Abstract
Elevated serum retinol-binding protein (RBP) concentration has been associated with obesity and insulin resistance, but accompanying retinol values have not been reported. Assessment of retinol is required to discriminate between apo-RBP, which may act as an adipokine, and holo-RBP, which transports vitamin A. The relations between serum RBP, retinol, retinyl esters, BMI, and measures of insulin resistance were determined in obese adults. Fasting blood (≥8 h) was collected from obese men and women (n = 76) and blood chemistries were obtained. Retinol and retinyl esters were quantified by HPLC and RBP by ELISA. RBP and retinol were determined in age and sex-matched, nonobese individuals (n = 41) for comparison. Serum apo-RBP was two-fold higher in obese (0.90 ± 0.62 µM) than nonobese subjects (0.44 ± 0.56 µM) (P < 0.001). The retinol to RBP ratio (retinol:RBP) was significantly lower in obese (0.73 ± 0.13) than nonobese subjects (0.90 ± 0.22) (P < 0.001) and RBP was strongly associated with retinol in both groups (r = 0.71 and 0.90, respectively, P < 0.0001). In obese subjects, RBP was associated with insulin (r = 0.26, P < 0.05), homeostatic model assessment of insulin resistance (r = 0.29, P < 0.05), and quantitative insulin sensitivity check index (r = −0.27, P < 0.05). RBP was associated with BMI only when obese and nonobese subjects were combined (r = 0.25, P < 0.01). Elevated serum RBP, derived in part from apo-RBP, was more strongly associated with retinol than with BMI or measures of insulin resistance in obese adults. Investigations into the role of RBP in obesity and insulin resistance should include retinol to facilitate the measurement of apo-RBP and retinol:RBP. When evaluating the therapeutic potential of lowering serum RBP, consideration of the consequences on vitamin A metabolism is paramount.
Keywords: obesity, RBP, RBP4, retinol, vitamin A
Introduction
In addition to its well established role as the major blood carrier of retinol, serum retinol-binding protein (RBP) has recently been referred to as “RBP4,” a new adipokine, by members of the diabetic research community (1). This development was instigated by a series of studies in mice and humans revealing a strong relationship between serum RBP and obesity-induced insulin resistance (2, 3). These novel findings spawned widespread fervor to understand the role of RBP in obesity and insulin resistance, generating a considerable pool of publications in a relatively short amount of time. While some studies have validated the original observations of elevated RBP in obesity and insulin resistance in humans (3–8), others have not (9–15). Often lacking in these publications are data for serum retinol, arguably RBP’s most important physiological companion, representing a possible explanation for conflicting results.
Retinol abundance influences RBP secretion from the liver (16), the primary storage site of vitamin A. Serum retinol concentration is commonly used to assess vitamin A status in humans because liver biopsy, the gold standard (17), is not feasible. In the fasted state, vitamin A circulates primarily as retinol bound to RBP (i.e., holo-RBP) in approximately a 1:1 molar ratio, and to a much lesser extent, as retinyl esters carried by lipoproteins (18, 19). Typically retinyl esters make up <5% of the total plasma retinol concentration (20, 21). However, during hypervitaminosis A, substantial retinyl esters can be detected in the serum and values >10% of total retinol are clinically relevant [see review (22)]. It is unclear how obesity affects the saturation of RBP with retinol, or the circulation of retinyl esters. No studies have reported the relation between serum retinol and retinyl ester concentrations in fasted obese adults. Nor have both of these retinol forms been correlated with RBP in the same study. Previous failure to include retinol in RBP association analyses has potentially hidden the true relationship between obesity, holo-RBP, apo-RBP, and altered vitamin A circulation. Thus, we examined the relations between RBP, retinol, retinyl esters, BMI, and measures of insulin sensitivity in a sample of fasted obese adults.
Materials and Methods
Subjects
Obese subject data were pooled from control or baseline groups from a cross-sectional and intervention study, respectively, conducted by our laboratory. A total of 76 (17 men, 59 women) adults (BMI > 30), aged 21–71 y were included in the obese group. Age and sex-matched, nonobese subject data (n = 41; 13 men, 28 women) were also pooled from previous studies conducted by our laboratory (21, 23, 24). All procedures for these studies were approved by the Health Sciences Institutional Review Board at the University of Wisconsin – Medical School.
Blood Collection and Serum Chemistry
Blood samples were drawn after an overnight fast (at least 8 h). Blood from all subjects was analyzed for retinol and RBP, while only blood from obese subjects was analyzed for hematocrit, serum lipids, insulin, glucose, C-reactive protein (CRP), and retinyl esters. For blood samples used to determine hematocrit, study phlebotomists drew whole blood samples into sterile-interior 3 mL Vacutainer® tubes containing 5.4 mg K2EDTA (Becton Dickinson, NJ). Samples used to determine serum lipid panels, insulin, glucose, CRP, retinol, retinyl esters, and RBP were drawn using 6 mL, sterile interior, Corvac brand serum separator tubes (Tyco Healthcare Group LP, MA). Whole blood samples sat at room temperature for 10 min, then were stored on ice until analysis within 18 h at the contract laboratory (Consultants Laboratory; Fond du Lac, WI). Blood for serum sat at room temperature for 10 – 20 min and then was centrifuged at 2200 × g for 10 min at 4 °C. Serum not used for analysis by the contract lab was transferred to cryotubes and stored under argon at −80°C until analysis for retinol, retinyl esters, and RBP.
Surrogate measures of insulin resistance utilizing fasting glucose and insulin concentrations were calculated for the obese group. The homeostasis model assessments of insulin resistance (HOMA-IR) and β-cell function (HOMA-β) were calculated as follows: HOMA-IR = insulin (µU/mL) * glucose (mM)/22.5 and HOMA-β = 20 * insulin (µU/mL)/(glucose (mM) −3.5) (25). The quantitative insulin sensitivity check index (QUICKI) was calculated as follows: QUICKI = 1/[log(insulin (µU/mL) + log(glucose (mg/dL)] (26). For interpretation, HOMA-IR and HOMA-β will increase, while QUICKI will decrease with increasing insulin resistance. Logarithmically transformed HOMA and QUICKI models are considered the best and most extensively validated minimal surrogate measures of insulin resistance (27, 28).
Serum Vitamin A and Retinol-Binding Protein Assays
Extraction and HPLC analyses were modified from Sowell et al. (29) to optimize the sensitivity of detection of retinol and retinyl esters. Briefly, retinol and retinyl esters were extracted from serum using a published procedure (30), with volume alterations. One mL serum was treated with 1 mL ethanol (0.1% butylated hydroxytoluene), and extracted 3 times with 1 mL hexanes. Retinyl butyrate was used as an internal standard to determine extraction efficiency (87 ± 5%). Dried samples were reconstituted in 80 µL methanol:dichloroethane (50:50, v:v) and 60 µL was analyzed by HPLC as published elsewhere (31).
Serum RBP was determined using a sandwich enzyme-linked immunosorbent assay (ELISA) as developed by Erhardt et al. (32). This sensitive ELISA was validated with HPLC-determined serum retinol and a commercially available RBP ELISA kit (ALPCO diagnostics) that has been used in several human studies (3, 33, 34). Greater serum dilution (1:9664) compared to the original protocol (1:6644) was required to accommodate the relatively higher RBP concentrations of our study population. Additionally, it was necessary to decrease the RBP standard dilutions (from 1:1661 to 1:1158) and use more per plate well (5, 10, 15, 20 and 25 µL) to generate a calibration curve with a higher range. The intra- and inter-assay coefficients of variation were 8.3 and 13.6%, respectively.
Statistical Analysis
Data are presented as means ± SDs. Differences in RBP, retinol, BMI, age, and the retinol to RBP molar ratio (retinol:RBP) between the obese and nonobese groups were determined by one-way ANOVA. Pearson’s correlation was performed to determine simple associations between analytes. Fisher’s r-to-Z transformation was used to test for significant differences between r values of obese and nonobese subjects. Multiple linear regression (MLR) analysis was performed to identify variables associated with RBP after adjustment for age and retinol. Age was included as a covariate because of the large range of ages in our sample, and retinol was included because of its physiological relationship with RBP. Stepwise MLR with backward selection was used to select the best multivariate models to explain RBP and total retinyl ester concentration. Prior to correlation and regression analyses, non-normally distributed variables (total retinyl ester concentration, glucose, insulin, triacylglycerols, VLDL cholesterol (VLDL-C), HDL cholesterol (HDL-C), CRP, hematocrit, HOMA-IR and HOMA-β) were logarithmically transformed. Bonferroni testing was applied to correct for multiple comparisons. Because hypervitaminosis A causes abnormally high circulating retinyl ester concentrations (>10% of the total circulating vitamin A in the fasted state), regression models with total retinyl ester concentration as the dependent variable were constructed with and without values from potentially hypervitaminotic subjects (n = 3). All statistical procedures (PROC CORR, PROC GLM and PROC REG) were performed with SAS software (version 8.2; SAS Institute Inc, Cary, NC). Statistical analyses were 2-sided and P < 0.05 was considered significant.
Results
Physical and vitamin A-related biochemical characteristics of obese and nonobese subjects were compared (Table 1). Mean age and serum retinol concentrations were not different between the two groups. Mean BMI and RBP concentrations were higher in obese subjects and retinol:RBP was lower, compared to nonobese subjects. RBP concentrations did not differ by gender in either group alone or in both groups combined. Fasting glucose, LDL cholesterol LDL-C), triacylglycerol and total cholesterol levels were slightly higher than recently reported values from a group of overweight and obese subjects without diabetes (11), while insulin, HOMA-IR and HOMA-β were slightly lower (Table 2). The mean QUICKI in our obese sample was similar to previously published values from obese, nondiabetic subjects (26). Importantly, none of the subjects in the current study had blood glucose concentrations above 126 mg/dL, the current diagnostic cut-off for diabetes, established by the American Diabetes Association. Diagnostic cut-off values for HOMA and QUICKI have been proposed, but are not used routinely to diagnose insulin resistance in individuals.
Table 1.
Physical and vitamin A-related biochemical characteristics of obese (n = 76; 17 men and 59 women) and nonobese fasted adults (n = 41; 13 men and 28 women)a
| Characteristic | Obese (n = 76) | Nonobese (n = 41) | Pb |
|---|---|---|---|
| Age (y) | 39.1 ± 13.5 (21–71) | 37.5 ± 17.7 (20–70)c | 0.60 |
| BMI (kg/m2) | 34.3 ± 4.0 (30.1–44.4) | 24.8 ±3.2(19.7–29.3) | <0.0001 |
| Retinol (µM) | 2.23 ± 0.57 (1.00–3.95) | 2.23 ± 0.58 (1.06–3.68) | 0.96 |
| RBP (µM) | 3.13 ± 0.88 (1.52–5.24) | 2.67 ± 1.02 (0.61–5.47) | 0.012 |
| Retinol:RBP | 0.73 ± 0.14 (0.49–1.16) | 0.90 ± 0.23 (0.66–1.72) | <0.0001 |
RBP, retinol-binding protein
Significant differences between nonobese and obese subjects were determined by one-way ANOVA (P < 0.05).
Values are mean ± SD; range in parentheses (all such values).
Table 2.
Blood chemistry characteristics of fasted obese adultsa
| Characteristic | Obese [n] |
|---|---|
| Glucose (mM) | 5.31 ± 0.90 [64]b |
| Insulin (pM) | 75.2 ± 48.9 [63] |
| Total Cholesterol (mM) | 5.12 ± 1.01 [75] |
| Triacylglycerols (mM) | 1.68 ± 1.24 [75] |
| HDL-C (mM) | 1.20 ± 0.36 [64] |
| VLDL-C (mM) | 0.53 ± 0.30 [62] |
| LDL-C (mM) | 3.31 ± 0.76 [62] |
| C-reactive protein (mg/dl) | 0.51 ± 0.51 [64] |
| Hematocrit (%) | 42.2 ± 5.2 [63] |
| HOMA-IR | 2.40 ± 1.20 [63] |
| HOMA-β | 129.7 ± 77.1 [63] |
| QUICKI | 0.34 ± 0.03 [63] |
| Retinyl esters (nM) | 64.2 ± 52.0 [76] |
| Retinyl esters (% of total vitamin A) | 2.93 ± 2.34 [76] |
HOMA-IR, homeostasis model assessment of insulin resistance; HOMA-β, HOMA of β cell function; QUICKI, quantitative insulin sensitivity check index.
Values are mean ± SD; n in brackets [all such values].
Independent associations between serum RBP and other characteristics related to obesity and insulin sensitivity were determined after control for either age alone, or age and serum retinol concentration in the obese group (Table 3). Total cholesterol, log HDL-C, LDL-C, log CRP, log glucose, and log HOMA-β were not associated with RBP. Log Insulin and log HOMA-IR were positively associated with RBP (r = 0.26, P = 0.04 and r = 0.29, P = 0.03, respectively), while QUICKI was inversely associated with RBP (r = −0.27, P = 0.03) before and after adjusting for age and retinol. BMI was associated with log CRP (P < 0.005) after control for age and log CRP was associated with age (P = 0.02) after control for BMI. Log VLDL-C, log triacylglycerols, log hematocrit, and log retinyl esters were associated with RBP after control for age, but not after control for age and retinol. BMI and RBP were not associated in the obese group, but a combined analysis of obese and nonobese subjects revealed a significant association before (r = 0.25, P = 0.01) and after control for age and retinol (P < 0.001). RBP was highly correlated with retinol before (r = 0.71, P < 0.0001) and after control for age (P < 0.001) and this association remained significant after Bonferroni correction for multiple comparisons (P < 0.005). The age-adjusted association between RBP and VLDL-C was the only other association to survive Bonferroni correction, but the relationship was not significant when retinol was included in the model.
Table 3.
Independent associations between serum retinol-binding protein (RBP) and other characteristics in fasted obese adults after adjustment for age alone or after adjustment for age and retinola
| Age-adjusted | Age and retinol-adjusted | |||
|---|---|---|---|---|
| Characteristic | βb | P | β | P |
| BMI (kg/m2) | −0.019 | 0.44 | 0.001 | 0.94 |
| Retinol (µM) | 1.04 | <0.0001c | NA | NA |
| Log Glucose (mM) | −0.013 | 0.99 | 0.036 | 0.96 |
| Log Insulin (pM) | 0.455 | 0.031d | 0.326 | 0.039d |
| Total cholesterol (mM) | 0.002 | 0.52 | −0.001 | 0.51 |
| Log Triacylglycerols (mM) | 0.479 | 0.0034d | 0.130 | 0.33 |
| Log HDL-C (mM) | −0.199 | 0.59 | −0.322 | 0.24 |
| Log VLDL-C (mM) | 0.748 | <0.0002c | 0.310 | 0.077 |
| LDL-C (mM) | −0.001 | 0.99 | −0.000 | 0.75 |
| Log C-reactive protein (mg/dl) | 0.019 | 0.87 | 0.036 | 0.67 |
| Log Hematocrit (%) | 2.26 | 0.031d | 1.36 | 0.089 |
| Log HOMA-IR | 0.506 | 0.021d | 0.337 | 0.042d |
| Log HOMA-β | 0.366 | 0.066 | 0.261 | 0.080 |
| QUICKI | −8.74 | 0.028d | −6.33 | 0.034d |
| Log Retinyl esters (nM) | 0.314 | 0.0055d | 0.066 | 0.47 |
RBP, retinol-binding protein; HOMA-IR, homeostasis model assessment of insulin resistance; HOMA-β, HOMA of β cell function; QUICKI, quantitative insulin sensitivity check index.
Regression coefficient.
P < 0.005 after Bonferroni correction.
Not significant after Bonferroni correction.
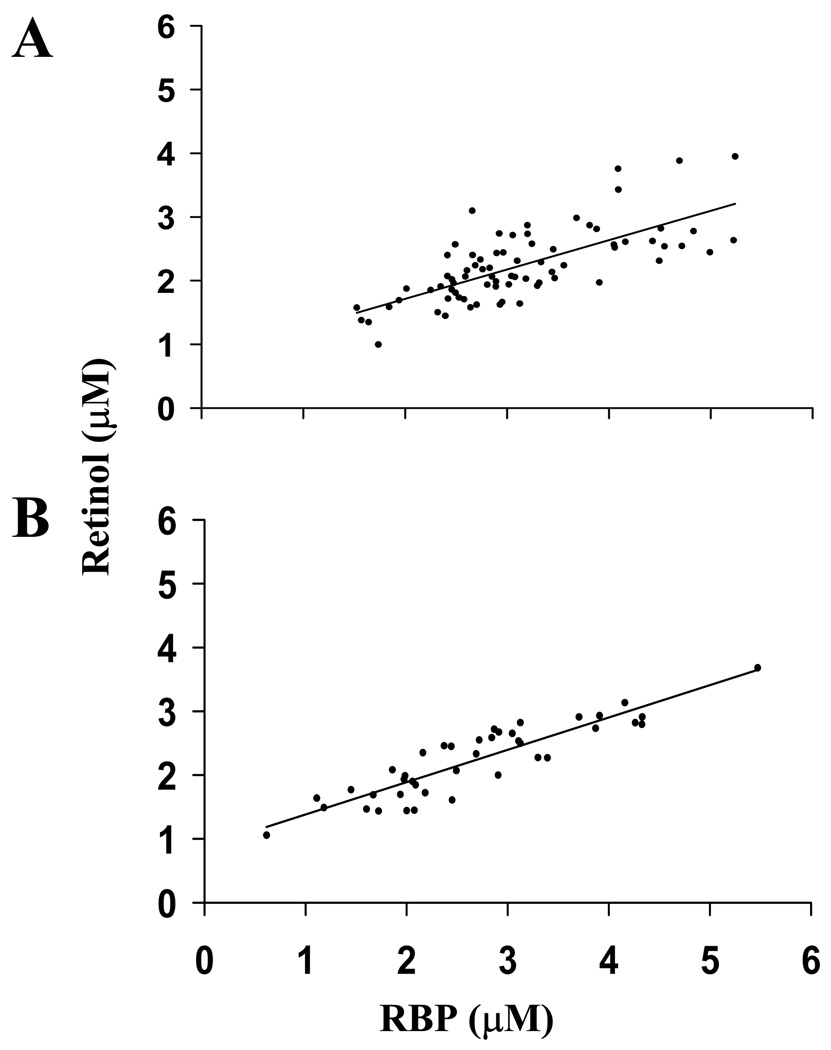
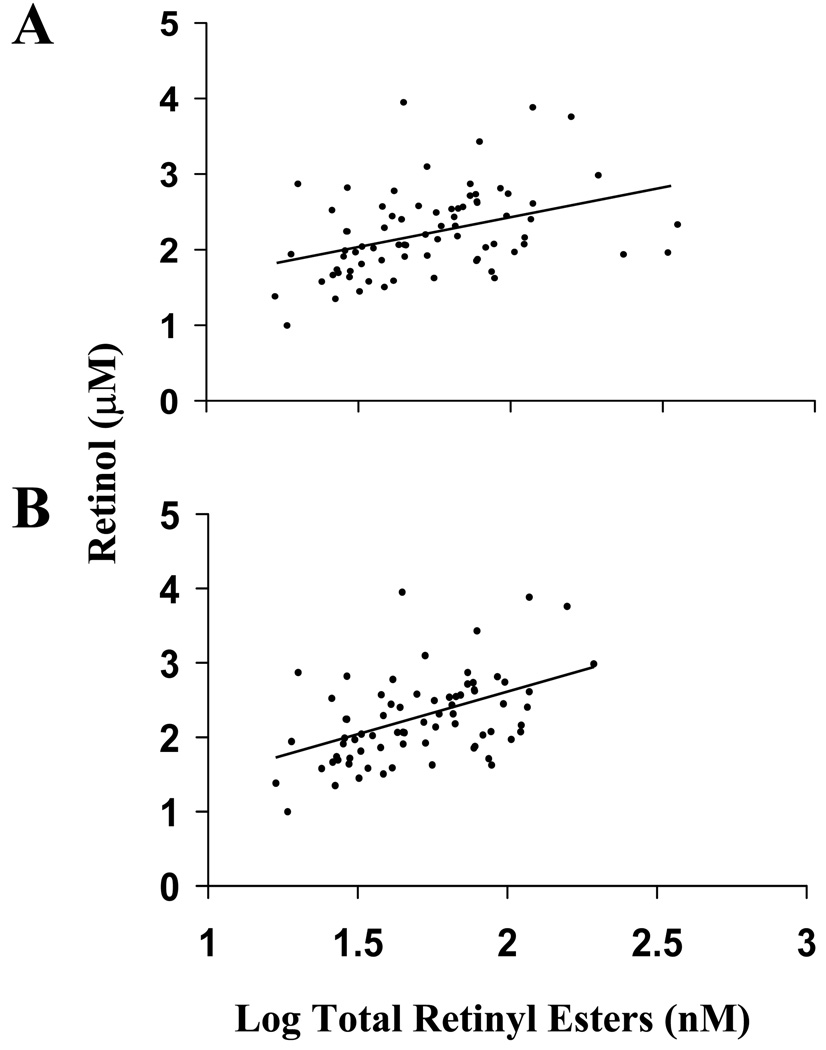
RBP and retinol were highly correlated in simple models in both the obese and nonobese groups (Fig. 1). Correlations were significantly different based on obesity status (P = 0.0035), according to Fisher’s r-to-Z transformation test. In obese subjects, retinol and log total retinyl ester concentration were associated in simple models (Fig. 2). The association was strengthened after removal of three potentially hypervitaminotic A subjects (retinyl ester ≥10% total retinol) from the sample. The association remained significant after adjustment for age, log VLDL-C and LDL-C, the major fasting retinyl ester carriers. Stepwise MLR analysis applying backward selection demonstrated that serum retinol concentration (P < 0.001), log VLDL-C (P < 0.002) and LDL-C (P < 0.05) were the only significant independent variables to explain log total retinyl ester concentration.
Figure 1.
Correlation between serum retinol-binding protein (RBP) and retinol in fasted obese adults (n = 76) (r = 0.71, P < 0.0001) (A) and fasted nonobese adults (n = 41) (r = 0.90, P < 0.0001) (B). Correlations were significantly different by Fisher’s r-to-Z transformation (P = 0.0035).
Figure 2.
Correlation between log total serum retinyl ester concentration and retinol in fasted obese adults including potentially hypervitaminotic subjects (>10% of total vitamin A as retinyl esters) (n = 76) (r = 0.34, P < 0.003) (A) and excluding potentially hypervitaminotic subjects (n = 73) (r = 0.49, P < 0.0001) (B).
The concentration of apo-RBP was determined by subtracting the serum retinol concentration from the total RBP concentration. The values for obese and nonobese individuals were 0.90 ± 0.62 and 0.44 ± 0.56 µM, respectively. These means are different (P < 0.001) and imply that adipose tissue may be secreting RBP that is not bound to retinol into the general circulation.
Discussion
Serum RBP is predominantly synthesized by hepatocytes, but extrahepatic tissues contain appreciable RBP mRNA (35). Notably, the kidney and adipocytes contain approximately 10 and 20% that of the liver RBP mRNA, respectively (35, 36). Yang et al. (2) observed that RBP mRNA expression increased in adipose tissue, but not in liver, of insulin-resistant adipose-Glut4−/− mice, suggesting a potential role for adipocyte-derived RBP as a signal in obesity-induced insulin resistance. Soon after, serum RBP, regardless of origin, joined the ranks of leptin, adiponectin, resistin, visfatin, and others as the newest member of the adipokine family. Elevated serum RBP concentrations have since been implicated in a host of comorbidities such as Polycystic Ovary Syndrome (37, 38), gestational diabetes (39), inflammation (7, 34), cardiovascular disease (10), metabolic syndrome (6), and cancer (40). However, much like past associations found between serum retinol concentrations and cancer incidence (41, 42), RBP associations with markers of chronic disease have not been consistently observed. For every report of elevated RBP concentrations in obesity or insulin resistance (3–8), there is a report to the contrary (9–15). A partial explanation for equivocal findings may be the past neglect of serum retinol as a covariate.
In the current study, we found that RBP was strongly associated with retinol and weakly or not at all associated with BMI and measures of insulin resistance in fasted obese adults. BMI was not associated with RBP in simple or multivariate models in the obese group, but when obese and nonobese subjects were combined, we saw a weak association after adjusting for age and retinol. RBP was associated positively with insulin and HOMA-IR and negatively with QUICKI after adjustment for age and retinol in the obese group. These associations were far weaker compared to the association between RBP and retinol, based on correlation strength and ability to survive Bonferroni correction. However, simple surrogate measures of insulin resistance such as HOMA and QUICKI lack the sensitivity of the more invasive hyperinsulinemic euglycemic glucose clamp technique, generally considered the gold standard for assessing insulin sensitivity (27). Future studies should utilize more direct measures of insulin sensitivity such as the glucose clamp or intravenous glucose tolerance testing to compare the association strength of RBP and retinol to that of RBP and magnitude of insulin resistance.
There is evidence to suggest that RBP expression is upregulated in obesity. In our study, the mean serum RBP concentration of the obese group was higher than that of the nonobese group despite identical mean serum retinol concentrations. Elevated RBP concentrations observed in obese subjects may be explained by greater amounts of RBP in circulation derived from adipose tissue (2, 3). Perhaps enough retinol is not available in adipose to bind to the RBP and therefore, apo-RBP is released from the adipose diluting the holo-RBP in plasma. Biochemically, vitamin A researchers have referred to apo-RBP as the protein without retinol bound to it. It is unknown if the elevated adipose-derived serum apo-RBP we and others have observed in obese individuals is a direct contributor to insulin resistance. Alternatively, apo-RBP from adipose may transport an unidentified ligand that is responsible for mediating insulin signaling. There is presently no data to favor one of these hypotheses over the other, thus future investigations are needed to elucidate the mechanism behind the proposed causative role of RBP in insulin resistance.
Retinol:RBP has been in use for decades in the vitamin A field as a more informative variable than either component alone (19). When serum retinol concentrations were taken into account with the use of retinol:RBP, the difference between obese and nonobese subjects was highly significant. In light of this evidence, we propose that retinol:RBP may provide greater utility than RBP concentrations alone in the characterization of abnormal RBP regulation in obese adults. At the very least, we recommend that serum retinol concentration be included as a covariate in association analyses involving RBP, in order to avoid spurious associations. In our analysis, RBP was significantly associated with VLDL-C, triacylglycerol, hematocrit, and retinyl ester concentrations, but after adjustment for retinol, none of these associations remained (Table 3).
RBP was more strongly correlated with retinol in nonobese subjects than in obese subjects (Fig. 1). This supports the notion that abnormal RBP regulation results from obesity-related factors apart from vitamin A status. Utilization of retinol:RBP could help isolate such influences on RBP regulation. In nonobese adults, retinol:RBP has been reported as 0.91 ± 0.24 (43). This is identical to the ratio we observed in nonobese subjects (i.e., 0.90 ± 0.22). In obese diabetic adults with and without microalbuminuria, retinol:RBP was significantly lower than in healthy controls (44). In overweight and obese children and adolescents, retinol:RBP was lower compared to lean controls (6). However, values for this ratio have not been reported in nondiabetic obese adults. We observed a significantly lower retinol:RBP in obese subjects without diabetes (0.73 ± 0.13) compared to age-matched nonobese subjects.
In addition to the retinol-RBP complex, vitamin A is found in circulation as retinyl esters in lipoproteins, particularly VLDL-C and LDL-C (18). However, retinyl esters comprise a small percentage of the total vitamin A in circulation and thus are often overlooked (19). Because measurement of post-prandial vitamin A circulation is complicated by chylomicron transport of newly ingested retinyl esters, we collected serum from fasted subjects. We found a significant correlation between total retinyl ester concentration and retinol, independent of VLDL-C, and LDL-C concentrations, suggesting a possible connection between the homeostatic control mechanisms responsible for maintaining serum retinol concentration and the metabolism of circulating retinyl esters. The correlation was strengthened by the removal of potentially hypervitaminotic subjects from the analysis. This observation supports the hypothesis that elevated circulating retinyl esters (>10% of total retinol) are a result of abnormal vitamin A metabolism due to hypervitaminosis A, usually caused by excessive intake. It is interesting to note that total retinyl ester concentration was independently associated with retinol, VLDL-C and LDL-C after backward selection of the best multivariate model to explain total retinyl ester concentration. To the extent of our knowledge, such a relationship has not been reported previously in fasted obese adults.
A novel therapeutic potential has been suggested to lower plasma RBP concentrations in patients with and at risk for type 2 diabetes (45). As etiological partners in the pathogenesis of the metabolic syndrome, obesity and insulin resistance are intimately linked, identifying obese individuals as potential candidates for therapeutic treatment with RBP-lowering drugs. It is unknown how such treatment strategies to lower RBP will affect vitamin A circulation and status. The ramifications, which may include altered vitamin A utilization and changes in liver reserves, of such a drug would need to be carefully monitored and evaluated. Significant weight loss by morbidly obese individuals through stomach banding (5), and by obese adolescents through lifestyle modification (7), resulted in decreased serum RBP concentrations. If the extra apo-RBP diluting the plasma pool is adipose derived, decreasing % body fat through diet and exercise may increase retinol:RBP and such possibilities should be investigated.
Acknowledgments
The authors thank Francisco Arredondo, Craft Technologies, for his assistance with the development of the RBP ELISA assay, as well as Ting-Li Lin and Peter Crump, UW-Madison College of Agriculture and Life Sciences Statistical Consulting Service, for statistical analysis consultation. This work partially fulfills the Ph.D. requirements for JPM.
This research was supported by the National Research Initiative of the USDA Cooperative State Research, Education and Extension Service, grant number 2003-35200-05377 and NIHNIDDK 61973.
Footnotes
Presented in part as an oral presentation at the 2008 Experimental Biology meeting: Mills JP, Furr HC, Tanumihardjo SA. Serum retinol binding protein is more strongly associated with retinol than measures of insulin sensitivity and BMI in fasted obese adults.
References
- 1.Tamori Y, Sakaue H, Kasuga M. RBP4, an unexpected adipokine. Nat Med. 2006;12:30–31. doi: 10.1038/nm0106-30. [DOI] [PubMed] [Google Scholar]
- 2.Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–362. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- 3.Graham TE, Yang Q, Bluher M, Hammarstedt A, Ciaraldi TP, Henry RR, Wason CJ, Oberbach A, Jansson PA, Smith U, Kahn BB. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med. 2006;354:2552–2563. doi: 10.1056/NEJMoa054862. [DOI] [PubMed] [Google Scholar]
- 4.Cho YM, Youn BS, Lee H, Lee N, Min SS, Kwak SH, Lee HK, Park KS. Plasma retinol-binding protein-4 concentrations are elevated in human subjects with impaired glucose tolerance and type 2 diabetes. Diabetes Care. 2006;29:2457–2461. doi: 10.2337/dc06-0360. [DOI] [PubMed] [Google Scholar]
- 5.Haider DG, Schindler K, Prager G, Bohdjalian A, Luger A, Wolzt M, Ludvik B. Serum retinol-binding protein 4 is reduced after weight loss in morbidly obese subjects. J Clin Endocrinol Metab. 2007;92:1168–1171. doi: 10.1210/jc.2006-1839. [DOI] [PubMed] [Google Scholar]
- 6.Aeberli I, Biebinger R, Lehmann R, l’Allemand D, Spinas GA, Zimmermann MB. Serum retinol-binding protein 4 concentration and its ratio to serum retinol are associated with obesity and metabolic syndrome components in children. J Clin Endocrinol Metab. 2007;92:4359–4365. doi: 10.1210/jc.2007-0468. [DOI] [PubMed] [Google Scholar]
- 7.Balagopal P, Graham TE, Kahn BB, Altomare A, Funanage V, George D. Reduction of elevated serum retinol binding protein in obese children by lifestyle intervention: Association with subclinical inflammation. J Clin Endocrinol Metab. 2007;92:1971–1974. doi: 10.1210/jc.2006-2712. [DOI] [PubMed] [Google Scholar]
- 8.Qi Q, Yu Z, Ye X, Zhao F, Huang P, Hu FB, Franco OH, Wang J, Li H, Liu Y, Lin X. Elevated retinol-binding protein 4 levels are associated with metabolic syndrome in Chinese people. J Clin Endocrinol Metab. 2007;92:4827–4834. doi: 10.1210/jc.2007-1219. [DOI] [PubMed] [Google Scholar]
- 9.Janke J, Engeli S, Boschmann M, Adams F, Bohnke J, Luft FC, Sharma AM, Jordan J. Retinol-binding protein 4 in human obesity. Diabetes. 2006;55:2805–2810. doi: 10.2337/db06-0616. [DOI] [PubMed] [Google Scholar]
- 10.von Eynatten M, Lepper PM, Liu D, Lang K, Baumann M, Naworth PP, Bierhaus A, Dugi KA, Heemann U, Allolio B, Humpert PM. Retinol-binding protein 4 is associated with components of the metabolic syndrome, but not with insulin resistance, in men with type 2 diabetes or coronary artery disease. Diabetologia. 2007;50:1930–1937. doi: 10.1007/s00125-007-0743-8. [DOI] [PubMed] [Google Scholar]
- 11.Shea J, Randell E, Vasdev S, Wang PP, Roebothan B, Sun G. Serum retinol-binding protein 4 concentrations in response to short-term overfeeding in normal-weight, overweight, and obese men. Am J Clin Nutr. 2007;86:1310–1315. doi: 10.1093/ajcn/86.5.1310. [DOI] [PubMed] [Google Scholar]
- 12.Lewis JG, Shand BI, Frampton CM, Elder PA. An ELISA for plasma retinol-binding protein using monoclonal and polyclonal antibodies: plasma variation in normal and insulin resistant subjects. Clin Biochem. 2007;40:828–834. doi: 10.1016/j.clinbiochem.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 13.Promintzer M, Krebs M, Todoric J, Luger A, Bischof MG, Nowotny P, Wagner O, Esterbauer H, Anderwald C. Insulin resistance is unrelated to circulating retinol binding protein and protein C inhibitor. J Clin Endocrinol Metab. 2007;92:4306–4312. doi: 10.1210/jc.2006-2522. [DOI] [PubMed] [Google Scholar]
- 14.Ziegelmeier M, Bachmann A, Seeger J, Lossner U, Kratzsch J, Bluher M, Stumvoll M, Fasshauer M. Serum levels of adipokine retinol-binding protein-4 in relation to renal function. Diabetes Care. 2007;30:2588–2592. doi: 10.2337/dc07-0275. [DOI] [PubMed] [Google Scholar]
- 15.Broch M, Vendrell J, Ricart W, Richart C, Fernandez-Real JM. Circulating retinol-binding protein-4, insulin sensitivity, insulin secretion, and insulin disposition index in obese and nonobese subjects. Diabetes Care. 2007;30:1802–1806. doi: 10.2337/dc06-2034. [DOI] [PubMed] [Google Scholar]
- 16.Dixon JL, Goodman DS. Studies on the metabolism of retinol-binding protein by primary hepatocytes from retinol-deficient rats. J Cell Physiol. 1987;130:14–20. doi: 10.1002/jcp.1041300104. [DOI] [PubMed] [Google Scholar]
- 17.Tanumihardjo SA. Assessing vitamin A status: past, present and future. J Nutr. 2004;134:290S–293S. doi: 10.1093/jn/134.1.290S. [DOI] [PubMed] [Google Scholar]
- 18.Krasinski SD, Cohn JS, Russell RM, Schaefer EJ. Postprandial plasma vitamin A metabolism in humans: a reassessment of the use of plasma retinyl esters as markers for intestinally derived chylomicrons and their remnants. Metabolism. 1990;39:357–465. doi: 10.1016/0026-0495(90)90249-c. [DOI] [PubMed] [Google Scholar]
- 19.Soprano DR, Blaner WS. Plasma retinol-binding protein. In: Sporn MB, Roberts AB, Goodman DS, editors. The retinoids: biology, chemistry, and medicine. New York: Raven Press; 1994. pp. 257–282. [Google Scholar]
- 20.Rosales FJ, Topping JD, Smith JE, Shankar AH, Ross AC. Relation of serum retinol to acute phase proteins and malarial morbidity in Papua New Guinea children. Am J Clin Nutr. 2000;71:1582–1588. doi: 10.1093/ajcn/71.6.1582. [DOI] [PubMed] [Google Scholar]
- 21.Penniston KL, Weng N, Binkley N, Tanumihardjo SA. Serum retinyl esters are not elevated in postmenopausal women with and without osteoporosis whose preformed vitamin A intakes are high. Am J Clin Nutr. 2006;84:1350–1356. doi: 10.1093/ajcn/84.6.1350. [DOI] [PubMed] [Google Scholar]
- 22.Penniston KL, Tanumihardjo SA. The acute and chronic toxic effects of vitamin A. Am J Clin Nutr. 2006;83:191–201. doi: 10.1093/ajcn/83.2.191. [DOI] [PubMed] [Google Scholar]
- 23.Molldrem KL, Li J, Simon PW, Tanumihardjo SA. Lutein and beta-carotene from lutein-containing yellow carrots are bioavailable in humans. Am J Clin Nutr. 2004;80:131–136. doi: 10.1093/ajcn/80.1.131. [DOI] [PubMed] [Google Scholar]
- 24.Horvitz MA, Simon PW, Tanumihardjo SA. Lycopene and beta-carotene are bioavailable from lycopene ‘red’ carrots in humans. Eur J Clin Nutr. 2004;58:803–811. doi: 10.1038/sj.ejcn.1601880. [DOI] [PubMed] [Google Scholar]
- 25.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DR, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 26.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–2410. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 27.Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294:E15–E26. doi: 10.1152/ajpendo.00645.2007. [DOI] [PubMed] [Google Scholar]
- 28.Chen H, Sullivan G, Quon MJ. Assessing the predictive accuracy of QUICKI as a surrogate index for insulin sensitivity using a calibration model. Diabetes. 2005;54:1914–1925. doi: 10.2337/diabetes.54.7.1914. [DOI] [PubMed] [Google Scholar]
- 29.Sowell AL, Huff DL, Yeager PR, Caudill SP, Gunter EW. Retinol, alpha-tocopherol, lutein/zeaxanthin, beta-cryptoxanthin, lycopene, alpha-carotene, trans-beta-carotene, and four retinyl esters in serum determined simultaneously by reversed-phase HPLC with multiwavelength detection. Clin Chem. 1994;40:411–416. [PubMed] [Google Scholar]
- 30.Tchum SK, Tanumihardjo SA, Newton S, de Benoist B, Owusu-Agyei S, Arthur FK, Tetteh A. Evaluation of vitamin A supplementation regimens in Ghanaian postpartum mothers with the use of the modified-relative-dose-response test. Am J Clin Nutr. 2006;84:1344–1349. doi: 10.1093/ajcn/84.6.1344. [DOI] [PubMed] [Google Scholar]
- 31.Mills JP, Penniston KL, Tanumihardjo SA. Extra-hepatic vitamin A concentrations in captive rhesus (Macaca mulatta) and marmoset (Callithrix jacchus) monkeys fed excess vitamin A. Int J Vitam Nutr Res. 2005;75:126–132. doi: 10.1024/0300-9831.75.2.126. [DOI] [PubMed] [Google Scholar]
- 32.Erhardt JG, Estes JE, Pfeiffer CM, Biesalski HK, Craft NE. Combined measurement of ferritin, soluble transferrin receptor, retinol binding protein, and C-reactive protein by an inexpensive, sensitive, and simple sandwich enzyme-linked immunosorbent assay technique. J Nutr. 2004;134:3127–3132. doi: 10.1093/jn/134.11.3127. [DOI] [PubMed] [Google Scholar]
- 33.Graham TE, Wason CJ, Bluher M, Kahn BB. Shortcomings in methodology complicate measurements of serum retinol binding protein (RBP4) in insulin-resistant human subjects. Diabetologia. 2007;50:814–823. doi: 10.1007/s00125-006-0557-0. [DOI] [PubMed] [Google Scholar]
- 34.Yao-Borengasser A, Varma V, Bodles AM, Rasouli N, Phanavanh B, Lee MJ, Starks T, Kern LM, Spencer HJ, 3rd, Rashidi AA, McGehee RE, Jr, Fried SK, Kern PA. Retinol binding protein 4 expression in humans: Relationship to insulin resistance, inflammation, and response to pioglitazone. J Clin Endocrinol Metab. 2007;92:2590–2597. doi: 10.1210/jc.2006-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soprano DR, Soprano KJ, Goodman DS. Retinol-binding protein messenger RNA levels in the liver and in extrahepatic tissues of the rat. J Lipid Res. 1986;27:166–171. [PubMed] [Google Scholar]
- 36.Tsutsumi C, Okuno M, Tannous L, Piantedosi R, Allan M, Goodman DS, Blaner WS. Retinoids and retinoid-binding protein expression in rat adipocytes. J Biol Chem. 1992;267:1805–1810. [PubMed] [Google Scholar]
- 37.Tan BK, Chen J, Lehnert H, Kennedy R, Randeva HS. Raised serum, adipocyte, and adipose tissue retinol-binding protein 4 in overweight women with polycystic ovary syndrome: Effects of gonadal and adrenal steroids. J Clin Endocrinol Metab. 2007;92:2764–2772. doi: 10.1210/jc.2007-0091. [DOI] [PubMed] [Google Scholar]
- 38.Hahn S, Backhaus M, Broecker-Preuss M, Tan S, Dietz T, Kimmig R, Schmidt M, Mann K, Janssen OE. Retinol-binding protein 4 levels are elevated in polycystic ovary syndrome women with obesity and impaired glucose metabolism. Eur J Endocrinol. 2007;157:201–207. doi: 10.1530/EJE-07-0143. [DOI] [PubMed] [Google Scholar]
- 39.Chan TF, Chen HS, Chen YC, Lee CH, Chou FH, Chen IJ, Chen SY, Jong SB, Tsai EM. Increased serum retinol-binding protein 4 concentrations in women with gestational diabetes mellitus. Reprod Sci. 2007;14:169–174. doi: 10.1177/1933719106298407. [DOI] [PubMed] [Google Scholar]
- 40.Patz EF, Jr, Campa MJ, Gottlin EB, Kusmartseya I, Guan XR, Herndon JE., 2nd Panel of serum biomarkers for the diagnosis of lung cancer. J Clin Oncol. 2007;25:5578–5583. doi: 10.1200/JCO.2007.13.5392. [DOI] [PubMed] [Google Scholar]
- 41.Harris RW, Forman D, Doll R, Vessey MP, Wald NJ. Cancer of the cervix uteri and vitamin A. Br J Cancer. 1986;53:653–659. doi: 10.1038/bjc.1986.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sato R, Helzlsouer KJ, Alberg AJ, Hoffman SC, Norkus EP, Comstock GW. Prospective study of carotenoids, tocopherols, and retinoid concentrations and the risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2002;11:451–457. [PubMed] [Google Scholar]
- 43.Schweigert FJ, Steinhagen B, Raila J, Siemann A, Peet D, Buscher U. Concentrations of carotenoids, retinol and alpha-tocopherol in plasma and follicular fluid of women undergoing IVF. Hum Reprod. 2003;18:1259–1264. doi: 10.1093/humrep/deg249. [DOI] [PubMed] [Google Scholar]
- 44.Raila J, Henze A, Spranger J, Mohlig M, Pfeiffer AFH, Schweigert FJ. Microalbuminuria is a major determinant of elevated plasma retinol-binding protein 4 in type 2 diabetic patients. Kidney Int. 2007;72:505–511. doi: 10.1038/sj.ki.5002372. [DOI] [PubMed] [Google Scholar]
- 45.Takebayashi K, Suetsugu M, Wakabayashi S, Aso Y, Inukai T. Retinol binding protein-4 levels and clinical features of type 2 diabetes patients. J Clin Endocrinol Metab. 2007;92:2712–2719. doi: 10.1210/jc.2006-1249. [DOI] [PubMed] [Google Scholar]