Abstract
Background
Although the rationale for earlier screening of persons with a family history of colorectal cancer is plausible, there is no direct evidence that earlier assessment is either effective or cost-effective.
Objective
To estimate the clinical and economic effect of using family history assessment to identify persons for colorectal cancer screening before age 50.
Methods
We developed a decision model to compare costs and outcomes for two scenarios: (a) standard population screening starting at age 50; (b) family history assessment at age 40, followed by screening colonoscopy at age 40 for those with a suggestive family history of colorectal cancer. The analysis was conducted using the health insurer perspective.
Results
Using U.S. population estimates, 22 million would be eligible for family history assessment, and one million would be eligible for early colonoscopy; 2,834 invasive cancers would be detected, and 29,331 life years would be gained. The initial program cost would be $900 million. The discounted cost per life year gained of family history assessment versus no assessment equals $58,228. The results were most sensitive to the life expectancy benefit from earlier screening, the cost of colonoscopy, and the relative risk of colon cancer in those with a family history.
Conclusions
The cost-effectiveness of family history assessment for colorectal cancer approaches that of other widely accepted technologies; yet, the results are sensitive to several assumptions where better data are needed. Because of the relatively high prevalence of family history in the population, careful analysis and empirical data are needed.
Introduction
Family history assessment is a potentially valuable tool for reducing the burden of colorectal cancer. People with a first-degree relative (parent, sibling, or child) with colon cancer diagnosed at age <60 years or with more than one first-degree relative diagnosed with colorectal cancer at any age are at increased risk for developing cancer and are more likely to be diagnosed at an earlier age (1). Traditional ascertainment of family history in practice is a gateway to find families at high risk for rare single-gene Mendelian disorders. Moreover, family history is also an independent risk factor, reflecting multiple genes of higher prevalence and environmental exposures that are as yet poorly characterized. More than identifying relatively rare families with extreme cancer risk, family history assessment’s major potential benefit may thus lie in identifying large numbers of persons who are at moderately increased risk for developing colorectal cancer. Accordingly, several clinical practice guidelines recommend that persons meeting family history criteria should be advised to begin colon cancer screening at an earlier age than the general population (2–5).
Although the epidemiologic and biological rationale for earlier screening of persons with a family history is plausible, there is no direct evidence that earlier screening is either effective or cost-effective. Family history assessment is not uniformly practiced in clinical settings, and there are no specific programs under way to increase family history assessment in the general population. Additionally, many more people in the population have suggestive family histories than those who carry single-gene disorders for major cancer susceptibility; thus, any assessment program will result in many more false than true positives. The clinical and economic implications of promoting population-wide family history assessment are great: tens of millions of adults would be assessed, and 10% to 15% of those evaluated would likely meet criteria for more aggressive screening programs (2, 3). Implementing such programs would also greatly affect primary care physicians, specialists, and public and private health care payers.
To better evaluate these issues, we developed a decision model to characterize the clinical and economic implications of implementing family history assessment programs in primary care settings to identify persons at increased risk for developing colorectal cancer. Our model addresses people at “moderate” risk of colorectal cancer because of their family history and does not address the costs and benefits of assessment for rare Mendelian disorders, such as familial adenomatous polyposis and hereditary nonpolyposis colorectal cancer, as they have been discussed elsewhere (6–9).
Materials and Methods
Conceptual Issues
The preclinical course of colorectal cancer in persons with a family history of colorectal cancer but do not carry high-risk mutations is not well known. There is widespread agreement that most colorectal cancers develop from adenomatous polyps, although the true proportion is a source of some debate (10, 11). Persons with a family history of colorectal cancer are more likely to develop cancer at a younger age (1, 12); yet, it is not clear whether this is due to differences in the risk of developing polyps, the age at which polyps develop, the risk and rate that polyps progress to invasive cancer, or a combination of these factors (13, 14). Some of the paucity of evidence is reflected in the level of variation in recommendations of the guidelines developed by leading organizations (Table 1).
Table 1.
Recommendations regarding use of family history to assess risk of colorectal cancer
| Source | Risk categories | Screening schedule |
|---|---|---|
| American Cancer Society (34) | CRC or AdP in ≥1 first-degree relative before age 60 or ≥2 first-degree relatives at any age (excluding HNPCC and FAP) | Colonoscopy every 5–10 y, starting at age 40 |
| American Gastroenterological Association (2) | ≥2 first-degree relatives with CRC/AdP, or ≥1 first degree-relative affected before age 60
1 first-degree relative affected with CRC/AdP at or after age 60* |
Colonoscopy every 5 y, beginning at age 40 or 10 y before youngest diagnosis in family, whichever came first
Same as average risk, but beginning at age 40 |
| Canadian Task Force on Preventive Health Care (35) | 1 or 2 first-degree relatives with CRC
>2 relatives with CRC |
Same as average risk
Consider genetic screening |
| U.S. Preventive Services Task Force | ≥1 first-degree relative with CRC onset before age 60 | Any screening modality, starting at age 40 |
Abbreviations: AdP, adenomatous polyp; CRC, colorectal cancer; FOBT, fecal occult blood testing; Flex sig, flexible sigmoidoscopy; HNPCC, hereditary nonpolyposis colorectal cancer; FAP, familial adenomatous polyposis.
Used for the current analysis.
For decision modeling, it is important to be precise in defining the family history criteria, as well as the age at onset, mode, and frequency of screening of persons with a “positive” family history. It is also necessary to make certain simplifying assumptions to make the modeling exercise tractable and to convey important concepts with clarity. We know that a certain fraction of healthy individuals in a population will develop colorectal polyps, and that the number of polyps observed in a given population increases with the age of the cohort. In the absence of screening, a fraction of the polyps that develop will transform into invasive cancers; current estimates suggest that about 5% will do so (15–17). The sojourn time from polyp onset to invasive cancer will vary but on average may be 5 to 10 years (13, 18, 19). Figure 1A displays this concept of polyp development and transformation. Polyp behavior may be different in persons who, because of their family history, are at increased risk for colorectal cancer. Below, we outline two possible approaches to characterizing polyp development and transformation in those with a family history.
Figure 1.
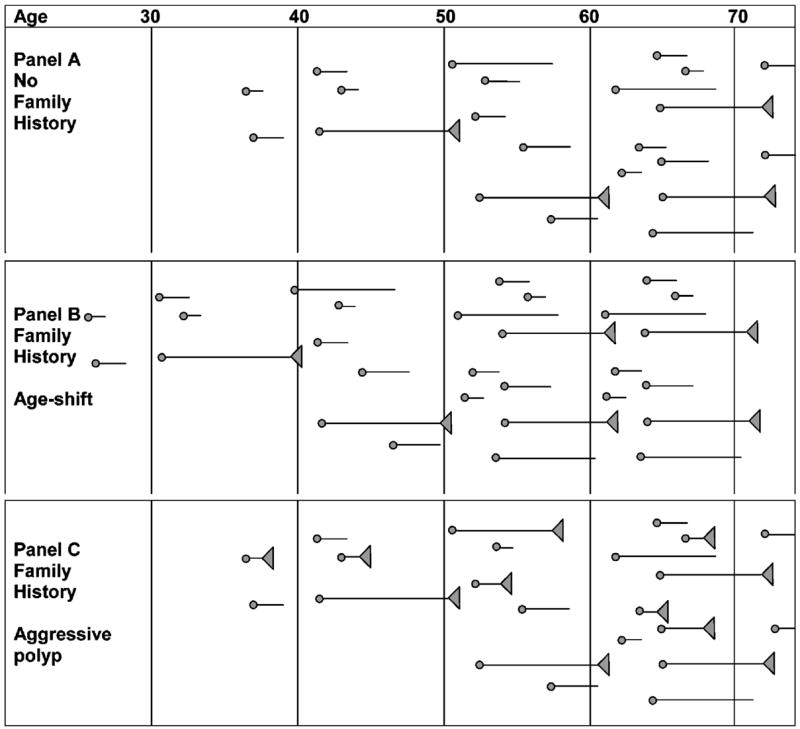
Conceptualization of polyp development under the “age shift” and “aggressive polyp” hypotheses. A. Polyp development in average-risk persons. B. Polyps (age shift) begin earlier, but the rate of transformation to malignancy is the same as for those without a family history. C. The age at onset is the same (aggressive polyp), but a higher proportion of polyps develop into invasive malignancies, and the sojourn time between onset and invasive disease is often shorter.
 , polyp onset times.
, polyp onset times.
 , polyps that transform into invasive cancers.
, polyps that transform into invasive cancers.
“Polyp Age Shift” Hypothesis
One plausible scenario is that persons with a family history develop more polyps at an earlier age. In this case, rate of development and risk of transformation into invasive cancer are the same as for those without a family history but because individuals have more polyps, more are available to transform into cancers at a younger age. (Fig. 1B) The rare inherited condition familial adenomatous polyposis, caused by a mutation in the adenomatous polyposis coli (APC) gene, is often offered as biological support of this hypothesis. Persons with familial adenomatous polyposis develop thousands of polyps throughout their colon at a very young age, and although the risk of any one polyp progressing to colorectal cancer is very modest, the sheer number of polyps results in affected persons having a near 100% chance of developing colon cancer before age 60 (20). Other evidence can be found in an analysis of two large cohorts involving >840,000 patient-years of follow-up, where a family history of colorectal cancer was associated with a significant increase in risk in younger persons (1.7- to 4-fold increase between ages 40 and 60 years) but not with a significantly increased risk for persons older than age 60 (21).
“Aggressive Polyp” Hypothesis
Another plausible scenario is that persons with a family history develop polyps at the same rate as those without family history, but the risk of transformation into invasive cancer is greater (Fig. 1C). In addition to higher rates of transformation, the dwell times for “aggressive polyps” may be shorter than polyps in those without a family history. There is some biological evidence that polyps behave more aggressively in persons with a family history. Expansion of the proliferative compartment of epithelial cells in colonic crypts and colonic adenomas have been described in individuals from families with high rates of colon cancer (22). It has been noted that DNA methylation is a common characteristic in colorectal tumors, and that patients with extensively methylated tumors are also more likely to have early onset cancer and affected family members (23–25). Epidemiologic studies have shown that adenomatous polyps diagnosed in a first-degree relative before age 60 increases the risk of colorectal cancer (1). This evidence suggests that genetic factors may predispose persons to such problems as DNA methylation and thus higher risk for neoplastic transformation, even if the number of adenomatous polyps that develop are similar to those without a family history.
The true pathophysiology of colorectal cancer in persons with a family history is likely to be a hybrid of both the stage shift and aggressive polyp hypotheses. Discovering this process (as well as the degree of heterogeneity) is critical to devising an efficient assessment strategy for this population.
Conceptual Issues and Simulation Modeling
Simulation models are useful when the clinical or policy decision is complex, and the information about risks, benefits, and costs of different therapy or screening options is uncertain (26). Models can identify important clinical, epidemiologic, and economic data that “drive” the outcome of the simulation. If the evidence supporting these variables is weak or nonexistent, models can be used to support requests for further study. The uncertainties surrounding early screening for persons with a family history of early colorectal cancer fits the modeling scenario well.
In addition to polyp behavior, several other issues are also important to factor into the simulation model. The accuracy of family history assessment (particularly specificity, as is true for other screening tests), the performance of the cancer screening modality at detecting precancerous lesions, and the screening schedule all influence screening efficacy.
Conceptual Issues for Cost-effectiveness Analysis
We wish to determine whether family history assessment is cost-effective compared with standard care (no risk stratification, universal screening starting at age 50) for the adult population. When comparing a new therapy with an existing therapy for a given population with a given medical condition, cost-effectiveness is measured as the change in costs of care for new technology compared with the existing therapy, relative to the change in effectiveness of new therapy compared with the existing therapy. The difference in costs over the difference in effectiveness of family history assessment versus standard care, known as the incremental cost-effectiveness of family history assessment, can be derived using the following formula:
where CFmHx and CSC refer to average total costs, and EFmHx and ESC refer to average total effectiveness for the family history assessment and standard care arms, respectively. In the family history assessment arm, individuals incur screening costs (and polypectomy costs) but are spared from the treatment costs of colon cancer. In the standard care arm, no screening costs are incurred, but care costs are incurred for those who present as clinically diagnosed colorectal cancers before age 50. Effectiveness can be defined as polyps detected, invasive cancers prevented, life years gained, or quality-adjusted life years gained. Costs and benefits are considered as they accrue over a lifetime starting from the point of assessment (or no assessment) family history.
Model Assumptions
Our model is designed to compare two scenarios: (a) population screening using colonoscopy beginning at age 50 (standard care); (b) family history assessment at age 40, with stratification into those who begin screening with colonoscopy at age 40 (“positive family history”) and the remainder who would begin screening at age 50 (Fig. 2). Models must make assumptions about the disease, the setting in which assessment for the disease will take place, and the perspective of the analysis; that is, from what (or who’s) point of view the analysis is structured. Our model starts with the assumption that disease progression follows the “polyp age shift” hypothesis, because this idea seems to underlie the majority of screening guidelines for persons with a family history. Specifically, the model characterizes persons with a suggestive family history of colon cancer as having a higher than average risk of developing a polyp at age 40 than an average risk person (no family history). We will later analyze the cost-effectiveness of assessment guidelines under the “aggressive polyp” hypothesis. In the aggressive polyp scenario, the model assigns a higher risk of malignant transformation for each polyp. Polyp sojourn times are based on estimates in the published literature. The setting includes all U.S. residents ages 40 to 45 years with a “positive” family history and therefore would be eligible for screening. In accordance with current guidelines, those with one or more first-degree relatives who were diagnosed with colorectal cancer before age 60 years or with two first-degree relatives diagnosed at any age are considered “positive” and therefore eligible for screening (2). We exclude those with histories suggestive of familial cancer syndromes (e.g., hereditary nonpolyposis colorectal cancer and familial adenomatous polyposis), as they are a small proportion of those having close relatives with colon cancer, screening guidelines are quite different (7, 27), and cost-effectiveness analyses have already been conducted for these syndromes (6, 8). Following guideline specifications for screening frequency for persons with the family history noted above, we assume that those who are eligible begin colonoscopy at age 40 are screened every 10 years thereafter (2).
Figure 2.
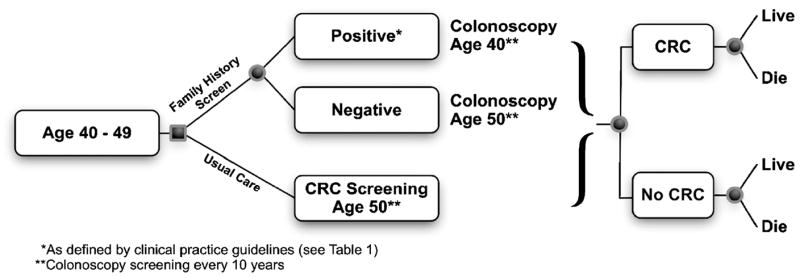
Decision tree and outcomes for family history assessment for colorectal cancer susceptibility.
Many epidemiologic and clinical factors will determine the efficacy and cost-effectiveness of family history assessment, and we must make assumptions about these for our base case. For example, the accuracy with which family histories are elicited and the choice of the screening modality and accuracy of that modality; individual adherence to screening recommendations, the clinical benefits for those who are screened (compared with no screening) in terms of number of advanced malignancies prevented, and years of life saved through screening. Table 2 lists factors that were explicitly considered for the model and values and ranges chosen for those factors. The analysis takes the perspective of assessment from a “best case” perspective (i.e., with variables that are generally favorable to assessment). If family history assessment is not cost-effective under favorable assumptions, it is unlikely to improve under less favorable (more realistic) assumptions. This is done to focus the analysis on the incremental benefits of having a national policy of beginning colon cancer assessment at age 40 (through risk stratification) rather than age 50. Later in the sensitivity analysis, we vary these estimates across a range of plausible values and determine how these changes influence the outcome of the analysis.
Table 2.
Data used to inform the decision model
| Item | Input | Low | High | Reference |
|---|---|---|---|---|
| Cost of family history screen | 1st/3rd of level III office visit, new patient ($40) | 32 | 100 | HCPC 99204 (36) |
| No. eligible screenees, U.S. | Adults age 40–44: 22,441,863 | — | — | U.S. Census (37) |
| Proportion with positive FmHx | 9% | 8% | 15% | PLCO trial (38) |
| Lead time for polyps | 6.4 y | 4 y | 9 y | Moss et al. (39), Launoy et al. (40) |
| Proportion of polyps that do not progress | 0.95 | 0.8 | 0.99 | Shinya et al. (15), Rex et al. (16), Gschwantler et al. (17) |
| Risk of perforation, screening colonoscopy | 1.96 per 1,000 | 0.5 per 1,000 | 2.5 per 1,000 | Gatto et al. (41) |
| Screening colonoscopy cost | $723 | $600 | $1,500 | HCPC G0105* (36, 42) |
| Proportion of positives receiving colonoscopy (adherence) | 75% | 50% | 100% | Estimate |
| Relative risk of developing colorectal cancer in person With family history, age 40–50 | 2.25 | 1.8 | 2.7 | Johns et al. (43) |
| Probability of developing colorectal cancer, age 40–50 | 0.185% | 0.148% | 0.222% | SEER STAT (44) |
| Life expectancy benefit for avoidance of colorectal cancer age 40–50 | 10.35 | 5 | 12 | Calculated (see article), Sonnenberg et al. (45) |
| Cost of treating perforation | $15,684 | $10,000 | $20,000 | |
| Cost of polypectomy and pathologic evaluation | $1,036 | $800 | $1,500 | HCPC 45385, 88305 † (36, 42) |
| Lifetime cost of treatment for colorectal cancer in age 40–50 | $51,600 | $40,000 | $60,000 | Brown et al. (46) |
| Efficacy of colonoscopy for removing precancerous polyps | 100% | Estimate | ||
| Discount rate for future costs and life years | 3% per annum | Gold et al. (47) |
Abbreviations: HCPC, Healthcare Common Procedure Code; PLCO, prostate, lung, colon, and ovary.
HCPC G0105 screening colonoscopy, high-risk individual (physician fee, $390; facility fee, $333).
HCPC 45385 lesion removal colonoscopy (physician fee, $599; facility fee, $333); 88305 Tissue exam by pathologist ($104).
Sensitivity Analysis
Sensitivity analysis tests the robustness of the model to changes in values for input variables. We performed one-way sensitivity analysis on all variables in the model and then arranged them in order based on the variables that had the greatest effect on the overall cost-effectiveness of the model. Ranges for each variable were based on 95% confidence intervals if available from the source data or by expert opinion.
We also did a multiway sensitivity analysis of the model. This was done by creating probability density functions for each variable and then randomly drawing observations from those distributions and rerunning the analysis while tracking descriptive statistics (mean, SD) of several key outputs. When these statistics converged (did not change more than ±1.5% between successive simulations), we halted the simulations. In cases where the distributions were unknown, we created triangular distributions with mean values equal to the base case value for the model, and the extremes at either end of the ranges used in the one-way analysis. The array of cost-effectiveness outcome points were then mapped onto a cost-effectiveness plane and 95% confidence intervals were tabulated.
Results
Among the 22 million persons ages 40 to 44 years, we assume half would receive family history assessment during a visit to physician offices. Family history assessment would identify ~1.1 million individuals who would be eligible for early screening with colonoscopy. Assuming 75% compliance with the initial recommended colonoscopy, screening would identify 2,834 polyps that would become symptomatic invasive cancers before age 50. Those who had these polyps removed before the onset of cancer are predicted to have 10.35 years added to their life expectancy, on average. Screening and polyp removal would add 29,331 years of life (undiscounted) to this group. The total cost of family history assessment, including colonoscopy, polypectomy, and rare perforations, would be $890 million per year, at an average cost per precancerous polyp detected of $314,500 (undiscounted). After discounting of future costs and life years that accrue to probands only, the incremental cost-effectiveness of family history assessment is $58,228 per life year gained.
Sensitivity Analysis
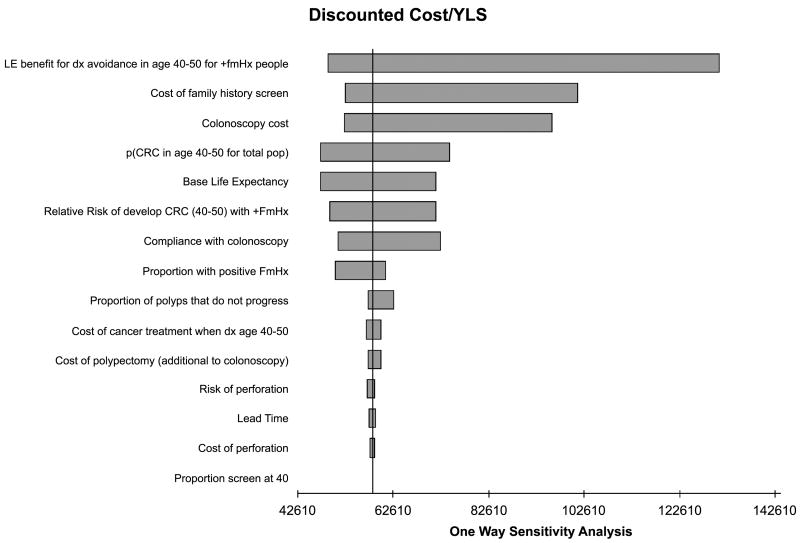
We varied individual inputs into the model to determine their effect on the overall cost-effectiveness of the assessment strategy. Figure 3 shows the results for the 15 variables that had the greatest effect on the outcome. By far the most important variable was the survival gain among those who have precancerous polyps removed. The next most influential factors, in declining order of importance, are the cost of colonoscopy, and the baseline risk of colorectal cancer between ages 40 to 49 in the average risk population.
Figure 3.
One-way sensitivity analysis.
We conducted an n-way probabilistic sensitivity analysis, based on replications of the analysis with values drawn from distributions for the 15 most sensitive variables from the one-way analysis. This analysis shows a mean cost-effectiveness of $94,428 per life year gained (median = $87,675), and a 90% confidence interval range from $53,705 per life year gained to $152,655 per life year gained. Based on these results, Fig. 4 shows a cost-effectiveness acceptability curve for family history assessment.
Figure 4.
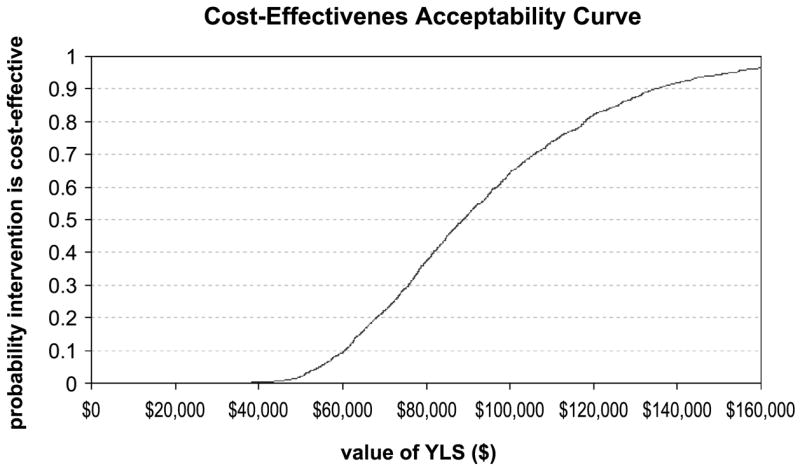
Cost-effectiveness acceptability curve for family history assessment. Probability that family history assessment is associated with a cost per life year gained that is lower than the corresponding cost-effectiveness ratios (x-axis). The value of the ceiling ratio at a probability of 0.5 is the expected cost per quality-adjusted life years for family history assessment.
Outcomes Under an Aggressive Polyp Scenario
Under the aggressive polyp scenario, most polyps arise after age 40, but a higher proportion transform into invasive cancers. In this case, screening colonoscopy at age 40 for those with suggestive family histories will identify fewer polyps that are destined to become invasive cancers. If we assume that 50% fewer polyps destined to become cancers are not detected at age 40 because they would develop after the initial screening but prior to age 50, the cost-effectiveness of assessment decreases to $121,922/life year saved.
Discussion
Family history assessment for colorectal cancer susceptibility is a costly but potentially beneficial intervention that has feasibility for implementation in clinical practice. We built a decision model to evaluate the cost-effectiveness of implementing such a program. Under generally favorable assumptions towards family risk assessment and using the early-onset hypothesis of polyp behavior in persons with family history, family history assessment may be modestly cost-effective. The most important factors influencing the outcome are the life expectancy gain for persons who undergo regular colonoscopy screening and the cost of colonoscopy. If polyp behavior is substantially different than theorized, simply shifting screening to an earlier age is much less cost-effective. Additional research that better defines the benefits of colonoscopy in 40-year-olds with suggestive family histories and clearer delineation of polyp behavior are the most critical information that is needed to better define the cost-effectiveness of assessment.
Our model does not address several other issues that ultimately will determine the success and cost-effectiveness of family history assessment. First, it is not clear how many individuals visit primary care physicians for preventive care near age 40, particularly men. Second, individuals need to have an opportunity to receive family history assessment. Acute care visits for acute or chronic medical care needs are often poor opportunities for assessment. Reducing the proportion who present for assessment does not influence cost-effectiveness of assessment itself; yet, it greatly diminishes the benefits that are realized for the population. Ultimately, other approaches for risk assessment, such as mailed surveys, might be more effective means of obtaining family risk information. Third, it is not certain that providing individuals with information about their risk of colorectal cancer will influence adherence to screening, both for those with and without suggestive family histories. Colon cancer screening is currently underused in the United States (28). Studies suggest that risk counseling can improve use of screening services both in those at increased and average risk for colorectal cancer (29–31); yet, it is not known whether counseling at age 40 would translate into higher rates of screening 10 years hence (for those at average risk). This model does not quantify the costs and benefits of identifying those who carry rare mutations with high disease penetrance, primarily hereditary nonpolyposis colorectal cancer and familial adenomatous polyposis. Cost-effectiveness analyses of screening for these conditions have been reported previously (8, 9, 32, 33). This model does not include administrative costs associated with starting and maintaining family history assessment programs. We only consider the effect of adherence to screening during age 40 to 50, because this is the place where practice changes in response to the guidelines. Although we do not explicitly address the issue, persons with a family history might adhere more closely to screening over their lifetimes compared with average risk persons. In this case, the model may underestimate the cost-effectiveness of family history screening. For the sake of simplicity, and because of lack of agreement among experts about the ideal schedule and efficacy of repeat screening after polypectomy, the model does not address the issue of interval follow-up for those who have adenomatous polyps removed, other than assuming that repeat screening occurs at age 50. Increases in use of screening of persons with suggestive family histories may affect both demand for and supply of colonoscopy over time. These issues, combined with changes in screening efficiency will ultimately influence the future price of the procedure. The costs of colorectal cancer are based on estimates for persons predominantly over age 65 and thus may underestimate to true lifetime costs for a younger person with this disease. Finally, the opportunity for family history assessment affords an opportunity to determine risks for several conditions that may benefit from screening and prevention, such as other cancers, diabetes, and coronary artery disease. It is reasonable to consider the costs and benefits of “global” risk assessment and counseling based on family history, and there are efficiencies of gathering all relevant information together. In addition, risk assessment offers an opportunity to counsel patients on other ways to reduce risk, such as reducing dietary fat and increasing exercise. On the other hand, such practice runs the risk of losing effectiveness by overwhelming the recipient with information about risks for multiple diseases, particularly for those without interventions that are known to be effective. The list of family history information that is useful will probably grow over time, with more precise genomic knowledge and practical and effective interventions.
Public and private health insurance plans generally do not cover costs associated with family history assessment, except (in some cases) as part of a general medical evaluation. Certainly, direct payment for service (i.e., billing codes) would encourage providers to take and document a family history. Health plans might argue that such payment is not justified until we have direct evidence of benefit, as was obtained for other screening procedures like mammography and colorectal cancer screening after age 50. Certainly, a prospective controlled trial of family history assessment combined with early colonoscopy for those with a suggestive family history would be costly, time consuming, and challenging to implement on a population level. As costly as such trials are in prevention, our analysis suggests that such an investment may be worthwhile, given the substantial potential costs and benefits involved in such programs.
This analysis considers guidelines and screening practices that are widely agreed upon in the United States. Other countries with greater constraints on health resource expenditures might consider less costly screening modalities (e.g., fecal occult blood testing and flexible sigmoidoscopy) or screening schedules. Simulation models can address the tradeoffs between cost, sensitivity, specificity, and feasibility in these situations.
Acknowledgments
We thank Jeremy Jass for helpful comments on previous versions of this article.
Grant support: National Cancer Institute grant R01 HG002941 and National Human Genome Research Institute grants R01 HG02263-01 and P50 HG 3374-01.
Footnotes
Note: A technical report detailing the model, inputs, and outputs is available upon request.
This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Winawer SJ, Zauber AG, Gerdes H, et al. Risk of colorectal cancer in the families of patients with adenomatous polyps. National Polyp Study Workgroup. N Engl J Med. 1996;334:82–7. doi: 10.1056/NEJM199601113340204. [DOI] [PubMed] [Google Scholar]
- 2.Winawer S, Fletcher R, Rex D, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale—update based on new evidence. Gastroenterology. 2003;124:544–60. doi: 10.1053/gast.2003.50044. [DOI] [PubMed] [Google Scholar]
- 3.Smith RA, von Eschenbach AC, Wender R, et al. American Cancer Society guidelines for the early detection of adenomatous polyps and colorectal cancer. American Cancer Society; 2001. [Google Scholar]
- 4.McLeod R. Screening strategies for colorectal cancer: systematic review and recommendations, 01 – 2. London (ON): Canadian Task Force; 2001. The Canadian Task Force on Preventive Health Care. [Google Scholar]
- 5.U.S. Preventive Services Task Force (USPSTF) Screening for colorectal cancer: recommendation and rationale. Ann Intern Med. 2002;137:129–31. doi: 10.7326/0003-4819-137-2-200207160-00014. [DOI] [PubMed] [Google Scholar]
- 6.Chikhaoui Y, Gelinas H, Joseph L, Lance JM. Cost-minimization analysis of genetic testing versus clinical screening of at-risk relatives for familial adenomatous polyposis. Int J Technol Assess Health Care. 2002;18:67–80. [PubMed] [Google Scholar]
- 7.Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96:261–8. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramsey SD, Clarke L, Etzioni R, Higashi M, Berry K, Urban N. Cost-effectiveness of microsatellite instability screening as a method for detecting hereditary nonpolyposis colorectal cancer. Ann Intern Med. 2001;135:577–88. doi: 10.7326/0003-4819-135-8_part_1-200110160-00008. [DOI] [PubMed] [Google Scholar]
- 9.Reyes CM, Allen BA, Terdiman JP, Wilson LS. Comparison of selection strategies for genetic testing of patients with hereditary nonpolyposis colorectal carcinoma: effectiveness and cost-effectiveness. Cancer. 2002;95:1848–56. doi: 10.1002/cncr.10910. [DOI] [PubMed] [Google Scholar]
- 10.Bond JH. Colon polyps and cancer. Endoscopy. 2003;35:27–35. doi: 10.1055/s-2003-36410. [DOI] [PubMed] [Google Scholar]
- 11.Jass JR. Pathogenesis of colorectal cancer. Surg Clin North Am. 2002;82:891–904. doi: 10.1016/s0039-6109(02)00047-6. [DOI] [PubMed] [Google Scholar]
- 12.Ahsan H, Neugut AI, Garbowski GC, et al. Family history of colorectal adenomatous polyps and increased risk for colorectal cancer. Ann Intern Med. 1998;128:900–5. doi: 10.7326/0003-4819-128-11-199806010-00006. [DOI] [PubMed] [Google Scholar]
- 13.Atkin WS, Morson BC, Cuzick J. Long-term risk of colorectal cancer after excision of rectosigmoid adenomas. N Engl J Med. 1992;326:658–62. doi: 10.1056/NEJM199203053261002. [DOI] [PubMed] [Google Scholar]
- 14.Cannon-Albright LA, Thomas A, Goldgar DE, et al. Familiality of cancer in Utah. Cancer Res. 1994;54:2378–85. [PubMed] [Google Scholar]
- 15.Shinya H, Wolff WI. Morphology, anatomic distribution and cancer potential of colonic polyps. Ann Surg. 1979;190:679–83. doi: 10.1097/00000658-197912000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rex DK, Lehman GA, Ulbright TM, et al. Colonic neoplasia in asymptomatic persons with negative fecal occult blood tests: influence of age, gender, and family history. Am J Gastroenterol. 1993;88:825–31. [PubMed] [Google Scholar]
- 17.Gschwantler M, Kriwanek S, Langner E, et al. High-grade dysplasia and invasive carcinoma in colorectal adenomas: a multivariate analysis of the impact of adenoma and patient characteristics. Eur J Gastroenterol Hepatol. 2002;14:183–8. doi: 10.1097/00042737-200202000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Morson BC. Evolution of cancer of the colon and rectum. Cancer. 1974;34:845–9. doi: 10.1002/1097-0142(197409)34:3+<845::aid-cncr2820340710>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 19.Hoff G, Sauar J, Vatn MH, et al. Polypectomy of adenomas in the prevention of colorectal cancer: 10 years’ follow-up of the Telemark Polyp Study I. A prospective, controlled population study. Scand J Gastroenterol. 1996;31:1006–10. doi: 10.3109/00365529609003121. [DOI] [PubMed] [Google Scholar]
- 20.Lal G, Gallinger S. Familial adenomatous polyposis. Semin Surg Oncol. 2000;18:314–23. doi: 10.1002/(sici)1098-2388(200006)18:4<314::aid-ssu6>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 21.Fuchs CS, Giovannucci EL, Colditz GA, Hunter DJ, Speizer FE, Willett WC. A prospective study of family history and the risk of colorectal cancer. N Engl J Med. 1994;331:1669–74. doi: 10.1056/NEJM199412223312501. [DOI] [PubMed] [Google Scholar]
- 22.Gerdes H, Gillin JS, Zimbalist E, Urmacher C, Lipkin M, Winawer SJ. Expansion of the epithelial cell proliferative compartment and frequency of adenomatous polyps in the colon correlate with the strength of family history of colorectal cancer. Cancer Res. 1993;53:279–82. [PubMed] [Google Scholar]
- 23.Jass JR, Whitehall VL, Young J, Leggett BA. Emerging concepts in colorectal neoplasia. Gastroenterology. 2002;123:862–76. doi: 10.1053/gast.2002.35392. [DOI] [PubMed] [Google Scholar]
- 24.Frazier ML, Xi L, Zong J, et al. Association of the CpG island methylator phenotype with family history of cancer in patients with colorectal cancer. Cancer Res. 2003;63:4805–8. [PubMed] [Google Scholar]
- 25.Chan AO, Issa JP, Morris JS, Hamilton SR, Rashid A. Concordant CpG island methylation in hyperplastic polyposis. Am J Pathol. 2002;160:529–36. doi: 10.1016/S0002-9440(10)64872-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramsey SD, McIntosh M, Etzioni R, Urban N. Simulation modeling of outcomes and cost effectiveness. Hematol Oncol Clin North Am. 2000;14:925–38. doi: 10.1016/s0889-8588(05)70319-1. [DOI] [PubMed] [Google Scholar]
- 27.King JE, Dozois RR, Lindor NM, Ahlquist DA. Care of patients and their families with familial adenomatous polyposis. Mayo Clin Proc. 2000;75:57–67. doi: 10.4065/75.1.57. [DOI] [PubMed] [Google Scholar]
- 28.From the Centers for Disease Control and Prevention. Colorectal cancer test use among persons aged > or = 50 years: United States, 2001. JAMA. 2003;289:2492–3. doi: 10.1001/jama.289.19.2492. [DOI] [PubMed] [Google Scholar]
- 29.Halbert CH, Lynch H, Lynch J, et al. Colon cancer screening practices following genetic testing for hereditary nonpolyposis colon cancer (HNPCC) mutations. Arch Intern Med. 2004;164:1881–7. doi: 10.1001/archinte.164.17.1881. [DOI] [PubMed] [Google Scholar]
- 30.Hadley DW, Jenkins JF, Dimond E, de Carvalho M, Kirsch I, Palmer CG. Colon cancer screening practices after genetic counseling and testing for hereditary nonpolyposis colorectal cancer. J Clin Oncol. 2004;22:39–44. doi: 10.1200/JCO.2004.06.128. [DOI] [PubMed] [Google Scholar]
- 31.Johnson KA, Trimbath JD, Petersen GM, Griffin CA, Giardiello FM. Impact of genetic counseling and testing on colorectal cancer screening behavior. Genet Test. 2002;6:303–6. doi: 10.1089/10906570260471831. [DOI] [PubMed] [Google Scholar]
- 32.Ramsey SD, Burke W, Clarke L. An economic viewpoint on alternative strategies for identifying persons with hereditary nonpolyposis colorectal cancer. Genet Med. 2003;5:353–63. doi: 10.1097/01.GIM.0000086626.03082.B5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown ML, Kessler LG. The use of gene tests to detect hereditary predisposition to cancer: economic considerations. J Natl Cancer Inst. 1995;87:1131–6. doi: 10.1093/jnci/87.15.1131. [DOI] [PubMed] [Google Scholar]
- 34.Smith RA, von Eschenbach AC, Wender R, et al. American Cancer Society guidelines for the early detection of cancer: update of early detection guidelines for prostate, colorectal, and endometrial cancers. Also: update 2001—testing for early lung cancer detection. CA Cancer J Clin. 2001;51:38–75. doi: 10.3322/canjclin.51.1.38. quiz 77 – 80. [DOI] [PubMed] [Google Scholar]
- 35.Colorectal cancer screening. Recommendation statement from the Canadian Task Force on Preventive Health Care. CMAJ. 2001;165:206–8. [PMC free article] [PubMed] [Google Scholar]
- 36.cms.hhs.gov [homepage on the internet] Baltimore: Department of Health and Human Services (DHHS), Centers for Medicaid and Medicare Services; [updated Thursday, September 16, 2004; cited 2005 May]. Available from: http://www.cms.hhs.gov/physicians/mpfsapp/step0.asp. [Google Scholar]
- 37.www.census.gov [homepage on the internet]. Washington DC: US Census Bureau Population Division; [updated 2004 December 22; cited 2005 February]. Available from: http://www.census.gov/popest/estimates.php.
- 38.Pinsky PF, Kramer BS, Reding D, Buys S. Reported family history of cancer in the prostate, lung, colorectal, and ovarian cancer screening trial. Am J Epidemiol. 2003;157:792–9. doi: 10.1093/aje/kwg043. [DOI] [PubMed] [Google Scholar]
- 39.Moss SM, Hardcastle JD, Coleman DA, Robinson MH, Rodrigues VC. Interval cancers in a randomized controlled trial of screening for colorectal cancer using a faecal occult blood test. Int J Epidemiol. 1999;28:386–90. doi: 10.1093/ije/28.3.386. [DOI] [PubMed] [Google Scholar]
- 40.Launoy G, Smith TC, Duffy SW, Bouvier V. Colorectal cancer mass-screening: estimation of faecal occult blood test sensitivity, taking into account cancer mean sojourn time. Int J Cancer. 1997;73:220–4. doi: 10.1002/(sici)1097-0215(19971009)73:2<220::aid-ijc10>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 41.Gatto NM, Frucht H, Sundararajan V, Jacobson JS, Grann VR, Neugut AI. Risk of perforation after colonoscopy and sigmoidoscopy: a population-based study. J Natl Cancer Inst. 2003;95:230–6. doi: 10.1093/jnci/95.3.230. [DOI] [PubMed] [Google Scholar]
- 42.Department of Health and Human Services (DHHS), Centers for Medicare and Medicaid Services (CMS). Update of HCPCS Costs and Payments for Ambulatory Surgical Centers (ASCs) and File Names, Descriptions, and Instructions for Retrieving the 2004 ASC HCPCS Additions, Deletions, and Master Listing, Change Request 2890, Transmittal AB 03 – 137: DHHS, CMS, 2003.
- 43.Johns LE, Houlston RS. A systematic review and meta-analysis of familial colorectal cancer risk. Am J Gastroenterol. 2001;96:2992–3003. doi: 10.1111/j.1572-0241.2001.04677.x. [DOI] [PubMed] [Google Scholar]
- 44.SEER*Stat 6.1 Software Version 6.1. Bethesda: http://seer.cancer.gov/seerstat/ [Google Scholar]
- 45.Sonnenberg A, Delco F, Inadomi JM. Cost-effectiveness of colonoscopy in screening for colorectal cancer. Ann Intern Med. 2000;133:573–84. doi: 10.7326/0003-4819-133-8-200010170-00007. [DOI] [PubMed] [Google Scholar]
- 46.Brown ML, Lipscomb J, Snyder C. The burden of illness of cancer: economic cost and quality of life. Annu Rev Public Health. 2001;22:91–113. doi: 10.1146/annurev.publhealth.22.1.91. [DOI] [PubMed] [Google Scholar]
- 47.Gold MR, Siegal JE, Russel LB, Weinstein MC, editors. Cost-effectiveness in health and medicine. New York: Oxford University Press; 1996. [Google Scholar]