Abstract
Resveratrol, a polyphenol compound with reported antioxidant and anti-carcinogenic effects, a wide range of molecular targets, and toxicity only at extreme doses, has received considerable attention. We evaluated the radioprotective effect of orally administered resveratrol on the frequencies of chromosome aberrations in irradiated mouse bone marrow cells. CBA/CaJ mice were divided into four groups: (1) no treatment, (2) resveratrol only, (3) radiation only, and (4) resveratrol and radiation. Resveratrol treatment (100 mg/kg daily) was initiated 2 days prior to irradiation. Bone marrow was then harvested at 1 and 30 days after a single dose of 3 Gy whole-body γ radiation. A statistically significant (P < 0.05) reduction in the mean total chromosome aberration frequency per metaphase at both times postirradiation in the resveratrol and radiation group compared to the radiation-only group was observed. This study is the first to demonstrate that resveratrol has radioprotective effects in vivo. These results support the use of resveratrol as a radioprotector with the potential for widespread application.
INTRODUCTION
Ionizing radiation has been shown to induce DNA damage, which can lead to mutagenesis and carcinogenesis, depending on the total dose, dose rate and animal species (1–3). Growing concern about radiation damage to normal tissues from occupational, therapeutic and accidental exposures has heightened the need for nontoxic protective compounds and has prompted interest in the use of dietary compounds and medicinal plants for radiation protection (4, 5). One dietary compound, resveratrol (trans-3,5,4′-trihydroxy-stilbene), which is found in a wide variety of plant species including grapes, peanuts, blueberries, bilberries, cranberries, lingonberries and the weed Polygonum cuspidatum (6, 7), has received considerable attention for its reputed health benefits as a cardioprotector and chemopreventive agent (8). Its antioxidant effects, its ability to induce apoptosis and cell cycle arrest, and its low toxicity make resveratrol an attractive candidate for radioprotection of normal cells and cancer prevention.
Resveratrol’s antioxidant properties are mediated by its ability to scavenge free radicals and to promote the activities of antioxidants such as glutathione and antioxidant enzymes such as superoxide dismutase and catalase (9, 10). Other studies have revealed resveratrol’s ability to induce apoptosis in cancer cells through caspase activation and upregulation of CD95-CD95L signaling (11); to activate p53-dependent transcription activity (12); to activate the MAP kinases, ERK, JNKs and p38 kinase (13); to decrease expression of the anti-apoptosis oncoprotein Bcl2 (14); and to induce Bax expression (15). Resveratrol also modulates cell cycle distribution in a concentration- and tissue-specific manner, resulting in suppression of cell cycle progression and cell cycle arrest at G0/G1 phase, the G1/S transition, S phase, the S/G2 transition, and G2/M phase (16). Further, resveratrol has been shown to inhibit expression of cyclins D1, D2 and E and to decrease expression of CDKs 2, 4 and 6 (16, 17); induce WAF1/p21 (17); decrease hyper-phosphorylation of the retinoblastoma protein (17); and inhibit ribonucelotide reductase (18). While some studies have reported a resveratrol-induced increase in DNA synthesis (16), one study reported a dose-dependent biphasic effect on DNA synthesis (19). It has been speculated that slowing the cell cycle would benefit the cell by allowing increased time for repair; however, to our knowledge, increased DNA repair has not been demonstrated using resveratrol.
A relatively nontoxic compound with multiple molecular targets, resveratrol has the potential to act as a radioprotector in normal cells exposed to the damaging effects of ionizing radiation. The goal of the study reported here was to determine whether resveratrol (100 mg/kg per day) initiated 2 days prior to 3 Gy whole-body γ irradiation would result in a reduction in radiation-induced damage to bone marrow cells in mice.
MATERIALS AND METHODS
Animals
Nine-week-old male CBA/CaJ mice weighing 24.7 ± 0.7 g obtained from Jackson Laboratory were used in this study. Mice were allowed to acclimate for approximately 1 week prior to use. The mice were housed and maintained according to the approved standards in the Laboratory Animal Resources facility at Colorado State University (CSU). During each phase of the study, mice were observed daily for general condition and activity levels. Any signs of illness or mortality were recorded and investigated. The Animal Care and Use Committee at CSU approved all animal handling and experimental protocols.
Treatment Groups
A total of 80 10-week-old male CBA/CaJ mice were divided into two sets (1 day and 30 days) of four groups each, 10 mice each, as follows: (1) no treatment, (2) resveratrol only (100 mg/kg), (3) radiation only (3Gy), and (4) resveratrol + radiation (100 mg/kg and 3 Gy). One and 30 days after whole-body irradiation, bone marrow from each mouse was individually harvested and processed; slides were prepared and metaphase chromosome aberrations scored as described below.
Radiation
For radiation exposure, mice were placed as a group of five into a well-ventilated plexiglass container. A total dose of 3 Gy γ radiation given at a dose rate of 1.18 Gy/min was delivered using a 137Cs irradiator (J. L. Shepherd model 81-14).
Resveratrol Dose and Administration
Resveratrol (3,4′,5-trihydroxy-trans-stilbene, Sigma-Aldrich) was dissolved in 100% ethanol to a concentration of 50 mg/ml. Prior to gavage, the resveratrol/ethanol dose was diluted as follows: 0.05 ml of resveratrol (50 mg/ml) in 0.2 ml distilled water for a final concentration of 10 mg/ml. Individual mice in each designated group received a resveratrol dose of 100 mg/kg administered by gavage every 24 h for 2 days prior to irradiation and again on the day of irradiation, 30 min prior to radiation exposure. For mice in the 30-day group, the resveratrol/ethanol mixture was added to the drinking water at a rate of 50 mg resveratrol per 100 ml, beginning on the morning after irradiation and continuing until the final day of the study period. The resveratrol stock and resveratrol water combination were protected from light by covering the container with aluminum foil. The resveratrol water combination was changed regularly.
Bone Marrow Collection
Colchicine (0.1 ml of a 0.5% solution) was administered by intraperitoneal (i.p.) injection into each mouse 1 h prior to bone marrow collection. Bone marrow was flushed from each femur, tibia and radius with PBS. The PBS bone marrow mixture was centrifuged at 1000 rpm for 5 min at 4°C. The cell concentrate was then placed into an incubation mixture (9 ml 0.075 M KCl, 1 ml EDTA trypsin, 200 µl colcemid), mixed and incubated for 30 min at 37°C. After incubation, 5 ml fresh fixative (3:1 methanol:acetic acid) was added, mixed and centrifuged at 1000 rpm for 5 min. Cells were washed with fresh fixative two more times. The supernatant was decanted, a small amount of fixative was added, and cells were dropped onto clean, wet microscope slides and allowed to dry.
Scoring of Chromosome Aberrations
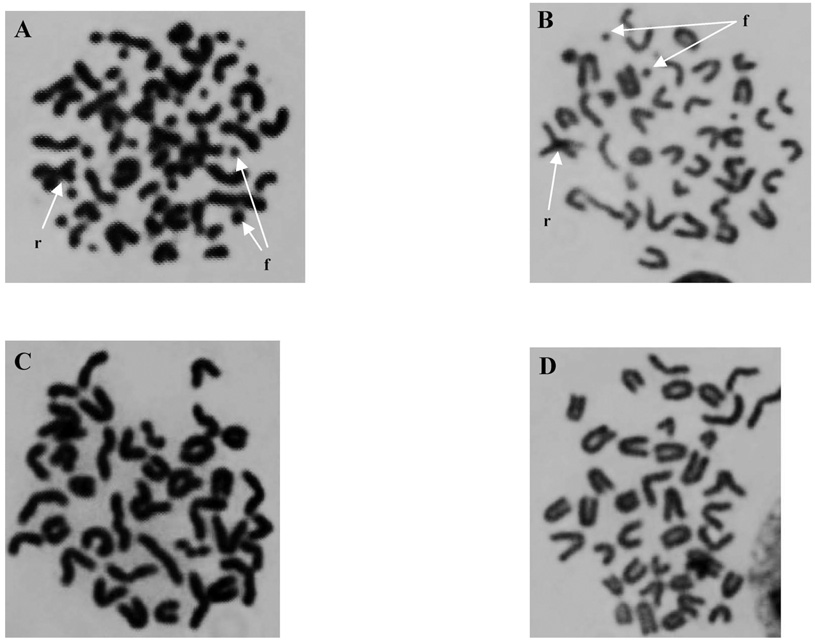
Slides with bone marrow metaphase cells were stained with 10% Giemsa (Sigma-Aldrich) for 7 min, rinsed, air dried and mounted with a glass cover slip. The slides were coded and blinded; then 25 metaphases with 38–40 chromosomes each were scored for aberrations. Chromosome aberrations observed in bone marrow metaphases were scored as follows: (1) fragments (chromosome and chromatid types), identified as un-rejoined acentric fragments derived from a chromosome or chromatid severance, including terminal deletions and interstitial deletions; (2) chromatid (or isochromatid) gaps, identified as an achromatic site along the length of a chromatid that was less than the width of the chromatid; (3) dicentrics, identified as a chromosome (or chromatid) end joined to another chromosome (or chromatid); and (4) Robertsonian translocations, identified as two chromosomes joined at their centromeres (chromosome type). All bone marrow metaphases were scored in the same fashion to allow consistent comparisons. Figure 1 shows representative photomicrographs of metaphase bone marrow cells from each treatment group 1 day postirradiation. After scoring, the slides were unblinded and results were compiled according to the respective treatment group. As a result of the relatively poor morphology of metaphase spreads derived directly from bone marrow, categories of aberrations were combined and tallied to give a total that was used for statistical calculations.
FIG. 1.
Photomicrographs of metaphase bone marrow cells 1 day postirradiation. Panel A: radiation only, panel B: radiation + resveratrol, panel C: no treatment, and panel D: resveratrol only. Arrows indicate chromosome fragments (f) and Robertsonian translocations (r).
Statistical Analysis
For statistical analysis, the following categories were compiled: (1) fragments: chromosome and chromatid types combined, (2) gaps: chromatid and isochromatid types combined, (3) dicentrics: only chromatid types observed, (4) Robertsonian translocations: chromosome type, and (5) total aberrations. Statistical analysis consisted of evaluation of these aberration frequencies per time for each treatment group. Total aberrations represent the sum of all chromosome and chromatid aberration types observed. The data are presented as the mean number of chromosome aberrations per bone marrow metaphase ± SD. Since the data exhibited non-normality and heteroscedasticity, the non-parametric Kruskal-Wallis ANOVA was used for overall comparisons between the four treatment groups at the two times, and Wilcoxon rank-sum tests were used to compare chromosome aberration frequencies between pairs of groups.
RESULTS
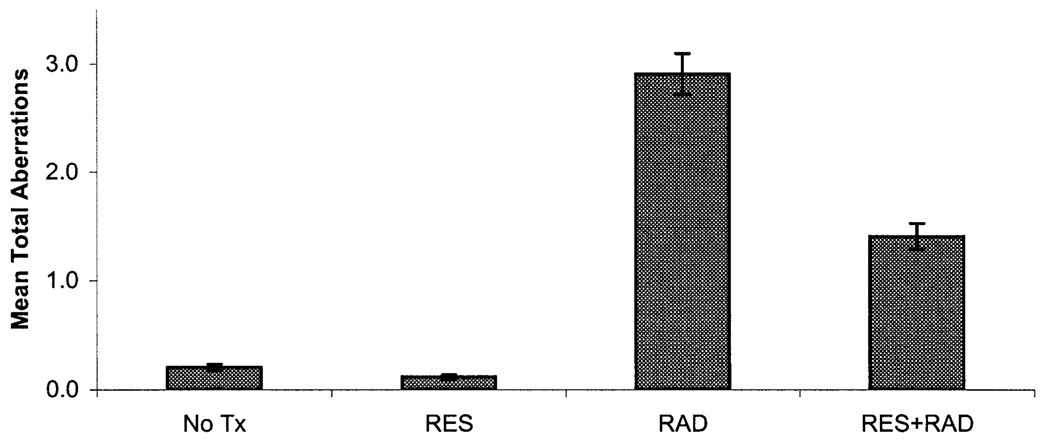
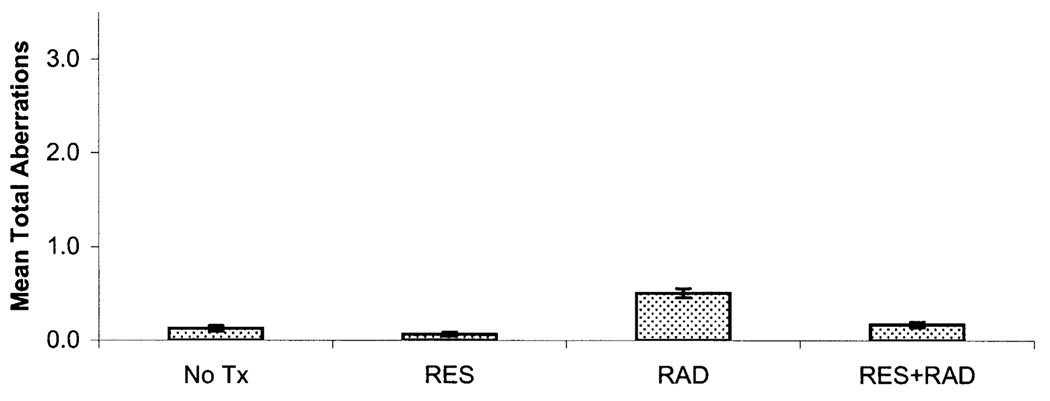
All chromosome aberration frequencies were found to vary by treatment group and time (Table 1 and Fig. 2 and Fig. 3). Fragments were the most common aberration type observed in the radiation and resveratrol + radiation groups, followed by gaps, dicentrics and Robertsonian translocations. For fragments and total chromosome aberration frequencies, the Kruskal-Wallis ANOVA P value was less than 0.0001 at days 1 and 30, while for gaps and dicentrics the P value was less than 0.001 at day 1 but not at day 30 (Table 2). Low mean levels of total background chromo-some aberrations, fragments, gaps, dicentrics and Robert-sonian translocations were observed in the bone marrow metaphase cells scored at days 1 and 30 for the untreated group. In the resveratrol group, lower mean numbers of background chromosome aberrations, fragments, gaps, dicentrics and Robertsonian translocations at day 1 and 30 were noted compared to the no treatment group. However, the difference between the no treatment and resveratrol groups did not rise to the level of statistical significance for any aberration category. The radiation group had the highest mean level of total chromosome aberrations, fragments, gaps, dicentrics and Robertsonian translocations at days 1 and 30 after radiation exposure. On day 1, the resveratrol + radiation group had the second highest mean levels of total chromosome aberrations, fragments, gaps, dicentrics and Robertsonian translocations; however, on day 30, the mean levels of total chromosome aberrations fragments, gaps, dicentrics and Robertsonian translocations for the resveratrol + radiation group were similar to those of the no treatment group (Fig. 2 and Fig. 3).
TABLE 1.
Mean Chromosome Aberrations per Bone Marrow Metaphase Cell Observed in Each Mouse Group at Each Time
| Total | Fragments | Gaps | Dicentrics | Robertsonian | |
|---|---|---|---|---|---|
| No treatment | |||||
| Day 1 | 0.20 ± 0.55 | 0.06 ± 0.28 | 0.07 ± 0.38 | 0.01 ± 0.11 | 0.06 ± 0.25 |
| Day 30 | 0.14 ± 0.45 | 0.02 ± 0.15 | 0.04 ± 0.27 | 0.01 ± 0.09 | 0.07 ± 0.25 |
| Resveratrol | |||||
| Day 1 | 0.12 ± 0.39 | 0.02 ± 0.18 | 0.04 ± 0.26 | 0.02 ± 0.13 | 0.03 ± 0.18 |
| Day 30 | 0.07 ± 0.27 | 0.01 ± 0.13 | 0.02 ± 0.15 | 0.00 ± 0.06 | 0.03 ± 0.18 |
| Radiation | |||||
| Day 1 | 2.90 ± 3.04 | 2.12 ± 2.65 | 0.33 ± 0.70 | 0.28 ± 0.28 | 0.17 ± 0.50 |
| Day 30 | 0.51 ± 0.87 | 0.22 ± 0.61 | 0.13 ± 0.55 | 0.01 ± 0.11 | 0.14 ± 0.39 |
| Resveratrol + radiation | |||||
| Day 1 | 1.40 ± 1.92 | 0.88 ± 1.67 | 0.22 ± 0.60 | 0.19 ± 0.49 | 0.12 ± 0.34 |
| Day 30 | 0.18 ± 0.47 | 0.02 ± 0.14 | 0.06 ± 0.30 | 0.00 ± 0.06 | 0.09 ± 0.31 |
Notes. Values are means ± SD based on 250 cells counted. Total includes fragments, gaps, dicentrics and Robertsonian translocations. Fragments include chromosome and chromatid types, including intersitital deletions, terminal deletions and breaks. Gaps include chromatid and isochromatid types. Dicentrics were of the chromatid type and Robertsonian translocations were of the chromosome type.
FIG. 2.
Total mean chromosome aberrations per bone marrow metaphase cell ± SE day 1 after 3 Gy whole-body irradiation. Statistically significant differences at the P < 0.05 level were found when comparing the no treatment (No Tx) and radiation (RAD) groups and the radiation (RAD) and resveratrol + radiation (RES + RAD) groups for mean total aberrations.
FIG. 3.
Total mean chromosome aberrations per bone marrow metaphase cell ± SE 30 days postirradiation. Statistically significant differences at the P < 0.05 level were found when comparing the no treatment (No Tx) and radiation (RAD) groups and the radiation (RAD) and resveratrol + radiation (RES + RAD) groups for mean total aberrations.
TABLE 2.
Statistical Comparisons between Mouse Treatment Groups and Aberration Categories at Each Time
| Wilcoxon rank-sum tests (P < 0.05) | ||||||
|---|---|---|---|---|---|---|
| Event | Day | Kruskal-Wallis ANOVA (P value) Overall | Resveratrol + radiation vs radiation | Radiation vs no treatment | Resveratrol + radiation vs resveratrol | Resveratrol + radiation vs no treatment |
| Total | 1 | <0.0001 | ✓ | ✓ | ✓ | ✓ |
| 30 | <0.0001 | ✓ | ✓ | ✓ | ns | |
| Fragments | 1 | <0.0001 | ✓ | ✓ | ✓ | ✓ |
| 30 | <0.0001 | ✓ | ✓ | ns | ns | |
| Gaps | 1 | <0.0001 | ✓ | ✓ | ✓ | ✓ |
| 30 | 0.0476 | ns | ✓ | ns | ns | |
| Dicentrics | 1 | <0.0001 | ns | ✓ | ✓ | ✓ |
| 30 | 0.6637 | ns | ns | ns | ns | |
| Robertsonian | 1 | <0.0001 | ns | ✓ | ✓ | ✓ |
| 30 | 0.0014 | ns | ✓ | ✓ | ns | |
Note. The ✓ indicates a statistically significant relationship while the ns indicates that a significant relationship was not found at P < 0.05.
Statistically significant differences (P < 0.05) were found on day 1 postirradiation when comparing the means for total chromosome aberrations, fragments, gaps, dicentrics and Robertsonian translocation frequencies between the no treatment and radiation groups, resveratrol + radiation and resveratrol groups, and resveratrol + radiation and no treatment groups. However, only the mean frequencies for total aberrations, fragments and gaps were significantly different when comparing the resveratrol + radiation and radiation groups (Table 2). On day 30, statistically significant differences (P < 0.05) were found between the means for total chromosome aberrations for the resveratrol + radiation and radiation, radiation and no treatment, and resveratrol + radiation and zesveratrol groups (Table 2). No statistically significant difference was found for any chromosome aberration category (total, fragments, gaps, dicentrics or Robertsonian translocations) between the resveratrol + radiation and no treatment groups at day 30.
DISCUSSION
Resveratrol has been shown to sensitize cancer cells to the damaging effects of radiation in vitro (20–22). One study examined the effect of resveratrol on ionizing radiation-induced damage in normal cells (23). Unfortunately, this study did not clearly document whether resveratrol combined with a single 5-Gy X-ray dose sensitized these normal cells to radiation-induced DNA damage. Currently, there are no reports concerning the effects of combining resveratrol and radiation in vivo. Our results indicate that resveratrol (100 mg/kg daily) administered as a bolus each day for 2 days, then 30 min prior to 3 Gy whole-body irradiation on the third day, and then daily in the drinking water until the end of the study period reduces the mean total chromosome aberration frequency observed in bone marrow metaphases at 1 and 30 days postirradiation. The mean numbers of total chromosome aberrations per metaphase were reduced 2.1-fold at 1 day and 2.8-fold at 30 days in the resveratrol + radiation group compared to the radiation-only group. At 30 days postirradiation, the mean numbers of total aberrations were not significantly different in the resveratrol + radiation group and the no treatment group, indicating that resveratrol is effective at reducing the mean number of total chromosome aberrations to nearly normal background levels within 30 days (Fig. 3). The difference in the mean total chromosome aberration frequencies between the no treatment and resveratrol groups was not statistically significant, indicating that resveratrol itself does not induce observable chromosome aberrations at the 100 mg/kg oral dose.
A review of the results of the experiments reported here provides three basic observations. First, radiation-induced chromosome aberrations (total, fragments, gaps, dicentrics and Robertsonian translocations) were significantly increased in the radiation-only group on day 1 compared to unirradiated mice at day 1. This was also true on day 30, except for dicentrics, which had a frequency that was similar to background levels. Second, resveratrol significantly reduced radiation-induced total aberrations, fragments and gaps on day 1 compared to the irradiated mice but not dicentrics or Robertsonian translocations. At 30 days, resveratrol significantly reduced radiation-induced total aberrations and fragments but not gaps, dicentrics or Robert-sonian translocations. Third, resveratrol reduced radiation-induced total aberrations, fragments, gaps and Robertsonian translocations to background levels (P > 0.05) at 30 days.
While the specific mechanism responsible for the observed effect of resveratrol on reducing the frequency of chromosome aberrations was not evaluated in this study, a number of possible mechanisms have been described in published studies. Since γ radiation is known to induce cellular damage in part though oxidative processes, it is possible that the significant reduction in observed chromosomal damage observed in the resveratrol + radiation group compared to the radiation group is the result of the direct antioxidant properties of resveratrol or that it occurs indirectly through the induction of antioxidants like glutathione and increases in enzymes like superoxide dismutase and catalase (9, 10). The ability of resveratrol at concentrations of 15–20, 30 and 100 µM to induce cell cycle arrest in S phase and/or at the G2/M transition in leukemia cells (24, 25) and potentially allow a longer period for chromosomal repair after a DNA-damaging event could have contributed to reducing the frequency of chromosome aberrations seen in metaphase spreads in the resveratrol + radiation group. Induction of apoptosis in damaged or abnormal cells at concentrations of 10–200 µM (11, 14) would also facilitate a reduction in the observed frequency of chromosome aberrations in the resveratrol + radiation group. It is likely that all these properties of resveratrol contribute to the observed reduction in chromosome aberrations observed at each time examined in this study.
The resveratrol dose selected in this study was expected to produce tissue concentrations consistent with those (10–50 µM) that have been shown to have effects in vitro on molecular targets. One study using an oral dose of 50 mg/kg achieved a liver and kidney concentration of 25–30 µM, and resveratrol conjugates were still present at 3 h (26).
A number of studies of the toxicity of resveratrol using extremely large doses (2000 and 4000/3000 mg/kg in mice and 3000 mg/kg in rats) have shown renal toxicity (27, 28), while a daily dose of 20 mg/kg in rats did not (29). In the study reported here, we found that resveratrol at a daily dose of 100 mg/kg did not induce chromosome aberrations after 30 days of ingestion.
Bone marrow is a complex grouping of different cell types, including leukocytes, erythrocytes, megakaryocytes and stromal cells at different stages of maturation. The exact kinetics of each of these types of bone marrow cells in the mouse is unclear. However, studies evaluating bone marrow cells and bone marrow stromal cells after irradiation (30–33) have shown that radiation-induced aberrations in bone marrow-derived cell populations can persist for months after irradiation (31) and that bone marrow stromal cells continue to show radiation-induced alterations in the bone marrow stromal compartment in vitro 6 months after 2 Gy irradiation (33). In addition, bone marrow stromal cells have reduced capacity for self-renewal and altered blocking of apoptosis in attached hematopoietic stem cells after irradiation (32). These factors likely contribute to the continued presence of bone marrow chromosome aberrations 30 days after whole-body irradiation. With its wide range of cellular targets, it is possible that resveratrol has beneficial effects not only on bone marrow hematopoietic cells but also on the stromal compartment.
In this study we determined that administration of resveratrol at a dose of 100 mg/kg daily, initiated 2 days prior to 3 Gy whole-body irradiation, resulted in a statistically significant reduction in the mean total chromosome aberration frequency in mouse bone marrow cells. This reduction in chromosome aberration frequency is thought to be the result of a combination of resveratrol’s previously identified cellular and molecular properties, including its antioxidant activities and its ability to induce apoptosis and cell cycle arrest. Future studies will investigate these possibilities using this in vivo model in an attempt to determine the specific contribution from each mechanism. The potential for resveratrol to exert its radioprotective and cancer-preventive properties to reduce or prevent radiation-induced acute myeloid leukemia in at risk populations will also be evaluated.
ACKNOWLEDGMENTS
The authors wish to acknowledge the generous support of the staff in the CSU Laboratory Animal Resources, especially Elisa French. Without their invaluable assistance this work would not have been possible. This work was supported by NIH/NCI Awards CA 09236 and CA 43322; U.S. Department of Energy Grant FG0201ER63239; and NASA Grant NNJ04HD83G.
REFERENCES
- 1.Sowa M, Arthurs BJ, Estes BJ, Morgan WF. Effects of ionizing radiation on cellular structures, induced instability and carcinogenesis. EXS. 2006;96:293–301. doi: 10.1007/3-7643-7378-4_12. [DOI] [PubMed] [Google Scholar]
- 2.Elmore E, Lao X-Y, Kapadia R, Redpath JL. The effect of dose rate on radiation-induced neoplastic transformation in vitro by low doses of low-LET radiation. Radiat. Res. 2006;166:832–838. doi: 10.1667/RR0682.1. [DOI] [PubMed] [Google Scholar]
- 3.Suit H, Goldberg S, Niemierko A, Ancukiewicz M, Hall E, Goitein M, Wong W, Paganetti H. Secondary carcinogenesis in patients treated with radiation: a review of data on radiation-induced cancers in human, non-human primate, canine and rodent subjects. Radiat. Res. 2007;167:12–42. doi: 10.1667/RR0527.1. [DOI] [PubMed] [Google Scholar]
- 4.Turner ND, Braby LA, Ford J, Lupton JR. Opportunities for nutritional amelioration of radiation-induced cellular damage. Nutrition. 2002;18:904–912. doi: 10.1016/s0899-9007(02)00945-0. [DOI] [PubMed] [Google Scholar]
- 5.Arora R, Gupta D, Chawla R, Sagar R, Sharma A, Kumar R, Prasad J, Singh S, Samanta N, Sharma RK. Radioprotection by plant products: Present status and future prospects. Phytother. Res. 2005;19:1–22. doi: 10.1002/ptr.1605. [DOI] [PubMed] [Google Scholar]
- 6.Rimando AM, Kalt W, Magee JB, Dewey J, Ballington JR. Resveratrol, pterostilbene, and piceatannol in Vaccinium berries. J. Agric. Food Chem. 2004;52:4713–4719. doi: 10.1021/jf040095e. [DOI] [PubMed] [Google Scholar]
- 7.Burns J, Yokota T, Ashihara H, Lean MEJ, Crozier A. Plant and herbal sources of resveratrol. J. Agric. Food Chem. 2002;50:3337–3340. doi: 10.1021/jf0112973. [DOI] [PubMed] [Google Scholar]
- 8.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat. Rev. Drug Disc. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Cao Z, Zhu H. Upregulation of endogenous antioxidants and phase 2 enzymes by the red wine polyphenol, resveratrol in cultured aortic smooth muscle cells leads to cytoprotection against oxidative and electrophilic stress. Pharmacol. Res. 2006;53:6–15. doi: 10.1016/j.phrs.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Losa GA. Resveratrol modulates apoptosis and oxidation in human blood mononuclear cells. Eur. J. Clin. Invest. 2003;33:818–823. doi: 10.1046/j.1365-2362.2003.01219.x. [DOI] [PubMed] [Google Scholar]
- 11.Clement M-V, Hirpara JL, Chawdhury S-H, Pervaiz S. Chemopreventive agent resveratrol, natural product derived from grapes, triggers CD95 signaling-dependent apoptosis in human tumor cells. Blood. 1998;92:996–1002. [PubMed] [Google Scholar]
- 12.Huang C, Ma W, Goranson A, Dong Z. Resveratrol suppresses cell transformation and induces apoptosis through a p53-dependent pathway. Carcinogenesis. 1999;20:237–242. doi: 10.1093/carcin/20.2.237. [DOI] [PubMed] [Google Scholar]
- 13.She Q-B, Bode AM, Ma W-Y, Chen N-Y, Dong Z. Resveratrol-induced activation of p53 and apoptosis is mediated by extra-cellular signal-regulated protein kinases and p38 kinase. Cancer Res. 2001;61:1604–1610. [PubMed] [Google Scholar]
- 14.Surh Y-J, Hurh Y-J, Kang J-Y, Lee E, Kong G, Lee SJ. Resveratrol, an antioxidant present in red wine, induces apoptosis in human promyelocytic leukemia (HL-60) cells. Cancer Lett. 1999;140:1–10. doi: 10.1016/s0304-3835(99)00039-7. [DOI] [PubMed] [Google Scholar]
- 15.Tessitore L, Davit A, Sarotto I, Caderni G. Resveratrol depresses the growth of colorectal aberrant crypt foci by affecting bax and p21 (CIP) expression. Carcinogenesis. 2000;21:1619–1622. [PubMed] [Google Scholar]
- 16.Delmas D, Lancon A, Colin D, Jannin B, Latruffe N. Resveratrol as a chemopreventive agent: a promising molecule for fighting cancer. Curr. Drug Targets. 2006;7:423–442. doi: 10.2174/138945006776359331. [DOI] [PubMed] [Google Scholar]
- 17.Kundu JK, Surh Y-J. Molecular basis of chemoprevention by resveratrol: NF-κB and AP-1 as potential targets. Mutat. Res. 2004;555:65–80. doi: 10.1016/j.mrfmmm.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 18.Pozo-Guisado E, Alvarez-Barrientos A, Mulero-Navarro S, Santiago-Josefat B, Fernandez-Salguero PM. The antiproliferative activity of resveratrol results in apoptosis in MCF-7 but not MDA-MB-231 human breast cancer cells: cell-specific alteration of the cell cycle. Biochem. Pharmacol. 2002;64:1375–1386. doi: 10.1016/s0006-2952(02)01296-0. [DOI] [PubMed] [Google Scholar]
- 19.Kuwajerwala N, Cifuentes E, Gautam S, Menon M, Barrack ER, Reddy GPV. Resveratrol induces prostate cancer cell entry into S phase and inhibits DNA synthesis. Cancer Res. 2002;62:2488–2492. [PubMed] [Google Scholar]
- 20.Baatout S, Derradji H, Jacquet P, Ooms D, Michaux A, Mergeay M. Enhanced radiation-induced apoptosis of cancer cell lines after treatment with resveratrol. Int. J. Mol. Med. 2004;13:895–902. [PubMed] [Google Scholar]
- 21.Zoberi I, Bradbury CM, Curry HA, Bisht KS, Goswami PC, Roti Roti JL, Gius D. Radiosensitizing and anti-proliferative effects of resveratrol in two human cervical tumor cell lines. Cancer Lett. 2002;175:165–173. doi: 10.1016/s0304-3835(01)00719-4. [DOI] [PubMed] [Google Scholar]
- 22.Scarlatti F, Sala G, Ricci C, Maioli C, Milani F, Minella M, Botturi M, Ghidoni R. Resveratrol sensitization of DU145 prostate cancer cells to ionizing radiation is associated with ceramide increase. Cancer Lett. 2007;253:124–130. doi: 10.1016/j.canlet.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 23.Fiore M, Festa F, Cornetta T, Ricordy R, Cozzi R. Resveratrol affects X-ray induced apoptosis and cell cycle delay in human cells in-vitro. Int. J. Mol. Med. 2005;15:1005–1012. [PubMed] [Google Scholar]
- 24.Duraj J, BoDo J, Sulikova M, Rauko P, Sedlak J. Diverse resveratrol sensitization to apoptosis induced by anticancer drugs in sensitive and resistant leukemia cells. Neoplasma. 2006;53:384–392. [PubMed] [Google Scholar]
- 25.Ferry-Dumazet H, Garnier O, Mamani-Matsuda M, Vercauteren J, Belloc F, Billiard C, Dupouy M, Thiolat D, Kolb JP, Mossalayi MD. Resveratrol inhibits the growth and induces apoptosis of both normal and leukemic hematopoietic cells. Carcinogenesis. 2002;23:1327–1333. doi: 10.1093/carcin/23.8.1327. [DOI] [PubMed] [Google Scholar]
- 26.Vitrac X, Desmouliere A, Brouillaud B, Krisa S, Deffieux G, Barthe N, Rosenbaum J, Merillon J-M. Distribution of [14C]-trans-resveratrol, a cancer chemopreventive polyphenol, in mouse tissues after oral administration. Life Sci. 2003;72:2219–2233. doi: 10.1016/s0024-3205(03)00096-1. [DOI] [PubMed] [Google Scholar]
- 27.Horn TL, Cwik MJ, Morrissey RL, Kapetanovic I, Crowell JA, Booth TD, McCormick DL. Oncogenicity evaluation of resveratrol in p53+ (p53 knockout) mice. Food Chem. Toxicol. 2007;45:55–63. doi: 10.1016/j.fct.2006.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crowell JA, Korytko PJ, Morrissey RL, Booth TD, Levine BS. Resveratrol-associated renal toxicity. Toxicol. Sci. 2004;82:614–619. doi: 10.1093/toxsci/kfh263. [DOI] [PubMed] [Google Scholar]
- 29.Juan ME, Vinardell MP, Planas JM. The daily oral administration of high doses of trans-resveratrol to rats for 28 days is not harmful. J. Nutr. 2002;132:257–260. doi: 10.1093/jn/132.2.257. [DOI] [PubMed] [Google Scholar]
- 30.Dai JM, Sun DC, Lin RX, Yang J, Lou S, Wang SQ. Microarray analysis of differentially expressed genes in mouse bone marrow tissues after ionizing radiation. Int. J. Radiat. Biol. 2006;82:511–521. doi: 10.1080/09553000600857389. [DOI] [PubMed] [Google Scholar]
- 31.Gridley DA, Pecaut MJ. Whole-body irradiation and long-term modification of bone marrow-derived cell populations by low-and high-LET radiation. In Vivo. 2006;20:781–790. [PubMed] [Google Scholar]
- 32.Greenberger JS, Epperly MW, Jahroudi N, Pogue-Geile KL, Berry LA, Bray J, Goltry KL. Role of bone marrow stromal cells in irradiation leukomogenesis. Acta Haematol. 1996;96:1–15. doi: 10.1159/000203708. [DOI] [PubMed] [Google Scholar]
- 33.Greenberger JS, Anderson J, Berry LA, Epperly M, Cronkite EP, Boggs SS. Effects of irradiation of CBA/CA mice on hematopoietic stem cells and stromal cells in long-term bone marrow cultures. Leukemia. 1996;10:514–527. [PubMed] [Google Scholar]