Abstract
The diterpene forskolin (FS) binds to, and activates, mammalian membranous adenylyl cyclase (AC) isoforms I–VIII. Diterpenes without C1-OH group do not activate ACs. The C1-OH group forms a hydrogen bond with the backbone oxygen of Val506 of the C1 catalytic subunit of AC (isoform V numbering). To better understand the mechanism of AC activation we examined the interactions of FS and eight FS analogs with purified catalytic AC subunits C1 (AC V) and C2 (AC II) by fluorescence spectroscopy, using 2′,3′-O-(N-methylanthraniloyl)-guanosine 5′-triphosphate (MANT-GTP) as fluorescent reporter probe, and by enzymatic activity. FS analogs induced C1/C2 assembly as assessed by fluorescence resonance energy transfer from Trp1020 of C2 to MANT-GTP and by increased direct MANT-GTP fluorescence in the order of efficacy FS ~ 7-deacetyl-FS ~ 6-acetyl-7-deacetyl-FS ~ 9-deoxy-FS > 7-deacetyl-7-(N-methylpiperazino-γ-butyryloxy)-FS > 1-deoxy-FS ~ 1,9-dideoxy-FS ~ 7-deacetyl-1-deoxy-FS ~ 7-deacetyl-1,9-dideoxy-FS. In contrast, FS analogs activated catalysis in the order of efficacy FS > 7-deacety-FS ~ 6-acetyl-7-deacetyl-FS ~ 9-deoxy-FS > 7-deacetyl-7-(N-methylpiperazino-γ-butyryloxy)-FS ≫ 1-deoxy-FS, 1,9-dideoxy-FS, 7-deacetyl-1-deoxy-FS and 7-deacetyl-1,9-dideoxy-FS (all ineffective). 1-Deoxy-FS analogs inhibited FS-stimulated catalysis by an apparently non-competitive mechanism. Our data suggest a two-step mechanism of AC activation by diterpenes. In the first step, diterpenes, regardless of their substitution pattern, promote C1/C2 assembly. In the second and yet poorly understood step, diterpenes that form a hydrogen bond between C1-OH and Val506 promote a conformational switch that results in activation of catalysis. The apparent non-competitive interaction of FS with 1-deoxy-FS analogs is explained by impaired ligand exchange due to strong hydrophobic interactions with C1/C2.
1. Introduction
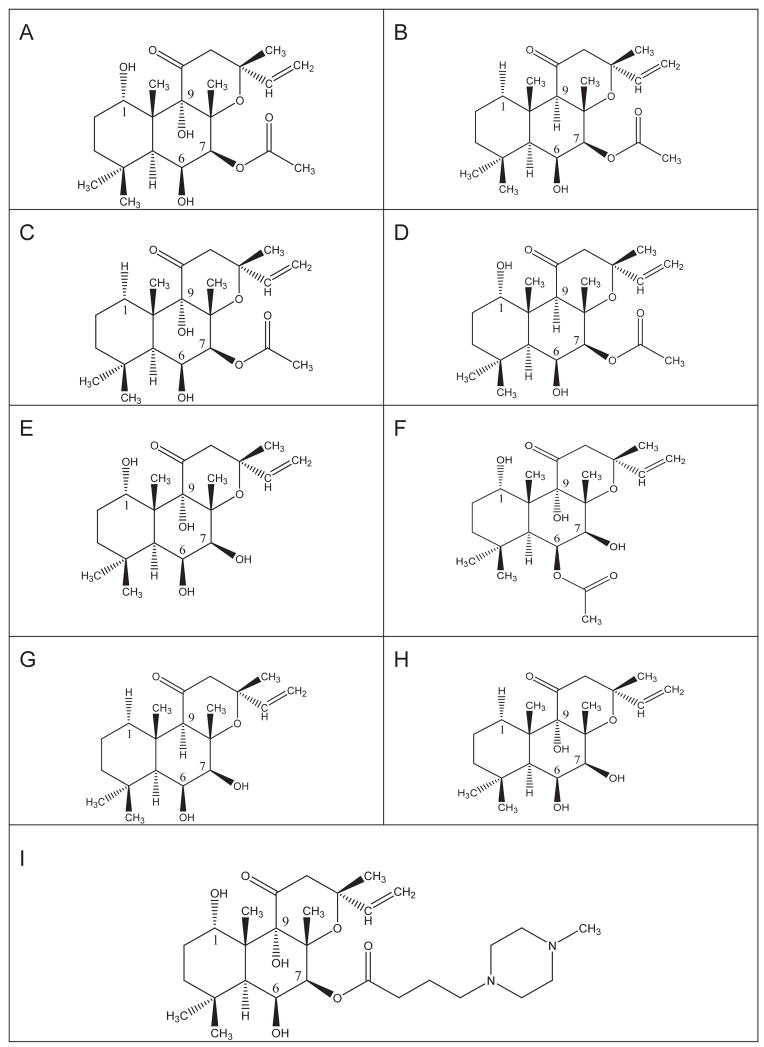
In mammals, nine membranous adenylyl cyclase (AC) isoforms are expressed [1–3]. ACs catalyze the conversion of ATP into the second messenger cAMP and play a central role in transmembrane signal transduction mediated by hormones and neurotransmitters. All AC isoforms are activated by the G-protein Gs, and AC isoforms I–VIII (but not AC IX) are also activated by the diterpene, forskolin (FS), which is produced in the roots of the Indian plant, Coleus forskohlii. Fig. 1 shows the structures of FS and various FS analogs. The FS analogs shown differ from each other in the OH-substitution of C1 and C9 and substitution at C6 or C7 [4–6]. FS is of interest for the treatment of various disorders including cardiovascular diseases, bronchial asthma, obesity and glaucoma [7]. As an important step towards the achievement of this ambitious goal, there has been recent progress in the development of isoform-specific FS analogs [8, 9]. However, the precise mechanism by which FS activates AC is still unknown.
Fig. 1. Structures of FS and FS analogs.
A , FS; B, 1,9dd-FS; C, 1d-FS; D, 9-d-FS; E, 7DA-FS; F, 6A7DA-FS; G, 7DA1,9dd-FS; H, 7DA1d-FS; I, DMB-FS.
A major advance to study AC at a molecular level was the establishment of a soluble reconstituted system consisting of the C1 subunit of AC V and the C2 subunit of AC II. This system is effectively activated by Gs and FS [10, 11]. Crystal structures of C1/C2 bound to various nucleotides including fluorescent 2′,3′-O-(N-methylanthraniloyl) (MANT)-substituted nucleotides have been resolved [12–14]. MANT-nucleotides are used as reporter probes to monitor conformational changes in C1/C2. Upon binding of FS to C1/C2, there is an increased fluorescence resonance energy transfer (FRET) from Trp1020 of C2 to the MANT-group of MANT-GTP and an increase in direct fluorescence [13, 14]. Those data indicate that FS promotes C1/C2 assembly, forming a hydrophobic pocket at the interface of the two subunits to which the MANT-group binds and gives rise to the fluorescence signals.
Crystallographic studies showed that FS and its water-soluble analog DMB-FS [15] (Fig. 1I) bind at the interface between C1 and C2, opposite to the catalytic site. FS interacts with C1/C2 mostly via hydrophobic interactions [12–14] (Fig. 2A). In addition, there are hydrogen bonds between the C1-OH group of FS and the backbone oxygen of Val506 of C1 (AC V numbering), the C7-acetyl group of FS and Ser942 (AC II numbering) via a water molecule and the C11-OH group of FS and Ser508 (AC V numbering) [12] (Fig. 2B). Differences between Kd values of FS equilibrium dialysis binding and EC50 values of FS for activation of catalysis by C1/C2 raised the question of two FS binding sites [10]. In accordance with this assumption, in the C2/C2 homodimer crystal, two FS molecules are found [16]. However, in the C1/C2 heterodimer crystal, there is only one FS binding site [12]. Moreover, dissociations between binding affinity and potency for activation of catalysis were observed for intact ACs [17, 18]. Accordingly, the aim of the present study was to improve our knowledge on the mechanism of AC activation by FS. In order to achieve our aim, we systematically examined the effects of FS and eight FS analogs (Fig. 1) on FRET to, and direct fluorescence of, MANT-GTP, as well as catalysis of C1/C2.
Fig. 2. FS binding site in mammalian AC.
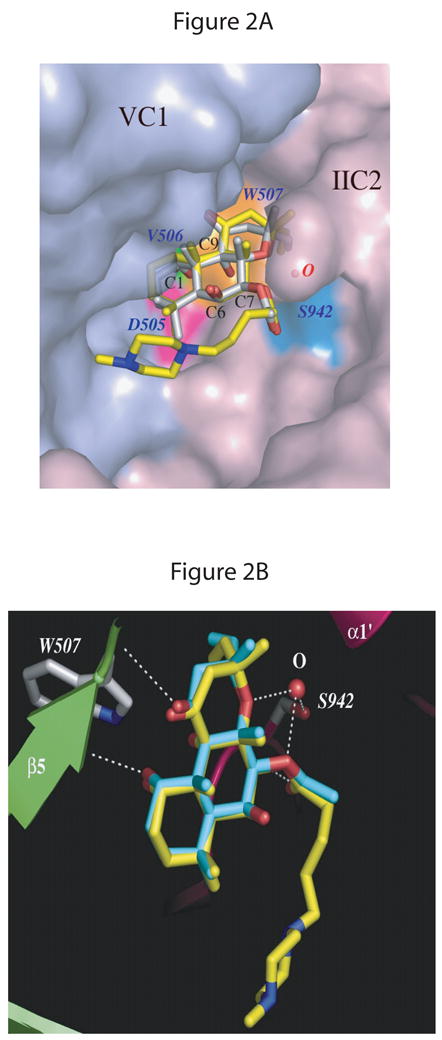
The AC structure with DMB-FS (PDB: 1AZS) is superimposed on both C1 and C2 domains of the AC structure with FS (PDB:1CS4). A, overview. The molecular surface of the VC1 and IIC2 proteins in the diterpene binding pocket is displayed in light-blue and light-pink, respectively. FS and DMB-FS are drawn as stick models; carbon atoms are gray for FS and yellow for DMB-FS, nitrogens blue, and oxygens red. Three conserved residues from VC1 forming a binding pocket in proximity to the C1-OH group of FS/DMB-FS are colored magenta for Asp505, green for Val506, and orange for Trp507. A water molecule bridges the side chain of Ser942 (cyan) from IIC2 and two oxygens of the FS/DMB-FS. B, detail view. VC1 and IIC2 are shown in lime and pink, respectively. Secondary structure elements are labeled as defined previously [12]. FS and DMB-FS are drawn as stick models; carbon atoms are cyan for FS, yellow for DMB-FS and gray for side chains of protein residues, nitrogens blue, and oxygens red. The white dashed lines depict the hydrogen bonds between amino acid residues Val506 of VC1 and C1-OH of FS, Ser942 (IIC2) and C7-OH of FS via a water molecule as well as Ser508 (VC1) and C11-OH of FS. Fig. 2 was generated using the PyMol program (DeLano Scientific, San Carlos, CA, USA).
2. Materials and Methods
2.1. Materials
FS was purchased from LC Laboratories (Woburn, MA, USA). DMB-FS was from Calbiochem (La Jolla, CA, USA). All other FS analogs were from Sigma-Aldrich (St. Louis, MO, USA). Stock solutions of FS and FS analogs (10 mM each) were prepared in DMSO. Dilutions of FS analogs were prepared in such a way that in all fluorescence and enzymatic activity assays a final DMSO concentration of 3% (vol/vol) was achieved. MANT-GTP was obtained from Jena Bioscience (Jena, Germany). VC1 and IIC2 and GTPγS-activated Gsα (Gsα-GTPγS) were expressed and purified as described [11]. [α-32P]ATP (800 Ci/mmol) was purchased from PerkinElmer (Wellesley, MA, USA). Neutral alumina (super I, WN-6) was from Sigma.
2.2. Fluorescence spectroscopy
All experiments were conducted using a Cary Eclipse fluorescence spectrophotometer equipped with a Peltier-thermostated multicell holder at 25°C (Varian, Walnut Creek, CA, USA). Measurements were performed in a quartz fluorescence microcuvette (Hellma, Plainview, NY, USA). The final assay volume was 150 μl. Reaction mixtures contained a buffer consisting of 100 mM KCl, 10 mM MnCl2 and 25 mM HEPES/NaOH, pH 7.4, and 1 μM MANT-GTP. Steady-state emission spectra were recorded at low speed with λex = 350 nm (λem = 370–500 nm) (direct fluorescence) and λex = 280 nm (λem = 300–500 nm) (FRET) in the presence of 5 μM VC1 plus 25 μM IIC2 without and with FS or FS analogs at various concentrations. The final DMSO concentration was 3% (vol/vol). DMSO at this concentration had no effect on MANT-GTP fluorescence (data not shown). Fluorescence recordings were analyzed with the spectrum package of the Cary Eclipse software (Varian, Walnut Creek, CA). In all experiments, baseline fluorescence (buffer alone) was subtracted.
2. 3. AC activity assay
AC activity was determined essentially as described in the literature [13]. Briefly, reaction mixtures (50 μl final volume) contained 100 μM ATP/Mn2+ and 10 mM MnCl2 or 100 μM ATP/Mg2+ and 10 mM MgCl2 and FS or FS analogs at various concentration in the presence of 3% (vol/vol) DMSO. Additionally, assay tubes contained VC1 (8 nM) and IIC2 (40 nM). For experiments with Gsα-GTPγS, tubes contained VCI (3 nM), IIC2 (15 nM) and Gsα-GTPγS (50 nM). Following a 2 min pre-incubation at 30°C, reactions were initiated by adding 20 μl of reaction mixture containing (final) 1.0–1.5 μCi/tube [α-32P]ATP, 0.1 mM cAMP and 100 mM KCl in 25 mM HEPES/NaOH, pH 7.4, and a regenerating system consisting of 2.7 mM mono(cyclohexyl)ammonium phosphoenolpyruvate, 0.125 IU pyruvate kinase and 1 IU myokinase. Reactions were conducted for 20 min at 30°C and were terminated by adding 20 μL of 2.2 N HCl. Denatured protein was precipitated by a 1 min centrifugation at 25°C and 15,000 × g. Sixty-five μl of the supernatant fluid were applied onto disposable columns filled with 1.3 g neutral alumina. [32P]cAMP was separated from [α-32P]ATP by elution of [32P]cAMP with 4 ml of 0.1 M ammonium acetate, pH 7.0. Recovery of [32P]cAMP was ~80% as assessed with [3H]cAMP as standard. [32P]cAMP was determined liquid scintillation counting using Ecolume scintillation cocktail (Fisher, Pittsburgh, PA, USA). Data were analyzed by non-linear regression using the Prism 4.02 program (GraphPad, San Diego, CA, USA).
2.4. Statistics
Statistical comparisons in Figs. 5 and 6 and Table 1 were performed using the t-test. Differences were considered as statistically significant with *p < 0.05 and ** p < 0.01.
Fig. 5. Efficacies of FS and FS analogs at stimulating direct MANT-GTP fluorescence and at inducing FRET in C1/C2.
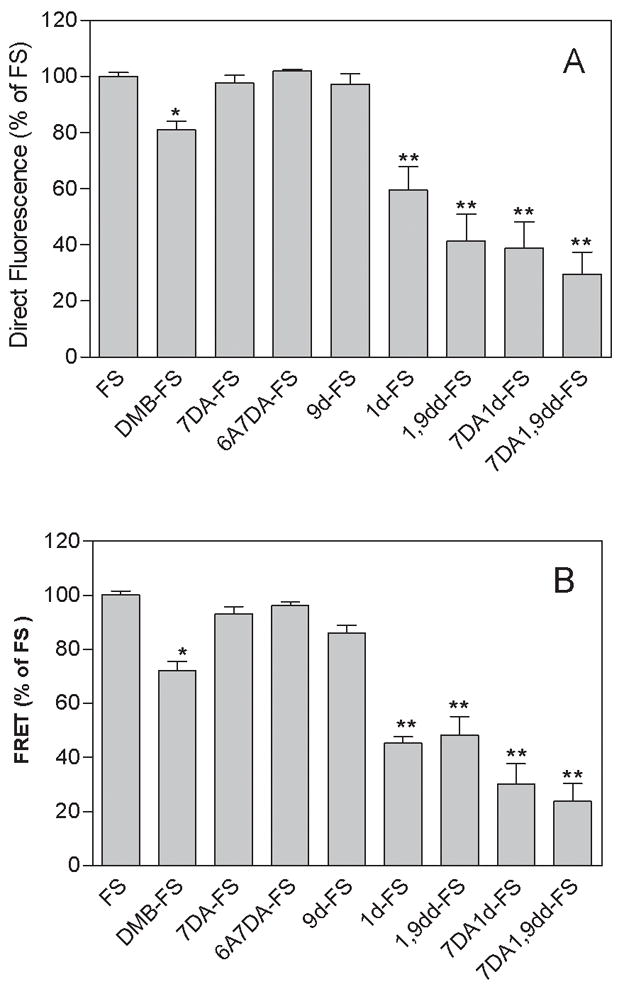
Fluorescence experiments were performed as illustrated in Fig. 4. Data were normalized relative to the effect of FS (100 μM, = 100%). All FS analogs were used at a concentration of 100 μM. Panel A shows normalized direct fluorescence increase of MANT-GTP at λex = 350 nm and λem = 420 nm induced by FS and FS derivatives in the presence of 10 mM Mn2+. Direct fluorescence was calculated subtracting basal fluorescence at λem = 420 nm (C1/C2 + MANT-GTP) from the corresponding fluorescence determined in the presence of FS analogs. Panel B shows normalized FRET from Trp1020 to MANT-GTP induced by FS and FS derivatives at λex = 280 nm and λem = 420 nm in the presence of 10 mM Mn2+. FRET was calculated by subtraction of the F420/F335 ratios of basal fluorescence from the corresponding ratio in the presence of FS analogs. Data from three to five experiments with at least two independent batches of protein were pooled and are shown as means ± SD. Statistical comparisons between FS and different analogs were performed using the t-test. * p < 0.05 and ** p < 0.01.
Fig. 6. Effect of FS and FS analogs on the catalytic activity of C1/C2.

Enzymatic studies were performed as described in “Materials and Methods”. Shown are representative concentration/response curves for the stimulatory activities of FS analogs relative to FS (panel A) and for the non-competitive antagonism of FS-stimulated C1/C2 catalysis by 7DA-1,9dd-FS, 1,9dd-FS or 1d-FS (panel B). Activities are expressed in % relative to the maximum activity induced by FS (100 μM). Experiments were conducted in the presence of Mn2+ (10 mM), C1 (3 nM), C2 (15 nM), Gsα-GTPγS (50 nM), ATP (100 μM), KCl (100 mM), [α-32P]ATP (1.0 – 1.5 μCi/tube), 3% (vol/vol) DMSO and increasing concentrations (300 nM - 100 μM) of different diterpenes as indicated. Data were analyzed by non-linear regression and best fitted to sigmoidal concentration/response curves. Data are the mean values ± SD of a representative experiment performed in duplicates. Similar results were obtained in 3–4 independent experiments. Basal catalytic activity of C1/C2 was 29 ± 0.5 nmol/mg C1/min, and maximum FS-stimulated catalytic activity was 680 ± 32 nmol/mg C1/min.
Table 1.
Potencies and efficacies of FS and FS analogs for activation/inhibition of C1/C2 catalytic activity under different experimental conditions
| Diterpene | C1/C2 + Gsα + Mg2+ | C1/C2 + Mn2+ | C1/C2 + Gsα + Mn2+ | |
|---|---|---|---|---|
| Efficacy (%) | Efficacy (%) | Efficacy (%) | EC50 (μM) | |
| FS | 100 | 100 | 100 | 7.4 ± 1.2 |
| DMB-FS | 33 ± 1 | 15 ± 4 | 41 ± 6** | 27.6 ± 7.0 ** |
| 7DA-FS | 83 ± 2 | 21 ± 6 | 86 ± 3 * | 3.1 ± 1.4 ** |
| 6A7DA-FS | 43 ± 2 | 36 ± 8 | 83 ± 14 | 2.6 ± 1.0 ** |
| 9d-FS | 33 ± 17 | 29 ± 5 | 79 ± 13 | 19.5 ± 0.7 ** |
| 1d-FS | ineffective | ineffective | ineffective | ineffective |
| 1,9dd-FS | ineffective | ineffective | ineffective | ineffective |
| 7DA1,9dd-FS | ineffective | ineffective | ineffective | ineffective |
| 7DA1d-FS | ineffective | ineffective | ineffective | ineffective |
| FS + 1d-FS | N.D. | N.D. | 46 ± 1 ** | 1.2 ± 0.7 * |
| FS + 1,9dd-FS | N.D. | N.D. | 52 ± 2 ** | 5.6 ± 1.7 |
| FS + 7DA1,9dd-FS | N.D. | N.D. | 47 ± 1 ** | 2.9 ± 0.9 * |
Catalytic activity of C1/C2 was determined as described under “Materials and Methods”. Experiments were conducted in the presence of Mg2+ or Mn2+ (10 mM each), and in presence of C1 (3 nM), C2 (15 nM) plus Gsα-GTPγS (50 nM) or in the presence of C1 (8 nM) plus C2 (40 nM) without Gsα. Assays contained ATP (100 μM), KCl (100 mM), ([α-32P] ATP (1.0–1.5 μCi/tube), 3% (vol/vol) DMSO and diterpenes at various concentrations. The efficacy for each analog was determined by dividing the fitted maximal stimulation obtained for the analog by the maximum stimulation obtained by treatment of C1/C2 with 100 μM FS. Basal catalytic activities with C1/C2 + Mg2+ + Gsα-GTPγS, C1/C2 + Mn2+ and C1/C2 + Mn2+ + Gsα-GTPγS were 4.3 ± 0.7, 1.2 ± 0.9 and 27 ± 0.3 nmol/mg C1/min respectively. Maximum FS-stimulated catalytic activities were 57 ± 2, 167 ± 29 and 676 ± 36 nmol/mg C1/min, respectively. Data are the mean values ± SD of 3–4 independent experiments performed in duplicates. Statistical comparisons between FS and different analogs were performed using the t-test.
p < 0.05 and
p < 0.01.
N.D., not determined.
3. Results
3.1. Effects of FS and FS analogs on fluorescence properties of MANT-GTP bound to C1/C2
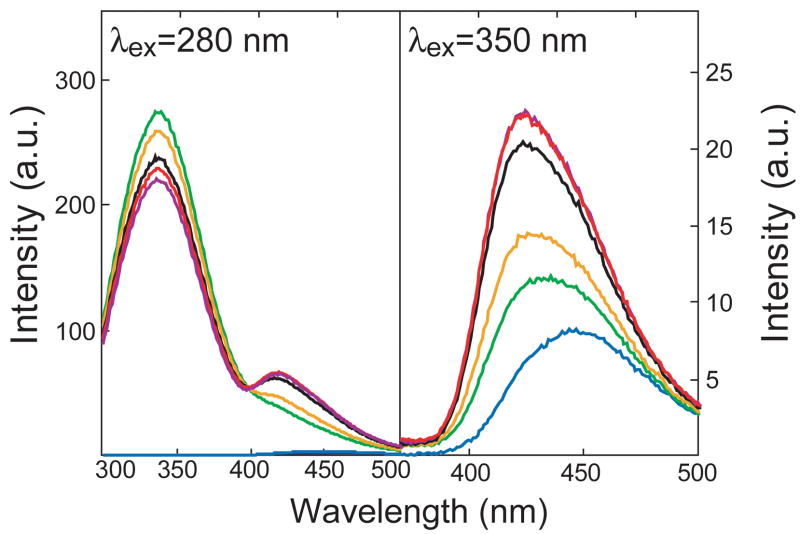
MANT-GTP is directly excited at λex = 350 nm [13, 14], resulting in emission with a maximum at λem = 450 nm (Fig. 3). Translocation of the MANT-group into a hydrophobic environment increases fluorescence and results into a shift of the emission maximum to shorter wavelengths. This phenomenon is also referred to as blue-shift. The addition of C1/C2 to cuvettes resulted in a moderate increase in direct fluorescence and a decrease of the emission maximum to λem = 430 nm. These data show that MANT-GTP binds to C1/C2 already in the absence of an activator, with the MANT-group residing in a hydrophobic pocket at the interface between the C1- and C2 subunit [13, 14]. FS enhanced this fluorescence increase and reduced the emission maximum to λem = 425 nm. The effect of FS was concentration-dependent and reached a maximum at 30 μM. These data show that FS enhances C1/C2 assembly and/or facilitates formation of the hydrophobic pocket.
Fig. 3. Cumulative concentration/response curves for the effects of FS on FRET and direct MANT-GTP fluorescence in C1/C2.
Fluorescence studies were performed as described in “Materials and Methods”. Fluorescence spectra are shown at λex= 280 nm (λem = 300–500 nm, left-hand graph) (FRET) and at λex = 350 nm (λem = 370–500 nm) (direct fluorescence, right-hand graph). Fluorescence measurements were performed in a quartz florescence microcuvette at 25°C. Reaction mixtures contained 100 mM KCl, 10 mM MnCl2 and 25 mM HEPES/NaOH, pH 7.4 and MANT-GTP (1 μM). The final assay volume was 150 μl and the final DMSO concentration was 3% (vol/vol). FS was added cumulatively to cuvettes to yield final concentrations of 1–151 μM. The C1 and C2 concentrations were 5 and 25 μM, respectively. Tracings are defined as follows. Blue, MANT-GTP; green, C1/C2; yellow, 1 μM FS; black, 11 μM FS; purple, 31 μM FS; red, 151 μM FS. Fluorescence intensities are shown in arbitrary units (a. u.). Fluorescence tracings are representative for three independent experiments.
We also examined FRET from tryptophan and tyrosine residues in C1/C2 to the MANT-group. At a wavelength of λex = 280 nm, MANT-GTP is only minimally excited, accordingly resulting in minimal emission at λem = 450 nm. In marked contrast, the trypophan residues, particularly Trp1020 of IIC2, and tyrosine residues of C1 and C2 are effectively excited at λex = 280 nm, resulting in a large fluorescence emission with a maximum at λem = 350 nm. Thus, the fluorescence light emitted at λem 350 nm can excite the MANT-group of MANT-GTP, provided sufficiently close proximity of donor and acceptor. In fact, the distance between Trp1020 of IIC2 and the MANT-group is less than 5 Å, allowing for FRET to occur [13, 14]. Basal FRET between Trp1020 and MANT-GTP, i.e. FRET in the absence of FS, is characterized by the emission shoulder between λem 420–450 nm. The addition of FS at increasing concentrations decreased the endogenous C1/C2 fluorescence at λem = 350 nm, reflecting absorption of energy by the MANT-group of MANT-GTP. The absorbed energy then excited the MANT-group and resulted in concomitant increases in fluorescence with a maximum at λem = 420–425 nm, again reflecting C1/C2 assembly or more efficient formation of the hydrophobic pocket. Like in the direct fluorescence experiments, the effect of FS on FRET reached a maximum at a concentration of 30 μM. Thus, both the direct fluorescence assay and the FRET assay allowed us to monitor binding of FS analogs to C1/C2.
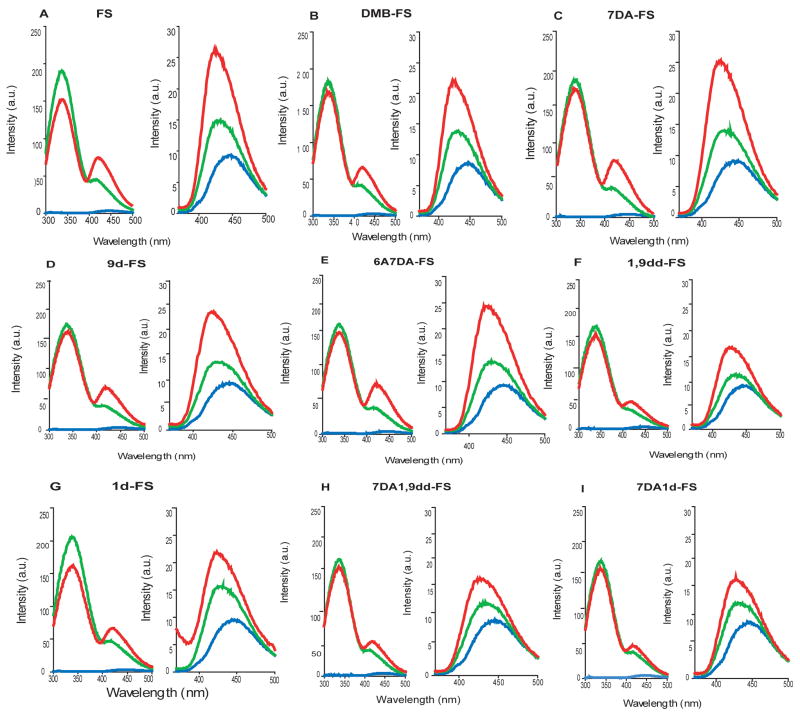
Fig. 4 shows representative original tracings for the stimulatory effects of FS and FS analogs (100 μM each) on FRET and direct MANT-GTP fluorescence with C1/C2, and Fig. 5 provides a statistical summary of the effects of the diterpenes on FRET and direct fluorescence. Diterpenes stimulated FRET and direct MANT-GTP fluorescence in the order of efficacy FS ~ 7DA-FS ~ 6A7DA-FS ~ 9d-FS > DMB-FS > 1d-FS ~ 1,9-dd-FS ~ 7DA1d-FS ~ 7DA1,9dd-FS.
Fig. 4. Effects of FS and FS analogs on FRET and direct MANT-GTP fluorescence in C1/C2.
Fluorescence studies were performed as described in “Materials and Methods”. Shown are the effects of FS, DMB-FS, 9d-FS, 7DA-FS, 6A7DA-FS, 1,9dd-FS, 1d-FS, 7DA1,9dd-FS and 7DA1d-FS (Panels A–I, respectively) on MANT-GTP fluorescence. Fluorescence spectra are shown at λex = 280 nm (λem = 300–500 nm) (FRET, left-hand graph) and at λex = 350 nm (λem = 370–500 nm) (direct fluorescence, right-hand graph). Fluorescence measurements were performed in a quartz fluorescence microcuvette at 25°C. Reaction mixtures contained 100 mM KCl, 10 mM MnCl2 and 25 mM HEPES/NaOH, pH 7.4, MANT-GTP (1 μM) and FS or FS analogs (100 μM). The final assay volume was 150 μl and the final DMSO concentration was 3% (vol/vol). The order of addition of the reagents was MANT-GTP (blue tracings), followed by C1/C2 (green tracings), followed by FS or FS analogs (red tracings). Fluorescence intensities are shown in arbitrary units (a. u.). Fluorescence tracings are representative for three to five independent experiments with at least two different batches of protein.
In order to assess the specificity of the effects of diterpenes on direct MANT-GTP fluorescence and FRET, we performed a number of control experiments. First, none of the diterpenes studied exhibited a fluorescence signal when excited at λex = 280 nm and λex = 350 nm in buffer in the absence and presence of MANT-GTP. Second, in the presence of MANT-GTP and C2 but in the absence of C1, diterpenes did not elicit an increase in direct MANT-GTP fluorescence or FRET. Third, in the presence of MANT-GTP and C1 but in the absence of C2, diterpenes failed to increase direct MANT-GTP fluorescence and FRET as well (data not shown). These data indicate that for the fluorescence signals reported in Figs. 3–5, both the C1- and the C2 subunit are required, ruling out non-specific diterpene effects.
3.2. Effects of FS and FS analogs on the catalytic activity of C1/C2
Fig. 6A shows representative concentration/response curves for the stimulatory effects of FS and FS analogs on the catalytic activity of C1/C2 in the presence of Mn2+ and Gsα, and Table 1 provides a statistical summary of the effects of diterpenes on catalysis. FS analogs activated catalysis in the order of potency 6A7DA-FS ~ 7DA-FS > FS > 9d-FS > DMB-FS. The order of efficacy was FS > 7DA-FS ~ 6A7DA-FS ~ 9d-FS > DMB-FS ≫ 1d-FS, 1,9dd-FS, 7DA1-FS and 7DA1,9dd-FS (ineffective).
We also examined the effects of some of the ineffective 1d-FS analogs at a fixed concentration on the concentration/response for FS. 1d-FS, 1,9dd-FS and 7DA1d-FS decreased the efficacy of FS by ~50% without decreasing the potency of FS, i.e. the FS analogs inhibited the effects of FS non-competitively.
Finally, the effects of FS and FS analogs were examined on catalysis by C1/C2 in the presence of Mn2+ and in the absence of Gs, and by C1/C2 in the presence of Gs and Mg2+ (Table 1). Under those conditions, it was impossible to obtain saturated concentration/response curves for the diterpenes so that we could only determine efficacies of compounds at a single high concentration (100 μM). Except for DMB-FS and 7DA-FS under Mg2+ conditions with Gs, efficacies of all diterpenes relative to FS substantially decreased compared to Mn2+ conditions with Gs. Again, all 1d-FS analogs did not activate catalysis by C1/C2.
4. Discussion
4. 1. Two-step mechanism of AC activation by diterpenes
The most important finding of this study is that all FS analogs, regardless of their substitution at C1, C6, C7 and C9 of the diterpene ring (Fig. 1), promote C1/C2 assembly as assessed by fluorescence spectroscopy (Figs. 3–5). Thus, we conclude that for diterpene binding to AC, hydrophobic interactions are critical. Given the large C1/C2 hydrophobic interface [12] (Fig. 2A), at first glance, this finding may not appear surprising. However, from previous [3H]FS binding studies and enzyme activity measurements with intact ACs it was concluded that the lack of the C1-OH group results in inactive FS analogs that do not bind to AC anymore [4, 5]. A major difference between our present study and the previous studies is that we examined purified AC subunits, whereas in previous studies, membranes expressing AC and multiple other proteins were used. Given the fact that AC is not the only protein that binds FS [5], the most likely explanation for the discrepancies is that previous [3H]FS binding studies did not monitor AC but rather proteins such as ion channels or glucose transporters. This conclusion is supported by the fact that previous binding studies with membranes reported on a high-affinity [3H]FS site (Kd in the 10 nM-range) [4, 5], whereas equilibrium dialysis binding studies and catalysis studies with purified C1/C2 show that the affinity of AC for FS is about three orders of magnitudes higher, i.e. in the 10 μM-range (Fig. 6 and Table 1) [10].
The second most important finding of our study is that deletion of the C1-OH group, regardless of the substitution of C7, C6 and C9, results in a complete loss of stimulatory effect of diterpenes on catalysis (Fig. 6 and Table 1). These findings support the previous suggestion that diterpene binding to AC is not sufficient for activation of catalysis [2, 12]. Rather, we propose that a conformational switch in AC, triggered by hydrogen bonding of the diterpene C1-OH group to the backbone oxygen of Val506 (AC V) [12] is essential for stimulating catalysis. Thus, AC activation occurs through a two-step mechanism. In the first step, diterpene binding to the hydrophobic site occurs, and in the second step, hydrogen bonding between C1-OH and Val506 triggers a crucial conformational change activating catalysis.
We would like to emphasize that presently, we do have direct evidence for the proposed activating conformational change in AC. In an effort to answer this crucial question, we have actually already undertaken substantial efforts to crystallize C1/C2 in complex with Gsα-GTPγS, MANT-GTP and 1d-FS or 1,9dd-FS, but so far, these efforts have not yet been successful for unknown reasons. However, the fact that 1d-FS derivatives reduce basal catalysis of holo-AC2 expressed in Sf9 cell membranes [9] supports the view that 1d-FS derivatives do bind to AC but do not trigger the supposed activating conformational change. In case of AC2, 1d-FS analogs may rather stabilize an inactive enzyme conformation (see also Discussion below). In view of the difficulties with crystallography, future molecular modelling studies, which are beyond the scope of the present study, may be helpful to elucidate the conformational change in AC.
4.2. Molecular analysis of the interaction of FS analogs with C1/C2
In previous studies it was often difficult to obtain saturated concentration/response curves for diterpenes, rendering calculation of potencies and efficacies arbitrary [4, 5, 8]. In the initial phase of this project, we also experienced substantial difficulties in obtaining saturated concentration/response curves for diterpenes since we limited the final DMSO concentration in assays to 1% (vol/vol). However, when increasing the DMSO concentration to 3% (vol/vol), saturated concentration/response curves could be achieved without compromising fluorescence signals and catalytic activity. Unfortunately, previous studies often only incompletely defined solvent conditions. None of the FS analogs studied herein surpassed FS in terms of efficacy for catalysis. Rather, depending on the specific experimental conditions chosen, FS analogs exhibited slightly to strongly reduced efficacies (Fig. 6 and Table 1). Thus, AC could be considered as a “FS receptor” with ligands exhibiting efficacies ranging from full agonism via partial agonism to antagonism. By analogy to the two-state model of receptor activation [19], one could have expected that non-stimulatory FS analogs actually decrease basal AC activity as inverse agonists, but no such inhibition was observed (Fig. 6 and Table 1). This is probably due to the low catalysis rate under those conditions. In contrast, holo-AC2 exhibits high basal catalytic activity, and under those conditions, non-stimulatory FS analogs actually decrease basal activity [9].
In contrast to the C1-OH group, the C9-OH group does not form a hydrogen bond with C1/C2 [12] (Fig. 2). Accordingly, it was not surprising to observe that deletion of the C9-OH group had only minor effects on the efficacy of 9d-FS to bind to, and activate catalysis of, C1/C2 (Figs. 4–6 and Table 1).
In addition to the hydrogen bond between the diterpene C1-OH group and Val506 of C1 (AC V), there is a hydrogen bond between the acetyl group of C7 and Ser942 of C2 (AC II) mediated via a water molecule [12] (Fig. 2). However, the latter hydrogen bond plays only a relatively small role in diterpene binding to, and activation of catalysis by, C1/C2, since potencies and efficacies of 7DA-FS in terms of fluorescence signals and catalysis were similar to those of FS (Figs. 4–6). In accordance with those findings is the fact that the exchange of the acetyl group from C7 to C6 is well tolerated in terms of binding and catalysis. However, in conjunction with a missing C1-OH group, deletion of the C7-acetyl group reduced diterpene efficacy at promoting C1/C2 assembly (Figs. 4 and 5), indicating that the hydrogen bonds stabilize diterpene/AC interactions under certain conditions.
FS and DMB-FS bind to AC very similarly, i.e. the root mean square deviation (RMSD) of FS/DMB-FS and the surrounding amino acids of C1/C2 was less than 1.1 Å (Fig. 2). Thus, we were surprised that the N-methylpiperazino-γ-butyryloxy substituent of DMB-FS was not well tolerated in terms of potency and efficacy for activation of catalysis although the substituent does not sterically interfere with ligand binding to AC (Figs. 2 and 6 and Table 1). We also noted significantly reduced efficacy of DMB-FS relative to FS with respect to C1/C2 assembly (Figs. 4 and 5). These data suggest that the minor differences in positioning of DMB-FS and FS in the binding pocket (Figs. 2, 4 and 5) are actually amplified in rather large differences with respect to catalysis (Fig. 6 and Table 1).
Under optimal conditions for catalysis, i.e. in the presence of Mn2+ and Gs [11, 13], the C7-acetyl group, the switch from C7-acetyl to C6-acetyl and the omission of the C9-OH group had only small effects on efficacy. In contrast, the exchange of Mn2+ against Mg2+ and the omission of Gs substantially reduced diterpene efficacies (and potencies) (Table 1), indicating that stabilizing interactions in AC provided by Mn2+and Gs can compensate for missing interactions of AC with FS analogs. Comparing the efficacies of diterpenes (100 μM each) at promoting C1/C2 assembly in the presence of Mn2+ and in the absence of Gs with the corresponding efficacies in terms of catalysis, the discrepancies between the two parameters become even more striking (Fig. 5 and Table 1). For example, 7DA-FS (100 μM) was as effective as FS at promoting C1/C2 assembly but exhibited just 20% of the efficacy of FS at stimulating catalysis. These dissociations further corroborate the concept that following diterpene binding to AC driven by hydrophobic interactions, an additional conformational switch is required to activate catalysis. The most important conformational change is afforded by the diterpene C1-OH-Val506 interaction (Fig. 2), but in the absence of Gs, interactions of the C7-acetyl group with Ser942 via a water molecule play a role in the activation process as well. Thus, activation of catalysis by diterpenes is regulated by multiple conformational switches which depend on the presence of other modulators such as Mg2+, Mn2+ and Gs.
4.3. 1d-FS derivatives as FS antagonists
If 1d-FS derivatives were FS antagonists in the sense of receptor theory [20], they should not only fail to activate catalysis, but they should also competitively inhibit the stimulatory effects of FS. In fact, all 1d-FS derivatives examined inhibited FS-stimulated catalysis (Fig. 6 and Table 1). However, the inhibitory effects of diterpenes on FS-stimulated catalysis were non-competitive, raising the question whether FS and 1d-FS derivatives bind to different sites. Based on the fluorescence studies, there is, however, no evidence for different binding sites for diterpenes with and without C1-OH group since the fluorescence signals were qualitatively comparable for the two classes of ligands (Fig. 4). In addition, based on the crystal structures for C1/C2 [12–14], there is no evidence for a second diterpene binding site. Rather, it is conceivable that strong hydrophobic interactions of diterpenes with C1/C2 impair the free exchange of FS and 1d-FS analogs, resulting in apparently non-competitive ligand interactions [9, 21]. Difficulties in obtaining true equilibrium conditions are also the most likely explanation for the inability to unequivocally identify a single FS binding site in C1/C2 by equilibrium dialysis experiments [10].
4.4. Comparison of C1/C2 with holo-ACs
The C1/C2 system allows for the analysis of diterpene/AC interactions by fluorescence spectroscopy, whereas such analysis is impossible with holo-ACs expressed in Sf9 cell membranes. This difference is mainly due to the fact that for fluorescence spectroscopy, very high protein concentrations, i.e. concentrations in the μM-range, are required that cannot be achieved in a membrane overexpression system. However, the C1/C2 system is artifical in the sense that it consists only of small fragments in relation to the intact AC protein, rendering the model much more sensitive to entropy-related effects than holo-ACs which are more rigid proteins [1–3]. Therefore, it is important to directly compare the pharmacological effects of diterpenes at C1/C2 and holo-ACs. Indeed, in terms of potency and efficacy, there are quantitative differences in the effects of FS, DMB-FS, 7DA-FS, 6A7DA-FS and 9d-FS at C1/C2 compared to holo-ACs (Table 1) [9]. However, one cannot readily attribute those differences to entropic differences between the two systems since C1/C2 is actually a hybrid with components from two AC isoforms (C1 from AC5 and C2 from AC2). To render issues even more complex, holo-AC2 and holo-AC5 differ from each other quite considerably with respect to the pharmacological profile of diterpenes and the inhibition pattern by MANT-nucleotides [9, 22]. Thus, future studies will have to compare a given holo-AC with the corresponding C1- and C2 subunits from the same AC isoform. We have already tried to express C2 from AC5 and C1 from AC2, but to this end, we have not yet obtained functionally active protein.
Despite these problems in the precise comparison of C1/C2 with holo-ACs, there are some findings that lend support to the notion that data obtained with C1/C2 are relevant for holo-ACs. Specifically, 1d-FS derivatives uniformly failed to activate C1/C2 and all recombinant holo-ACs studied, including ACs 2 and 5 (Fig. 6 and Table 1) [9]. Furthermore, in native mouse heart membranes, 1d-FS does not increase AC activity [23]. Moreover, 1d-FS derivatives showed apparent non-competitive interaction with FS in all systems studied so far (Fig. 6) [9]. These data indicate that the two-step mechanism of AC activation proposed for C1/C2 can also be applied to holo-ACs. This concept is further corroborated by the inhibitory effect of 1d-FS derivatives on the high basal activity of AC2 [9].
4.5. Conclusions
Binding of diterpenes to the purified catalytic subunits of AC occurs mainly via hydrophobic interactions. However, activation of catalysis requires an additional conformational switch triggered by a hydrogen bond between the C1-OH group of diterpenes and the backbone oxygen of Val506 of C1. Additional hydrogen-bonding substituents of diterpenes modulate activation of catalysis. 1d-FS analogs inhibit FS-stimulated catalysis by an apparently non-competitive interaction, which is presumably due to strong hydrophobic diterpene/AC interactions, impairing free ligand exchange. Future studies will have to elucidate the precise nature of the proposed second conformational switch. With repect to potential therapeutic applications, it will also be important to examine the effects of diterpenes in native systems such as organ membranes and intact organs. In fact, a recent study on mouse heart AC showed that in this system, multiple AC isoforms contribute to the overall effects of diterpenes, rendering the pharmacological profile very complex [23].
Acknowledgments
This work was supported by Deutsche Forschungsgemeinschaft research grant Se 529/5-1 to R.S, NIH grant RO1 DK 46371 to S.R.S. and a predoctoral fellowship from the Elite Graduate Student program of Free State of Bavaria to M.H. We also thank the reviewers of this paper for their constructive critique.
Abbreviations
- AC
adenylyl cyclase
- C1
C1 catalytic subunit of adenylyl cyclase isoform V
- C2
C2 catalytic subunit of adenylyl cyclase isoform II
- DMSO
dimethyl sulfoxide
- FRET
fluorescence resonance energy transfer
- GTPγS
guanosine 5′-[γ-thio]triphosphate
- MANT-GTP
2′,3′-O-(N-methylanthraniloyl)-guanosine 5′-triphosphate
- FS
forskolin
- 1d-FS
1-deoxy-forskolin
- 9d-FS
9-deoxy-forskolin
- 1,9dd-FS
1,9-dideoxy-forskolin
- 7DA-FS
7-deacetyl-forskolin
- 6A7DA-FS
6-acetyl-7-deacetyl-forskolin
- 7DA1d-FS
7-deacetyl-1-deoxy-forskolin
- 7DA1
9dd-FS, 7-deacetyl-1,9-dideoxy-forskolin
- DMB-FS
7-deacetyl-7-(N-methylpiperazino-γ-butyryloxy)-forskolin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sunahara RK, Dessauer CW, Gilman AG. Complexity and diversity of mammalian adenylyl cyclases. Annu Rev Pharmacol Toxicol. 1996;36:461–80. doi: 10.1146/annurev.pa.36.040196.002333. [DOI] [PubMed] [Google Scholar]
- 2.Tang WJ, Hurley JH. Catalytic mechanism and regulation of mammalian adenylyl cyclases. Mol Pharmacol. 1998;54:231–40. doi: 10.1124/mol.54.2.231. [DOI] [PubMed] [Google Scholar]
- 3.Hanoune J, Defer N. Regulation and role of adenylyl cyclase isoforms. Annu Rev Pharmacol Toxicol. 2001;41:145–74. doi: 10.1146/annurev.pharmtox.41.1.145. [DOI] [PubMed] [Google Scholar]
- 4.Seamon KB, Daly JW. Forskolin: its biological and chemical properties. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1986;20:1–150. [PubMed] [Google Scholar]
- 5.Laurenza A, Sutkowski EM, Seamon KB. Forskolin: a specific stimulator of adenylyl cyclase or a diterpene with multiple sites of action? Trends Pharmacol Sci. 1989;10:442–7. doi: 10.1016/S0165-6147(89)80008-2. [DOI] [PubMed] [Google Scholar]
- 6.Robbins JD, Boring DL, Tang WJ, Shank R, Seamon KB. Forskolin carbamates: binding and activation studies with type I adenylyl cyclase. J Med Chem. 1996;39:2745–52. doi: 10.1021/jm960191+. [DOI] [PubMed] [Google Scholar]
- 7.Iwatsubo K, Tsunematsu T, Ishikawa Y. Isoform-specific regulation of adenylyl cyclase: a potential target in future pharmacotherapy. Expert Opin Ther Targets. 2003;7:441–51. doi: 10.1517/14728222.7.3.441. [DOI] [PubMed] [Google Scholar]
- 8.Onda T, Hashimoto Y, Nagai M, Kuramochi H, Saito S, Yamazaki H, Toya Y, Sakai I, Homcy CJ, Nishikawa K, Ishikawa Y. Type-specific regulation of adenylyl cyclase. Selective pharmacological stimulation and inhibition of adenylyl cyclase isoforms. J Biol Chem. 2001;276:47785–93. doi: 10.1074/jbc.M107233200. [DOI] [PubMed] [Google Scholar]
- 9.Pinto C, Papa D, Hübner M, Mou TC, Lushington GH, Seifert R. Activation and inhibition of adenylyl cyclase isoforms by forskolin analogs. J Pharmacol Exp Ther. 2008;325:27–36. doi: 10.1124/jpet.107.131904. [DOI] [PubMed] [Google Scholar]
- 10.Dessauer CW, Scully TT, Gilman AG. Interactions of forskolin and ATP with the cytosolic domains of mammalian adenylyl cyclase. J Biol Chem. 1997;272:22272–7. doi: 10.1074/jbc.272.35.22272. [DOI] [PubMed] [Google Scholar]
- 11.Sunahara RK, Dessauer CW, Whisnant RE, Kleuss C, Gilman AG. Interaction of Gsα with the cytosolic domains of mammalian adenylyl cyclase. J Biol Chem. 1997;272:22265–71. doi: 10.1074/jbc.272.35.22265. [DOI] [PubMed] [Google Scholar]
- 12.Tesmer JJ, Sunahara RK, Gilman AG, Sprang SR. Crystal structure of the catalytic domains of adenylyl cyclase in a complex with Gsα-GTPγS. Science. 1997;278:1907–16. doi: 10.1126/science.278.5345.1907. [DOI] [PubMed] [Google Scholar]
- 13.Mou TC, Gille A, Fancy DA, Seifert R, Sprang SR. Structural basis for the inhibition of mammalian membrane adenylyl cyclase by 2′ (3′)-O-(N-methylanthraniloyl)-guanosine 5′-triphosphate. J Biol Chem. 2005;280:7253–61. doi: 10.1074/jbc.M409076200. [DOI] [PubMed] [Google Scholar]
- 14.Mou TC, Gille A, Suryanarayana S, Richter M, Seifert R, Sprang SR. Broad specificity of mammalian adenylyl cyclase for interaction with 2′,3′-substituted purine- and pyrimidine nucleotide inhibitors. Mol Pharmacol. 2006;70:878–86. doi: 10.1124/mol.106.026427. [DOI] [PubMed] [Google Scholar]
- 15.Laurenza A, Khandelwal Y, De Souza NJ, Rupp RH, Metzger H, Seamon KB. Stimulation of adenylate cyclase by water-soluble analogues of forskolin. Mol Pharmacol. 1987;32:133–9. [PubMed] [Google Scholar]
- 16.Zhang G, Liu Y, Ruoho AE, Hurley JH. Structure of the adenylyl cyclase catalytic core. Nature. 1997;386:247–53. doi: 10.1038/386247a0. [DOI] [PubMed] [Google Scholar]
- 17.Seamon KB, Daly JW, Metzger H, de Souza NJ, Reden J. Structure-activity relationships for activation of adenylate cyclase by the diterpene forskolin and its derivatives. J Med Chem. 1983;26:436–9. doi: 10.1021/jm00357a021. [DOI] [PubMed] [Google Scholar]
- 18.Seamon KB, Vaillancourt R, Edwards M, Daly JW. Binding of [3H]forskolin to rat brain membranes. Proc Natl Acad Sci USA. 1984;81:5081–5. doi: 10.1073/pnas.81.16.5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seifert R, Wenzel-Seifert K. Constitutive activity of G-protein-coupled receptors: cause of disease and common property of wild-type receptors. Naunyn-Schmiedeberg’s Arch Pharmacol. 2002;366:381–416. doi: 10.1007/s00210-002-0588-0. [DOI] [PubMed] [Google Scholar]
- 20.Neubig RR, Spedding M, Kenakin T, Christopoulos A International Union of Pharmacology Committee on Receptor Nomenclature and Drug Classification. International Union of Pharmacology Committee on Receptor Nomenclature and Drug Classification. XXXVIII. Update on terms and symbols in quantitative pharmacology. Pharmacol Rev. 2003;55:597–606. doi: 10.1124/pr.55.4.4. [DOI] [PubMed] [Google Scholar]
- 21.Kenakin T, Jenkinson S, Watson C. Determining the potency and molecular mechanism of action of insurmountable antagonists. J Pharmacol Exp Ther. 2006;319:710–23. doi: 10.1124/jpet.106.107375. [DOI] [PubMed] [Google Scholar]
- 22.Gille A, Lushington GH, Mou TC, Doughty MB, Johnson RA, Seifert R. Differential inhibition of adenylyl cyclase isoforms and soluble guanylyl cyclase by purine and pyrimidine nucleotides. J Biol Chem. 2004;279:19955–69. doi: 10.1074/jbc.M312560200. [DOI] [PubMed] [Google Scholar]
- 23.Göttle M, Geduhn J, König B, Gille A, Höcherl K, Seifert R. Characterization of mouse heart adenylyl cyclase. J Pharmacol Exp Ther. 2009 doi: 10.1124/jpet.109.150953. in press. [DOI] [PubMed] [Google Scholar]