Abstract
Our understanding of picornavirus RNA replication has improved over the past 10 years, due in large part to the discovery of cis-active RNA elements (CREs) within picornavirus RNA genomes. CREs function as templates for the conversion of VPg, the Viral Protein of the genome, into VPgpUpUOH. These so called CREs are different from the previously recognized cis-active RNA sequences and structures within the 5′ and 3′ NTRs of picornavirus genomes. Two adenosine residues in the loop of the CRE RNA structures allow the viral RNA-dependent RNA polymerase 3DPol to add two uridine residues to the tyrosine residue of VPg. Because VPg and/or VPgpUpUOH prime the initiation of viral RNA replication, the asymmetric replication of viral RNA could not be explained without an understanding of the viral RNA template involved in the conversion of VPg into VPgpUpUOH primers. We review the growing body of knowledge regarding picornavirus CREs and discuss how CRE RNAs work coordinately with viral replication proteins and other cis-active RNAs in the 5′ and 3′ NTRs during RNA replication.
1. Introduction
Picornavirus RNA genomes are mRNAs that also function as templates for viral RNA replication. Considering the RNA World hypothesis (Gesteland et al., 1999), picornaviruses represent modern day organisms with evolutionarily ancient replication strategies. Picornaviruses replicate via RNA intermediates within replication complexes assembled on lipid membranes in the cytoplasm of eukaryotic host cells. The replication of viral RNA within oligomeric viral protein particles or membranous replication complexes containing oligomerized viral proteins (Kopek et al., 2007) reveals common features of positive-strand RNA viruses, dsRNA viruses, and retroviruses (Ahlquist, 2006). Co-localization of viral proteins and viral nucleic acid templates within replication complexes facilitates interactions between viral proteins and viral RNA templates and also sequesters viral replication intermediates (like dsRNA) from innate antiviral host proteins. When picornavirus RNA genomes like that of poliovirus are introduced into cell-free reactions containing cytoplasmic extracts from uninfected host cells the viral RNA is translated, the viral polyproteins are processed, viral RNA replication complexes are assembled, viral RNA is replicated, and newly synthesized RNA genomes are packaged into progeny virus (Barton and Flanegan, 1993; Molla et al., 1991). Thus, all of the metabolic steps of picornavirus mRNA translation, RNA replication, and virus assembly are faithfully recapitulated in cell-free reactions containing cytoplasmic extracts. In addition to poliovirus, cell-free reactions containing cytoplasmic extracts support the translation and replication of EMCV (Svitkin and Sonenberg, 2003), rhinovirus 14 (Todd et al., 1997b), Aichi virus (Nagashima et al., 2005), and Coxsackievirus B3 RNAs (van Ooij et al., 2006). These data document the incredible ability of picornavirus RNA genomes, via cis-active RNA sequences and structures, to co-opt the cellular translation machinery of the cytoplasm, to express viral proteins, to build viral replication complexes anchored on host lipid membranes, to efficiently replicate viral RNA, and to assemble progeny virus.
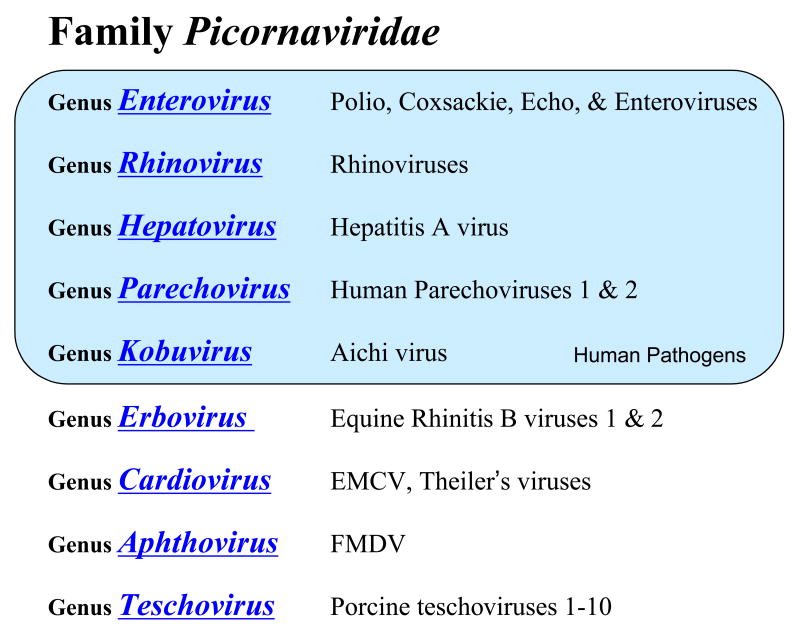
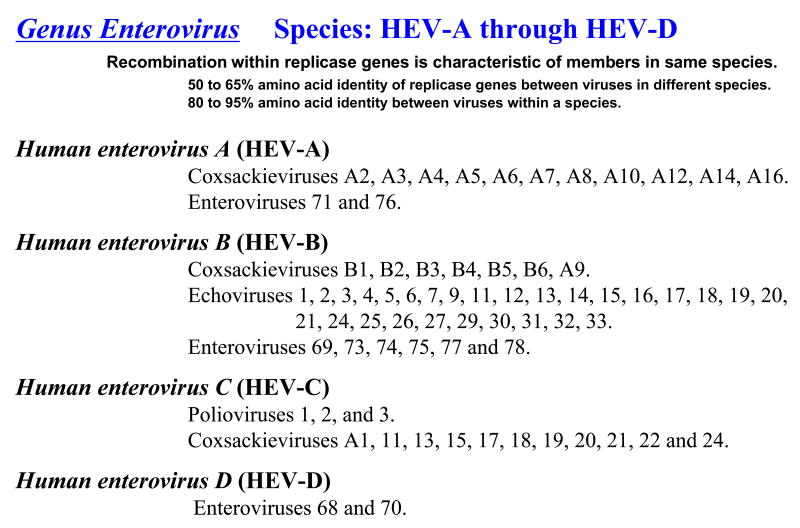
Picornaviruses are a family of positive-strand RNA viruses in the order Picornavirales (Le Gall et al., 2008). There are nine genera in the Picornaviridae family: Enterovirus, Rhinovirus, Hepatovirus, Parechovirus, Kobuvirus, Erbovirus, Cardiovirus, Aphthovirus, and Teschovirus (Figure 1) (Stanway et al., 2002). Viruses from these genera include many notorious human and agricultural pathogens. Polioviruses, rhinoviruses, and hepatitis A virus are well known human pathogens. Foot and mouth disease virus (FMDV), encephalomyocarditis virus (EMCV), and Theiler's virus are notable non-human pathogens. As exemplified by the four human enterovirus species (HEV-A, HEV-B, HEV-C, and HEV-D) (Figure 2), each picornavirus genus often includes several species and there are often many individual serotypes of virus within each species. In addition to the viruses mentioned above, there are many new picornaviruses being discovered, including simian enteroviruses (Oberste et al., 2007b), a new genus of human rhinoviruses (Briese et al., 2008; Lau et al., 2007), and new human (Oberste et al., 2007a) and non-human picornaviruses (Hales et al., 2008).
Figure 1. The Picornaviridae family.
Figure 2. Virus in the genus Enterovirus: human enterovirus species A-D.
RNA recombination between more than one member of a particular picornavirus species leads to viable recombinants, providing a biologically meaningful relationship between individual viruses within each species (Brown et al., 2003; Oberste et al., 2004a; Oberste et al., 2004b; Oberste et al., 2004c). RNA recombination between individual viruses within a species occurs in the regions of the genome encoding for picornavirus replication proteins (Brown et al., 2003). Replication genes within this region of the genome have a high degree of identity (80-95% identity between viruses within a species) which allows for viable chimeric virus. Recombination between viruses in alternate species is restricted because the chimeric viral proteins are too unrelated to function effectively with the chimeric RNA genome. While RNA recombination can occur within intratypic capsid genes (i.e., the same serotype), the degree of variation between different (intertypic) serotypes, even those within a species, restricts the production of viable progeny. RNA recombination during viral RNA replication in co-infected cells is important in the poliovirus eradication campaign, where Sabin strains of the live-attenuated oral poliovirus vaccine recombine with non-polio group C enteroviruses in vaccinees and their contacts leading to progeny vaccine-derived polioviruses that have the capacity to circulate in human populations and cause paralytic poliomyelitis (reviewed in (Kew et al., 2005)). Thus, the production of viable chimeric progeny from RNA recombination helps define picornaviral species and is also biologically relevant in nature.
Although there are many different picornaviruses with various degrees of relatedness, all picornaviruses share several common features. All picornaviruses have single-stranded RNA genomes of positive polarity covalently linked to 5′ terminal viral proteins. The RNA genome contains a 5′ nontranslated region (NTR) with an internal ribosome entry site (IRES), an open reading frame encoding the viral capsid proteins and the viral replication proteins, a 3′ NTR and a 3′ poly(A) tail. The contribution of particular cis-active RNA elements to viral mRNA stability (Kempf and Barton, 2008), viral mRNA translation (Trono et al., 1988), and viral RNA replication have been determined for several representative picornaviruses. While some cis-active RNA elements like 3′ poly(A) sequences are functionally relevant during several steps of virus replication, including viral mRNA stability (Sachs, 1990), viral mRNA translation (Thoma et al., 2004), and viral RNA replication (Silvestri et al., 2006); this review will highlight the cis-active RNA elements of picornavirus RNA genomes involved only in RNA replication. Other chapters in this special edition highlight the role of Picornavirales IRES RNAs in viral mRNA translation.
2. Picornavirus RNA genomes and viral replication proteins
Picornavirus RNA genomes, including poliovirus (Figure 3), are composed of a covalently linked 5′ terminal protein called VPg (Viral Protein of the genome), a 5′ nontranslated region (NTR), an open reading frame (ORF), a 3′ NTR and a poly(A) tail of variable length (∼ 20 to 150 adenosine residues long). The capsid proteins are encoded within the 5′ end of the ORF while the nonstructural viral proteins required for RNA replication are encoded within the 3′ two thirds of the viral ORF (Figure 3). The chemical formula for poliovirus (C322,652 H492,368 N98,245 O131,196 P7,501 S2,340), as reported by Eckard Wimmer (Wimmer, 2006), reveals the presence of 7501 phosphorus atoms in poliovirus. These phosphorus atoms correspond to the phosphodiester moieties of ribonucleotides in the 7,500 base long PV RNA which is packaged within PV particles (Figure 3).
Figure 3. Poliovirus and the poliovirus RNA genome.
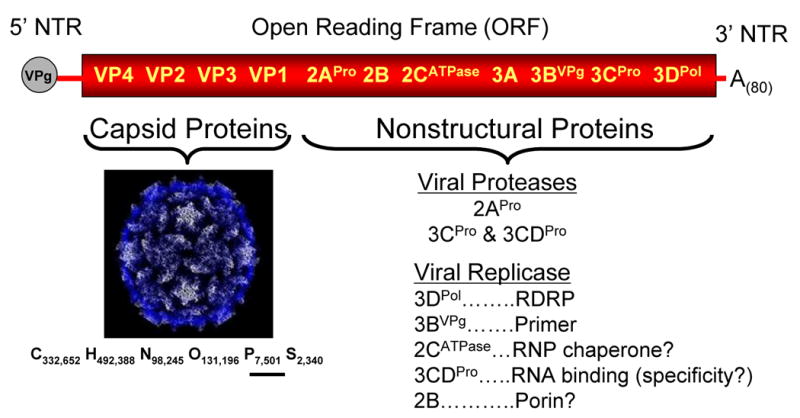
Poliovirus RNA genome (illustrated at the top of the figure) is packaged within virus particles (bottom left). The RNA genome is an mRNA encoding the capsid proteins and nonstructural proteins. Nonstructural proteins include the viral proteases and viral replicase proteins. Chemical formula for poliovirus from (Wimmer, 2006).
Translation of PV RNA in the cytoplasm of infected cells followed by polyprotein processing by viral proteases 2APro and 3CDPro results in the synthesis of several PV proteins, all of which contribute to the formation and function of RNA replication complexes. 3DPolymerase (3DPol) is a primer-dependent RNA-dependent RNA polymerase (Figure 3) (Flanegan and Baltimore, 1977; Neufeld et al., 1991). VPg (also called viral protein 3B) and the uridylylated form of VPg (VPgpUpUOH) function as primers for PV RNA replication (Paul, 2002; Paul et al., 1998), thereby becoming covalently linked to the 5′ ends of PV RNA (Lee et al., 1977; Nomoto et al., 1977). 2CATPase (and its larger polyprotein precursor 2BC) has several critical roles associated with viral RNA replication including host membrane rearrangements associated with the formation of RNA replication complexes (Cho et al., 1994; Teterina et al., 1997), RNA binding domains that interact with viral RNA (Banerjee et al., 2001; Rodriguez and Carrasco, 1995), and ATPase activity required for the initiation of negative-strand RNA synthesis (Barton and Flanegan, 1997) as well as the conversion of VPg into VPgpUpUOH (Lyons et al., 2001)(as discussed in more detail below). 2B is a porin that alters the permeability of host cell membranes (Madan et al., 2007; Nieva et al., 2003), however its precise role(s) in viral replication are unclear. Viral protein 3CD is a protease involved in viral polyprotein processing (Harris et al., 1992), specific host protein cleavages (Kundu et al., 2005; Sharma et al., 2004), as well as an RNA binding protein with important functions during RNA replication (Andino et al., 1993; Andino et al., 1990b; Gamarnik and Andino, 2000; Harris et al., 1994; Shen et al., 2007; Yang et al., 2004) (as discussed in more detail below). 2APro functions during RNA replication (Li et al., 2001); however, its mechanistic role(s) are unclear.
3. Cis-active RNA elements required for viral RNA replication reside in the 5′ NTR, the 3′ NTR and the ORF of picornavirus RNA genomes
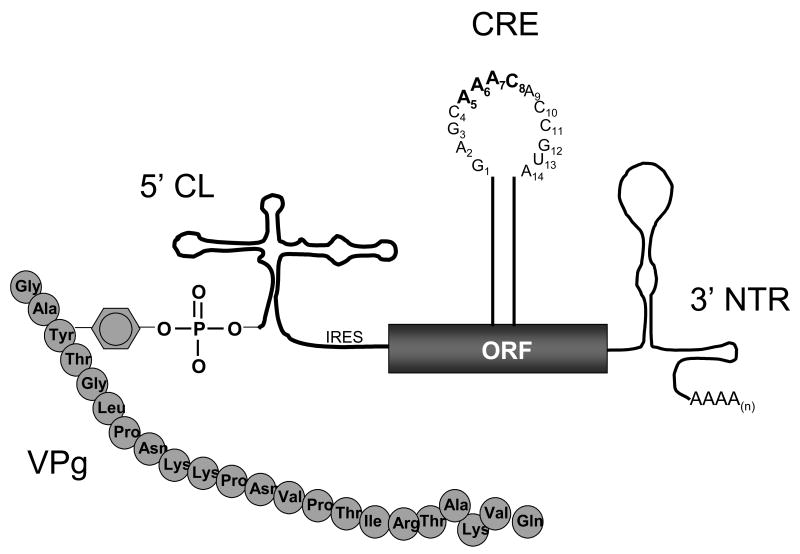
In the case of poliovirus, there are four distinct cis-active RNA elements required for viral RNA replication (Figure 4); a cloverleaf RNA structure at the 5′ terminus of PV RNA (5′ CL), a cis-replication element in the ORF (CRE), the 3′ NTR, and the 3′ poly(A) tail. The 5′ cloverleaf RNA forms ribonucleoprotein complexes (RNPs) containing poly r(C) binding protein (PCBP) and 3CD that are required for RNA replication (Barton et al., 2001; Herold and Andino, 2001). The CRE RNA located in the ORF templates the uridylylation of VPg to make VPgpUpUOH and is the primary focus of this review (as discussed below). The 3′ NTR can be deleted from poliovirus without completely preventing replication (Todd et al., 1997a); however, the 3′ NTR is required for efficient RNA replication (Brown et al., 2005). The poly(A) tail is required for RNA replication (Sarnow, 1989). A poly(A) tail ≥20 bases long supports wildtype levels of viral RNA replication (Silvestri et al., 2006). When the poly(A) tail is reduced to 12 nucleotides in length viral negative-strand RNA synthesis is reduced and when the poly(A) tail is ≤ 8 bases long negative-strand RNA synthesis ceases (Herold and Andino, 2001; Silvestri et al., 2006). Comparable and/or related cis-active RNAs are present in the 5′ NTR, ORF, and 3′ NTR of other picornavirus RNA genomes. Together, these cis-active elements mediate the specific replication of viral RNA genomes via interactions with specific viral replication proteins. Furthermore, these cis-active RNAs mediate particular mechanistic steps of RNA replication, like the conversion of VPg into VPgpUpUOH as discussed below.
Figure 4. VPg and cis-active RNA sequences and structures required for poliovirus RNA replication.
VPg, a 22 amino acid long viral protein, is covalently linked by the tyrosine hydroxyl to the 5′ terminus of viral RNA via a phosphodiester (Ambros and Baltimore, 1978). There are four distinct cis-active RNA sequences and structures required for RNA replication: a cloverleaf RNA sequence and structure at the 5′ terminus (5′ CL), a cis-replication element (CRE) in the open reading frame, a 3′ nontranslated region (3′ NTR), and a 3′ poly(A) tail.
The IRES within the 5′ NTR of PV RNA is not directly required for viral RNA replication, and is therefore not illustrated in detail in Figure 4. When the PV IRES was replaced by heterologous IRES RNAs from EMCV and HCV, the chimeric viral RNAs replicated efficiently (Alexander et al., 1994; Lu and Wimmer, 1996; Zhao et al., 2000; Zhao et al., 1999). Furthermore, when IRES RNA sequences were deleted from PV RNA templates the viral RNAs were still efficient templates for RNA replication when PV proteins were provided in trans (Murray et al., 2004). These investigations revealed that the IRES RNA sequences could be substituted with heterologous sequences or deleted from PV RNA templates without preventing RNA replication. Together these experiments establish that IRES RNA sequences (and the proteins that interact with them) are not directly involved in viral RNA replication.
4. VPg and VPgpUpUOH
There are two forms of VPg within infected cells, VPg and VPgpUpUOH (Crawford and Baltimore, 1983). These two forms of VPg function as primers for the primer-dependent RNA-dependent RNA polymerase 3DPol. Much of the research in the area of picornavirus RNA replication over the past 10 years has been work designed to determine:
how VPg is converted into VPgpUpUOH,
which of these two primers prime the initiation of negative-strand RNA synthesis, and
which of these two primers prime the initiation of positive-strand RNA synthesis.
The goals of this research are to understand and to explain the mechanisms by which PV RNA is replicated in an asymmetric manner, wherein positive-strand RNA is made to great excess relative to negative-strand RNA (reviewed in (Paul, 2002; Sean and Semler, 2008)).
5. The discovery of CRE
While RNA elements in the 5′ NTRs (Andino et al., 1993; Andino et al., 1990a; Andino et al., 1990b) and 3′ NTRs (Pilipenko et al., 1992; Rohll et al., 1995) of picornavirus RNA genomes were known to be required for RNA replication for many years, until more recently no one had investigated whether there were RNA elements within the ORF of picornavirus RNA genomes which were required for RNA replication. Replication competent defective interfering particles with large in-frame deletions in the capsid encoding region of poliovirus suggested that capsid proteins and the RNA sequences encoding them were not required for RNA replication (Cole and Baltimore, 1973; Cole et al., 1971; Nomoto et al., 1979). Since deleting capsid protein encoding sequences also resulted in deletion of all RNA elements in this region, these studies suggested that there were no RNA elements in the capsid encoding region of the poliovirus ORF required for RNA replication. These observations inspired the construction of poliovirus RNA replicons without capsid encoding sequences that retained RNA sequences encoding the non-structural replication proteins and the necessary cis-active RNA sequences required for RNA replication (Collis et al., 1992; Hagino-Yamagishi and Nomoto, 1989; Kaplan and Racaniello, 1988). In contrast, when McKnight and Lemon deleted the capsid encoding region of the ORF of human rhinovirus-14 (HRV-14) in attempts to make RNA replicons like those for poliovirus, such rhinovirus RNAs were completely defective for RNA replication (McKnight and Lemon, 1996). It was subsequently discovered that there was a cis-acting replication element (CRE) within the HRV-14 VP1 capsid encoding region that was required for RNA replication (McKnight and Lemon, 1998).
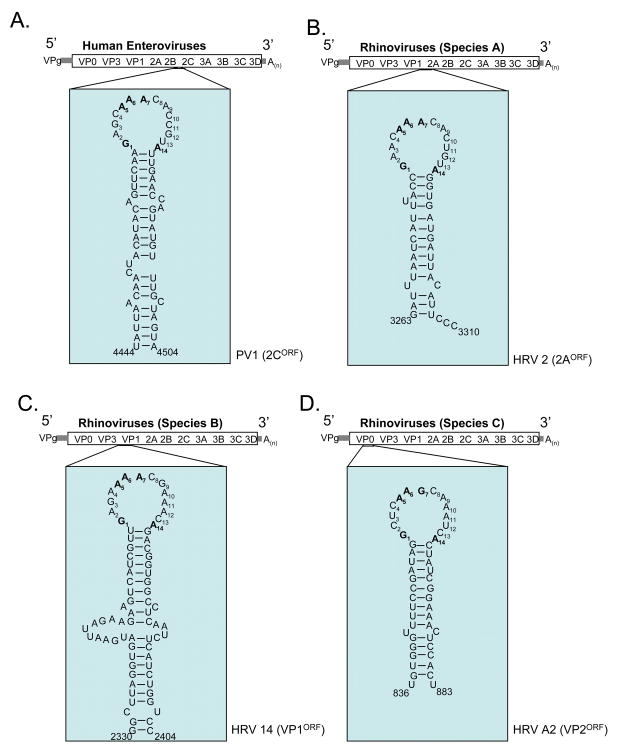
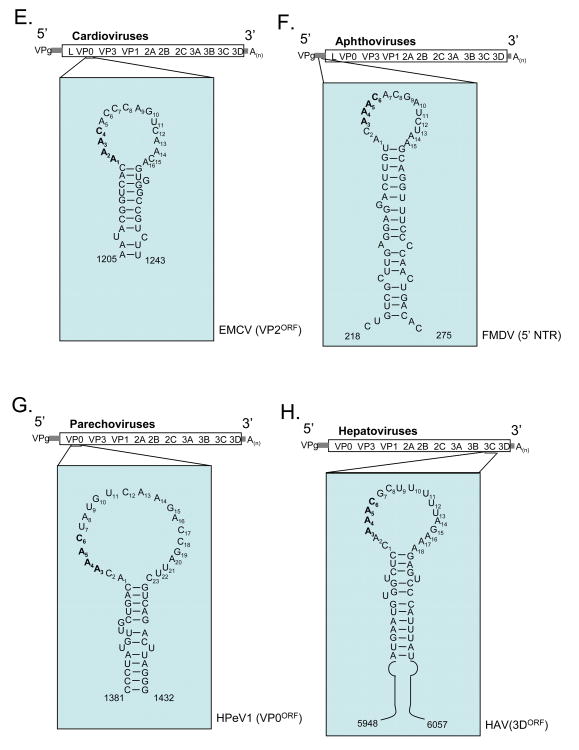
Of the nine genera in the Picornaviridae family, a CRE like that found in HRV-14 has been identified in six of them: Enterovirus (Goodfellow et al., 2000; Paul et al., 2000), Rhinovirus (Gerber et al., 2001; McKnight and Lemon, 1998), Cardiovirus (Lobert et al., 1999), Aphthovirus (Mason et al., 2002), Parechovirus (Al-Sunaidi et al., 2007), and Hepatovirus (Yang et al., 2008) (Fig. 5). All but the Parechovirus and species C rhinovirus CREs have been shown to be required for RNA replication. The Parechovirus and species C rhinovirus CREs have yet to be experimentally tested and confirmed. A putative CRE has not been identified or reported for virus in the Kobu-, Erbo- or Teschovirus genera. While a CRE is presumably present within all picornaviral genomes, its location in the genomic RNA varies substantially depending on the picornavirus genus or species (Fig. 5). CRE is located in the 2C open reading frame of enteroviruses (Goodfellow et al., 2000; Paul et al., 2000), the 2A open reading frame of species A rhinoviruses (Gerber et al., 2001), the VP1 open reading frame of species B rhinoviruses (McKnight and Lemon, 1998), the VP2 open reading frame of species C rhinoviruses (Cordey et al., 2008), the VP2 region of cardioviruses (Lobert et al., 1999), just upstream of the IRES in the Aphthovirus foot-and-mouth disease virus (FMDV) (Mason et al., 2002), the VP0 region of parechoviruses (Al-Sunaidi et al., 2007) and the open reading frame of 3D for hepatoviruses (Yang et al., 2008). (Fig. 5A-H). FMDV is the only picornavirus thus far that has CRE located outside the viral open reading frame. The variable location of CRE within distinct picornaviruses suggests that its position within the genome may not be of particular importance and indeed several studies have demonstrated the positional independence of the CRE (Goodfellow et al., 2000; Goodfellow et al., 2003a; Mason et al., 2002; Yin et al., 2003) (Yang et al., 2008). Nonetheless, the natural location of CRE (as described above and in Fig. 5A-H) is conserved within the RNA genomes of particular picornaviral species (Cordey et al., 2008).
Figure 5. Location and secondary structures of CREs.
The location in the viral genome and the secondary structures of representative CREs. Nucleotide numbers from full-length RNA genome sequences. (A) PV1 CRE, representative of CREs in human enterovirus species A-D (Goodfellow et al., 2000; van Ooij et al., 2006) (B) Human rhinovirus 2 (HRV 2) CRE, representative of species A rhinoviruses (Gerber et al., 2001). (C) HRV 14 CRE, representative of species B rhinoviruses (McKnight and Lemon, 1998). (D) HRV A2 CRE, representative of species C rhinoviruses (Cordey et al., 2008). (E) EMCV CRE, representative of Cardioviruses (Lobert et al., 1999). (F) FMDV CRE, representative of Apthoviruses (Mason et al., 2002). The FMDV CRE is located 218 to 275 bases downstream from the variable length poly(C) tract in the 5′ NTR. (G) Human parechovirus 1 (HPeV1) CRE, representative of Parechoviruses (Al-Sunaidi et al., 2007). (H) Hepatitis A virus (HAV) CRE, representative of Hepatoviruses (Yang et al., 2008). Only sequences from the apex of the 110 nt long HAV CRE are presented due to space constraints.
The CREs of human enteroviruses and rhinoviruses have characteristic 14 base loops where the 1st base is a purine, the 5th and 6th bases are A residues involved in templating the addition of uridine onto VPg (as discussed below), the 7th residue is a purine, and the 14th residue is a purine (Fig. 5A-D). Therefore, human enterovirus and rhinovirus CREs share a common sequence motif within the loop, RNNNAARNNNNNNR (Fig. 5A-D, conserved residues of loops in bold) (Cordey et al., 2008; Yang et al., 2002), which likely corresponds to a common structure (Thiviyanathan et al., 2004). The loop structures at the apex of Cardio-, Aphtho-, Parecho-, and Hepatovirus CREs are more variable in length (15 to 23 bases long) (Fig. 5E-H). Nonetheless, the AAAC sequence common in the loop of picornavirus CREs is evident (Fig. 5E-H, AAAC sequence in bold). The dsRNA stems of CRE RNA structures, with various internal loops and bulges, are more variable in sequence and length. Therefore, the more important feature of CREs is the loop structure containing the conserved adenosine residues. Below we discuss how CRE RNA sequences and structures participate in the conversion of VPg into VPgpUpUOH.
6. Conversion of VPg into VPgpUpUOH
Following the discovery of the HRV14 CRE by Kevin McKnight in the Lemon laboratory (McKnight and Lemon, 1998), Ian Goodfellow in the laboratory of David Evans discovered the CRE of poliovirus (Goodfellow et al., 2000). The poliovirus CRE is a stem-loop RNA structure of ∼61 nucleotides in the 2CATPase portion of the ORF (Figure 5A). This CRE RNA structure is conserved in the 2CATPase portion of the ORF of all human enteroviruses (HEV-A, HEV-B, HEV-C, & HEV-D).
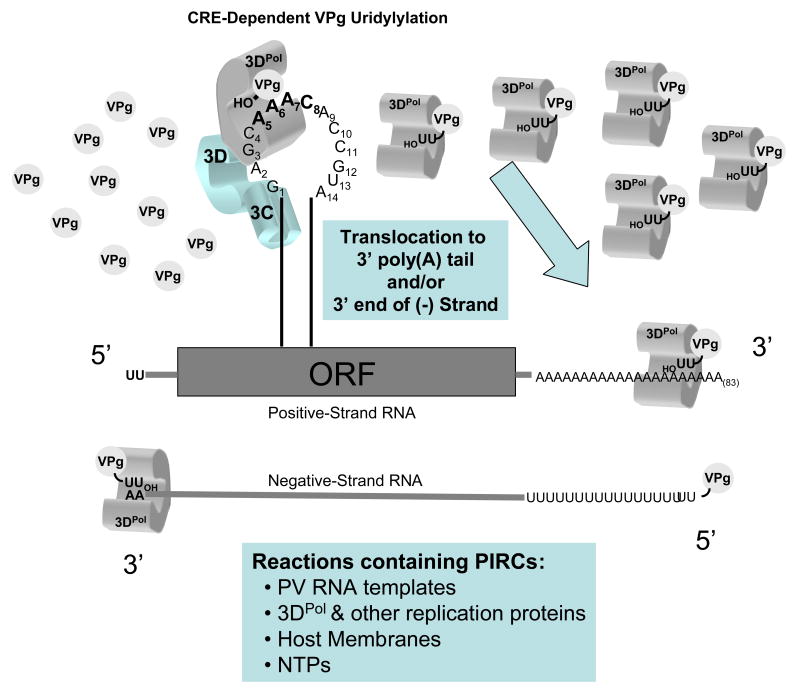
Aniko Paul in the Wimmer lab subsequently established that the poliovirus CRE RNA functioned as a template for the uridylylation of VPg, resulting in the synthesis of VPgpUpUOH (VPg with two uridines covalently attached via a phosphodiester linkage to the tyrosine hydroxyl of VPg) (Paul et al., 2003a; Paul et al., 2000; Paul et al., 2003b). Conversion of VPg into VPgpUpUOH was accomplished in defined reactions containing purified components including the CRE RNA template, 3DPol, VPg, and UTP (Figure 6) (Paul et al., 2003a; Paul et al., 2000; Paul et al., 2003b). Investigations using these defined reactions established that the viral RNA-dependent RNA polymerase 3DPol was responsible for catalyzing the addition of two uridine residues to the tyrosine residue of VPg and that the A5 residue, in conjunction with the A6 residue in the loop of the CRE RNA structure, functioned in a “slide-back” mechanism to template the addition of UTP onto the tyrosine residue of VPg (Figure 6 and reference (Paul et al., 2003b)). Each VPgpUpUOH product probably remains associated with the 3DPol molecule involved in its synthesis (as illustrated in Fig. 6).
Figure 6. CRE-dependent VPg uridylylation.
VPg is converted into VPgpUpUOH in defined reactions containing CRE RNA templates, 3DPol, VPg, and UTP (Paul et al., 2003a; Paul et al., 2000; Paul et al., 2003b). Viral protein 3CD stimulates these reactions (Nayak et al., 2005; Nayak et al., 2006; Pathak et al., 2007; Pathak et al., 2002; Yang et al., 2004; Yin et al., 2003).
Viral protein 3CD stimulates the CRE-dependent conversion of VPg into VPgpUpUOH; however, the mechanism by which 3CD stimulates the reaction is unclear (Figure 6). An RNA binding domain within 3CPro is required for stimulation of CRE-dependent VPgpUpUOH synthesis and has been shown to bind to the upper portion of the CRE stem (Nayak et al., 2005; Nayak et al., 2006; Pathak et al., 2007; Pathak et al., 2002; Yang et al., 2004; Yin et al., 2003). While 3CPro alone can stimulate the reaction, it does not do so as well as 3CDPro, suggesting that the 3DPol portion of 3CDPro is also contributing to the uridylylation reaction (Nayak et al., 2006; Pathak et al., 2007). The involvement of the 3DPol moiety was confirmed by mutations within 3DPol of HRV14 that allow HRV14 to adapt to using a PV CRE for RNA replication and also by the presence of 3DPol in 3CDPro contributing to specificity of CRE binding by 3CDPro (Shen et al., 2007).
CRE-dependent VPg uridylylation was confirmed for several other picornaviruses using cognate 3DPol and CRE RNA templates from human rhinovirus 2 (Gerber et al., 2001), foot and mouth disease virus (Nayak et al., 2005; Nayak et al., 2006), Coxsackievirus B3 (van Ooij et al., 2006), and human rhinovirus 14 (Shen et al., 2007). Together, the experimental investigations using defined reactions have allowed for detailed analyses of the minimal components required for conversion of VPg into VPgpUpUOH, however, these defined reactions do not support normal viral RNA replication wherein the VPg and/or VPgpUpUOH primers support asymmetric RNA replication. Below we will discuss how CRE RNAs function coordinately with other cis-active RNAs in picornavirus RNA templates and the roles of VPg and VPgpUpUOH primers during the asymmetric replication of picornavirus RNA.
7. PV RNA Replication
CRE RNAs do not function independently during viral RNA replication. Rather, CRE RNAs function coordinately with the other cis-active RNA sequences within picornavirus RNA and with the full complement of viral replication proteins within membranous RNA replication complexes (Bienz et al., 1992). Sequestering components of replication within membranous structures is an efficient way to keep multiple copies of viral proteins, like the membrane-associated precursors of VPg primers (Fujita et al., 2007), in close proximity to viral RNA templates. During RNA replication, each viral positive-strand RNA is copied into a complementary negative-strand RNA and the negative-strand RNA is then copied into 40 to 70 positive-strand RNA progeny (Novak and Kirkegaard, 1991). A VPg molecule is covalently attached to the 5′ end of each negative- and positive-strand RNA molecule (Pettersson et al., 1978). In addition, hundreds of excess VPgpUpUOH molecules accumulate for each negative-strand RNA intermediate (Lyons et al., 2001). Therefore, each membranous RNA replication complex must accumulate many copies of VPg precursors before RNA replication commences. In contrast to the VPg molecules used in defined VPg uridylylation reactions (Figure 6), VPg is thought to accumulate within membranous RNA replication complexes as larger precursor polyproteins (3ABCD, 3BCD, 3ABC, and/or 3AB) (Towner et al., 1998). The actual composition of membranous RNA replication complexes is unknown; however, from the discussion above one can begin to imagine that many copies of particular viral replication proteins are likely present in each membranous RNA replication complex, along with one or more viral RNA templates.
Preinitiation RNA replication complexes (PIRCs) have proven to be a particularly useful tool to identify the coordinate interactions of distinct cis-active RNAs and viral proteins during poliovirus RNA replication. Guanidine HCl, a reversible inhibitor of viral protein 2CATPase, is used to allow PIRCs to accumulate within cell-free translation-replication reactions containing cytoplasmic extracts from HeLa cells (Barton et al., 1995). Removal of the reversible inhibitor guanidine from the accumulated PIRCs allows for the initiation of viral RNA replication. There are several advantageous features of this experimental system:
viral RNA replication is synchronous and sequential, with negative-strand RNA being made before positive-strand RNA (Barton and Flanegan, 1997),
viral RNA replication is asymmetric, with an excess of positive-strand RNA being made from each negative-strand template,
viral RNA replication proteins can be provided in trans to viral RNA templates (Barton et al., 2002; Murray et al., 2004),
lethal and non-lethal mutations in viral proteins and viral RNA templates can be phenotypically associated with defects in specific steps of viral RNA replication including the conversion of VPg into VPgpUpUOH, the synthesis of negative-strand RNA, and the synthesis of positive-strand RNA.
The first two important insights made using PIRCs was that the 5′ cloverleaf RNA was required in cis for negative-strand RNA synthesis (Barton et al., 2001) and that the guanidine-inhibited 2CATPase was required immediately before or at the moment of initiation of negative-strand RNA synthesis (Barton and Flanegan, 1997). We and others interpreted these results to indicate that ribonucleoproteins (RNPs) at the 5′ and 3′ termini of viral RNA templates worked coordinately via proximal orientation to mediate PV RNA replication within membranous replication complexes and that 2CATPase functions were directly involved in mediating these long range RNP interactions. Cis-active RNA at the 5′ terminus of Aichi virus also appears to be required for negative-strand RNA synthesis, providing evidence that other picornaviruses with unrelated 5′ RNA sequences share common strategies for negative-strand RNA synthesis (Nagashima et al., 2005; Nagashima et al., 2008). Another discovery made using PIRCs was that the 5′ cloverleaf of poliovirus RNA templates was required in cis for CRE-dependent VPg uridylylation within membranous RNA replication complexes and that guanidine inhibited the conversion of VPg into VPgpUpUOH (Lyons et al., 2001). These data suggested that viral 2CATPase activity, the target of guanidine inhibition (Pfister and Wimmer, 1999), is required immediately before CRE-dependent VPg uridylylation and that RNPs associated with the 5′ cloverleaf RNA may function coordinately with the CRE RNPs to help convert VPg into VPgpUpUOH in the context of membranous RNA replication complexes.
The next important insight made using PIRCs was that CRE RNAs were not required for viral negative-strand RNA synthesis (Goodfellow et al., 2003b; Morasco et al., 2003; Murray and Barton, 2003). Mutations that destroyed CRE RNA structures within viral RNA templates did not prevent negative-strand RNA synthesis, however, positive-strand RNA replication was absolutely dependent upon CRE-dependent VPg uridylylation (Goodfellow et al., 2003b; Morasco et al., 2003; Murray and Barton, 2003). Because the tyrosine hydroxyl of VPg was required for negative-strand RNA synthesis, independent of CRE or VPgpUpUOH, the Barton and Flanegan labs concluded that VPg could prime the initiation of negative-strand RNA synthesis and that VPgpUpUOH was required to prime the initiation of positive-strand RNA synthesis (Morasco et al., 2003; Murray and Barton, 2003). This was theoretically satisfying because the asymmetric replication of picornavirus RNA demands that the mechanisms of positive-strand RNA synthesis be different from the mechanisms of negative-strand RNA synthesis (to account for the synthesis of more positive-strand RNAs relative to negative-strand RNAs). The Flanegan lab went on to show that two adenosines at the 3′ end of negative-strand RNA were the key cis-active RNA sequences allowing VPgpUpUOH priming of positive-strand RNA synthesis (Figure 6, note complementarity of uridine residues in VPgpUpUOH with the two A residues at the 3′ end of negative-strand RNA templates) (Sharma et al., 2005). Importantly, VPgpUpUOH cannot prime the initiation of positive-strand RNA replication when the two adenosine residues are internal from the 3′ end of negative-strand RNA (Herold and Andino, 2000). Thus, 3DPol and VPgpUpUOH, via unknown mechanisms, are translocated from the CRE RNA templates where they are made to the 3′ terminus of negative-strand RNA templates to prime the reiterative initiation of positive-strand RNA synthesis (Figure 7). Because the 3DPol/VPgpUpUOH molecules are sequestered within membranous replication complexes they may function by mass action, where free diffusion within the confines of the replication complex favor interactions with the 3′ ends of viral RNA templates.
Figure 7. CRE-dependent VPgpUpUOH synthesis and translocation to the 3′ termini of viral RNA templates for RNA replication.
3DPol and VPgpUpUOH must translocate from the CRE portion of viral RNA templates to the 3′ ends of positive- and negative-strand RNA templates to prime the initiation of RNA synthesis. These processes are supported within viral RNA replication complexes formed within cell-free translation-replication reactions (Goodfellow et al., 2003b; Lyons et al., 2001; Morasco et al., 2003; Murray and Barton, 2003; van Ooij et al., 2006).
Another important discovery made using PIRCs and tissue culture studies was that CRE RNAs with point mutations, particularly point mutations of the A5 template residue, functioned as dominant negative inhibitors of viral RNA replication (Crowder and Kirkegaard, 2005). These mutations blocked both VPg uridylylation and negative-strand RNA synthesis (van Ooij et al., 2006). Thus, CRE RNP complexes inactivated by point mutations (especially in the templating A5 residue) somehow prevent VPg from priming negative-strand RNA synthesis. Since these point mutations also block VPgpUpUOH synthesis, some investigators interpret these studies to suggest that CRE-dependent VPgpUpUOH is the normal primer for negative-strand RNA synthesis whereas others still consider VPg as a potential primer for negative-strand RNA synthesis. Despite these alternate interpretations, there is emerging consensus that CRE RNPs work coordinately with other cis-active RNAs at the 5′ and 3′ termini of picornavirus RNA templates to mediate PV RNA replication. These potential RNP interactions provide plenty of opportunity for mechanistic coordination between membrane-associated replication proteins and seemingly distal cis-active RNA elements.
8. CREs, in conjunction with VPgpUpUOH, lower the concentrations of UTP required for viral RNA replication
De novo initiation of RNA synthesis by polymerases requires a relatively high concentration of the initiating NTP (NTPi) when compared with the concentration of NTPs required for the elongation of RNA synthesis (Km of ∼75 to 150 μM NTP for initiation and ∼5-10 μM for elongation) (van Dijk et al., 2004). In contrast, poliovirus 3DPol requires relatively low concentrations of UTP (∼4 μM Km) to uridylylate VPg in reactions containing CRE RNA templates (Korneeva and Cameron, 2007). Furthermore, when CRE-dependent VPgpUpUOH priming and CRE-independent VPg priming of poliovirus negative-strand RNA synthesis were compared a functional CRE RNA was found to lower the Km of UTP required for negative-strand RNA synthesis (Steil and Barton, 2008). VPg primed the initiation of negative-strand RNA synthesis with a Km of 103 μM UTP whereas (CRE-dependent) VPgpUpUOH primed the initiation of negative-strand RNA synthesis with a Km of 12 μM UTP (Steil and Barton, 2008). These results suggest that CRE RNAs, by virtue of their interactions with 3DPol, can lower the Km of UTP required for de novo RNA polymerization (initiation), as compared to VPg priming by 3DPol on poly(A) templates. Thus, CRE-dependent VPg uridylylation may serve two important purposes: 1) providing VPgpUpUOH primers for both negative- and positive-strand RNA replication and 2) overcoming the rate-limiting effects of NTPi concentrations, which in the absence of VPg uridylylation would otherwise restrict the initiation of RNA replication. In this manner, CRE-dependent PV RNA replication mechanisms circumvent the requirement of a high initiating NTP concentration which typically constrains RNA synthesis by prokaryotic and viral RNA polymerases (Amiott and Jaehning, 2006a; Amiott and Jaehning, 2006b; Gaal et al., 1997; Jia and Patel, 1997; van Dijk et al., 2004).
9. Unresolved aspects of RNA replication
There are several important unresolved aspects of RNA replication (Figure 7):
When is VPg converted into VPgpUpUOH (before, after, and/or during negative-strand RNA synthesis)?
When are 3AB and/or other polyprotein precursors of VPg proteolytically processed into VPg (before, after, and/or during CRE-dependent uridylylation)?
How are 3DPol proteins (and VPg) reiteratively delivered to CRE RNA templates within membranous replication complexes to allow for the synthesis of hundreds of VPgpUpUOH molecules?
What is the mechanistic contribution of 5′ cis-active RNPs to the initiation of negative-strand RNA synthesis and CRE-dependent VPg uridylylation?
How is VPgpUpUOH translocated from CRE RNA templates to the 3′ termini of viral RNA templates (Figure 7)?
How do CRE RNA-VPg-3DPol interactions lower the concentration of UTP required for protein-primed RNA polymerization (initiation)?
The evidence at hand indicates that the processes of CRE-dependent VPg uridylylation must involve dynamic events where multiple copies of 3DPol and VPg are delivered to the CRE RNA portion of viral RNA templates within membranous RNA replication complexes. The mechanistic details of these dynamic processes probably involve interactions between RNP complexes containing 5′ cis-active RNA sequences, CRE and 3′ NTR/poly(A) sequences (as proposed in (Murray and Barton, 2003)). Because precursor proteins of VPg are membrane-anchored and substrates of 3CDPro, it is easy to speculate that 5′ cloverleaf, CRE, and 3′ NTR RNPs (which all contain 3CDPro) may work coordinately to proteolytically cleave membrane-associated VPg precursors and 3CDPro precursors to deliver both VPg and 3DPol to CRE RNA templates near membranes within the replication complexes.
10. Structural studies
Atomic structures of CRE (Thiviyanathan et al., 2004), 3DPol (Appleby et al., 2005; Thompson et al., 2007; Thompson and Peersen, 2004), VPg/3DPol co-crystals (Ferrer-Orta et al., 2006), and 3CD molecules (Marcotte et al., 2007) provide insights into the manner in which these molecules would interact with cis-active viral RNA structures and with other viral proteins. The Cameron lab has proposed models for how these proteins interact with CRE (Pathak et al., 2007). In this model, two 3CD molecules bind the CRE stem and stimulate VPg uridylylation, presumably through interactions of 3CD with 3DPol. Nonetheless, it remains to be determined how 3CD and other viral proteins interact coordinately and dynamically with the 5′ cloverleaf, CRE and 3′ NTR/poly(A) to initiate negative-strand RNA synthesis and VPg uridylylation. It will be particularly interesting to determine how CRE RNA templates and VPg molecules interact with the catalytic site of 3DPol and how such interactions are much more efficient at uridylylating VPg than 3DPol interactions with other templates such as poly(A), which under some circumstances can function as a template for VPgpUpUOH synthesis (Paul et al., 1998).
11. Potential contributions of viral 2CATPase
Evidence suggests that CRE RNAs function coordinately with the other cis-active RNA sequences and structures at the 5′ and 3′ termini of picornavirus RNA templates, presumably via RNP interactions that may be facilitated by 2CATPase activity. The mechanistic function of 2CATPase activity is unclear. While it has long been considered a putative helicase (Kadare and Haenni, 1997), diligent efforts failed to detect 2C-mediated helicase activity (Pfister et al., 2000; Pfister and Wimmer, 1999) and the requirement of 2CATPase activity before and/or at the moment of initiation of RNA replication (Barton and Flanegan, 1997; Lyons et al., 2001; Murray and Barton, 2003) made us consider other possible mechanistic roles of 2CATPase activity. We currently favor the possibility that 2CATPase functions as a RNP chaperone to mediate the formation of functional RNP complexes on viral cis-active RNA structures. We consider this function to be similar to the RNP chaperone activities of SM-like proteins, which are required to assemble functional RNP complexes involved in RNA splicing (Pellizzoni et al., 2002b).
Dreyfuss et al. show that snRNP complexes can self-assemble by combining individual protein and RNA subunits in vitro (in the absence of SMN complexes) but that the ATPase activity of SMN complexes is required for the assembly and activity of snRNPs in cells (Pellizzoni et al., 2002b). snRNPs can self assemble inappropriately in vitro on U-rich RNAs other than snRNAs, leading to nonfunctional RNPs. Dreyfuss et al. show that SMN complexes are machines capable of preventing assembly of snRNPs on U-rich RNAs other than snRNAs (Pellizzoni et al., 2002b). The ATPase activity of particular subunits within SMN complexes is necessary for this ability to prevent illicit assembly of nonfunctional snRNPs (Baccon et al., 2002; Friesen et al., 2002; Gubitz et al., 2002; Pellizzoni et al., 2002a; Pellizzoni et al., 2002b; Wang and Dreyfuss, 2001a; Wang and Dreyfuss, 2001b). Thus, SMN complexes, through ATPase activity, function as RNP chaperones to prevent illicit assembly of snRNPs (Pellizzoni et al., 2002b). We hypothesize that ATPases within viral replication complexes function in a similar manner. In the case of poliovirus, we speculate that 2CATPase may function as a RNP chaperone within membranous replication complexes to coordinately assemble 5′ cloverleaf, 2C-CRE, and 3′NTR RNPs at the appropriate points in time with the appropriate protein subunits. This hypothesis accounts for some of the apparent paradoxes observed between experiments using purified subunits (reconstitution experiments) and work using preinitiation RNA replication complexes (Agol et al., 1999). Purified 3DPol can be used to make negative-strand RNA in vitro; however, 3DPol will use virtually any nonviral template RNA and any primer in vitro (Tuschall et al., 1982). Using purified 3DPol, VPg can be uridylylated in vitro in the absence of membranes or 2CATPase on various authentic or inauthentic templates (Paul et al., 2000; Paul et al., 1998; Rieder et al., 2000), However, within preinitiation RNA replication complexes, VPg uridylylation requires the 5′ cloverleaf and 2CATPase activity and VPg uridylylation occurs exclusively on CRE RNA sequences within PV RNA templates rather than on 3′ poly(A) sequences of PV RNA templates (Morasco et al., 2003; Murray and Barton, 2003). VPg uridylylation requires membranes when natural poliovirus replication complexes are isolated from infected cells (Takegami et al., 1983). Thus, preinitiation RNA replication complexes support authentic replication and 2CATPase activity is required for steps of RNA replication, including CRE-dependent VPg uridylylation, that require seemingly distal cis-active RNA elements (Barton and Flanegan, 1997; Barton et al., 2001; Lyons et al., 2001; Morasco et al., 2003; Murray and Barton, 2003). 2CATPase may act to prevent the formation of non-functional RNPs within membranous poliovirus RNA replication complexes in a manner analogous to that of ATPase subunits within SMN complexes. Studies of SV40 T antigen, an ATPase protein involved in managing RNP complexes at SV40 DNA origins of replication, may also provide insights into the regulation of dynamic RNPs associated with replication of viral nucleic acids (Li et al., 2003). The possible roles of 2CATPase and the manner in which picornaviral cis-active RNAs function coordinately to mediate RNA replication await further investigation.
12. CREs yet to be discovered?
CREs will likely be found in the genomes of the remaining picornaviruses where such elements have yet to be described; the Kobu-, Erbo-, and Teschoviruses. Furthermore, the advantages of CREs and nucleotidylylated protein primers described in this review suggest that other positive-strand RNA viruses, like those in the Dicistroviridae, Comoviridae, and Sequiviridae families, may employ CRE RNAs. Like picornaviruses, the viruses in the Dicistroviridae, Comoviridae, and Sequiviridae families have small VPg proteins covalently linked to the 5′ ends of their RNA genomes. CREs that are used as templates to nucleotidylylate the protein primers of these viruses have not been described, but may be discovered in the coming years.
13. Conclusions
Cis-active RNA sequences and structures within picornavirus RNAs coordinately interact with viral replication proteins to mediate the replication of viral RNA. One of the cis-active RNAs within the genomes of picornaviruses, the CRE, is a template for the conversion of VPg into VPgpUpUOH. VPg and/or VPgpUpUOH prime the replication of viral RNA, thereby becoming covalently linked to the 5′ ends of viral RNA. Progress in understanding the replication mechanisms of picornavirus RNAs will likely be informative and relevant to related viruses in the Dicistroviridae, Comoviridae, and Sequiviridae families of the Picornavirales order (Le Gall et al., 2007). We anxiously anticipate the new discoveries that will ultimately lead to a clear and theoretically satisfying explanation for the asymmetric replication of picornavirus RNA.
Acknowledgments
We thank Brian Kempf for critical evaluation of the manuscript. The authors were supported by grants from the National Institutes of Health; T32-AI052066 (BPS) and AI42189 (DJB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agol VI, Paul AV, Wimmer E. Paradoxes of the replication of picornaviral genomes. Virus Res. 1999;62(2):129–47. doi: 10.1016/s0168-1702(99)00037-4. [DOI] [PubMed] [Google Scholar]
- Ahlquist P. Parallels among positive-strand RNA viruses, reverse-transcribing viruses and double-stranded RNA viruses. Nat Rev Microbiol. 2006;4(5):371–82. doi: 10.1038/nrmicro1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sunaidi M, Williams CH, Hughes PJ, Schnurr DP, Stanway G. Analysis of a new human parechovirus allows the definition of parechovirus types and the identification of RNA structural domains. J Virol. 2007;81(2):1013–21. doi: 10.1128/JVI.00584-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander L, Lu HH, Wimmer E. Polioviruses containing picornavirus type 1 and/or type 2 internal ribosomal entry site elements: genetic hybrids and the expression of a foreign gene. Proc Natl Acad Sci U S A. 1994;91(4):1406–10. doi: 10.1073/pnas.91.4.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambros V, Baltimore D. Protein is linked to the 5′ end of poliovirus RNA by a phosphodiester linkage to tyrosine. J Biol Chem. 1978;253(15):5263–6. [PubMed] [Google Scholar]
- Amiott EA, Jaehning JA. Mitochondrial transcription is regulated via an ATP “sensing” mechanism that couples RNA abundance to respiration. Mol Cell. 2006a;22(3):329–38. doi: 10.1016/j.molcel.2006.03.031. [DOI] [PubMed] [Google Scholar]
- Amiott EA, Jaehning JA. Sensitivity of the yeast mitochondrial RNA polymerase to +1 and +2 initiating nucleotides. J Biol Chem. 2006b;281(46):34982–8. doi: 10.1074/jbc.M608638200. [DOI] [PubMed] [Google Scholar]
- Andino R, Rieckhof GE, Achacoso PL, Baltimore D. Poliovirus RNA synthesis utilizes an RNP complex formed around the 5′-end of viral RNA. EMBO J. 1993;12(9):3587–98. doi: 10.1002/j.1460-2075.1993.tb06032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andino R, Rieckhof GE, Baltimore D. A functional ribonucleoprotein complex forms around the 5′ end of poliovirus RNA. Cell. 1990a;63(2):369–80. doi: 10.1016/0092-8674(90)90170-j. [DOI] [PubMed] [Google Scholar]
- Andino R, Rieckhof GE, Trono D, Baltimore D. Substitutions in the protease (3Cpro) gene of poliovirus can suppress a mutation in the 5′ noncoding region. J Virol. 1990b;64(2):607–12. doi: 10.1128/jvi.64.2.607-612.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleby TC, Luecke H, Shim JH, Wu JZ, Cheney IW, Zhong W, Vogeley L, Hong Z, Yao N. Crystal structure of complete rhinovirus RNA polymerase suggests front loading of protein primer. J Virol. 2005;79(1):277–88. doi: 10.1128/JVI.79.1.277-288.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccon J, Pellizzoni L, Rappsilber J, Mann M, Dreyfuss G. Identification and characterization of Gemin7, a novel component of the survival of motor neuron complex. J Biol Chem. 2002;277(35):31957–62. doi: 10.1074/jbc.M203478200. [DOI] [PubMed] [Google Scholar]
- Banerjee R, Tsai W, Kim W, Dasgupta A. Interaction of poliovirus-encoded 2C/2BC polypeptides with the 3′ terminus negative-strand cloverleaf requires an intact stem-loop b. Virology. 2001;280(1):41–51. doi: 10.1006/viro.2000.0770. [DOI] [PubMed] [Google Scholar]
- Barton DJ, Black EP, Flanegan JB. Complete replication of poliovirus in vitro: preinitiation RNA replication complexes require soluble cellular factors for the synthesis of VPg-linked RNA. J Virol. 1995;69(9):5516–27. doi: 10.1128/jvi.69.9.5516-5527.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton DJ, Flanegan JB. Coupled translation and replication of poliovirus RNA in vitro: synthesis of functional 3D polymerase and infectious virus. J Virol. 1993;67(2):822–31. doi: 10.1128/jvi.67.2.822-831.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton DJ, Flanegan JB. Synchronous replication of poliovirus RNA: initiation of negative-strand RNA synthesis requires the guanidine-inhibited activity of protein 2C. J Virol. 1997;71(11):8482–9. doi: 10.1128/jvi.71.11.8482-8489.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton DJ, Morasco BJ, Eisner-Smerage L, Flanegan JB. Poliovirus RNA replication and genetic complementation in cell-free reactions. In: Semler BL, Wimmer E, editors. Molecular Biology of Picornaviruses. ASM Press; Washington, D.C.: 2002. pp. 461–469. [Google Scholar]
- Barton DJ, O'Donnell BJ, Flanegan JB. 5′ cloverleaf in poliovirus RNA is a cis-acting replication element required for negative-strand synthesis. EMBO J. 2001;20(6):1439–48. doi: 10.1093/emboj/20.6.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienz K, Egger D, Pfister T, Troxler M. Structural and functional characterization of the poliovirus replication complex. J Virol. 1992;66(5):2740–7. doi: 10.1128/jvi.66.5.2740-2747.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briese T, Renwick N, Venter M, Jarman RG, Ghosh D, Kondgen S, Shrestha SK, Hoegh AM, Casas I, Adjogoua EV, Akoua-Koffi C, Myint KS, Williams DT, Chidlow G, van den Berg R, Calvo C, Koch O, Palacios G, Kapoor V, Villari J, Dominguez SR, Holmes KV, Harnett G, Smith D, Mackenzie JS, Ellerbrok H, Schweiger B, Schonning K, Chadha MS, Leendertz FH, Mishra AC, Gibbons RV, Holmes EC, Lipkin WI. Global distribution of novel rhinovirus genotype. Emerg Infect Dis. 2008;14(6):944–7. doi: 10.3201/eid1406.080271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown B, Oberste MS, Maher K, Pallansch MA. Complete genomic sequencing shows that polioviruses and members of human enterovirus species C are closely related in the noncapsid coding region. J Virol. 2003;77(16):8973–84. doi: 10.1128/JVI.77.16.8973-8984.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DM, Cornell CT, Tran GP, Nguyen JH, Semler BL. An authentic 3′ noncoding region is necessary for efficient poliovirus replication. J Virol. 2005;79(18):11962–73. doi: 10.1128/JVI.79.18.11962-11973.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho MW, Teterina N, Egger D, Bienz K, Ehrenfeld E. Membrane rearrangement and vesicle induction by recombinant poliovirus 2C and 2BC in human cells. Virology. 1994;202(1):129–45. doi: 10.1006/viro.1994.1329. [DOI] [PubMed] [Google Scholar]
- Cole CN, Baltimore D. Defective interfering particles of poliovirus. II. Nature of the defect. J Mol Biol. 1973;76(3):325–43. doi: 10.1016/0022-2836(73)90508-1. [DOI] [PubMed] [Google Scholar]
- Cole CN, Smoler D, Wimmer E, Baltimore D. Defective interfering particles of poliovirus. I. Isolation and physical properties. J Virol. 1971;7(4):478–85. doi: 10.1128/jvi.7.4.478-485.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collis PS, O'Donnell BJ, Barton DJ, Rogers JA, Flanegan JB. Replication of poliovirus RNA and subgenomic RNA transcripts in transfected cells. J Virol. 1992;66(11):6480–8. doi: 10.1128/jvi.66.11.6480-6488.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordey S, Gerlach D, Junier T, Zdobnov EM, Kaiser L, Tapparel C. The cis-acting replication elements define human enterovirus and rhinovirus species. RNA. 2008;14(8):1568–78. doi: 10.1261/rna.1031408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford NM, Baltimore D. Genome-linked protein VPg of poliovirus is present as free VPg and VPg-pUpU in poliovirus-infected cells. Proc Natl Acad Sci U S A. 1983;80(24):7452–5. doi: 10.1073/pnas.80.24.7452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowder S, Kirkegaard K. Trans-dominant inhibition of RNA viral replication can slow growth of drug-resistant viruses. Nat Genet. 2005;37(7):701–9. doi: 10.1038/ng1583. [DOI] [PubMed] [Google Scholar]
- Ferrer-Orta C, Arias A, Agudo R, Perez-Luque R, Escarmis C, Domingo E, Verdaguer N. The structure of a protein primer-polymerase complex in the initiation of genome replication. EMBO J. 2006;25(4):880–8. doi: 10.1038/sj.emboj.7600971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanegan JB, Baltimore D. Poliovirus-specific primer-dependent RNA polymerase able to copy poly(A) Proc Natl Acad Sci U S A. 1977;74(9):3677–80. doi: 10.1073/pnas.74.9.3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen WJ, Wyce A, Paushkin S, Abel L, Rappsilber J, Mann M, Dreyfuss G. A novel WD repeat protein component of the methylosome binds Sm proteins. J Biol Chem. 2002;277(10):8243–7. doi: 10.1074/jbc.M109984200. [DOI] [PubMed] [Google Scholar]
- Fujita K, Krishnakumar SS, Franco D, Paul AV, London E, Wimmer E. Membrane topography of the hydrophobic anchor sequence of poliovirus 3A and 3AB proteins and the functional effect of 3A/3AB membrane association upon RNA replication. Biochemistry. 2007;46(17):5185–99. doi: 10.1021/bi6024758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaal T, Bartlett MS, Ross W, Turnbough CL, Jr, Gourse RL. Transcription regulation by initiating NTP concentration: rRNA synthesis in bacteria. Science. 1997;278(5346):2092–7. doi: 10.1126/science.278.5346.2092. [DOI] [PubMed] [Google Scholar]
- Gamarnik AV, Andino R. Interactions of viral protein 3CD and poly(rC) binding protein with the 5′ untranslated region of the poliovirus genome. J Virol. 2000;74(5):2219–26. doi: 10.1128/jvi.74.5.2219-2226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber K, Wimmer E, Paul AV. Biochemical and genetic studies of the initiation of human rhinovirus 2 RNA replication: identification of a cis-replicating element in the coding sequence of 2A(pro) J Virol. 2001;75(22):10979–90. doi: 10.1128/JVI.75.22.10979-10990.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesteland RF, Cech TR, Atkins JF, editors. The RNA World. Second Edition. Cold Spring Harbor Laboratory Press; Cold Spring Harbor: 1999. [Google Scholar]
- Goodfellow I, Chaudhry Y, Richardson A, Meredith J, Almond JW, Barclay W, Evans DJ. Identification of a cis-acting replication element within the poliovirus coding region. J Virol. 2000;74(10):4590–600. doi: 10.1128/jvi.74.10.4590-4600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodfellow IG, Kerrigan D, Evans DJ. Structure and function analysis of the poliovirus cis-acting replication element (CRE) RNA. 2003a;9(1):124–37. doi: 10.1261/rna.2950603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodfellow IG, Polacek C, Andino R, Evans DJ. The poliovirus 2C cis-acting replication element-mediated uridylylation of VPg is not required for synthesis of negative-sense genomes. J Gen Virol. 2003b;84(Pt 9):2359–63. doi: 10.1099/vir.0.19132-0. [DOI] [PubMed] [Google Scholar]
- Gubitz AK, Mourelatos Z, Abel L, Rappsilber J, Mann M, Dreyfuss G. Gemin5, a novel WD repeat protein component of the SMN complex that binds Sm proteins. J Biol Chem. 2002;277(7):5631–6. doi: 10.1074/jbc.M109448200. [DOI] [PubMed] [Google Scholar]
- Hagino-Yamagishi K, Nomoto A. In vitro construction of poliovirus defective interfering particles. J Virol. 1989;63(12):5386–92. doi: 10.1128/jvi.63.12.5386-5392.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales LM, Knowles NJ, Reddy PS, Xu L, Hay C, Hallenbeck PL. Complete genome sequence analysis of Seneca Valley virus-001, a novel oncolytic picornavirus. J Gen Virol. 2008;89(Pt 5):1265–75. doi: 10.1099/vir.0.83570-0. [DOI] [PubMed] [Google Scholar]
- Harris KS, Reddigari SR, Nicklin MJ, Hammerle T, Wimmer E. Purification and characterization of poliovirus polypeptide 3CD, a proteinase and a precursor for RNA polymerase. J Virol. 1992;66(12):7481–9. doi: 10.1128/jvi.66.12.7481-7489.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KS, Xiang W, Alexander L, Lane WS, Paul AV, Wimmer E. Interaction of poliovirus polypeptide 3CDpro with the 5′ and 3′ termini of the poliovirus genome. Identification of viral and cellular cofactors needed for efficient binding. J Biol Chem. 1994;269(43):27004–14. [PubMed] [Google Scholar]
- Herold J, Andino R. Poliovirus requires a precise 5′ end for efficient positive-strand RNA synthesis. J Virol. 2000;74(14):6394–400. doi: 10.1128/jvi.74.14.6394-6400.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold J, Andino R. Poliovirus RNA replication requires genome circularization through a protein-protein bridge. Mol Cell. 2001;7(3):581–91. doi: 10.1016/S1097-2765(01)00205-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Patel SS. Kinetic mechanism of GTP binding and RNA synthesis during transcription initiation by bacteriophage T7 RNA polymerase. J Biol Chem. 1997;272(48):30147–53. doi: 10.1074/jbc.272.48.30147. [DOI] [PubMed] [Google Scholar]
- Kadare G, Haenni AL. Virus-encoded RNA helicases. J Virol. 1997;71(4):2583–90. doi: 10.1128/jvi.71.4.2583-2590.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan G, Racaniello VR. Construction and characterization of poliovirus subgenomic replicons. J Virol. 1988;62(5):1687–96. doi: 10.1128/jvi.62.5.1687-1696.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempf BJ, Barton DJ. Poly (rC) binding proteins and the 5′ cloverleaf of uncapped poliovirus mRNA function during de novo assembly of polysomes. J Virol. 2008;82(12):5835–46. doi: 10.1128/JVI.01513-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kew OM, Sutter RW, de Gourville EM, Dowdle WR, Pallansch MA. Vaccine-derived polioviruses and the endgame strategy for global polio eradication. Annu Rev Microbiol. 2005;59:587–635. doi: 10.1146/annurev.micro.58.030603.123625. [DOI] [PubMed] [Google Scholar]
- Kopek BG, Perkins G, Miller DJ, Ellisman MH, Ahlquist P. Three-Dimensional Analysis of a Viral RNA Replication Complex Reveals a Virus-Induced Mini-Organelle. PLoS Biol. 2007;5(9):e220. doi: 10.1371/journal.pbio.0050220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korneeva VS, Cameron CE. Structure-function relationships of the viral RNA-dependent RNA polymerase: fidelity, replication speed, and initiation mechanism determined by a residue in the ribose-binding pocket. J Biol Chem. 2007;282(22):16135–45. doi: 10.1074/jbc.M610090200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu P, Raychaudhuri S, Tsai W, Dasgupta A. Shutoff of RNA polymerase II transcription by poliovirus involves 3C protease-mediated cleavage of the TATA-binding protein at an alternative site: incomplete shutoff of transcription interferes with efficient viral replication. J Virol. 2005;79(15):9702–13. doi: 10.1128/JVI.79.15.9702-9713.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau SK, Yip CC, Tsoi HW, Lee RA, So LY, Lau YL, Chan KH, Woo PC, Yuen KY. Clinical features and complete genome characterization of a distinct human rhinovirus (HRV) genetic cluster, probably representing a previously undetected HRV species, HRV-C, associated with acute respiratory illness in children. J Clin Microbiol. 2007;45(11):3655–64. doi: 10.1128/JCM.01254-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gall O, Christian P, Fauquet CM, King AM, Knowles NJ, Nakashima N, Stanway G, Gorbalenya AE. Picornavirales, a proposed order of positive-sense single-stranded RNA viruses with a pseudo-T = 3 virion architecture. Arch Virol. 2008;153(4):715–727. doi: 10.1007/s00705-008-0041-x. [DOI] [PubMed] [Google Scholar]
- Lee YF, Nomoto A, Detjen BM, Wimmer E. A protein covalently linked to poliovirus genome RNA. Proc Natl Acad Sci U S A. 1977;74(1):59–63. doi: 10.1073/pnas.74.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Zhao R, Lilyestrom W, Gai D, Zhang R, DeCaprio JA, Fanning E, Jochimiak A, Szakonyi G, Chen XS. Structure of the replicative helicase of the oncoprotein SV40 large tumour antigen. Nature. 2003;423(6939):512–8. doi: 10.1038/nature01691. [DOI] [PubMed] [Google Scholar]
- Li X, Lu HH, Mueller S, Wimmer E. The C-terminal residues of poliovirus proteinase 2A(pro) are critical for viral RNA replication but not for cis- or trans-proteolytic cleavage. J Gen Virol. 2001;82(Pt 2):397–408. doi: 10.1099/0022-1317-82-2-397. [DOI] [PubMed] [Google Scholar]
- Lobert PE, Escriou N, Ruelle J, Michiels T. A coding RNA sequence acts as a replication signal in cardioviruses. Proc Natl Acad Sci U S A. 1999;96(20):11560–5. doi: 10.1073/pnas.96.20.11560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu HH, Wimmer E. Poliovirus chimeras replicating under the translational control of genetic elements of hepatitis C virus reveal unusual properties of the internal ribosomal entry site of hepatitis C virus. Proc Natl Acad Sci U S A. 1996;93(4):1412–7. doi: 10.1073/pnas.93.4.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons T, Murray KE, Roberts AW, Barton DJ. Poliovirus 5′-terminal cloverleaf RNA is required in cis for VPg uridylylation and the initiation of negative-strand RNA synthesis. J Virol. 2001;75(22):10696–708. doi: 10.1128/JVI.75.22.10696-10708.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan V, Sanchez-Martinez S, Vedovato N, Rispoli G, Carrasco L, Nieva JL. Plasma membrane-porating domain in poliovirus 2B protein. A short peptide mimics viroporin activity. J Mol Biol. 2007;374(4):951–64. doi: 10.1016/j.jmb.2007.09.058. [DOI] [PubMed] [Google Scholar]
- Marcotte LL, Wass AB, Gohara DW, Pathak HB, Arnold JJ, Filman DJ, Cameron CE, Hogle JM. Crystal structure of poliovirus 3CD protein: virally encoded protease and precursor to the RNA-dependent RNA polymerase. J Virol. 2007;81(7):3583–96. doi: 10.1128/JVI.02306-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason PW, Bezborodova SV, Henry TM. Identification and characterization of a cis-acting replication element (cre) adjacent to the internal ribosome entry site of foot-and-mouth disease virus. J Virol. 2002;76(19):9686–94. doi: 10.1128/JVI.76.19.9686-9694.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight KL, Lemon SM. Capsid coding sequence is required for efficient replication of human rhinovirus 14 RNA. J Virol. 1996;70(3):1941–52. doi: 10.1128/jvi.70.3.1941-1952.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight KL, Lemon SM. The rhinovirus type 14 genome contains an internally located RNA structure that is required for viral replication. RNA. 1998;4(12):1569–84. doi: 10.1017/s1355838298981006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molla A, Paul AV, Wimmer E. Cell-free, de novo synthesis of poliovirus. Science. 1991;254(5038):1647–51. doi: 10.1126/science.1661029. [DOI] [PubMed] [Google Scholar]
- Morasco BJ, Sharma N, Parilla J, Flanegan JB. Poliovirus cre(2C)-dependent synthesis of VPgpUpU is required for positive- but not negative-strand RNA synthesis. J Virol. 2003;77(9):5136–44. doi: 10.1128/JVI.77.9.5136-5144.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray KE, Barton DJ. Poliovirus CRE-dependent VPg uridylylation is required for positive-strand RNA synthesis but not for negative-strand RNA synthesis. J Virol. 2003;77(8):4739–50. doi: 10.1128/JVI.77.8.4739-4750.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray KE, Steil BP, Roberts AW, Barton DJ. Replication of poliovirus RNA with complete internal ribosome entry site deletions. J Virol. 2004;78(3):1393–402. doi: 10.1128/JVI.78.3.1393-1402.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima S, Sasaki J, Taniguchi K. The 5′-terminal region of the Aichi virus genome encodes cis-acting replication elements required for positive- and negative-strand RNA synthesis. J Virol. 2005;79(11):6918–31. doi: 10.1128/JVI.79.11.6918-6931.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima S, Sasaki J, Taniguchi K. Interaction between polypeptide 3ABC and the 5′-terminal structural elements of the genome of Aichi virus: implication for negative-strand RNA synthesis. J Virol. 2008;82(13):6161–71. doi: 10.1128/JVI.02151-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak A, Goodfellow IG, Belsham GJ. Factors required for the Uridylylation of the foot-and-mouth disease virus 3B1, 3B2, and 3B3 peptides by the RNA-dependent RNA polymerase (3Dpol) in vitro. J Virol. 2005;79(12):7698–706. doi: 10.1128/JVI.79.12.7698-7706.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak A, Goodfellow IG, Woolaway KE, Birtley J, Curry S, Belsham GJ. Role of RNA structure and RNA binding activity of foot-and-mouth disease virus 3C protein in VPg uridylylation and virus replication. J Virol. 2006;80(19):9865–75. doi: 10.1128/JVI.00561-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld KL, Richards OC, Ehrenfeld E. Purification, characterization, and comparison of poliovirus RNA polymerase from native and recombinant sources. J Biol Chem. 1991;266(35):24212–9. [PubMed] [Google Scholar]
- Nieva JL, Agirre A, Nir S, Carrasco L. Mechanisms of membrane permeabilization by picornavirus 2B viroporin. FEBS Lett. 2003;552(1):68–73. doi: 10.1016/s0014-5793(03)00852-4. [DOI] [PubMed] [Google Scholar]
- Nomoto A, Jacobson A, Lee YF, Dunn J, Wimmer E. Defective interfering particles of poliovirus: mapping of the deletion and evidence that the deletions in the genomes of DI(1), (2) and (3) are located in the same region. J Mol Biol. 1979;128(2):179–96. doi: 10.1016/0022-2836(79)90125-6. [DOI] [PubMed] [Google Scholar]
- Nomoto A, Kitamura N, Golini F, Wimmer E. The 5′-terminal structures of poliovirion RNA and poliovirus mRNA differ only in the genome-linked protein VPg. Proc Natl Acad Sci U S A. 1977;74(12):5345–9. doi: 10.1073/pnas.74.12.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak JE, Kirkegaard K. Improved method for detecting poliovirus negative strands used to demonstrate specificity of positive-strand encapsidation and the ratio of positive to negative strands in infected cells. J Virol. 1991;65(6):3384–7. doi: 10.1128/jvi.65.6.3384-3387.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberste MS, Maher K, Nix WA, Michele SM, Uddin M, Schnurr D, al-Busaidy S, Akoua-Koffi C, Pallansch MA. Molecular identification of 13 new enterovirus types, EV79-88, EV97, and EV100-101, members of the species Human Enterovirus B. Virus Res. 2007a;128(12):34–42. doi: 10.1016/j.virusres.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Oberste MS, Maher K, Pallansch MA. Evidence for frequent recombination within species human enterovirus B based on complete genomic sequences of all thirty-seven serotypes. J Virol. 2004a;78(2):855–67. doi: 10.1128/JVI.78.2.855-867.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberste MS, Maher K, Pallansch MA. Complete genome sequences for nine simian enteroviruses. J Gen Virol. 2007b;88(Pt 12):3360–72. doi: 10.1099/vir.0.83124-0. [DOI] [PubMed] [Google Scholar]
- Oberste MS, Penaranda S, Maher K, Pallansch MA. Complete genome sequences of all members of the species Human enterovirus A. J Gen Virol. 2004b;85(Pt 6):1597–607. doi: 10.1099/vir.0.79789-0. [DOI] [PubMed] [Google Scholar]
- Oberste MS, Penaranda S, Pallansch MA. RNA recombination plays a major role in genomic change during circulation of coxsackie B viruses. J Virol. 2004c;78(6):2948–55. doi: 10.1128/JVI.78.6.2948-2955.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak HB, Arnold JJ, Wiegand PN, Hargittai MR, Cameron CE. Picornavirus genome replication: assembly and organization of the VPg uridylylation ribonucleoprotein (initiation) complex. J Biol Chem. 2007;282(22):16202–13. doi: 10.1074/jbc.M610608200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak HB, Ghosh SK, Roberts AW, Sharma SD, Yoder JD, Arnold JJ, Gohara DW, Barton DJ, Paul AV, Cameron CE. Structure-function relationships of the RNA-dependent RNA polymerase from poliovirus (3Dpol). A surface of the primary oligomerization domain functions in capsid precursor processing and VPg uridylylation. J Biol Chem. 2002;277(35):31551–62. doi: 10.1074/jbc.M204408200. [DOI] [PubMed] [Google Scholar]
- Paul A. Possible unifying mechanism of picornavirus genome replication. In: Semler BL, Wimmer E, editors. Molecular Biology of Picornaviruses. ASM Press; Washington, D.C.: 2002. pp. 227–246. [Google Scholar]
- Paul AV, Peters J, Mugavero J, Yin J, van Boom JH, Wimmer E. Biochemical and genetic studies of the VPg uridylylation reaction catalyzed by the RNA polymerase of poliovirus. J Virol. 2003a;77(2):891–904. doi: 10.1128/JVI.77.2.891-904.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul AV, Rieder E, Kim DW, van Boom JH, Wimmer E. Identification of an RNA hairpin in poliovirus RNA that serves as the primary template in the in vitro uridylylation of VPg. J Virol. 2000;74(22):10359–70. doi: 10.1128/jvi.74.22.10359-10370.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul AV, van Boom JH, Filippov D, Wimmer E. Protein-primed RNA synthesis by purified poliovirus RNA polymerase. Nature. 1998;393(6682):280–4. doi: 10.1038/30529. [DOI] [PubMed] [Google Scholar]
- Paul AV, Yin J, Mugavero J, Rieder E, Liu Y, Wimmer E. A “slide-back” mechanism for the initiation of protein-primed RNA synthesis by the RNA polymerase of poliovirus. J Biol Chem. 2003b;278(45):43951–60. doi: 10.1074/jbc.M307441200. [DOI] [PubMed] [Google Scholar]
- Pellizzoni L, Baccon J, Rappsilber J, Mann M, Dreyfuss G. Purification of native survival of motor neurons complexes and identification of Gemin6 as a novel component. J Biol Chem. 2002a;277(9):7540–5. doi: 10.1074/jbc.M110141200. [DOI] [PubMed] [Google Scholar]
- Pellizzoni L, Yong J, Dreyfuss G. Essential role for the SMN complex in the specificity of snRNP assembly. Science. 2002b;298(5599):1775–9. doi: 10.1126/science.1074962. [DOI] [PubMed] [Google Scholar]
- Pettersson RF, Ambros V, Baltimore D. Identification of a protein linked to nascent poliovirus RNA and to the polyuridylic acid of negative-strand RNA. J Virol. 1978;27(2):357–65. doi: 10.1128/jvi.27.2.357-365.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister T, Jones KW, Wimmer E. A cysteine-rich motif in poliovirus protein 2C(ATPase) is involved in RNA replication and binds zinc in vitro. J Virol. 2000;74(1):334–43. doi: 10.1128/jvi.74.1.334-343.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister T, Wimmer E. Characterization of the nucleoside triphosphatase activity of poliovirus protein 2C reveals a mechanism by which guanidine inhibits poliovirus replication. J Biol Chem. 1999;274(11):6992–7001. doi: 10.1074/jbc.274.11.6992. [DOI] [PubMed] [Google Scholar]
- Pilipenko EV, Maslova SV, Sinyakov AN, Agol VI. Towards identification of cis-acting elements involved in the replication of enterovirus and rhinovirus RNAs: a proposal for the existence of tRNA-like terminal structures. Nucleic Acids Res. 1992;20(7):1739–45. doi: 10.1093/nar/20.7.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder E, Paul AV, Kim DW, van Boom JH, Wimmer E. Genetic and biochemical studies of poliovirus cis-acting replication element cre in relation to VPg uridylylation. J Virol. 2000;74(22):10371–80. doi: 10.1128/jvi.74.22.10371-10380.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez PL, Carrasco L. Poliovirus protein 2C contains two regions involved in RNA binding activity. J Biol Chem. 1995;270(17):10105–12. doi: 10.1074/jbc.270.17.10105. [DOI] [PubMed] [Google Scholar]
- Rohll JB, Moon DH, Evans DJ, Almond JW. The 3′ untranslated region of picornavirus RNA: features required for efficient genome replication. J Virol. 1995;69(12):7835–44. doi: 10.1128/jvi.69.12.7835-7844.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs A. The role of poly(A) in the translation and stability of mRNA. Curr Opin Cell Biol. 1990;2(6):1092–8. doi: 10.1016/0955-0674(90)90161-7. [DOI] [PubMed] [Google Scholar]
- Sarnow P. Role of 3′-end sequences in infectivity of poliovirus transcripts made in vitro. J Virol. 1989;63(1):467–70. doi: 10.1128/jvi.63.1.467-470.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sean P, Semler BL. Coxsackievirus B RNA replication: lessons from poliovirus. Curr Top Microbiol Immunol. 2008;323:89–121. doi: 10.1007/978-3-540-75546-3_5. [DOI] [PubMed] [Google Scholar]
- Sharma N, O'Donnell BJ, Flanegan JB. 3′-Terminal sequence in poliovirus negative-strand templates is the primary cis-acting element required for VPgpUpU-primed positive-strand initiation. J Virol. 2005;79(6):3565–77. doi: 10.1128/JVI.79.6.3565-3577.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Raychaudhuri S, Dasgupta A. Nuclear entry of poliovirus protease-polymerase precursor 3CD: implications for host cell transcription shut-off. Virology. 2004;320(2):195–205. doi: 10.1016/j.virol.2003.10.020. [DOI] [PubMed] [Google Scholar]
- Shen M, Wang Q, Yang Y, Pathak HB, Arnold JJ, Castro C, Lemon SM, Cameron CE. Human rhinovirus type 14 gain-of-function mutants for oriI utilization define residues of 3C(D) and 3Dpol that contribute to assembly and stability of the picornavirus VPg uridylylation complex. J Virol. 2007;81(22):12485–95. doi: 10.1128/JVI.00972-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestri LS, Parilla JM, Morasco BJ, Ogram SA, Flanegan JB. Relationship between poliovirus negative-strand RNA synthesis and the length of the 3′ poly(A) tail. Virology. 2006;345(2):509–19. doi: 10.1016/j.virol.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Stanway G, Hovi T, Knowles NJ, Hyypia T. Molecular and Biological Basis of Picornavirus Taxonomy. In: Semler BL, Wimmer E, editors. Molecular Biology of Picornaviruses. ASM Press; Washington, D.C.: 2002. pp. 17–24. [Google Scholar]
- Steil BP, Barton DJ. Poliovirus CRE-dependent VPg uridylylation lowers the Km of the initiating NTP for viral RNA replication. Journal of Virology. 2008 doi: 10.1128/JVI.00427-08. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitkin YV, Sonenberg N. Cell-free synthesis of encephalomyocarditis virus. J Virol. 2003;77(11):6551–5. doi: 10.1128/JVI.77.11.6551-6555.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takegami T, Kuhn RJ, Anderson CW, Wimmer E. Membrane-dependent uridylylation of the genome-linked protein VPg of poliovirus. Proc Natl Acad Sci U S A. 1983;80(24):7447–51. doi: 10.1073/pnas.80.24.7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teterina NL, Gorbalenya AE, Egger D, Bienz K, Ehrenfeld E. Poliovirus 2C protein determinants of membrane binding and rearrangements in mammalian cells. J Virol. 1997;71(12):8962–72. doi: 10.1128/jvi.71.12.8962-8972.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiviyanathan V, Yang Y, Kaluarachchi K, Rijnbrand R, Gorenstein DG, Lemon SM. High-resolution structure of a picornaviral internal cis-acting RNA replication element (cre) Proc Natl Acad Sci U S A. 2004;101(34):12688–93. doi: 10.1073/pnas.0403079101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma C, Ostareck-Lederer A, Hentze MW. A poly(A) tail-responsive in vitro system for cap- or IRES-driven translation from HeLa cells. Methods Mol Biol. 2004;257:171–80. doi: 10.1385/1-59259-750-5:171. [DOI] [PubMed] [Google Scholar]
- Thompson AA, Albertini RA, Peersen OB. Stabilization of poliovirus polymerase by NTP binding and fingers-thumb interactions. J Mol Biol. 2007;366(5):1459–74. doi: 10.1016/j.jmb.2006.11.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AA, Peersen OB. Structural basis for proteolysis-dependent activation of the poliovirus RNA-dependent RNA polymerase. EMBO J. 2004;23(17):3462–71. doi: 10.1038/sj.emboj.7600357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd S, Towner JS, Brown DM, Semler BL. Replication-competent picornaviruses with complete genomic RNA 3′ noncoding region deletions. J Virol. 1997a;71(11):8868–74. doi: 10.1128/jvi.71.11.8868-8874.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd S, Towner JS, Semler BL. Translation and replication properties of the human rhinovirus genome in vivo and in vitro. Virology. 1997b;229(1):90–7. doi: 10.1006/viro.1996.8416. [DOI] [PubMed] [Google Scholar]
- Towner JS, Mazanet MM, Semler BL. Rescue of defective poliovirus RNA replication by 3AB-containing precursor polyproteins. J Virol. 1998;72(9):7191–200. doi: 10.1128/jvi.72.9.7191-7200.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trono D, Andino R, Baltimore D. An RNA sequence of hundreds of nucleotides at the 5′ end of poliovirus RNA is involved in allowing viral protein synthesis. J Virol. 1988;62(7):2291–9. doi: 10.1128/jvi.62.7.2291-2299.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuschall DM, Hiebert E, Flanegan JB. Poliovirus RNA-dependent RNA polymerase synthesizes full-length copies of poliovirion RNA, cellular mRNA, and several plant virus RNAs in vitro. J Virol. 1982;44(1):209–16. doi: 10.1128/jvi.44.1.209-216.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk AA, Makeyev EV, Bamford DH. Initiation of viral RNA-dependent RNA polymerization. J Gen Virol. 2004;85(Pt 5):1077–93. doi: 10.1099/vir.0.19731-0. [DOI] [PubMed] [Google Scholar]
- van Ooij MJ, Vogt DA, Paul A, Castro C, Kuijpers J, van Kuppeveld FJ, Cameron CE, Wimmer E, Andino R, Melchers WJ. Structural and functional characterization of the coxsackievirus B3 CRE(2C): role of CRE(2C) in negative- and positive-strand RNA synthesis. J Gen Virol. 2006;87(Pt 1):103–13. doi: 10.1099/vir.0.81297-0. [DOI] [PubMed] [Google Scholar]
- Wang J, Dreyfuss G. A cell system with targeted disruption of the SMN gene: functional conservation of the SMN protein and dependence of Gemin2 on SMN. J Biol Chem. 2001a;276(13):9599–605. doi: 10.1074/jbc.M009162200. [DOI] [PubMed] [Google Scholar]
- Wang J, Dreyfuss G. Characterization of functional domains of the SMN protein in vivo. J Biol Chem. 2001b;276(48):45387–93. doi: 10.1074/jbc.M105059200. [DOI] [PubMed] [Google Scholar]
- Wimmer E. The test-tube synthesis of a chemical called poliovirus. The simple synthesis of a virus has far-reaching societal implications. EMBO Rep. 2006:S3–9. doi: 10.1038/sj.embor.7400728. 7 Spec No. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Rijnbrand R, McKnight KL, Wimmer E, Paul A, Martin A, Lemon SM. Sequence requirements for viral RNA replication and VPg uridylylation directed by the internal cis-acting replication element (cre) of human rhinovirus type 14. J Virol. 2002;76(15):7485–94. doi: 10.1128/JVI.76.15.7485-7494.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Rijnbrand R, Watowich S, Lemon SM. Genetic evidence for an interaction between a picornaviral cis-acting RNA replication element and 3CD protein. J Biol Chem. 2004;279(13):12659–67. doi: 10.1074/jbc.M312992200. [DOI] [PubMed] [Google Scholar]
- Yang Y, Yi MK, Simmonds P, Evans DJ, Lemon SM. Identification of a conserved replication element (cre) within the 3Dpol-coding sequence of Hepatoviruses. 2008 doi: 10.1128/JVI.00787-08. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J, Paul AV, Wimmer E, Rieder E. Functional dissection of a poliovirus cis-acting replication element [PV-cre(2C)]: analysis of single-and dual-cre viral genomes and proteins that bind specifically to PV-cre RNA. J Virol. 2003;77(9):5152–66. doi: 10.1128/JVI.77.9.5152-5166.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao WD, Lahser FC, Wimmer E. Genetic analysis of a poliovirus/hepatitis C virus (HCV) chimera: interaction between the poliovirus cloverleaf and a sequence in the HCV 5′ nontranslated region results in a replication phenotype. J Virol. 2000;74(13):6223–6. doi: 10.1128/jvi.74.13.6223-6226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao WD, Wimmer E, Lahser FC. Poliovirus/Hepatitis C virus (internal ribosomal entry site-core) chimeric viruses: improved growth properties through modification of a proteolytic cleavage site and requirement for core RNA sequences but not for core-related polypeptides. J Virol. 1999;73(2):1546–54. doi: 10.1128/jvi.73.2.1546-1554.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]