Abstract
Purpose
Interstitial cystitis/painful bladder syndrome and chronic prostatitis/chronic pelvic pain syndrome, collectively renamed urological chronic pelvic pain syndromes, are a group of medically unexplained physical symptoms. Diagnosis depends on excluding all possible causes of pain and treatment targets symptoms alone. An emerging body of research implicates systemic factors in the pathogenesis of urological chronic pelvic pain syndromes including abnormal sympathetic nervous system and hypothalamic-pituitary-adrenal axis activity. Several new lines of evidence also suggest a genetic component to disease pathogenesis. Despite ongoing efforts, neither effective treatments nor mechanistic under-standing of the pathogenesis of urological chronic pelvic pain syndromes exists.
Materials and Methods
We performed a survey of the available literature on urological chronic pelvic pain syndromes. We reviewed recent research implicating genetic mechanisms in the development of urological chronic pelvic pain syndromes to find a systematic approach of rigorous phenotyping on which to base further investigation of these chronic pain conditions.
Results
Three studies revealed identifying genetic risk factors for disease. In addition, increasing lines of evidence of familial clustering and twin studies suggested a genetic basis for disease.
Conclusions
Given the success of genome-wide association studies in quantifying genetic risk in several polygenic diseases, we suggest a similar genome-wide approach to the study of urological chronic pelvic pain syndromes. As genome-wide association studies depend on carefully defined patient populations, we provide an outline for a thorough and multidisciplinary characterization of patient phenotypes. Although urological chronic pelvic pain syndromes continue to mystify clinicians and researchers alike, we believe the powerful new methods of unbiased interrogation of the whole genome based on systematically grouped patients possess enormous potential for progress in treating and understanding this disease.
Keywords: prostatitis; cystitis, interstitial; genetics; genome, human
Interstitial cystitis/painful bladder syndrome and chronic prostatitis/chronic pelvic pain syndrome were recently renamed urological chronic pelvic pain syndromes (UCPPS).1,2 We adhere to that nomenclature in reference to IC/PBS and CP/CPPS. Physicians have traditionally diagnosed and treated UCPPS based solely on symptoms such as the presence of chronic pelvic pain or discomfort and significantly impaired quality of life without evidence of local infection.3 A major obstacle in undertaking research on UCPPS is the absence of any kind of molecular aspect of the disease. Studies on tissue are difficult because tissue procurement from patients is limited and no single animal model recapitulates all symptoms of the disease. Competing theories about the origin and even the definition of UCPPS abound. However, hypothesis driven studies of a disease with no identifiable underlying pathology are inherently limited in the amount of information they can provide.
Recently rapid advances in understanding the patterns of human genetic variation and maturing high throughput, cost-effective methods for genotyping have provided powerful research tools for unbiased identification of genetic variants that contribute to health and disease. Thus, we provide an overview of genetic approaches to UCPPS. It must be noted that despite intriguing clues into pathogenesis, UCPPS remains poorly understood. Consequently we also identify promising new genetic tools, and propose a clinical phenotyping and research strategy for elucidating the pathogenesis of UCPPS.
METHODS
Articles were identified by a search of the databases PubMed® (1966 to October 2008), CINAHL® (1981 to October 2008), Cochrane Collaboration Reviews (1993 to October 2008), Current Contents® (2000 to October 2008), EBSCO® Academic Search Premier (1975 to October 2008), EMBASE® (1974 to October 2008), ISI Web of Science® (1980 to October 2008), PsycINFO® (1967 to October 2008) and Science Citation Indexes® (1996 to October 2008), as well as Scirus®, Scopus™ and Google™ Scholar (October 2008). We used the key words genetics, interstitial cystitis, painful bladder syndrome, chronic prostatitis and chronic pelvic pain syndrome.
RESULTS
Hypotheses of Peripheral Disease Origin
Historically hypothesis driven research into UCPPS has explored peripheral, organ based mechanisms. Several recent reviews provide extensive overviews of the major theories.4,5 Inflammation may have a critical role in the disease process based on the presence of cytokines and chemokines in the pathogenesis of UCPPS.6 Isolation of autoantibodies from the bladder of patients with IC provides circumstantial but nonspecific and nondiagnostic evidence of autoimmunity. Several lines of research suggest that mast cells have an important role in the inflammatory component of UCPPS, although treatment with antihistamines fails to alleviate symptoms.7
Knowledge of inflammation induced neuroplasticity has led to exploration of the hypothesis that the pain of UCPPS may be of neuropathic origin. Changes in mucosal surface glycosaminoglycans and the resultant increased epithelial permeability have been hypothesized as a potential source of bladder pain. Antiproliferative factor, a frizzled-8 peptide, was found to be a sensitive and specific urine biomarker of IC8 but a recent expert states that “the use of APF as a diagnostic marker and a part of the clinical definition of the syndrome remains tantalizing but not clinically accessible.”9
Hypotheses of Systemic Disease Origin
The important reality is that no postulated molecular mechanism explains the symptoms of UCPPS. Several case series show lack of UCPPS symptomatic improvement after end organ removal (cystectomy or prostatectomy), thus providing support against the prostatocentric or bladder centric view of the disease.10–12 Likewise, to our knowledge no treatment has been shown consistently to alleviate the symptoms, much less elucidate the root cause.13–15 With mounting evidence that UCPPS may be a peripheral manifestation of a central disorder, attention has recently turned to systemic origins of disease. Potential systemic causes include hypothalamic-pituitary-adrenal axis dysregulation and abnormal sympathetic nervous system activity. 16,17 We recently described a cohort of patients with CP/CPPS and reduced activity of CYP21A2, the enzyme that converts progesterone to corticosterone and 17-hydroxyprogesterone to 11-deoxycortisol, suggesting a possible biological explanation for abnormal sympathetic activity.16 Most recently results from observational and interventional case series as well as studies of several cohorts have implicated genetic mechanisms in the pathogenesis of symptoms that patients with UCPPS experience.18–21
Genetic Approaches to UCPPS
Genetic studies of UCPPS can be hypothesis driven or hypothesis generating. While the underlying pathology of UCPPS remains unknown, 3 studies have pursued hypothesis driven queries for genetic differences between cases and controls in specific target genes as identified by pathophysiological observations of phenotypes. The recent sequencing of the human genome has made hypothesis generating studies possible, which examine differences between cases and controls across the entire genome without bias toward previously identified genes, providing a powerful means of objective discovery of new candidate genes to uncover the pathogenetic basis of UCPPS.
Hypothesis driven studies
Results from the Interstitial Cystitis Database show that up to 40% of patients with IC may also suffer from allergic diseases, a number twice that of the general population. 22 Autoimmune conditions such as systemic lupus erythematosus and Sjogren’s syndrome are also observed in the IC population at significantly increased rates.23 Based on evidence that patient susceptibility to autoimmune diseases may be affected by specific allelic variants in IL-4/IL-4 receptor and adrenergic receptor genes, and the hypothesis that IC may result from abnormal autoimmune activity, Sugaya et al explored the risk of IC in patients with polymorphisms known to increase risk for other autoimmune conditions.24 They found that the frequencies of the Arg/Arg genotype of ADRB2 and the TT genotype of the IL-4 gene were significantly higher in patients with IC than in controls. The authors did not exclude patients with allergies or autoimmune comorbidities from the IC pool, and so it is not clear to what degree these genotypes contribute to the risk of IC independent of their known association with immune diseases. In addition, treatment with antihistamines, an effective therapy for many allergies, failed to provide significant clinical benefit in the NIDDK ICCTG trial.7 These data provide further support for the notion that IC has genetic origins, although the findings are not yet clinically useful.
Shoskes et al explored polymorphisms in the promoter sites of cytokines involved in inflammation in men with CP/CPPS, including TNF-α, transforming growth factor-β, IL-10 and IL-6.25 Patients with CPPS were more likely to have the low IL-10 genotype. IL-10 is a known inhibitor of activated macrophages and a product of T regulatory cells to reduce Th1 and Th2 activity. In addition, anti-inflammatory phytotherapy failed in men with high IL-10 and low TNF-α genotypes. These genetic findings suggest a possible autoimmune etiology in the low IL-10 group, for which anti-inflammatory treatment may be most effective. The authors also identified a subset of patients with high IL-10 and low TNF-α who, based on genotype, do not fit a model of CPPS consistent with autoimmune mechanisms and, therefore, require alternative therapeutic options. Their study provides evidence for genetic risk factors in CPPS and points to the importance of accurate patient stratification based on genotype and phenotype when considering effective treatment options.
Tricyclic antidepressants are a commonly prescribed treatment for IC. In pursuit of genetic susceptibility to this treatment Nishijima et al reported on the Arg16Gly polymorphism in the β2-adrenoceptor ADRB2.26 The study revealed that Arg/Arg had a significantly greater prevalence in patients with IC than Arg/Gly or Gly/Gly, and that patients with Arg/Arg responded less favorably to TCA therapy than those carrying Arg/Gly or Gly/Gly. The authors postulated that the Arg16Gly polymorphism is associated with down-regulation of ADRB2 in the detrusor muscle and that effectiveness of TCA therapy depends on this polymorphism. This finding has interesting clinical implications when considering TCA treatment.
While hypothesis driven searches for genetic risk factors in UCPPS have produced some associations and contribute to an increasing body of literature citing genetics as a risk factor for disease, the findings to date are difficult to generalize and not yet clinically useful. Importantly the lack of mechanistic understanding of the disease process undermines the foundation of this approach and severely impairs its chances of uncovering significant genetic contributions to disease.
Genome-wide association studies
On the other hand, recent discoveries from the Human Genome Project and the International HapMap Project allow an unbiased genome-wide comparison of gene variant prevalence between cases and controls, obviating the need to guess which genes are likely to harbor variants affecting risk. GWAS use single nucleotide polymorphisms or simple sequence length polymorphisms to identify specific regions of the genome inherited in greater than expected frequencies in patients with disease, narrowing investigative focus to defined regions of the genome for further sequencing. GWAS allow comprehensive interrogation of the genome that can uncover regions of interest, especially when little is known about disease etiology. For diseases with complex, polygenic inheritance in which each contributing allele adds incremental risk of disease development, GWAS can be particularly powerful in identifying high risk haplotypes.
Indeed this approach has proved successful for many diseases with unknown underlying biology, including age related macular degeneration, in which GWAS revealed complement factor H to be a significant risk factor for disease development. Identification of complement factor H spurred investigation of other components of the complement activation pathway, leading to the discovery of specific alleles that are protective against age related macular degeneration. Thus, GWAS proved to be an essential component of detecting high risk alleles and using that information to understand the fundamental biology of disease.27 Application of GWAS to UCPPS makes logical sense given the lack of a defined mechanism or even objective diagnostic confirmation of disease.
Linkage analysis
Similar to the GWAS approach is the linkage study, in which diseases approximating mendelian inheritance are tracked through generations of families and high risk haplotypes are located through the use of single nucleotide polymorphisms. Linkage studies require genetic information from affected and unaffected members of a family, and use recombination events to map the probable location of the loci of interest. The linkage method applies only when a disease appears to track as a single gene. Application of linkage studies and GWAS to UCPPS is consistent with evidence that the disease can track with mendelian and complex inheritance patterns. Establishing the genetic basis of UCPPS requires fulfillment of the 3 criteria of 1) evidence of familial clustering, 2) increased concordance in monozygotic compared to dizygotic twins and 3) increased recurrence risk (see table).
Table.
Summary of published studies on the genetics of UCPPS
| References | Results |
|---|---|
| Familial clustering: | |
| Koziol et al29 | 27/374 (7%) Pts with IC had family members with IC |
| Simon et al22 | 19/352 (5%) Pts reported at least 1 other family member with IC |
| Warren et al18 | 5/50 (10%) Pts with IC had FDR with IC |
| Dimitrakov et al28 | 2 Generations with 4 members affected with IC and CP/CPPS |
| Warren JW: Urology 2004; 63: 17 | Prevalence of NIDDK confirmed IC in female FDRs was 995/100,000 |
| Weissman et al20 | FDRs of probands with IC (vs those without) significantly more likely to have PD, thyroid disorder or urological problems (adjusted OR 1.95, 95% CI 1.13–3.38, p = 0.02) |
| Concordance in twin studies: | |
| Warren et al31 | 5/8 MZ twins and 0/10 DZ twins with confirmed IC |
| Increased recurrence risk: | |
| Warren JW: Urology 2004; 63: 17 | IC risk ratio (lambda) of 17 in adult female FDRs |
| Weissman20 and Talati19 et al | 4-Fold increased lifetime risk of IC in FDRs of pts with IC vs general population |
Familial clustering
We described for the first time a single family with familial clustering of IC/PBS and CP/CPPS.28 Subsequently we identified several other families with evidence of familial clustering. Analysis of this group of families indicated that our data were consistent with the published results of increased familial clustering of IC/PBS in 19 of 352 (5%) patients with IC in the Interstitial Cystitis Database,22 as well as results from Koziol29 and Warren18 et al. Warren et al reported that the prevalence of NIDDK confirmed IC in female FDRs 31 to 73 years old was 995/100,000 (95% CI 653–1,337).18 This represents a lambda (the ratio of the risk of having IC as a FDR compared to the risk of IC in the general population) of 17 in adult female FDRs of affected patients compared to the IC prevalence of 60/100,000 from the Nurses’ Health Study.30 Familial clustering is a necessary but insufficient criterion for the genetic basis of disease.
Further evidence for a genetic basis of IC derives from its association with other comorbidities. Weissman et al reported a possible genetic syndrome linking PD, IC, anxiety disorders and thyroid problems. 20 Patients with IC were 4 times more likely to have PD (95% CI 1.22–13.40). FDRs of probands had a 2-fold increased lifetime risk of any of the syndromic conditions compared with the general population (95% CI 1.13–3.38). In addition, this group located a source of genetic risk at marker D13S779 on chromosome 13 with a LOD score of more than 4 when patients with any of the aforementioned conditions were considered affected. Notably the greatest association (highest LOD score) was found in families with bladder problems, and case review by a urologist confirmed that these patients probably had IC. Furthermore, Talati19 and Hamilton21 et al showed that patients with PD or social anxiety disorder had a 5-fold increased incidence of IC, and that FDR of probands had an increased risk of all comorbidities regardless of the specific disease of the proband. These findings are consistent with previous data indicating a genetic basis for PD, and suggest that PD, IC, social anxiety disorder and thyroid problems may be manifestations of a common genetic syndrome.
Twin studies
To measure the extent to which this increased risk is attributable to shared genetic factors as opposed to shared environmental factors, preliminary twin studies have been conducted to measure the heritability of UCPPS (criterion 2). Heritability is measured by comparing concordance in MZ twins with that in DZ twins. In the Interstitial Cystitis Association/Fishbein series 12 of 18 MZ twins and 0 of 35 DZ twins with IC were co-affected.31 The authors reported a lambda of 17 in adult FDRs of patients with IC.18 This satisfies criterion 3 and is consistent with evidence from other studies revealing significantly increased recurrence risk.18–21
Family based studies and mendelian UCPPS
Based on the criteria presented, inherited genetic susceptibility is undoubtedly a key component of UCPPS etiology but the lack of a mendelian inheritance pattern suggests that multiple genes in combination with environmental factors are responsible for the majority of cases. While such complex traits are difficult to study, mendelian traits are generally caused by a single gene disorder with a strong effect and, thus, they are amenable to standard methods of linkage analysis. Fortuitously we have identified 20 Bulgarian families with apparent autosomal dominant (mendelian) inheritance of IC, presenting a unique opportunity to uncover candidate genes for UCPPS inherited from a single individual, the so-called founder effect.32 Most promisingly it fulfills the first and foremost prerequisite for successful linkage mapping by providing a set of families in which the disease phenotype (UCPPS) is segregating and the assessment of the phenotypes has been made with minimal ambiguity. While such families probably represent individuals with UCPPS at the extreme of the distribution, the results may nevertheless be generalizable to the broader UCPPS community, especially inasmuch as these findings elucidate the mechanism of disease. Identification of candidate genes in these families will provide a roadmap for candidate genes in UCPPS, which will help in the development of biomarkers to study the clinical manifestations and symptom severity of UCPPS, and eventually develop effective treatments. This approach has proved fruitful in Parkinson’s disease,33,34 Alzheimer’s disease35 and even hypertension.36–38
Challenges in UCPPS genetics
Observations from our cohort of families as well as the aforementioned studies suggest that the current diagnostic criteria for UCPPS detect something real, even if they do so imprecisely. A major challenge for genetic studies of the disease is the identification of appropriate patient populations for genotyping. The complex underlying biology of UCPPS has undermined attempts to develop a gold standard diagnostic test for the disease. UCPPS is probably better defined as a quantitative rather than a qualitative or categorical trait. Quantitative traits fall along a spectrum of severity with states of normal, a model that is consistent with the polygenic mode of inheritance thought to characterize most complex genetic disorders. The number or expression pattern of risk gene variants carried by a given individual and the interaction of these variants with nongenetic factors of varying strength might produce more or fewer symptoms of greater or lesser severity.
Patients with UCPPS present with a wide array of symptoms. Under the model of systemic mechanism of disease, numerous local (dysfunctional voiding, trauma, infection, inflammation, nerve damage or abnormal autoimmune activity) or systemic factors (HPA dysregulation, SNS, immune or endocrine dysfunction) trigger initial symptoms in a genetically predisposed individual. In essence peripheral and central stimuli may cause symptoms of UCPPS, and the heterogeneity of each patient response on a biological level may reflect individual genetics for disease, as determined by specific allelic makeup.
Phenotyping
Recent advances in characterization of intermediate phenotypes in psychiatric diseases may provide a powerful tool for identifying specific genetic causes of UCPPS. An intermediate phenotype is a “mechanism-related manifestation of a complex phenotype.” 39 Those intermediate phenotypes found to be heritable are called endophenotypes. Intermediate phenotypes are especially useful when characterizing diseases of polygenic inheritance in which a single allele confers measurable but incremental risk for disease development.40
UCPPS intermediate phenotyping
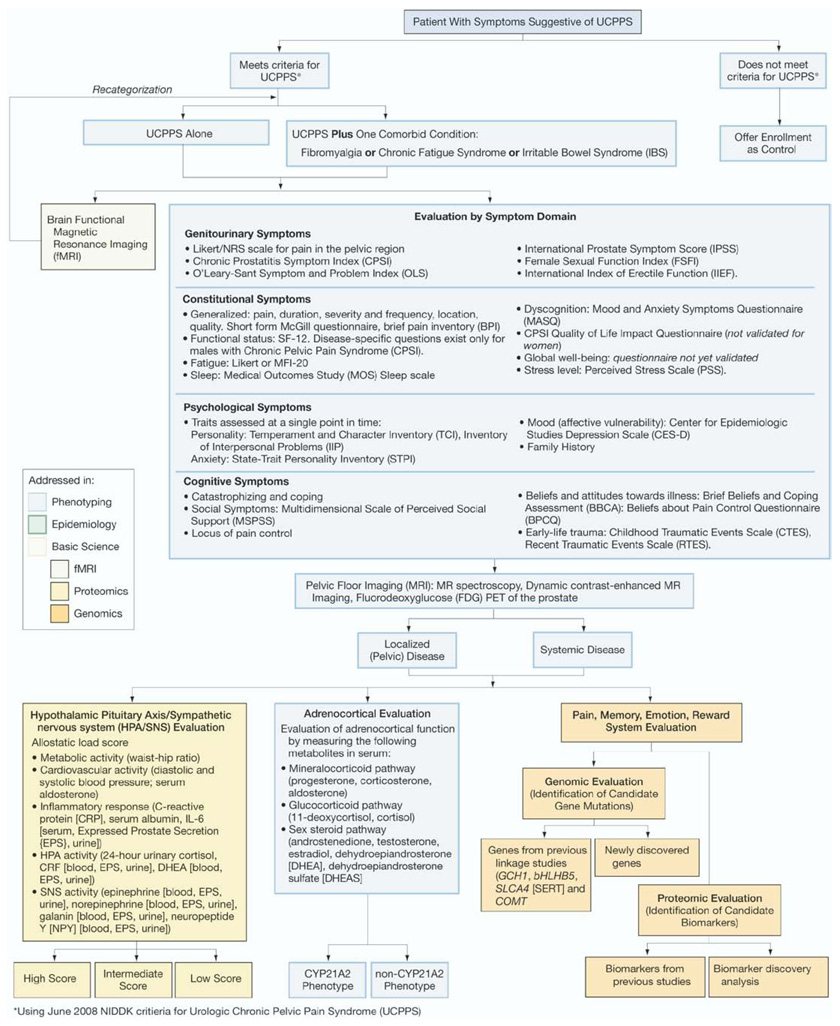
It is hypothesized that UCPPS may originate from many genes, each conferring variable and usually incremental risk. It is further hypothesized that patients with a similar allelic makeup may also exhibit a similar range of clinically defined symptoms. Thus, patient stratification based on careful and objective phenotyping may identify pools of patients with similar genetic risk profiles, thereby increasing the chances of identifying specific allelic determinants associated with disease. The figure shows a possible approach to UCPPS in this context.
Figure.
Schematic representation of patient phenotyping in UCPPS. Patients meeting standard diagnostic criteria should be characterized as objectively as possible according to brain fMRI, complete review of symptoms, HPA/SNS score, adrenocortical evaluation and protein biomarkers identified in previous studies. Selective grouping of patients according to this system will increase power of GWAS for new genetic targets. CRF, corticotropin-releasing factor. MFI-20, Multidimensional Fatigue Inventory. MR, magnetic resonance. PET, positron emission tomography.
Examples of intermediate phenotypes in UCPPS include but are not limited to hypothalamic-pituitary axis/sympathetic nervous system dysfunction, presence of localized or systemic disease, comorbidities and brain fMRI pattern. In recognition of the likely systemic mechanism of disease, characterization of patient phenotypes should take a broadly comprehensive view of symptoms rather than solely a urological perspective. To this end an ideal phenotype would reveal symptoms in urology, gastroenterology, neurology, gynecology, psychiatry and rheumatology. Each of these quantifiable phenotypes may be used to group patients according to shared characteristics. Importantly this approach will produce valuable results whether UCPPS is an umbrella term for a set of mendelian disorders or it arises in polygenic fashion as described previously.
The success of intermediate phenotyping has been documented for a variety of psychiatric conditions such as schizophrenia, alcoholism and bipolar disorder, which have genetic and environmental risk factors associated with disease pathogenesis.41,42 Notably, because UCPPS likely shares the characteristic of having genetic and environmental triggers, it may be especially appropriate for intermediate phenotyping as a means of interrogating the genome for specific risk factors. In essence the success of future genetic investigation of UCPPS hinges on accurate, objective phenotyping of defined patient populations.
Phenomics
Borrowing from the success of psychiatry in identifying the genetic determinants of polygenic and multifactorial diseases by intermediate phenotypes and endophenotypes, we propose by analogy a similar approach to study genetic determinants of UCPPS. Rather than shy away from potentially confounding variables in UCPPS, this tact derives power by embracing the multiplicity of conditions.
Comorbidity
Comorbidities, which are exhibited by a large fraction of patients with UCPPS, present a problem to traditional genetic approaches to disease by confounding the relationship between diseases. However, comorbidities might reflect different patterns of symptoms that result from shared genetic risk factors. This is a well documented phenomenon in neurogenetics where major depression and generalized anxiety disorder are thought to co-occur at high rates because they represent different faces of the same underlying risk genes and, thus, perhaps the same underlying disease processes.
Comorbidity in UCPPS could also be an artifact that arises from errors in the lumping and splitting of symptoms such that a single pathophysiological process can cause symptoms that meet the criteria for multiple diseases, as appears to be the case for many personality disorder diagnoses. Our phenomics proposal embraces the presence of comorbidities as reflective of distinctive underlying biology carrying the potential to further refine knowledge of UCPPS.
Patient Stratification
The difficulty in identifying risk genes for UCPPS results partly from the lack of objective tests to narrow populations for genetic study and, in large part, from the complexity of genetic risk. What we now call a single disorder, UCPPS, might result from interaction of a large number of common genetic variants, with no variant proving necessary or sufficient for developing the disorder. This situation is thought to characterize the common forms of cardiovascular, endocrinological and neurological disorders. Alternatively, UCPPS might result from diverse individual mutations and, thus, actually represent a large family of rare mendelian diseases with a similar pathophysiology, as has been shown to be the case in retinitis pigmentosa.42–44 Regardless of the inheritance pattern of the disease, this approach may discriminate with powerful specificity the relative contributions of a variety of genetic risk factors whether mendelian or polygenic.
CONCLUSIONS
The investigative trail left by UCPPS has led urologists from the understanding of the disease as a peripheral disorder to a systemic disorder to an umbrella term for disorders with fundamentally genetic determinants. An increasing body of evidence showing familial clustering of UCPPS supports the notion of it being a genetic disease. Indeed, familial clustering appears to apply to IC in families with other comorbidities such as PD, social anxiety disorder and thyroid problems. The well established prevalence of comorbidities of IC, such as fibromyalgia, allergies and Sjogren’s, irritable bowel and chronic fatigue syndromes, present an enticing picture of UCPPS as a set of conditions with significant pathophysiological over-lap with many other diseases due to central, genetically defined risk factors. Twin studies and increased recurrence risk add to the claim that UCPPS has genetic origins. We also identified a cohort of families in which UCPPS appears to track in autosomal dominant fashion, which provides a roadmap for candidate gene identification.
Because successful treatments often fail and because of the wide variability in patient reported symptoms of UCPPS, we support a phenomics approach to research of this disease (see figure). Patient stratification through careful phenotyping may allow grouping of patients based on shared physical manifestations of disease and, therefore, a shared genetic predisposition to those symptoms. By combining patient symptoms from a variety of disciplines, it is possible to avoid the artificial creation of discrete syndromes when it is possible that a common set of genetic risk factors creates a multiplicity of symptoms cutting across medical specialties.
Abbreviations and Acronyms
- ADRB2
β-2-adrenergic receptor
- APF
antiproliferative factor
- CP/CPPS
chronic prostatitis/chronic pelvic pain syndrome
- DZ
dizygotic
- FDR
first-degree relative
- fMRI
functional magnetic resonance imaging
- GWAS
genome-wide association studies
- HPA
hypothalamic-pituitary axis
- IC
interstitial cystitis
- IC/PBS
interstitial cystitis/painful bladder syndrome
- ICCTG
Interstitial Cystitis Clinical Trials Group
- IL
interleukin
- MZ
monozygotic
- NIDDK
National Institute of Diabetes and Digestive and Kidney Diseases
- PD
panic disorder
- SNS
sympathetic nervous system
- TCA
tricyclic antidepressant
- TNF
tumor necrosis factor
- UCPPS
urological chronic pelvic pain syndromes
REFERENCES
- 1.Schaeffer AJ. Clinical practice. Chronic prostatitis and the chronic pelvic pain syndrome. N Engl J Med. 2006;355:1690. doi: 10.1056/NEJMcp060423. [DOI] [PubMed] [Google Scholar]
- 2.Multi-disciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network (U01) [Accessed June 1, 2008];National Institutes of Diabetes and Digestive and Kidney Diseases, National Institutes of Health. Available at http://grants.nih.gov/grants/guide/rfa-files/RFA-DK-07-003.html.
- 3.Krieger J, Nyberg L, Jr, Nickel J. NIH consensus definition and classification of prostatitis. JAMA. 1999;282:236. doi: 10.1001/jama.282.3.236. [DOI] [PubMed] [Google Scholar]
- 4.Buffington CA. Comorbidity of interstitial cystitis with other unexplained clinical conditions. J Urol. 2004;172:1242. doi: 10.1097/01.ju.0000137953.49304.6c. [DOI] [PubMed] [Google Scholar]
- 5.Pontari M. Chronic prostatitis/chronic pelvic pain syndrome. Urol Clin North Am. 2008;35:81. doi: 10.1016/j.ucl.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Desireddi NV, Campbell PL, Stern JA, Sobkoviak R, Chuai S, Shahrara S, et al. Monocyte chemoattractant protein-1 and macrophage inflammatory protein-1alpha as possible biomarkers for the chronic pelvic pain syndrome. J Urol. 2008;179:1857. doi: 10.1016/j.juro.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sant GR, Propert KJ, Hanno PM, Burks D, Culkin D, Diokno AC, et al. A pilot clinical trial of oral pentosan polysulfate and oral hydroxyzine in patients with interstitial cystitis. J Urol. 2003;170:810. doi: 10.1097/01.ju.0000083020.06212.3d. [DOI] [PubMed] [Google Scholar]
- 8.Keay S, Zhang C, Shoenfelt J, Erickson DR, Whitmore K, Warren JW, et al. Sensitivity and specificity of antiproliferative factor, heparin-binding epidermal growth factor-like growth factor, and epidermal growth factor as urine markers for interstitial cystitis. Urology. 2001;57:9. doi: 10.1016/s0090-4295(01)01127-x. [DOI] [PubMed] [Google Scholar]
- 9.Hanno P. Re-imagining interstitial cystitis. Urol Clin North Am. 2008;35:91. doi: 10.1016/j.ucl.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Blaivas JG, Weiss JP, Desai P, Flisser AJ, Stember DS, Stahl PJ. Long-term followup of augmentation enterocystoplasty and continent diversion in patients with benign disease. J Urol. 2005;173:1631. doi: 10.1097/01.ju.0000154891.40110.08. [DOI] [PubMed] [Google Scholar]
- 11.Badenoch AW. Chronic interstitial cystitis. Br J Urol. 1971;43:718. doi: 10.1111/j.1464-410x.1971.tb12092.x. [DOI] [PubMed] [Google Scholar]
- 12.Flood HD, Malhotra SJ, O’Connell HE, Ritchey MJ, Bloom DA, McGuire EJ. Long-term results and complications using augmentation cystoplasty in reconstructive urology. Neurourol Urodyn. 1995;14:297. doi: 10.1002/nau.1930140402. [DOI] [PubMed] [Google Scholar]
- 13.Nickel JC. Inflammatory conditions of the male genitourinary tract: prostatitis and related conditions, orchitis, and epididymitis. In: Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Campbell-Walsh Urology. 9th ed. Philadelphia: Elsevier Saunders; 2006. [Google Scholar]
- 14.Dimitrakov J, Kroenke K, Steers WD, Berde C, Zurakowski D, Freeman MR, et al. Pharmacologic management of painful bladder syndrome/interstitial cystitis: a systematic review. Arch Intern Med. 2007;167:1922. doi: 10.1001/archinte.167.18.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dimitrakov JD, Kaplan SA, Kroenke K, Jackson JL, Freeman MR. Management of chronic prostatitis/chronic pelvic pain syndrome: an evidence-based approach. Urology. 2006;67:881. doi: 10.1016/j.urology.2005.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dimitrakov J, Joffe HV, Soldin SJ, Bolus R, Buffington CA, Nickel JC. Adrenocortical hormone abnormalities in men with chronic prostatitis/chronic pelvic pain syndrome. Urology. 2008;71:261. doi: 10.1016/j.urology.2007.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson RU, Orenberg EK, Chan CA, Morey A, Flores V. Psychometric profiles and hypothalamic-pituitary-adrenal axis function in men with chronic prostatitis/chronic pelvic pain syndrome. J Urol. 2008;179:956. doi: 10.1016/j.juro.2007.10.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warren J, Jackson T, Meyers D, Xu J. Fishbein/Interstitial Cystitis Association (ICA) survey of interstitial cystitis among family members of ICA members: preliminary analysis. Urology. 2001;57:126. doi: 10.1016/s0090-4295(01)01095-0. [DOI] [PubMed] [Google Scholar]
- 19.Talati A, Ponniah K, Strug LJ, Hodge SE, Fyer AJ, Weissman MM. Panic disorder, social anxiety disorder, and a possible medical syndrome previously linked to chromosome 13. Biol Psychiatry. 2008;63:594. doi: 10.1016/j.biopsych.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weissman MM, Gross R, Fyer A, Heiman CA, Gareroff MJ, Hodge SE, et al. Interstitial cystitis and panic disorder: a potential genetic syndrome. Arch Gen Psychiatry. 2004;61:273. doi: 10.1001/archpsyc.61.3.273. [DOI] [PubMed] [Google Scholar]
- 21.Hamilton SP, Fyer AJ, Durner M, Heiman GA, Baisre De Leon A, Hodge SE, et al. Further genetic evidence for a panic disorder syndrome mapping to chromosome 13q. Proc Natl Acad Sci U S A. 2003;100:2550. doi: 10.1073/pnas.0335669100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simon LJ, Landis JR, Erickson DR, Nyberg LM. The Interstitial Cystitis Data Base Study: concepts and preliminary baseline descriptive statistics. Urology. 1997;49:64. doi: 10.1016/s0090-4295(99)80334-3. [DOI] [PubMed] [Google Scholar]
- 23.van de Merwe JP, Yamada T, Sakamoto Y. Systemic aspects of interstitial cystitis, immunology and linkage with autoimmune disorders. Int J Urol, suppl. 2003;10:S35. doi: 10.1046/j.1442-2042.10.s1.10.x. [DOI] [PubMed] [Google Scholar]
- 24.Sugaya K, Nishijima S, Yamada T, Miyazato M, Hatano T, Ogawa Y. Molecular analysis of adrenergic receptor genes and interleukin-4/interleukin-4 receptor genes in patients with interstitial cystitis. J Urol. 2002;168:2668. doi: 10.1016/S0022-5347(05)64241-3. [DOI] [PubMed] [Google Scholar]
- 25.Shoskes DA, Albakri Q, Thomas K, Cook D. Cytokine polymorphisms in men with chronic prostatitis/chronic pelvic pain syndrome: association with diagnosis and treatment response. J Urol. 2002;168:331. [PubMed] [Google Scholar]
- 26.Nishijima S, Sugaya K, Yamada T, Miyazato M, Ogawa Y. Efficacy of tricyclic antidepressant is associated with beta2-adrenoceptor genotype in patients with interstitial cystitis. Biomed Res. 2006;27:163. doi: 10.2220/biomedres.27.163. [DOI] [PubMed] [Google Scholar]
- 27.Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dimitrakov JD. A case of familial clustering of interstitial cystitis and chronic pelvic pain syndrome. Urology. 2001;58:281. doi: 10.1016/s0090-4295(01)01138-4. [DOI] [PubMed] [Google Scholar]
- 29.Koziol JA, Clark DC, Gittes RF, Tan EM. The natural history of interstitial cystitis: a survey of 374 patients. J Urol. 1993;149:465. doi: 10.1016/s0022-5347(17)36120-7. [DOI] [PubMed] [Google Scholar]
- 30.Curhan GC, Speizer FE, Hunter DJ, Curhan SG, Stampfer MJ. Epidemiology of interstitial cystitis: a population based study. J Urol. 1999;161:549. [PubMed] [Google Scholar]
- 31.Warren JW, Keay SK, Meyers D, Xu J. Concordance of interstitial cystitis in monozygotic and dizygotic twin pairs. Urology. 2001;57:22. doi: 10.1016/s0090-4295(01)01120-7. [DOI] [PubMed] [Google Scholar]
- 32.Dimitrakov JD, Boyden SE, Freeman MR, Detchev IY, Tchitalov JI, Kunkel L. Single nucleotide polymorphism (SNP) analysis of autosomal dominant interstitial cystitis: a family-based study. J Urol, suppl. 2005;173:84. abstract 303. [Google Scholar]
- 33.Polymeropoulos MH, Higgins JJ, Golbe LI, Johnson WE, Ide SE, Di Iorio G, et al. Mapping of a gene for Parkinson’ disease to chromosome 4q21–3. Science. 1996;274:1197. doi: 10.1126/science.274.5290.1197. [DOI] [PubMed] [Google Scholar]
- 34.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehajia A, Dutra A, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 35.Janssen JC, Beck JA, Campbell TA, Dickinson A, Fox NC, Harvey RJ, et al. Early onset familial Alzheimer’s disease: mutation frequency in 31 families. Neurology. 2003;60:235. doi: 10.1212/01.wnl.0000042088.22694.e3. [DOI] [PubMed] [Google Scholar]
- 36.Luft FC. Mendelian forms of human hypertension and mechanisms of disease. Clin Med Res. 2003;1:291. doi: 10.3121/cmr.1.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luft FC, Toka O, Toka HR, Jordan J, Bahring S. Mendelian hypertension with brachydactyly as a molecular genetic lesson in regulatory physiology. Am J Physiol Regul Integr Comp Physiol. 2003;285:R709. doi: 10.1152/ajpregu.00174.2003. [DOI] [PubMed] [Google Scholar]
- 38.Luft FC. Present status of genetic mechanisms in hypertension. Med Clin North Am. 2004;88:1. doi: 10.1016/s0025-7125(03)00118-4. [DOI] [PubMed] [Google Scholar]
- 39.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 40.Meyer-Lindenberg A, Weinberger DR. Inter-mediate phenotypes and genetic mechanisms of psychiatric disorders. Nat Rev Neurosci. 2006;7:818. doi: 10.1038/nrn1993. [DOI] [PubMed] [Google Scholar]
- 41.Goldman D, Ducci F. Deconstruction of vulnerability to complex diseases: enhanced effect sizes and power of intermediate phenotypes. Sci World J. 2007;7:124. doi: 10.1100/tsw.2007.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jin ZB, Mandai M, Yokota T, Higuchi K, Ohmori K, Ohtsuki F, et al. Identifying pathogenic genetic background of simplex or multiplex retinitis pigmentosa patients: a large scale mutation screening study. J Med Genet. 2008;45:465. doi: 10.1136/jmg.2007.056416. [DOI] [PubMed] [Google Scholar]
- 43.Jordan SA, Farrar GJ, Kenna P, Humphries MM, Sheils DM, Kumar-Singh R, et al. Localization of an autosomal dominant retinitis pigmentosa gene to chromosome 7q. Nat Genet. 1993;4:54. doi: 10.1038/ng0593-54. [DOI] [PubMed] [Google Scholar]
- 44.Kumar-Singh R, Farrar GJ, Mansergh F, Kenna P, Bhattacharya S, Gal A, et al. Exclusion of the involvement of all known retinitis pigmentosa loci in the disease present in a family of Irish origin provides evidence for a sixth autosomal dominant locus (RP8) Hum Mol Genet. 1993;2:875. doi: 10.1093/hmg/2.7.875. [DOI] [PubMed] [Google Scholar]