Abstract
Detection of antibodies to human immunodeficiency virus (HIV) by enzyme-linked immunosorbent assay (ELISA) is the accepted method to screen blood products at risk to transmit infection. The presence of antibodies to HIV in 565 serum specimens from 274 patients with acquired immunodeficiency syndrome (AIDS) or AIDS-related complex, symptomatic and asymptomatic subjects at risk for AIDS, and controls was determined with an ELISA that incorporates synthetic peptides (designated E32/E34) representing sequences in the envelope glycoprotein gp41. Of 105 specimens from patients with AIDS or AIDS-related complex, 3 specimens that were negative by commercially licensed ELISA and immunoblot test were similarly unreactive in the E32/E34 ELISA. For homosexual men with generalized lymphadenopathy, 186 specimens were positive by the E32/E34 ELISA and 63 specimens were negative. In comparison, with the licensed ELISA, 184 of these samples were positive and 65 samples were negative. The two samples that were positive in the E32/E34 ELISA but not the commercial kit were also positive by immunoblotting. Sequential sera from one individual who apparently underwent seroconversion according to the commercial assays were all positive by E32/E34 ELISA and immunoblotting. Thus, the ELISA with synthetic peptides is an extremely sensitive and specific test of antibody response to HIV and has not yet yielded a negative result with a Western blot (immunoblot)-confirmed antibody-positive serum.
Full text
PDF
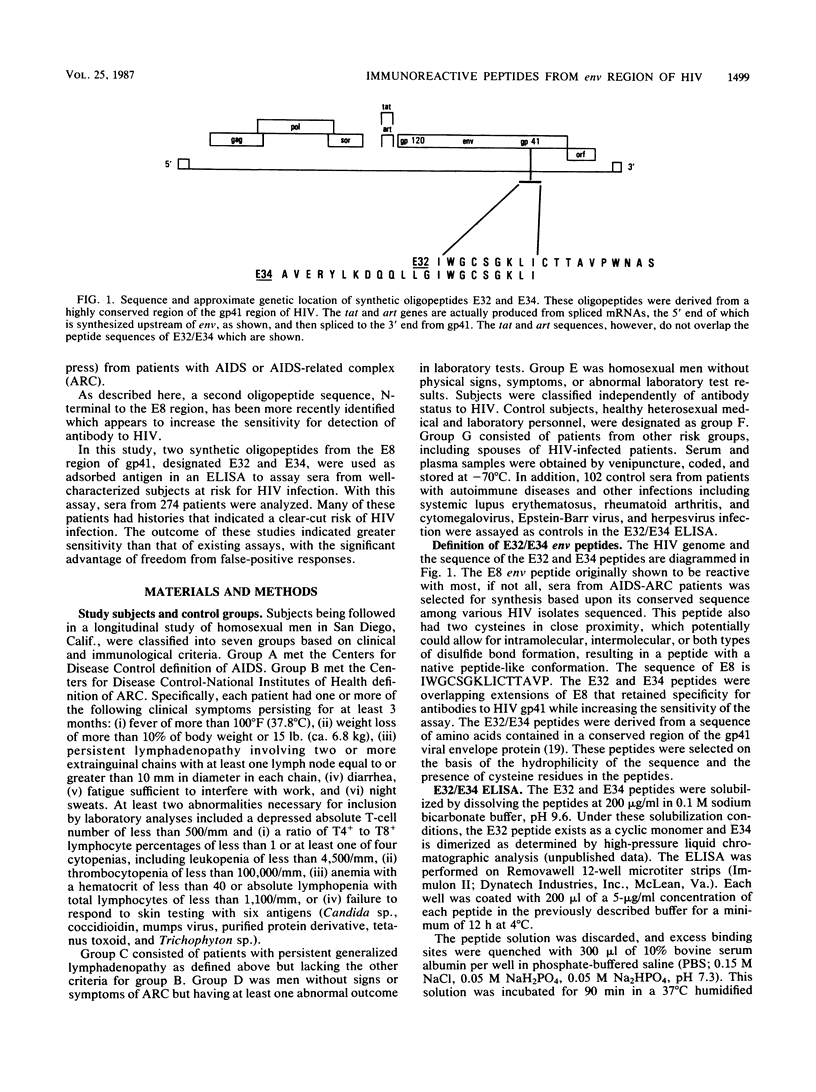
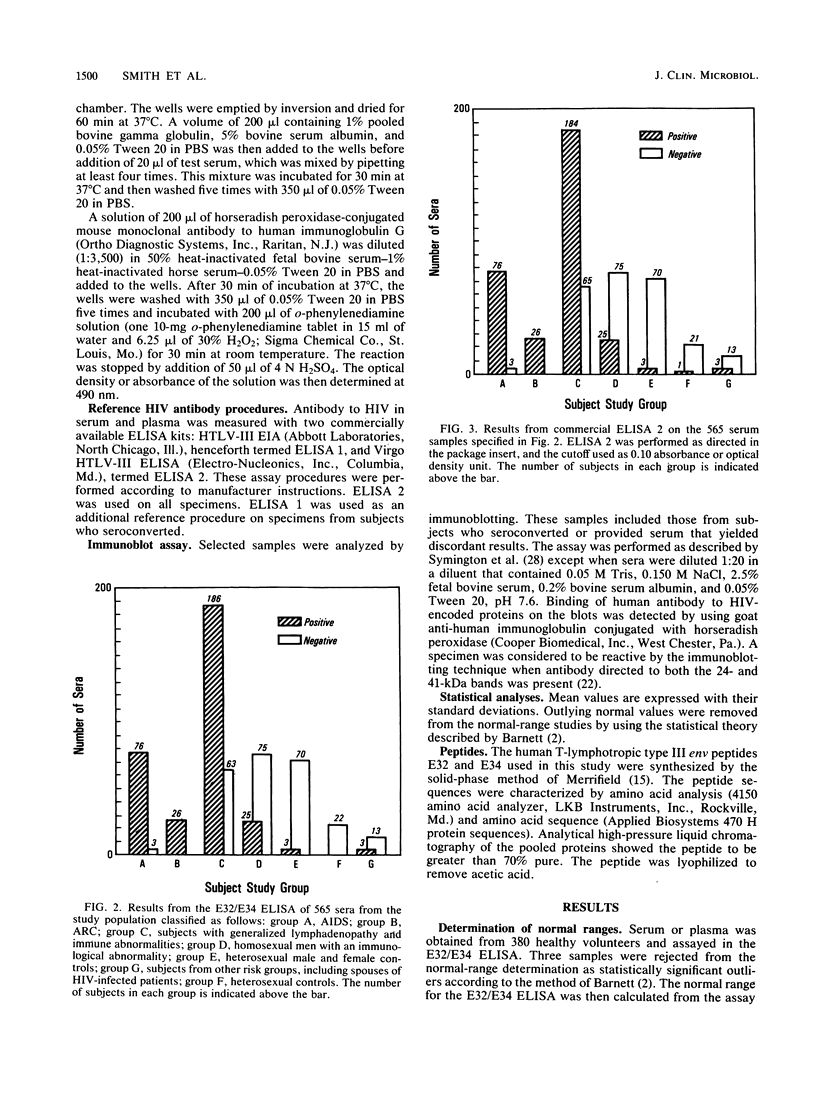




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barin F., McLane M. F., Allan J. S., Lee T. H., Groopman J. E., Essex M. Virus envelope protein of HTLV-III represents major target antigen for antibodies in AIDS patients. Science. 1985 May 31;228(4703):1094–1096. doi: 10.1126/science.2986291. [DOI] [PubMed] [Google Scholar]
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Biberfeld G., Bredberg-Rådén U., Böttiger B., Putkonen P. O., Blomberg J., Juto P., Wadell G. Blood donor sera with false-positive Western blot reactions to human immunodeficiency virus. Lancet. 1986 Aug 2;2(8501):289–290. doi: 10.1016/s0140-6736(86)92111-2. [DOI] [PubMed] [Google Scholar]
- Franchini G., Robert-Guroff M., Wong-Staal F., Ghrayeb J., Kato I., Chang T. W., Chang N. T. Expression of the protein encoded by the 3' open reading frame of human T-cell lymphotropic virus type III in bacteria: demonstration of its immunoreactivity with human sera. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5282–5285. doi: 10.1073/pnas.83.14.5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith M. F. HTLV-III testing of donor blood imminent; complex issues remain. JAMA. 1985 Jan 11;253(2):173-5, 179-81. doi: 10.1001/jama.253.2.173. [DOI] [PubMed] [Google Scholar]
- Groopman J. E., Chen F. W., Hope J. A., Andrews J. M., Swift R. L., Benton C. V., Sullivan J. L., Volberding P. A., Sites D. P., Landesman S. Serological characterization of HTLV-III infection in AIDS and related disorders. J Infect Dis. 1986 Apr;153(4):736–742. doi: 10.1093/infdis/153.4.736. [DOI] [PubMed] [Google Scholar]
- Hirose S., Uemura Y., Fujishita M., Kitagawa T., Yamashita M., Imamura J., Ohtsuki Y., Taguchi H., Miyoshi I. Isolation of HTLV-I from cerebrospinal fluid of a patient with myelopathy. Lancet. 1986 Aug 16;2(8503):397–398. doi: 10.1016/s0140-6736(86)90081-4. [DOI] [PubMed] [Google Scholar]
- Kennedy R. C., Henkel R. D., Pauletti D., Allan J. S., Lee T. H., Essex M., Dreesman G. R. Antiserum to a synthetic peptide recognizes the HTLV-III envelope glycoprotein. Science. 1986 Mar 28;231(4745):1556–1559. doi: 10.1126/science.3006246. [DOI] [PubMed] [Google Scholar]
- Landesman S. H., Ginzburg H. M., Weiss S. H. The AIDS epidemic. N Engl J Med. 1985 Feb 21;312(8):521–525. doi: 10.1056/nejm198502213120829. [DOI] [PubMed] [Google Scholar]
- Lange J. M., van den Berg H., Dooren L. J., Vossen J. M., Kuis W., Goudsmit J. HTLV-III/LAV infection in nine children infected by a single plasma donor: clinical outcome and recognition patterns of viral proteins. J Infect Dis. 1986 Jul;154(1):171–174. doi: 10.1093/infdis/154.1.171. [DOI] [PubMed] [Google Scholar]
- Lerner R. A. Antibodies of predetermined specificity in biology and medicine. Adv Immunol. 1984;36:1–44. doi: 10.1016/s0065-2776(08)60898-6. [DOI] [PubMed] [Google Scholar]
- Muesing M. A., Smith D. H., Cabradilla C. D., Benton C. V., Lasky L. A., Capon D. J. Nucleic acid structure and expression of the human AIDS/lymphadenopathy retrovirus. Nature. 1985 Feb 7;313(6002):450–458. doi: 10.1038/313450a0. [DOI] [PubMed] [Google Scholar]
- Osterholm M. T., Bowman R. J., Chopek M. W., McCullough J. J., Korlath J. A., Polesky H. F. Screening donated blood and plasma for HTLV-III antibody. Facing more than one crisis? N Engl J Med. 1985 May 2;312(18):1185–1189. doi: 10.1056/NEJM198505023121810. [DOI] [PubMed] [Google Scholar]
- Popovic M., Sarngadharan M. G., Read E., Gallo R. C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984 May 4;224(4648):497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- Ratner L., Haseltine W., Patarca R., Livak K. J., Starcich B., Josephs S. F., Doran E. R., Rafalski J. A., Whitehorn E. A., Baumeister K. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature. 1985 Jan 24;313(6000):277–284. doi: 10.1038/313277a0. [DOI] [PubMed] [Google Scholar]
- Saag M. S., Britz J. Asymptomatic blood donor with a false positive HTLV-III Western blot. N Engl J Med. 1986 Jan 9;314(2):118–118. doi: 10.1056/nejm198601093140212. [DOI] [PubMed] [Google Scholar]
- Salahuddin S. Z., Groopman J. E., Markham P. D., Sarngadharan M. G., Redfield R. R., McLane M. F., Essex M., Sliski A., Gallo R. C. HTLV-III in symptom-free seronegative persons. Lancet. 1984 Dec 22;2(8417-8418):1418–1420. doi: 10.1016/s0140-6736(84)91619-2. [DOI] [PubMed] [Google Scholar]
- Sarngadharan M. G., Popovic M., Bruch L., Schüpbach J., Gallo R. C. Antibodies reactive with human T-lymphotropic retroviruses (HTLV-III) in the serum of patients with AIDS. Science. 1984 May 4;224(4648):506–508. doi: 10.1126/science.6324345. [DOI] [PubMed] [Google Scholar]
- Saxinger C., Gallo R. C. Methods in laboratory investigation. Application of the indirect enzyme-linked immunosorbent assay microtest to the detection and surveillance of human t cell leukemia-lymphoma virus. Lab Invest. 1983 Sep;49(3):371–377. [PubMed] [Google Scholar]
- Schüpbach J., Haller O., Vogt M., Lüthy R., Joller H., Oelz O., Popovic M., Sarngadharan M. G., Gallo R. C. Antibodies to HTLV-III in Swiss patients with AIDS and pre-AIDS and in groups at risk for AIDS. N Engl J Med. 1985 Jan 31;312(5):265–270. doi: 10.1056/NEJM198501313120502. [DOI] [PubMed] [Google Scholar]
- Schüpbach J., Popovic M., Gilden R. V., Gonda M. A., Sarngadharan M. G., Gallo R. C. Serological analysis of a subgroup of human T-lymphotropic retroviruses (HTLV-III) associated with AIDS. Science. 1984 May 4;224(4648):503–505. doi: 10.1126/science.6200937. [DOI] [PubMed] [Google Scholar]
- Shaw G. M., Broder S., Essex M., Gallo R. C. Human T-cell leukemia virus: its discovery and role in leukemogenesis and immunosuppression. Adv Intern Med. 1984;30:1–27. [PubMed] [Google Scholar]
- Symington J., Green M., Brackmann K. Immunoautoradiographic detection of proteins after electrophoretic transfer from gels to diazo-paper: analysis of adenovirus encoded proteins. Proc Natl Acad Sci U S A. 1981 Jan;78(1):177–181. doi: 10.1073/pnas.78.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veronese F. D., DeVico A. L., Copeland T. D., Oroszlan S., Gallo R. C., Sarngadharan M. G. Characterization of gp41 as the transmembrane protein coded by the HTLV-III/LAV envelope gene. Science. 1985 Sep 27;229(4720):1402–1405. doi: 10.1126/science.2994223. [DOI] [PubMed] [Google Scholar]
- Wang J. J., Steel S., Wisniewolski R., Wang C. Y. Detection of antibodies to human T-lymphotropic virus type III by using a synthetic peptide of 21 amino acid residues corresponding to a highly antigenic segment of gp41 envelope protein. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6159–6163. doi: 10.1073/pnas.83.16.6159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. H., Goedert J. J., Sarngadharan M. G., Bodner A. J., Gallo R. C., Blattner W. A. Screening test for HTLV-III (AIDS agent) antibodies. Specificity, sensitivity, and applications. JAMA. 1985 Jan 11;253(2):221–225. [PubMed] [Google Scholar]


