Abstract
A variety of stressors can cause the collapse of mitochondrial membrane potential (Δψm), but the events leading up to this catastrophic cellular event are not well understood at the mechanistic level. Based on our recent studies of oscillations in mitochondrial energetics, we have coined the term “mitochondrial criticality” to describe the state in which the mitochondrial network of cardiomyocytes becomes very sensitive to small perturbations in reactive oxygen species (ROS), resulting in the scaling of local mitochondrial uncoupling and Δψm loss to the whole cell, and the myocardial syncytium. At the point of criticality, the dynamics of the mitochondrial network bifurcate to oscillatory behavior. These energetic changes are translated into effects on the electrical excitability of the cell, inducing dramatic changes in the morphology and the threshold for activating an action potential. Emerging evidence suggests that this mechanism, by creating spatial and temporal heterogeneity of excitability in the heart during ischemia and reperfusion, underlies the genesis of potentially lethal cardiac arrhythmias.
Keywords: Mitochondrial oscillation, Oxidative stress, Inner membrane anion channel, Ischemia–reperfusion injury, Arrhythmias
1. Introduction
1.1. The physiological and pathophysiological consequences of mitochondrial inner membrane ion transport
One of the basic postulates of the chemiosmotic hypothesis [1] is that the mitochondrial inner membrane is generally impermeable to ions other than H+. As originally pointed out by Mitchell, this impermeability of the inner membrane is necessary for tight coupling between proton translocation, to generate the protonmotive force, and its dissipation through the F1F0 ATPase to synthesize ATP. The degree of coupling of oxidative phosphorylation will, therefore, be expected to vary with the degree of leakiness of the membrane to protons, or, as we expand this view, to other ions indirectly coupled to the proton gradient (e.g., Ca2+, K+, Na+).
To move an ion across a membrane requires both a driving force and a transport pathway [2], as well as a mechanism for maintaining charge balance. It has become increasingly clear in recent years that a variety of ion channels are present on the mitochondrial inner membrane [3-5]. The opening of these ion channels will partially or totally dissipate the proton motive force stored by contributing inward current and/or by expending part of the electrochemical gradient to eject those ions, e.g., K+ extrusion via the K+/H+ exchanger.
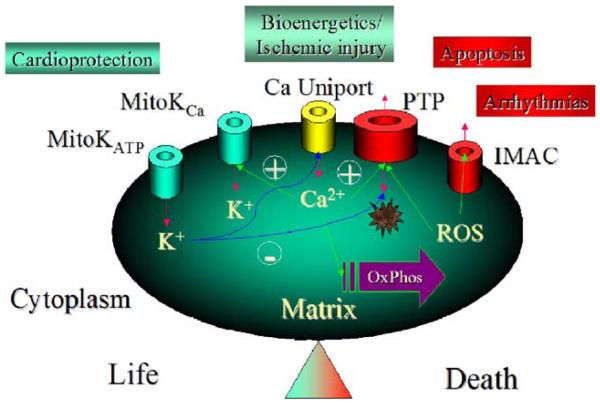
Although there is very little information about the molecular structure of most of the ion transporting proteins of the mitochondrial inner membrane, the use of multiple techniques such as patch-clamp, swelling assays, reconstitution of proteins in lipid bilayers and proteoliposomes, proteomics, and classical pharmacological methods, is beginning to allow the characterization of the functional effects of mitochondrial inner membrane channels. A picture of the role of these channels in pathophysiology is emerging. Fig. 1 summarizes the present knowledge concerning ion channels present in the mitochondrial inner membrane and the beneficial or detrimental consequences associated with their function. As emphasized by Fig. 1, one can group mitochondrial ion channels based on their pro-life or pro-death effects in cardiac cells. Mitochondrial K+ channels (mitoKATP and mitoKCa) are mediators of protection against ischemic injury (as recently reviewed elsewhere [4]), while the classical permeability transition pore (PTP) has been strongly associated with cellular necrosis and the induction of programmed cell death (apoptosis) [6,7]. The mitochondrial Ca2+ uniporter has a dual role as the main pathway for mitochondrial Ca2+ influx. This function is essential for the stimulation of energy metabolism during changes in cardiac work [8], but could also contribute to Ca2+ overload during ischemia [9]. This short review will focus on the phenomenon of mitochondrial ROS-induced ROS release (originally coined by Zorov et al. [10]) and the role of an inner membrane anion channel (IMAC) in the mechanism of Δψm oscillation and depolarization in cardiac cells subjected to metabolic and/or oxidative stress.
Fig. 1.
Mitochondrial inner membrane ion channels grouped according to their pro-life or pro-death effects on cardiac cells. MitoKATP: An inner membrane ATP-sensitive K+ channel activated by ischemia or K+ channel openers that is thought to play a central role in protection against ischemic injury. Multiple independent lines of evidence have characterized the properties of this channel, although the molecular structure of the pore is still unknown. MitoKCa: a toxin-sensitive (charybdotoxin, iberiotoxin) mitochondrial Ca2+-activated K+ channel has been detected in the cardiac mitochondrial inner membrane that has properties similar to KCa channels found in the plasmalemma of other cell types. KCa openers confer cardioprotection and have effects on mitochondrial function similar to those of mitoKATP channel openers. Ca Uniport: the Ca2+ uniporter is considered to be the major Ca2+ uptake pathway of the mitochondria, thus playing a role in the stimulation of NADH production and oxidative phosphorylation when workload increases. It may also contribute to mitochondrial Ca2+ overload during ischemia–reperfusion injury. PTP: the Permeability Transition Pore is responsible for the catastrophic increase in inner membrane permeability induced by Ca2+ and/or ROS. This large non-selective ion channel is capable of transporting solutes up to 1500 Da and has been linked to reperfusion injury and the induction of apoptosis. IMAC: the inner membrane anion channel is extensively discussed in the present review.
The central hypothesis is that stressors, including ischemia and reperfusion, promote the approach of the mitochondrial network to a critical state as a consequence of the accumulation of superoxide anion (O2•−) in the matrix as a byproduct of respiration. At the threshold of criticality, positive feedback of mitochondrial ROS on the open probability of IMAC causes rapid depolarization of Δψm that is transmitted to neighboring mitochondria, which scales to the entire mitochondrial network, affecting electrical excitability of the myocyte, and ultimately, the whole heart.
1.2. Mitochondrial physiology, oxidative stress, and cell death
In the context of the continuously high energetic demand of the heart, the loss of Δψm causes a rapid impairment of mitochondrial and cellular function that can lead to necrotic or apoptotic cell death. Thus, maintaining Δψm is of paramount importance. Oxidative stress is a major pathophysiological route to the collapse of ΔΨm, but, when kept under control, ROS serve as important signaling molecules [11,12]. ROS-mediated signaling can occur through a number of ROS-sensitive kinases [13] to bring about post-translational modification of key target proteins, or through the activation of redox-sensitive transcription factors that will affect the expression of multiple genes [14-16]. ROS-mediated signaling of gene expression is highly dependent on the ability of cells to maintain a reductive intracellular environment to counterbalance the highly oxidizing extracellular environment.
Throughout our lives, the toxic effects of ROS are kept in check by balancing the natural rates of ROS production with sophisticated antioxidant defense systems, including the enzymes superoxide dismutase, catalase, glutaredoxin thioltransferase, and glutathione peroxidase. The latter serves as a bridge between ROS and the reactive thiol pool (buffered by reduced glutathione and thioredoxin), which modulates the activity of vast number of cysteine-containing proteins. If this balance between ROS production and ROS scavenging is disrupted, serious and often irreversible cell damage occurs [17]. One such important pathological situation in the heart is reperfusion following ischemia, when ROS production accelerates and the detoxification systems are overwhelmed, resulting in the consumption of antioxidants and an increase in free radical concentrations [18,19]. This is the period during which ΔΨm is most likely to become unstable, representing the major decision point between cell life and death. Two channels located in the mitochondrial inner membrane have been implicated in ΔΨm depolarization; the PTP and the IMAC. With respect to the PTP, its role in necrotic and apoptotic cell death has been explored extensively. It suffices to say, for the present discussion, that there is evidence, primarily based on cyclosporin A (CsA) sensitivity, that the PTP opens during reperfusion, but not during ischemia [20], and that it represents a terminal cellular event contributing to myocardial injury. For the rest of this review, we will focus on the role of IMAC in ΔΨm depolarization, which we believe represents the first responder to oxidative stress in the mitochondrial inner membrane of the cardiac myocyte, and therefore might be an important target for therapeutic intervention.
1.3. IMAC and metabolic stress
Compelling evidence exists that there is a partially anion-selective channel present in mitochondria [21-23]. From studies of mitochondrial swelling, this channel has been shown to be activated by matrix Mg2+ depletion or alkalinization, and is inhibited by various cationic amphiphiles including Ca2+ channel blockers, antiarrhythmics, β-adrenergic antagonists, local anesthetics, tricyclic antidepressants, and anticonvulsants [22]. Although the molecular entity carrying anion fluxes in mitochondria has yet to be found, patch-clamp studies of isolated mitoplasts have identified a so-called centum picosiemen anion channel (conductance approximately 100–110 pS) that carries the predominant outwardly rectifying background conductance of the inner membrane [24-26]. This channel has many of the same properties as the IMAC described in swelling studies [24], although other, low conductance, anion channels have also been proposed as candidates to account for the macroscopic flux [27]. For convenience, based on its pharmacology, we have referred to the channel underlying ΔΨm depolarization as IMAC, and preliminary single channel data suggests that the relevant ROS-activated channel resembles the centum pS channel (data not shown).
We first postulated that IMAC was involved in oscillations in ΔΨm induced by substrate deprivation [3]. In more recent studies, the mechanism of mitochondrial ΔΨm and redox oscillation was rigorously explored using a well-defined trigger of local oxidative stress induced by a laser flash in approximately fifty mitochondria (see Fig. 2) [28]. We provided further evidence that this channel may be the primary player involved in the mitochondrial ROS-induced ROS release mechanism [28]. Our study and the work of Van den Hoek, et al. [29], were the first to suggest that IMAC might be a transport pathway for superoxide anion (O2•−), which is membrane impermeant [30], to move from the mitochondrial matrix to the intermembrane space. How and why this channel opens was explored in several studies that are discussed below.
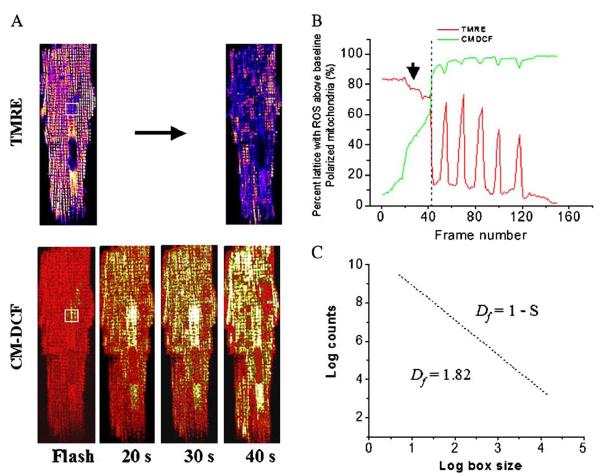
Fig. 2.
Mitochondrial criticality and fractal organization at the percolation threshold. (A) The mitochondrial lattice of cardiomyocytes, as imaged by two-photon laser scanning microscopy, was labeled with a membrane potential marker (top panels; TMRE labeled cell, intensity scaled from blue to white;) and a reactive oxygen species (ROS)-sensitive fluorescent probe (bottom panels; CM-H2DCFDA signal, scaled from red [reduced] to yellow [oxidized]) [28,36]. (B) The time course of ΔΨm depolarization (indicated by the arrow) and the development of the mitochondrial spanning cluster as mitochondrial ROS accumulates (lower panels of A) after a local flash (indicated by the white square). Criticality is reached when about 60% of the mitochondria have CM-DCF fluorescence levels ∼20% above baseline. This coincides with the expected theoretical percolation threshold. (C) The fractal dimension, Df, of the mitochondrial cluster, as revealed by the ROS probe (lower panels of A), was also consistent with percolation theory. Adapted from [36].
2. Mitochondrial criticality
2.1. Definition
Under normal conditions, metabolic networks are robust, and are able to adapt when the system is perturbed. The mitochondrial network of the heart cell must have properties of both constancy and flexibility, first providing a steady supply of ATP to fuel contraction, and second, to adapt the rate of energy production to meet the changing metabolic demand as workload varies [31,32]. The global response of the cardiac myocyte depends on the coordinated action of thousands of mitochondria arranged in a lattice-like network of nonlinearly coupled elements. When the normal function of the heart cell becomes severely compromised under pathophysiological conditions (e.g., ischemic injury) or metabolic stress (e.g., oxidative stress, substrate deprivation) the inherent vulnerability of the mitochondrial network is revealed. Several examples reported in the literature illustrate this point [33-35] (for review, see [6,7]).
It is under these circumstances that the mitochondrial network behaves, in many ways, like other physical, chemical, or man-made connected systems. Nonlinear networks subjected to excessive loads or the failure of parts of the system can approach a critical state, which is characterized by an extremely large susceptibility to external factors and strong correlation between individual components. Consequently, new emergent macroscopic behavior can appear, including spatio-temporal patterns of self-organization visualized as oscillations and/or waves in activity or the level of an intermediate. Our recent experimental and theoretical work shows that such conditions are met when mitochondrial ROS accumulates to a critical threshold in a well-defined fraction of the mitochondrial network (Fig. 2). At this point of mitochondrial criticality [36,37], bioenergetics becomes unstable and can undergo a rapid transition to ΔΨm oscillation or depolarization that involves a coordinated response of almost the entire population of mitochondria in the network.
The idea that biological systems can operate at the edge of dynamic instability, which might be an adaptation that favors a quick response to environmental change, was proposed previously [38,39] (for a review see [40]). However, critical behavior in cells experiencing pathological circumstances can lead to deadly outcomes such as apoptosis or necrosis [41,42]. The unique response of cardiac myocytes subjected to metabolic stress produces a remarkably reproducible pattern of synchronized autonomous oscillations of ΔΨm, NADH, ROS, and glutathione [28,43], which are closely coupled to oscillations in the electrical excitability and Ca2+ handling functions of the cell [28,44].
2.2. Spatial aspects of mitochondrial criticality: percolation cluster formation
As the mitochondria approach criticality, several questions arise as to (1) what determines the extent of the depolarized region; (2) how does the signal propagate throughout the network; and (3) how does ΔΨm depolarization occur almost simultaneously in distant regions of the cell. We have addressed these questions by applying percolation theory to the problem [36]. Percolation describes how local neighbor–neighbor interactions among elements in a lattice can scale to produce a macroscopic response spanning from one end of the array to the other [45]. Such a “spanning cluster” forms when there is a critical density of elements close to the threshold for a transition (the percolation threshold). In the case of the mitochondrial network stressed by a local oxidative challenge, we found that, prior to the first global ΔΨm depolarization, approximately 60% of the mitochondria had accumulated ROS to a level roughly 20% above baseline (Fig. 2A), which was the threshold for activation of the oscillator at the whole cell level. This critical density of mitochondria was consistent with that predicted by percolation theory (Fig. 2B). Moreover, the spatial distribution of mitochondria at the threshold had a fractal dimension in agreement with theory [36] (Fig. 2C).
Several interesting properties of the mitochondrial response can be explained when the network is considered as a percolation cluster. First, the question of the limited diffusivity and lifetime of O2·− as the triggering molecule is answered, since the only relevant diffusion distance is the intermitochondrial spacing (<0.5 μm). As long as there are enough neighboring mitochondria belonging to the spanning cluster (i.e., they have accumulated enough O2·− to approach the percolation threshold) a universal phase transition [45-47] will occur and mitochondria will depolarize for the first time throughout the cell. At this point, self-sustained oscillations in ΔΨm continue as a consequence of a bifurcation in the dynamics of the system (see below); however, the spatial pattern of subsequent depolarizations of the network will typically follow that of the original percolation cluster, with some mitochondria always remaining outside the cluster. In addition, the initiation site of the ΔΨm depolarization can arise from anywhere within the spanning cluster yet still follow the same stereotypical pattern. Another important feature of the percolation model is that the global transition can be prevented if the O2·− concentration reaching the neighboring mitochondrion is decreased below threshold, either by decreasing O2·− production (inhibiting respiration), decreasing O2·− release (inhibiting IMAC), or increasing the local ROS scavenging capacity (increasing the reduced glutathione pool) [36]. Similar interventions that partially decreased the local coupling between mitochondria slowed the rate of propagation of mitochondrial depolarization, which under normal conditions was ∼22 μm/s, facilitating the detection of waves of ΔΨm depolarization and repolarization [36].
3. Mechanistic hypothesis and computational model
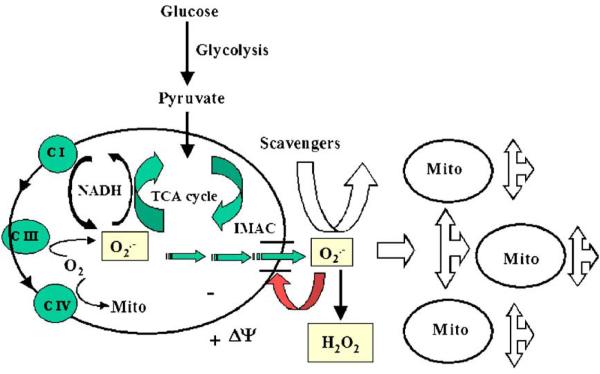
Our mechanistic hypothesis describing oscillations in mitochondrial energetics is based on experimental and theoretical data (Fig. 3) [28,36,43]. According to the postulated mechanism, under oxidative stress, the exacerbated ROS production from the electron transport chain accumulates in the mitochondrial matrix up to critical levels [28], triggering the opening of IMAC in a positive feedback loop (see below). The mitochondrial oscillator behaves as a relaxation oscillator, composed of both slow (ROS accumulation in the mitochondrial matrix) and fast (the IMAC opening and rapid ROS release) processes, which could be described in a single mitochondrion computational model [43]. The simple addition of a leak of electrons from the respiratory chain to O2·−, a formulation for an O2·−-activated IMAC conductance in the inner membrane, and a cytoplasmic ROS scavenging system in the cytoplasm was sufficient to support limit cycle oscillations within certain parametric domains of the model.
Fig. 3.
Mechanism of the mitochondrial oscillator. The mitochondrial and extramitochondrial processes involved in ROS-induced ROS release and the synchronization of mitochondrial activity (see text for further details). Adapted from [28].
An extensive stability analysis of the model revealed that the balance between the production and scavenging of ROS defines whether the system oscillates [43]. It is important to keep in mind the difference between the global qualitative properties of the model (i.e., the bifurcation diagram in Fig. 4A) and the dynamic behavior during an oscillatory cycle (Fig. 4B). The bifurcation diagram describes which parameters (e.g., the percentage of respiration leaking into ROS production, and superoxide dismutase, SOD, concentration) over which range of values will promote the appearance of oscillations in the context of a broad span of possible dynamic behaviors (Fig. 4A). According to this analysis, and in agreement with our working hypothesis, the simulations suggest that ROS build-up results from an increase in the rate of ROS production relative to the rate of ROS scavenging. This process leads to a bifurcation (discontinuity) in the dynamics that drives the system to either limit-cycle oscillation or a jump to the stable depolarized state.
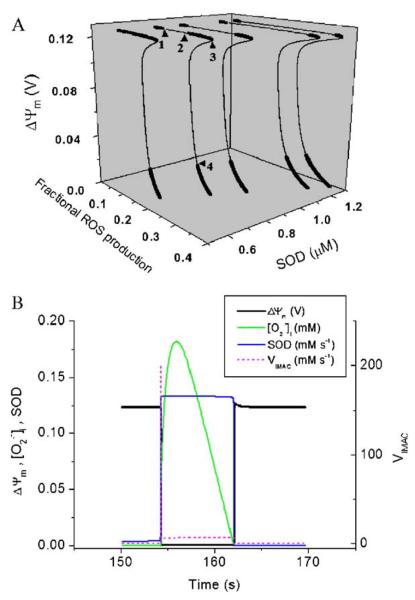
Fig. 4.
Global steady-state behavior of the mitochondrial oscillator (A) and the sequence of events during an oscillatory cycle (B). (A) Bifurcation diagram of ΔΨm as a function of ROS production and scavenging. The dynamic behavior of the computational model shows an upper branch of steady states in which ΔΨm is predominantly polarized, and a lower branch, in which ΔΨm is mainly depolarized. Transitions between both branches happen at arrowheads 3 and 4. Thick lines indicate domains of stable steady-state behavior whereas thin lines denote either unstable or oscillatory states. A stable oscillatory domain, embedded within the upper branch, emerges as SOD concentration increases. Arrowheads 1 and 2 in the upper branch indicate Hopf bifurcations delimiting the oscillatory region (thin line). Adapted from [43]. (B) The time course of change in ΔΨm, IMAC flux, SOD activity, and cytosolic superoxide ([O2·−]i) during a single cycle of the mitochondrial oscillator. At a critical level of mitochondrial ROS accumulation (see Fig. 2B), the IMAC channel rapidly opens, provoking the sudden release of O2S·− from the mitochondria into the intermembrane space (see Fig. 3). The current through IMAC quickly declines due to the loss of ΔΨm. The rate of SOD increases in parallel with the burst of available O2·− and stays high until the O2S·− is consumed, at which point IMAC closes, allowing ΔΨm to repolarize, initiating the next cycle.
Once we have established where in the parametric space the oscillations are possible, an analysis of an oscillatory cycle, for a certain parametric combination, is required to understand the inner workings of the oscillator. For each abrupt ΔΨm depolarization observed experimentally, the computational model predicts the release of O2·− through IMAC. The O2·− release is autocatalytic and produces a spike of O2·− into the intermembrane space. The abrupt increase in the open probability of the IMAC, at the onset of the rapid ΔΨm depolarization (Fig. 4B), agrees with the observed experimental behavior. The current amplitude of the IMAC decreases as a result of a reduction in the electrochemical driving force as ΔΨm nears the equilibrium potential for anions. The released O2S·− is quickly consumed by SOD (Fig. 4B), which terminates the self-amplification phase by closing IMAC (Fig. 4B). This allows ΔΨm to be restored by the TCA cycle and the respiration-driven proton pumps, while accumulation of O2·− begins again. A new cycle is initiated once matrix O2·− approaches the threshold for the regenerative response.
4. Pharmacological manipulation of IMAC and the mitochondrial benzodiazepine receptor
A number of amphiphilic compounds have been reported to block IMAC in mitochondrial swelling experiments [22]. Ligands of the mitochondrial benzodiazepine receptor (mBzR) are among the more potent inhibitors of IMAC [22], and we have demonstrated that they can acutely interrupt mitochondrial ΔΨm oscillations by stabilizing mitochondria in the polarized state (Fig. 5 and ref. [28]). The structure and functional role of the mBzR is not well understood, but it was first reported to be a multiprotein complex comprised of an 18-kDa isoquinoline binding protein, the outer membrane voltage-dependent anion channel (VDAC), and the adenine nucleotide translocator (ANT) of the inner membrane, located at the mitochondrial contact site [48]. A subsequent study challenged the requirement for the VDAC and ANT as components of the benzodiazepine binding site, and found that the 18-kDa protein was sufficient to reconstitute all of the specific binding properties when expressed in yeast [49]. Importantly, using patch clamp methods in isolated mitoplasts, mBzR ligands were shown to block mitochondrial inner membrane ion channels in mitoplasts with high affinity, including both a CsA-sensitive, large conductance channel (MCC) and a CsA-insensitive ∼100 pS anion channel (mCtS) [25]. It is currently unknown how the 18-kDa protein mBzR subunit might participate in modulating inner membrane ion conductance.
Fig. 5.
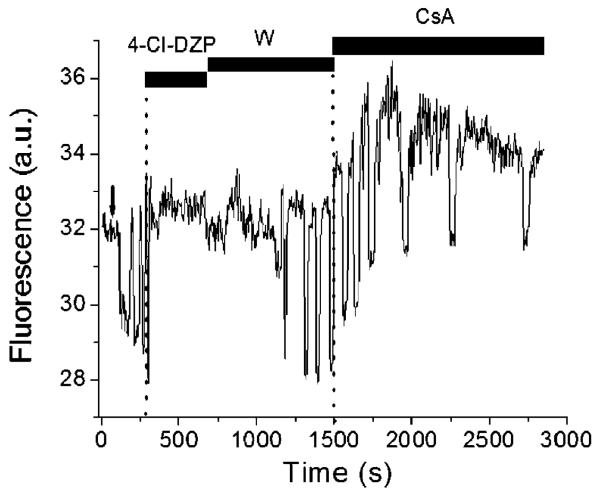
Experimental demonstration of the involvement of IMAC in ΔΨm oscillations and lack of effect by cyclosporin A. A freshly isolated cardiomyocyte loaded with the potential sensitive indicator TMRM and displaying flash-induced (arrow) mitochondrial oscillations in a continuous flow chamber, was exposed to 4-chlorodiazepam (4-Cl-DZP; 32 μM) followed by washout (W), at which time the oscillations resumed. Subsequent application of cyclosporin A (CsA; 1 μM) had no effect on the mitochondrial ΔΨm oscillations. The experimental evidence shows that, in the same cell, the antagonist of the mBzR is able to block the oscillations, and stabilizing ΔΨm in the polarized state, in a reversible manner whereas the PTP inhibitor does not. See [28] for numerous other lines of evidence excluding the involvement of PTP and Ca2+ in the response.
Since the approach to mitochondrial criticality (Fig. 2), and the subsequent oscillations in mitochondrial energetics [28], were insensitive to CsA, but inhibited by PK11195 (an isoquinoline carboxamide that binds to the mBzR), 4′Cldiazepam (a benzodiazepine antagonist), and DIDS (a general anion transport inhibitor that was previously shown to inhibit IMAC [50]), we hypothesize that the centum picosiemen channel could be the relevant single channel target responsible for cell-wide synchronized ΔΨm depolarization. Further investigation will be required to test whether this channel is ROS sensitive, and whether it can transport O2·−, as we have postulated based on experimental [28] and modeling [43] results.
In support of this general scheme, we have also shown than an agonist of the mBzR, FGIN-1–27 [51], produced an effect opposite to that of the mBzR blockers, that is, it favored permanent ΔΨm depolarization [28].
Importantly, the oscillatory depolarizations of ΔΨm did not involve the classical PTP (see Fig. 5 and numerous other lines of evidence in [28]) or depend on intracellular Ca2+ [28], underscoring the unique features of the mitochondrial oscillator in cardiac myocytes. Although both the PTP and the IMAC are capable of depolarizing ΔΨm, we hypothesize that IMAC is an early and reversible (no mitochondrial constituents are lost) target of ROS that is also likely to play a role in normal physiology, perhaps involved in the regulation of mitochondrial volume [22], or in intracellular ROS signaling [52]. It remains to be determined whether, under more severe conditions involving multiple noxious stimuli (e.g. Ca2+ overload, acidosis, lipid degradation), there is a progression from IMAC-mediated depolarization to PTP-mediated mitochondrial injury. Early evidence in the whole heart suggests that this may be true in the case of ischemia and reperfusion.
5. Links between mitochondrial depolarization and electrical excitability
5.1. Effects on the cellular action potential
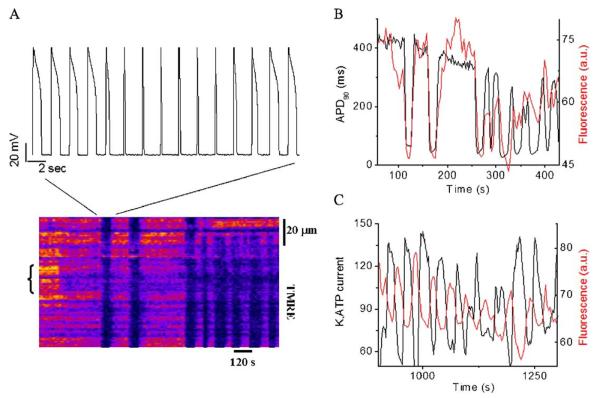
Mitochondrial criticality has profound effects on excitation-contraction coupling, the primary function of the cardiac cell. The correlation between ΔΨm depolarization and the shortening and elimination of the cellular action potential is unequivocal (Fig. 6A and B). This effect is a consequence of the uncoupled mitochondria consuming energy stores and activating sarcolemmal ATP-sensitive K+ current (IKATP) (Fig. 6C). This renders the cell electrically inexcitable at the nadir of the ΔΨm oscillation. Even when this limitation is overcome by artificially depolarizing the myocyte with whole-cell voltage clamp pulses, the effects of metabolic oscillation on the processes of Ca2+ handling and contraction are still evident [3], due to the inherent dependence of these processes on the energy state of the cell.
Fig. 6.
Mitochondrial oscillations drive the electrical excitability of the cardiomyocyte. (A) Action potentials (AP) (upper panel) evoked by brief current injections were recorded in current-clamp mode during whole-cell patch-clamp while simultaneously imaging ΔΨm with TMRE (lower panel). During a synchronized cell-wide depolarization-repolarization cycle, the AP shortens in synchrony with fast mitochondrial depolarization, and the cell becomes inexcitable when the mitochondria are in the depolarized state (remaining upward spikes are from the stimulus only). Recovery of ΔΨm coincided with AP restoration. (B) Correlation between the action potential duration (APD) at 90% repolarization and ΔΨm. (C) Correlation between sarcolemmal KATP current measured at 0 mV (from voltage ramps) and ΔΨm. Adapted from [28].
Thus far, we have described how the failure of individual mitochondria can scale to involve whole-cell bioenergetics and the integrated function of the cardiac myocyte. The next all-important question is whether these observations in isolated myocytes are relevant to the pathophysiology of the whole heart under conditions of ischemia and reperfusion.
5.2. Scaling of mitochondrial criticality to the whole heart: ischemia-related arrhythmias
When subjected to stress, the normally well-coordinated function of the heart degenerates and the synchronized dynamics of electrical and contractile activity become unstable [53,54]. Asynchrony arises in part from spatial heterogeneity in the electrical properties of the cells in the myocardial syncytium. While well studied at a descriptive level, abnormalities in electrical conduction and excitability during ischemia and reperfusion are not well understood mechanistically. Three leading hypotheses are that (i) membrane excitability is decreased as a consequence of sarcolemmal depolarization and partial inactivation of Na+ currents related to extracellular K+ accumulation, (ii) action potential shortening occurs as a result of KATP channel opening, and (iii) gap junctional conductance is decreased, resulting in conduction block. While action potential shortening occurs within the first 10 min of ischemia, longitudinal junctional resistance increases abruptly only after 15–20 min of global ischemia [55-57].
In light of the studies described above, we propose another mechanism that sets the stage for ischemia-related arrhythmias. The failure of the mitochondrial network of myocytes in the ischemic zone, either during ischemia or upon reperfusion, could underlie the electrical dysfunction. The early reperfusion phase would be particularly conducive to the mitochondrial critical state, since a burst of ROS production and antioxidant depletion is known to occur [58,59]. The tight linkage between the mitochondrial energetic state and cellular electrical excitability suggests that both spatial and temporal heterogeneities of mitochondrial ΔΨm polarization should be taken into consideration. Dramatic bistable or oscillatory changes in the duration of the cardiac action potential could potentially introduce enormous gradients of electrical excitability and repolarization across individual cells or groups of cells that have reached mitochondrial criticality. These “islands” of inexcitability could form the substrate for reentry underlying ventricular fibrillation. In ongoing experiments, we have begun to explore this possibility in isolated perfused guinea pig hearts, in which epicardial action potentials were optically mapped using the fluorescent voltage-sensitive indicator di-4-ANEPPS and a high-resolution photodiode array. In hearts subjected to 30 min ischemia followed by reperfusion, our recent experiments indicate that reperfusion arrhythmias can be almost completely eliminated by agents that we have shown block the ROS-induced ROS release mechanism and ΔΨm depolarization. These effects were distinct from that of the PTP blocker, CsA [60].
6. Conclusion
We have introduced the concept of mitochondrial criticality to describe how oxidative stress can, at the lowest level, collapse ΔΨm in individual mitochondria, and then scale to the whole cell depending on the state of all of the elements in the mitochondrial network. If a sufficient number of mitochondria are near threshold, the depolarization of ΔΨm can be synchronized and widespread. This originates a “cascade of failures” that scale up to the whole organ level in the form of arrhythmias, ultimately causing the death of the organism.
In terms of preventing mitochondrial criticality, apart from eliminating the metabolic stress in the first place, the most effective intervention is likely to be suppression of the source of ROS production, a difficult task given the inseparability of electron transport from mitochondrial ROS production. Alternatively, a prime therapeutic target could be IMAC, which we propose mediates both ROS transport and mitochondrial depolarization, and is conveniently modulated by a specific mitochondrial benzodiazepine receptor. Interruption of the ROS-induced ROS release mechanism could also be achieved by antioxidant therapy. However, in our experience, there is a marked time lag for these agents to take effect, as compared to inhibition of the ROS source or IMAC [28]. Antioxidant therapy required prolonged preincubation with extracellularly applied antioxidants (e.g., SOD mimetics or glutathione), which may render this strategy ineffective as a clinical application. A full understanding of the mechanism underlying mitochondrial criticality will, hopefully, lead to other effective ways of circumventing the contractile and electrical dysfunction of the post-ischemic heart in the future.
Acknowledgment
This work was supported by a grant from the National Institutes of Health: NIH R01 HL54598 (B.O'R.).
References
- 1.Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- 2.Nicholls DG, Ferguson SJ. Bioenergetics. 3rd ed. Vol. 3. Academic Press; 2002. [Google Scholar]
- 3.O'Rourke B. Pathophysiological and protective roles of mitochondrial ion channels. J. Physiol. 2000;529(Pt 1):23–36. doi: 10.1111/j.1469-7793.2000.00023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Rourke B. Evidence for mitochondrial K+ channels and their role in cardioprotection. Circ. Res. 2004;94:420–432. doi: 10.1161/01.RES.0000117583.66950.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gunter TE, Pfeiffer DR. Mechanisms by which mitochondria transport calcium. Am. J. Physiol. 1990;258:C755–C786. doi: 10.1152/ajpcell.1990.258.5.C755. [DOI] [PubMed] [Google Scholar]
- 6.Crompton M, Virji S, Doyle V, Johnson N, Ward JM. The mitochondrial permeability transition pore. Biochem. Soc. Symp. 1999;66:167–179. doi: 10.1042/bss0660167. [DOI] [PubMed] [Google Scholar]
- 7.Duchen MR. Contributions of mitochondria to animal physiology: from homeostatic sensor to calcium signalling and cell death. J. Physiol. 1999;516(Pt 1):1–17. doi: 10.1111/j.1469-7793.1999.001aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denton RM, McCormack JG. Ca2+ as a second messenger within mitochondria of the heart and other tissues. Ann. Rev. Physiol. 1990;52:451–466. doi: 10.1146/annurev.ph.52.030190.002315. [DOI] [PubMed] [Google Scholar]
- 9.Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am. J. Physiol.: Cell Physiol. 2004;287:C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 10.Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J. Exp. Med. 2000;192:1001–1014. doi: 10.1084/jem.192.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Droge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 12.Martin KR, Barrett JC. Reactive oxygen species as double-edged swords in cellular processes: low-dose cell signaling versus high-dose toxicity. Hum. Exp. Toxicol. 2002;21:71–75. doi: 10.1191/0960327102ht213oa. [DOI] [PubMed] [Google Scholar]
- 13.Otani H. Reactive oxygen species as mediators of signal transduction in ischemic preconditioning. Antioxid. Redox Signal. 2004;6:449–469. doi: 10.1089/152308604322899521. [DOI] [PubMed] [Google Scholar]
- 14.Haddad JJ. Oxygen sensing and oxidant/redox-related pathways. Biochem. Biophys. Res. Commun. 2004;316:969–977. doi: 10.1016/j.bbrc.2004.02.162. [DOI] [PubMed] [Google Scholar]
- 15.Arrigo AP. Gene expression and the thiol redox state. Free Radical Biol. Med. 1999;27:936–944. doi: 10.1016/s0891-5849(99)00175-6. [DOI] [PubMed] [Google Scholar]
- 16.Morel Y, Barouki R. Repression of gene expression by oxidative stress. Biochem. J. 1999;3:481–496. [PMC free article] [PubMed] [Google Scholar]
- 17.Halliwell B. Introduction: Free Radicals and Human Disease—Trick or Treat? Harwood Academic; Amsterdam: 1997. [Google Scholar]
- 18.Lucas DT, Szweda LI. Cardiac reperfusion injury: aging, lipid peroxidation, and mitochondrial dysfunction. Proc. Natl. Acad. Sci. U. S. A. 1998;95:510–514. doi: 10.1073/pnas.95.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marczin N, El-Habashi N, Hoare GS, Bundy RE, Yacoub M. Antioxidants in myocardial ischemia–reperfusion injury: therapeutic potential and basic mechanisms. Arch. Biochem. Biophys. 2003;420:222–236. doi: 10.1016/j.abb.2003.08.037. [DOI] [PubMed] [Google Scholar]
- 20.Griffiths EJ, Halestrap AP. Mitochondrial non-specific pores remain closed during cardiac ischaemia, but open upon reperfusion. Biochem. J. 1995;307(Pt 1):93–98. doi: 10.1042/bj3070093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garlid KD, Beavis AD. Evidence for the existence of an inner membrane anion channel in mitochondria. Biochim. Biophys. Acta. 1986;853:187–204. doi: 10.1016/0304-4173(87)90001-2. [DOI] [PubMed] [Google Scholar]
- 22.Beavis AD. Properties of the inner membrane anion channel in intact mitochondria. J. Bioenerg. Biomembr. 1992;24:77–90. doi: 10.1007/BF00769534. [DOI] [PubMed] [Google Scholar]
- 23.Beavis AD, Garlid KD. The mitochondrial inner membrane anion channel. Regulation by divalent cations and protons. J. Biol. Chem. 1987;262:15085–15093. [PubMed] [Google Scholar]
- 24.Borecky J, Jezek P, Siemen D. 108-pS channel in brown fat mitochondria might be identical to the inner membrane anion channel. J. Biol. Chem. 1997;272:19282–19289. [PubMed] [Google Scholar]
- 25.Kinnally KW, Zorov DB, Antonenko YN, Snyder SH, McEnery MW, Tedeschi H. Mitochondrial benzodiazepine receptor linked to inner membrane ion channels by nanomolar actions of ligands. Proc. Natl. Acad. Sci. U. S. A. 1993;90:1374–1378. doi: 10.1073/pnas.90.4.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sorgato MC, Keller BU, Stuhmer W. Patch-clamping of the inner mitochondrial membrane reveals a voltage-dependent ion channel. Nature. 1987;330:498–500. doi: 10.1038/330498a0. [DOI] [PubMed] [Google Scholar]
- 27.Antonenko YN, Kinnally KW, Tedeschi H. Identification of anion and cation pathways in the inner mitochondrial membrane by patch clamping of mouse liver mitoplasts. J. Membr. Biol. 1991;124:151–158. doi: 10.1007/BF01870459. [DOI] [PubMed] [Google Scholar]
- 28.Aon MA, Cortassa S, Marban E, O'Rourke B. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J. Biol. Chem. 2003;278:44735–44744. doi: 10.1074/jbc.M302673200. [DOI] [PubMed] [Google Scholar]
- 29.Vanden Hoek TL, Becker LB, Shao Z, Li C, Schumacker PT. Reactive oxygen species released from mitochondria during brief hypoxia induce preconditioning in cardiomyocytes. J. Biol. Chem. 1998;273:18092–18098. doi: 10.1074/jbc.273.29.18092. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi MA, Asada K. Superoxide anion permeability of phospholipid membranes and chloroplast thylakoids. Arch. Biochem. Biophys. 1983;226:558–566. doi: 10.1016/0003-9861(83)90325-9. [DOI] [PubMed] [Google Scholar]
- 31.Cortassa S, Aon MA, Marban E, Winslow RL, O'Rourke B. An integrated model of cardiac mitochondrial energy metabolism and calcium dynamics. Biophys. J. 2003;84:2734–2755. doi: 10.1016/S0006-3495(03)75079-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ingwall JS, Weiss RG. Is the failing heart energy starved? On using chemical energy to support cardiac function. Circ. Res. 2004;95:135–145. doi: 10.1161/01.RES.0000137170.41939.d9. [DOI] [PubMed] [Google Scholar]
- 33.Siemens A, Walter R, Liaw LH, Berns MW. Laser-stimulated fluorescence of submicrometer regions within single mitochondria of rhodamine-treated myocardial cells in culture. Proc. Natl. Acad. Sci. U. S. A. 1982;79:466–470. doi: 10.1073/pnas.79.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ichas F, Jouaville LS, Mazat JP. Mitochondria are excitable organelles capable of generating and conveying electrical and calcium signals. Cell. 1997;89:1145–1153. doi: 10.1016/s0092-8674(00)80301-3. [DOI] [PubMed] [Google Scholar]
- 35.Huser J, Blatter LA. Fluctuations in mitochondrial membrane potential caused by repetitive gating of the permeability transition pore. Biochem. J. 1999;343(Pt 2):311–317. [PMC free article] [PubMed] [Google Scholar]
- 36.Aon MA, Cortassa S, O'Rourke B. Percolation and criticality in a mitochondrial network. Proc. Natl. Acad. Sci. U. S. A. 2004;101:4447–4452. doi: 10.1073/pnas.0307156101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aon MA, O'Rourke B, Cortassa S. The fractal architecture of cytoplasmic organization: scaling, kinetics and emergence in metabolic networks. Mol. Cell. Biochem. 2004;256–257:169–184. doi: 10.1023/b:mcbi.0000009867.54552.09. [DOI] [PubMed] [Google Scholar]
- 38.Bak P. How Nature Works: The Science of Self-Organized Criticality. Copernicus; New York: 1996. [Google Scholar]
- 39.Kauffman SA. Origins of Order: Self-Organization and Selection in Evolution. Oxford Univ. Press; New York: 1993. [Google Scholar]
- 40.Sornette D. Chaos, Fractals, Self-Organization and Disorder: Concepts and Tools. Springer; Berlin: 2000. Critical Phenomena in Natural Sciences. [Google Scholar]
- 41.Kroemer G, Dallaporta B, Resche-Rigon M. The mitochondrial death/life regulator in apoptosis and necrosis. Annu. Rev. Physiol. 1998;60:619–642. doi: 10.1146/annurev.physiol.60.1.619. [DOI] [PubMed] [Google Scholar]
- 42.Plas DR, Thompson CB. Cell metabolism in the regulation of programmed cell death. Trends Endocrinol. Metab. 2002;13:75–78. doi: 10.1016/s1043-2760(01)00528-8. [DOI] [PubMed] [Google Scholar]
- 43.Cortassa S, Aon MA, Winslow RL, O'Rourke B. A mitochondrial oscillator dependent on reactive oxygen species. Biophys. J. 2004;87:2060–2073. doi: 10.1529/biophysj.104.041749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O'Rourke B, Ramza BM, Marban E. Oscillations of membrane current and excitability driven by metabolic oscillations in heart cells. Science. 1994;265:962–966. doi: 10.1126/science.8052856. [DOI] [PubMed] [Google Scholar]
- 45.Stauffer D, Aharony A. Introduction to Percolation Theory. Taylor and Francis; London: 1994. [Google Scholar]
- 46.Feder J. Fractals. Plenum Press; New York: 1988. [Google Scholar]
- 47.Schroeder M. Fractals, chaos, power laws, Minutes from an Infinite Paradise. W.H. Freeman and Company; New York: 1991. [Google Scholar]
- 48.McEnery MW, Snowman AM, Trifiletti RR, Snyder SH. Isolation of the mitochondrial benzodiazepine receptor: association with the voltage-dependent anion channel and the adenine nucleotide carrier. Proc. Natl. Acad. Sci. U. S. A. 1992;89:3170–3174. doi: 10.1073/pnas.89.8.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Joseph-Liauzun E, Farges R, Delmas P, Ferrara P, Loison G. The Mr 18,000 subunit of the peripheral-type benzodiazepine receptor exhibits both benzodiazepine and isoquinoline carboxamide binding sites in the absence of the voltage-dependent anion channel or of the adenine nucleotide carrier. J. Biol. Chem. 1997;272:28102–28106. doi: 10.1074/jbc.272.44.28102. [DOI] [PubMed] [Google Scholar]
- 50.Beavis AD, Davatol-Hag H. The mitochondrial inner membrane anion channel is inhibited by DIDS. J. Bioenerg. Biomembr. 1996;28:207–214. doi: 10.1007/BF02110652. [DOI] [PubMed] [Google Scholar]
- 51.Freeman FM, Young IG. The mitochondrial benzodiazepine receptor and avoidance learning in the day-old chick. Pharmacol. Biochem. Behav. 2000;67:355–362. doi: 10.1016/s0091-3057(00)00373-7. [DOI] [PubMed] [Google Scholar]
- 52.Aon MA, Cortassa S, O'Rourke B. Experimental demonstration of ultrafast behavior of the ROS-dependent mitochondrial oscillator of heart cells in the physiological domain. Biophys. J. 2005;88:446A. [Google Scholar]
- 53.Glass L. Cardiac electrophysiology. In: Zipes D.P.a.J., J., editors. From Cell to Bedside. W.B. Saunders Company; Philadelphia: 1995. pp. 363–370. [Google Scholar]
- 54.Garfinkel A, Chen PS, Walter DO, Karagueuzian HS, Kogan B, Evans SJ, Karpoukhin M, Hwang C, Uchida T, Gotoh M, Nwasokwa O, Sager P, Weiss JN. Quasiperiodicity and chaos in cardiac fibrillation. J. Clin. Invest. 1997;99:305–314. doi: 10.1172/JCI119159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kleber AG, Fleischhauer J, Cascio WE. Cardiac electrophysiology. In: Zipes DPAJ, J., editors. From Cell to Bedside. W.B. Saunders Company; Philadelphia: 1995. pp. 174–182. [Google Scholar]
- 56.Kleber AG, Riegger CB, Janse MJ. Electrical uncoupling and increase of extracellular resistance after induction of ischemia in isolated, arterially perfused rabbit papillary muscle. Circ. Res. 1987;61:271–279. doi: 10.1161/01.res.61.2.271. [DOI] [PubMed] [Google Scholar]
- 57.Riegger CB, Alperovich G, Kleber AG. Effect of oxygen withdrawal on active and passive electrical properties of arterially perfused rabbit ventricular muscle. Circ. Res. 1989;64:532–541. doi: 10.1161/01.res.64.3.532. [DOI] [PubMed] [Google Scholar]
- 58.Bolli R, Marban E. Molecular and cellular mechanisms of myocardial stunning. Physiol. Rev. 1999;79:609–634. doi: 10.1152/physrev.1999.79.2.609. [DOI] [PubMed] [Google Scholar]
- 59.Weiss JN, Korge P, Honda HM, Ping P. Role of the mitochondrial permeability transition in myocardial disease. Circ. Res. 2003;93:292–301. doi: 10.1161/01.RES.0000087542.26971.D4. [DOI] [PubMed] [Google Scholar]
- 60.Akar FG, Aon MA, Tomaselli GF, O'Rourke B. The mitochondrial origin of postischemic arrhythmias. J. Clin. Invest. doi: 10.1172/JCI25371. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]