Abstract
Smooth muscle contractile activity is a major regulator of function of the vascular system, respiratory system, gastrointestinal system and the genitourinary systems. Malfunction of contractility in these systems leads to a host of clinical disorders, and yet, we still have major gaps in our understanding of the molecular mechanisms by which contractility of the differentiated smooth muscle cell is regulated. This review will summarize recent advances in the molecular understanding of the regulation of smooth muscle myosin activity via phosphorylation/dephosphorylation of myosin, the regulation of the accessibility of actin to myosin via the actin-binding proteins calponin and caldesmon, and the remodelling of the actin cytoskeleton. Understanding of the molecular ‘players’ should identify target molecules that could point the way to novel drug discovery programs for the treatment of smooth muscle disorders such as cardiovascular disease, asthma, functional bowel disease and pre-term labour.
Keywords: myosin light chain phosphorylation, calponin, caldesmon, myosin phosphatase, CaMKII, actin cytoskeleton
Introduction
-
Mechanisms that regulate LC20 phosphorylation
Regulation of myosin phosphatase
CaMKII
-
Mechanisms that regulate the access of myosin to actin
Caldesmon
bCaP function in regulation of PKC and ERK signalling
Mechanisms that regulate cytoskeletal remodelling
Conclusions
Introduction
All contractility is initiated by changes in the activity of, or interactions of, actin and myosin. In recent years, a multitude of signalling pathways have been suggested to regulate smooth muscle contractility; however, these pathways can be broken down into three major types of mechanisms (Fig.1): (1) mechanisms that regulate actin-activated myosin ATPase activity via changes in the phosphorylation state of the 20-kD myosin light chain (LC20); (2) mechanisms that regulate the availability of actin to interact with myosin via the action of inhibitory actin-binding proteins such as caldesmon (CaD) and possibly calponin (CaP) and (3) the less well-studied possibility of mechanisms by which the cytoskeleton is remodelled to facilitate the transmission or maintenance of force developed by actomyosin interactions.
Figure 1.
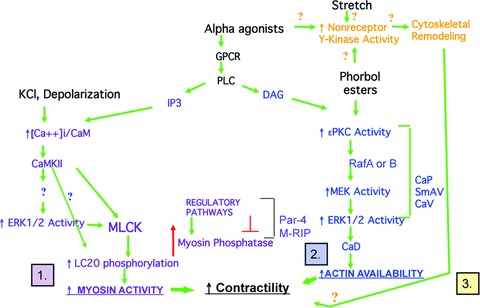
Pathways that regulate contractility (demonstrated and putative).
The first set of pathways is known to be involved in pathologies such as traumatic brain injury and post-haemorrhagic cerebral vasospasm [1, 2] (discussed in detail in a review by Jose Rafols in this series), and in pulmonary hypertension [3]. The second set of pathways is strongly implicated in pre-term labour [4]. The third set of pathways is implicated in asthma [5]. Thus, the diversity of signalling pathways that regulate contractility may well offer opportunities for discovery of potential disease- and organ-specific therapies.
The first set of mechanisms is illustrated on the left-hand side of Fig.1 (purple type), where depolarization of a smooth muscle cell, for example, by exposure to a physiological saline solution in which NaCl is replaced by KCl, opens voltage-dependent Ca2+ channels and increases intracellular ionized calcium (Ca2+i) levels. This leads to a Ca2+i Vcalmodulin (CaM)-dependent activation of myosin light chain kinase (MLCK), phosphorylation of myosin LC20 and an increase in myosin ATPase activity [6, 7]. However, as soon as intracellular Ca2+ indicators were successfully applied to smooth muscle cells and cell permeabilization techniques were developed, it became clear that many agonists increase the relative amount of force produced at a constant Ca2+i, i.e. cause ‘Ca2+ sensitization’ of force [8–17]. Ca2+ sensitization of force can be caused by a Ca2+ sensitization of LC20 phosphorylation mechanisms and this is now known to involve pathways that inhibit myosin phosphatase [18, 19] as well as pathways that increase the Ca2+ sensitivity of MLCK, such as the ERK1/2-mediated phosphorylation of MLCK [20–22], or, possibly by the direct phosphorylation of Ser19 on LC20 by kinases other than MLCK. These mechanisms are described in more detail below.
An observed ‘Ca2+ sensitization’ of force can also occur in the absence of changes in LC20 phosphorylation [23]. In this case most evidence points to the second type of pathway –i.e. those that regulate the activity of inhibitory actin-binding proteins that regulate the availability of actin to interact with myosin (Fig.1 right-hand side, blue type). These pathways include possible roles for ERK1/2, CaP and CaD [24]. These pathways are described below in more detail.
In recent years, the third possibility, (Fig.1, top, gold type), i.e. that contractility may be modulated by remodelling of the cytoskeleton has been suggested, although the molecular mechanisms are far less well defined. Remodelling of the cytoskeleton is well know to occur in airway smooth muscle, and there is growing evidence that this also happens in some blood vessels, (see below); however, it is not yet clear exactly how cytoskeletal remodelling modulates contractility of either the airway or vascular cells. This topic will be discussed in detail below. Little is known regarding the molecular mechanism of the remodelling but several groups have suggested that, as occurs in non-muscle cells, the process involves not only actin polymerization but also turnover of adhesion plaque proteins and activation of non-receptor tyrosine kinases such as those in the Src family [25].
Mechanisms that regulate LC20 phosphorylation
Smooth muscle myosin, unlike striated muscle myosin, requires phosphorylation at Ser 19 in order to show significant levels of actin-activated myosin ATPase activity. Ca2+/CaM-dependent activation of MLCK is the primary and best-known pathway by which changes in the phosphorylation level of smooth muscle myosin occur. However, it is worth mentioning that smooth muscle myosin, at least in vitro, is also known to be capable of being phosphorylated in a calcium-independent manner by additional kinases like Rho kinase [26], integrin-linked kinase [27], and zipper-interacting protein kinase (ZIPK) [28, 29]; however, the relative in vivo significance of these pathways is not yet entirely determined [18, 30, 31]. MLCK itself has been recently reviewed elsewhere [32] and will not be focused on here. Recent studies have uncovered evidence for a multitude of additional complex pathways by which smooth muscle myosin phosphorylation levels can be regulated in vivo, including both regulation of dephosphorylation and the activation of a second Ca2+/CaM-dependent kinase, CaMKII described below.
Regulation of myosin phosphatase
LC20 dephosphorylation is catalysed by a single myosin light chain phosphatase (MP). The smooth muscle MP complex consists of the catalytic subunit, PP1c-δ, a regulatory subunit, myosin phosphatase target subunit 1 (MYPT1), also referred to as myosin-binding subunit (MBS), and a small, approximately 20-kD subunit of unknown function. Alternative splicing of two exons gives rise to four major MYPT1 isoforms, that differ in the presence of a central insert and/or a leucine zipper motif at the C-terminal end of the protein (LZ+ and LZ− isoforms) [33–36].
Initially, MP was assumed to be constitutively active and not subject to regulation. However, in recent years, an array of regulatory pathways leading to MP inhibition and activation has been described and is summarized diagrammatically in Fig.2. The various MP regulators can be divided roughly into two groups: (/) protein scaffolds and (//) substrates of upstream kinases (or. phosphoproteins), with some regulators having a dual function.
Figure 2.
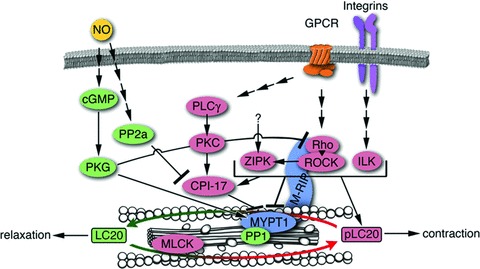
Regulatory pathways leading to myosin light chain phosphatase inhibition and activation. Activating signalling molecules (leading to relaxation) are shown in green, inhibitory molecules (supporting contractility) are shown in pink. Molecules shown in blue are bimodal and can be both, an inhibitor or an activator.
The most prominent MP regulator is the regulatory subunit, MYPT1, which behaves as both a scaffolding protein and a substrate of upstream kinases. In its function as a scaffold, MYPT1 can be classified as an activator of MP since it targets the MP complex to its substrate, LC20 [37]. Moreover, binding of MYPT1 to PP1c-8 also enhances its catalytic activity towards LC20 [38, 39]. On the other hand, phosphorylation of MYPT1 on one or both of the two major inhibitory phosphorylation sites (corresponding to threonine-696 and threonine-853 in mammalian MYPT1), leads to inactivation of MP [40, 41]. MYPT1 also contains an activating phosphorylation site – serine-695 – that inhibits subsequent inhibitory phosphorylation of threonine-696 [42].
Another phosphoprotein-type inhibitor of MP is CPI-17 (PKC-potentiated PP1 inhibitory protein of 17 kD) [43]. This small protein acts as an MP pseudosubstrate when phosphorylated, and binds to the catalytic site of MP, thereby competing with LC20 for phosphorylation. A bimodal MP regulator of the scaffold-type is the myosin phosphatase-Rho interacting protein (M-RIP), which targets MYPT1, Rho and Rho kinase to actomyosin filaments. As far as the targeting of MYPT1 to the actomyosin filaments is concerned, this protein can be an activator of MP; however, since it also targets Rho kinase to the MP complex, it can also have an inhibitory effect on MP activity. These different possibilities are reflected by controversial reports about M-RIP function [44–48].
The immediate MP regulators are, themselves, downstream elements of extracellular cascades. Inhibitory phosphorylation of MYPT1 is primarily mediated by the Rho pathway, via Rho kinase directly and/or via Rho kinase-mediated activation of ZIPK [41, 49–51]. Whether kinases other than Rho kinase can activate ZIPK in smooth muscle is not known. The inhibitory effect of activated protein kinase C (PKC) on MP is primarily mediated by CPI-17 [52]. Apart from PKC, CPI-17 can also be phosphorylated by ZIPK and integrin-linked kinase [53, 54]. As CPI-17 is more rapidly phosphorylated and dephosphorylated than MYPT1, these apparently redundant pathways might be necessary for fine-tuning of contraction; furthermore, each pathway provides contact points for offset signalling [55, 56].
Counterbalancing the Rho and PKC pathways, which support contractility, the nitric oxide pathway leads to relaxation. Nitric oxide elevates intracellular cGMP, which activates the type la cGMP-dependent protein kinase (PKG). PKG phosphorylates RhoA at Serine-188, an inhibitory phosphorylation site, thus disrupting RhoA signalling [57, 58]. Furthermore, PKG phosphorylates MYPT1 at the activating phosphorylation site, serine-695, thus blocking subsequent inhibitory phosphorylation [42, 59]. This effect on MYPT1 depends on the expression of the LZ+ iso-form of MYPT1, however, it is controversial whether the interaction between MYPT1 and PKG is mediated by a leucine zipper–leucine zipper interaction [60–62]. Other downstream effectors of nitric oxide include protein phosphatase 2a (PP2a) [63], one of the phosphatases that dephosphorylate and thus inactivate CPI-17 [64, 65].
These regulatory pathways form a network that tightly regulates MP and, therefore, has a significant impact on LC20 phosphorylation and contractility. Disturbance of the balance between LC20 phosphorylation and dephosphorylation is expected to, and is known to lead to pathologies. For example, vascular hypercon–striction of pulmonary arteries, caused by an overstimulated Rho pathway, leads to pulmonary artery hypertension [3]. MYPT1 phosphorylation in particular has been shown to be sensitive to hypoxia, thus probably playing an essential role in hypoxic relaxation [66]. In hypertensive rat models, inhibition of the Rho pathway corrected the hypertension [67]. In the setting of neonatal circulatory transition and in persistent pulmonary hypertension of the newborn, the Rho pathway appeared to be less important, but instead a role for CPI-17 was shown [68].
CaMKII
Ca2+/CaM-dependent kinase II (CaMKII) is a Serine/Threonine kinase that is ubiquitously expressed. It is a family of four closely related isoforms; α, β, y and δ, the products of four separate genes. The α- and β-isoforms are primarily restricted to neural tissues, but the γ- and δ-isoforms are widely distributed. We have identified six variants of CaMKII-γ from an aorta cDNA library raising the possibility that each could be differentially targeted, have different substrate specificity and, thus, have different functions [69].
CaMKII consists of an N-terminal catalytic/regulatory domain and a C-terminal association domain. A linker domain connects these two conserved domains, which is variable in sequence (Fig.3). The association domains of all isoforms of CaMKII interact to form large wheel-shaped holoenzymes [70]. Crystallographic data indicate that the holoenzyme is dodecameric [71].
Figure 3.
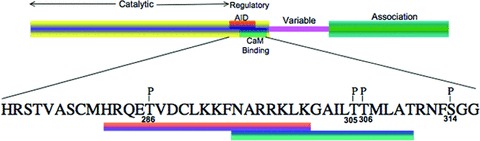
Domain structure of CaMKII. The phos-phosites are indicated with ‘P’ on top of the amino acid residues. The numbering is according to the a-isoform.
As the name implies CaMKII is activated by Ca2+ and CaM. In the inactive state, an autoinhibitory domain blocks the active site of the molecule, but the binding of Ca2+/CaM disrupts the autoinhibitory domain and the kinase becomes active. In vitro studies have shown that CaMKII can autophosphorylate at several sites [72, 73]. However, autophosphorylation of Thr286 (numbering according to α-isoform, Thr287 for β, 7 and δ) is the best explored site and two important consequences have been proposed for autophosphorylation at this site [74]. First, autophosphorylation of Thr286 disables the autoinhibitory domain; as a result CaM kinase acquires ‘autonomous activity’, activity that is retained even after removal of Ca2+. Secondly, the affinity of CaMKII for CaM increases about 1000-fold, also called ‘CaM trapping’. The ability of the kinase to retain activity after being activated Ca2+ at a previous point in time has been referred to as a ‘molecular memory’[75] that has been linked, for the α- and β-isoforms to the processes of synaptic plasticity, learning, and memory per se, supported, most convincingly by the CaMKII α-knockout mouse model [76]. In vascular smooth muscle a modified form of memory has been linked to a prolongation of vascular tone [77].
A distinct phase of autophosphorylation occurs at Thr305, Thr306 and Ser314 when Ca2+/CaM dissociates from the Thr286 phosphorylated protein [78]. Autophosphorylation of Thr305 and Thr306 are inhibitory as they prevent further binding of CaM. However, autophosphorylation at Ser314 has no effect on binding of CaM [73, 79]. In vascular smooth muscle it appears that Thr305/306 phosphorylation is a significant regulator of vascular tone in that this site, which inhibits CaMKII activity, is, itself, inhibited by the action of α-agonists [77] leading to a delayed reactivation of kinase activity, and increased vascular tone.
Both pharmacological inhibitors and antisense knockdown of CaMKII in vascular tissue and cells have clearly shown that CaMKII activation is a significant regulator of vascular tone [20, 80, 81]. In vitro, CaMKII has been shown to phosphorylate many key proteins involved in smooth muscle regulation, including MLCK [82, 83], LC20 [84], CaD and CaP [85–87], phospholipase-α2 [88] and the α-subunit of Ca2+ channel [89]. The in vivo significance of most of these proteins is unclear; however, in vivo studies have indicated that CaMKII increases the activity of the voltage-dependent K+ channel [90], the voltage-dependent Ca2+ channel [91] and decreases the activity of the Ca2+ activated Cl channel [92]. The transcription factor, cAMP-responsive element-binding protein (CREB) also appears to be an in vivo substrate of CaMKII and the elevation of c-fos by CREB phosphorylation regulates gene expression in smooth muscle [93]. Phospholamban, a negative regulator of sarcoplasmic reticulum Ca2+-ATPase is also a substrate of CaMKII that seems to have in vivo relevance; however, because of the apparent low abundance of phospholamban in smooth muscle, the magnitude of the effect may be less than that in cardiac muscle [94].
CaMKII-mediated regulation of MLCK appears also to have in vivo relevance but is not due to direct phosphorylation of MLCK but rather to the recruitment of a more complex pathway involving activation of ERK and the presumed ERK-mediated phosphorylation of and activation of MLCK at constant Ca2+ levels [20, 81, 95] (pathway 1, Fig.1).
It is of interest that the knockdown of a specific variant of CaMKII-7, CaMKII-7G-2, inhibits depolarization-mediated contractions and the ERK-MLCK pathway described above. Whether this one variant is the only variant of the six known to be present in vascular tissue that regulates contractility, or whether other variants share this mechanism, is not yet known. Obviously, if G-2 were the sole variant effective in regulating vascular tone, it would be a potentially specific candidate molecule for drug discovery research. This variant is unusual in having a unique sequence of 99 amino acids that target the variant, when activated, to adhesion plaques. The prevention of CaMKII-7G-2 targeting to adhesion plaques leads to significant inhibition of ERK activation as well as contractility [81].
A specific G-2 phosphatase, a small C-terminal domain phosphatase-3 (SCP3) homologue, a PP2C-type phosphatase has also been reported [96]. This phosphatase is primarily expressed in vascular smooth muscle tissues and specifically binds to the association domain of the CaMKII-γ G-2. SCP3 dephosphorylation of CaMKII-7 G-2 is site specific, excluding the Thr287 site associated with Ca2+/CaM-independent activation of the kinase. Thus, the selective dephosphorylation by SCP3 creates a constitutively active kinase which is regulated by phosphorylation-dependent targeting mechanisms [96].
Recent work on the δ-isoform of CaMKII has shown a role for CaMKII-δ in PDGF- stimulated vascular smooth muscle migration [97] and wound healing [98]. Vascular injury induced by balloon angioplasty has been shown to increase CaMKII-δ isoform expression in smooth muscle cells and in fibroblasts [99]. Trafficking of iNOS was also shown to be dependent on the activation of CaMKII-δ isoform [100]. Thus, the 7- and δ-isoforms of CaMKII may have distinct, but equally important roles in vascular function.
Mechanisms that regulate the access of myosin to actin
Caldesmon
CaD is a highly conserved, actin- [101] and myosin- [102] binding protein that exists in two isoforms which are generated by alternative splicing. Whereas the heavy isoform (h-CaD) is restricted to smooth muscle cells, the light isoform (I-CaD) is expressed in non-muscle and de-differentiated smooth muscle cells [103]. This thin-filament associated protein is capable of stabilizing actin filaments [104], blocking Arp2/3 mediated actin polymerization [105], inhibiting actomyosin ATPase activity [106] as well as actin-myosin interaction [107] and thereby regulates contractility of smooth muscle cells. The inhibitory effect of h-CaD can be reversed by either binding of (CaM)/Ca2+ to the C-terminal domain [108] or by phosphorylation of Serine residues 759 and 789 by either ERK1/2 [109] or cdc2 [110]. These events lead to a conformational change in the CaD structure which allows interaction between actin and myosin [111]. While cdc2 phosphorylation plays an important role in cytokinesis [112], the phosphorylation of CaD by ERK1/2 appears to regulate smooth muscle contractility [113]. In this latter process ERK1/2 is activated by PKC (Fig.1) which in turn can be stimulated by phorbol esters or GPCR receptor agonists. Interestingly, CaD is found in podosomes [114], structures that are known to represent sites of PKC activation and signalling and that are involved in actin cytoskeleton remodelling processes [115]. Thus CaD is an important mediator of smooth muscle contractility, regulated, among other mechanisms, by a PKC/ERK signalling pathway.
bCaP function in regulation of PKC "/e and ERK1/2 signalling
CaP is an actin-binding protein that was first isolated from gizzard smooth muscle cells and that can regulate myosin ATPase activity [116]. To date there are known three isoforms of this putative actin regulatory protein, called hi CaP (smooth muscle-specific basic CaP, bCaP) [117], h2 CaP (neutral CaP) [118] and acidic CaP [119] which are encoded by three different genes. The most abundant isoform in differentiated smooth muscle cell (dSMC) is bCaP [120,121], although the other isoforms are also expressed in this cell type in a lower extent [119,122]. Moreover bCaP can be used as a differentiation marker of smooth muscle due to its down-regulation in proliferating cells [87]. CaP proteins consist of a conserved so called CaP homology (CH) domain in their N-Terminus, a Troponin I (Tnl) – like actin-binding domain and three C-terminal repeats [123,124]. The very C-terminus is the variable region in the three CaP-isoforms, whereas the N-terminal fragment is highly conserved (see Fig.4). The CH-domain has been shown to bind acidic phospholipids [125] and ERK1/2 [126], although this structural motif is often implicated in actin binding in many other cytoskeleton proteins [127]. PKC-α and -ɛ can interact with bCaP through the C-terminal repeats [128]. Actin binding of CaP occurs through the Tnl-like domain and in a weaker fashion through the C-terminal repeats [124] and is regulatable by phosphorylation of PKC at Serine 175 and Threonine 184 residues located within the C-terminal repeats [129,130].
Figure 4.

Domain structure of calponin (CaP). CaP proteins consist of a conserved N-terminus including the CH-domain (blue), the Tnl-like domain (yellow) as well as the three C-terminal repeats (green). However, the very C-terminal end is a highly variable region (red) that differs in size and amino acid sequence within the three CaP isoforms.
The functional role of bCaP in regulation of smooth muscle contractility is controversial. One theory is that bCaP may directly regulate contractility by inhibiting actomyosin ATPase activity of myosin heads cross-linked to actin [116] and indeed experiments have shown that bCaP is able to negatively influence in vitro motility of actin filaments [131]. On the other hand, bCaP has been suggested to facilitate agonist-induced signal transduction perhaps by acting as a scaffold protein. This latter hypothesis is based on the findings in vascular smooth muscle cells that bCaP acts as an adaptor protein to directly interact with the signalling proteins PKC [128] and ERK1/2 [126], cotranslocates to the cell cortex upon stimulation together with ERK1/2 and PKC [132] and, in addition, seems to promote PKC activation [128]. Moreover it was shown that a knockdown of bCaP in differentiated vascular smooth muscle cells results in impaired ERK1/2 activity, h-CaD phosphorylation and contractility [133].
Extracellular regulated kinase 1/2 (ERK1/2) is a member of the mitogen-activated protein kinase (MAPK) family, kinases that posses serine/threonine activity. Stimulation of most cell surface receptors by mitogens or GPCR agonists causes activation of MAPK signalling pathways where MAPKs such as ERK1/2 become phosphorylated at Thr and Tyr residues. Phosphorylated ERK1/2 proteins are able to form dimers, enter the nucleus, phosphorylate transcription factors and thereby promote cell proliferation of undifferentiated cells [134–136]. On the other hand, active MAPKs have also been found in differentiated, non-proliferating contractile smooth muscle cells [137,138], but their function in this cell types is still not fully understood. Furthermore it is interesting that an intact actin cytoskeleton seems to be necessary for ERK signalling [139] and that ERK1/2 itself shows actin-binding properties [126], suggesting that the actin cytoskeleton is involved in ERK1/2 signalling. More evidence for ERK1/2 playing a significant role in regulation of smooth muscle contractility comes from studies in pregnant rats showing that ERK1/2 activity is connected to onset of labour [140]. In uterine smooth muscle, the kinase is activated in late pregnancy, possibly due to an increased cortical tension, after translocation to the cell surface. This event is followed by an increased h-CaD phosphorylation which contributes to the initiation of contractions [4, 25].
Another interesting interaction partner of bCaP that seems to be involved in the ERK1/2 signalling pathway is the smooth muscle Archvillin (SmAV) protein. Recent findings show that SmAV binds to bCaP, ERK1/2 and Raf [141, 142], translocates to the cell periphery in smooth muscle cells after agonist stimulation and colocalizes with ERK1/2 and bCaP Furthermore, an antisense-mediated knockdown of SmAV decreases ERK1/2 activation and contractility of smooth muscle, similar to a bCaP knockdown [141].
The model shown in Fig.5 brings these findings together. In this model bCaP acts as a scaffold, connecting ERK1/2 and PKC pathways to regulation of smooth muscle cell contractility [24] (Fig.5): (1) In the first step a, yet unclear, stimulus leads to subsequent activation of PKC-α/ɛ, which is facilitated by bCaP bCaP has been shown in vitroto be able to stimulate PKC-α/ɛ activation in the absence of lipids [128]. It can be speculated that either cell permeant phorbol esters bind to PKC-α/ɛ located at the actin filaments to activate the molecule or, in the case of GPCR activation, that another PKC isoform such as PKC-δ activates PKC-α/ɛ by phosphorylation. Activated PKC-α/ɛ would then be able to phosphorylate bCaP and attenuate its binding affinity to actin, so that (2) the PKC-a/8-ERK1/2-bCaP complex translocates to the cell cortex, where it comes in contact with SmAV, acting as a scaffold for Raf and MEK. PKC-α/ɛ at the surface membrane could then undergo a full activation through binding of diacylglycerol, produced by active phospholipase C at the cell membrane. Active PKC-α/ɛ can phosphorylate Raf [143], which in turn activates MEK, followed by activation of the ERK1/2 molecule. (3) Whereas PKC-α/ɛ, bCaP and SmAV stay at the cell cortex, (4) phosphorylated ERK1/2 moves back to the actin cytoskeleton where it comes into proximity with the actin-bound h-CaD molecule and phosphorylates its substrate [144]. Phosphorylation of h-CaD results in a conformational change of the molecule, leading to partial dissociation of h-CaD from actin and, hence, to actomyosin interaction and contraction [113].
Figure 5.
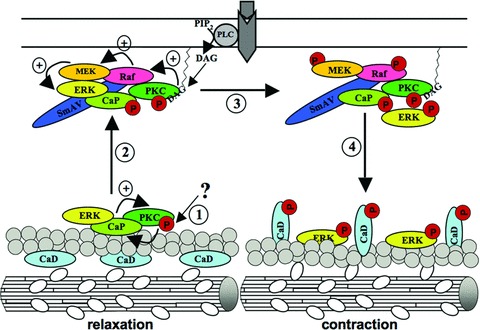
Model of bCaP function in regulation of smooth muscle cell contractility. (1) Starting with a yet unidentified stimulus, PKC-α/ɛ gets subsequently activated, an event that is further supported by bCaP binding. PKC-α/ɛ may now phosphorylate bCaP, leading to an impaired actin-binding property of bCaP (2) Hence the ERK1/2-PKC-a/e-bCaP complex translocates to the cell cortex where it binds to SmAV, a protein acting as a scaffold for Raf and MEK. Moreover the PKC-α/ɛ molecule gets fully activated by membrane bound diacylglycerol that is produced by activated phospholipase C coupled to GPCR. The activated PKC-α/ɛ molecule phosphorylates Raf, which in turn phosphorylates MEK that now activates ERK1/2. Whereas the SmAV-bCaP-PKC-α/ɛ complex stays at the membrane, (4) activated ERK1/2 moves back to the actin filaments where it comes in contact with its substrate h-CaD. Phosphorylation of the h-CaD molecule leads to its conformational change, resulting in enabled actin-myosin interaction and hence to contraction. For detailed information see text/article.
Less is known about bCaP and its involvement in vascular diseases, but hints come from mice with a mutated bCaP locus which express a C-terminal truncated form of the protein lacking the Tnl-like domain as well as the three C-terminal repeats and the variable C-terminus. Surprisingly the most conspicuous phenotype of these mice was an increased bone formation, leading to the hypothesis that bCaP is involved in regulation of osteogenesis [145]. Further examination has revealed that these mice display a faster shortening velocity [146], a lower heart beat rate, an impaired α-adrenergic vasoconstriction, an enhanced arterial baroreflex sensitivity [147], a lower active isometric force [148] as well as impaired arterial blood pressure regulation during exercise due to an enhanced muscular vasodilation [149]. Particularly the enhanced vasodilation in mice expressing the truncated bCaP form is consistent with our hypothesis that bCaP is required to activate ERK1/2 which in turn inactivates h-CaD, thereby facilitating contraction. However, it should be considered that the truncated bCaP protein expressed in the mouse model lacks only the actin-binding domains and the CH-domain is still expressed. So it should be examined if this truncated bCaP form is still able to interact with ERK1/2 and could therefore substitute for the wild-type bCaP in some of its functions.
In other studies, it has been shown that blood vessels of mice with a mutated bCaP locus are more fragile [150] and therefore less resistant to metastasis of tumour cells [151, 152]. Indeed down-regulated bCaP expression is a poor diagnostic marker in many cancers [153–158], leading to the hypothesis that misregu-lated bCaP levels are connected to cancerous diseases. It has been speculated that down-regulated bCaP levels in smooth muscle cells of blood vessel tumours leads to a destabilization of actin filaments, thereby weakening adhesion of cells to neighbouring cells as well as to the extracellular matrix. This in turn could be a reason for the fragility and penetrability of metastatic tumour cells [150]. On the other hand it was also shown that restoration of bCaP expression in transformed cells can lead to a reduced proliferation rate, tumourigenicity and metastatic cell motility. It has also been speculated that a loss of bCaP leads to an unstable actin filament system, thereby facilitating cytoskeleton reorganisation which is necessary for cells to assume an invasive phenotype [159,160]. Finally, given the evidence (mentioned above) that CaP interacts with MAPKs and the known association of MAPKs with regulation of proliferation, this pathway could also contribute to this property of CaP. Thus, the exact mechanisms by which bCaP can act as a tumour suppressor remains to be elucidated.
Mechanisms that regulate cytoskeletal remodelling
The actin cytoskeleton is defined as the collection of actin filaments and actin filament associated proteins, including adhesion plaque proteins. It has previously been assumed that the actin cytoskeleton in dSMC is largely static, performing a solely structural role, in comparison to the dynamic cytoskeleton of migrating, proliferating vascular smooth muscle cells [161]. Although this topic is still controversial, a body of evidence is growing to support the idea that the actin cytoskeleton is remodelled during contractile agonist activation and that this remodelling might modulate vascular contractility [162–164].
Actin can exist as either filamentous actin (F-actin) or globular actin (G-actin) in cells. G-actin spontaneously polymerizes to form F-actin above its critical concentration (∼8 μg/ml) [165]. The G-actin concentration in the cytoplasm of dVSM cells is above the critical concentration. However, the polymerization of actin is tightly controlled by a large number of actin-binding proteins such as profilin, ADF/cofilin, capping proteins, and sequestering proteins, etc. Signalling pathways that regulate these processes therefore regulate the actual ratio between G- and F-actin resulting in assembly or disassembly of the actin filament (Fig.6) [165,166].
Figure 6.
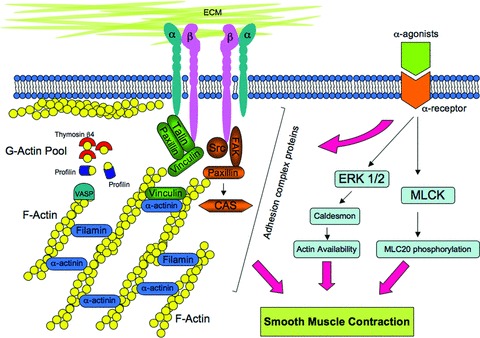
Cytoskeletal remodelling at focal adhesions and regulation of smooth muscle contraction. Integrins connect the extracellular matrix to actin filaments within the cell. Actin filaments are linked to cytoplasmic domain of integrin by linker proteins (green). Mechanical and/or contractile stimuli induce the cytoskeletal remodelling by recruiting signalling proteins (orange) to focal adhesions.
Actin exists primarily as F-actin (∼80% of total actin) in unstimu-lated contractile intact vascular smooth muscle. The percentage of F-actin can go above 90% in α-adrenergic receptor stimulated smooth muscle, indicating dynamic remodelling of the actin cytoskeleton [164]. This result is consistent with the findings reported by Gunst and colleagues for airway smooth muscle [167,168].
Actin is the most abundant protein in smooth muscle and it is highly conserved throughout evolution and across species [169]. Six actin isoforms, products of separate genes, are present in vertebrate tissues [170]. Among these isoforms, two smooth muscle type isoforms (α and γ) and two cytoplasmic isoforms (β and γ) coexist in differentiated vascular smooth muscle [164, 171]. The cytoplasmic β- and 7-actin isoforms are also sometimes referred to as non-muscle isoforms in smooth muscle tissues [169]. Evidence exists, largely in cultured non-muscle cells that the different actin isoforms perform different cellular functions [169]. The amino acid sequences of these actin isoforms are remarkably conserved and most sequence differences are clustered at the NF2-terminal ends [172]. The NF2-terminal region of actin is known not to be involved in actin–actin monomer binding and hence does not directly regulate actin filament polymerization; however, this region is known to be the binding site of many actin-binding proteins including myosin [173] and many actin polymerization regulatory proteins. Recently, we showed that the NFb-terminal of actin isoforms also modulates the contractile function of vascular smooth muscle by using the NH2-terminal decoy peptides of actin isoforms [164]. This result supports the concept that different actin isoforms perform different functions, even though the detailed mechanisms involved are not clear yet.
The actin filaments that connect to either dense bodies or dense plaques (also known as focal adhesion complexes) in smooth muscle are likely to include both the contractile filaments, i.e. actomyosin-containing myofilaments, as well as actin filaments that are not associated with myosin, i.e. the non-muscle actin cytoskeleton. However, the arrangement of the contractile filaments with respect to the overall cytoskeleton and the mechanism by which the non-muscle cytoskeleton is coupled to the contractile apparatus are still a matter of debate [174]. A differential subcellular distribution of α-smooth muscle and β-cytoplasmic actin in vascular smooth muscle [175] and a differential distribution for β-cytoplasmic actin from actomyosin containing contractile bundles in chicken gizzard smooth muscle [174] have been reported. A separation of cytoskeletal actin from actomyosin-containing contractile actin has been reported by using immunoprecipitation [176]. In contrast, others have argued against any actin isoform-specific domains in vascular smooth muscle [177–179]. It is quite likely that the connection between the two actin cytoskeletons is dynamic perhaps regulated by ‘a hierarchical slippage clutch’ that is, itself, regulated by signalling pathways [180].
Focal adhesion complexes are large structures, often a micron in size, containing, in different cell types, over a hundred proteins including integrin, actin, actin-binding proteins, protein kinases, and signalling proteins (Fig.6) [181]. At focal adhesions, the force generated by the contractile proteins is conveyed to the extracellular matrix (ECM) via integrin receptors. The integrin family consists of 18 α- and 8 β-subunits which can form 24 unique integrin heterodimers [182]. The short intracellular domains of integrins are indirectly connected to the actin cytoskeleton by several proteins such as talin, vinculin, paxillin and by these connections, the extracellular events can be communicated into the cell and vice versa [183]. Further details on this topic can be found in other recent review articles [181,183–185].
There are several reports indicating that the connection between the actin cytoskeleton and components of focal adhesion complexes are dynamic and are remodelled during agonist-induced activation in differentiated airway smooth muscle. Integrin mechanotransduction and consequent signal transduc-tion-dependent alterations in the adhesion plaque proteins, paxillin, talin, vinculin and FAK has been reported for both vascular and airway smooth muscle [163, 186–195].
Actin cytoskeletal remodelling also has been reported to occur in vascular smooth muscle [162, 164, 189, 190, 196], However, what has not been clear is exactly how, or if, cytoskeletal remodelling modulates contractility of smooth muscle. Different possible mechanisms have been suggested by different groups. Some groups have suggested that cytoskeletal remodelling may alter the transmission of cross-bridge generated force to the cell membrane [192]. Dynamic cytoskeletal processes within the cell may enhance the strength of the connections between membrane adhesion junctions and actin filament within the contractile apparatus and cytoskeletal network, thus providing a strong and rigid framework for the transmission of force generated by the interaction of myosin and actin filaments to the outside of the cell [167]. However, Seowand his colleagues have published that the length of total contractile filament complex is changed by addition or removal of myofilament units, suggesting the possibility of dynamic remodelling in actomyosin containing myofilament [197–202]. Other groups have suggested that cytoskeletal remodelling modulates force maintenance or optimizes the energetic cost of tension in the vascular smooth muscle cell [203].
Recently, we found that the actin cytoskeleton in vascular smooth muscle is differentially remodelled in a stimulus- and pathway-dependent manner. Interestingly, γ-actin, the least abundant actin isoform in vascular smooth muscle, is the most dynamically remodelled by α-adrenergic receptor stimulation [164]. This result suggests that the non-muscle actin cytoskeleton is more dynamic than the actin cytoskeleton in contractile apparatus. The reorganization of the cytoskeleton was shown to correlate with mechanisms for regulation of smooth muscle contraction.
To understand the functional specificity of individual actin iso-forms and to identify the isoforms related diseases, several investigations utilized manipulation of the isoform-specific genes [204–206]. Ablation of γ-cytoplasmic actin in skeletal muscle causes progressive muscle necrosis and regeneration in mouse [207–209]. Vascular smooth muscle contractility and blood pressure homeostasis are impaired in the a smooth muscle actin null mouse [206].
It is conceivable that abnormal contractility in vascular smooth muscle may result from the perturbation of normal cytoskeletal remodelling processes. Recently, Guo et al.[210] published that 14% of inherited familial ascending thoracic aorta aneurysms leading to acute aortic dissections are caused by missense mutations in ACTA2 (encodes α smooth muscle actin). By using structural and immunofluorescence analysis, this study show that interference with actin filament polymerization caused by this mutation leads to impaired smooth muscle contraction [210]. There is much experimental evidence supporting the concept that remodelling of the actin cytoskeleton is a crucial regulator of smooth muscle function. However, much remains to be determined regarding the mechanistic basis for the actin cytoskeletal remodelling in smooth muscle.
Conclusions
Undoubtedly, the information above has communicated the concept that smooth muscle ‘excitation-contraction coupling’ consists of far more than a simple calcium switch. Although the complexity of the multitude of, apparently, redundant signalling pathways which regulate smooth muscle contractility can be overwhelming, clearly the importance of proper functioning of smooth muscle systems requires the fine tuning made possible by this sort of complex system.
The importance of pathways that regulate plasticity of the cytoskeleton and those that regulate the availability of actin to interact with myosin is only now becoming clear. Similarly, the relative importance of the mix of pathways that regulate myosin phosphorylation levels in dSM tissues is just now playing out. The good news is that this complex network of pathways offers a multitude of possible leads for novel drug targets, with the hundreds of proteins present in adhesion plaques representing, potentially, quite accessible targets. Added to this the well-known tissue- and organ-specific nature of smooth muscle signalling molecules and pathways, it is feasible that such targets could lead to quite specific, as well as effective, new therapeutics.
Acknowledgments
We are grateful for all the excellent previous articles that have helped shape this review and we also apologize to those authors whose articles were not cited due to space limitations. The present work is supported by National Heart, Lung, and Blood Institute Grants HL-86655, HL-80003 and HL-31704 and by National Institute of Child Health and Human Development Grant HD-043054 (to K.G.M.).
References
- 1.Krelpke CW, Morgan R, Roberts G, Bagchi M, Rafols JA. Calponin phosphorylation in cerebral cortex microvessels mediates sustained vasoconstriction after brain trauma. Neurol Res. 2007;29:369–74. doi: 10.1179/016164107X204684. [DOI] [PubMed] [Google Scholar]
- 2.Kim I, Leinweber BD, Morgalla M, Butler WE, Seto M, Sasaki Y, Peterson JW, Morgan KG. Thin and thick filament regulation of contractility in experimental cerebral vasospasm. Neurosurgery. 2000;46:440–7. doi: 10.1097/00006123-200002000-00033. [DOI] [PubMed] [Google Scholar]
- 3.Fukumoto Y. Tawara S, Shlmokawa H. Recent progress in the treatment of pulmonary arterial hypertension: expectation for rho-kinase inhibitors. Tohoku J Exp Med. 2007;211:309–20. doi: 10.1620/tjem.211.309. [DOI] [PubMed] [Google Scholar]
- 4.Lì Y, Je H-D, Malek S, Morgan KG. ERK1/2-mediated phosphorylation of myometrial caldesmon during pregnancy and labor. Am J Physiol Regul Integr Comp Physiol. 2003;284:R192–9. doi: 10.1152/ajpregu.00290.2002. [DOI] [PubMed] [Google Scholar]
- 5.An SS, Bai TR, Bates JH, Black JL, Brown RH, Brusasco V, Chltano P, Deng L, Dowell M, Eidelman DH, Fabry B, Falrbank NJ, Ford LE, Fredberg JJ, Gerthoffer WT, Gilbert SH, Gosens R, Gunst SJ, Halayko AJ, Ingram RH, Irvin CG, James AL, Janssen LJ, King GG, Knight DA, Lauzon AM, Lakser OJ, Ludwlg MS, Lutchen KR, Maksym GN, Martin JG, Mauad T, McParland BE, Mljalloviacute;ch SM, Mitchell HW, Mitchell RW, Mltzner W, Murphy TM, Pare PD, Pellegrlno R, Sanderson MJ, Schellenberg RR, Seow CY, Sllvelra PS, Smith PG, Solway J, Stephens NL, Sterk PJ, Stewart AG, Tang DD, Tepper RS, Tran T, Wang L. Airway smooth muscle dynamics: a common pathway of airway obstruction in asthma. Eur Respir J. 2007;29:834–60. doi: 10.1183/09031936.00112606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sobleszek A. Vertebrate smooth muscle myosin: Enzymatic and structural properties (Winnipeg Symposium August 1975) Biochem Smooth Muscle. 1977:413–43. [Google Scholar]
- 7.Kamm KE, Stull JT. The function of myosin and myosin light chain kinase phosphorylation in smooth muscle. Ann Rev Pharmacol Toxicol. 1985;25:593–620. doi: 10.1146/annurev.pa.25.040185.003113. [DOI] [PubMed] [Google Scholar]
- 8.Morgan JP, Morgan KG. Vascular smooth muscle: the first recorded Ca2+ transients. Pflug Archiv. 1982;395:75–7. doi: 10.1007/BF00584972. [DOI] [PubMed] [Google Scholar]
- 9.Morgan JP, DeFeo TT, Morgan KG. A chemical procedure for loading the calcium indicator aequorin into mammalian working myocardium. PflugArchiv. 1984;400:338–40. doi: 10.1007/BF00581571. [DOI] [PubMed] [Google Scholar]
- 10.Hlmpens B, Matthljs G, Somlyo AV, Butler TM, Somlyo AP. Cytoplasmic free calcium, myosin light chain phosphorylation, and force in phasic and tonic smooth muscle. J Gen Physiol. 1988;92:713–29. doi: 10.1085/jgp.92.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brozovlch FV, Walsh MP, Morgan KG. Regulation of force in skinned, single cells of ferret aortic smooth muscle. Pflug Arch. 1990;416:742–9. doi: 10.1007/BF00370624. [DOI] [PubMed] [Google Scholar]
- 12.Collins EM, Walsh MP, Morgan KG. Contraction of single vascular smooth muscle cells by phenylephrine at constant [Ca2+]i. Am J Physiol Heart Circ Physiol. 1992;262:H754–62. doi: 10.1152/ajpheart.1992.262.3.H754. [DOI] [PubMed] [Google Scholar]
- 13.Nlshlmura J, Kolber M, van Breemen C. Norepinephrine and GTP-gamma-S increase myofilament Ca2 +sensitivity in alpha-toxin permeabilized arterial smooth muscle. Biochem Biophys Res Commun. 1988;157:677–83. doi: 10.1016/s0006-291x(88)80303-6. [DOI] [PubMed] [Google Scholar]
- 14.Moreland S, Moreland RS. Effects of dihydropyridines on stress, myosin phosphorylation, and V0 in smooth muscle. Am J Physiol. 1987;252:H1049–58. doi: 10.1152/ajpheart.1987.252.6.H1049. [DOI] [PubMed] [Google Scholar]
- 15.Moreland SJ, Nlshlmura J, Van Breemen C, Ahn HY, Moreland RS. Transient myosin phosphorylation at constant Ca2+ during agonist activation of permeabilized arteries. Am J Physiol. 1992;263:C540–4. doi: 10.1152/ajpcell.1992.263.2.C540. [DOI] [PubMed] [Google Scholar]
- 16.Katsuyama H, Wang CL, Morgan KG. Regulation of vascular smooth muscle tone by caldesmon. J Biol Chem. 1992;267:14555–8. [PubMed] [Google Scholar]
- 17.Bradley AB, Morgan KG. Alteration in cytoplasmic calcium sensitivity during porcine coronary artery contractions as detected by aequorin. J Physiol (Lond) 1987;385:437–48. doi: 10.1113/jphysiol.1987.sp016500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sward K, Mlta M, Wilson DP, Deng JT, Susnjar M, Walsh MP. The role of RhoA and Rho-associated kinase in vascular smooth muscle contraction. Curr Hypertens Rep. 2003;5:66–72. doi: 10.1007/s11906-003-0013-1. [DOI] [PubMed] [Google Scholar]
- 19.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–58. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- 20.Kim I, Je H-D, Gallant C, Zhan Q, Van Riper D, Badwey JA, Singer HA, Morgan KG. Ca2+-calmodulin-dependent protein kinase ll-dependent activation of contractility in ferret aorta. J Physiol. 2000;526:367–74. doi: 10.1111/j.1469-7793.2000.00367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen DHD, Catling AD, Webb DJ, Sankovlc M, Walker LA, Somlyo AV, Weber MJ, Gonlas SL. Myosin light chain kinase functions downstream of Ras/ERK to promote migration of urokinase-type plasminogen activator-stimulated cells in an integrin-selective manner. J Cell Biol. 1999;146:149–64. doi: 10.1083/jcb.146.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glnnan R, Singer HA. CaM kinase ll-dependent activation of tyrosine kinases and ERK1/2 in vascular smooth muscle. Am J Physiol Cell Physiol. 2002;282:C754–61. doi: 10.1152/ajpcell.00335.2001. [DOI] [PubMed] [Google Scholar]
- 23.Jiang MJ, Morgan KG. Agonist-specific myosin phosphorylation and intracellular calcium during isometric contractions of arterial smooth muscle. Pflug Archiv. 1989;413:637–43. doi: 10.1007/BF00581814. [DOI] [PubMed] [Google Scholar]
- 24.Morgan KG, Gangopadhyay SS. Invited Review: Cross-bridge regulation by thin filament-associated proteins. J Appl Physiol. 2001;91:953–62. doi: 10.1152/jappl.2001.91.2.953. [DOI] [PubMed] [Google Scholar]
- 25.L Y, Gallant C, Malek S, Morgan KG. Focal adhesion signaling is required for myometrial ERK activation and contractile phenotype switch before labor. J Cell Biochem. 2007;100:129–40. doi: 10.1002/jcb.21033. [DOI] [PubMed] [Google Scholar]
- 26.Kurelshl Y, Kobayashi S, Amano M, Klmura K, Kanalde H, Nakano T, Kalbuchi K, Ito M. Rho-associated kinase directly induces smooth muscle contraction through myosin light chain phosphorylation. J Biol Chem. 1997;272:12257–60. doi: 10.1074/jbc.272.19.12257. [DOI] [PubMed] [Google Scholar]
- 27.Deng JT, Van Llerop JE, Sutherland C, Walsh MP. Ca2+-independent smooth muscle contraction, a novel function for integrin-linked kinase. J Biol Chem. 2001;276:16365–73. doi: 10.1074/jbc.M011634200. [DOI] [PubMed] [Google Scholar]
- 28.Kogel D, Plottner O, Landsberg G, Christian S, Scheldtmann KH. Cloning and characterization of Dlk, a novel ser-ine/threonine kinase that is tightly associated with chromatin and phosphorylates core histones. Oncogene. 1998;17:2645–54. doi: 10.1038/sj.onc.1202204. [DOI] [PubMed] [Google Scholar]
- 29.Murata-Horl M, Sulzu F, Iwasakl T, Klkuchl A, Hosoya H. ZIP kinase identified as a novel myosin regulatory light chain kinase in HeLa cells. FEBS Lett. 1999;451:81–4. doi: 10.1016/s0014-5793(99)00550-5. [DOI] [PubMed] [Google Scholar]
- 30.Braun AP, Schulman H. The multifunctional calcium/calmodulin-dependent protein kinase: from form to function. Annu Rev Physiol. 1995;57:417–45. doi: 10.1146/annurev.ph.57.030195.002221. [DOI] [PubMed] [Google Scholar]
- 31.Weber LP, Van Llerop JE, Walsh MP. Ca2+-independent phosphorylation of myosin in rat caudal artery and chicken gizzard myofilaments. J Physiol. 1999;516:805–24. doi: 10.1111/j.1469-7793.1999.0805u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herring BP, El-Mounayr IO, Gallagher PJ, Yin F, Zhou J. Regulation of myosin light chain kinase and telokin expression in smooth muscle tissues. Am J Physiol Cell Physiol. 2006;291:C817–27. doi: 10.1152/ajpcell.00198.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen YH, Chen MX, Alessl DR, Campbell DG, Shanahan C, Cohen P, Cohen PT. Molecular cloning of cDNA encoding the 110 kDa and 21 kDa regulatory subunits of smooth muscle protein phosphatase 1M. FEBS Lett. 1994;356:51–5. doi: 10.1016/0014-5793(94)01231-8. [DOI] [PubMed] [Google Scholar]
- 34.Dlrksen WP, Vladlc F, Fisher SA. A myosin phosphatase targeting subunit iso-form transition defines a smooth muscle developmental phenotypic switch. Am J Physiol Cell Physiol. 2000;278:C589–600. doi: 10.1152/ajpcell.2000.278.3.C589. [DOI] [PubMed] [Google Scholar]
- 35.Hartshorne DJ, Ito M, Erdodl F. Myosin light chain phosphatase: subunit composition, interactions and regulation. J Muscle Res Cell Motil. 1998;19:325–41. doi: 10.1023/a:1005385302064. [DOI] [PubMed] [Google Scholar]
- 36.Khatrl JJ, Joyce KM, Brozovlch FV, Fisher SA. Role of myosin phosphatase isoforms in cGMP-mediated smooth muscle relaxation. J Biol Chem. 2001;276:37250–7. doi: 10.1074/jbc.M105275200. [DOI] [PubMed] [Google Scholar]
- 37.Shimizu H, Ito M, Mlyahara M, Ichlkawa K, Okubo S, Konlshl T, Naka M, Tanaka T, Hlrano K, Hartshorne DJ, Nakano T. Characterization of the myosin-binding sub-unit of smooth muscle myosin phosphatase. J Biol Chem. 1994;269:30407–11. [PubMed] [Google Scholar]
- 38.Alessl D, MacDougall LK, Sola MM, Ikebe M, Cohen P. The control of protein phosphatase-1 by targeting subunits. The major myosin phosphatase in avian smooth muscle is a novel form of protein phosphatase-1. Eur JBiochem. 1992;210:1023–35. doi: 10.1111/j.1432-1033.1992.tb17508.x. [DOI] [PubMed] [Google Scholar]
- 39.Shorazl A, lizuka K, Fadden P, Mosse C, Somlyo AP, Somlyo AV, Haystead TA. Purification and characterization of the mammalian myosin light chain phosphatase holoenzyme. The differential effects of the holoenzyme and its subunits on smooth muscle. J Biol Chem. 1994;269:31598–606. [PubMed] [Google Scholar]
- 40.Feng J, Ito M, Ichlkawa K, Isaka N, Nishikawa M, Hartshorne DJ, Nakano T. Inhibitory phosphorylation site for Rho-associated kinase on smooth muscle myosin phosphatase. J Biol Chem. 1999;274:37385–90. doi: 10.1074/jbc.274.52.37385. [DOI] [PubMed] [Google Scholar]
- 41.Klmura K, Ito M, Amano M, Chlhara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kalbuchl K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–8. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 42.Wooldrldge AA, MacDonald JA, Erdodi F, Ma C, Borman MA, Hartshorne DJ, Haystead TA. Smooth muscle phosphatase is regulated in vivo by exclusion of phosphorylation of threonine 696 of MYPT1 by phosphorylation of Serine 695 in response to cyclic nucleotides. J Biol Chem. 2004;279:34496–504. doi: 10.1074/jbc.M405957200. [DOI] [PubMed] [Google Scholar]
- 43.Eto M, Ohmorl T, Suzuki M, Furuya K, Morita F. A novel protein phosphatase-1 inhibitory protein potentiated by protein kinase C Isolation from porcine aorta media and characterization. J Biochem. 1995;118:1104–7. doi: 10.1093/oxfordjournals.jbchem.a124993. [DOI] [PubMed] [Google Scholar]
- 44.Koga Y, Ikebe M. p116Rip decreases myosin II phosphorylation by activating myosin light chain phosphatase and by inactivating RhoA. J Biol Chem. 2005;280:4983–91. doi: 10.1074/jbc.M410909200. [DOI] [PubMed] [Google Scholar]
- 45.Mulder J, Ariaens A, van den Boomen D, Moolenaar WH. p116Rip targets myosin phosphatase to the actin cytoskeleton and is essential for RhoA/ROCK-regulated neu-ritogenesis. Mol Biol Cell. 2004;15:5516–27. doi: 10.1091/mbc.E04-04-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rlddlck N, Ohtani K, Surks HK. Targeting by myosin phosphatase-RhoA interacting protein mediates RhoA/ROCK regulation of myosin phosphatase. J Cell Biochem. 2008;103:1158–70. doi: 10.1002/jcb.21488. [DOI] [PubMed] [Google Scholar]
- 47.Surks HK, Richards CT, Mendelsohn ME. Myosin phosphatase-Rho interacting protein. A new member of the myosin phosphatase complex that directly binds RhoA. J Biol Chem. 2003;278:51484–93. doi: 10.1074/jbc.M305622200. [DOI] [PubMed] [Google Scholar]
- 48.Surks HK, Rlddlck N, Ohtanl K. M-RIP targets myosin phosphatase to stress fibers to regulate myosin light chain phosphorylation in vascular smooth muscle cells. J Biol Chem. 2005;280:42543–51. doi: 10.1074/jbc.M506863200. [DOI] [PubMed] [Google Scholar]
- 49.Endo A, Surks HK, Mochizuki S, Mochlzuki N, Mendelsohn ME. Identification and characterization of zipper-interacting protein kinase as the unique vascular smooth muscle myosin phosphatase-associated kinase. J Biol Chem. 2004;279:42055–61. doi: 10.1074/jbc.M403676200. [DOI] [PubMed] [Google Scholar]
- 50.Hagerty L, Weltzel DH, Chambers J, Fortner CN, Brush MH, Loiselle D, Hosoya H, Haystead TA. R0CK1 phospho-rylates and activates zipper-interacting protein kinase. J Biol Chem. 2007;282:4884–93. doi: 10.1074/jbc.M609990200. [DOI] [PubMed] [Google Scholar]
- 51.MacDonald JA, Borman MA, Muranyi A, Somlyo AV, Hartshorne DJ, Haystead TA. Identification of the endogenous smooth muscle myosin phosphatase-associated kinase. Proc Natl Acad Sci USA. 2001;98:2419–24. doi: 10.1073/pnas.041331498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.LI L, Eto M, Lee MR, Morita F, Yazawa M, Kltazawa T. Possible involvement of the novel CPI-17 protein in protein kinase C signal transduction of rabbit arterial smooth muscle. J Physiol. 1998;508:871–81. doi: 10.1111/j.1469-7793.1998.871bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deng JT, Sutherland C, Brautigan DL, Eto M, Walsh MP. Phosphorylation of the myosin phosphatase inhibitors, CPI-17 and PHI-1, by integrin-linked kinase. Biochem J. 2002;367:517–24. doi: 10.1042/BJ20020522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.MacDonald JA, Eto M, Borman MA, Brautigan DL, Haystead TA. Dual Ser and Thr phosphorylation of CPI-17, an inhibitor of myosin phosphatase, by MYPT-associated kinase. FEBS Lett. 2001;493:91–4. doi: 10.1016/s0014-5793(01)02277-3. [DOI] [PubMed] [Google Scholar]
- 55.Dimopoulos GJ, Semba S, Kitazawa K, Eto M, Kitazawa T. Ca2+-dependent rapid Ca2+ sensitization of contraction in arterial smooth muscle. Circ Res. 2007;100:121–9. doi: 10.1161/01.RES.0000253902.90489.df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lincoln TM. Myosin phosphatase regulatory pathways: different functions or redundant functions? Circ Res. 2007;100:10–2. doi: 10.1161/01.RES.0000255894.25293.82. [DOI] [PubMed] [Google Scholar]
- 57.Sauzeau V, Le Jeune H, Carlo-Toumaniantz C, Smolenski A, Lohmann SM, Bertoglio J, Chardln P, Pacaud P, Loirand G. Cyclic GMP-dependent protein kinase signaling pathway inhibits RhoA-induced Ca2+ sensitization of contraction in vascular smooth muscle. J Biol Chem. 2000;275:21722–9. doi: 10.1074/jbc.M000753200. [DOI] [PubMed] [Google Scholar]
- 58.Sawada N, Itoh H, Yamashlta J, Dol K, Inoue M, Masatsugu K, Fukunaga Y, Sakaguchi S, Sone M, Yamahara K, Yurugi T, Nakao K. cGMP-dependent protein kinase phosphorylates and inactivates RhoA. Biochem Biophys Res Commun. 2001;280:798–805. doi: 10.1006/bbrc.2000.4194. [DOI] [PubMed] [Google Scholar]
- 59.Nakamura M, Ichikawa K, Ito M, Yamamori B, Okinaka T, Isaka N, Yoshida Y, Fujita S, Nakano T. Effects of the phosphorylation of myosin phosphatase by cyclic GMP-dependent protein kinase. Cell Signal. 1999;11:671–6. doi: 10.1016/s0898-6568(99)00036-4. [DOI] [PubMed] [Google Scholar]
- 60.Huang QQ, Fisher SA, Brozovlch FV. Unzipping the role of myosin light chain phosphatase in smooth muscle cell relaxation. J Biol Chem. 2004;279:597–603. doi: 10.1074/jbc.M308496200. [DOI] [PubMed] [Google Scholar]
- 61.Lee E, Hayes DB, Langsetmo K, Sundberg EJ, Tao TC. Interactions between the leucine-zipper motif of cGMP-dependent protein kinase and the C-termi-nal region of the targeting subunit of myosin light chain phosphatase. J Mol Biol. 2007;373:1198–212. doi: 10.1016/j.jmb.2007.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Surks HK, Mochizukl N, Kasai Y, Georgescu SP, Tang KM, Ito M, Lincoln TM, Mendelsohn ME. Regulation of myosin phosphatase by a specific interaction with cGMP- dependent protein kinase lalpha. Science. 1999;286:1583–7. doi: 10.1126/science.286.5444.1583. [DOI] [PubMed] [Google Scholar]
- 63.Illi B, Dello Russo C, Colussi C, Rodati J, Pallaoro M, Spallotta F, Rotili D, Valente S, Ragone G, Martelli F, Biglioli P, Steinkuhler C, Gallinari P, Mai A, Capogrossi MC, Gaetano C. Nitric oxide modulates chromatin folding in human endothelial cells via protein phosphatase 2A activation and class II histone deacetylases nuclear shuttling. Circ Res. 2008;102:51–8. doi: 10.1161/CIRCRESAHA.107.157305. [DOI] [PubMed] [Google Scholar]
- 64.Bonnevier J, Arner A. Actions downstream of cyclic GMP/protein kinase G can reverse protein kinase C-mediated phosphorylation of CPI-17 and Ca(2+) sensitization in smooth muscle. J Biol Chem. 2004;279:28998–9003. doi: 10.1074/jbc.M404259200. [DOI] [PubMed] [Google Scholar]
- 65.Takizawa N, Niiro N, Ikebe M. Dephosphorylation of the two regulatory components of myosin phosphatase, MBS and CPI17. FEBS Lett. 2002;515:127–32. doi: 10.1016/s0014-5793(02)02451-1. [DOI] [PubMed] [Google Scholar]
- 66.Wardle RL, Gu M, Ishida Y, Paul RJ. Ca2+-desensitizing hypoxic vasorelaxation: pivotal role for the myosin binding subunit of myosin phosphatase (MYPT1) in porcine coronary artery. J Physiol. 2006;572:259–67. doi: 10.1113/jphysiol.2005.104083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morlshlta T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–4. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 68.Dakshinamurtl S, Mellow L, Stephens NL. Regulation of pulmonary arterial myosin phosphatase activity in neonatal circulatory transition and in hypoxic pulmonary hypertension: a role for CPI-17. Pediatr Pulinonol. 2005;40:398–407. doi: 10.1002/ppul.20290. [DOI] [PubMed] [Google Scholar]
- 69.Gangopadhyay SS, Barber AL, Gallant C, Grabarek Z, Smith JL, Morgan KG. Differential functional properties of calmodulin-dependent protein kinase ligamma variants isolated from smooth muscle. Biochein J. 2003;372:347–57. doi: 10.1042/BJ20030015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gaertner TR, Kolodzlej SJ, Wang D, Kobayashl R, Koomen JM, Stoops JK, Waxham MN. Comparative analyses of the three-dimensional structures and enzymatic properties of alpha, beta, gamma and delta isoforms of Ca2+-calmodulin-dependent protein kinase II. J Biol Chem. 2004;279:12484–94. doi: 10.1074/jbc.M313597200. [DOI] [PubMed] [Google Scholar]
- 71.Rosenberg OS, Delndl S, Sung RJ, Nairn AC, Kurlyan J. Structure of the autoinhibited kinase domain of CaMKII and SAXS analysis of the holoenzyme. Cell. 2005;123:849–60. doi: 10.1016/j.cell.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 72.Miller SG, Patton BL, Kennedy MB. Sequences of autophosphorylation sites in neuronal type II CaM kinase that control Ca2(+)-independent activity. Neuron. 1988;1:593–604. doi: 10.1016/0896-6273(88)90109-2. [DOI] [PubMed] [Google Scholar]
- 73.Hanson PI, Schulman H. Inhibitory autophosphorylation of multifunctional Ca2+/calmodulin-dependent protein kinase analyzed by site-directed mutagenesis. J Biol Chem. 1992;267:17216–24. [PubMed] [Google Scholar]
- 74.Schworer CM, Colbran RJ, Keefer JR, Soderllng TR. Ca2+/calmodulin-dependent protein kinase II. Identification of a regulatory autophosphorylation site adjacent to the inhibitory and calmodulin-binding domains. J Biol Chem. 1988;263:13486–9. [PubMed] [Google Scholar]
- 75.De Konlnck P, Schulman H. Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations. Science. 1998;279:227–30. doi: 10.1126/science.279.5348.227. [DOI] [PubMed] [Google Scholar]
- 76.Frankland PW, O'Brien C, Ohno M, Klrkwood A, Sllva AJ. Alpha-CaMKII-dependent plasticity in the cortex is required for permanent memory. Nature. 2001;411:309–13. doi: 10.1038/35077089. [DOI] [PubMed] [Google Scholar]
- 77.Munevar S, Gangopadhyay SS, Gallant C, Colombo B, Sellke FW, Morgan KG. CaMKIIT287 and T305 regulate history-dependent increases in alpha agonist-induced vascular tone. J Cell Mol Med. 2008;12:219–26. doi: 10.1111/j.1582-4934.2007.00202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Patton BL, Miller SG, Kennedy MB. Activation of type II calcium/calmodulin-dependent protein kinase by Ca2+/ calmodulin is inhibited by autophosphorylation of threonine within the calmodulin-binding domain. J Biol Chem. 1990;265:11204–12. [PubMed] [Google Scholar]
- 79.Colbran RJ, Soderllng TR. Calcium/ calmodulin-independent autophosphorylation sites of calcium/calmodulin-dependent protein kinase II. Studies on the effect of phosphorylation of threonine 305/306 and serine 314 on calmodulin binding using synthetic peptides. J Biol Chem. 1990;265:11213–9. [PubMed] [Google Scholar]
- 80.Rokolya A, Singer HA. Inhibition of CaM kinase II activation and force maintenance by KN-93 in arterial smooth muscle. Am J Physiol Cell Physiol. 2000;278:C537–45. doi: 10.1152/ajpcell.2000.278.3.C537. [DOI] [PubMed] [Google Scholar]
- 81.Marganskl WA, Gangopadhyay SS, Je H-D, Gallant C, Morgan KG. Targeting of a novel Ca+2/calmodulin-dependent protein kinase II is essential for extracellular signal-regulated kinase-mediated signaling in differentiated smooth muscle cells. Circ Res. 2005;97:541–9. doi: 10.1161/01.RES.0000182630.29093.0d. [DOI] [PubMed] [Google Scholar]
- 82.Ikebe M, Reardon S. Phosphorylation of smooth myosin light chain kinase by smooth muscle Ca2+/calmodulin-dependent multifunctional protein kinase. J Biol Chem. 1990;265:8975–8. [PubMed] [Google Scholar]
- 83.Hashimoto Y, Soderllng TR. Phosphorylation of smooth muscle myosin light chain kinase by Ca2+/calmodulin-dependent protein kinase II: comparative study of the phosphorylation sites. Arch Biochem Biophys. 1990;278:41–5. doi: 10.1016/0003-9861(90)90228-q. [DOI] [PubMed] [Google Scholar]
- 84.Edelman AM, Lin W-H, Osterhout DJ, Bennett MK, Kennedy MB, Krebs EG. Phosphorylation of smooth muscle myosin by type II Ca2+/calmodulin-dependent protein kinase. Mol Cell Biochem. 1990;97:87–98. doi: 10.1007/BF00231704. [DOI] [PubMed] [Google Scholar]
- 85.Ngai PK, Walsh MP. Inhibition of smooth muscle actin-activated myosin Mg2+-ATPase activity by caldesmon. J Biol Chem. 1984;259:13656–9. [PubMed] [Google Scholar]
- 86.Ikebe M, Reardon S. Phosphorylation of smooth muscle caldesmon by calmodulin-dependent protein kinase II. J Biol Chem. 1990;265:17607–12. [PubMed] [Google Scholar]
- 87.Winder SJ, Walsh MP. Calponin: thin filament-linked regulation of smooth muscle contraction. Cell Signal. 1993;5:677–86. doi: 10.1016/0898-6568(93)90029-l. [DOI] [PubMed] [Google Scholar]
- 88.Muthalif MM, Hefner Y, Canaan S, Harper J, Zhou H, Parmentler JH, Aebersold R, Gelb MH, Malik KU. Functional interaction of calcium-/calmodulin-dependent protein kinase II and cytosolic phospholipase A(2) J Biol Chem. 2001;276:39653–60. doi: 10.1074/jbc.M103136200. [DOI] [PubMed] [Google Scholar]
- 89.Wolfe JT, Wang H, Perez-Reyes E, Barrett PQ. Stimulation of recombinant Ca(v)3.2, T-type, Ca(2+) channel currents by CaMKIIgamma(C) J Physiol. 2002;538:343–55. doi: 10.1113/jphysiol.2001.012839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ledoux J, Chartier D, Leblanc N. Inhibitors of calmodulin-dependent protein kinase are nonspecific blockers of voltage-dependent K+ channels in vascular myocytes. J Pharmacol Exp Ther. 1999;290:1165–74. [PubMed] [Google Scholar]
- 91.McCarron JG, McGeown JG, Reardon S, Ikebe M, Fay FS, Walsh JV., Jr Calcium-dependent enhancement of calcium current in smooth muscle by calmodulin-dependent protein kinase II. Nature. 1992;357:74–7. doi: 10.1038/357074a0. [DOI] [PubMed] [Google Scholar]
- 92.Greenwood IA, Ledoux J, Leblanc N. Differential regulation of Ca(2+(-activated Cl(-) currents in rabbit arterial and portal vein smooth muscle cells by Ca(2+)-calmodulin-dependent kinase. J Physiol. 2001;534:395–408. doi: 10.1111/j.1469-7793.2001.00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cartln L, Lounsbury KM, Nelson MT. Coupling of Ca(2+) to CREB activation and gene expression in intact cerebral arteries from mouse: roles of ryanodine receptors and voltage- dependent Ca(2+) channels. Circ Res. 2000;86:760–7. doi: 10.1161/01.res.86.7.760. [DOI] [PubMed] [Google Scholar]
- 94.Chen W, Lah M, Robinson PJ, Kemp BE. Phosphorylation of phospholamban in aortic smooth muscle cells and heart by cal-cium/calmodulin-dependent protein kinase II. Cell Signal. 1994;6:617–30. doi: 10.1016/0898-6568(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 95.Abraham ST, Benscoter H, Schworer CM, Singer HA. A role for Ca2+/calmoduin-dependent protein kinase II in the mito-gen-activated protein kinase signaling cascade of cultured rat aortic vascular smooth muscle cells. Circ Res. 1997;81:575–84. doi: 10.1161/01.res.81.4.575. [DOI] [PubMed] [Google Scholar]
- 96.Gangopadhyay SS, Gallant C, Sundberg EJ, Lane WS, Morgan KG. Regulation of Ca2+/calmodulin kinase II by a small C-terminal domain phosphatase. Biochem J. 2008;412:507–16. doi: 10.1042/BJ20071582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pflelderer PJ, Lu KK, Crow MT, Keller RS, Singer HA. Modulation of Vascular Smooth Muscle Cell Migration by CaM Kinase ll-{delta}2. Am J Physiol Cell Physiol. 2004;286:C1238–45. doi: 10.1152/ajpcell.00536.2003. [DOI] [PubMed] [Google Scholar]
- 98.Mercure MZ, Ginnan R, Singer HA. CaM kinase II delta2-dependent regulation of vascular smooth muscle cell polarization and migration. Am J Physiol Cell Physiol. 2008;294:C1465–75. doi: 10.1152/ajpcell.90638.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.House SJ, Singer HA. CaMKII-delta iso-form regulation of neointima formation after vascular injury. Arterioscler Thromb Vasc Biol. 2008;28:441–7. doi: 10.1161/ATVBAHA.107.156810. [DOI] [PubMed] [Google Scholar]
- 100.Jones RJ, Jourd'heuil D, Salerno JC, Smith SM, Singer HA. iNOS regulation by calcium/calmodulin-dependent protein kinase II in vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2007;292:H2634–42. doi: 10.1152/ajpheart.01247.2006. [DOI] [PubMed] [Google Scholar]
- 101.Sobue K, Muramoto Y, Fujita M, Kakiuchi S. Purification of a calmodulin-binding protein from chicken gizzard that interacts with F-actin. Proc Natl Acad Sci USA. 1981;78:5652–5. doi: 10.1073/pnas.78.9.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang Z, Jiang H, Yang Z-Q, Chacko S. Both N-terminal myosin-binding and C-terminal actin-binding sites on smooth muscle caldesmon are required for caldesmon-mediated inhibition of actin filament velocity. Proc Natl Acad Sci USA. 1997;94:11899–904. doi: 10.1073/pnas.94.22.11899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Humphrey MB, Herrera-Sosa H, Gonzalez G, Lee R, Bryan J. Cloning of cDNAs encoding human caldesmons. Gene. 1992;112:197–204. doi: 10.1016/0378-1119(92)90376-z. [DOI] [PubMed] [Google Scholar]
- 104.Dabrowska R, Hinssen H, Gatazkiewicz B, Nowak E. Modulation of gelsolin-induced actin-filament severing by caldesmon and tropomyosin and the effect of these proteins on the actin activation of myosin Mg(2+)-ATPase activity. Biochem J. 1996;315:753–9. doi: 10.1042/bj3150753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yamakita Y, Oosawa F, Yamashiro S, Matsumura F. Caldesmon inhibits Arp2/3-mediated actin nucleation. J Biol Chem. 2003;278:17937–44. doi: 10.1074/jbc.M208739200. [DOI] [PubMed] [Google Scholar]
- 106.Marston SB, Redwood CS. Inhibition of actin-tropomyosin activation of myosin MgATPase activity by the smooth muscle regulatory protein caldesmon. J Biol Chem. 1992;267:16796–800. [PubMed] [Google Scholar]
- 107.Lee Y-H, Gallant C, Guo H, Li Y, Wang CL, Morgan KG. Regulation of vascular smooth muscle tone by N-terminal region of caldesmon: possible role of tethering actin to myosin. J Biol Chem. 2000;275:3213–20. doi: 10.1074/jbc.275.5.3213. [DOI] [PubMed] [Google Scholar]
- 108.Horiuchi KY, Miyata H, Chacko S. Modulation of smooth muscle actomyosin ATPase by thin filament associated proteins. Biochem Biophys Res Commun. 1986;136:962–8. doi: 10.1016/0006-291x(86)90426-2. [DOI] [PubMed] [Google Scholar]
- 109.Adam LP, Hathaway DR. Identification of mitogen-activated protein kinase phosphorylation sequences in mammalian h-Caldesmon. FEBS Letters. 1993;322:56–60. doi: 10.1016/0014-5793(93)81110-l. [DOI] [PubMed] [Google Scholar]
- 110.Yamashiro S, Yamakita Y, Hosoya H, Matsumura F. Phosphorylation of non-muscle caldesmon By P34cdc2 kinase during mitosis. Nature. 1991;349:169–72. doi: 10.1038/349169a0. [DOI] [PubMed] [Google Scholar]
- 111.Huang R, Li L, Guo H, Wang CL. Caldesmon binding to actin is regulated by calmodulin and phosphorylation via different mechanisms. Biochemistry. 2003;42:2513–23. doi: 10.1021/bi0268605. [DOI] [PubMed] [Google Scholar]
- 112.Yamakita Y, Yamashiro S, Matsumura F. Characterization of mitotically phosphorylated caldesmon. J Biol Chem. 1992;267:12022–9. [PubMed] [Google Scholar]
- 113.Kordowska J, Huang R, Wang CL. Phosphorylation of caldesmon during smooth muscle contraction and cell migration or proliferation. J Biomed Sci. 2006;13:159–72. doi: 10.1007/s11373-005-9060-8. [DOI] [PubMed] [Google Scholar]
- 114.Eves R, Webb BA, Zhou S, Mak AS. Caldesmon is an integral component of podosomes in smooth muscle cells. J Cell Sci. 2006;119:1691–702. doi: 10.1242/jcs.02881. [DOI] [PubMed] [Google Scholar]
- 115.Gu Z, Kordowska J, Williams GL, Wang CL, Hai C-M. Erk1/2 MAPK and caldesmon differentially regulate podosome dynamics in A7r5 vascular smooth muscle cells. Exp Cell Res. 2007;313:849–66. doi: 10.1016/j.yexcr.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Winder SJ, Walsh MP. Smooth muscle calponin. Inhibition of actomyosin MgATPase and regulation by phosphorylation. J Biol Chem. 1990;265:10148–55. [PubMed] [Google Scholar]
- 117.Takahashi K, Abe M, Hiwada K, Kokubu T. A novel troponin T-like protein (calponin) in vascular smooth muscle: interaction with tropomyosin paracrystals. J Hypertens Suppl. 1988;6:S40–3. doi: 10.1097/00004872-198812040-00008. [DOI] [PubMed] [Google Scholar]
- 118.Strasser P, Gimona M, Moessler H, Herzog M, Small JV. Mammalian calponin. Identification and expression of genetic variants. FEBS Lett. 1993;330:13–8. doi: 10.1016/0014-5793(93)80909-e. [DOI] [PubMed] [Google Scholar]
- 119.Applegate D, Feng W, Green RS, Taubman MB. Cloning and expression of a novel acidic calponin isoform from rat aortic vascular smooth muscle. J Biol Chem. 1994;269:10683–90. [PubMed] [Google Scholar]
- 120.Jin JP, Walsh MP, Resek ME, McMartin GA. Expression and epitopic conservation of calponin in different smooth muscles and during development. Biochem Cell Biol. 1996;74:187–96. doi: 10.1139/o96-019. [DOI] [PubMed] [Google Scholar]
- 121.Gimona M, Herzog M, Vandekerckhove J, Small JV. Smooth muscle specific expression of calponin. FEBS Lett. 1990;274:159–62. doi: 10.1016/0014-5793(90)81353-p. [DOI] [PubMed] [Google Scholar]
- 122.Hossain MM, Hwang DY, Huang QQ, Sasaki Y, Jin JP. Developmentally regulated expression of calponin isoforms and the effect of h2-calponin on cell proliferation. Am J Physiol Cell Physiol. 2003;284:C156–67. doi: 10.1152/ajpcell.00233.2002. [DOI] [PubMed] [Google Scholar]
- 123.Mezgueldi M, Mendre C, Calas B, Kassab R, Fattoum A. Characterization of the regulatory domain of gizzard calponin. Interactions of the 145–163 region with F-actin, calcium-binding proteins, and tropomyosin. J Biol Chem. 1995;270:8867–76. doi: 10.1074/jbc.270.15.8867. [DOI] [PubMed] [Google Scholar]
- 124.Mino T, Yuasa U, Nakamura F, Naka M, Tanaka T. Two distinct actin-binding sites of smooth muscle calponin. Eur J Biochem. 1998;251:262–8. doi: 10.1046/j.1432-1327.1998.2510262.x. [DOI] [PubMed] [Google Scholar]
- 125.Bogatcheva NV, Gusev NB. Interaction of smooth muscle calponin with phospholipids. FFSS Leff. 1995;371:123–6. doi: 10.1016/0014-5793(95)00868-a. [DOI] [PubMed] [Google Scholar]
- 126.Leinweber BD, Leavis PC, Grabarek Z, Wang CL, Morgan KG. Extracellular regulated kinase (ERK) interaction with actin and the calponin homology (CH) domain of actin-binding proteins. Biochem J. 1999;344:117–23. [PMC free article] [PubMed] [Google Scholar]
- 127.Gimona M, Djinovic-Carugo K, Kranewitter WJ, Winder SJ. Functional plasticity of CH domains. FEBS Letters. 2002;513:98–106. doi: 10.1016/s0014-5793(01)03240-9. [DOI] [PubMed] [Google Scholar]
- 128.Leinweber B, Parissenti AM, Gallant C, Gangopadhyay SS, Kirwan-Rhude A, Leavis PC, Morgan KG. Regulation of protein kinase C by the cytoskeletal protein calponin. J Biol Chem. 2000;275:40329–36. doi: 10.1074/jbc.M008257200. [DOI] [PubMed] [Google Scholar]
- 129.Winder SJ, Allen BG, Fraser ED, Kang HM, Kargacin GJ, Walsh MP. Calponin phosphorylation vitro and in intact muscle. Biochem J. 1993;296:827–36. doi: 10.1042/bj2960827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nakamura F, Mino T, Yamamoto J, Naka M, Tanaka T. Identification of the regulatory site in smooth muscle calponin that is phosphorylated by protein kinase C. J Biol Chem. 1993;268:6194–201. [PubMed] [Google Scholar]
- 131.Shirinsky VP, Biryukov KG, Hettasch JM, Sellers JR. Inhibition of the relative movement of actin and myosin by caldesmon and calponin. J Biol Chem. 1992;267:15886–92. [PubMed] [Google Scholar]
- 132.Menice CB, Hulvershorn J, Adam LP, Wang CL, Morgan KG. Calponin and mitogen-activated protein kinase signaling in differentiated vascular smooth muscle. J Biol Chem. 1997;272:25157–61. doi: 10.1074/jbc.272.40.25157. [DOI] [PubMed] [Google Scholar]
- 133.Je H-D, Gangopadhyay SS, Ashworth TD, Morgan KG. Calponin is required for agonist-induced signal transduction-evidence from an antisense approach in ferret smooth muscle. J Physiol. 2001;537:567–77. doi: 10.1111/j.1469-7793.2001.00567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Khokhlatchev AV, Canagarajah B, Wilsbacher J, Robinson M, Atkinson M, Goldsmith E, Cobb MH. Phosphorylation of the MAP kinase ERK2 promotes its homodimerization and nuclear translocation. Cell. 1998;93:605–15. doi: 10.1016/s0092-8674(00)81189-7. [DOI] [PubMed] [Google Scholar]
- 135.English J, Pearson G, Wllsbacher J, Swantek J, Karandlkar M, Schulchan X, Cobb MH. New insights into the control of MAP kinase pathways. Exp Cell Res. 1999;253:255–70. doi: 10.1006/excr.1999.4687. [DOI] [PubMed] [Google Scholar]
- 136.Lewis TS, Shapiro PS, Ahn NG. Signal transduction through MAP kinase cascades. Adv Cancer Res. 1998;74:49–139. doi: 10.1016/s0065-230x(08)60765-4. [DOI] [PubMed] [Google Scholar]
- 137.Adam LP. Gaplnskl CJ, Hathaway DR. Phosphorylation sequences in h-caldesmon from phorbol ester-stimulated canine aortas. FEBS Letters. 1992;302:223–6. doi: 10.1016/0014-5793(92)80446-n. [DOI] [PubMed] [Google Scholar]
- 138.Chiltls TJ, Watson MH, Sanghera JS, Campbell DL, Pelech SL, Mak AS. Phosphorylation of smooth muscle caldesmon by mitogen-activated protein (MAP) kinase and expression of MAP kinase in differentiated smooth muscle cells. J Biol Chem. 1992;267:22853–9. [PubMed] [Google Scholar]
- 139.Tsakiridis T, Bergman A, Somwar R, Taha C, Aktorles K, Cruz TF, Klip A, Downey GP. Actin filaments facilitate insulin activation of the src and collagen homologous/mitogen-activated protein kinase pathway leading to DNA synthesis and c-fos expression. J Biol Chem. 1998;273:28322–31. doi: 10.1074/jbc.273.43.28322. [DOI] [PubMed] [Google Scholar]
- 140.LI Y, Je H-D, Malek S, Morgan KG. Role of ERK1/2 in uterine contractility and preterm labor in rats. Am J Physiol Regul Integr Comp Physiol. 2004;287:R328–35. doi: 10.1152/ajpregu.00042.2004. [DOI] [PubMed] [Google Scholar]
- 141.Gangopadhyay SS, Takizawa N, Gallant C, Barber AL, Je HD, Smith TC, Luna EJ, Morgan KG. Smooth muscle archvillin: a novel regulator of signaling and contractility in vascular smooth muscle. J Cell Sci. 2004;117:5043–57. doi: 10.1242/jcs.01378. [DOI] [PubMed] [Google Scholar]
- 142.Gangopadhyay SS, Kengnl E, Gallant C, Kim HR, Leavls P, DeGnore J, Appel S, Morgan KG. 2008. SmAV is an ERK scaffolding protein. ASCB Annual Meeting. San Francisco, CA,
- 143.Morrison DK, Davis RJ. Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu Rev Cell Dev Biol. 2003;19:91–118. doi: 10.1146/annurev.cellbio.19.111401.091942. [DOI] [PubMed] [Google Scholar]
- 144.Khalil RA, Morgan KG. Enzyme translo-cations during smooth muscle activation. In: Barany M, editor. Biochemistry of Smooth Muscle Contraction. Academic Press; 1996. pp. 307–18. . In:, editor.. San Diego, CA:. [Google Scholar]
- 145.Yoshikawa H, Taniguchi SI, Yamamura H, Mori S, Sugimoto M, Miyado K, Nakamura K, Nakao K, Katsuki M, Shibata N, Takahashi K. Mice lacking smooth muscle calponin display increased bone formation that is associated with enhancement of bone morphogenetic protein responses. Genes Cells. 1998;3:685–95. doi: 10.1046/j.1365-2443.1998.00214.x. [DOI] [PubMed] [Google Scholar]
- 146.Matthew JD, Khromov AS, McDuffie MJ, Somlyo AV, Somlyo AP, Taniguchi S, Takahashi K. Contractile properties and proteins of smooth muscles of a calponin knockout mouse. J Physiol. 2000;529:811–24. doi: 10.1111/j.1469-7793.2000.00811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Masuki S, Takeoka M, Taniguchi S, Nose H. Enhanced baroreflex sensitivity in free-moving calponin knockout mice. Am J Physiol Heart Circ Physiol. 2003;284:H939–46. doi: 10.1152/ajpheart.00610.2002. [DOI] [PubMed] [Google Scholar]
- 148.Fujishige A, Takahashi K, Tsuchiya T. Altered mechanical properties in smooth muscle of mice with a mutated calponin locus. Zoolog Sci. 2002;19:167–74. doi: 10.2108/zsj.19.167. [DOI] [PubMed] [Google Scholar]
- 149.Masuki S, Takeoka M, Taniguchi S, Yokoyama M, Nose H. Impaired arterial pressure regulation during exercise due to enhanced muscular vasodilatation in calponin knockout mice. J Physiol. 2003;553:203–12. doi: 10.1113/jphysiol.2003.047803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Taniguchi S, Takeoka M, Ehara T, Hashimoto S, Shibuki H, Yoshimura N, Shigematsu H, Takahashi K, Katsuki M. Structural fragility of blood vessels and peritoneum in calponin hi-deficient mice, resulting in an increase in hematogenous metastasis and peritoneal dissemination of malignant tumor cells. Cancer Res. 2001;61:7627–34. [PubMed] [Google Scholar]
- 151.Taniguchi S. Suppression of cancer phenotypes through a multifunctional actin-binding protein, calponin, that attacks cancer cells and simultaneously protects the host from invasion. Cancer Sci. 2005;96:738–46. doi: 10.1111/j.1349-7006.2005.00118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ogura T, Kobayashi H, Ueoka Y, Okugawa K, Kato K, Hirakawa T, Hashimoto S, Taniguchi S, Wake N, Nakano H. Adenovirus-mediated calponin hi gene therapy directed against peritoneal dissemination of ovarian cancer: bifunctional therapeutic effects on peritoneal cell layer and cancer cells. Clin Cancer Res. 2006;12:5216–23. doi: 10.1158/1078-0432.CCR-06-0674. [DOI] [PubMed] [Google Scholar]
- 153.Yamamura H, Yoshikawa H, Tatsuta M, Akedo H, Takahashi K. Expression of the smooth muscle calponin gene in human osteosarcoma and its possible association with prognosis. Int J Cancer. 1998;79:245–50. doi: 10.1002/(sici)1097-0215(19980619)79:3<245::aid-ijc6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 154.Koganehira Y, Takeoka M, Ehara T, Sasaki K, Murata H, Saida T, Taniguchi S. Reduced expression of actin-binding proteins, h-caldesmon and calponin hi, in the vascular smooth muscle inside melanoma lesions: an adverse prognostic factor for malignant melanoma. Br J Dermatol. 2003;148:971–80. doi: 10.1046/j.1365-2133.2003.05238.x. [DOI] [PubMed] [Google Scholar]
- 155.Fisher C, Montgomery E, Healy V. Calponin and h-caldesmon expression in synovial sarcoma; the use of calponin in diagnosis. Histopathology. 2003;42:588–93. doi: 10.1046/j.1365-2559.2003.01652.x. [DOI] [PubMed] [Google Scholar]
- 156.Sasaki Y, Yamamura H, Kawakami Y, Yamada T, Hiratsuka M, Kameyama M, Ohigashi H, Ishikawa O, Imaoka S, Ishiguro S, Takahashi K. Expression of smooth muscle calponin in tumor vessels of human hepatocellular carcinoma and its possible association with prognosis. Cancer. 2002;94:1777–86. doi: 10.1002/cncr.10402. [DOI] [PubMed] [Google Scholar]
- 157.Islam AH, Ehara T, Kato H, Hayama M, Nishizawa O. Loss of calponin hi in renal angiomyolipoma correlates with aggressive clinical behavior. Urology. 2004;64:468–73. doi: 10.1016/j.urology.2004.03.054. [DOI] [PubMed] [Google Scholar]
- 158.Yanagisawa Y, Takeoka M, Ehara T, Itano N, Miyagawa S, Taniguchi S. Reduction of Calponin hi expression in human colon cancer blood vessels. Eur J Surg Oncol. 2008;34:531–7. doi: 10.1016/j.ejso.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 159.Horiuchi A, Nikaido T, Taniguchi S, Fujii S. Possible role of calponin hi as a tumor suppressor in human uterine leiomyosarcoma. J Natl Cancer Inst. 1999;91:790–6. doi: 10.1093/jnci/91.9.790. [DOI] [PubMed] [Google Scholar]
- 160.Lener T, Burgstaller G, Gimona M. The role of calponin in the gene profile of metastatic cells: inhibition of metastatic cell motility by multiple calponin repeats. FEBS Lett. 2004;556:221–6. doi: 10.1016/s0014-5793(03)01401-7. [DOI] [PubMed] [Google Scholar]
- 161.Somlyo AV. Ultrastructure of vascular smooth muscle. In: Geiger SR, editor. Handbook of physiology. American Physiological Society; 1980. pp. 33–67. . In:, editor. . Bethesda:. [Google Scholar]
- 162.Cipolla M, Gokina N, Osol G. Pressure-induced actin polymerization in vascular smooth muscle as a mechanism underlying myogenic behavior. FASEB J. 2002;16:72–6. doi: 10.1096/cj.01-0104hyp. [DOI] [PubMed] [Google Scholar]
- 163.Opazo Saez A, Zhang W, Wu Y, Turner CE, Tang DD, Gunst SJ. Tension development during contractile stimulation of smooth muscle requires recruitment of paxillin and vinculin to the membrane. Am J Physiol Cell Physiol. 2004;286:C433–47. doi: 10.1152/ajpcell.00030.2003. [DOI] [PubMed] [Google Scholar]
- 164.Kim HR, Gallant C, Leavis PC, Gunst SJ, Morgan KG. Cytoskeletal remodeling in differentiated vascular smooth muscle is actin isoform-dependent and stimulus-dependent. Am J Physiol Cell Physiol. 2008;295:C768–78. doi: 10.1152/ajpcell.00174.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–65. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- 166.Stossel TP, Fenteany G, Hartwig JH. Cell surface actin remodeling. J Cell Scl. 2006;119:3261–4. doi: 10.1242/jcs.02994. [DOI] [PubMed] [Google Scholar]
- 167.Zhang W, Gunst SJ. Interactions of Airway Smooth Muscle Cells with Their Tissue Matrix: Implications for Contraction. Proc Am Thorac Soc. 2008;5:32–9. doi: 10.1513/pats.200704-048VS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhang W, Wu Y, Du L, Tang DD, Gunst SJ. Activation of the Arp2/3 complex by N-WASp is required for actin polymerization and contraction in smooth muscle. Am J Physiol Cell Physiol. 2005;288:C1145–60. doi: 10.1152/ajpcell.00387.2004. [DOI] [PubMed] [Google Scholar]
- 169.Herman IM. Actin isoforms. Curr Opin CellBiol. 1993;5:48–55. doi: 10.1016/s0955-0674(05)80007-9. [DOI] [PubMed] [Google Scholar]
- 170.Vandekerckhove J, Weber K. At least six different actins are expressed in a higher mammal: an analysis based on the amino acid sequence of the amino-terminal tryptic peptide. J Mol Biol. 1978;126:783–802. doi: 10.1016/0022-2836(78)90020-7. [DOI] [PubMed] [Google Scholar]
- 171.Fatigati V, Murphy R. Actin and tropomyosin variants in smooth muscles Dependence on tissue type. J Biol Chem. 1984;259:14383–8. [PubMed] [Google Scholar]
- 172.Vandekerckhove J, Weber K. The complete amino acid sequence of actins from bovine aorta, bovine heart, bovine fast skeletal muscle, and rabbit slow skeletal muscle. Differentiation. 1979;14:123–33. doi: 10.1111/j.1432-0436.1979.tb01021.x. [DOI] [PubMed] [Google Scholar]
- 173.Prochniewicz E, Thomas DD. Site-specific mutations in the myosin binding sites of actin affect structural transitions that control myosin binding. Biochemistry. 2001;40:13933–40. doi: 10.1021/bi010893n. [DOI] [PubMed] [Google Scholar]
- 174.North AJ, Gimona M, Lando Z, Small JV. Actin isoform compartments in chicken gizzard smooth muscle cells. J Cell Sci. 1994;107:445–55. doi: 10.1242/jcs.107.3.445. [DOI] [PubMed] [Google Scholar]
- 175.Parker CA, Takahashi K, Tang JX, Tao T, Morgan KG. Cytoskeletal targeting of calponin in differentiated, contractile smooth muscle cells of the ferret. J Physiol. 1998;508:187–98. doi: 10.1111/j.1469-7793.1998.187br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lehman W. Calponin and the composition of smooth muscle thin filaments. J Muscle Res Cell Motil. 1991;12:221–4. doi: 10.1007/BF01745110. [DOI] [PubMed] [Google Scholar]
- 177.Stromer MH, Mayes MS, Bellin RM. Use of actin isoform-specific antibodies to probe the domain structure in three smooth muscles. Histomchem Cell Biol. 2002;118:291–9. doi: 10.1007/s00418-002-0453-8. [DOI] [PubMed] [Google Scholar]
- 178.Drew JS, Moos C, Murphy R. Localization of isoactins in isolated smooth muscle thin filaments by double gold immunolabeling. Am J Physiol. 1991;260:C1332–40. doi: 10.1152/ajpcell.1991.260.6.C1332. [DOI] [PubMed] [Google Scholar]
- 179.Drew JS, Murphy R. Actin Isoform expression, cellular heterogeneity, and contractile function in smooth muscle. Can J Physiol Pharmacol. 1997;75:869–77. [PubMed] [Google Scholar]
- 180.Hu K, Ji L, Applegate KT, Danuser G, Waterman-Storer CM. Differential transmission of actin motion within focal adhesions. Science. 2007;315:111–5. doi: 10.1126/science.1135085. [DOI] [PubMed] [Google Scholar]
- 181.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 182.Avraamides CJ, Garmy-Susini B, Varner JA. Integrins in angiogenesis and lymphangiogenesis. Nat Rev Cancer. 2008;8:604–17. doi: 10.1038/nrc2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hynes RO. Integrins; bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 184.Arnaout MA, Goodman SL, Xiong J. Structure and mechanics of integrin-based cell adhesion. Curr Opin Cell Biol. 2007;19:495–507. doi: 10.1016/j.ceb.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Morgan MR, Humphries MJ, Bass MD. Synergistic control of cell adhesion by integrins and syndecans. Nat Rev Mol Cell Biol. 2007;8:957–69. doi: 10.1038/nrm2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tang DD, Gunst SJ. Depletion of focal adhesion kinase by antisense depresses contractile activation of smooth muscle. Am J Physiol Cell Physiol. 2001;280:C874–83. doi: 10.1152/ajpcell.2001.280.4.C874. [DOI] [PubMed] [Google Scholar]
- 187.Tang DD, Gunst SJ. Signal transduction in smooth muscle: selected contribution: roles of focal adhesion kinase and paxillin in the mechanosensitive regulation of myosin phosphorylation in smooth muscle. J Appl Physiol. 2001;91:1452–9. doi: 10.1152/jappl.2001.91.3.1452. [DOI] [PubMed] [Google Scholar]
- 188.Tang DD, Wu M-F, Opazo Saez AM, Gunst SJ. The focal adhesion protein paxillin regulates contraction in canine tracheal smooth muscle. J Physiol. 2002;542:501–13. doi: 10.1113/jphysiol.2002.021006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Tang DD, Tan J. Downregulation of profilin with antisense oligodeoxynu-cleotides inhibits force development during stimulation of smooth muscle. Am J Physiol Heart Circ Physiol. 2003;285:H1528–36. doi: 10.1152/ajpheart.00188.2003. [DOI] [PubMed] [Google Scholar]
- 190.Tang DD, Tan J. Role of Crk-Associated substrate in the regulation of vascular smooth muscle contraction. Hypertension. 2003;42:858–63. doi: 10.1161/01.HYP.0000085333.76141.33. [DOI] [PubMed] [Google Scholar]
- 191.Tang DD, Turner CE, Gunst SJ. Expression of non-phosphorylatable paxillin mutants in canine tracheal smooth muscle inhibits tension development. J Physiol. 2003;553:21–35. doi: 10.1113/jphysiol.2003.045047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Gerthoffer WT, Gunst SJ. Signal transduction in smooth muscle: invited review: focal adhesion and small heat shock proteins in the regulation of actin remodeling and contractility in smooth muscle. J Appl Physiol. 2001;91:963–72. doi: 10.1152/jappl.2001.91.2.963. [DOI] [PubMed] [Google Scholar]
- 193.Gunst SJ, Fredberg JJ. The first three minutes: smooth muscle contraction cytoskeletal events, and soft glasses. J Appl Physiol. 2003;95:413–25. doi: 10.1152/japplphysiol.00277.2003. [DOI] [PubMed] [Google Scholar]
- 194.Gunst SJ, Tang DD, Opazo Saez A. Cytoskeletal remodeling of the airway smooth muscle cell: a mechanism for adaptation to mechanical forces in the lung. Respir Physiol Neurobiol. 2003;137:151–68. doi: 10.1016/s1569-9048(03)00144-7. [DOI] [PubMed] [Google Scholar]
- 195.Kim HR, Hoque M, Hai C-M. Cholinergic receptor-mediated differential cytoskeletal recruitment of actin- and integrin-binding proteins in intact airway smooth muscle. Am J Physiol Cell Physiol. 2004;287:C1375–83. doi: 10.1152/ajpcell.00100.2004. [DOI] [PubMed] [Google Scholar]
- 196.Ohanian V, Gatfield K, Ohanian J. Role of the actin cytoskeleton in G-protein-coupled receptor activation of PYK2 and paxillin in vascular smooth muscle. Hypertension. 2005;46:93–9. doi: 10.1161/01.HYP.0000167990.82235.3c. [DOI] [PubMed] [Google Scholar]
- 197.Ali F, Pare P, Seow C. Models of contractile units and their assembly in smooth muscle. Can J Physiol Pharmacol. 2005;83:825–31. doi: 10.1139/y05-052. [DOI] [PubMed] [Google Scholar]
- 198.Herrera AM, Martinez EC, Seow CY. Electron microscopic study of actin polymerization in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1161–8. doi: 10.1152/ajplung.00298.2003. [DOI] [PubMed] [Google Scholar]
- 199.Herrera AM, McParland BE, Bienkowska A, Tait R, Pare PD, Seow CY. ‘Sarcomeres’ of smooth muscle: functional characteristics and ultrastructural evidence. J Cell Sci. 2005;118:2381–92. doi: 10.1242/jcs.02368. [DOI] [PubMed] [Google Scholar]
- 200.Kuo KH, Seow CY. Contractile filament architecture and force transmission in swine airway smooth muscle. J Cell Scl. 2004;117:1503–11. doi: 10.1242/jcs.00996. [DOI] [PubMed] [Google Scholar]
- 201.Kuo KH, Herrera AM, Seow CY. Ultrastructure of airway smooth muscle. Respir Physiol Neurobiol. 2003;137:197–208. doi: 10.1016/s1569-9048(03)00147-2. [DOI] [PubMed] [Google Scholar]
- 202.Seow CY. Myosin filament assembly in an ever-changing myofilament lattice of smooth muscle. Am J Physiol Cell Physiol. 2005;289:C1363–8. doi: 10.1152/ajpcell.00329.2005. [DOI] [PubMed] [Google Scholar]
- 203.Jones KA, Perkins WJ, Lorenz RR, Prakash YS, Sieck GC, Warner DO. F-actin stabilization increases tension cost during contraction of permeabilized airway smooth muscle in dogs. J Physiol. 1999;519:527–38. doi: 10.1111/j.1469-7793.1999.0527m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kumar A, Crawford K, Close L, Madison M, Lorenz J, Doetschman T, Pawlowski S, Duffy J, Neumann J, Robbins J, Boivin GP, O'Toole BA, Lessard JL. Rescue of cardiac alpha-actin-deficient mice by enteric smooth muscle gamma-actin. Proc Natl Acad Sci USA. 1997;94:4406–11. doi: 10.1073/pnas.94.9.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Crawford K, Flick R, Close L, Shelly D, Paul R, Bove K, Kumar A, Lessard J. Mice lacking skeletal muscle actin show reduced muscle strength and growth deficits and die during the neonatal period. Mol Cell Biol. 2002;22:5887–96. doi: 10.1128/MCB.22.16.5887-5896.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Schildmeyer LA, Braun R, Taffet G, Debiasi M, Burns AE, Bradley A, Schwartz RJ. Impaired vascular contractility and blood pressure homeostasis in the smooth muscle {alpha}-actin null mouse. FASEB J. 2000;14:2213–20. doi: 10.1096/fj.99-0927com. [DOI] [PubMed] [Google Scholar]
- 207.Sonnemann KJ, Fitzsimons DP, Patel JR, Liu Y, Schneider MF, Moss RL, Ervasti JM. Cytoplasmic [gamma]-actin is not required for skeletal muscle development but its absence leads to a progressive myopathy. Dev Cell. 2006;11:387–97. doi: 10.1016/j.devcel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 208.Hanft LM, Rybakova IN, Patel JR, Rafael-Fortney JA, Ervasti JM. Cytoplasmic gamma-actin contributes to a compensatory remodeling response in dystrophin-deficient muscle. Proc Natl Acad Sci USA. 2006;103:5385–90. doi: 10.1073/pnas.0600980103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Prins KW, Lowe DA, Ervasti JM. Skeletal muscle-specific ablation of gamma cyto-actin does not exacerbate the MDX pheno-type. PLoS ONE. 2008;3:e2419. doi: 10.1371/journal.pone.0002419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Guo D-C, Pannu H, Tran-Fadulu V, Papke CL, Yu RK, Avidan N, Bourgeois S, Estrera AL, Safi HJ, Sparks E, Amor D, Ades L, McConnell V, Willoughby CE, Abuelo D, Willing M, Lewis RA, Kim DH, Scherer S, Tung PP, Ahn C, Buja LM, Raman CS, Shete SS, Milewicz DM. Mutations in smooth muscle [alpha]-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat Genet. 2007;39:1488–93. doi: 10.1038/ng.2007.6. [DOI] [PubMed] [Google Scholar]


