Abstract
Monkeys separated from their mothers soon after birth and raised with peers display many disturbances in emotional behavior that are similar to human mood and anxiety disorders. In addition to emotional disturbances, both mood and anxiety disorders are often characterized by disruptions in normal sleep-wake cycles, a behavior that has not been well characterized in adversely-reared non-human primates. Because polysomnographic measures are difficult to obtain in unrestrained monkeys we used 24-hour actigraphy measures to assess probable sleep-wake patterns in juvenile nursery- and mother-reared rhesus macaques (Macaca mulatta, N=16) over several days in the home cage. In addition we assayed plasma cortisol in the morning, afternoon, and evening. Relative to mother-reared (MR) monkeys, actigraphic algorhithms indicated that nursery-reared (NR) animals had shorter durations of nocturnal sleep, earlier morning waking, and longer periods of sleep during the active period, specifically in the mid morning. No shift in diurnal patterns of cortisol was observed, but NR animals displayed an overall elevation in cortisol. Finally a significant interaction was found between cortisol and actigraphic determination of sleep efficiency in the two groups. A strong positive relationship (r2 > 0.8) was found between mean cortisol levels and sleep efficiency for the MR monkeys, but a significant negative relationship was found between these same variables for the NR monkeys, indicating a fundamentally different relationship between waking cortisol and actigraphy patterns in these two groups.
Keywords: circadian, sleep, sleep-wake cycle, cortisol, hypothalamic-pituitary-adrenal axis, rearing, mood, development
1. INTRODUCTION
Dysfunctional social and emotional behavior has been observed in many species, including both nonhuman primates and humans, following perturbed mother-infant relationships (Caldji et al., 2000; Levine, 2001; Sanchez et al., 2001; Rutter and O’Connor, 2004). In non-human primates, young monkeys removed from their mothers and reared with peers (NR) often develop an emotional profile that can be characterized as hypervigilant. This pattern includes a potentiated acoustic startle response, altered function of the hypothalamic-pituitary-adrenal (HPA) axis, more aggression in social interactions, and a general pattern of excessive fearfulness (Parr et al., 2002; Sanchez, 2006; Winslow, 2005). NR monkeys also display reductions in affiliative behavior, lowered dominance rank, increased alcohol consumption, and changes in central serotonin and amygdala activity (Suomi, 1991; Higley et al., 1996; Bastian et al., 2003; Sabatini et al., 2007) as compared to animals that remained with their mothers and larger social group during the same period of development (MR). The emotional profile of NR monkeys shares many features with the patterns of hypervigilance and hyper-responsivity to stress that is often seen in human mood disorders (Pine et al, in press), and suggest that peer rearing may be a good model for key features of human mood and anxiety disorders.
While NR monkeys display disruptions in many processes characteristic of mood and anxiety disorders, alterations in sleep-wake patterns represent one area in which disturbances are commonly observed in human psychopathology but remain relatively unexplored in NR monkeys. Sleep disruption is one of the most common symptoms of mood and anxiety disorders. Sleep problems are among the diagnostic criteria for unipolar and bipolar depression, posttraumatic stress disorder, and generalized anxiety disorder (APA, 1994). Sleep disorders are also frequently observed in panic disorder and are common in many other mood dysphoric states (Mellman, 2006; Peterson and Benca, 2006), and such disturbances take many forms (Peterson and Benca, 2006). Moreover, such perturbations have been linked to disruption in the HPA axis which may be overactive or dysregulated in mood and anxiety disorders (Steiger, 2007). Finally, sleep disturbance might also represent a risk factor in healthy individuals for subsequent onset of depression (Peterson and Benca, 2006), increasing the importance of work in non-human primate models in which longitudinal studies can be performed under tightly regulated conditions.
The sleep-wake patterns of juvenile NR monkeys have not been well characterized, however. Only two studies (Reite and Short, 1978; Kaemingk and Reite, 1987) have examined sleep patterns in adversely-reared nonhuman primates and both focused on nighttime sleep disturbances during the infancy period. These studies did show that NR monkeys and monkeys experiencing repeated maternal separations have more nighttime arousals and less REM sleep than is typical of MR animals in a social group, however no direct comparisons between NR and MR monkeys were made, and data were not systematically collected during the daytime hours, making circadian patterns difficult to assess.
In the present study, because of our desire to obtain measures across the 24h cycle in animals from their home environment we used actigraphy to get a continuous measure of motor activity in juvenile nursery- and mother-reared rhesus macaques across several testing days. Morning, afternoon, and evening cortisol samples were also obtained. Although actigraphic determinants of motor activity do not provide the same definitive measure of sleep as polysomnographic indices, algorithms of motor activity across time have been developed and validated with polysomnography in human models and these models are thought to provide a good proxy for sleep measures.
We predicted that relative to MR monkeys, juvenile NR rhesus monkeys would display disruptions in the daily pattern of motor activity reflective of sleep–wake disturbances and exhibit alterations in the diurnal pattern of cortisol secretion. These disruptions were expected given that a.) preliminary signs of abnormal sleep patterns already have been observed in NR infant monkeys (Kaemingk and Reite, 1987); b.) circadian cortisol secretion and central serotonergic functioning, both previously shown to be disrupted by NR, play an important role in sleep-wake regulation (Zajicek et al., 1997; Mehlman et al., 2000; Steiger, 2002; Markov and Goldman, 2006); and c.) many patterns of physiological and behavioral dysfunction tend to intensify through development and persist through adulthood (Shannon et al., 2005; Sanchez, 2006; Shannon et al., 1998; Caspi et al., 2003; Ichise et al., 2006; Jans et al., 2007).
2. METHODS
2.1 Animals & Rearing
Sixteen male rhesus monkeys (Macaca mulatta) from two annual birth cohorts (eight animals per cohort) were randomly assigned to either a nursery- or mother-rearing condition at birth. Nursery-reared (NR) animals were separated from their mothers within 48h of birth. Infants were housed in incubators (50 × 40 × 42 cm) and cared for daily by human caregivers for the first 14 days of life. At 2 weeks of age monkeys were transferred from the incubator to a larger cage (74 × 65 × 84 cm) until they were 4–6 weeks of age, at which point they were transferred to group housing with three other nursery-reared animals of approximately the same age. At 6–10 months of age NR monkeys were then moved into a large indoor-outdoor housing facility that contained both adults and juveniles that had been raised under semi-natural conditions. Finally, at approximately 12 months of age pairs of NR monkeys were removed from the social group and pair-housed in indoor cages for the duration of this study. In contrast, mother-reared (MR) monkeys remained with their mothers and an extended social group in 2.44 × 3.05 × 2.21m indoor-outdoor runs until 1 year of age, at which point they were moved to a separate cage and paired with another MR animal. For a more detailed description of rearing procedures, see Shannon et al. (1998).
Permanent home cages were standard wire mesh stainless steel primate housing (4.3 ft3). Animals had ad libitum access to water, and were fed daily with primate chow supplemented with fresh fruit and vegetables. Cages were cleaned daily between 7–9 AM and veterinary care was provided as needed. Throughout the study monkeys were pair housed in a room with 12–16 other monkeys that were both visible and audible. The home rooms were maintained at ~74°F and 35% humidity and were maintained on a 12h light-dark cycle with lights on at 0600h EST. All animal care and experimental methods adhered to the standards specified by the NIH guidelines for Animal Care and Use, and all experimental procedures were approved by the NIMH animal care and use committee. All efforts were made to minimize animal suffering and to reduce the number of animals used.
2.2 Actigraphy
Small lightweight activity monitors (Mini-Mitter, Bend, OR) were attached to the animals’ primate collars at approximately 23 months of age (range 20–25mos). Subjects were fully habituated to the collars several months before testing. The activity monitors (Actiwatch-64) consisted of accelerometers which detected displacement in any direction and generated activity counts. Sampling frequency was 32 Hz and data points were acquired in 15-second epochs. Monitors remained on the collars for 9 days, but due to battery failure and interference by the animals, the actual length of recorded activity ranged between 4 and 9 days.
Actigraphy is widely used in human sleep research and has been validated with other measures in several reports (Ancoli-Israel et al., 2003; Kushida et al., 2001; Oakley, 1997; Sadeh et al., 1995, Sadeh and Acebo, 2002). Generally agreement rates between actigraphic assessements and traditional polysomnographic (PSG) measures are above 90 percent and considered a valid index of human sleep (Sadeh et al., 1995; Kushida et al. 2001). Although no studies have yet reported validation of actigraphy with PSG recordings in monkeys, several studies have used actigraphy to measure 24h activity in nonhuman primates as well (Zhdanova et al., 2002; Sri Kantha and Suzuki, 2006) and comparison with video-taped behavior has found good correlation between actigraphic measures and whole body movements in rhesus monkeys (Papailiu, 2007). This indicates that actigraphic methods are a good way of determining gross behavioral states such as mobility and immobility in rhesus monkeys and when combined with scoring algorithms provide a reasonable estimate of sleep and wake states. However small motor activity not involving the entire body, such as isolated head, neck, or arm movements, is not well reflected by actigraphy (Papailiu, 2007), and it is possible that monkeys performing such sedentary movement may be falsely coded as sleep leading to inaccurately long reported sleep times. Similar problems have been reported in some human populations (Kushida et al., 2001; Sadeh and Acebo, 2002). Therefore, although we refer to actigraphically determined measures here as sleep all sleep measures reported hereon should be regarded as ‘probable sleep.’
After collection, actigraphy data was binned into 10min intervals and analyzed for rearing differences in raw activity counts across time. Data was also interpolated with algorithms contained within the Mini Mitter software (Actiware ® Software, Version 5.0), which generated sleep-wake indices for each animal: a categorical definition of each 15s epoch as either asleep or awake; and a categorization of the consecutive period of nighttime sleep (sleep or wake epochs occur both in and outside of the sleep period – see below). The epoch based sleep categorization was used to determine measures of total sleep time and sleep percent. The phasic sleep measure was used identify a night-time sleep period by measuring sleep onset and sleep offset phases of each animal.
The epoch based sleep-wake categorization was based on the temporally smoothed activity count in the focal epoch. If the activity count of the focal epoch exceeded the threshold that epoch was scored as asleep otherwise it was scored as awake. The temporal smoothing consisted of the focal epoch plus a weighted average of activity in the surrounding 16 epochs (8 epochs on either side), with more weight given to counts immediately preceding or following the focal epoch. Specifically, the eight epochs immediately before and after the focal epoch were multiplied by 1/5, the eight counts at either end of the 16-epoch section were multiplied by 1/25, and the activity count of the epoch in question was multiplied by four. This smoothing algorithm and sleep threshold determination is a part of the Mini Mitter software package and has been validated in prior human studies (Oakley, 1997; Kushida et al., 2001). The Mini Mitter software was set to an automatic wake-threshold calculation (medium level or 40 counts).
The sleep period is a more rigorous categorization of the phasic state of night-time sleep, and was calculated using the epoch-based sleep-wake categorization. Sleep period onset was defined as the first epoch of a section with at least 10 consecutive minutes with all but one epoch (15s) categorized as completely immobile (activity count = 0). Similarly, sleep period termination (i.e. the wake onset) was defined as the last epoch of the last section of 10 consecutive minutes with all by one epoch scored as immobile. This method of sleep categorization has also been validated in previous studies (Oakley, 1997).
Multi-day actigraphy data were averaged within each subject across days to generate a mean sleep-wake profile of each animal for the entire 24h day, the 12h lights-on active period, the 12h lights-off dark period, and the sleep period (sleep onset to sleep end of individual subjects), using the Mini Mitter software. The active period was further divided into a morning period (0600h–1200h) and an afternoon/evening period (1200h–1800h) for post-hoc testing. Within each analytic period, data were analyzed for (1) total sleep time and (2) total motor activity (activity count value). Within the sleep period time, data were also analyzed for (3) entire sleep period length (time from sleep period onset to offset), (4) sleep efficiency defined as total time asleep/sleep period length (note that this definition is different from the ‘sleep efficiency’ measure output by the Mini Mitter software and which we deemed more appropriate for research animals), (5) number of sleep bouts per hour, an indication of sleep quality (6) an early-wake index, defined as the number of minutes between the end of the sleep period and time lights are turned on, and (7) sleep-onset latency defined as the amount of time between lights off (1800h) and sleep onset. These measures (sleep time, activity counts, sleep efficiency, sleep bouts/h, early-wake index, and sleep-onset latency) were output directly from the Mini Mitter software for each animal across all days of collection.
The variability of these measures within subject and across days was analyzed in order to obtain an indication of their consistency or stability. The coefficient of variation (CV), the standard deviation divided by the mean, was obtained for each measure across repeated testing days. The stability for each actigraphic measure was then calculated as one minus the CV, The stability measure was obtained for each subject and a mean for each rearing group were then calculated across subject means.
2.3 Plasma Cortisol
Within 4 weeks (range 2–8wks) of actigraphy testing, plasma cortisol levels were measured at 0600h, 1200h, and 1800h in all monkeys. To ensure that blood samples did not reflect stress from handling, each sample was collected on a different day, and plasma samples were not collected during actigraphy testing. Animals were briefly anesthetized with .05ml/kg ketamine (IM) in their home cages and brought into a separate room for blood drawing. Within 15 minutes of contact with experimenters (average of 8min), blood was collected from the femoral vein using an EDTA-coated vacutainer collection tubes. Although both ketamine injection and handling may have led to slightly elevated cortisol levels, we have developed this method to obtain a measure of cortisol levels while minimizing effects of stress (Sanchez et al, 2005). Aliquots were placed on ice until centrifugation then stored at −80°C until assayed. Cortisol levels were measured using a commercially available cortisol ELISA (Calbiotech™; Catalog No. CO103S). Serum samples were undiluted and analyzed in duplicate in a single assay. Intra-assay coefficient of variation was 4.42%. Plasma cortisol levels were computed using a log-logit equation based on standards and optical density computations. Due to blood clotting, one mother-reared 0600h sample is absent from the dataset.
3.4 Statistical Analyses
Total raw activity counts were first binned into 10 min points (each point was a mean of the 40 15s epochs of which it was comprised) and a mean for each animal was generated across the multiple testing days. These data were then subjected to an omnibus repeated measures ANOVA with a between subjects factor for rearing and 144 repeated time points. A repeated measures ANOVA was also performed on the cortisol measures with rearing as a between subjects factor and 3 time points as repeated measures. For the cortisol data we also conducted separate planned t-tests to compare group differences at each time point because some studies have found rearing effects to have a stronger effect on morning than afternoon cortisol levels (Sanchez et al, 2006). Because of the large number of time points contained within the activity data we did not perform planned t tests on the individual time points but planned to follow up significant interaction with post-hoc analyses (significant interactions were not found). Group comparisons of all sleep parameters including sleep time, sleep efficiency, sleep bouts/h, early-wake index, and sleep-onset latency were conducted with two sample t-tests performed on the individual subject mean profiles. Relationships between sleep-wake measures and plasma cortisol levels were assessed using linear regressions. All statistics were conducted with Statistica verson 6.0 (StatSoft, Tulsa, OK), and significance was set at p<.05 throughout.
4. RESULTS
4.1 TOTAL ACTIVITY COUNTS ARE DISTRIBUTED DIFFERENTLY ACROSS 24h PERIOD IN ALL MONKEYS AND ACTIGRAPHIC MEASURES ARE RELATIVELY STABLE WITHIN SUBJECTS
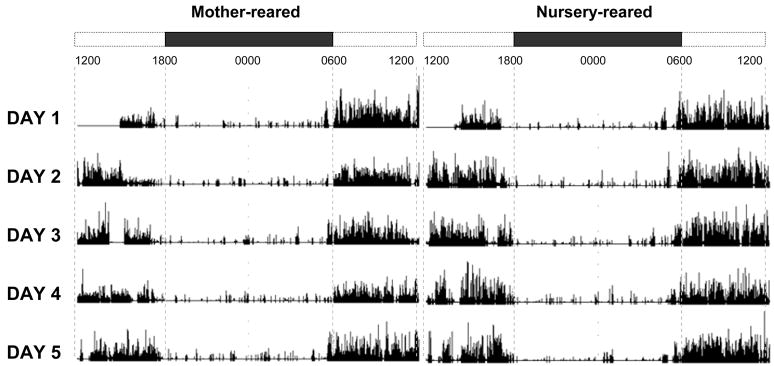
Representative actigrams of a nursery-reared and mother-reared animal can be seen in Fig 1. Total activity was measured in 15s epochs in the home cage across 4–9 consecutive days with lights off at 1800h and lights on at 0600h.
Figure 1.
Mother- and nursery-reared representative 24-hr actigrams from Mini Mitter ‘Actiware’ software. Data is representative of the raw activity counts of a single MR and NR animal. Bars at the top represent time of day starting at 1200h. Open sections represent lights-on (0600h–1800h) and black bars represent lights-off (1800h–0600h).
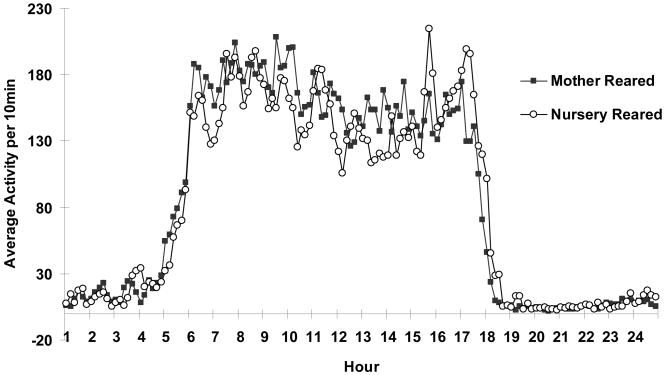
Mean daily activity measures of the 10-min activity count averages across the 24h cycle for the two groups were subjected to a repeated measures ANOVA. No main effect of rearing was observed for overall levels of motor activity collapsed across time (F1, 144=0.011, ns), and although NR monkeys generally appear to have a rightward shift in overall activity across the day, with increased activity occurring later in the day, the interaction of rearing by time did not reach significance (F1, 144=1.11, ns).
The mean, range, and stability of actigraphic sleep and wake indices for each group are detailed in Table I. The mean, minimum, maximum and standard deviation of these measures are based on averages of individual profiles. The coefficient of variation and stability (1-CV) are based on the multiple days of recording for each individual. With the exception of the early-morning wake index, all measures showed good stability within subject. The early-morning wake index did show substantial variability within subject, indicating a fair amount of day to day variability. Group differences on this measure should be viewed with caution, however the fact that averages were taken across multiple days mitigates the variability to some extent.
Table I. Descriptive statistics.
Descriptive statistics of actigraphic measures throughout the daily intervals. Lights-off is from 1800h-0600h. Lights-on is from 0600h–1800h. The morning and evening lights-on periods are from 0600h–1200h and 1200h–1800h, respectively. The stability measure was calculated as one minus the coefficient of variation calculated on all measures within each individual subject. Asterisks indicate measures for which significant group differences were found.
| Interval | Data Type | Mean | Min. | Max. | Std. Dev | CV % | Stability | |
|---|---|---|---|---|---|---|---|---|
| Mother-reared | Daily | Activity Count * | 85.42 | 71.08 | 100.57 | 10.55 | 0.12 | 0.88 |
| Lights-on | Sleep time (min) * | 226.34 | 165.88 | 301.00 | 49.96 | 0.22 | 0.78 | |
| Daily | Sleep time (min) | 929.94 | 853.22 | 1101.50 | 88.06 | 0.09 | 0.91 | |
| Evening lights-on | Sleep time (min) | 144.75 | 104.78 | 213.91 | 38.06 | 0.26 | 0.74 | |
| Morning lights-on | Sleep time (min) * | 82.72 | 50.31 | 124.09 | 25.77 | 0.31 | 0.69 | |
| Lights-off | Sleep time (min) | 684.18 | 656.72 | 700.66 | 15.17 | 0.02 | 0.98 | |
| Sleep | Sleep time (min) * | 608.98 | 530.72 | 671.31 | 49.39 | 0.08 | 0.92 | |
| Sleep | Sleep efficiency | 97.50 | 95.32 | 99.16 | 1.42 | 0.01 | 0.99 | |
| Sleep | Early Wake (min) * | 64.38 | 8.34 | 127.31 | 41.08 | 0.64 | 0.36 | |
| Nursery-reared | Daily | Activity Count * | 85.98 | 69.58 | 98.57 | 10.65 | 0.12 | 0.88 |
| Lights-on | Sleep time (min) * | 277.08 | 218.06 | 356.84 | 48.43 | 0.17 | 0.83 | |
| Daily | Sleep time (min) | 960.10 | 895.72 | 1047.75 | 53.56 | 0.06 | 0.94 | |
| Evening lights-on | Sleep time (min) | 154.04 | 115.44 | 186.19 | 25.15 | 0.16 | 0.84 | |
| Morning lights-on | Sleep time (min) * | 123.05 | 85.44 | 179.56 | 34.24 | 0.28 | 0.72 | |
| Lights-off | Sleep time (min) | 677.94 | 661.84 | 693.53 | 10.28 | 0.02 | 0.98 | |
| Sleep | Sleep time (min) * | 580.13 | 510.78 | 639.13 | 46.44 | 0.08 | 0.92 | |
| Sleep | Sleep efficiency | 97.30 | 95.66 | 98.53 | 0.94 | 0.01 | 0.99 | |
| Sleep | Early Wake (min) * | 85.51 | 30.31 | 157.78 | 44.35 | 0.52 | 0.48 | |
4.2 ALL MONKEYS SLEEP MORE AT NIGHT THAN DURING THE DAY AND MORE IN THE LATE AFTERNOON THAN IN THE MORNING
The raw activity data were then converted to expected sleep-wake states for each 15s bin. The number and distribution of the sleep-wake states across the 24h period was then assessed for each group. Across rearing groups, probable sleep durations during the lights-off period were longer than durations during the lights-on (active) period (t30=−41.51, p<0.0001; lights-off mean 681.06min, lights-on mean 251.71min). Within the lights-on period, animals slept less in the morning than in the evening (t30=4.93, p<0.0001; morning mean 102.89min, evening mean 148.83min). The time spent sleeping across the entire day, within the lights-on and -off periods, and within morning and afternoon portions of the light cycle are listed in Table II. Of note, both the total amount of actigraphically inferred sleep observed and the distribution of sleep across the 24-hour cycle is very similar to a recently published study using telemetric PSG measures of sleep in the home cage (Hsieh et al, 2008).
Table II. T-tests of sleep time (min) during daily periods.
Mean and group comparisons of sleep time in the two groups of animals across different time intervals. The daily interval is the entire 24hr period. Lights-off is from 1800h-0600h. Lights-on is from 0600h–1800h. The morning and evening lights-on periods are from 0600h–1200h and 1200h–1800h, respectively. NS indicates a nonsignificant (p>0.05) two-sample t-test.
| Mother-reared | Nursery-reared | T-value | df | p-value | |
|---|---|---|---|---|---|
| Daily Period | 929.9 | 960.1 | −1.30 | 14 | NS |
| Lights-off Period | 684.2 | 677.9 | 1.02 | 14 | NS |
| Lights-on Period | 226.3 | 277.1 | −3.32 | 14 | <0.01 |
| Morning Lights-on Period | 82.7 | 123.0 | −3.28 | 14 | <0.01 |
| Evening Lights-on Period | 143.6 | 154.0 | −1.06 | 14 | NS |
4.3 NR MONKEYS HAVE EARLY MORNING WAKING AND SLEEP LESS DURING THE NIGHT
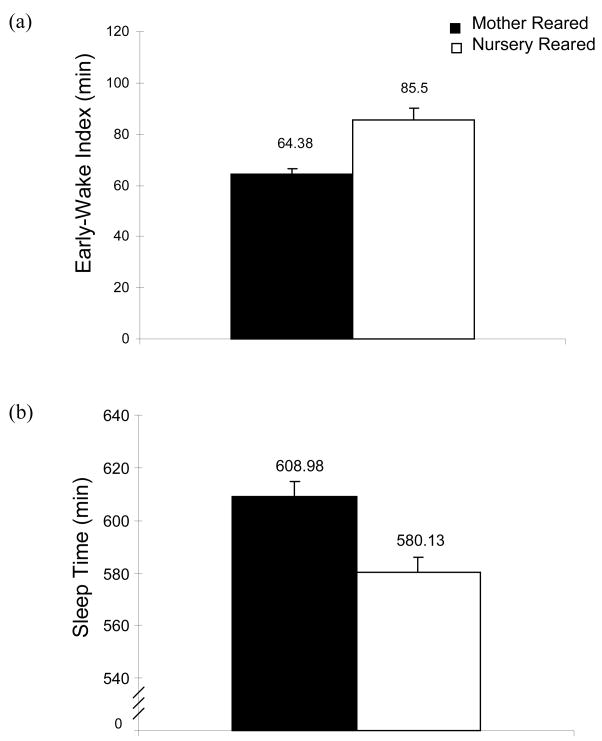
Comparison of the patterns of sleep epochs revealed several differences between the two groups. First NR monkeys transitioned out of a motor pattern indicative of the sleep period (39/40 15s epochs of complete immobility) earlier in the day than MR monkeys did. This is reflected in a significantly longer early-wake index for NR monkeys than MR monkeys. The early-wake index is a measure of the time between transition out of a period of immobility and the time ambient lights are turned on. NR animals transitioned out of sleep immobility 21.13min earlier than MR monkeys (Fig 3a, Table III, t14=-4.27, p<0.01). A reduction in immobility during the lights-off period is also reflected in findings that NR animals also had a shorter sleep period (39/40 consecutive immobile epochs) during lights off, 624.86min versus 596.48 (t14=2.81, p<0.05) and less time categorized as asleep within the sleep period than did MR animals, 608.98min versus 580.13min (Fig 3b, Table III, t14=3.44, p<0.01). However, all three measures (shorter sleep period, total sleep duration and early waking index) may be a reflection of the same underlying phenomenon in the NR group which can best be characterized as early morning motor restlessness. The length of the sleep period, the total sleep duration, the early wake index, and indices of sleep efficiency are depicted in Table III. Rearing groups did not differ significantly in sleep-onset latencies, measured as the time between lights-off and motor patterns consistent with sleep onset. Nor were any differences detected in sleep quality between rearing groups as sleep efficiency and sleep bouts/h were not significantly different between groups (see Table III).
Figure 3.
Mean early-wake index (a) & mean total sleep time during the sleep period (b) for MR and NR monkeys. The early-wake index is the elapsed time in min between waking and light-on. Sleep time is the time spent sleeping, excluding waking periods within the sleep period (time from sleep onset to end). Both two-sample t-tests are significant (p<0.05). NR and MR animals are represented by open bars and black bars, respectively.
Table III. T-tests of activity during the sleep period.
Group statistics of various sleep related indices from the actigraphic analysis are shown in Table III. . The sleep period is the time between sleep onset to sleep end (i.e., wake onset). Total sleep is the time spent sleeping, excluding periods of waking, within the sleep period. The early-wake index is the elapsed time between sleep end to lights-on in the morning. Onset latency is the time between lights-off and sleep onset at night. Sleep efficiency is the percentage of time sleeping (‘total sleep’) divided by the sleep period length. Sleep bouts/h is the frequency of sleeping within the sleep period with a sleep bout defined as one or more consecutive epochs scored as asleep. NS indicates a nonsignificant (p>0.05) two-sample t-test.
| Mother-reared | Nursery-reared | T-value | df | p-value | |
|---|---|---|---|---|---|
| Sleep Period Length (min) | 624.86 | 596.48 | 2.81 | 14 | < 0.05 |
| Total Sleep (min) | 608.98 | 580.13 | 3.44 | 14 | < 0.01 |
| Early-Wake Index (min) | 64.38 | 85.51 | −4.27 | 14 | < 0.01 |
| Onset Latency (min) | 30.8 | 38.0 | −0.84 | 14 | NS |
| Sleep efficiency (%) | 97.50 | 97.26 | 0.32 | 14 | NS |
| Sleep bouts/h | 3.03 | 2.97 | 0.12 | 14 | NS |
4.4 NR MONKEYS SLEEP MORE DURING THE MORNING
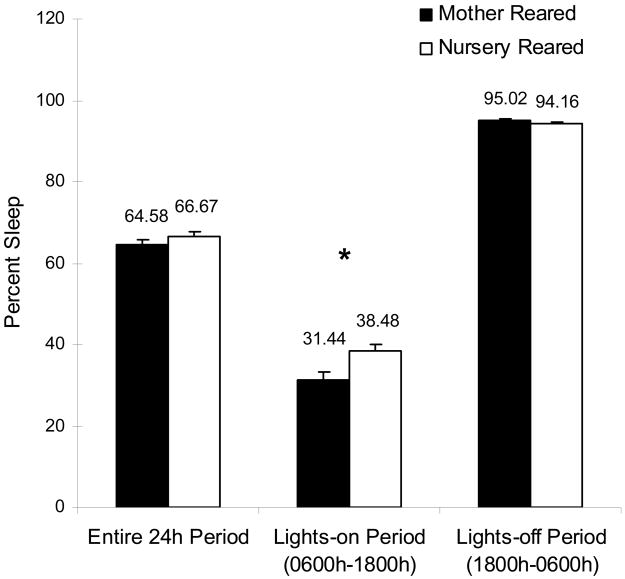
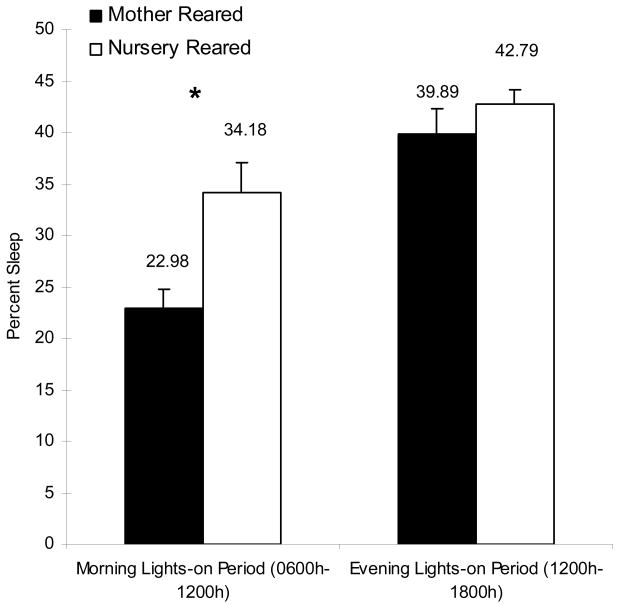
In contrast to having increased motor restlessness during the nighttime sleep period, NR animals spent more time in motor patterns reflective of sleep during the active (lights-on) period than did MR animals (0600h–1800h; Fig 4a, Table I, t14=3.32, p<0.01). This increase was most pronounced in the morning as dividing the active period into two intervals (morning, 0600h–1200h; afternoon/evening, 1200h–1800h) revealed that this difference in actigraphic indices of sleep were only significantly different between the groups in the first interval (Fig 4b, Table I; t14=3.28, p<0.01).
Figure 4.
Percent sleep within daily intervals. a. Mean percent sleep during the entire 24hr period, the lights-on period (0600h–1800h), and the lights-off period (1800h–0600h). b. Mean percent sleep during the morning lights-on period (0600h–1200h) and the evening lights-on period (1200h 1800h). NR and MR animals are represented by open bars and black bars, respectively. The asterisk indicates a significant (p<0.05) effect as measured by a two-sample t-test.
4.5 NR MONKEYS HAVE ELEVATED PLASMA CORTISOL BUT NORMAL PATTERNS
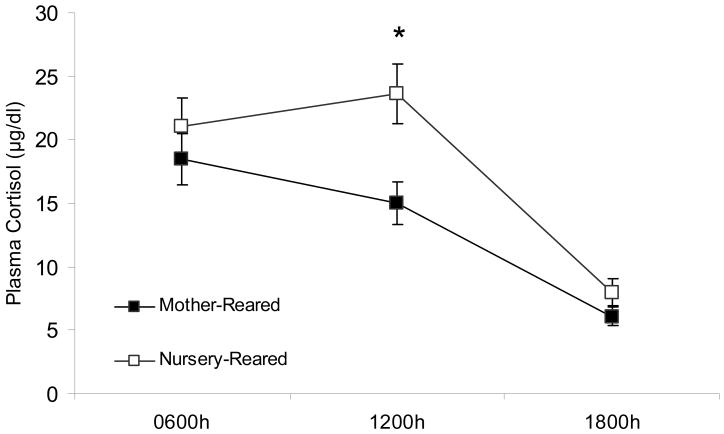
Repeated-measures ANOVA on cortisol levels revealed a significant main effect for time of day, F2,26=41.48, p<0.01 collapsed across rearing group. There also was a main effect of rearing with NR animals having overall higher cortisol levels than MR animals, F1,13=5.33, p<0.05. There was not, however, an interaction between rearing group and time of day (F2,26=2.06, ns), indicating that the pattern of cortisol secretion remained intact across the three time points in the two rearing conditions, with a consistent overall elevation in the NR monkeys across time points. Although the group by time interaction was not significant, planned t-tests conducted at each time point revealed significant group differences at 1200h (t14=−3.02, p<0.05), but not at the 0600h or 1800h samplings (t13=−0.890, ns; t14=−1.45, ns; Fig 5). Taken together these data indicate that the NR group displayed a significant elevation of cortisol levels at 1200h time point, but the intra-individual patterns across the three time points did not differ across groups.
Figure 5.
Mean circadian basal plasma cortisol levels (μg/dl) over time for MR and NR animals. Asterisks indicate a significant (p<0.05) rearing effect at that time point. Standard error bars are depicted. NR and MR animals are represented by open squares and black squares, respectively.
4.4 MEAN CORTISOL LEVELS PREDICT SLEEP EFFICIENCY AT NIGHT
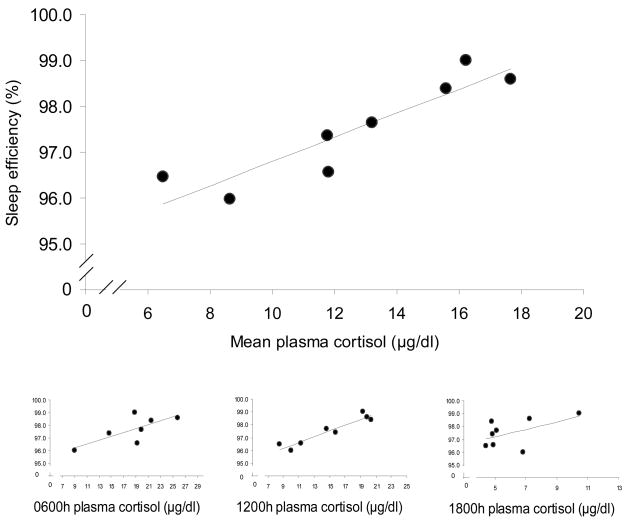
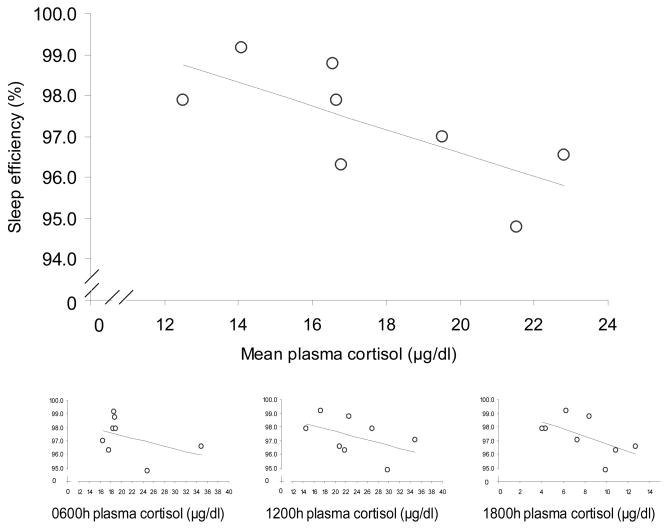
Finally, in order to compare the cortisol and sleep indices across animals, the mean cortisol level for each animal across all time points (0600h, 1200h & 1800h) was compared to actigraphic estimates of sleep behavior: early-morning wake index, sleep duration and sleep efficiency during the sleep period, and the total sleep time during the morning lights-on period using linear regression. Cortisol levels significantly predicted sleep efficiency (percent sleep within nighttime sleep period), but did not relate to any other activity indices. In the MR group, animals with higher mean daytime plasma cortisol concentrations had greater sleep efficiencies (i.e. percent time sleeping) than animals with lower mean daytime cortisol levels (Fig 6a; r2 = 0.831, p<0.01). In contrast, within the NR group, animals with higher cortisol concentrations had lower sleep efficiencies (Fig 6b; r2 = 0.506, p<0.05). A multiple regression model with group and mean cortisol levels as predictors of sleep efficiency revealed a significant interaction effect (F1,14=20.16, p<0.01). Analyzing each time point of cortisol collection revealed only the MR 1200h cortisol levels to correlate significantly with sleep efficiency (Figure 6a inset; MR: r2 = 0.898, p<0.001), while the early morning MR level was a trend (r2 =0.531, p=0.063), and evening levels did not relate (r2 =0.287, ns). For the NR subjects although the mean level correlated negatively with sleep efficiency, none of the individual cortisol time points related significantly to sleep efficiency (NR 0600h: r2 = 0.180; 1200h: r2 = 0.226; 1800h: r2 =0.343).
Figure 6.
Mean basal plasma cortisol levels (μg/dl) over all three time points (0600h, 1200h, 1800h) and mean sleep efficiencies (%) during the sleep interval for MR and NR animals. (a) MR, black circles, r2 = 0.831, p<0.01; (b) NR, open circles, r2 = 0.506, p<0.05. Insets are 0600h, 1200h, and 1800h plasma cortisol levels vs. mean sleep efficienies (%). (MR 0600h:, r2 =0.531, p=0.063, 1200h: r2 =0.898, p<0.001, 1800h: r2 =0.287, ns; NR 0600h: r2 =0.180, ns, 1200h: r2 =0.226, ns, 1800h: r2 =0.343, ns)
5. DISCUSSION
Actigraphy is a relatively new and promising analytic tool in nonhuman primate research. Using this noninvasive technique, we were able to quantify movement patterns and estimate durations of sleep and wake behavior in rhesus macaques over several days in animals with differing rearing histories while they were in their home cages. Importantly, because actigraphy data can be acquired with minimal stress, this approach enables acquisition of semi-naturalistic behavioral expression in a well-controlled laboratory environment.
The present study revealed several novel findings. First, during the nighttime lights-off period, actigraphic algorithms indicated that NR animals had shorter sleep period durations and earlier morning wake times than MR animals. These algorithms also indicated that NR animals had more epochs characterized as asleep during the active period, particularly in the morning during the lights-on period, than MR animals. These findings indicate that the transition from a low motor activity phase characteristic of sleep to the high motor activity phase characteristic of waking is more gradual in the NR animals than the MR animals and extend previous reports of sleep disturbances in infant NR monkeys (Kaemingk and Reite, 1987).
In addition to finding alterations characteristic of sleep disturbances, we also found significant differences in plasma cortisol levels between rearing groups. In line with previous reports (Champoux et al., 1989; Higley et al., 1992; Sanchez, 2006), NR monkeys displayed an overall increase in cortisol levels, across three daily time points. However, in contrast to our sleep findings and to some previous reports (Sanchez et al., 2005), maternal deprivation did not result in a detectable change in the diurnal pattern of cortisol across the day. Although the fact that the biggest difference between the groups occurred at 1200h, just after the period of increased sleep in the NR animals might be further indication of a rightward shift in circadian rhymicity, but the fact that there was not a group by time interaction indicates caution in this interpretation.
It is notable that these sleep disruptions are occurring in 2 year old monkeys which is a developmental phase equivalent to late childhood (pre-puberty) in humans. Marked changes in sleep typically occur in humans during puberty and these rearing effects may become more pronounced as the animals progress through puberty. Likewise, both hypersecretion of cortisol (Dahl et al., 1991; Goodyer et al., 1996) and sleep disturbance (Ivanenko et al., 2005) have been associated with adolescent mood and anxiety disorders previously, but the relationship between both of these variables and depression is relatively weak in adolescence and becomes stronger in adulthood (Knowles and MacLean, 1990; Zalsman et al., 2006). It is possible that the sleep disturbances of prepubescent monkeys in the present study might be an early phase of sleep problems which will shift or intensify during the pubertal transition period. Tracking such a shift in relation to other behavioral measures may provide useful insight into developmental changes in states of psychopathology and coping responses in organisms that have had early adversity as they traverse across the pubertal transition.
In addition to overall higher levels of cortisol, NR and MR animals appeared to have a different relationship between basal cortisol and actigraphic indices of sleep. NR animals displaying greater mean basal cortisol levels during the day had lower sleep efficiencies compared to NR animals with lower cortisol levels. Similar findings have been reported between sleep difficulty and increased afternoon cortisol in young children in two recent studies (el-Shiekh et al, 2008; Hatzinger et al., 2008) and indicate that hypersecretion of cortisol during the day may be linked to sleep difficulties at night even relatively early in life. While the precise reason for the relationship between heightened diurnal levels of cortisol and sleep difficulties is impossible to discern from the present dataset, past research has emphasized the negative influence that heightened arousal and stress has on sleep quality, and has indicated that elevated levels of cortisol during the day are an index of hyper-arousal and pathogenic levels of allostatic load (el-Sheikh et al, 2008). However it is also possible that the direction is reversed and poor sleep quality is driving increased diurnal cortisol secretion (Buckley & Schatzberg, 2005), or that a third factor underlies dysregulation in a number of systems independently in the NR animals.
Perhaps the most striking finding in this study, however, was that, in contrast to the adversely-reared animals, higher daily cortisol levels were associated with better sleep efficiency in the MR animals. Separate analyses of each time point suggests that the mid-day cortisol level (a time when activities of other animals and humans are at their peak) was the strongest predictor of sleep efficiency in the MR animals. This finding may reflect the fact that moderate levels of arousal are good for sleep efficiency in MR animals but disruptive of sleep in NR animals. Although cortisol is often considered a proxy for stress, there are many behavioral and psychological functions for which cortisol at low to moderate levels is beneficial. For example many previous studies have found an inverted-u relationship between cortisol secretion and both memory performance and attention (eg Lupien & McEwen, 1997; Davis, 2002), with intermediate levels being superior on many measures to either excessively low or excessively high circulating levels. In addition several studies have found a dose-response relationship for exogenous cortisol administration and sleep in which moderate levels of cortisol are sleep conducive relative to either low or high levels (Buckley & Schatzberg, 2005). This relationship may reflect activation of the two types of cortisol receptors - with increased occupancy of the high affinity mineralocorticoid receptors which occurs at low to moderate circulating levels being responsible for sleep induction, and occupancy of the low affinity glucocorticoid receptors (GR) occurring at moderate to high circulating levels being responsible for hyperemotionality and sleep disruptions (Buckley & Schatzberg, 2005; de Kloet et al., 2001) While the complete circadian picture of cortisol is not known in this sample, this pattern suggests a typical inverted-u function for sleep regulation may exist as well. Moderate levels of circulating cortisol such as are present in the higher end of the diurnal cortisol distribution in the MR animals may represent maximal efficiency of the HPA axis and optimal functioning of other circadian processes such as sleep, whereas the higher end of cortisol in NR monkeys may represent levels that reach the pathological level where HPA and circadian dysregulation occur.
While the etiology underlying human psychopathology is complex, and likely involves interactions between genetic and environmental factors on many levels, the similarities between nursery-reared monkeys and the state of hypervigilance in humans with affective disorders (Pine et al, in press) suggests some commonalities at a neuronal circuit level. Identifying common behavioral and neurobiological abnormalities in prepubescent monkeys affords an opportunity to follow the progression of behavioral and neurobiological development through adulthood and as a consequence of treatment in a controlled environment.
At present the literature suggest two likely systems of dysregulation in NR monkeys that might account for sleep disturbances seen here. First, several studies have shown that the effects of adverse rearing in monkeys appear to be more pronounced in animals with a genetic variant in the serotonin transporter (Bennett et al., 2002; Barr et al., 2004a; Barr et al., 2004b). The finding that sleep disruptions in NR monkeys might also relate to alterations in serotonergic functioning is further suggested by findings that altered levels of serotonin have been linked to aberrant sleep-wake cycles (Zajicek et al., 1997; Mehlman et al., 2000) and hyperactivation of the HPA axis in monkeys (Barr et al., 2004b).
Another potential neurobiological site that might relate to the patterns observed here is the amygdala, which is clearly involved in pathologic mood and anxiety states in humans (Southwick et al., 2005). Previous studies have shown that amygdala lesions can alter mother-infant interactions and diminish social learning in infant rhesus monkeys (Prather et al., 2001). Moreover, it has recently been shown that activity of a single gene in the amygdala is related to differential social behavior expressed by monkeys housed with the mother for the first month versus the first week of life (Sabatini et al., 2007). Increases in acoustic startle response also suggest differential functioning of the amygdala in adversely reared monkeys (Parr et al., 2002). Overall, these studies suggest that important developmental changes are taking place in the amygdala during early postnatal life and are mediated by contact with the mother. Finally, Benca et al. (2000) showed that sleep was less disrupted in chair-restrained rhesus monkeys with amygdala lesions than in non-lesioned monkeys, suggesting that the amygdala may mediate stress induced alterations in sleep patterns in rhesus monkeys.
Given the important role that serotonin and the amygdala are thought to play in mood disorders, linking these neurobiological processes to patterns of sleep disruption in juvenile nursery-reared monkeys may be important because sleep alterations could serve as a much less invasive and more easily detectable probe of risk for psychopathology than other probes. This may be especially useful for pediatric populations for whom verbal reports are not always reliable and extensive behavioral analyses not always possible.
One of the limitations of the present study was the variability observed in the early wake index (see Table 1), which was one of the major differences observed between rearing groups. Future studies will need to address whether this variability was the result of specific conditions of the study or if this measure is inherently variable. Future studies should also focus on clarifying the potential link between alterations in sleep and such underlying neurobiological dysfunctions. In particular, studies focusing on sleep patterns in NR monkeys that are undergoing treatment with selective serotonin reuptake inhibitors would be warranted given the potential role of serotonin in sleep function and the well documented relationship between NR and serotonergic activity. Although not possible with actigraphy methods, characterizing sleep stages, particularly REM sleep, in NR monkeys would be important to pursue future studies.
In sum the present study used actigraphic assessment of motor activity in the home cage to document several alterations in sleep behavior in NR monkeys. These sleep disturbances were accompanied by hypersecretion of cortisol, and together suggest an underlying state of hypervigilance that may be related to anxious or dysphoric affect.
Figure 2.
Mean activity counts across the 24 hour period in 10 min bins for NR and MR animals. Analysis of 10 min epochs across the 24 hour period revealed no main or interaction effects of total activity counts for rearing. NR and MR animals are represented by open circles and black squares, respectively.
Acknowledgments
The authors wish to thank Dr. Steven Suomi and Dee Higley for providing expertise with rearing procedures, and all the animal care staff at the NIH animal center for providing excellent care of the monkeys.
ROLE OF FUNDING SOURCE
This research was supported in full by the Intramural Research Program of the NIH, National Institute of Mental Health. No private agency participated in the design, collection, analysis or interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest to report.
CONTRIBUTORS (not published)
Catherine Barrett conducted statistical analysis of the data, generated all figures and drafted the manuscript. Pamela Noble and Erin Hanson supervised all data collection procedures and assisted with data processing and analysis of both activity and cortisol data. Daniel Pine, James Winslow, and Eric Nelson oversaw experimental design, project funding, and manuscript editing and development.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–92. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. American Psychiatric Press; 1994. [Google Scholar]
- Barr CS, Newman TK, Lindell S, Shannon C, Champoux M, Lesch KP, Suomi SJ, Goldman D, Higley JD. Interaction between serotonin transporter gene variation and rearing condition in alcohol preference and consumption in female primates. Arch Gen Psychiatry. 2004a;61:1146–52. doi: 10.1001/archpsyc.61.11.1146. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Shannon C, Parker C, Dvoskin RL, Becker ML, Schwandt M, Champoux M, Lesch KP, Goldman D, Suomi SJ, Higley JD. Rearing condition and rh5-HTTLPR interact to influence limbic-hypothalamic-pituitary-adrenal axis response to stress in infant macaques. Biol Psychiatry. 2004b;55:733–8. doi: 10.1016/j.biopsych.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Bastian ML, Sponberg AC, Sponberg AC, Suomi SJ, Higley JD. Long-term effects of infant rearing condition on the acquisition of dominance rank in juvenile and adult rhesus macaques (Macaca mulatta) Dev Psychobiol. 2003;42:44–51. doi: 10.1002/dev.10091. [DOI] [PubMed] [Google Scholar]
- Benca RM, Obermeyer WH, Shelton SE, Droster J, Kalin NH. Effects of amygdala lesions on sleep in rhesus monkeys. Brain Res. 2000;879:130–8. doi: 10.1016/s0006-8993(00)02761-x. [DOI] [PubMed] [Google Scholar]
- Bennett AJ, Lesch KP, Heils A, Long JC, Lorenz JG, Shoaf SE, Champoux M, Suomi SJ, Linnoila MV, Higley JD. Early experience and serotonin transporter gene variation interact to influence primate CNS function. Mol Psychiatry. 2002;7:118–22. doi: 10.1038/sj.mp.4000949. [DOI] [PubMed] [Google Scholar]
- Buckley TM, Schatzberg AF. Review: on the interactions of the hypothalamic-pituitary-adrenal (HPA) axis and sleep: normal HPA axis activity and circadian rhythm, exemplary sleep disorders, J. Clin Endocrin Metab: Clin Exp. 2005;90:3106–3114. doi: 10.1210/jc.2004-1056. [DOI] [PubMed] [Google Scholar]
- Caldji C, Diorio J, Meaney MJ. Variations in maternal care in infancy regulate the development of stress reactivity. Biol Psychiatry. 2000;48:1164–74. doi: 10.1016/s0006-3223(00)01084-2. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–9. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Champoux M, Coe CL, Schanberg SM, Kuhn CM, Suomi SJ. Hormonal Effects of Early Rearing Conditions in the Infant Rhesus Monkey. Am J of Primatol. 1989;19:111–117. doi: 10.1002/ajp.1350190204. [DOI] [PubMed] [Google Scholar]
- Dahl RE, Ryan ND, Puig-Antich J, Nguyen NA, al-Shabbout M, Meyer VA, Perel J. 24-hour cortisol measures in adolescents with major depression: a controlled study. Biol Psychiatry. 1991;30:25–36. doi: 10.1016/0006-3223(91)90067-v. [DOI] [PubMed] [Google Scholar]
- Davis EP, Bruce J, Gunnar MR. The anterior attention network: associations with temperament and neuroendocrine activity in 6-year-old children. Dev Psychobiol. 2002;40:43–56. doi: 10.1002/dev.10012. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Oltzl MS, Joels M. Stress and cognition: are corticosteroids good or bad guys? Trends Neurosci. 1999;22:422–426. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Buckhalt JA, Keller PS, Granger DA. Children’s objective and subjective sleep disruptions: links with afternoon cortisol levels. Health Psychol. 2008;27:26–33. doi: 10.1037/0278-6133.27.1.26. [DOI] [PubMed] [Google Scholar]
- Goodyer IM, Herbert J, Altham PM, Pearson J, Secher SM, Shiers HM. Adrenal secretion during major depression in 8- to 16-year-olds, I. Altered diurnal rhythms in salivary cortisol and dehydroepiandrosterone (DHEA) at presentation. Psychol Med. 1996;26:245–56. doi: 10.1017/s0033291700034644. [DOI] [PubMed] [Google Scholar]
- Hatzinger M, Brand S, Perren S, Stadelmann S, von Wyl A, von Klitzing K, Holsboer-Trachsler E. Electroencephalographic sleep profles and hypothalamic-pituitary-adrenocortical (HPA)-activity in kindergarten children: early indication of poor sleep quality associated with increase cortisol secretion. J Psychiat Res. 2008;42:542–543. doi: 10.1016/j.jpsychires.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Higley JD, Suomi SJ, Linnoila M. A longitudinal assessment of CSF monoamine metabolite and plasma cortisol concentrations in young rhesus monkeys. Biol Psychiatry. 1992;32:127–45. doi: 10.1016/0006-3223(92)90016-s. [DOI] [PubMed] [Google Scholar]
- Higley JD, Suomi SJ, Linnoila M. A nonhuman primate model of type II alcoholism? Part 2. Diminished social competence and excessive aggression correlates with low cerebrospinal fluid 5-hydroxyindoleacetic acid concentrations. Alcohol Clin Exp Res. 1996;20:643–50. doi: 10.1111/j.1530-0277.1996.tb01666.x. [DOI] [PubMed] [Google Scholar]
- Hsieh KC, Robinson EL, Fuller CA. Sleep architecture in unrestrained rhesus onkeys (Macaca mulatta) synchronized to 24-hour light-dark cycles. Sleep. 2008;31:1239–1250. [PMC free article] [PubMed] [Google Scholar]
- Ichise M, Vines DC, Gura T, Anderson GM, Suomi SJ, Higley JD, Innis RB. Effects of early life stress on [11C]DASB positron emission tomography imaging of serotonin transporters in adolescent peer- and mother-reared rhesus monkeys. J Neurosci. 2006;26:4638–43. doi: 10.1523/JNEUROSCI.5199-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanenko A, Crabtree VM, Gozal D. Sleep and depression in children and adolescents. Sleep Med Rev. 2005;9:115–29. doi: 10.1016/j.smrv.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Jans LA, Riedel WJ, Markus CR, Blokland A. Serotonergic vulnerability and depression: assumptions, experimental evidence and implications. Mol Psychiatry. 2007;12:522–43. doi: 10.1038/sj.mp.4001920. [DOI] [PubMed] [Google Scholar]
- Kaemingk K, Reite M. Social environment and nocturnal sleep: studies in peer-reared monkeys. Sleep. 1987;10:542–50. doi: 10.1093/sleep/10.6.542. [DOI] [PubMed] [Google Scholar]
- Knowles JB, MacLean AW. Age-related changes in sleep in depressed and healthy subjects. A meta-analysis. Neuropsychopharmacology. 1990;3:251–9. [PubMed] [Google Scholar]
- Kushida CA, Chang A, Gadkary C, Guilleminault C, Carrillo O, Dement WC. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med. 2001;2:389–96. doi: 10.1016/s1389-9457(00)00098-8. [DOI] [PubMed] [Google Scholar]
- Levine S. Primary social relationships influence the development of the hypothalamic--pituitary--adrenal axis in the rat. Physiol Behav. 2001;73:255–60. doi: 10.1016/s0031-9384(01)00496-6. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS. The acute effects of corticosteroids on cognition: integration of animal and human model studies. Brain Res Rev. 1997;24:1–27. doi: 10.1016/s0165-0173(97)00004-0. [DOI] [PubMed] [Google Scholar]
- Markov D, Goldman M. Normal sleep and circadian rhythms: neurobiologic mechanisms underlying sleep and wakefulness. Psychiatr Clin North Am. 2006;29:841–53. doi: 10.1016/j.psc.2006.09.008. abstract vii. [DOI] [PubMed] [Google Scholar]
- Mehlman PT, Westergaard GC, Hoos BJ, Sallee FR, Marsh S, Suomi SJ, Linnoila M, Higley JD. CSF 5-HIAA and nighttime activity in free-ranging primates. Neuropsychopharmacology. 2000;22:210–8. doi: 10.1016/S0893-133X(99)00101-3. [DOI] [PubMed] [Google Scholar]
- Mellman TA. Sleep and anxiety disorders. Psychiatr Clin North Am. 2006;29:1047–58. doi: 10.1016/j.psc.2006.08.005. abstract x. [DOI] [PubMed] [Google Scholar]
- Oakley NR. Technical report to Mini Mitter Co., Inc. 1997. Validation with polysomnography of the sleep-watch sleep/wake scoring algorithm used by the actiwatch activity monitoring system. [Google Scholar]
- Papailiu A, Sullivan E, Cameron JL. Behaviors in rhesus monkeys (Macaca mulatta) associated with activity counts measured with accelerometer. American Journal of Primatology. 2007;69:1–9. doi: 10.1002/ajp.20476. [DOI] [PubMed] [Google Scholar]
- Parr LA, Winslow JT, Davis M. Rearing experience differentially affects somatic and cardiac startle responses in rhesus monkeys (Macaca mulatta) Behav Neurosci. 2002;51:859–66. doi: 10.1037//0735-7044.116.3.378. [DOI] [PubMed] [Google Scholar]
- Peterson MJ, Benca RM. Sleep in mood disorders. Psychiatr Clin North Am. 2006;29:1009–32. doi: 10.1016/j.psc.2006.09.003. abstract ix. [DOI] [PubMed] [Google Scholar]
- Pine DS, Helfenstein SM, Bar-Haim Y, Nelson EE, Fox NA. Challenges for developing novel treatments for childhood disorders: lessons from research on anxiety. Neuropsychopharmacology. doi: 10.1038/npp.2008.113. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather MD, Lavenex P, Mauldin-Jourdain ML, Mason WA, Capitanio JP, Mendoza SP, Amaral DG. Increased social fear and decreased fear of objects in monkeys with neonatal amygdala lesions. Neuroscience. 2001;106:653–8. doi: 10.1016/s0306-4522(01)00445-6. [DOI] [PubMed] [Google Scholar]
- Reite MD, Short MS. Nocturnal sleep in separated monkey infants. Arch Gen Psychiatry. 1978:1247–1253. doi: 10.1001/archpsyc.1978.01770340097011. [DOI] [PubMed] [Google Scholar]
- Rutter M, O’Connor TG. Are there biological programming effects for psychological development? Findings from a study of Romanian adoptees. Dev Psychol. 2004;40:81–94. doi: 10.1037/0012-1649.40.1.81. [DOI] [PubMed] [Google Scholar]
- Sabatini MJ, Ebert P, Lewis DA, Levitt P, Cameron JL, Mirnics K. Amygdala gene expression correlates of social behavior in monkeys experiencing maternal separation. J Neurosci. 2007;27:3295–304. doi: 10.1523/JNEUROSCI.4765-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh A, Hauri PJ, Kripke DF, Lavie P. The role of actigraphy in the evaluation of sleep disorders. Sleep. 1995;18:288–302. doi: 10.1093/sleep/18.4.288. [DOI] [PubMed] [Google Scholar]
- Sadeh A, Acebo C. The role of actigraphy in sleep medicine. Sleep Med Rev. 2002;6:113–24. doi: 10.1053/smrv.2001.0182. [DOI] [PubMed] [Google Scholar]
- Sanchez MM. The impact of early adverse care on HPA axis development: nonhuman primate models. Horm Behav. 2006;50:623–31. doi: 10.1016/j.yhbeh.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev Psychopathol. 2001;13:419–49. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Noble PM, Lyon CK, Plotsky PM, Davis M, Nemeroff CB, Winslow JT. Alterations in diurnal cortisol rhythm and acoustic startle response in nonhuman primates with adverse rearing. Biol Psychiatry. 2005;57:373–81. doi: 10.1016/j.biopsych.2004.11.032. [DOI] [PubMed] [Google Scholar]
- Shannon C, Champoux M, Suomi SJ. Rearing condition and plasma cortisol in rhesus monkey infants. Am J Primatol. 1998;46:311–21. doi: 10.1002/(SICI)1098-2345(1998)46:4<311::AID-AJP3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Shannon C, Schwandt ML, Champoux M, Shoaf SE, Suomi SJ, Linnoila M, Higley JD. Maternal absence and stability of individual differences in CSF 5-HIAA concentrations in rhesus monkey infants. Am J Psychiatry. 2005;162:1658–64. doi: 10.1176/appi.ajp.162.9.1658. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Vythilingam M, Charney DS. The psychobiology of depression and resilience to stress: implications for prevention and treatment. Annu Rev Clin Psychol. 2005;1:255–91. doi: 10.1146/annurev.clinpsy.1.102803.143948. [DOI] [PubMed] [Google Scholar]
- Sri Kantha S, Suzuki J. Sleep quantitation in common marmoset, cotton top tamarin and squirrel monkey by non-invasive actigraphy. Comp Biochem Physiol A Mol Integr Physiol. 2006;144:203–10. doi: 10.1016/j.cbpa.2006.02.043. [DOI] [PubMed] [Google Scholar]
- Steiger A. Sleep and the hypothalamo-pituitary-adrenocortical system. Sleep Med Rev. 2002;6:125–38. doi: 10.1053/smrv.2001.0159. [DOI] [PubMed] [Google Scholar]
- Steiger A. Neurochemical regulation of sleep. J Psychiatr Res. 2007;41:537–52. doi: 10.1016/j.jpsychires.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Suomi SJ. Early stress and adult emotional reactivity in rhesus monkeys. Ciba Found Symp. 1991;156:171–83. doi: 10.1002/9780470514047.ch11. discussion 183–8. [DOI] [PubMed] [Google Scholar]
- Winslow JT. Neuropeptides and non-human primate social deficits associated with pathogenic rearing experience. Int J Dev Neurosci. 2005;23:245–51. doi: 10.1016/j.ijdevneu.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Zajicek KB, Higley JD, Suomi SJ, Linnoila M. Rhesus macaques with high CSF 5-HIAA concentrations exhibit early sleep onset. Psychiatry Res. 1997;73:15–25. doi: 10.1016/s0165-1781(97)00112-1. [DOI] [PubMed] [Google Scholar]
- Zalsman G, Oquendo MA, Greenhill L, Goldberg PH, Kamali M, Martin A, Mann JJ. Neurobiology of depression in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2006;15:843–68. vii–viii. doi: 10.1016/j.chc.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Zhdanova IV, Geiger DA, Schwagerl AL, Leclair OU, Killiany R, Taylor JA, Rosene DL, Moss MB, Madras BK. Melatonin promotes sleep in three species of diurnal nonhuman primates. Physiol Behav. 2002;75:523–9. doi: 10.1016/s0031-9384(02)00654-6. [DOI] [PubMed] [Google Scholar]