Introduction
Research over the past decade on the role of intraflagellar transport (IFT) in ciliogenesis has led to the realization that cilia throughout the body are sensory antennae that coordinate development and function of many tissues and organs (Rosenbaum and Witman, 2002; Snell et al., 2004; Singla and Reiter, 2006). The sensory role of cilia evolved in early eukaryotes and is seen in simple organisms (Wang et al., 2006; Inglis et al., 2007) as well as in mammals (Michaud and Yoder, 2006; Eggenschwiler and Anderson, 2007). Thus, it is not surprising that one of the best understood sensory signaling systems, the outer segment (OS) of vertebrate rod and cone photoreceptors, depends on IFT for both development and maintenance (Marszalek et al., 2000; Pazour et al., 2002a; Baker et al., 2003; Besharse et al., 2003). The goal of this brief overview is to illustrate how the photoreceptor OS can be viewed as a highly elaborate sensory cilium and model for analysis of IFT. We bring attention to the problem of rod and cone OS morphogenesis as a special case in the rapidly emerging field of ciliogenesis. We also distinguish between general features of ciliogenesis common to all sensory cilia and the unique features relevant to photoreceptors. Finally, we introduce emerging data that suggest the existence of an alternative IFT kinesin motor that is involved in photoreceptor OS formation.
Phototransduction Occurs in a Sensory Cilium
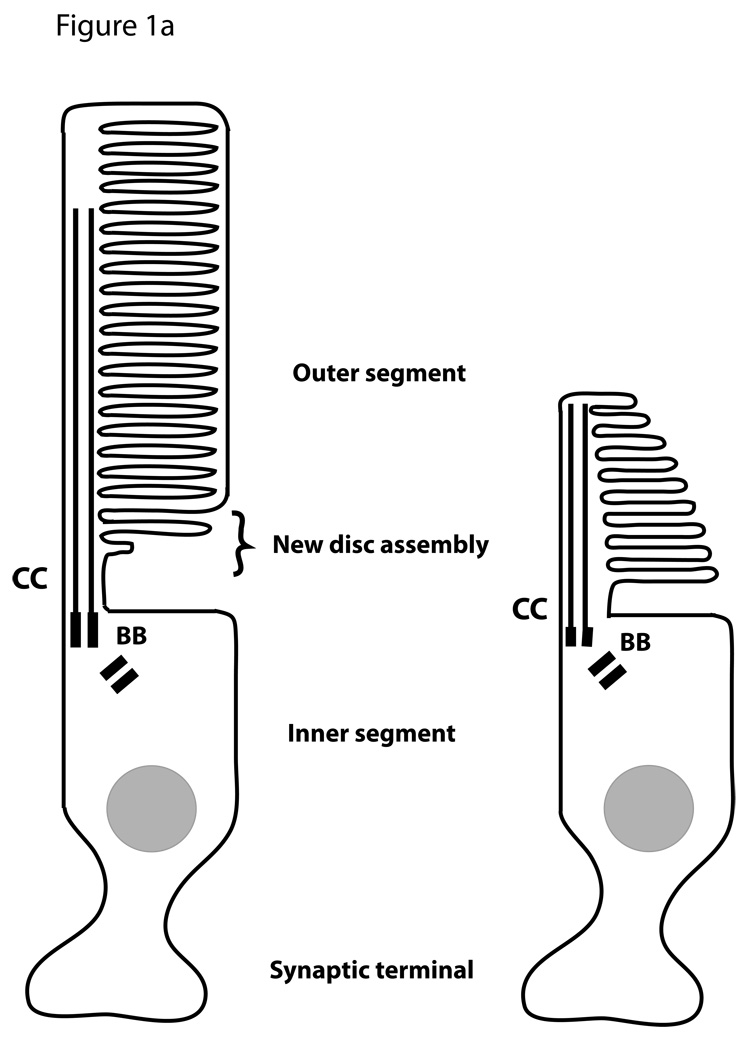
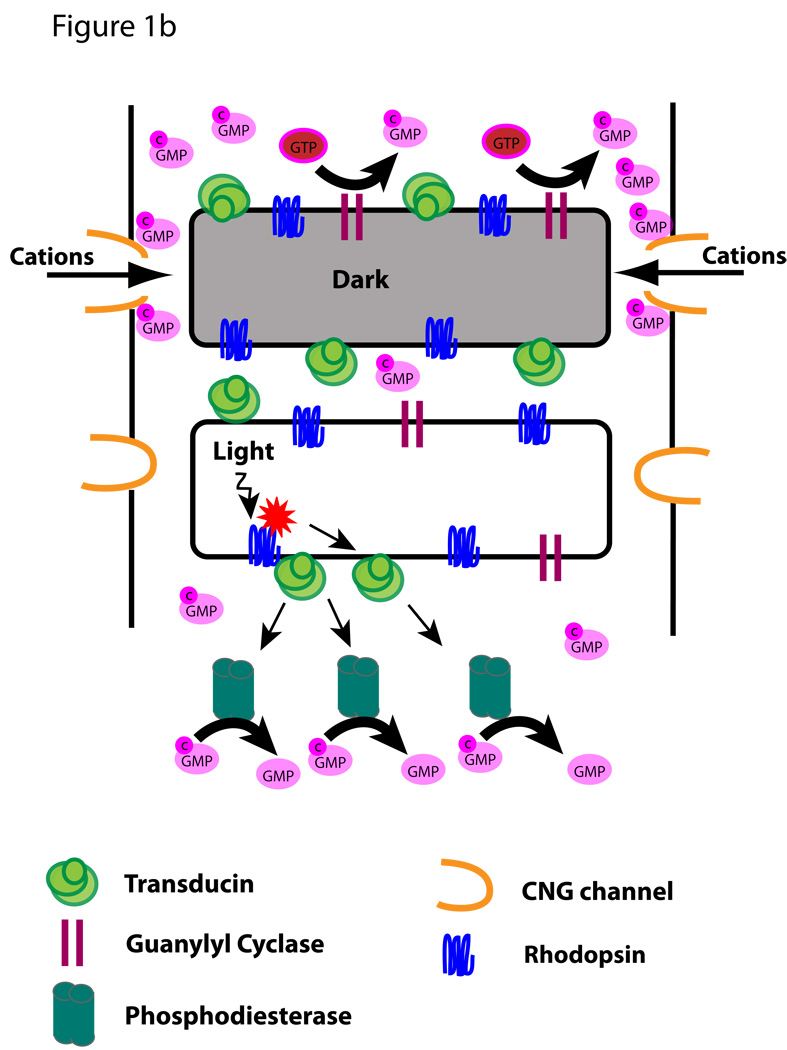
Vertebrate rod and cone photoreceptors are sensory neurons whose function in vision depends on formation of a complex sensory cilium (Figure 1A). The cell body of each photoreceptor extends a short axon, which makes synaptic contact with second order neurons (bipolar and horizontal cells), and a short dendrite, which terminates in a phototransduction organelle called the outer segment (OS). The dendritic region between the OS and nucleus is called the inner segment. Extensive analysis of the photoreceptor sensory OS over several decades has yielded a reasonably clear picture of the key molecular events of phototransduction and deactivation of the photoresponse (Burns and Arshavsky, 2005). At the most fundamental level (Figure 1B) these events depend on the presence of rhodopsin (R) to detect photons, membrane guanylyl cyclase (GC) to produce cGMP (cG), and a cyclic nucleotide gated channel (CNG) to regulate movement of cations. Light absorbed by rhodopsin activates the heterotrimeric G protein, transducin, which in turn activates cGMP phosphodiesterase (PDE) to reduce cGMP levels. In the dark cGMP keeps the CNG channel open, whereas in the light reduced cGMP levels result in channel closing and cell hyperpolarization. Further mechanisms requiring additional OS proteins (not shown in Figure 1) include deactivation of rhodopsin through phosphorylation and binding of arrestin, as well as regulation of transducin activity by a regulator of G-protein signaling complex.
Figure 1. The photoreceptor sensory OS.
A. Schematic illustrating the structure of rod and cone photoreceptors. The sensory organelle is composed of a stack of discs that initiated from the ciliary membrane at the base of the cilium. The dendritic portion extending from the basal body (BB) to the nucleus is called the inner segment. The cell body includes the nucleus and a short axon that terminates into a synapse. B. Simplified diagram of the phototransduction cascade taking place in the OS in the dark or upon light activation. This version does not include events involved in the deactivation of rhodopsin.
The numerous membrane and membrane-associated proteins involved in phototransduction are synthesized in the inner segment and then concentrated in the OS. Some such as arrestin and transducin move dramatically between the segments in response to light (Calvert et al., 2006). This, along with fact that mammalian photoreceptors exhibit turnover of the OS at rates approximating 10% of their length per day (LaVail, 1973), means that mechanisms for trafficking of phototransduction proteins into the OS are highly relevant for both initial development and maintenance of the cell. Although multiple mechanisms including random diffusion and binding of soluble proteins are likely to play a role, intraflagellar transport (IFT) has been identified as an important mechanism (Rosenbaum et al., 1999; Marszalek et al., 2000; Pazour et al., 2002a; Jimeno et al., 2006).
Membrane Proteins are Required for Sensory OS Development
The sensory OS of photoreceptors is similar to that of other sensory cilia, but photoreceptor specific mechanisms are required for the morphogenesis and turnover of the photosensitive disc membranes. The OS arises through transformation of the plasma membrane with the axoneme forming a structural backbone (De Robertis, 1956; Tokuyasu and Yamada, 1959; De Robertis, 1960; Besharse et al., 1985; Knabe and Kuhn, 1997). At the earliest stages, the precursors of disc membranes are formed as a disorderly array of vesicular and tubular structures, but quickly become organized into discs that are oriented perpendicular to the axoneme. Even before disc assembly begins, however, the cilium has a distinct proximal transition zone and a distal domain containing its dominant membrane proteins, rhodopsin (see Figure 2, (Besharse, 1986)) and peripherin-2 (Lee et al., 2006). This finding allows one to conceptually distinguish between ciliary transport mechanisms for membrane proteins, which may be common to many different sensory cilia, and separate mechanisms underlying morphogenesis and alignment of discs. Neither is well understood, but some progress has been made recently with a proposed role for IFT in rhodopsin trafficking (Marszalek et al., 2000; Pazour et al., 2002a; Jimeno et al., 2006).
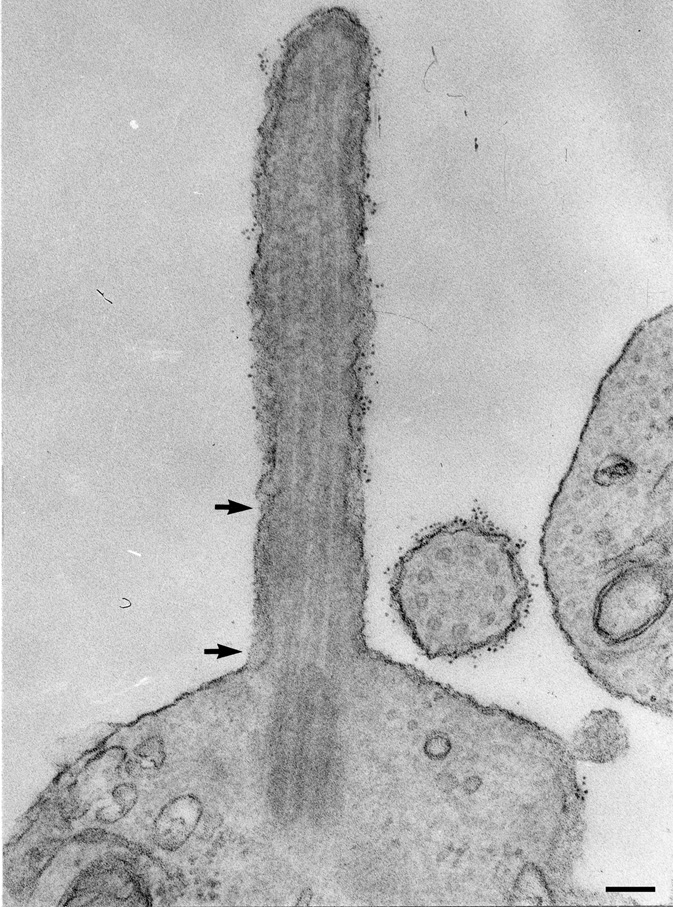
Figure 2. The photoreceptor cilium has a distinct transition zone and distal domain enriched in rhodopsin prior to the formation of discs.
Rhodopsin labeling indicated by ferritin is enriched in the distal end and is much less abundant in the transition zone (between the arrows). Scale bar: 0.5 µm. Image was published previously (Besharse, 1986) and is reproduced here with permission of the publisher.
An important feature of the photoreceptor OS is that at least two of its membrane proteins are required for OS assembly. Disc membranes in rods (see Figure 3) have expanded, flat domains containing two membrane guanylyl cyclases (Dizhoor et al., 1994; Liu et al., 1994; Hallett et al., 1996) along with rhodopsin at a concentration of about 25,000 µm2 (Calvert et al., 2001) and an edge or rim domain containing tetramers and higher order oligomers of the tetraspanin proteins, peripherin-2 and Rom1 (Molday and Molday, 1987; Arikawa et al., 1992; Loewen and Molday, 2000). The disc rim also contains the ABC transporter, ABCA4 (Illing et al., 1997). Analysis of mutant mice shows that both peripherin-2 (Jansen and Sanyal, 1984; Kedzierski et al., 1998) and rhodopsin (Humphries et al., 1997; Lem et al., 1999) are essential for OS formation. In both cases initial ciliogenesis occurs and a distal membrane expansion similar to that in wildtype cells is formed, but disc assembly and OS elongation fail entirely (Lee et al., 2006; Lee and Flannery, 2007). This suggests that both proteins play important morphogenetic and/or structural roles. In contrast, deletion of the two photoreceptor membrane guanylyl cyclases (Baehr et al., 2007) or ABCA4 (Weng et al., 1999) does not block OS formation, although the abnormal and unstable OS eventually degenerates. It has been suggested that rhodopsin and peripherin-2 are independently transported to the OS (Fariss et al., 1997; Lee et al., 2006), and it is known that both peripherin-2 and Rom1 can reach the distal cilium in the absence of rhodopsin (Lee et al., 2006). However, the mechanisms that underlie protein sorting between disc and rim domains are unknown. The problem is further complicated by the fact that another OS membrane protein, the cyclic nucleotide gated channel (CNG), is targeted to the plasma membrane (Cook et al., 1989), which requires additional sorting mechanisms between the disc and plasma membrane.
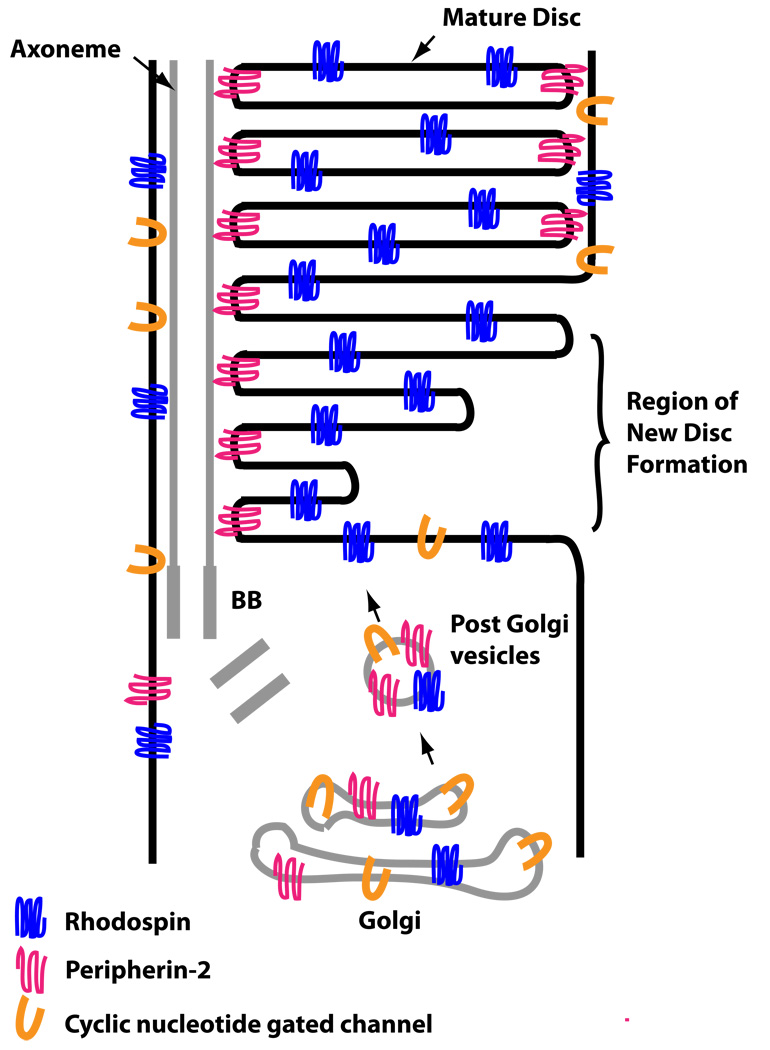
Figure 3. Simplified diagram illustrating the sorting problem for OS membrane proteins during disc formation.
Membrane proteins are transported into vesicles from the Golgi complex to the base of the cilium. Once in the OS, they segregate to distinct compartments of the discs or the plasma membrane.
The Connecting Cilium is a Transition Zone Between Membrane Domains
The early stages of the photoreceptor cilium development are similar to other developing cilia in that a 9 + 0 axoneme emerges from a basal body associated with apical cell surface (Greiner et al., 1981; Knabe and Kuhn, 1997). In photoreceptors, the portion of the cilium between the basal body and the disc-forming region, generally referred to as the connecting cilium, is actually the structural equivalent of the transition zone of other cilia and flagella (reviewed in (Besharse and Horst, 1990). Here Y-shaped cross-linkers (see Figure 4) connect the doublet microtubules with integral membrane structures comprising the ciliary necklaces (Rohlich, 1975; Besharse et al., 1985; Horst et al., 1987; Besharse and Horst, 1990). In photoreceptors, ciliary necklaces seen in freeze fracture images and globular membrane structures seen in the plasma membrane (Figure 4) have an average longitudinal spacing of about 32 nm (Besharse et al., 1985) suggesting that they are both part of the same structure. The cytosolic space in the transition zone between inner and outer segments is reduced to ~0.25 µm in diameter, and the axoneme and cross-linking structures further crowd that space (Figure 4). Since all movement between the segments must proceed through this narrow and structurally complex space, the connecting cilium is effectively a bottleneck and a potential regulatory site. For example, it has been suggested that centrins, which localize within the transition zone and exhibit Ca++-activated binding to the α/β subunits of photoreceptor transducin, regulate translocation of transducin between the segments (Pulvermüller et al., 2002; Giessl et al., 2006).
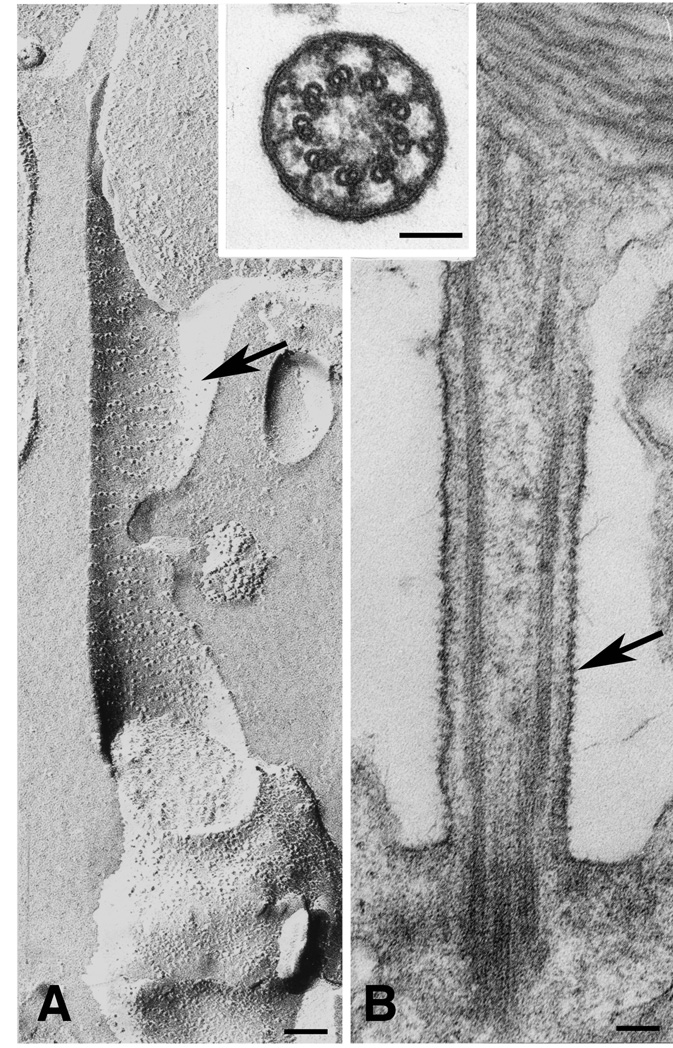
Figure 4. The structure of the connecting cilium is that of a transition zone.
Images of a freeze fracture replica (A) and a conventionally stained EM section (B) oriented with the OS above and the inner segment below. Arrows indicate ciliary necklaces in A and bead-like membrane structures in B. Note the presence of fibrils in the extracellular space extending from the cilium membrane. The inset is a cross section showing doublet microtubules and the Y-shaped microtubule-membrane cross-linkers. Magnifcation bars are 0.1 µm for all three images. Image was published previously (Besharse, 1986) and is reproduced here with permission of the publisher.
Early work on the structural organization of the Y-shaped cross-linkers showed that they consist of a trans-membrane assemblage containing glycoproteins with extracellular domains that maintain their association with the axoneme after extraction with detergent (Horst et al., 1987; Horst et al., 1990), high salt, and chaotropic agents (Muresan and Besharse, 1994). Interest in these structures has re-emerged recently with the finding that nephrocystin 1 and RPGR, both proteins associated with photoreceptor degenerative diseases, are concentrated in the connecting cilium (Hong et al., 2003; Fliegauf et al., 2006). Furthermore, Ush2a and Ush2c, two proteins associated with Usher's syndrome, a combination of hearing impairment and blindness, have long ectodomains extending between the inner segment plasma membrane and ciliary surface suggesting that they may be associated with the cross-linkers (Liu et al., 2007; Maerker et al., 2008). Both proteins have been proposed to form fibrous links between the two membranes, analogous to the ankle links between adjacent stereocilia in hair cells of the inner ear (Adato et al., 2005; McGee et al., 2006). This could explain how mutations in these genes lead to reduced stability and loss of both hair cells and photoreceptors in humans.
The structural order within the transition zone is likely to be directly related to its role as a gatekeeper between the distinct inner and outer segment membrane domains (Horst et al., 1987; Muresan and Besharse, 1994). Membrane proteins such as the CNG channel (Huttl et al., 2005), membrane guanylyl cyclase (Baehr et al., 2007), and rhodopsin are highly enriched in the OS (Papermaster et al., 1985) but are found at negligible levels in the inner segment. On the other hand, the inner segment membrane Na+/K+ ATPase is not present in the OS (Schneider et al., 1991). Although membrane continuity exists at the connecting cilium, mixing between the two domains does not appear to occur unless the ciliary barrier is disrupted (Spencer et al., 1988). This suggests that the transition zone can serve as both a gatekeeper between the inner outer segment membrane domains and as a corridor for transport of membrane proteins to the OS.
The Photoreceptor Axoneme Extends Deep into the OS
In mature rods the axoneme extends more than half the length of the OS (Brown et al., 1963; Kaplan et al., 1987; Sale et al., 1988), and in some cases may extend most of its length (Luby-Phelps et al., 2008). In cones the axoneme extends the full length of the OS and turns over during the process of disc shedding of the distal OS tip (Eckmiller, 1996). Within the proximal one third of the OS, the axoneme looses the B subfiber of the doublet to form singlet microtubules (Brown et al., 1963; Steinberg and Wood, 1975; Roof et al., 1991; Knabe and Kuhn, 1997; Insinna et al., 2008). Singlets are found through much of the length of the OS. Photoreceptor OS resemble sensory cilia in the olfactory epithelium (Reese, 1965), kidney (Webber and Lee, 1975), and C. elegans sensory neurons (Inglis et al., 2007) in the use of singlet extensions. This structural similarity is hypothesized to be related to common molecular mechanisms dedicated to extend and maintain distal extensions of the cilium (Evans et al., 2006; Insinna et al., 2008).
Rp1, a doublecortin domain containing protein that is mutated in a form of human retinitis pigmentosa, binds to singlet microtubules in cultured cells and is uniquely localized to the distal axoneme (Liu et al., 2004) suggesting that it may associate with and stabilize distal singlets in the OS. Since Rp1 also binds to disc rims, this protein appears to play an important role in OS assembly by mediating vertical alignment of discs along the axoneme (Liu et al., 2004).
Intraflagellar Transport in Photoreceptors
A critical role for IFT in OS assembly is supported by both cell biological and genetic data. First, multiple subunits of kinesin II (Beech et al., 1996; Muresan et al., 1997; Muresan et al., 1999; Whitehead et al., 1999; Marszalek et al., 2000) are associated with photoreceptor axonemes, and all three subunits of the kinesin II motor co-immunoprecipitate with IFT proteins in retinal extracts (Baker et al., 2003). Furthermore, both Dhc1b/Dhc2 heavy chain and the light intermediate chain (Lic3/D2Lic) of the dynein 2 retrograde motor are localized along bovine photoreceptor cilia (Mikami et al., 2002). In addition to the IFT motors, multiple IFT proteins are associated with OS axonemes in mouse, frog and zebrafish photoreceptors (Pazour et al., 2002a; Tsujikawa and Malicki, 2004; Insinna et al., 2008; Luby-Phelps et al., 2008). These findings are also supported by co-immunoprecipitation and sucrose density gradient sedimentation data (Baker et al., 2003) showing that multiple IFT complex B proteins from bovine photoreceptor OS fractionate as a large ~17S particle with properties similar to that of the IFT B complex of Chlamydomonas(Cole et al., 1998; Lucker et al., 2005). Finally, the functional importance of these findings has been demonstrated in mice deficient in the Kif3A subunit of kinesin II (Marszalek et al., 2000; Jimeno et al., 2006) and in Tg737orpk mice deficient in the IFT complex B protein, IFT88 (Pazour et al., 2002a). In both cases, photoreceptors are progressively lost after failure of OS morphogenesis and mislocalization of rhodopsin. Furthermore, similar results have been reported in zebrafish photoreceptors carrying the ovl mutation in IFT88, or after antisense morpholino knockdown of IFT52 or IFT57 (Tsujikawa and Malicki, 2004).
Although current data strongly support a role for anterograde IFT in photoreceptors, direct evidence for retrograde IFT, which would return OS components to the inner segment, has not been reported. Furthermore, the fact that the photoreceptor OS turns over about 10% of its volume daily by disc shedding at its distal tip (LaVail, 1976) implies that a retrograde pathway would not play a major role in the transport of essential disc membrane components. Nonetheless, the cytoplasmic dynein 2 motor is associated with OS axonemes (Mikami et al., 2002), and there is ample reason to think that a retrograde process would be required in photoreceptors. Retrograde IFT could play a role in support of photoreceptor axoneme dynamics at the distal tip (Roof et al., 1991; Pedersen et al., 2005), return of anterograde IFT kinesins to the inner segment (Signor et al., 1999), transport of a soluble component of the phototransduction machinery to the inner segment (Calvert et al., 2006), or for IFT mediated signaling (Wang et al., 2006). IFT mediated signaling could be used to coordinate OS trafficking and OS length. Further studies on the retrograde IFT motor and its most closely associated proteins, the IFT complex A (Pedersen et al., 2005), are needed in photoreceptors.
Kif17, a homologue of OSM-3, is Required for OS Assembly
Kinesin II, the canonical anterograde IFT motor, (Kozminski et al., 1995; Rosenbaum and Witman, 2002; Scholey, 2008), is clearly required for OS assembly (Marszalek et al., 2000; Jimeno et al., 2006). However, we have recently found that an additional kinesin called Kif17 is also required for OS formation (Insinna et al., 2008). To put this into perspective the terminology becomes important. Kinesin II is a heterotrimeric member of the kinesin 2 family that in vertebrates is composed of Kif3A, Kif3B, and Kap3 subunits (Lawrence et al., 2004). A conditional deletion approach in photoreceptors involved deletion of the Kif3A subunit (Marszalek et al., 2000), and work at the protein level shows that all three kinesin II subunits co-IP with IFT proteins from retina (Baker et al., 2003). Kif17, also a kinesin 2 family member, is a homodimeric kinesin that is known in mammals for its involvement in dendritic trafficking in neurons (Setou et al., 2000; Guillaud et al., 2003). However, Kif17 is the homologue of the homodimeric OSM-3 kinesin in C. elegans, which serves as an accessory IFT motor (Snow et al., 2004; Evans et al., 2006; Pan et al., 2006). Work in C. elegans amphid channel cilia revealed a functional coordination between kinesin II and OSM-3 in which both kinesins are involved in elongation of the middle segment composed of doublet microtubules (MT). However, OSM-3 was found to be essential for elongation of MT singlets in the distal segment. In this model, cargo is moved along the middle segment by both IFT motors, while OSM-3 alone is responsible for transport in the distal segment.
Since photoreceptor OS (see above), like C. elegans sensory cilia, have singlet extensions of their axoneme (Figure 5), we have begun to test the potential role of Kif17, the Osm-3 homologue, in OS formation (Insinna et al., 2008). Kif17 co-localizes with IFT proteins along the axoneme and co-immunoprecipitates with IFT proteins. Furthermore, depletion of Kif17 using a morpholino knockdown approach in zebrafish embryos prevents OS development (Figure 6). In the most severe cases, extension of the connecting cilium was stopped at the level of the basal body. In less severe cases small, disorganized OS formed and the cells showed mislocalization of the cone visual pigment. These results demonstrate that Kif17 is associated with IFT proteins and is necessary for OS assembly. Although a role for Kif17 in singlet extension is a plausible explanation for short OS phenotype in cases of partial knockdown of Kif17, the complete failure of OS formation with more complete knockdown suggests that Kif17 is required for some additional feature of early OS formation prior to OS elongation. Further experiments are needed to define which steps involve Kif17.
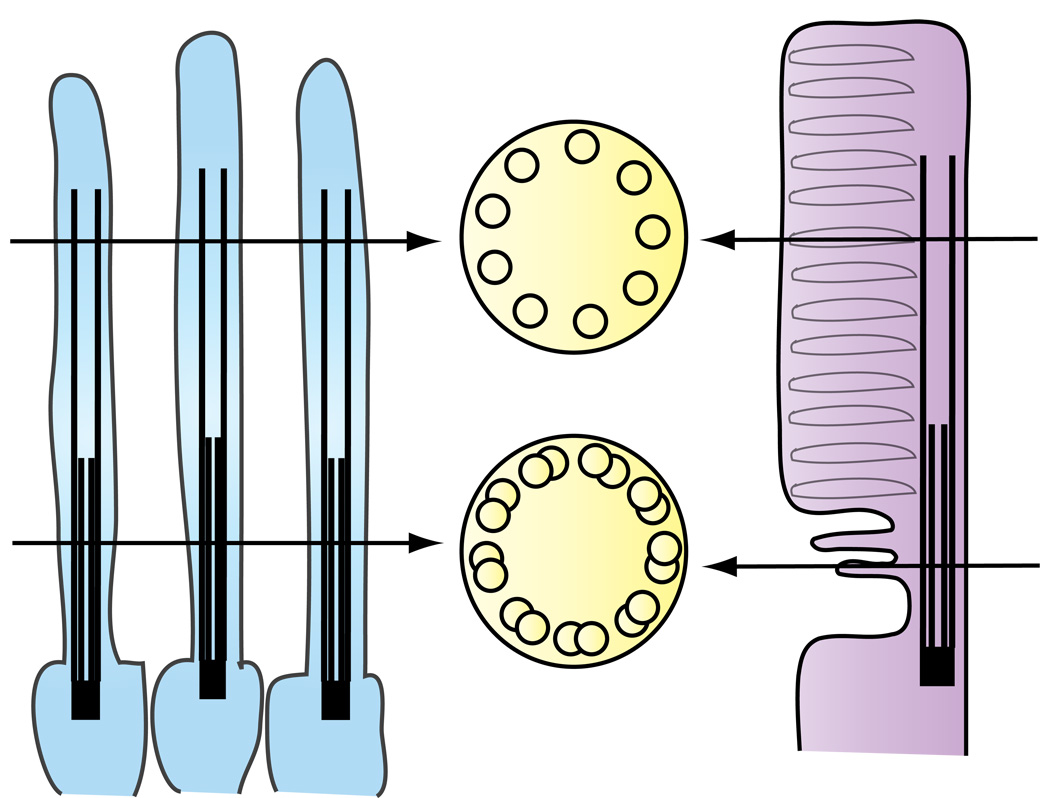
Figure 5. Diagram showing similarities between C.elegans amphid channel cilia (left) and the photoreceptor cilium (right).
Both sensory cilia present a bipartite structure with a proximal segment composed of nine microtubule doublets with singlets towards the distal end.
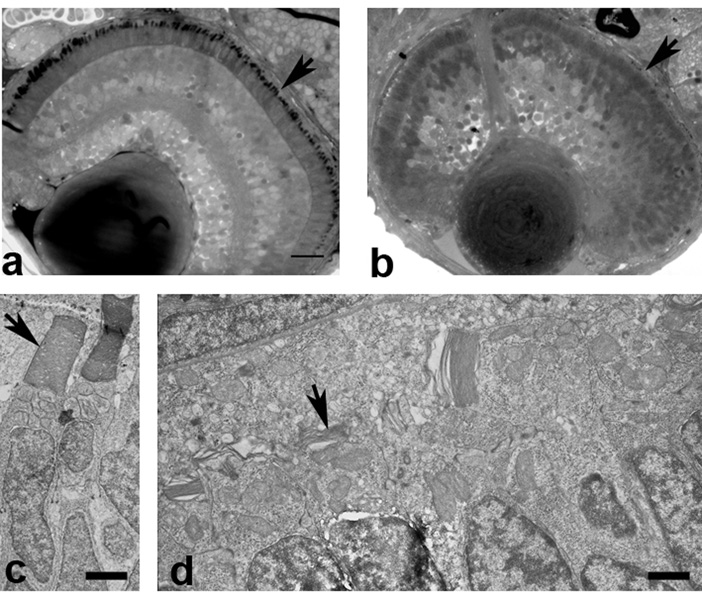
Figure 6. Failure of OS formation in zebrafish embryos depleted of Kif17 protein.
A. The retina of a 3 days old wild type embryo develops normal OS (arrowhead). B. Embryos injected with an antisense morpholino directed against Kif17 mRNA fail to develop OS (arrowhead) and present a small degree of disorganization of the retina layers. Scale bar in A 10 µm. C. EM picture of wild type photoreceptors with normal OS (arrowhead). Scale bar: 3.4 µm. D. EM picture of a morphant with very short and abnormal OS. Scale bar: 1.4 µm. Similar images are presented in Insinna, et al. (2008).
An interesting feature of the Kif17 knockdown phenotype in zebrafish embryos is that the pronephros showed normal development with formation of motile cilia of normal length (Insinna et al., 2008). This indicates that Kif17 is not necessary for elongation of kidney epithelial cilia. In cultured mammalian kidney epithelial cells a dominant negative Kif17 protein blocked delivery of an exogenous olfactory cyclic nucleotide gated channel (CNG) into the distal tip of the cilium (Jenkins et al., 2006). Although this suggests a role for Kif17 in trafficking of the membrane protein, the dominant negative construct had no apparent effect on cilium elongation. Normal cilium elongation in both zebrafish and cultured cells would be expected according to the C.elegans model if their axonemes are dominated by doublet MT. Future analysis evaluating a role for Kif17 would be aided by a thorough structural analysis of the distal axoneme and evaluation of the lengths of singlet extensions.
Regulation of Photoreceptor IFT
Emerging data suggest the existence of multiple IFT accessory proteins of direct relevance to photoreceptor IFT. For example, the fleer (flr) mutation in zebrafish results in multiple cilium related defects including failure of photoreceptor OS formation (Pathak et al., 2007). At the EM level, these phenotypes are associated with disorganization of the B-subfiber of doublets and a deficiency in polyglutamylation of tubulin. Positional cloning has shown that flr, which encodes a tetratricopeptide repeat protein, is the homologue of C. elegans DYF-1. This is an interesting connection because in C. elegans DYF-1 is thought to be necessary for the association of OSM-3 kinesin and the IFT particle; in the absence of DYF-1 OSM-3 is inactive and IFT is driven by kinesin II alone (Ou et al., 2005). This raises the possibility that flr may be directly associated with the function of the homologue of OSM-3, Kif17, in photoreceptor OS formation. The nature of the association is unclear, however. Unlike the Kif17 knockdown (Insinna et al., 2008), the flr mutation results in cilium defects in multiple cilium types including the pronephros (Pathak et al., 2007). The fact that flr is associated with doublets that are deficient in polyglutamylated tubulin has led to the suggestion that the low activity of OSM-3 kinesin in DYF-1 mutants could be the result of its sensitivity to reduced levels of polyglutamylated tubulin. Alternatively, it is also possible that flr functions as both a cohesion factor and a regulator of polyglutamylation in axonemes.
Proteins encoded by genes associated with Bardet-Biedl syndrome (BBS) are thought to play a role at critical steps of IFT transport and are likely to be relevant in photoreceptors because retinal degeneration, along with obesity and polydactyly, are key components in the syndrome. Seven of the 12 BBS proteins have been shown to form a core protein complex, called the BBsome, that associates with the basal body, promotes trafficking of vesicles, and is associated with ciliary membrane expansion (Nachury et al., 2007). It is of some interest that BBS7 and BBS8 are among the BBS proteins associated with the BBsome because mutations in the homologous genes in C. elegans lead to defective IFT (Blacque et al., 2004). Furthermore, functional analysis suggests that they serve as cohesion factors between IFT sub-complexes A and B and the associated kinesin motors (Ou et al., 2005). The additional BBsome components, BBS4 (Abd-El-Barr et al., 2007) and BBS1 (Davis et al., 2007), also exhibit a photoreceptor OS degeneration in mice. Although BBS proteins are not stable components of IFT proteins complexes, current data strongly suggests that they play an important accessory role in trafficking to the OS. In the case of mice with mutant BBS4 (Abd-El-Barr et al., 2007) vesicles accumulate in the inner segment at the base of the cilium and rhodopsin is mis-localized. These findings suggest a direct relationship between IFT, BBS proteins, and OS trafficking in photoreceptors.
OS Membrane Proteins as IFT Cargo
Although the components of the OS axoneme are expected to be transported by IFT (Qin et al., 2004), a major question is whether OS specific proteins are IFT cargo? Current data favors the idea that at least two OS membrane proteins, rhodopsin and GC1, are transported by IFT. Rhodopsin is mislocalized in mice deficient in both Kif3A subunit of kinesin II (Marszalek et al., 2000) and in Tg737orpk mice deficient in IFT88 (Pazour et al., 2002a). Similar results have been obtained for membrane guanylyl cyclase 1 (GC1) in Tg737orpk mice (unpublished data). These data are in keeping with a growing body of evidence showing a role for IFT in the trafficking ciliary membrane proteins such as polycystins 1 and 2 (Pazour et al., 2002b; Bae et al., 2006; Follit et al., 2006), the TRPV channel (Qin et al., 2005), and the olfactory cyclic nucleotide gated channel (Jenkins et al., 2006). However, mis-localization data alone does not provide molecular insight into the details of coupling of cargo to IFT particles. Accordingly, we have used immunoprecipitation and pull down assays to identify IFT associated proteins. This approach shows that both rhodopsin and GC1 co-immunoprecipitate with IFT proteins and that the co-chaperones, HSC70 and MRJ (DnajB6), are associated with these complexes (unpublished data). The chaperone complex may play a role in loading or stabilization of IFT cargo complexes.
IFT is Not the Only Pathway into the OS
Although the idea that IFT can deliver OS membrane proteins is attractive and consistent with emerging data from other sensory cilia, other pathways into the OS including diffusion and additional facilitated mechanisms are likely. Mutations in myosin VIIa (Ush1B) underlie a form of Usher's syndrome. Myosin VIIa and an actin filament network are associated with the photoreceptor cilium (Liu et al., 1997). Mice carrying a mutation in myosin VIIa have altered OS disc assembly kinetics and accumulate large amounts of rhodopsin in the connecting cilium. This has led to the proposal that myosin VIIa, in addition to kinesin II, is involved in OS trafficking of rhodopsin (Liu et al., 1999; Williams, 2002). How these two distinct motors and cytoskeletal systems are coordinated remains unknown. It is likely, however, that they work successively. For example, kinesin II could play a role in transport to the disc-forming region with myosin VIIa playing a role in reshaping disc membranes or sorting disc proteins at the base of the OS. Consistent with this idea, disruption of actin filaments with cytochalasin D disrupts the formation of closed discs in rod cells without disrupting the expansion of the OS membrane (Williams et al., 1988).
Simple diffusion may be sufficient for some soluble proteins such as transducin and arrestin and provides an attractive alternative to IFT for their rapid translocation between the segments in response to light (Sokolov et al., 2002; Calvert et al., 2006; Strissel et al., 2006). Green fluorescent protein which does not bind to other cellular components readily equilibrates between the segments via the connecting cilium (Peet et al., 2004). The high diffusion coefficient for soluble, globular proteins would be sufficient to account for the magnitude and rate of translocation seen in photoreceptors (Calvert et al., 2006). Furthermore, it has been pointed out that the extraordinary large number of arrestin molecules moving into the OS in light would likely saturate any motor driven mechanism (Calvert et al., 2006; Strissel et al., 2006).
Diffusion alone would lead to an equilibrium distribution and would still require a mechanism such as binding to a cellular compartment to establish a non-equilibrium condition. This idea is implicit in a recent suggestion that Ca++ regulated binding of centrins to the βγ subunit of transducin regulates their translocation (Pulvermüller et al., 2002; Giessl et al., 2006). Centrins are found in the connecting cilium where their binding to transducin βγ subunit could reduce diffusion. Quantitative analysis of GFP-arrestin distribution compared to free GFP in darkness shows that arrestin is in a non-equilibrium distribution in the inner segment (Peet et al., 2004), and its redistribution to the OS in light has been reported to be energy independent (Nair et al., 2005). Arrestin is known to bind to both tubulin and photo-activated rhodopsin, which has led to the idea that in light it redistributes by diffusion from microtubule binding sites in the inner segment to photo-activated rhodopsin binding sites in the OS (Nair et al., 2005). Although the idea that diffusion coupled with an exchange of binding sites remains attractive, the identity of the binding sites remains in question. Quantitative analysis has shown that photo-activated rhodopsin cannot account for the full magnitude of translocation because the amount of arrestin would exceed the available rhodopsin binding sites by more than an order of magnitude (Strissel et al., 2006).
Summary and Perspective
Rapid progress is being made in understanding the molecular details of IFT transport pathways and ciliogenesis in multiple cell types. Each system has a combination of general features along with cell specific features. The cell specific features of the photoreceptor sensory OS illustrated in this overview make it an important system that can provide new insights into the function of IFT in trafficking of cell specific cargo. Further studies of photoreceptors will likely yield new molecular insights into the details of how membrane proteins are moved into cilia and how two different kinesin motors, kinesin II and Kif17, are coordinated. In addition to providing insight into membrane protein trafficking by IFT, the high degree of conservation of the IFT through evolution suggests that use of simple IFT model systems will aid in understanding how defects in trafficking can lead to photoreceptor degeneration, and more broadly, to other cilium based diseases.
Acknowledgments
This work was supported by NIH grant EY03222 (JCB), a NEI core grant for Vision Research, and Medical College of Wisconsin Development Funds (JCB). Christine Insinna was supported by NIH NRSA Training grant T32-EY014537.
References
- Abd-El-Barr MM, Sykoudis K, Andrabi S, Eichers ER, Pennesi ME, Tan PL, Wilson JH, Katsanis N, Lupski JR, Wu SM. Impaired photoreceptor protein transport and synaptic transmission in a mouse model of Bardet-Biedl syndrome. Vision Research. 2007;47:3394–3407. doi: 10.1016/j.visres.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adato A, Lefevre G, Delprat B, Michel V, Michalski N, Chardenoux S, Weil D, El-Amraoui A, Petit C. Usherin, the defective protein in Usher syndrome type IIA, is likely to be a component of interstereocilia ankle links in the inner ear sensory cells. Hum Mol Genet. 2005;14:3921–3932. doi: 10.1093/hmg/ddi416. [DOI] [PubMed] [Google Scholar]
- Arikawa K, Molday LL, Molday RS, Williams DS. Localization of peripherin/rds in the disk membranes of cone and rod photoreceptors: Relationship to disk membrane morphogenesis and retinal degeneration. JCB. 1992;116:659–667. doi: 10.1083/jcb.116.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae YK, Qin H, Knobel KM, Hu J, Rosenbaum JL, Barr MM. General and cell-type specific mechanisms target TRPP2/PKD-2 to cilia. Development. 2006;133:3859–3870. doi: 10.1242/dev.02555. [DOI] [PubMed] [Google Scholar]
- Baehr W, Karan S, Maeda T, Luo DG, Li S, Bronson JD, Watt CB, Yau KW, Frederick JM, Palczewski K. The function of guanylate cyclase 1 and guanylate cyclase 2 in rod and cone photoreceptors. J Biol Chem. 2007;282:8837–8847. doi: 10.1074/jbc.M610369200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SA, Freeman K, Luby-Phelps K, Pazour GJ, Besharse JC. IFT20 links kinesin II with a mammalian intraflagellar transport complex that is conserved in motile flagella and sensory cilia. J Biol Chem. 2003;278:34211–34218. doi: 10.1074/jbc.M300156200. [DOI] [PubMed] [Google Scholar]
- Beech PL, Pagh-Roehl K, Noda Y, Hirokawa N, Burnside B, Rosenbaum JL. Localization of kinesin superfamily proteins to the connecting cilium of fish photoreceptors. Journal of Cell Science. 1996;109:889–897. doi: 10.1242/jcs.109.4.889. [DOI] [PubMed] [Google Scholar]
- Besharse JC. Photosensitive membrane turnover: differentiated membrane domains and cell-cell interaction. In: Adler R, Farber D, editors. The Retina: A Model for Cell Biological Studies, Part I. New York: Academic Press; 1986. pp. 297–352. [Google Scholar]
- Besharse JC, Baker SA, Luby-Phelps K, Pazour GJ. Photoreceptor intersegmental transport and retinal degeneration: a conserved pathway common to motile and sensory cilia. Adv Exp Med Biol. 2003;533:157–164. [PubMed] [Google Scholar]
- Besharse JC, Forestner DM, Defoe DM. Membrane assembly in retinal photoreceptors III. distinct membrane domains of the connnecting cilium of developing rods. Journal of Neuroscience. 1985;5:1035–1048. doi: 10.1523/JNEUROSCI.05-04-01035.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besharse JC, Horst CJ. The photoreceptor connecting cilium. A model for the transition zone. In: Bloodgood RA, editor. Ciliary and Flagellar Membranes. New York: Plenum Publishing Corp.; 1990. pp. 389–417. [Google Scholar]
- Blacque OE, Reardon MJ, Li C, McCarthy J, Mahjoub MR, Ansley SJ, Badano JL, Mah AK, Beales PL, Davidson WS, Johnsen RC, Audeh M, Plasterk RH, Baillie DL, Katsanis N, Quarmby LM, Wicks SR, Leroux MR. Loss of C. elegans BBS-7 and BBS-8 protein function results in cilia defects and compromised intraflagellar transport. Genes Dev. 2004;18:1630–1642. doi: 10.1101/gad.1194004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PK, Gibbons IR, Wald G. The visual cells and visual pigment of the mudpuppy, Necturus. JCB. 1963;19:79–106. doi: 10.1083/jcb.19.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns ME, Arshavsky VY. Beyond counting photons: trials and trends in vertebrate visual transduction. Neuron. 2005;48:387–401. doi: 10.1016/j.neuron.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Calvert PD, Govardovskii VI, Krasnoperova N, Anderson RE, Lem J, Makino CL. Membrane protein diffusion sets the speed of rod phototransduction. Nature. 2001;411:90–94. doi: 10.1038/35075083. [DOI] [PubMed] [Google Scholar]
- Calvert PD, Strissel KJ, Schiesser WE, Pugh EN, Jr, Arshavsky VY. Light-driven translocation of signaling proteins in vertebrate photoreceptors. Trends Cell Biol. 2006;16:560–568. doi: 10.1016/j.tcb.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Cole DG, Diener DR, Himelblau AL, Beech PL, Fuster JC, Rosenbaum JL. Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. JCB. 1998;141:993–1008. doi: 10.1083/jcb.141.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook NJ, Molday LL, Reid D, Kaupp UB, Molday RS. The cGMP-gated channel of bovine rod photoreceptors is localized exclusively in the plasma membrane. J Biol Chem. 1989;264:6996–6999. [PubMed] [Google Scholar]
- Davis RE, Swiderski RE, Rahmouni K, Nishimura DY, Mullins RF, Agassandian K, Philp AR, Searby CC, Andrews MP, Thompson S, Berry CJ, Thedens DR, Yang B, Weiss RM, Cassell MD, Stone EM, Sheffield VC. A knockin mouse model of the Bardet-Biedl syndrome 1 M390R mutation has cilia defects, ventriculomegaly, retinopathy, and obesity. Proc Natl Acad Sci U S A. 2007;104:19422–19427. doi: 10.1073/pnas.0708571104. Epub 12007 Nov 19421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis E. Morphogenesis of the retinal rods; an electron microscope study. J Biophys Biochem Cytol. 1956;2:209–218. doi: 10.1083/jcb.2.4.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis E. Some observations on the ultrastructure and morphogenesis of photoreceptors. J Gen Physiol. 1960;43(6) Suppl:1–13. doi: 10.1085/jgp.43.6.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dizhoor AM, Lowe DG, Olshevskaya EV, Laura RP, Hurley JB. The human photoreceptor membrane guanylyl cyclase, RetGC, is present in outer segments and is regulated by calcium and a soluble activator. Neuron. 1994;12:1345–1352. doi: 10.1016/0896-6273(94)90449-9. [DOI] [PubMed] [Google Scholar]
- Eckmiller MS. Renewal of the ciliary axoneme in cone outer segments of the retina of Xenopus laevis. Cell Tissue Res. 1996;285:165–169. doi: 10.1007/s004410050632. [DOI] [PubMed] [Google Scholar]
- Eggenschwiler JT, Anderson KV. Cilia and developmental signaling. Annu Rev Cell Dev Biol. 2007;23:345–373. doi: 10.1146/annurev.cellbio.23.090506.123249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JE, Snow JJ, Gunnarson AL, Ou G, Stahlberg H, McDonald KL, Scholey JM. Functional modulation of IFT kinesins extends the sensory repertoire of ciliated neurons in Caenorhabditis elegans. J Cell Biol. 2006;172:663–669. doi: 10.1083/jcb.200509115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fariss RN, Molday RS, Fisher SK, Matsumoto B. Evidence from normal and degenerating photoreceptors that two outer segment integral membrane proteins have separate transport pathways. J Comp Neurol. 1997;387:148–156. doi: 10.1002/(sici)1096-9861(19971013)387:1<148::aid-cne12>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Fliegauf M, Horvath J, von Schnakenburg C, Olbrich H, Muller D, Thumfart J, Schermer B, Pazour GJ, Neumann HP, Zentgraf H, Benzing T, Omran H. Nephrocystin specifically localizes to the transition zone of renal and respiratory cilia and photoreceptor connecting cilia. J Am Soc Nephrol. 2006;17:2424–2433. doi: 10.1681/ASN.2005121351. [DOI] [PubMed] [Google Scholar]
- Follit JA, Tuft RA, Fogarty KE, Pazour GJ. The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly. Mol Biol Cell. 2006;17:3781–3792. doi: 10.1091/mbc.E06-02-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giessl A, Trojan P, Rausch S, Pulvermuller A, Wolfrum U. Centrins, gatekeepers for the light-dependent translocation of transducin through the photoreceptor cell connecting cilium. Vision Research. 2006;46:4502–4509. doi: 10.1016/j.visres.2006.07.029. Epub 2006 Oct 4506. [DOI] [PubMed] [Google Scholar]
- Greiner JV, Weidman TA, Bodley HD, Greiner CAM. Ciliogenesis in photoreceptor cells of the retina. Experimental Eye Research. 1981;33:433–446. doi: 10.1016/s0014-4835(81)80094-2. [DOI] [PubMed] [Google Scholar]
- Guillaud L, Setou M, Hirokawa N. KIF17 dynamics and regulation of NR2B trafficking in hippocampal neurons. J Neurosci. 2003;23:131–140. doi: 10.1523/JNEUROSCI.23-01-00131.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett MA, Delaat JL, Arikawa K, Schlamp CL, Kong F, Williams DS. Distribution of guanylate cyclase within photoreceptor outer segments. J Cell Sci. 1996;109(Pt 7):1803–1812. doi: 10.1242/jcs.109.7.1803. [DOI] [PubMed] [Google Scholar]
- Hong DH, Pawlyk B, Sokolov M, Strissel KJ, Yang J, Tulloch B, Wright AF, Arshavsky VY, Li TS. RPGR isoforms in photoreceptor connecting cilia and the transitional zone of motile cilia. Invest Ophthalmol Vis Sci. 2003;44:2413–2421. doi: 10.1167/iovs.02-1206. [DOI] [PubMed] [Google Scholar]
- Horst CJ, Forestner DM, Besharse JC. Cytoskeletal-membrane interactions: a stable interaction between cell surface glycoconjugates and doublet microtubules of the photoreceptor connecting cilium. JCB. 1987;105:2973–2987. doi: 10.1083/jcb.105.6.2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst CJ, Johnson LV, Besharse JC. Transmembrane assemblage of the photoreceptor connecting cilium and motile cilium transition zone contain a common immunologic epitope. Cell Motility and the Cytoskeleton. 1990;17:329–344. doi: 10.1002/cm.970170408. [DOI] [PubMed] [Google Scholar]
- Humphries MM, Rancourt D, Farrar GJ, Kenna P, Hazel M, Bush RA, Sieving PA, Sheils DM, McNally N, Creighton P, Erven A, Boros A, Gulya K, Capecchi MR, Humphries P. Retinopathy induced in mice by targeted disruption of the rhodopsin gene. Nat Genet. 1997;15:216–219. doi: 10.1038/ng0297-216. [DOI] [PubMed] [Google Scholar]
- Huttl S, Michalakis S, Seeliger M, Luo DG, Acar N, Geiger H, Hudl K, Mader R, Haverkamp S, Moser M, Pfeifer A, Gerstner A, Yau KW, Biel M. Impaired channel targeting and retinal degeneration in mice lacking the cyclic nucleotide-gated channel subunit CNGB1. J Neurosci. 2005;25:130–138. doi: 10.1523/JNEUROSCI.3764-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illing M, Molday LL, Molday RS. The 220-kDa Rim protein of retinal rod outer segments is a member of the ABC transporter superfamily. JBC. 1997;272:10303–10310. doi: 10.1074/jbc.272.15.10303. [DOI] [PubMed] [Google Scholar]
- Inglis PN, Ou G, Leroux MR, Scholey JM. The sensory cilia of Caenorhabditis elegans. WormBook. 2007:1–22. doi: 10.1895/wormbook.1.126.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insinna C, Pathak P, Perkins B, Drummond I, Besharse JC. The homodimeric kinesin, Kif17, is essential for vertebrate photoreceptor sensory outer segment development. Developmental Biology (published online) 2008 doi: 10.1016/j.ydbio.2008.01.025. http://dx.doi.org/10.1016/j.ydbio.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen HG, Sanyal S. Development and degeneration of retina in rds mutant mice: electron microscopy. J Comp Neurol. 1984;224:71–84. doi: 10.1002/cne.902240107. [DOI] [PubMed] [Google Scholar]
- Jenkins PM, Hurd TW, Zhang L, McEwen DP, Brown RL, Margolis B, Verhey KJ, Martens JR. Ciliary targeting of olfactory CNG channels requires the CNGB1b subunit and the kinesin-2 motor protein, KIF17. Curr Biol. 2006;16:1211–1216. doi: 10.1016/j.cub.2006.04.034. [DOI] [PubMed] [Google Scholar]
- Jimeno D, Feiner L, Lillo C, Teofilo K, Goldstein LS, Pierce EA, Williams DS. Analysis of kinesin-2 function in photoreceptor cells using synchronous Cre-loxP knockout of Kif3a with RHO-Cre. Invest Ophthalmol Vis Sci. 2006;47:5039–5046. doi: 10.1167/iovs.06-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan MW, Iwata RT, Sears RC. Lengths of immunolabeled ciliary microtubules in frog photoreceptor outer segments. Exp Eye Res. 1987;44:623–632. doi: 10.1016/s0014-4835(87)80134-3. [DOI] [PubMed] [Google Scholar]
- Kedzierski W, Bok D, Travis GH. Non-cell-autonomous photoreceptor degeneration in rds mutant mice mosaic for expression of a rescue transgene. J Neurosci. 1998;18:4076–4082. doi: 10.1523/JNEUROSCI.18-11-04076.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knabe W, Kuhn HJ. Ciliogenesis in photoreceptor cells of the tree shrew retina. Anat.Embryol.(Berl) 1997;196:123–131. doi: 10.1007/s004290050085. [DOI] [PubMed] [Google Scholar]
- Kozminski KG, Beech PL, Rosenbaum JL. The Chlamydomonas kinesin-like protein FLA10 is involved in motility associated with the flagellar membrane. JCB. 1995;131:1517–1527. doi: 10.1083/jcb.131.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVail MM. Kinetics of rod outer segment renewal in the developing mouse retina. JCB. 1973;58:650–661. doi: 10.1083/jcb.58.3.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVail MM. Rod outer segment disc shedding in rat retina: relationship to cyclic lighting. Science. 1976;194:1071–1074. doi: 10.1126/science.982063. [DOI] [PubMed] [Google Scholar]
- Lawrence CJ, Dawe RK, Christie KR, Cleveland DW, Dawson SC, Endow SA, Goldstein LS, Goodson HV, Hirokawa N, Howard J, Malmberg RL, McIntosh JR, Miki H, Mitchison TJ, Okada Y, Reddy AS, Saxton WM, Schliwa M, Scholey JM, Vale RD, Walczak CE, Wordeman L. A standardized kinesin nomenclature. J Cell Biol. 2004;167:19–22. doi: 10.1083/jcb.200408113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ES, Burnside B, Flannery JG. Characterization of peripherin/rds and rom-1 transport in rod photoreceptors of transgenic and knockout animals. Invest Ophthalmol Vis Sci. 2006;47:2150–2160. doi: 10.1167/iovs.05-0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ES, Flannery JG. Transport of truncated rhodopsin and its effects on rod function and degeneration. Invest Ophthalmol Vis Sci. 2007;48:2868–2876. doi: 10.1167/iovs.06-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lem J, Krasnoperova NV, Calvert PD, Kosaras B, Cameron DA, Nicolo M, Makino CL, Sidman RL. Morphological, physiological, and biochemical changes in rhodopsin knockout mice. Proc Natl Acad Sci U S A. 1999;96:736–741. doi: 10.1073/pnas.96.2.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Zuo J, Pierce EA. The retinitis pigmentosa 1 protein is a photoreceptor microtubule-associated protein. J Neurosci. 2004;24:6427–6436. doi: 10.1523/JNEUROSCI.1335-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Bulgakov OV, Darrow KN, Pawlyk B, Adamian M, Liberman MC, Li T. Usherin is required for maintenance of retinal photoreceptors and normal development of cochlear hair cells. Proc Natl Acad Sci U S A. 2007;104:4413–4418. doi: 10.1073/pnas.0610950104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Seno K, Nishizawa Y, Hayashi F, Yamazaki A, Matsumoto H, Wakabayashi T, Usukura J. Ultrastructural localization of retinal guanylate cyclase in human and monkey retinas. Exp Eye Res. 1994;59:761–768. doi: 10.1006/exer.1994.1162. [DOI] [PubMed] [Google Scholar]
- Liu XR, Udovichenko IP, Brown SDM, Steel KP, Williams DS. Myosin VIIa participates in opsin transport through the photoreceptor cilium. Journal of Neuroscience. 1999;19:6267–6274. doi: 10.1523/JNEUROSCI.19-15-06267.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XR, Vansant G, Udovichenko IP, Wolfrum U, Williams DS. Myosin VIIa, the product of the Usher 1B syndrome gene, is concentrated in the connecting cilia of photoreceptor cells. Cell Motility and the Cytoskeleton. 1997;37:240–252. doi: 10.1002/(SICI)1097-0169(1997)37:3<240::AID-CM6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Loewen CJ, Molday RS. Disulfide-mediated oligomerization of Peripherin/Rds and Rom-1 in photoreceptor disk membranes. Implications for photoreceptor outer segment morphogenesis and degeneration. J Biol Chem. 2000;275:5370–5378. doi: 10.1074/jbc.275.8.5370. [DOI] [PubMed] [Google Scholar]
- Luby-Phelps K, Fogerty J, Baker SA, Pazour GJ, Besharse JC. Spatial Distribution of Intraflagellar Transport Proteins in Vertebrate Photoreceptors. Vision Research. 2008;48:413–423. doi: 10.1016/j.visres.2007.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucker BF, Behal RH, Qin H, Siron LC, Taggart WD, Rosenbaum JL, Cole DG. Characterization of the intraflagellar transport complex B core: direct interaction of the IFT81 and IFT74/72 subunits. J Biol Chem. 2005;280:27688–27696. doi: 10.1074/jbc.M505062200. [DOI] [PubMed] [Google Scholar]
- Maerker T, van Wijk E, Overlack N, Kersten FF, McGee J, Goldmann T, Sehn E, Roepman R, Walsh EJ, Kremer H, Wolfrum U. A novel Usher protein network at the periciliary reloading point between molecular transport machineries in vertebrate photoreceptor cells. Hum Mol Genet. 2008;17:71–86. doi: 10.1093/hmg/ddm285. [DOI] [PubMed] [Google Scholar]
- Marszalek JR, Liu X, Roberts EA, Chui D, Marth JD, Williams DS, Goldstein LS. Genetic evidence for selective transport of opsin and arrestin by kinesin-II in mammalian photoreceptors. Cell. 2000;102:175–187. doi: 10.1016/s0092-8674(00)00023-4. [DOI] [PubMed] [Google Scholar]
- McGee J, Goodyear RJ, McMillan DR, Stauffer EA, Holt JR, Locke KG, Birch DG, Legan PK, White PC, Walsh EJ, Richardson GP. The very large G-protein-coupled receptor VLGR1: a component of the ankle link complex required for the normal development of auditory hair bundles. J Neurosci. 2006;26:6543–6553. doi: 10.1523/JNEUROSCI.0693-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud EJ, Yoder BK. The primary cilium in cell signaling and cancer. Cancer Research. 2006;66:6463–6467. doi: 10.1158/0008-5472.CAN-06-0462. [DOI] [PubMed] [Google Scholar]
- Mikami A, Tynan SH, Hama T, Luby-Phelps K, Saito T, Crandall JE, Besharse JC, Vallee RB. Molecular structure of cytoplasmic dynein 2 and its distribution in neuronal and ciliated cells. J Cell Sci. 2002;115:4801–4808. doi: 10.1242/jcs.00168. [DOI] [PubMed] [Google Scholar]
- Molday RS, Molday LL. Differences in the protein composition of bovine retinal rod outer segment disk and plasma membranes isolated by a ricin-gold-dextran density perturbation method. JCB. 1987;105:2589–2601. doi: 10.1083/jcb.105.6.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muresan V, Bendala-Tufanisco E, Hollander BA, Besharse JC. Evidence for kinesin-related proteins associated with the axoneme of retinal photoreceptors. Experimental Eye Research. 1997;64:895–903. doi: 10.1006/exer.1996.0261. [DOI] [PubMed] [Google Scholar]
- Muresan V, Besharse JC. Complex intermolecular interactions maintain a stable linkage between the photoreceptor connecting cilium axoneme and plasma membrane. Cell Motility and the Cytoskeleton. 1994;28:213–230. doi: 10.1002/cm.970280305. [DOI] [PubMed] [Google Scholar]
- Muresan V, Lyass A, Schnapp BJ. The kinesin motor KIF3A is a component of the presynaptic ribbon in vertebrate photoreceptors. Journal of Neuroscience. 1999;19:1027–1037. doi: 10.1523/JNEUROSCI.19-03-01027.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peranen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, Jackson PK. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–1213. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- Nair KS, Hanson SM, Mendez A, Gurevich EV, Kennedy MJ, Shestopalov VI, Vishnivetskiy SA, Chen J, Hurley JB, Gurevich VV, Slepak VZ. Light-dependent redistribution of arrestin in vertebrate rods is an energy-independent process governed by protein-protein interactions. Neuron. 2005;46:555–567. doi: 10.1016/j.neuron.2005.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou G, Blacque OE, Snow JJ, Leroux MR, Scholey JM. Functional coordination of intraflagellar transport motors. Nature. 2005;436:583–587. doi: 10.1038/nature03818. [DOI] [PubMed] [Google Scholar]
- Pan X, Ou G, Civelekoglu-Scholey G, Blacque OE, Endres NF, Tao L, Mogilner A, Leroux MR, Vale RD, Scholey JM. Mechanism of transport of IFT particles in C. elegans cilia by the concerted action of kinesin-II and OSM-3 motors. J Cell Biol. 2006;174:1035–1045. doi: 10.1083/jcb.200606003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papermaster DS, Schneider BG, Besharse JC. Vesicular transport of newly synthesized opsin from the Golgi apparatus toward the rod outer segment: Ultrastructural immunocytochemical and autoradiographic evidence in Xenopus retinas. IOVS. 1985;26:1386–1404. [PubMed] [Google Scholar]
- Pathak N, Obara T, Mangos S, Liu Y, Drummond IA. The zebrafish fleer gene encodes an essential regulator of cilia tubulin polyglutamylation. Mol Biol Cell. 2007;18:4353–4364. doi: 10.1091/mbc.E07-06-0537. Epub 2007 Aug 4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Baker SA, Deane JA, Cole DG, Dickert BL, Rosenbaum JL, Witman GB, Besharse JC. The intraflagellar transport protein, IFT88, is essential for vertebrate photoreceptor assembly and maintenance. J Cell Biol. 2002a;157:103–113. doi: 10.1083/jcb.200107108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, San Agustin JT, Follit JA, Rosenbaum JL, Witman GB. Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in orpk mice with polycystic kidney disease. Curr Biol. 2002b;12:R378–R380. doi: 10.1016/s0960-9822(02)00877-1. [DOI] [PubMed] [Google Scholar]
- Pedersen LB, Miller MS, Geimer S, Leitch JM, Rosenbaum JL, Cole DG. Chlamydomonas IFT172 is encoded by FLA11, interacts with CrEB1, and regulates IFT at the flagellar tip. Curr Biol. 2005;15:262–266. doi: 10.1016/j.cub.2005.01.037. [DOI] [PubMed] [Google Scholar]
- Peet JA, Bragin A, Calvert PD, Nikonov SS, Mani S, Zhao X, Besharse JC, Pierce EA, Knox BE, Pugh EN., Jr Quantification of the cytoplasmic spaces of living cells with EGFP reveals arrestin-EGFP to be in disequilibrium in dark adapted rod photoreceptors. J Cell Sci. 2004;117:3049–3059. doi: 10.1242/jcs.01167. [DOI] [PubMed] [Google Scholar]
- Pulvermüller A, Giessl A, Heck M, Wottrich R, Schmitt A, Ernst OP, Choe HW, Hofmann KP, Wolfrum U. Calcium-dependent assembly of centrin-G-protein complex in photoreceptor cells. Mol Cell Biol. 2002;22:2194–2203. doi: 10.1128/MCB.22.7.2194-2203.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H, Burnette DT, Bae YK, Forscher P, Barr MM, Rosenbaum JL. Intraflagellar transport is required for the vectorial movement of TRPV channels in the ciliary membrane. Curr Biol. 2005;15:1695–1699. doi: 10.1016/j.cub.2005.08.047. [DOI] [PubMed] [Google Scholar]
- Qin H, Diener DR, Geimer S, Cole DG, Rosenbaum JL. Intraflagellar transport (IFT) cargo: IFT transports flagellar precursors to the tip and turnover products to the cell body. J Cell Biol. 2004;164:255–266. doi: 10.1083/jcb.200308132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese TS. Olfactory cilia in the frog. J. Cell Biol. 1965;25:209–230. doi: 10.1083/jcb.25.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlich P. The sensory cilium of retinal rods is analogous to the transitional zone of motile cilia. Cell and Tissue Research. 1975;161:421–430. doi: 10.1007/BF00220009. [DOI] [PubMed] [Google Scholar]
- Roof D, Adamian M, Jacobs D, Hayes A. Cytoskeletal specializations at the rod photoreceptor distal tip. JCN. 1991;305:289–303. doi: 10.1002/cne.903050210. [DOI] [PubMed] [Google Scholar]
- Rosenbaum JL, Cole DG, Diener DR. Intraflagellar transport: The eyes have it. JCB. 1999;144:385–388. doi: 10.1083/jcb.144.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev Mol Cell Biol. 2002;3:813–825. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- Sale WS, Besharse JC, Piperno G. Distribution of acetylated α-tubulin in retina and in in vitro-assembled microtubules. Cell Motility and the Cytoskeleton. 1988;9:243–253. doi: 10.1002/cm.970090306. [DOI] [PubMed] [Google Scholar]
- Schneider BG, Shyjan AW, Levenson R. Co-localization and polarized distribution of Na,K-ATPase alpha 3 and beta 2 subunits in photoreceptor cells. J Histochem Cytochem. 1991;39:507–517. doi: 10.1177/39.4.1848572. [DOI] [PubMed] [Google Scholar]
- Scholey JM. Intraflagellar transport motors in cilia: moving along the cell's antenna. J Cell Biol. 2008;180:23–29. doi: 10.1083/jcb.200709133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setou M, Nakagawa T, Seog DH, Hirokawa N. Kinesin superfamily motor protein KIF17 and mLin-10 in NMDA receptor-containing vesicle transport. Science. 2000;288:1796–1802. doi: 10.1126/science.288.5472.1796. [DOI] [PubMed] [Google Scholar]
- Signor D, Wedaman KP, Orozco JT, Dwyer ND, Bargmann CI, Rose LS, Scholey JM. Role of a class DHC1b dynein in retrograde transport of IFT motors and IFT raft particles along cilia, but not dendrites, in chemosensory neurons of living Caenorhabditis elegans. JCB. 1999;147:519–530. doi: 10.1083/jcb.147.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla V, Reiter JF. The primary cilium as the cell's antenna: signaling at a sensory organelle. Science. 2006;313:629–633. doi: 10.1126/science.1124534. [DOI] [PubMed] [Google Scholar]
- Snell WJ, Pan J, Wang Q. Cilia and flagella revealed: from flagellar assembly in Chlamydomonas to human obesity disorders. Cell. 2004;117:693–697. doi: 10.1016/j.cell.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Snow JJ, Ou G, Gunnarson AL, Walker MR, Zhou HM, Brust-Mascher I, Scholey JM. Two anterograde intraflagellar transport motors cooperate to build sensory cilia on C. elegans neurons. Nat Cell Biol. 2004;6:1109–1113. doi: 10.1038/ncb1186. [DOI] [PubMed] [Google Scholar]
- Sokolov M, Lyubarsky AL, Strissel KJ, Savchenko AB, Govardovskii VI, Pugh EN, Jr, Arshavsky VY. Massive light-driven translocation of transducin between the two major compartments of rod cells: a novel mechanism of light adaptation. Neuron. 2002;34:95–106. doi: 10.1016/s0896-6273(02)00636-0. [DOI] [PubMed] [Google Scholar]
- Spencer M, Detwiler PB, Bunt-Milam AH. Distribution of membrane proteins in mechanically dissociated retinal rods. IOVS. 1988;29:1012–1020. [PubMed] [Google Scholar]
- Steinberg RH, Wood I. Clefts and microtubules of photoreceptor outer segments in the retina of the domestic cat. J Ultrastruct Res. 1975;51:307–403. doi: 10.1016/s0022-5320(75)80102-x. [DOI] [PubMed] [Google Scholar]
- Strissel KJ, Sokolov M, Trieu LH, Arshavsky VY. Arrestin translocation is induced at a critical threshold of visual signaling and is superstoichiometric to bleached rhodopsin. J Neurosci. 2006;26:1146–1153. doi: 10.1523/JNEUROSCI.4289-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyasu K, Yamada E. The fine structure of the retina studied with the electron microscope. IV. Morphogenesis of outer segments of retinal rods. J Biophys Biochem Cytol. 1959;6:225–230. doi: 10.1083/jcb.6.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujikawa M, Malicki J. Intraflagellar transport genes are essential for differentiation and survival of vertebrate sensory neurons. Neuron. 2004;42:703–716. doi: 10.1016/s0896-6273(04)00268-5. [DOI] [PubMed] [Google Scholar]
- Wang Q, Pan J, Snell WJ. Intraflagellar transport particles participate directly in cilium-generated signaling in Chlamydomonas. Cell. 2006;125:549–562. doi: 10.1016/j.cell.2006.02.044. [DOI] [PubMed] [Google Scholar]
- Webber WA, Lee J. Fine structure of mammalian renal cilia. Anat Rec. 1975;182:339–343. doi: 10.1002/ar.1091820307. [DOI] [PubMed] [Google Scholar]
- Weng J, Mata NL, Azarian SM, Tzekov RT, Birch DG, Travis GH. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt's disease from the phenotype in abcr knockout mice. Cell. 1999;98:13–23. doi: 10.1016/S0092-8674(00)80602-9. [DOI] [PubMed] [Google Scholar]
- Whitehead JL, Wang SY, Bost-Usinger L, Hoang E, Frazer KA, Burnside B. Photoreceptor localization of the KIF3A and KIF3B subunits of the heterotrimeric microtubule motor kinesin II in vertebrate retina. Experimental Eye Research. 1999;69:491–503. doi: 10.1006/exer.1999.0724. [DOI] [PubMed] [Google Scholar]
- Williams DS. Transport to the photoreceptor outer segment by myosin VIIa and kinesin II. Vision Res. 2002;42:455–462. doi: 10.1016/s0042-6989(01)00228-0. [DOI] [PubMed] [Google Scholar]
- Williams DS, Linberg KA, Vaughan DK, Fariss RN, Fisher SK. Disruption of microfilament organization and deregulation of disk membrane morphogenesis by cytochalasin D in rod and cone photoreceptors. JCN. 1988;272:161–176. doi: 10.1002/cne.902720202. [DOI] [PubMed] [Google Scholar]