Abstract
Rationale
Psychostimulants, such as yohimbine and amphetamine, can enhance learning and memory. Extinction of conditioned fear involves new learning, so we asked whether psychostimulants could enhance this learning. Previous work suggests that yohimbine facilitates extinction, using freezing as a fear measure. However, psychostimulant-induced alterations in locomotion can confound freezing measurements. Furthermore, the effects of amphetamine on fear extinction have never been examined.
Objective
We evaluated the effectiveness of yohimbine and amphetamine in enhancing fear extinction. In addition to freezing, we measured bar-press suppression, which is less sensitive to changes in locomotion. We asked: Do psychostimulants reduce fear during extinction training, when drug is present? Does learning extinction with psychostimulants result in better extinction retention?
Methods
Rats received fear conditioning on day 1 followed by partial extinction training on days 2 and 3. Yohimbine (1.0, 2.0 or 5.0 mg/kg, i.p.), amphetamine (1.0 mg/kg, i.p.), or vehicle were injected prior to extinction on day 2.
Results
Yohimbine dose-dependently reduced freezing during extinction training on day 2, whereas bar-press suppression was reduced at the highest dose only. When tested drug-free, yohimbine-treated rats showed equivalent levels of freezing and suppression to controls. Amphetamine also decreased freezing during extinction, but did not decrease suppression. During the drug-free test, there was no difference between amphetamine-treated rats and controls in either measure.
Conclusions
Although yohimbine and amphetamine are capable of decreasing freezing, neither drug strengthens retention of fear extinction. Based on these rodent findings, psychostimulants may not be suitable adjuncts to extinction-based therapies for treatment of anxiety disorders.
Keywords: fear conditioning, psychostimulant, norepinephrine, fear extinction, bar-press suppression, freezing, alpha2-adrenoceptor antagonist, reuptake blocker, anxiety disorders
Introduction
Psychostimulants can enhance learning and memory in a variety of tasks. Two psychostimulant drugs known to strengthen learning and retention are yohimbine, an α2-adrenoceptor antagonist, and amphetamine, a monoaminergic reuptake blocker. In humans, administration of yohimbine during presentation of an emotional story enhances subsequent free recall and recognition memory in a drug-free test (O'Carroll et al., 1999). Similarly, amphetamine given to people before or after a verbal learning task enhances later recall and recognition (Soetens et al., 1993; Soetens et al., 1995). In rats, amphetamine facilitates learning and retention of aversive memories such as conditioned taste aversion (Fenu and Di Chiara, 2003) and conditioned avoidance (Davies et al., 1974; Blaiss and Janak, 2007).
Although the formation of aversive memories is enhanced by psychostimulants, the question remains whether these drugs can enhance extinction of these memories. In fear extinction, fear responses are reduced upon repeated presentation of conditioned stimuli, resulting in the formation of a new inhibitory memory (Rescorla, 2004; Myers and Davis, 2007; Quirk and Mueller, 2008). Previous work has shown that yohimbine administered prior to extinction can enhance subsequent recall of extinction (Cain et al., 2004; Morris and Bouton, 2007), an effect attributed to increased norepinephrine release. In addition, pre-extinction administration of tricyclic antidepressants, which block reuptake of norepinephrine, enhances recall of extinction the next day (Telegdy et al., 1983; Kikusui et al., 2001). Amphetamine also blocks reuptake of norepinephrine, as well as other monoamines, and is already in use clinically to treat attention disorders. Decades of research implicate noradrenergic signaling in enhancing memory formation (McGaugh, 2000), and memory for extinction is impaired by lesions of the locus coeruleus which deprive the cortex of norepinephrine (Mason and Fibiger, 1979; McCormick and Thompson, 1982). Recently, we have shown that fear extinction is dependent on noradrenergic signaling in the prefrontal cortex (Mueller et al., 2008). Because psychostimulants increase extracellular concentrations of norepinephrine (Goldberg and Robertson, 1983; Berridge, 2006), we asked whether systemic administration of psychostimulants could enhance acquisition and/or retention of fear extinction.
Most prior work on conditioned fear has used freezing as a measure of fear. Amphetamine has been shown to increase locomotor activity (Dews, 1953), whereas yohimbine has been observed to increase (Mason et al., 1998) or decrease (Chopin et al., 1986) locomotor activity), which could confound freezing measurements. To determine whether these drugs enhance extinction acquisition or simply reduce freezing responses, we trained rats to bar press for food and assessed both freezing and bar-press suppression to a conditioned auditory stimulus. Because bar-press suppression is normalized for baseline press rates (Bouton and Bolles, 1980; Mast et al., 1982), it is less likely to be confounded by psychostimulant-induced changes in baseline activity. We administered yohimbine or amphetamine prior to extinction training, and then tested the rats drug-free the following day, thus allowing us to evaluate whether the drugs accelerated the acquisition of extinction and/or improved retention of extinction. Characterizing the effects of psychostimulants in rodent studies of fear extinction is a first step in determining whether these drugs might be useful as pharmacological adjuncts to exposure therapy for anxiety disorders (Pine and Cohen, 2002; Myers and Davis, 2007).
Materials and Methods
Subjects
104 male Sprague-Dawley rats weighing 270−320 g were housed and handled as previously described (Quirk et al., 2000). Rats were restricted to 18 g of standard rat chow daily, and were subsequently trained to press a bar for food on a variable interval schedule (VI 60 s). All procedures were approved by the local Institutional Animal Care and Use Committee in accordance with the National Institute of Health guidelines.
Apparatus
Fear conditioning and extinction were carried out in four identical operant conditioning chambers (Coulbourn Instruments, Allentown, PA). The floor consisted of stainless steel bars separated by 1.8 cm, connected to a shock scrambler (Coulbourn). A response lever was located 6.5 cm above the floor, and a speaker was positioned on the opposite wall. The chamber was ventilated and illuminated by a single house light, and housed in a sound-attenuating box (Med Associates, Burlington, VT). Locomotor activity was measured in an open field (91.5 × 91.5 × 61 cm), divided into peripheral (within 15.25 cm of the walls) and central (61 × 61 cm) regions of approximately equal area.
Drugs
All drugs were purchased from Sigma (St. Louis, MO). Yohimbine was dissolved in distilled water to avoid the formation of a precipitate, and d-amphetamine was dissolved in physiological saline. Solutions were prepared the same day they were used, and administered in a volume of 1.0 ml/kg.
Fear Conditioning and Extinction
On day 1, rats were presented with five tones (30 s, 75 dB, 4 kHz) in the absence of shocks, and were subsequently fear conditioned with seven presentations of the tone that co-terminated with a footshock (0.43 mA, 0.5 s). The intertrial interval varied and averaged 3 minutes. Groups of rats were matched on freezing during conditioning. On day 2, rats were injected with yohimbine (5.0, 2.0, or 1.0 mg/kg, i.p.), amphetamine (1.0 mg/kg, i.p.), or vehicle 20−30 min prior to extinction training. Extinction consisted of either 6 or 8 presentations of the tone in the absence of shock, administered in the same chamber as conditioning. On days 3 and 4, rats were given additional extinction trials (drug-free) to test for extinction memory, again in the same chamber (AAA design). A variant of this design was used to test whether yohimbine would enhance fear extinction when extinction and testing occurred in a novel environment. Rats were fear conditioned in context A (as above) on day 1. On day 2, rats were administered yohimbine (1.0 mg/hg, i.p.) prior to 8 trials of extinction in a novel chamber (context B). On day 3, rats were tested drug free in context B, (ABB design). Context A consisted of the operant conditioning chamber with grid floors. Context B differed in that the flooring consisted of vinyl sheets, the walls of the operant conditioning chamber were striped, and a novel almond odor was present.
Locomotor Activity
Following fear conditioning and extinction procedures, rats were no longer food restricted and had access to food in their homecages ad libitum. After 2−3 days, rats were assigned to either the drug or vehicle condition based on drug exposure during extinction, such that each group consisted of 50% drug-experienced and 50% drug-naïve rats. Rats were then assessed for drug-induced locomotor activity in an open field for 10 min. Open field testing was performed during the light cycle under lit conditions, and was recorded onto video tapes.
Data Analysis
Digital video files were analyzed offline with Freezescan software (Clever Systems, Reston, VA), which calculated the percent time rats were motionless during tone presentations. We also assessed suppression of bar pressing as an additional measure of conditioned fear. The rate of bar pressing during the 30 sec tone was compared to the rate in the 60 seconds prior to the tone, to calculate a suppression ratio as follows: Suppression = (pretone rate – tone rate)/(pretone rate + tone rate). A suppression ratio of 0 indicates no suppression of bar pressing, and a ratio of 1.0 indicates maximal suppression. For the open-field experiments, locomotion (line crossings) was scored manually from videotape by observers blind to the treatment of the rats. All group comparisons were made using ANOVA and Student's t-tests. Significant main effects of ANOVA were followed by Tukey's post hoc comparisons.
Results
Yohimbine decreased freezing, but did not enhance fear extinction
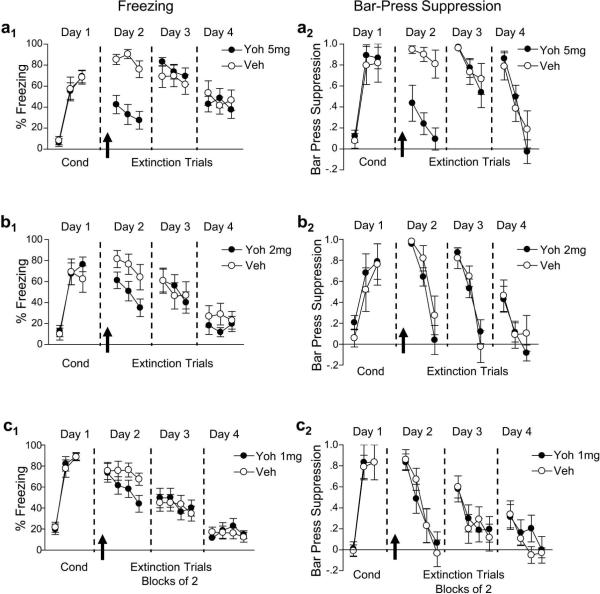
Fear conditioning was conducted on day 1, and on day 2 we injected 5.0 mg/kg (i.p.) of yohimbine prior to extinction training. This dose of yohimbine was selected to match that used in a previous study of fear extinction in mice (Cain et al., 2004). Rats injected with yohimbine expressed significantly lower levels of freezing on average than rats injected with distilled water (vehicle) throughout the extinction session (yohimbine: 34%, vehicle: 84%, n = 12 and 11; Fig. 1a1). ANOVA revealed a main effect of group (F1,21 = 24.0, p < 0.001) and a group by trial interaction (F5,105 = 2.6, p = 0.029) indicating that yohimbine-treated rats expressed less freezing overall and a higher rate of extinction during the session than vehicle-treated controls. Yohimbine-treated rats also showed significantly less bar-press suppression on average than vehicle-treated rats during extinction training (yohimbine: 0.26, vehicle: 0.88; effect of group: F1,21 = 27.0, p < 0.001; Fig. 1a2). Thus, yohimbine decreased fear expression and appeared to facilitate extinction learning. In a drug-free test the following day, however, both groups expressed similar levels of freezing (yohimbine: 77%, vehicle: 66%; F1,21 = 0.6, p = 0.45), and similar bar-press suppression (yohimbine: 0.76, vehicle: 0.82; F1,21 = 0.1, p = 0.82). These findings indicate that this dose of yohimbine does not improve long-term retention of extinction.
Figure 1.
Yohimbine dose-dependently decreases conditioned freezing and bar-press suppression, but does not enhance long-term extinction retention. (a) A high dose of yohimbine (5.0 mg/kg, i.p.) significantly reduced freezing and bar-press suppression during extinction. (b) A reduced dose of yohimbine (2.0 mg/kg, i.p.) decreased freezing, but not bar-press suppression, during extinction. (c) The lowest dose of yohimbine (1.0 mg/kg, i.p.) decreased freezing, but not bar-press suppression, during extinction. No dose of yohimbine enhanced long-term retention of extinction on either measure of fear. Data are shown in blocks of 2 trials. * p < 0.05.
It is possible that the high dose of yohimbine we used affects both pre- and postsynaptic α2-adrenoceptors, whereas lower doses may be more selective for presynaptic autoreceptors, preventing negative feedback and enhancing noradrenergic transmission. To address this, we repeated the experiment with a lower dose of yohimbine (2.0 mg/kg, i.p.; Fig. 1b). Yohimbine-treated rats expressed significantly less freezing on average than vehicle-treated rats throughout extinction training (yohimbine: 49%, vehicle: 74%, n = 10 and 12; F1,20 = 4.39, p = 0.049). In contrast, bar press suppression did not differ between the groups (yohimbine: 0.55 vehicle: 0.69 ;F1,20 = 1.66, p = 0.21). In a drug-free test the following day, both the yohimbine- and vehicle-injected groups showed equivalent levels of freezing (yohimbine: 62%, vehicle: 61%; F1,20 = 0.00, p = 0.95) and bar press suppression (yohimbine: 0.51, vehicle: 0.48; F1,20 = 0.07, p = 0.80). Thus, a dose of 2.0 mg/kg yohimbine reduced fear expression in only one of two measures, and did not enhance long-term extinction retention.
Recently, Morris and Bouton (2007) showed that an even lower dose of yohimbine (1.0 mg/kg) given prior to fear extinction strengthened extinction memory in rats. We therefore repeated the experiment with this dose, and increased the number of extinction trials from 6 to 8 in order to match Bouton and Morris (2007). Rats that were injected with 1.0 mg/kg yohimbine prior to extinction training did not show significantly reduced freezing compared to controls (yohimbine: 59%, vehicle: 74%, n = 12 and 12; F1,22 = 2.14, p = 0.16). However, extinction of the freezing response proceeded at a faster rate in yohimbine-treated rats, as evidenced by a significant group by trial interaction (F7,154 = 2.47, p = 0.02; Fig. 1c). In contrast, bar-press suppression was similar between groups (yohimbine: 0.40, vehicle: 0.43; F1,22 = 0.06, p = 0.82) and no group by trial interaction was observed (F7,154 = 0.39, p = 0.91), suggesting that fear expression was still intact. During a drug-free test the following day, there was no difference between groups in freezing (yohimbine: 44%, vehicle: 42%; F1,22 = 0.04, p = 0.85), or bar-press suppression (yohimbine: 0.32, vehicle: 0.30; F1,22 = 0.02, p = 0.90).
These yohimbine results are summarized in Figure 2. Yohimbine dose-dependently reduced freezing during extinction training (Fig. 2a). In addition, we assessed the effects of yohimbine on locomotion in an open field. Yohimbine did not reduce locomotor activity at any one dose, although we observed an overall significant reduction across all doses tested (F3,30 = 3.67, p = 0.02; Fig. 2b), suggesting that yohimbine induced a mild motor impairment. To assess this possibility further, we examined the effect of yohimbine on spontaneous bar pressing prior to the first extinction tone. Yohimbine significantly reduced bar pressing only at the highest dose (F3,65 = 4.74, p = 0.005; Fig. 2c, Tukey post hoc, p = 0.005). Thus, the pronounced reduction in freezing and suppression with the 5.0 mg/kg dose of yohimbine were likely due to non-specific effects on motivational state, rather than to a reduction in fear expression. Under no conditions did yohimbine enhance long-term extinction retention.
Figure 2.
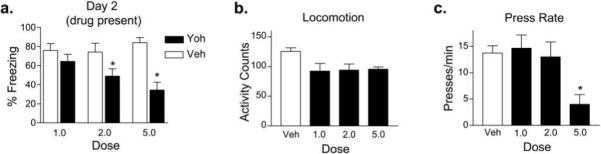
Summary of yohimbine-induced reduction of freezing responses and locomotor activity. (a) Average freezing across trials during extinction. (b) Yohimbine reduces locomotor activity at all doses and (c) impairs bar-pressing for food at the highest dose.
One possible reason for the apparent disagreement between our findings and those of Morris and Bouton (2007) is the contextual design used. To address this, we ran an additional experiment examining the effects of yohimbine when extinction and testing were done in a novel chamber (ABB design). On day 1, rats were fear conditioned in context A, and on day 2 were injected with 1.0 mg/kg yohimbine prior to extinction training in a novel context B. Rats injected with yohimbine did not show significantly reduced freezing compared to controls (yohimbine: 63%, vehicle: 69%, n = 8 and 8; F1,14 = 0.52, p = 0.48), although extinction of the freezing response proceeded at a faster rate in yohimbine-treated rats, as evidenced by a significant group by trial interaction (F7,98 = 2.72, p = 0.013). In contrast, bar-press suppression was similar between groups (yohimbine: 0.32, vehicle: 0.45; F1,14 = 0.64, p = 0.44; Fig. 3) and no group by trial interaction was observed (F7,98 = 1.39, p = 0.22), suggesting that fear expression was still intact. During a drug-free test the following day, there was no difference between groups in freezing (yohimbine: 55%, vehicle: 54%; F1,14 = 0.01, p = 0.91), or bar-press suppression (yohimbine: 0.28, vehicle: 0.25; F1,14 = 0.05, p = 0.83). Thus, yohimbine did not enhance long-term extinction retention regardless of the contextual design of the experiment.
Figure 3.
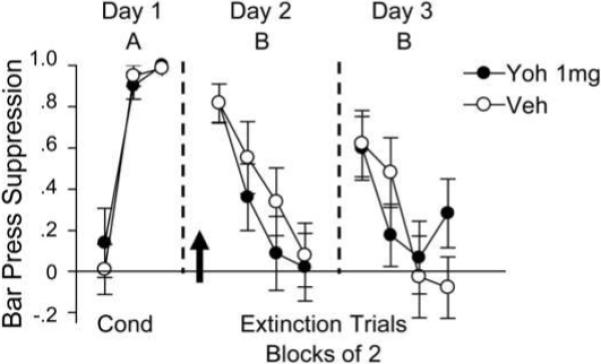
Yohimbine does not enhance extinction retention when fear conditioning and extinction occur in different contexts. A low dose of yohimbine (1.0 mg/kg, i.p.) did not decrease bar-press suppression during extinction and had no effect on long-term retention of extinction. Data are shown in blocks of 2 trials.
Amphetamine decreased freezing, but did not enhance fear extinction
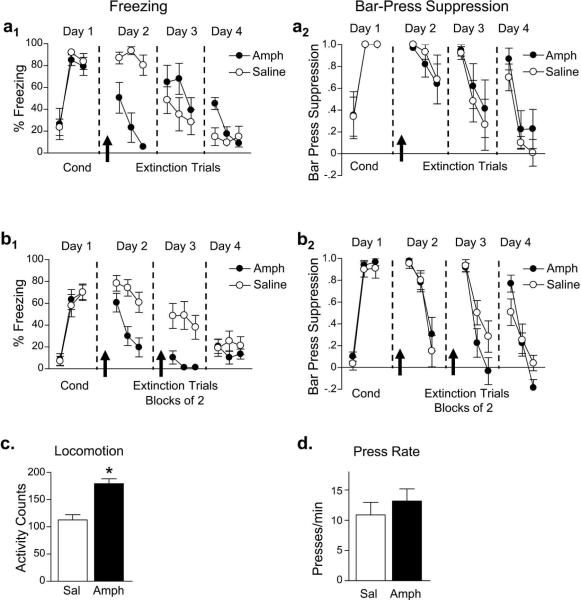
Because previous work has shown that a dose of 1.0 mg/kg (i.p.) amphetamine enhances consolidation of inhibitory avoidance learning (Martinez, Jr. et al., 1980), we examined whether this dose would enhance memory for fear extinction. We injected amphetamine prior to extinction training, and observed that amphetamine-treated rats expressed lower levels of freezing on average than saline-treated rats throughout the extinction session (amphetamine: 27%, saline: 87%, n = 6 and 6; Fig. 4a). ANOVA confirmed that amphetamine decreased freezing as compared to saline (F1,10 = 34.67, p < 0.001), and showed that amphetamine decreased freezing responses at a faster rate than saline as indicated by a significant treatment by trial interaction (F5,50 = 3.66, p = 0.007). In contrast to freezing, bar press suppression showed no effect of amphetamine, as there were no differences between treatment groups during extinction training (amphetamine: 0.81, saline: 0.87; F1,10 = 0.21, p = 0.66). Preserved bar-press suppression suggests that amphetamine-induced reductions in freezing are not due to reduced fear expression. In a drug-free test the next day, no differences were observed between the amphetamine- and saline-treated groups for freezing (amphetamine: 57%, saline: 37%, F1,10 = 1.28, p = 0.28) or suppression (amphetamine: 0.66, saline: 0.56, F1,10 = 0.23, p = 0.64). In an additional drug-free test (day 4), however, amphetamine-treated animals froze significantly more than saline-treated animals during the first block of two tones (amphetamine: 45%, saline: 15%; t10 = 3.10, p = 0.011). Thus, a single injection of amphetamine prior to extinction did not enhance long-term extinction retention, but rather partially impaired it.
Figure 4.
Amphetamine decreases conditioned freezing but does not reduce fear expression or enhance long-term extinction retention. (a) A single exposure of amphetamine during extinction decreased freezing, but did not affect bar-press suppression. (b) Amphetamine administered prior to each of two days of extinction reduced freezing on both days, but did not affect bar-press suppression. (c) Amphetamine increased locomotor activity in an open field, but did not affect bar pressing for food (d). Data are shown in blocks of 2 trials. * p < 0.05.
Although a single injection of amphetamine did not enhance extinction, we asked whether repeated injections across multiple extinction training sessions might facilitate extinction learning. Amphetamine was administered before each of the first two extinction sessions (Fig. 4b). Amphetamine-injected rats showed significantly less freezing than controls in both the first extinction session (amphetamine: 37%, saline: 71%, n = 12 and 11) and the second extinction session (amphetamine: 4%, saline: 45%; F1,21 = 14.27, p = 0.001). In contrast, no effects were observed for bar-press suppression during the first (amphetamine: 0.69, saline: 0.63; F1,21 = 0.25, p = 0.62) or second (amphetamine: 0.37, saline: 0.57; F1,21 = 3.39, p = 0.08) extinction sessions. When tested drug-free the following day, there was no difference in freezing (amphetamine: 15%, saline: 22%; F1,21 = 0.60, p = 0.45) or bar-press suppression (amphetamine: 0.27, saline: 0.27; F1,21 = 0.001, p = 0.98). Thus, amphetamine administered across two days of extinction did not enhance long-term extinction retention.
To determine whether this dose of amphetamine affected locomotion, we performed an open field test (Fig. 4c). Amphetamine-treated rats exhibited significantly greater locomotor activity than saline-treated rats (t21 = 5.15, p < 0.001). We also examined the effect of amphetamine on bar pressing prior to the first extinction tone (Fig. 4d). Amphetamine did not alter bar pressing as compared to saline treatment (t33 = 0.79, p = 0.44). These results, together with the lack of effect on bar-press suppression, suggest that amphetamine simply interfered with freezing responses rather than facilitating extinction learning.
Discussion
We examined the effects of two psychostimulant drugs, yohimbine and amphetamine, on the acquisition and retention of fear extinction. In agreement with previous findings (Cain et al., 2004; Morris and Bouton, 2007), we found that yohimbine reduces freezing during extinction training, suggesting a decrease in fear expression. Upon further analysis, however, we found that the apparent decrease in fear expression was not evident when examining a second measure of fear (bar-press suppression) that is less affected by baseline locomotion. At high doses, both locomotion and bar pressing were impaired by yohimbine, suggesting that this drug induces a mild ataxia (Majczynski et al., 2006) and/or reduces motivation for exploration and food reinforcement. A reduction in exploration is consistent with the known anxiogenic-like effects of yohimbine. For example, systemic administration of yohimbine has previously been shown to hinder exploration of the open arms of an elevated plus-maze (Johnston and File, 1989).
In our hands, yohimbine failed to enhance long-term retention of extinction at all doses examined, and regardless of the contextual design. Our finding that the highest dose of yohimbine (5.0 mg/kg) was ineffective at enhancing extinction is in agreement with Morris and Bouton (2007). In that study, however, a lower dose of 1.0 mg/kg of yohimbine facilitated both acquisition and retention of extinction. We observed that this lower dose was ineffective in enhancing retention of extinction regardless of contextual design. Yohimbine has also been shown to effectively facilitate retention of extinction in mice after five extinction trials (Cain et al., 2004), but not after 10 or more trials. The discrepancy between our results and those showing enhanced extinction with yohimbine (Cain et al., 2004; Morris and Bouton, 2007; Hefner et al., 2008) does not appear to be due to the contextual design of the experiments. Unlike those previous studies, however, we incorporated a second measure of fear (bar-press suppression). It is possible that the appetitive motivation to bar press for food could affect extinction learning. It has been suggested that yohimbine increases the learning of contextual inhibition of responding to a fear conditioned stimulus (Morris and Bouton, 2007). Thus, yohimbine may be less effective in situations where the context is ambiguous. Clinically, however, it has been argued that extinction-based exposure therapy should be conducted in the original context in which the person was exposed to trauma to maximally protect them from context-induced renewal (Bouton, 2002; Tobena et al., 1993).
Similar to yohimbine, amphetamine appeared to facilitate extinction acquisition, as tone-evoked freezing was rapidly reduced across trials. The lack of an effect on bar-press suppression and the large increase in locomotion, however, suggests that the reduction in freezing did not reflect a reduction in fear. Rather, freezing behavior was likely masked by amphetamine-induced hyperactivity. Furthermore, amphetamine treatment during extinction did not enhance subsequent extinction recall, indicating that this drug does not facilitate extinction. In agreement with our findings, previous research has demonstrated that amphetamine either has no effect or impairs retention of extinction in other paradigms. For instance, amphetamine did not affect retention of extinction of Pavlovian conditioned approach when only five trials of extinction were given (Blaiss and Janak, 2007). Furthermore, amphetamine was found to impair extinction retention of passive avoidance (Kumar, 1971), and of fear-potentiated startle (Borowski and Kokkinidis, 1998). Thus, we conclude that amphetamine does not enhance extinction acquisition or retention at the dose used in the present study. Recently, it was shown that much lower doses enhance acquisition of fear to a tone in mice (Wood and Anagnostaras, 2008). Perhaps very low doses of amphetamine could be used to enhance extinction.
Psychostimulant drugs enhance norepinephrine release, and high levels of norepinephrine have been associated with anxiety disorders such as post-traumatic stress disorder (Geracioti, Jr. et al., 2001). Patients with this disorder exhibit resistance to extinction (Orr et al., 2000; Milad et al., 2008) and exaggerated norepinephrine release in response to traumatic stimuli (Geracioti, Jr. et al., 2006), suggesting that heightened norepinephrine release may be responsible for impairing extinction learning. Furthermore, yohimbine can cause panic attacks in PTSD patients (Southwick et al., 1997; Southwick et al., 1999). Thus, increasing circulating levels of norepinephrine with psychostimulants may hinder extinction memory formation. In support of this, our data suggest that amphetamine exposure during extinction, which augments circulating norepinephrine levels, partially impairs long-term retention of extinction.
Although our data suggest that psychostimulants are not able to enhance extinction in rats, whether these drugs can serve as adjuncts for extinction-based therapy in humans remains unknown. A number of other drugs have been shown to enhance extinction retention in rodents, although research in humans is lagging. These include PEPA, an AMPA receptor potentiator (Zushida et al., 2007), and AM404, an inhibitor of endocannabinoid breakdown and reuptake (Chhatwal et al., 2005). A growing body of evidence supports the use of dcycloserine, a partial agonist of the NMDA receptor, to enhance extinction retention in rats (Walker et al., 2002; Ledgerwood et al., 2003), and exposure therapy in humans (Ressler et al., 2004; Hofmann et al., 2006; Guastella et al., 2008). Thus, research on fear extinction is paving the way for use of pharmacological adjuncts to exposure therapy to treat anxiety disorders.
Acknowledgements
This work was supported by R01-MH058883, S06-GM008224, G12-RR03051 (RCMI) and the UPR President's Office to GJQ, and a FQRNT (Quebec, Canada) postdoctoral fellowship to DM.
Footnotes
Conflict of Interest
The authors have no competing conflict of interest.
References
- Berridge CW. Neural substrates of psychostimulant-induced arousal. Neuropsychopharmacology. 2006;31:2332–2340. doi: 10.1038/sj.npp.1301159. [DOI] [PubMed] [Google Scholar]
- Blaiss CA, Janak PH. Post-training, but not post-reactivation, administration of amphetamine and anisomycin modulates Pavlovian conditioned approach. Neurobiol Learn Mem. 2007;87:644–658. doi: 10.1016/j.nlm.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowski TB, Kokkinidis L. The effects of cocaine, amphetamine, and the dopamine D1 receptor agonist SKF 38393 on fear extinction as measured with potentiated startle: implications for psychomotor stimulant psychosis. Behav Neurosci. 1998;112:952–965. doi: 10.1037//0735-7044.112.4.952. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol Psychiatry. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Bolles RC. Conditioned fear assessed by freezing and by the suppression of three different baselines. Animal Learning and Behavior. 1980;8:429–434. [Google Scholar]
- Cain CK, Blouin AM, Barad M. Adrenergic transmission facilitates extinction of conditional fear in mice. Learn Mem. 2004;11:179–187. doi: 10.1101/lm.71504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhatwal JP, Davis M, Maguschak KA, Ressler KJ. Enhancing cannabinoid neurotransmission augments the extinction of conditioned fear. Neuropsychopharmacology. 2005;30:516–524. doi: 10.1038/sj.npp.1300655. [DOI] [PubMed] [Google Scholar]
- Chopin P, Pellow S, File SE. The effects of yohimbine on exploratory and locomotor behavior are attributable to its effects at noradrenaline and not at benzodiazepine receptors. Neuropharmacology. 1986;25:53–57. doi: 10.1016/0028-3908(86)90058-4. [DOI] [PubMed] [Google Scholar]
- Davies JA, Jackson B, Redfern PH. The effect of amantadine, L-dopa, (plus)-amphetamine and apomorphine on the acquisition of the conditioned avoidance response. Neuropharmacology. 1974;13:199–204. doi: 10.1016/0028-3908(74)90107-5. [DOI] [PubMed] [Google Scholar]
- Dews PB. The measurement of the influence of drugs on voluntary activity in mice. Brit J Pharmacol. 1953;8:46–48. doi: 10.1111/j.1476-5381.1953.tb00749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenu S, Di Chiara G. Facilitation of conditioned taste aversion learning by systemic amphetamine: role of nucleus accumbens shell dopamine D1 receptors. Eur J Neurosci. 2003;18:2025–2030. doi: 10.1046/j.1460-9568.2003.02899.x. [DOI] [PubMed] [Google Scholar]
- Geracioti TD, Jr., Baker DG, Ekhator NN, West SA, Hill KK, Bruce AB, Schmidt D, Rounds-Kugler B, Yehuda R, Keck PE, Jr., Kasckow JW. CSF norepinephrine concentrations in posttraumatic stress disorder. Am J Psychiatry. 2001;158:1227–1230. doi: 10.1176/appi.ajp.158.8.1227. [DOI] [PubMed] [Google Scholar]
- Geracioti TD, Jr., Carpenter LL, Owens MJ, Baker DG, Ekhator NN, Horn PS, Strawn JR, Sanacora G, Kinkead B, Price LH, Nemeroff CB. Elevated cerebrospinal fluid substance p concentrations in posttraumatic stress disorder and major depression. Am J Psychiatry. 2006;163:637–643. doi: 10.1176/ajp.2006.163.4.637. [DOI] [PubMed] [Google Scholar]
- Goldberg MR, Robertson D. Yohimbine: a pharmacological probe for study of the alpha 2-adrenoreceptor. Pharmacol Rev. 1983;35:143–180. [PubMed] [Google Scholar]
- Guastella AJ, Richardson R, Lovibond PF, Rapee RM, Gaston JE, Mitchell P, Dadds MR. A randomized controlled trial of D-cycloserine enhancement of exposure therapy for social anxiety disorder. Biol Psychiatry. 2008;63:544–549. doi: 10.1016/j.biopsych.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Hefner K, Whittle N, Juhasz J, Norcross M, Karlsson RM, Saksida LM, Bussey TJ, Singewald N, Holmes A. Impaired fear extinction learning and cortico-amygdala circuit abnormailities in a common genetic mouse train. J Neurosci. 2008;28:8074–8085. doi: 10.1523/JNEUROSCI.4904-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Pollack MH, Otto MW. Augmentation treatment of psychotherapy for anxiety disorders with D-cycloserine. CNS Drug Rev. 2006;12:208–217. doi: 10.1111/j.1527-3458.2006.00208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston AL, File SE. Yohimbine's anxiogenic action: evidence for noradrenergic and dopaminergic sites. Pharmacol Biochem Behav. 1989;32:151–156. doi: 10.1016/0091-3057(89)90225-6. [DOI] [PubMed] [Google Scholar]
- Kikusui T, Takeuchi Y, Mori Y. Pharmacological manipulations of the extinction process of fear-induced ultrasonic vocalization in rats. J Vet Med Sci. 2001;63:591–595. doi: 10.1292/jvms.63.591. [DOI] [PubMed] [Google Scholar]
- Kumar R. Extinction of fear. I. Effects of amylobarbitone and dexamphetamine given separately and in combination on fear and exploratory behaviour in rats. Psychopharmacologia. 1971;19:163–187. doi: 10.1007/BF00402640. [DOI] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J. Effects of D-cycloserine on extinction of conditioned freezing. Behav Neurosci. 2003;117:341–349. doi: 10.1037/0735-7044.117.2.341. [DOI] [PubMed] [Google Scholar]
- Majczynski H, Cabaj A, Slawinska U, Gorska T. Intrathecal administration of yohimbine impairs locomotion in intact rats. Behav Brain Res. 2006;175:315–322. doi: 10.1016/j.bbr.2006.08.040. [DOI] [PubMed] [Google Scholar]
- Martinez JL, Jr., Jensen RA, Messing RB, Vasquez BJ, Soumireu-Mourat B, Geddes D, Liang KC, McGaugh JL. Central and peripheral actions of amphetamine on memory storage. Brain Res. 1980;182:157–166. doi: 10.1016/0006-8993(80)90838-0. [DOI] [PubMed] [Google Scholar]
- Mason K, Heal DJ, Stanford SC. The anxiogenic agents, yohimbine and FG 7142, disrupt the noradrenergic response to novelty. Pharmacol Biochem Behav. 1998;60:321–327. doi: 10.1016/s0091-3057(97)00564-9. [DOI] [PubMed] [Google Scholar]
- Mason ST, Fibiger H. Noradrenaline, fear and extinction. Brain Res. 1979;165:47–56. doi: 10.1016/0006-8993(79)90043-x. [DOI] [PubMed] [Google Scholar]
- Mast M, Blanchard RJ, Blanchard DC. The relationship of freezing and response suppression in a CER situation. Psychol Rec. 1982;32:151–167. [Google Scholar]
- McCormick DA, Thompson RF. Locus coeruleus lesions and resistance to extinction of a classically conditioned response: involvement of the neocortex and hippocampus. Brain Res. 1982;245:239–249. doi: 10.1016/0006-8993(82)90806-x. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory--a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK. Presence and acquired origin of reduced recall for fear extinction in PTSD: Results of a twin study. J Psychiatr Res. 2008;42:515–520. doi: 10.1016/j.jpsychires.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RW, Bouton ME. The effect of yohimbine on the extinction of conditioned fear: a role for context. Behav Neurosci. 2007;121:501–514. doi: 10.1037/0735-7044.121.3.501. [DOI] [PubMed] [Google Scholar]
- Mueller D, Porter JT, Quirk GJ. Noradrenergic signaling in infralimbic cortex increases cell excitability and strengthens memory for fear extinction. J Neurosci. 2008;28:369–375. doi: 10.1523/JNEUROSCI.3248-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- O'Carroll RE, Drysdale E, Cahill L, Shajahan P, Ebmeier KP. Stimulation of the noradrenergic system enhances and blockade reduces memory for emotional material in man. Psychol Med. 1999;29:1083–1088. doi: 10.1017/s0033291799008703. [DOI] [PubMed] [Google Scholar]
- Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. J Abnorm Psychol. 2000;109:290–298. [PubMed] [Google Scholar]
- Pine DS, Cohen JA. Trauma in children and adolescents: risk and treatment of psychiatric sequelae. Biol Psychiatry. 2002;51:519–531. doi: 10.1016/s0006-3223(01)01352-x. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA. Spontaneous recovery. Learn Mem. 2004;11:501–509. doi: 10.1101/lm.77504. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, Hodges L, Davis M. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61:1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- Soetens E, Casaer S, D'Hooge R, Hueting JE. Effect of amphetamine on long-term retention of verbal material. Psychopharmacology (Berl) 1995;119:155–162. doi: 10.1007/BF02246156. [DOI] [PubMed] [Google Scholar]
- Soetens E, D'Hooge R, Hueting JE. Amphetamine enhances human-memory consolidation. Neurosci Lett. 1993;161:9–12. doi: 10.1016/0304-3940(93)90127-7. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Krystal JH, Bremner JD, Morgan CA, III, Nicolaou AL, Nagy LM, Johnson DR, Heninger GR, Charney DS. Noradrenergic and serotonergic function in posttraumatic stress disorder. Arch Gen Psychiatry. 1997;54:749–758. doi: 10.1001/archpsyc.1997.01830200083012. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Morgan CA, III, Charney DS, High JR. Yohimbine use in a natural setting: effects on posttraumatic stress disorder. Biol Psychiatry. 1999;46:442–444. doi: 10.1016/s0006-3223(99)00107-9. [DOI] [PubMed] [Google Scholar]
- Telegdy G, Fekete M, Balazs M, Kadar T. Effects of a new antidepressant drug on active avoidance behavior in rats. Comparative study with tricyclic antidepressants. Arch Int Pharmacodyn Ther. 1983;266:50–59. [PubMed] [Google Scholar]
- Tobena A, Fernandez-Terual A, Escorihuela RM, Nunez JF, Ferre P, Sanchez R. Limits of habituation and extinction: implications for relapse prevention programs in addictions. Drug Alcohol Depend. 1993;32:209–217. doi: 10.1016/0376-8716(93)90085-5. [DOI] [PubMed] [Google Scholar]
- Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J Neurosci. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SC, Anagnostaras SG. Memory and psychostimulants: modulation of Pavlovian fear conditioning by amphetamine in C57?BL/6 mice. Psychopharmacology. 2008 doi: 10.1007/s00213-008-1185-9. (advance online publication, 15 May 2008). doi:10.1007/s00213-008-1185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zushida K, Sakurai M, Wada K, Sekiguchi M. Facilitation of extinction learning for contextual fear memory by PEPA: a potentiator of AMPA receptors. J Neurosci. 2007;27:158–166. doi: 10.1523/JNEUROSCI.3842-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]