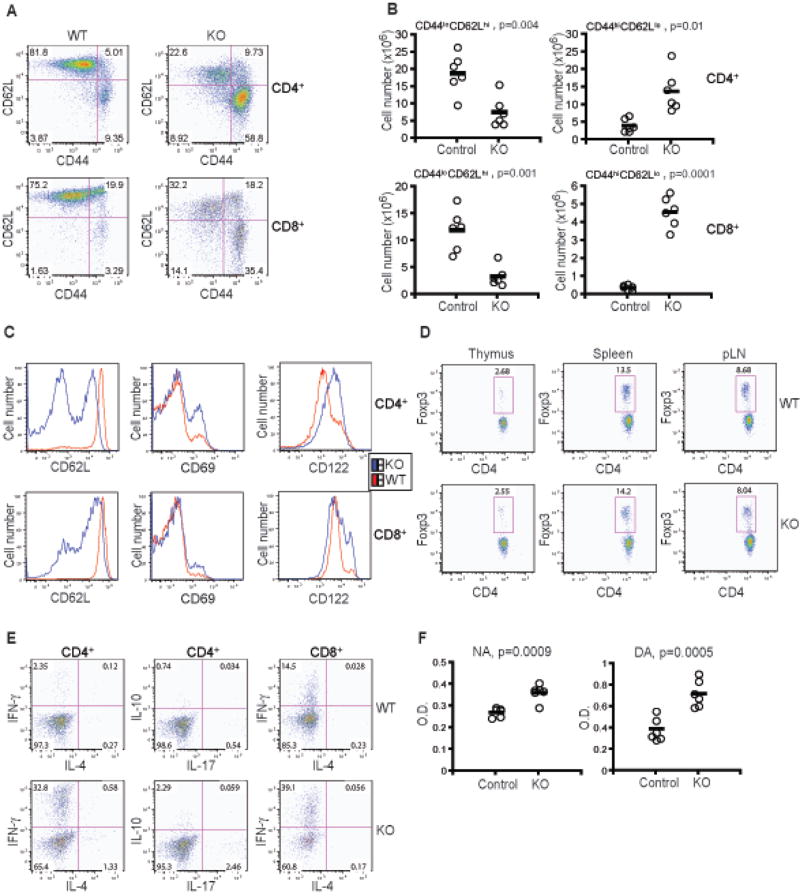
A previous study of Foxo3a-deficient mice showed that Foxo3a is essential for the inhibition of T cell activation and effector T cell differentiation (Lin et al., 2004). To investigate the function of Foxo1 in control of peripheral T cells, we first examined the expression of T cell activation markers CD44, CD62L and CD69 in CD4+ and CD8+ T cells isolated from the spleens of WT and KO mice. Compared to WT T cells, a higher percentage of KO T cells exhibited an activated CD44hiCD62Llo or CD69+ phenotype (Figure 3A and 3C). Notably, similar to KO thymic mature T cells, the CD44lo naïve CD4+ and CD8+ T cells from KO mice expressed lower levels of CD62L than control T cells from WT mice (Figure 3A and 3C). Increased T cell activation and decreased CD62L expression on naïve T cells was also observed in the lymph nodes of KO mice (Figure S1 and S2). In addition, KO mice developed lymphadenopathy associated with the expansion of CD4+ T cells that expressed high levels of the proliferating cell marker Ki-67 antigen (Figure S1 and S2). The cellularity of KO spleens was not significantly different from that of WT spleens (data not shown). However, spleens from KO mice had increased number of CD4+ and CD8+ T cells with the activated CD44hiCD62Llo phenotype, whereas the number of CD44loCD62Lhi naïve CD4+ and CD8+ T cells was decreased (Figure 3B). In addition to conventional CD4+ and CD8+ T cells, CD4+CD8+ immature T cells also give rise to a CD4+CD25+ regulatory T (Treg) cell lineage that expresses the transcription factor Foxp3 (Sakaguchi et al., 2008). CD4+CD25+Foxp3+ Treg cells are essential regulators of peripheral T cell tolerance. To determine whether enhanced T cell activation in Foxo1 KO mice was caused by the depletion of Treg cells, we examined these cells in the thymus and in the periphery. A comparable percentage of Foxp3-positive Treg cells was found in both WT and KO mice in all organs examined (Figure 3D and Figure S1). To investigate whether Foxo1-deficiency affected Treg cell function, we crossed KO mice with Foxp3-RFP reporter mice (Wan and Flavell, 2005), and isolated Treg cells on the basis of RFP expression. KO Treg cells inhibited naïve T cell proliferation as potently as WT Treg cells in an in vitro assay (Figure S3). Taken together, these findings suggest a cell-intrinsic function for Foxo1 in the maintenance of naïve phenotype T cells, and in the prevention of T cell activation.
Figure 3. T Cell Activation and Differentiation in Foxo1-deficient Mice.
(A) Expression of CD44 and CD62L in splenic CD4+ and CD8+ T cells from WT and KO mice at 6 weeks old. These are representative results of six independent experiments.
(B) The number of splenic CD4+ and CD8+ naïve (CD44loCD62Lhi) and activated (CD44hiCD62Llo) T cells from control (Foxo1/Foxo1) and KO mice (n=6) at 6–8 weeks old. The p values between two groups of T cell number are shown.
(C) Expression of CD62L, CD69, and CD122 in splenic CD4+ and CD8+ T cells from WT and KO mice at 6 weeks old. These are representative results of six mice per group analyzed.
(D) The percentage of thymic, splenic, and peripheral lymph node (pLN) CD4+Foxp3+ regulatory T (Treg) cells in WT and KO mice at 6 weeks old. These are representative results of six mice per group analyzed.
(E) CD4+ and CD8+ T cells isolated from the spleens of WT and KO mice were stimulated with PMA and ionomycin for 4 hr and analyzed for the expression of IFN-γ, IL-4, IL-10, and IL-17 by intracellular cytokine staining. These are representative results of two independent experiments.
(F) Titers of nuclear antibody (NA) and dsDNA antibody (DA) in the sera of control (Foxo1/Foxo1) and KO mice aged between 5 and 6 months (n = 6). The p values between the two groups of antibody titers are shown.
Foxo1 KO CD4+ and CD8+ T cells also expressed higher amounts of surface CD122 (Figure 3C), the shared receptor for IL-2 and IL-15 (Ma et al., 2006). We and others have shown that CD122 expression is controlled by transcription factors T-bet and eomesodermin that also regulates Th1 and cytotoxic T lymphocyte (CTL) differentiation (Intlekofer et al., 2005; Li et al., 2007). To determine effector T cell differentiation in Foxo1-deficient mice, we stimulated T cells from spleens with PMA and ionomycin for 4 hr and performed intracellular cytokine staining. Compared to T cells from WT mice, which had only a few CD4+ and CD8+ T cells capable of producing effector cytokines IFN-γ, IL-4, IL-10, and IL-17, a higher percentage of CD4+ and CD8+ T cells from KO mice produced these cytokines (Figure 3E). A similar increase in the number of cytokine-producing T cells was also observed in the lymph nodes of KO mice (Figure S1 and S2). To determine whether increased cytokine production of KO T cells was a consequence of enhanced T cell activation, we determined the frequency of cytokine-producing T cells among the activated CD44hi T cells from WT and KO mice. A higher percentage of KO CD4+CD44hi T cells produced IFN-γ or IL-17 than WT CD4+CD44hi T cells, whereas IL-4- or IL-10-producing CD4+ T cells or IFN-γ-producing CD8+ T cells were comparable among the activated WT and KO T cells (Figure S4). These observations suggest that in addition to Foxo1 control of T cell activation, it plays a major role in inhibiting Th1 and Th17 cell differentiation. To investigate whether this enhanced T cell differentiation would trigger immunopathology, we aged a cohort of Foxo1 KO mice for 5–6 months. Histopathological examination did not reveal drastic inflammation in all major organs (data not shown). We also measured the amounts of autoreactive antibodies in these mice. Increased titers of both nuclear and dsDNA antibodies were detected in the sera of Foxo1 KO mice (Figure 3F). These observations demonstrate that T cell Foxo1 is essential for the inhibition of effector T cell differentiation, and for the maintenance of B cell tolerance to self antigens.
Foxo1-dependent Transcriptional Program in Naïve T Cells
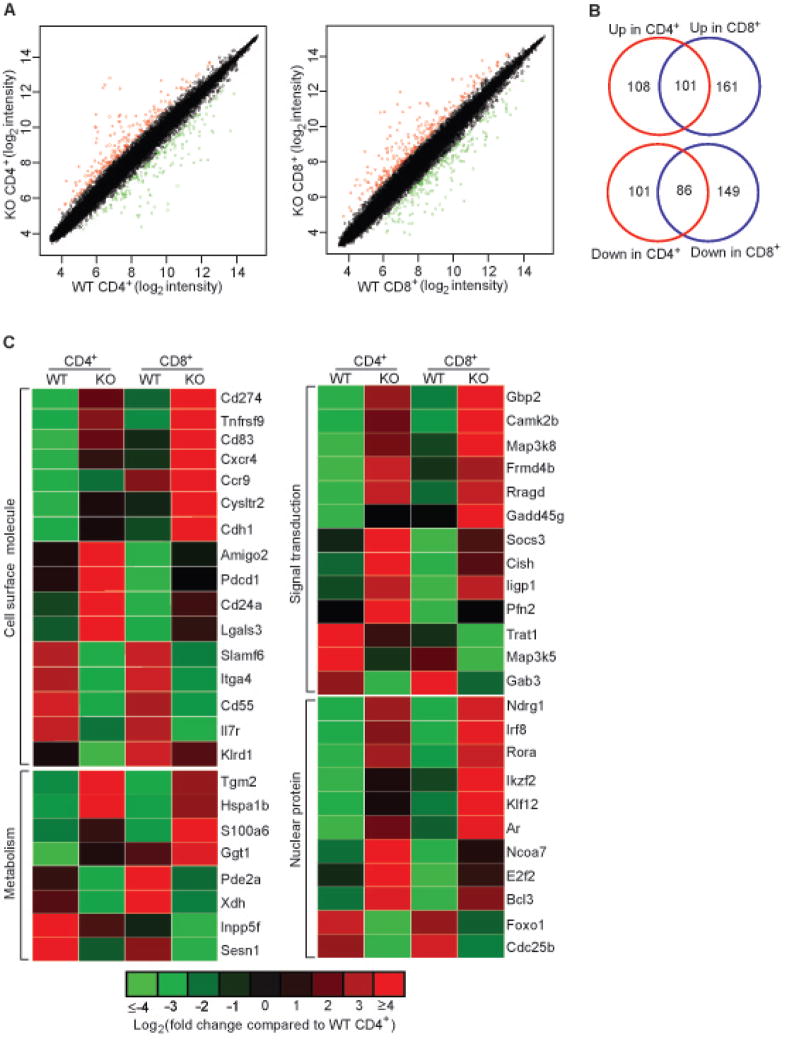
We wished to identify Foxo1 target genes that are involved in the control of T cell homeostasis and tolerance. To this end, we purified naïve phenotype (CD44loCD62LhiCD25−) CD4+ and CD8+ T cells from WT and Foxo1 KO mice by FACS sorting. RNA was prepared from these cells, and was analyzed by global gene expression profiling with the Affymetrix oligonucleotide arrays. Comparing WT and KO CD4+ T cells, 396 entries showed equal or greater than 2-fold change, whereas 497 entries differed in CD8+ T cells (Figure 4A, for a complete list see Table S1 and S2). Among the differentially expressed entries, 187 were shared between CD4+ and CD8+ T cells (Figure 4B and 4C, for a complete list see Table S3). We initially focused our analysis on these co-regulated genes, which encode among others, cell surface molecules, nuclear factors, and proteins involved in the signal transduction and metabolism (Figure 4C). Notably, the expression of genes encoding positive regulators of T cell activation and differentiation such as Tnfrsf9, Gadd45g and Rora was increased in Foxo1-deficient T cells (Lu et al., 2001; Vinay and Kwon, 1998; Yang et al., 2008) (Figure 4C). In addition, Foxo1 controls the expression of genes involved in cell adhesion, cell migration, and cellular stress responses (Figure 4C). These findings reveal diverse Foxo1 target genes in T cells which may collectively control T cell homeostasis and tolerance.
Figure 4. Differential Gene Expression between Wild-type and Foxo1-deficient Naïve T Cells.
(A) Comparison of gene expression values between WT and KO naïve CD4+ and CD8+ T cells.
(B) Overlapping of Foxo1-regulated genes in naïve CD4+ and CD8+ T cells. Genes with expression values equal or more than 2-fold different between WT and KO T cells are considered significant. The numbers of shared or uniquely regulated genes between CD4+ and CD8+ T cells are indicated.
(C) A sublist of co-regulated genes between CD4+ and CD8+ T cells. The genes are grouped as cell surface molecules, signaling proteins, nuclear factors, and molecules involved in cell metabolism.
Foxo1 Regulation of IL-7R Expression and IL-7 Signaling in T Cells
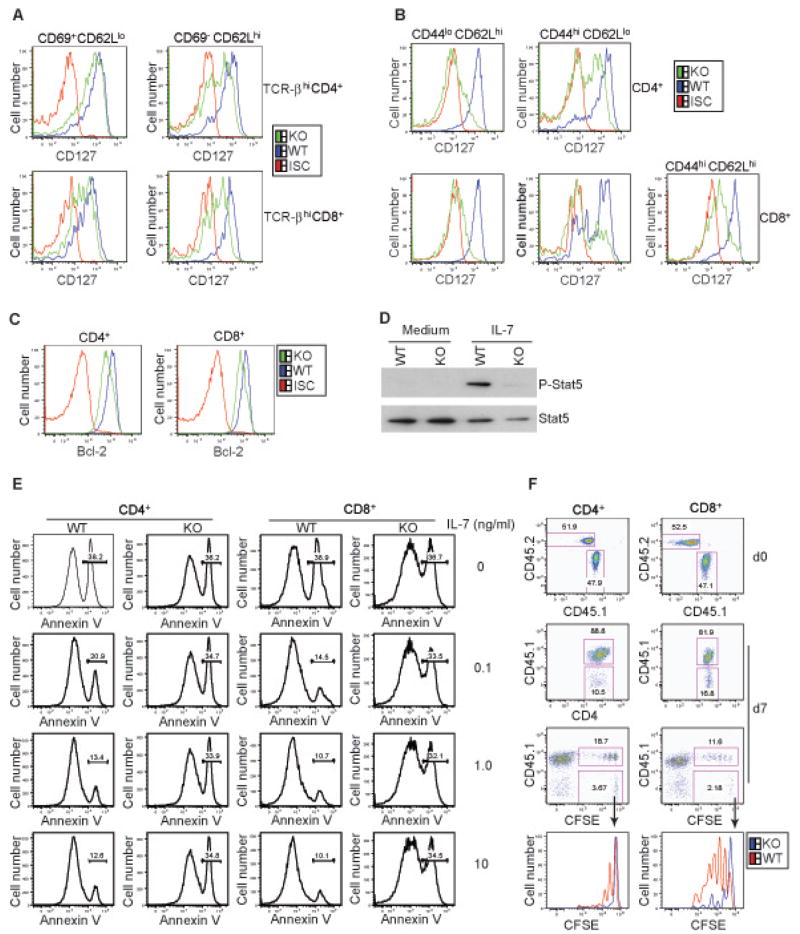
We focused on one of the Foxo1 target genes, Il7r, which was down-regulated in both CD4+ and CD8+ Foxo1-deficient T cells (Figure 4C). In line with the gene expression results, Foxo1-deficient CD4+ and CD8+ CD44loCD62Lhi naïve T cells expressed low to undetectable amounts of IL-7R protein (Figure 5B, left panel). IL-7R expression is induced in the thymocytes that undergo positive selection (Mazzucchelli and Durum, 2007). In contrast to the up-regulation of IL-7R on WT thymocytes, Foxo1-deficient CD4+ and CD8+ T cells expressed increasingly lower amounts of IL-7R when they matured from CD69+CD62Llo to CD69−CD62Lhi T cells (Figure 5A). IL-7R level was also greatly diminished in the activated CD44hi Foxo1 KO T cells (Figure 5B, right panel). These observations reveal a critical role for Foxo1 in control of IL-7R expression at multiple stages of T cell differentiation.
Figure 5. Defective IL-7R Expression and IL-7 Signaling in Foxo1-deficient T Cells.
(A) Expression of CD127 (IL-7R) in TCR-βhiCD4+ and TCR-βhiCD8+ T cells from WT and KO mice at 6 weeks old. CD69+CD62Llo or CD69−CD62Lhi T cells represent immature and mature populations respectively. ISC stands for the iso-type control antibody. These are representative results of five independent experiments.
(B) Expression of CD127 in splenic CD4+ and CD8+ T cells from WT and KO mice at 6 weeks old. CD44loCD62Lhi, CD44hiCD62Llo or CD44hiCD62Lhi T cells represent naïve, effecor and central memory subsets respectively. These are representative results of five independent experiments.
(C) Expression of Bcl-2 in splenic CD4+ and CD8+ naïve T cells from WT and KO mice at 8 weeks old. Intracellular staining was performed following the instructions of the manufacturer.
(D) Naïve CD4+ T cells from WT and KO mice were left untreated (medium) or treated with 10 ng/ml IL-7 for 20 min. The amounts of phosphorylated Stat5 and total Stat5 protein was determined by immunoblotting.
(E) Naïve CD4+ and CD8+ T cells from WT and KO mice were cultured in the absence or presence of increasing doses of IL-7 for 24 hr. Cell apoptosis was examined by Annexin-V staining.
(F) Naïve CD4+ and CD8+ T cells isolated from KO (CD45.2) and the congenic WT (CD45.1) mice were labeled with CFSE, and co-transferred into Rag1-deficient mice. The percentage of WT and KO T cells before (d0) and after transfer (d7) was shown. CFSE dilution indicates cell proliferation. The histograms showed homeostatic proliferation of the transferred T cells. These are representative results of three independent experiments.
The IL-7/IL-7R pathway is a pivotal regulator of T cell homeostasis, which is in part mediated by its induction of the pro-survival Bcl2 gene expression (Akashi et al., 1997; Maraskovsky et al., 1997). Consistent with the reduced IL-7R expression, Foxo1 KO CD4+ and CD8+ T cells expressed lower amounts of Bcl-2 protein than WT T cells (Figure 5C). IL-7 engagement of IL-7R activates JAK3 and JAK1 kinases that phosphorylate the Stat5 transcription factor (Jiang et al., 2005). Unlike WT naïve T cells, IL-7 stimulation of KO T cells failed to induce Stat5 phosphorylation (Figure 5D). IL-7 is a potent regulator of naïve T cell survival. Stimulation of WT CD4+ or CD8+ naïve T cells with IL-7 triggered dose-dependent inhibition of cell apoptosis assessed with Annexin V staining (Figure 5E). However, both CD4+ and CD8+ Foxo1 KO naïve T cells were refractory to IL-7-induced survival in vitro (Figure 5E). In vivo, IL-7 regulates the survival and homeostatic proliferation of naïve T cells (Ma et al., 2006). To investigate the proliferation potential of Foxo1 KO T cells, we performed a transfer experiment. We purified wild-type naïve CD4+ or CD8+ T cells from C57BL/6 mice that expressed the congenic marker CD45.1. These T cells were mixed with Foxo1 KO naïve T cells expressing the congenic marker CD45.2 at approximately 1:1 ratio (Figure 5F, top panel), labeled with CFSE, and transferred to Rag1−/− recipients. The usage of the CD45 marker enabled us to differentiate WT and KO T cells. After 7 days, T cells were recovered from the spleens and lymph nodes of the recipient mice, and assessed for cell proliferation by CFSE dilution. We found that the recovery of Foxo1 KO T cells was about 10–20% of the WT T cells, which was associated with the compromised homeostatic proliferation of KO T cells (Figure 5F). These observations further corroborated that the IL-7R expression defect of Foxo1-deficient T cells caused compromised IL-7 signaling and IL-7-induced T cell survival and proliferation.
A Cell-intrinsic Role for Foxo1 in Control of IL-7R Expression in T Cells
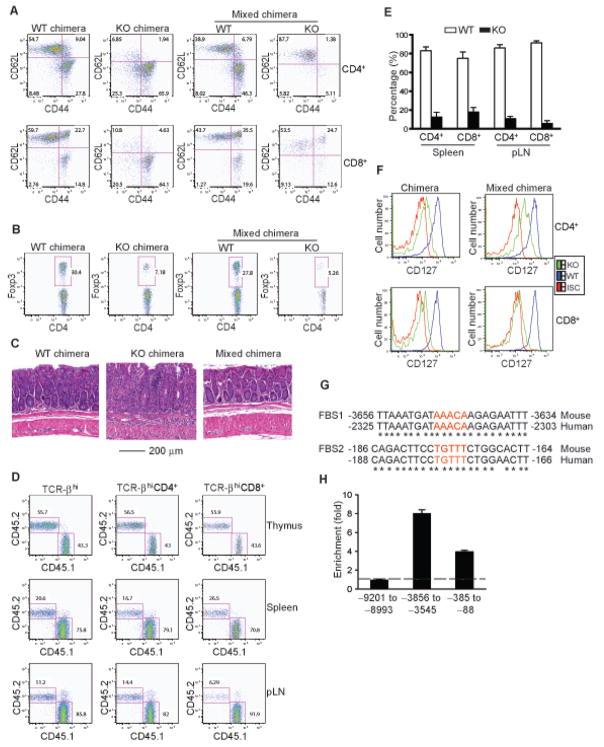
IL-7R expression is subjected to the regulation by multiple environmental cues such as the presence of other pro-survival cytokines including IL-2, IL-4, IL-6, and IL-15 (Park et al., 2004). This has been postulated as a mechanism to promote survival of the maximum possible number of T cells for the limited amount of IL-7 available (Mazzucchelli and Durum, 2007). Because a large fraction of Foxo1-deficient T cells were activated and produced cytokines (Figure 3), it was possible that the down-regulation of IL-7R expression in Foxo1 KO T cells was a consequence of the heightened cytokine stimulation. To study whether Foxo1 control of IL-7R expression was via cell-intrinsic or cell-extrinsic pathways, we generated mixed bone marrow chimeric mice. T cell-depleted bone marrow cells from CD45.2+ Foxo1 KO mice and CD45.1+ WT mice were transferred either separately or in combination into sublethally irradiated Rag1−/− recipients. All chimeric mice reconstituted with KO bone marrow cells developed severe wasting disease 8 weeks after the transfer. Upon histological examination, we found heavy mononuclear cell infiltration in the mucosal lamina propria and the subglandular area of the colons of these mice (Figure 6C). In contrast, mice reconstituted with WT bone marrow cells did not develop colitis (Figure 6C). A higher proportion of splenic CD4+ and CD8+ T cells from the KO chimera exhibited an activated phenotype than T cells from the WT chimera (Figure 6A), and differentiated to cytokine-producing effector T cells (data not shown). To determine whether Foxo1 deficiency affected Treg cell homeostasis under these conditions, we assessed Treg cell frequencies in these mice. Approximate 30% CD4+ T cells from the WT chimera were Treg cells, compared to about 7% KO CD4+ T cells (Figure 6B). These observations demonstrate an important role for Foxo1 in control of T cell tolerance, T cell activation, and Treg cell homeostasis in the bone-marrow chimeric mice.
Figure 6. An Cell-intrinsic Role for Foxo1 in Control of IL-7R Expression.
(A–F) Chimeras were generated by reconstitution of Rag1−/− recipients with WT (CD45.1), KO (CD45.2), or 1:1 mixed WT and KO bone marrow cells. The chimeric mice were analyzed 8 weeks after the bone marrow transfer. These are representative results of three independent experiments.
(A) Expression of CD44 and CD62L in splenic CD4+ and CD8+ T cells from WT, KO or mixed chimeras.
(B) Percentage of CD4+Foxp3+ regulatory T cells in the spleens of WT, KO or mixed chimeras.
(C) Development of inflammatory bowel disease in KO chimeric mice. Hematoxylin and eosin staining of the colons of WT, KO or mixed chimeric mice. These are representative results of four mice per group analyzed.
(D) Percentage of WT (CD45.1+) and KO (CD45.2+) T cells in the thymus, spleen and peripheral lymph nodes (pLN) of a mixed chimeric mouse.
(E) Percentage of WT and KO T cells in the spleens and pLNs of the mixed chimeras that are normalized to thymic T cells (n=8). The p values of percentage between the two groups of mice are less than 10−7.
(F) Expression of CD127 (IL-7R) in splenic naïve (CD44loCD62Lhi) CD4+ and CD8+ T cells from WT, KO or mixed chimeras. Expression of CD127 in T cells from WT and KO chimeras was plotted (left). Expression of CD127 in WT and KO T cell populations from the same mixed chimeric mouse was plotted (right). ISC stands for the iso-type control antibody.
(G) Alignment of the conserved Foxo1-binding sites (FBS) in mouse and human Il7r gene promoter regions. The consensus Foxo1-binding sequences were marked. The nucleotide numbers are in reference to the translation start site (ATG, A is +1). Asterisks show the conserved nucleotides.
(H) Chromatin immunoprecipitation analysis of Foxo1 binding to the mouse Il7r promoter region. Immunoprecipates from T cells were analyzed by quantitative PCR. The results were presented as fold of template enrichment in immunoprecipitates of Foxo1 antibody relative to those of an isotype control antibody (mean and s.d. of triplicate immunoprecipitations).
Mixed chimeric mice, however, did not develop colitis (Figure 6C). To examine the reconstitution efficiency of WT and KO bone marrow, we examined the distribution of CD45.1+ WT T cells and CD45.2+ KO T cells in these mice. In the thymus of one of the mixed chimera, WT and KO T precursor cells produced comparable number of TCR-βhi mature T cells (Figure 6D, top panel). However, in the spleen and lymph nodes of this mouse, the number of CD45.2+ KO T cells was greatly diminished compared to that of CD45.1+ WT T cells (Figure 6D, middle and bottom panels). Normalized to the reconstitution efficiency of the thymus, the number of KO CD4+ and CD8+ T cells in the periphery were 3–15 fold lower than that of WT T cells (Figure 6E). Similar to T cells from WT or KO chimera, the KO population of CD4+ and CD8+ T cells from mixed chimeras expressed lower levels of IL-7R than the WT population in the same mouse (Figure 6F). These observations reveal a T cell-intrinsic role for Foxo1 in promoting IL7-R expression, which was associated with the out competition of KO T cells by WT T cells in the periphery. To determine whether Foxo1 directly controlled Il7r gene transcription, we searched for evolutionarily conserved Foxo1-binding sites in the mouse Il7r promoter. Using rVista program, we found three putative Foxo1-binding sites within the 10 kb Il7r promoter region that were conserved between mouse and human (Figure S5). To investigate whether Foxo1 directly bound to these DNA elements, we performed chromatin immunoprecipitation of WT T cells with Foxo1 or control antibodies. Genomic fragments containing the proximal Il7r promoter, the 3.7 kb but not the 9.1 kb DNA regions upstream of the translation start site were selectively enriched with the Foxo1 antibody (Figure 6G, 6H). These findings support Il7r as a direct Foxo1 target gene in T cells.
A Critical Function of IL-7R in Foxo1 Control of Naïve OT-II T Cell Homeostasis
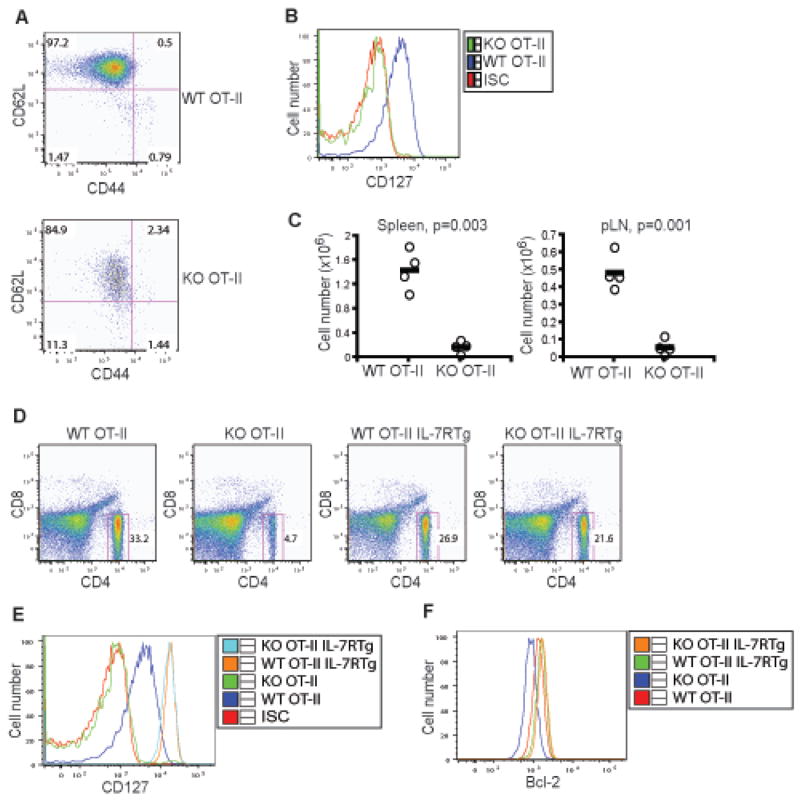
Foxo1 deficiency in T cells resulted in the activation and differentiation of a fraction of the peripheral T cells (Figure 3). We wished to determine how the antigen specificity of T cell receptor (TCR) would influence T cell responses in the absence of Foxo1. To this end, we crossed Foxo1 KO mice with OT-II (CD4+ TCR specific for an OVA peptide) transgenic mice onto Rag1−/− background, in order to exclude the T cell repertoire as a variable. Similar to T cells on the polyclonal background (Figure 2), deficiency of Foxo1 did not compromise OT-II T cell positive selection (Figure S6). In addition, mature thymic OT-II T cells expressed lower levels of CD62L and IL-7R (Figure S6), which was also in line with the polyclonal T cells (Figure 2 and 5). However, unlike T cells on the polyclonal background, splenic Foxo1 KO OT-II T cells maintained a naïve CD44lo phenotype (Figure 7A). Compared to WT OT-II mice, the number of mature OT-II T cells was greatly reduced in the spleens and lymph nodes of KO OT-II mice by 80–90% (Figure 7C). These observations reveal that Foxo1 is essential for the maintenance of naïve OT-II T cells in vivo.
Figure 7. Foxo1 Control of IL-7R Expression and Homeostasis of OT-II T Cells.
(A) Expression of CD44 and CD62L in splenic OT-II T cells from WT and KO mice at 8 weeks old. These are representative results of three independent experiments.
(B) Expression of CD127 (IL-7R) in splenic OT-II T cells from WT and KO mice. These are representative results of three independent experiments. ISC stands for the iso-type control antibody.
(C) Number of splenic and lymph node (pLN) OT-II T cells from WT and KO mice aged between 8 and 12 weeks. The p values of cell number between the two groups of mice are shown.
(D) Percentage of splenic OT-II T cells from WT, KO, WT IL-7R transgenic (IL-7RTg), and KO IL-7RTg mice at 8 weeks old. These are representative results of three independent experiments.
(E) CD127 expression in splenic OT-II T cells from WT, KO, WT IL-7RTg, and KO IL-7RTg mice at 8 weeks old. ISC stands for the iso-type control antibody.
(F) Bcl-2 expression in splenic OT-II T cells from WT, KO, WT IL-7RTg, and KO IL-7RTg mice at 8 weeks old.
As expected, Foxo1 KO OT-II T cells failed to express IL-7R (Figure 7B). To determine the functional consequences of diminished IL-7R expression on Foxo1-deficient T cells, we crossed KO or KO OT-II mice with a strain of IL-7R transgenic mice (Park et al., 2004). Restoration of IL-7R expression did not correct the T cell activation phenotype or significantly affect the number of Foxo1-deficient T cells on the polyclonal background (Figure S7 and data not shown). However, the restored IL-7R expression rescued peripheral T cell number in KO OT-II IL-7RTg mice (Figure 7D and 7E), which was associated with the recovery of Bcl-2 gene expression on KO OT-II T cells (Figure 7F). These findings establish a central role for IL-7R in Foxo1 control of naïve T cell homeostasis.
Discussion
Because of the embryonic lethal phenotype of Foxo1-deficient mice, the function of Foxo1 in T cells has not been studied in vivo. We have developed a novel mouse strain that enabled cell-type specific deletion of Foxo1 gene using the cre-loxP system. In this report, we used CD4-Cre transgenic mice to delete Foxo1 gene in T cells and explored its role in thymic T cell development and peripheral T cell activity. We found that Foxo1 was not essential for the positive selection of CD4+ and CD8+ T cells, but was required for the expression of IL-7R and CD62L in mature thymocytes. Foxo1 deficiency also led to the compromised IL-7R and CD62L expression in naïve T cells in the peripheral lymphoid organs. Diminished expression of IL-7R was associated with failed IL-7 signaling in Foxo1 knockout T cells, which resulted in the compromised IL-7-induced T cell survival in vitro and reduced IL-7-dependent homeostatic proliferation in vivo. Using a strain of IL-7R transgenic mouse, we showed that reduced IL-7R expression was responsible for the homeostasis defects of naïve Foxo1-deficient OT-II T cells. In addition, Foxo1 deficiency caused spontaneous T cell activation, effector T cell differentiation, and the production of autoantibodies in mice. In a bone marrow transfer model, lack of Foxo1 expression in T cells resulted in colitis. These observations reveal previously undefined potent and pleiotropic roles for Foxo1 in the control of T cell homeostasis and tolerance in vivo.
A major finding of the present study was that Foxo1 controlled naïve T cell homeostasis via its regulation of IL-7R expression. As a transcription factor, Foxo1 can bind to regulatory DNA sequences on target genes (Obsil and Obsilova, 2008). Indeed, using rVista program, we identified consensus Foxo1-binding sites in the promoter region of Il7r gene. We further found direct Foxo1 association with the proximal Il7r promoter and an evolutionarily conserved non-coding region 3.7 kb upstream of the translation start site. Future studies will be needed to test the importance of these Foxo1 binding site in control of IL-7R expression in T cells. In addition, it has been reported that Foxo1 can regulate gene expression independent of its DNA-binding domain (Ramaswamy et al., 2002). In this case, Foxo1 may interact with other nuclear factors involved in the control of IL-7R expression. Previous studies have revealed that IL-7R transcription in T cells is positively regulated through proximal promoter region that contains binding motifs for the transcription factor GABP (Xue et al., 2004). IL-7R transcription is also subjected to repression by the transcription repressor Gfi-1 (Park et al., 2004), which binds to an intronic region of Il7r gene. How Foxo1 interacts with these transcription factors in control of IL-7R transcription will be an interesting area for future exploration.
The expression of IL-7R is dynamically regulated at multiple stages of T cell differentiation (Mazzucchelli and Durum, 2007). When naïve T cells encounter antigen during infection, they undergo expansion and differentiation. This is associated with the down-regulation of IL-7R expression on most effector T cells (Huster et al., 2004; Kaech et al., 2003). Stimulation of T cells via the TCR, co-stimulatory receptor, and cytokine signaling pathways also inactivates Foxo1 via PKB-induced phosphorylation (Peng, 2008). It remains to be determined whether the down-regulation of IL-7R expression on effector T cells is a consequence of Foxo1 inactivation. It has been shown that a small subset of the effecor CD8+ T cells express high amounts of IL-7R, and differentiate into long-lived memory CD8+ T cells (Huster et al., 2004; Kaech et al., 2003). The function of Foxo1 in control of IL-7R expression in memory T cells warrants further investigation.
In addition to the control of naïve OT-II T cell homeostasis, Foxo1 was required for the inhibition of T cell activation and differentiation on T cell polyclonal background. It has been proposed that T cell activation and development of autoimmune diseases can be caused by T cell lymphopenia (King et al., 2004; Marleau and Sarvetnick, 2005), which is associated with IL-7-driven homeostatic T cell proliferation (Calzascia et al., 2008). Foxo1-deficient naïve T cells were depleted, and expressed significantly lower levels of IL-7R than Foxo1-deficient T cells with the activated phenotype, raising the possibility that T cell activation was a consequence of enhanced IL-7 stimulation. Overexpression of IL-7R via an IL-7R transgene in Foxo1-deficient T cells largely nullified IL-7R expression difference between naïve and activated T cells, but did not correct the T cell activation phenotype. These observations suggest that T cell activation in the absence of Foxo1 was not caused by defective IL-7R expression. Treg cell number was not reduced in un-manipulated Foxo1-deficient mice, which is consistent with a dispensable role for the IL-7R signaling pathway in control of Treg cell homeostasis (Bayer et al., 2008; Mazzucchelli et al., 2008; Vang et al., 2008). These findings imply that Foxo1 functions as a T cell intrinsic regulator of tolerance in these mice. The mechanisms by which Foxo1 regulates T cell activation remain to be determined. Gene expression profiling experiment revealed hundreds of putative Foxo1 target genes in naïve T cells. However, it is still an open question whether Foxo1 controls another master regulator of T cell tolerance, or alternatively Foxo1 regulates multiple signaling pathways that collectively ensure naïve T cell quiescence.
Reconstitution of sublethally irradiated Rag1−/− mice with Foxo1-deficinet bone marrow cells resulted in severe colitis that was not observed in un-manipulated KO mice aged for 5–6 months. Whole body irradiation induces tissue damage, and triggers the release of microbes and microbial products that cause systemic inflammation (Paulos et al., 2007). It remains to be determined whether the heightened inflammatory response associated with irradiation contributes to the development of colitis in the KO chimeras. In the KO chimeric mice, the number of CD4+Foxp3+ cells was diminished compared to that in the WT chimeras. Reduced percentage of KO Treg cells was also observed in the mixed chimeric mice that had received both wild-type and knockout bone marrows. These findings reveal a cell-intrinsic role for Foxo1 in control of Treg cell homeostasis in irradiated mice. Active immune suppression by Treg cells is essential for T cell tolerance (Sakaguchi et al., 2008). How Foxo1 cross-talks with Treg cells in control of T cell responses will be an interesting area for future study. In contrast to T cells from the KO chimeras, KO T cell populations from the mixed chimeric mice exhibited a naïve T cell phenotype. Replenishment of WT Treg cell in the mixed chimeras might suppress KO T cell activation. KO T cells expressed low levels of IL-7R, and were not competitive to WT T cells in the periphery. Therefore, it is also possible that KO T cells were rapidly depleted upon release from the thymus, before they could be activated by peripheral antigens. Since T cell activation in un-manipulated Foxo1-deficient mice was not associated with observable Treg cell defects, Foxo1 likely played an autonomous role in control of T cell activation.
The nature of the antigens that drive the expansion and differentiation of effector T cells in T cell-specific Foxo1-deficient mice remains to be fully characterized. Interestingly, Foxo1-deficient OT-II T cells on the Rag1−/− background were not activated. Because OT-II T cells are specific for the foreign ovalbumin antigen, these results imply that cognate antigen stimulation is needed for the activation of Foxo1-deficient T cells. Increased production of nuclear and dsDNA antibodies in Foxo1-deficient mice further suggested that self-antigens might be involved in the activation of T cells. Although we did not observe spontaneous colitis in Foxo1-deficient mice aged up to 6 months, T cells isolated from the gut-draining mesenteric lymph node exhibited more pronounced T cell activation than T cells from the other peripheral lymph nodes. Importantly, transfer of bone marrow cells isolated from T cell-specific Foxo1-deficient mice into irradiated Rag1−/− mice led to the development of colitis in recipient mice. These observations imply that Foxo1 is also critical to prevent the activation of T cells reactive to commensal bacterium antigens.
In conclusion, in this report, we have uncovered critical functions for Foxo1 in regulation of T cell homeostasis and tolerance. IL-7R was identified as a novel Foxo1 target gene involved in Foxo1 maintenance of naïve T cells. These findings will advance our knowledge on the function of Foxo family proteins in the immune system and might, on the long term, be exploited for finding cures for autoimmune diseases and cancer.
Experimental Procedures
Mice
Mouse genomic DNA of the Foxo1 gene was isolated from a 129SV BAC library (Genome Systems). The targeting vector was constructed by cloning three genomic fragments into the plasmid of pEasy-FLIRT. Linearized targeting vector was transfected into ES cells (TC1). Homologous recombinants were identified by Southern-blot analysis, and were implanted into foster mothers. Chimeric mice were bred to C57BL/6 mice, and the F1 generation was screened for germline transmission. The Neo gene was removed by breeding F1 mice with a strain of actin promoter-driven Flipase transgenic mice (JAX). Mice carrying the floxed allele of Foxo1 were backcrossed to C57BL/6 for five to six generations. CD4-Cre transgenic (4Cre), OT-II TCR transgenic (OT-II) mice, and Foxp3-RFP knockin mice were described previously (Li et al., 2006a; Wan and Flavell, 2005). IL-7R transgenic (IL-7RTg) mice (Park et al., 2004) were kindly provided to us by Dr. A. Singer (National Cancer Institute). All mice were maintained under specific pathogen-free conditions. All animal experimentation was conducted in accordance with institutional guidelines.
Histopathology
Tissues from sacrificed animals were fixed in Safefix II (Protocol) and embedded in paraffin. 5 μm sections were stained with hematoxylin and eosin.
Immunoblotting
For the analysis of Foxo1 protein expression, FACS-sorted CD4+, CD8+ and B cells were extracted with 1 × SDS sample buffer. To analyze IL-7-stimulated Stat5 phosphorylation, FACS-sorted naïve (CD44loCD62LhiCD25−) CD4+ and CD8+ from WT and KO mice were left untreated or treated with 10 ng/ml IL-7 for 20 min, and were lyzed with 1 × SDS sample buffer. Protein extracts were separated on 8% SDS PAGE gels and transferred to PVDF membrane (Millipore). The membranes were probed with antibodies against Foxo1, p38, Stat5, and phosphorylated Stat5 (Cell Signaling).
Chromatin Immunoprecipitation
The chromatin immunoprecipitation analysis was performed as described previously (Li et al., 2006a). Briefly, CD4+ T cells were fixed for 10 min at room temperature with 10% formaldehyde. After incubation, glycine was added to a final concentration of 0.125 M to “quench” the formaldehyde. Cells were pelleted, washed once with ice-cold PBS, and then lysed. The lysates were pelleted, resuspended, and sonicated to reduce DNA length to between 500 and 1000 base pairs. The chromatin was pre-cleared with protein A agarose beads for 1 hr, and then incubated with 5 μg of Foxo1 antibody (Abcam) or control rabbit Ig overnight. The immune complexes were precipitated with protein A agarose beads, washed, and eluted in 100 μl of TE with 0.5% SDS and 200 μg/ml proteinase K. Precipitated DNA was further purified with phenol/chloroform extranction and ethanol precipitation and was analyzed by quantitative PCR (qPCR). The primers used in the analysis of binding included: −9201 to −8993: 5′-GCC CAC TTT CTC CCT ACC TT-3′ and 5′-TCC AGC TAC TTC ACC GAA GC-3′; −3856 to −3545: 5′-TCT TTA AGC TTC CCG CAC TC-3′ and 5′-ACC TCA TCA GCC TTT CAT GG-3′; −385 to −88: 5′-AAG TGT GGA TTT TGG CCT TG-3′ and 5′-GAG AGA GGG AGA CCC AAA CC-3′.
Flow Cytometry
Cells from spleens, lymph nodes, or thymus were depleted of erythrocytes by hypotonic lysis. Cells were incubated with specific antibodies for 15 min on ice in the presence of 2.4G2 mAb to block FcγR binding. Samples were analyzed with LSR II (Becton Dickinson) and FloJo (Tree Star) software. Antibodies against cell surface markers and Foxp3 were obtained from eBiosciences. For intracellular cytokine staining, single-cell suspensions of spleens, peripheral lymph nodes (pLN) and mesenteric lymph nodes (mLN) were stimulated with 50 ng/ml phorbol 12-myristate 13-acetate (PMA, Sigma), 1 μM ionomycin (Sigma) and GolgiStop (BD Biosciences) for 4 hr. After stimulation, cells were first stained with CD4, CD8 and TCR-β antibodies, fixed and permeabilized with a Cytofix/Cytoperm kit (BD Biosciences), and stained with IFN-γ and IL-4, or IL-10 and IL-17 antibodies. Intracellular Foxp3 staining was carried out with a kit from the eBiosciences. Intracellar Ki-67 staining was performed with a kit from BD Biosciences. To analyze cell apoptosis, FACS-sorted naïve T cells were cultured in the absence or presence of IL-7 for 24 hr, and were stained with FITC-labeled annexin V (BD Biosciences) according to the manufacturer s instructions.
ELISA
The amounts of dsDNA and nuclear antibodies in mouse sera were determined with an ELISA kit from Alpha Diagnostic International. Sera from six pairs of WT and KO mice aged 5–6 months were assayed individually with 1:100 dilution in 1% BSA PBS.
Gene Expression Profiling
Splenic and LN CD44loCD62LhiCD25− naïve CD4+ and CD8+ T cells from WT and KO mice at 6–8 weeks old were purified by FACS-sorting and lyzed in QIAzol reagent. RNA was isolated with miRNeasy Mini Kit according to the manufacturer s instructions (Qiagen). Two-rounds of RNA amplification, labeling and hybridization to M430 2.0 chips (Affymetrix) were carried out at the Core Facility of Memorial Sloan-Kettering Cancer Center. All data analyses were done with R Console. The genes with 2-fold or more change of expression were considered as Foxo1-dependent genes. The Foxo1-dependent genes shared by CD4+ and CD8+ T cells were divided into four categories of cell surface proteins, signal transduction molecules, nuclear factors and protein involved in metabolism by Gene Ontology analysis at website of David Bioinformatics Resource (http://david.abcc.ncifcrf.gov/). The heat maps were made with R Console.
Treg Cell Suppression Assay
CD4+Foxp3+ regulatory T cells (Treg) were isolated from WT and KO mice that were bred to the Foxp3-RFP background by FACS sorting of CD4+RFP+ cells. CD44loCD4+RFP− cells sorted from WT mice were labeled with CFSE and used as responding T cells (Tresp). 5×104 Tresp cells were cultured in 96-well plates with 105 irradiated splenocytes and 2 μg/ml CD3 antibody in the presence or absence of 5×104 Treg cells for 72 hr. CFSE dilution was analyzed by FACS.
Adoptive Transfer of T Cells
Splenic and LN CD44loCD62LhiCD25− naïve CD4+ and CD8+ T cells from 6–8 weeks old CD45.1+ congenic C57BL/6 (WT) mice or CD45.2+ 4Cre-Foxo1/Foxo1 (KO) mice were purified by FACS-sorting. These cells were labeled with 4 μM of Carboxyfluorescein diacetate succinimidyl ester (CFSE, Sigma) at 37°C for 10 min. 2 × 106 of 1:1 mixed WT and KO T cells were injected i.v. to 6–8 week-old Rag1−/− mice. Mice were sacrificed 7 days after the transfer. CFSE dilution and the percentage of WT and KO T cells in spleens and pLNs were determined by FACS staining and analysis.
Generation of Bone Marrow Chimeras
Bone marrow cells isolated from 6–8 weeks old CD45.1+ congenic C57BL/6 (WT) mice or CD45.2+ 4Cre-Foxo1/Foxo1 (KO) mice were depleted of erythrocytes by hypotonic lysis and T cells and antigen-presenting cells by complement-mediated cell lysis. 2 × 106 WT, KO, or 1:1 mixed WT and KO bone marrow cells were injected i.v. to 6–8 week-old Rag1−/− mice that were sublethally irradiated (600 rad).
Statistical Analyses
Student s t test was used to calculate statistical significance for difference in a particular measurement between groups. p value of < 0.05 was considered statistically significant.
Supplementary Material
Figure S1. Analysis of Foxo1-deficient T Cells in the Mesenteric Lymph Nodes
(a) Expression of CD44 and CD62L in CD4+ and CD8+ T cells from the mesenteric lymph nodes (mLNs) of WT and KO mice at 6 weeks old. These are representative results of six independent experiments.
(b) Percentage of mLN CD4+Foxp3+ regulatory T (Treg) cells from WT and KO mice at 6 weeks old.
(c) Number of mLN CD4+ and CD8+ T cells from control (Foxo1/Foxo1) and KO mice aged between 6 and 8 weeks (n=6). The p values between the cell number of the two groups of mice are shown.
(d) CD4+ and CD8+ T cells isolated from the mLNs of WT and KO mice were stimulated with PMA and ionomycin for 4 hr and analyzed for the expression of IFN-γ, IL-4, IL-10, and IL-17 by intracellular cytokine staining. These are representative results of two independent experiments.
(e) Expression of Ki-67 in CD4+ and CD8+ T cells from the mLNs of WT and KO mice aged between 6 and 8 weeks.
Figure S2. Analysis of Foxo1-deficient T Cells in the Peripheral Lymph Nodes
(a) Expression of CD44 and CD62L in CD4+ and CD8+ T cells from the peripheral lymph nodes (pLNs) of WT and KO mice at 6 weeks old. These are representative results of six independent experiments.
(b) Number of pLN CD4+ and CD8+ T cells from control (Foxo1/Foxo1) and KO mice aged between 6 and 8 weeks (n=6). The p values between the cell number of the two groups of mice are shown.
(c) CD4+ and CD8+ T cells isolated from the pLNs of WT and KO mice were stimulated with PMA and ionomycin for 4 hr and analyzed for the expression of IFN-γ, IL-4, IL-10, and IL-17 by intracellular cytokine staining. These are representative results of two independent experiments.
(d) Expression of Ki-67 in CD4+ and CD8+ T cells from the pLNs of WT and KO mice aged between 6 and 8 weeks.
Figure S3. Suppressive Function of Foxo1-deficient Treg Cells
CD4+Foxp3+ regulatory T cells (Treg) were isolated from WT and KO mice. The CD44loCD4+ cells from WT mice were labeled with CFSE and used as responding T cells (Tresp). The suppression was shown by comparison of CFSE dilution in the Tresp cells cultured with or without 1:1 Tregs.
Figure S4. Cytokine Production of CD44hi WT and KO Splenic T cells
T cells isolated from the spleens of WT and KO mice were stimulated with PMA and ionomycin for 4 hr and analyzed for the expression of IFN-γ, IL-4, IL-10, and IL-17 in CD44hiCD4 and CD44hiCD8 T cells. These are representative results of two independent experiments.
Figure S5. Conserved Foxo1-Binding Sites in the Promoter Region of Il7r Gene
The conserved putative Foxo1-binding sites in the Il7r locus were analyzed by rVISTA online (http://genome.lbl.gov/vista/rvista/submit.shtml). Ten kilobase pairs (10 kb) of nucleotides upstream of transcription start site (indicated by red arrow) from mouse and human Il7r genes were used for rVISTA analysis. Three conserved noncoding sequences (CNSs, marked by pink color) and three conserved Foxo1 binding sites (shown as green bars) were depicted.
Figure S6. Development of Foxo1-deficient OT-II T cells
Thymic CD4 and CD8 profile of WT and KO OT-II mice at the age of 8 weeks (top left). Expression of CD69 and CD62L in the CD4+ OT-II thymocytes from WT and KO mice (bottom left). Expression of CD127 (IL-7R) in the immature (CD69+CD62Llo) and mature (CD69−CD62Lhi) CD4+ OT-II thymocytes from WT and KO mice. ISC stands for the iso-type control antibody. These are representative results of four mice per group analyzed.
Figure S7. Expression of CD44, CD62L, and CD127 in T cells on IL-7R Transgenic Background
(a) CD4+ and CD8+ T cells from the spleens of WT IL-7RTg and KO IL-7RTg mice at 8 weeks old were stained with CD44 and CD62L antibodies and analyzed by FACS. (b) Expression of CD127 in splenic CD44loCD62Lhi and CD44hiCD62Llo T cells from WT IL-7RTg and KO IL-7RTg. These are representative results of two independent experiments.
Acknowledgments
We are indebted to A. Singer (National Cancer Institute) for providing us IL-7R transgenic mice. We thank L. Evangelisti, C. Hughes, and J. Stein for their help in creating the Foxo1 mutant mice; X. Zhang for analysis of gene expression data; and X. Wang for critical commentary on the manuscript. M.O.L. is a Rita Allen Foundation Scholar. R.A.F. is an investigator of the Howard Hughes Medical Institute. This work is supported by a NIAMS-NIH KO1 grant (M.O.L.), an Arthritis Foundation Investigator Award (M.O.L.), the American Diabetes Association (R.A.F.), and NIH DK51665 (R.A.F.).
Footnotes
Competing interest statement
The authors declare that they have no competing financial interests.
Note Added in Proof: During revision of the current manuscript, an article by Kerdiles et al. used a different strain of T cell-specific Foxo1-deficient mice to reveal Foxo1 regulation of IL-7R and L-selectin expression similar to findings in our study (Nat. Immunol. 2, 176-184, 2009). Their study also showed Foxo1 regulation of CCR7 expression, suggesting a role for Foxo1 in control of T cell trafficking.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akashi K, Kondo M, von Freeden-Jeffry U, Murray R, Weissman IL. Bcl-2 rescues T lymphopoiesis in interleukin-7 receptor-deficient mice. Cell. 1997;89:1033–1041. doi: 10.1016/s0092-8674(00)80291-3. [DOI] [PubMed] [Google Scholar]
- Barata JT, Cardoso AA, Nadler LM, Boussiotis VA. Interleukin-7 promotes survival and cell cycle progression of T-cell acute lymphoblastic leukemia cells by down-regulating the cyclin-dependent kinase inhibitor p27(kip1) Blood. 2001;98:1524–1531. doi: 10.1182/blood.v98.5.1524. [DOI] [PubMed] [Google Scholar]
- Bayer AL, Lee JY, de la Barrera A, Surh CD, Malek TR. A function for IL-7R for CD4+CD25+Foxp3+ T regulatory cells. J Immunol. 2008;181:225–234. doi: 10.4049/jimmunol.181.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Burgering BM. A brief introduction to FOXOlogy. Oncogene. 2008;27:2258–2262. doi: 10.1038/onc.2008.29. [DOI] [PubMed] [Google Scholar]
- Calzascia T, Pellegrini M, Lin A, Garza KM, Elford AR, Shahinian A, Ohashi PS, Mak TW. CD4 T cells, lymphopenia, and IL-7 in a multistep pathway to autoimmunity. Proc Natl Acad Sci U S A. 2008;105:2999–3004. doi: 10.1073/pnas.0712135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson CM, Endrizzi BT, Wu J, Ding X, Weinreich MA, Walsh ER, Wani MA, Lingrel JB, Hogquist KA, Jameson SC. Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature. 2006;442:299–302. doi: 10.1038/nature04882. [DOI] [PubMed] [Google Scholar]
- Chandele A, Joshi NS, Zhu J, Paul WE, Leonard WJ, Kaech SM. Formation of IL-7Ralphahigh and IL-7Ralphalow CD8 T cells during infection is regulated by the opposing functions of GABPalpha and Gfi-1. J Immunol. 2008;180:5309–5319. doi: 10.4049/jimmunol.180.8.5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre S, Carrette F, Chen J, Lang V, Semichon M, Denoyelle C, Lazar V, Cagnard N, Dubart-Kupperschmitt A, Mangeney M, et al. FOXO1 regulates L-Selectin and a network of human T cell homing molecules downstream of phosphatidylinositol 3-kinase. J Immunol. 2008;181:2980–2989. doi: 10.4049/jimmunol.181.5.2980. [DOI] [PubMed] [Google Scholar]
- Fabre S, Lang V, Harriague J, Jobart A, Unterman TG, Trautmann A, Bismuth G. Stable activation of phosphatidylinositol 3-kinase in the T cell immunological synapse stimulates Akt signaling to FoxO1 nuclear exclusion and cell growth control. J Immunol. 2005;174:4161–4171. doi: 10.4049/jimmunol.174.7.4161. [DOI] [PubMed] [Google Scholar]
- Furuyama T, Kitayama K, Shimoda Y, Ogawa M, Sone K, Yoshida-Araki K, Hisatsune H, Nishikawa S, Nakayama K, Nakayama K, et al. Abnormal angiogenesis in Foxo1 (Fkhr)-deficient mice. J Biol Chem. 2004;279:34741–34749. doi: 10.1074/jbc.M314214200. [DOI] [PubMed] [Google Scholar]
- Hosaka T, Biggs WH, 3rd, Tieu D, Boyer AD, Varki NM, Cavenee WK, Arden KC. Disruption of forkhead transcription factor (FOXO) family members in mice reveals their functional diversification. Proc Natl Acad Sci U S A. 2004;101:2975–2980. doi: 10.1073/pnas.0400093101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Tindall DJ. Dynamic FoxO transcription factors. J Cell Sci. 2007;120:2479–2487. doi: 10.1242/jcs.001222. [DOI] [PubMed] [Google Scholar]
- Huster KM, Busch V, Schiemann M, Linkemann K, Kerksiek KM, Wagner H, Busch DH. Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets. Proc Natl Acad Sci U S A. 2004;101:5610–5615. doi: 10.1073/pnas.0308054101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- Jameson SC. Maintaining the norm: T-cell homeostasis. Nat Rev Immunol. 2002;2:547–556. doi: 10.1038/nri853. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Li WQ, Aiello FB, Mazzucchelli R, Asefa B, Khaled AR, Durum SK. Cell biology of IL-7, a key lymphotrophin. Cytokine Growth Factor Rev. 2005;16:513–533. doi: 10.1016/j.cytogfr.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- Kieper WC, Tan JT, Bondi-Boyd B, Gapin L, Sprent J, Ceredig R, Surh CD. Overexpression of interleukin (IL)-7 leads to IL-15-independent generation of memory phenotype CD8+ T cells. J Exp Med. 2002;195:1533–1539. doi: 10.1084/jem.20020067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C, Ilic A, Koelsch K, Sarvetnick N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell. 2004;117:265–277. doi: 10.1016/s0092-8674(04)00335-6. [DOI] [PubMed] [Google Scholar]
- Kondrack RM, Harbertson J, Tan JT, McBreen ME, Surh CD, Bradley LM. Interleukin 7 regulates the survival and generation of memory CD4 cells. J Exp Med. 2003;198:1797–1806. doi: 10.1084/jem.20030735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PP, Fitzpatrick DR, Beard C, Jessup HK, Lehar S, Makar KW, Perez-Melgosa M, Sweetser MT, Schlissel MS, Nguyen S, et al. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity. 2001;15:763–774. doi: 10.1016/s1074-7613(01)00227-8. [DOI] [PubMed] [Google Scholar]
- Leenders H, Whiffield S, Benoist C, Mathis D. Role of the forkhead transcription family member, FKHR, in thymocyte differentiation. Eur J Immunol. 2000;30:2980–2990. doi: 10.1002/1521-4141(200010)30:10<2980::AID-IMMU2980>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Li J, Huston G, Swain SL. IL-7 promotes the transition of CD4 effectors to persistent memory cells. J Exp Med. 2003;198:1807–1815. doi: 10.1084/jem.20030725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MO, Flavell RA. TGF-beta: a master of all T cell trades. Cell. 2008;134:392–404. doi: 10.1016/j.cell.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MO, Sanjabi S, Flavell RA. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity. 2006a;25:455–471. doi: 10.1016/j.immuni.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Li MO, Wan YY, Flavell RA. T cell-produced transforming growth factor-beta1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity. 2007;26:579–591. doi: 10.1016/j.immuni.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Li WQ, Jiang Q, Aleem E, Kaldis P, Khaled AR, Durum SK. IL-7 promotes T cell proliferation through destabilization of p27Kip1. J Exp Med. 2006b;203:573–582. doi: 10.1084/jem.20051520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Hron JD, Peng SL. Regulation of NF-kappaB, Th activation, and autoinflammation by the forkhead transcription factor Foxo3a. Immunity. 2004;21:203–213. doi: 10.1016/j.immuni.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Lu B, Yu H, Chow C, Li B, Zheng W, Davis RJ, Flavell RA. GADD45gamma mediates the activation of the p38 and JNK MAP kinase pathways and cytokine production in effector TH1 cells. Immunity. 2001;14:583–590. doi: 10.1016/s1074-7613(01)00141-8. [DOI] [PubMed] [Google Scholar]
- Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006;24:657–679. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- Maraskovsky E, O’Reilly LA, Teepe M, Corcoran LM, Peschon JJ, Strasser A. Bcl-2 can rescue T lymphocyte development in interleukin-7 receptor-deficient mice but not in mutant rag-1−/− mice. Cell. 1997;89:1011–1019. doi: 10.1016/s0092-8674(00)80289-5. [DOI] [PubMed] [Google Scholar]
- Marleau AM, Sarvetnick N. T cell homeostasis in tolerance and immunity. J Leukoc Biol. 2005;78:575–584. doi: 10.1189/jlb.0105050. [DOI] [PubMed] [Google Scholar]
- Mathis D, Benoist C. Back to central tolerance. Immunity. 2004;20:509–516. doi: 10.1016/s1074-7613(04)00111-6. [DOI] [PubMed] [Google Scholar]
- Mazzucchelli R, Durum SK. Interleukin-7 receptor expression: intelligent design. Nat Rev Immunol. 2007;7:144–154. doi: 10.1038/nri2023. [DOI] [PubMed] [Google Scholar]
- Mazzucchelli R, Hixon JA, Spolski R, Chen X, Li WQ, Hall VL, Willette-Brown J, Hurwitz AA, Leonard WJ, Durum SK. Development of regulatory T cells requires IL-7Ralpha stimulation by IL-7 or TSLP. Blood. 2008;112:3283–3292. doi: 10.1182/blood-2008-02-137414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema RH, Kops GJ, Bos JL, Burgering BM. AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature. 2000;404:782–787. doi: 10.1038/35008115. [DOI] [PubMed] [Google Scholar]
- Obsil T, Obsilova V. Structure/function relationships underlying regulation of FOXO transcription factors. Oncogene. 2008;27:2263–2275. doi: 10.1038/onc.2008.20. [DOI] [PubMed] [Google Scholar]
- Park JH, Yu Q, Erman B, Appelbaum JS, Montoya-Durango D, Grimes HL, Singer A. Suppression of IL7Ralpha transcription by IL-7 and other prosurvival cytokines: a novel mechanism for maximizing IL-7-dependent T cell survival. Immunity. 2004;21:289–302. doi: 10.1016/j.immuni.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Paulos CM, Wrzesinski C, Kaiser A, Hinrichs CS, Chieppa M, Cassard L, Palmer DC, Boni A, Muranski P, Yu Z, et al. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J Clin Invest. 2007;117:2197–2204. doi: 10.1172/JCI32205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng SL. Foxo in the immune system. Oncogene. 2008;27:2337–2344. doi: 10.1038/onc.2008.26. [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Nakamura N, Sansal I, Bergeron L, Sellers WR. A novel mechanism of gene regulation and tumor suppression by the transcription factor FKHR. Cancer Cell. 2002;2:81–91. doi: 10.1016/s1535-6108(02)00086-7. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T Cells and Immune Tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- Schluns KS, Lefrancois L. Cytokine control of memory T-cell development and survival. Nat Rev Immunol. 2003;3:269–279. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- Sebzda E, Zou Z, Lee JS, Wang T, Kahn ML. Transcription factor KLF2 regulates the migration of naive T cells by restricting chemokine receptor expression patterns. Nat Immunol. 2008;9:292–300. doi: 10.1038/ni1565. [DOI] [PubMed] [Google Scholar]
- Seddon B, Tomlinson P, Zamoyska R. Interleukin 7 and T cell receptor signals regulate homeostasis of CD4 memory cells. Nat Immunol. 2003;4:680–686. doi: 10.1038/ni946. [DOI] [PubMed] [Google Scholar]
- Stahl M, Dijkers PF, Kops GJ, Lens SM, Coffer PJ, Burgering BM, Medema RH. The forkhead transcription factor FoxO regulates transcription of p27Kip1 and Bim in response to IL-2. J Immunol. 2002;168:5024–5031. doi: 10.4049/jimmunol.168.10.5024. [DOI] [PubMed] [Google Scholar]
- Surh CD, Sprent J. Regulation of mature T cell homeostasis. Semin Immunol. 2005;17:183–191. doi: 10.1016/j.smim.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Tan JT, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg KI, Surh CD. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci U S A. 2001;98:8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vang KB, Yang J, Mahmud SA, Burchill MA, Vegoe AL, Farrar MA. IL-2, -7, and -15, but not thymic stromal lymphopoeitin, redundantly govern CD4+Foxp3+ regulatory T cell development. J Immunol. 2008;181:3285–3290. doi: 10.4049/jimmunol.181.5.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinay DS, Kwon BS. Role of 4-1BB in immune responses. Semin Immunol. 1998;10:481–489. doi: 10.1006/smim.1998.0157. [DOI] [PubMed] [Google Scholar]
- Wan YY, Flavell RA. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proc Natl Acad Sci U S A. 2005;102:5126–5131. doi: 10.1073/pnas.0501701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue HH, Bollenbacher J, Rovella V, Tripuraneni R, Du YB, Liu CY, Williams A, McCoy JP, Leonard WJ. GA binding protein regulates interleukin 7 receptor alpha-chain gene expression in T cells. Nat Immunol. 2004;5:1036–1044. doi: 10.1038/ni1117. [DOI] [PubMed] [Google Scholar]
- Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel R, Karsunky H, Klein-Hitpass L, Moroy T. The transcriptional repressor Gfi1 affects development of early, uncommitted c-Kit+ T cell progenitors and CD4/CD8 lineage decision in the thymus. J Exp Med. 2003;197:831–844. doi: 10.1084/jem.20021417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf I, Fruman DA. Regulation of quiescence in lymphocytes. Trends Immunol. 2003;24:380–386. doi: 10.1016/s1471-4906(03)00141-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Analysis of Foxo1-deficient T Cells in the Mesenteric Lymph Nodes
(a) Expression of CD44 and CD62L in CD4+ and CD8+ T cells from the mesenteric lymph nodes (mLNs) of WT and KO mice at 6 weeks old. These are representative results of six independent experiments.
(b) Percentage of mLN CD4+Foxp3+ regulatory T (Treg) cells from WT and KO mice at 6 weeks old.
(c) Number of mLN CD4+ and CD8+ T cells from control (Foxo1/Foxo1) and KO mice aged between 6 and 8 weeks (n=6). The p values between the cell number of the two groups of mice are shown.
(d) CD4+ and CD8+ T cells isolated from the mLNs of WT and KO mice were stimulated with PMA and ionomycin for 4 hr and analyzed for the expression of IFN-γ, IL-4, IL-10, and IL-17 by intracellular cytokine staining. These are representative results of two independent experiments.
(e) Expression of Ki-67 in CD4+ and CD8+ T cells from the mLNs of WT and KO mice aged between 6 and 8 weeks.
Figure S2. Analysis of Foxo1-deficient T Cells in the Peripheral Lymph Nodes
(a) Expression of CD44 and CD62L in CD4+ and CD8+ T cells from the peripheral lymph nodes (pLNs) of WT and KO mice at 6 weeks old. These are representative results of six independent experiments.
(b) Number of pLN CD4+ and CD8+ T cells from control (Foxo1/Foxo1) and KO mice aged between 6 and 8 weeks (n=6). The p values between the cell number of the two groups of mice are shown.
(c) CD4+ and CD8+ T cells isolated from the pLNs of WT and KO mice were stimulated with PMA and ionomycin for 4 hr and analyzed for the expression of IFN-γ, IL-4, IL-10, and IL-17 by intracellular cytokine staining. These are representative results of two independent experiments.
(d) Expression of Ki-67 in CD4+ and CD8+ T cells from the pLNs of WT and KO mice aged between 6 and 8 weeks.
Figure S3. Suppressive Function of Foxo1-deficient Treg Cells
CD4+Foxp3+ regulatory T cells (Treg) were isolated from WT and KO mice. The CD44loCD4+ cells from WT mice were labeled with CFSE and used as responding T cells (Tresp). The suppression was shown by comparison of CFSE dilution in the Tresp cells cultured with or without 1:1 Tregs.
Figure S4. Cytokine Production of CD44hi WT and KO Splenic T cells
T cells isolated from the spleens of WT and KO mice were stimulated with PMA and ionomycin for 4 hr and analyzed for the expression of IFN-γ, IL-4, IL-10, and IL-17 in CD44hiCD4 and CD44hiCD8 T cells. These are representative results of two independent experiments.
Figure S5. Conserved Foxo1-Binding Sites in the Promoter Region of Il7r Gene
The conserved putative Foxo1-binding sites in the Il7r locus were analyzed by rVISTA online (http://genome.lbl.gov/vista/rvista/submit.shtml). Ten kilobase pairs (10 kb) of nucleotides upstream of transcription start site (indicated by red arrow) from mouse and human Il7r genes were used for rVISTA analysis. Three conserved noncoding sequences (CNSs, marked by pink color) and three conserved Foxo1 binding sites (shown as green bars) were depicted.
Figure S6. Development of Foxo1-deficient OT-II T cells
Thymic CD4 and CD8 profile of WT and KO OT-II mice at the age of 8 weeks (top left). Expression of CD69 and CD62L in the CD4+ OT-II thymocytes from WT and KO mice (bottom left). Expression of CD127 (IL-7R) in the immature (CD69+CD62Llo) and mature (CD69−CD62Lhi) CD4+ OT-II thymocytes from WT and KO mice. ISC stands for the iso-type control antibody. These are representative results of four mice per group analyzed.
Figure S7. Expression of CD44, CD62L, and CD127 in T cells on IL-7R Transgenic Background
(a) CD4+ and CD8+ T cells from the spleens of WT IL-7RTg and KO IL-7RTg mice at 8 weeks old were stained with CD44 and CD62L antibodies and analyzed by FACS. (b) Expression of CD127 in splenic CD44loCD62Lhi and CD44hiCD62Llo T cells from WT IL-7RTg and KO IL-7RTg. These are representative results of two independent experiments.