Abstract
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease in the United States. The association between NAFLD and quality of life (QOL) remains unclear. These data are important to estimate the burden of illness in NAFLD. The aim was to report QOL scores of adults with NAFLD, and examine the association between NAFLD severity and QOL. QOL data were collected from adults with NAFLD enrolled in the NASH Clinical Research Network using the SF-36 survey and scores were compared to normative U.S. population scores. Liver biopsy histology was reviewed by a central pathology committee. A total of 713 subjects with NAFLD (M=269, F=444) were included. Mean age of subjects was 48.3 years; 61% had definite NASH, and 28% had bridging fibrosis or cirrhosis. Diabetes was present in 27% of subjects. Subjects with NAFLD had worse physical (mean=45.2) and mental health scores (mean=47.6) compared to the U.S. population with (mean=50) and without (physical: 55.8, mental: 52.5) chronic illness. Subjects with NASH reported lower physical health compared to subjects with fatty liver disease without NASH (44.5 vs. 47.1, p=.02). Subjects with cirrhosis had significantly (P<0.001) poorer physical health scores (38.4) vs. subjects with no (47.6), mild (46.2), moderate (44.6) or bridging fibrosis (44.6). Cirrhosis was associated with poorer physical health after adjusting for potential confounders. Mental health scores did not differ between participants with and without NASH or by degree of fibrosis.
Conclusion
Adults with NAFLD have a significant decrement in QOL. Treatment of NAFLD should incorporate strategies to improve QOL, especially physical health.
Keywords: Nonalcoholic steatohepatitis, SF-36, diabetes, obesity, physical health
The increasing prevalence of obesity in North America has had significant effects on the prevalence of obesity-related conditions, including nonalcoholic fatty liver disease (NAFLD) (1). NAFLD is now the most common chronic liver disease in the United States (2,3). Epidemiological surveys estimate that 3% to 34% of the general population has NAFLD (2, 4–6) and that 2% to 5% have nonalcoholic steatohepatitis (NASH) (6–8).
Because of the increasing importance of NAFLD, many studies have focused on understanding the epidemiology, natural history, and associated comorbidities of NAFLD. Quality of life (QOL) has been less well evaluated in patients with NAFLD, but is a key element in assessing the burden of a disease and the personal impact of the disease on daily living. QOL measurement also allows for a common metric to compare the burden of different diseases. Previous studies of QOL in NAFLD have been characterized by mixed populations of liver disease with relatively small numbers of patients with NAFLD (9,10). The one study with a larger number of patients with NAFLD used solely a disease-specific, rather than generic, QOL measure (11). Notwithstanding the limitations, these previous studies suggest that NAFLD is associated with impaired QOL. Patients with multiple other forms of chronic liver disease, including hepatitis C virus (HCV) (12), symptomatic hepatitis B virus (HBV) (13), and cholestatic liver disease (14), have also been shown to have decrements in QOL.
Using a large, multi-center sample, this study tested the hypothesis that individuals with NAFLD have lower QOL compared to individuals without NAFLD. QOL scores of individuals with NAFLD were compared to both a general reference U.S. population which included individuals with chronic illnesses, and to a healthy subset of the general population that excluded individuals with chronic illnesses. Because liver biopsies were available, a special focus of this study was the association between histologic severity of NAFLD and QOL in order to assess if increasing severity of disease was associated with lower QOL.
METHODS
Subjects
The Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN) was established by the National Institute of Diabetes & Digestive & Kidney Diseases (NIDDK) in 2002 to assess the natural history, pathogenesis, and therapy of NAFLD in the United States (15). The baseline QOL data were obtained from adult subjects enrolled in two NASH CRN studies: (1) the NAFLD Database, an observational cohort study; and (2) a randomized, placebo-controlled clinical trial, PIVENS (Pioglitazone versus Vitamin E versus Placebo for the Treatment of Nondiabetic Patients with Nonalcoholic Steatohepatitis; Clinical Trial number NCT00063622). These studies were conducted by 8 Clinical Centers and a central Data Coordinating Center (see acknowledgments for roster). Both study protocols were approved by all participating center IRBs and an independent Data and Safety Monitoring Board. Each participant provided written informed consent.
Measures
Demographics
Demographic information collected during screening interviews as part of the registration process included age, gender, self-reported ethnic and racial affiliation, highest educational level achieved, marital status, employment status, and annual income.
Anthropometrics
Weight and height were measured with participants standing wearing light clothing. Body mass index (BMI) was calculated as the weight (kg) divided by the height (meters) squared. Weight status was defined as normal (BMI 18.5–24.9), overweight (BMI 25–29.9), mild obesity (BMI 30–34.9), moderate obesity (BMI 35–39.9), and severe obesity (BMI > 40).
Histology
The NASH CRN Pathology Committee developed and validated a feature-based histological scoring system that encompasses the spectrum of lesions of NAFLD (16). Liver biopsy slides from subjects were read centrally by the Pathology Committee during which biopsies were rigorously evaluated according to the published scoring system (16). Steatosis was scored according to amount (%) of biopsy occupied using a four point scale. A diagnosis of NAFLD required the presence of ≥ 5% steatosis. Fibrosis was staged from 0 to 4, with 0 = none; 1a = mild zone 3 (central) perisinusoidal fibrosis, 1b = moderate zone 3 perisinuosidal fibrosis, 1c = periportal and portal fibrosis (zone 1) only; 2 = both perisinusoidal and periportal or portal fibrosis; 3 = bridging fibrosis and 4 = cirrhosis. Diagnostic determinations of each biopsy were also assigned. Categories utilized were: definite steatohepatitis, definitely not steatohepatitis, borderline steatohepatitis (zone 3 pattern) and borderline steatohepatitis (zone 1 pattern).
Quality of life
The Short form 36 (SF-36) is a generic QOL assessment survey that is validated, widely used, and has shown good psychometric properties in diverse disease states including patients with advanced liver disease (17–21). The SF-36 consists of 36 questions that make up eight sub-scales (physical function, physical role limitation, bodily pain, general health, vitality, social function, emotional role limitation, and mental health). Within each dimension, 0 is the worst and 100 is the best possible score (22). An overall physical health score (physical component scale [PCS]) and mental health score (mental component scale [MCS]) are derived from the subscale scores. Norm-based scoring of the SF-36 was done according to the instructions provided in the SF-36 users manual (23). Norm-based scoring involves a linear transformation to transform scores to a mean of 50 and standard deviation of 10. Norm-based scoring results in a straightforward interpretation of the QOL scores; that is, since the subscales have different ceilings and floors each score is affected by not only the disease being measured but also by arbitrary differences in the ceilings and floors of the particular scale. Therefore, familiarity with the mean of each scale is necessary when interpreting a particular scale score. With norm-based scoring, since each scale is scored to have a mean of 50 and the same standard deviation of 10, interpretation of a particular scale is clear-cut, greater than 50 equals better health than the U.S. population and less than 50 equals poorer health; additionally, one can easily see how many standard deviations a score is above or below the mean.
Data analysis
Descriptive statistics (mean, median, percentiles, standard deviation, range) were used to characterize the sample population. A student’s t-test (continuous data) or chi-square test (categorical data) was used to compare characteristics between individuals with and without NASH. A student’s t-test was used to compare SF-36 scores between individuals with and without NASH. Univariable regression analyses were conducted to examine the unadjusted association between subjects’ characteristics and SF-36 scores. Multivariable regression analyses were conducted to identify factors independently associated with PCS and MCS SF-36 scores. Analysis of variance (ANOVA) was used to compare SF-36 scores by degree of fibrosis; Tukey’s post-hoc test was used to identify where specific differences, identified globally in the ANOVA, occurred. Dummy variables were created for categorical variables with more than two levels. For analyses age was categorized as 18–30 years, 31–40, 41–50, 51–60, and 61–76 years. Data were assessed, as appropriate, for meeting regression assumptions prior to running analyses (i.e., linearity [assessed by partial regression plot], lack of multicollinearity [multicollinearity defined as Tolerance < .20 and/or Variance-inflation factor ≥4], homoscedasticity [assessed by simple regression plot], and absence of outliers [defined as standardized residuals greater than 3.3]). A p-value of <0.05 was considered statistically significant.
RESULTS
Subjects’ Baseline Characteristics
There were 713 adults enrolled in the Database and/or PIVENS trial of the NASH CRN between October 27, 2004 and October 30, 2007 who had complete biopsy results available at the time of analysis, ≥5% steatosis, and complete QOL data. The mean age of the sample was 48 ± 12 years and 62% were female. The majority of participants were non-Hispanic White (76%), followed by Hispanic (13%), and non-Hispanic, non-White (8%). The majority of participants had some college education (71%), and one third had completed a bachelors degree or higher. More than half (58%) of the sample had a household income of at least $50,000 per year and 70% were currently employed. The majority of adults were married (69%). The mean BMI was 34.3 ± 6.5 Kg/m2, 30% of subjects were mildly obese, 25% were moderately obese and 17% were severely obese. Type 2 diabetes was present in 27%. Sixty-one percent (436/713) had definite NASH, 20% (141/713) had borderline NASH, and 19% (136/713) had NAFLD but definitely not NASH. Twenty-three (167/713) percent had no fibrosis while 28% had bridging fibrosis or cirrhosis (197/713) (Table 1).
Table 1.
Baseline Characteristics
| All Subjects N=713 | NASH N=436 | No NASH N=136 | P-Value* | |
|---|---|---|---|---|
| Age | ||||
| Mean ± SD (range) yrs. | 48.2 ± 12.0 (18–76) | 49.3 ± 11.9 | 47.3 ± 12.1 | 0.076 |
|
| ||||
| Gender [n (%)] | ||||
| Female | 444 (62.3%) | 292 (67.0%) | 73 (53.7%) | 0.006 |
| Male | 269 (37.7%) | 144 (33.0%) | 63 (46.3%) | |
|
| ||||
| Race [n (%)] | ||||
| White, non-Hispanic | 543 (76.2%) | 342 (78.4%) | 97 (71.3%) | 0.324 |
| Non-White, non-Hispanic | 58 (8.1%) | 26 (6.0%) | 13 (9.6%) | |
| Multi-racial or missing, non-Hispanic | 21 (2.9%) | 15 (3.4%) | 3 (2.2%) | |
| Hispanic | 91 (12.8%) | 53 (12.2%) | 23 (16.9%) | |
|
| ||||
| Education [n (%)] | ||||
| Kindergarten – 8th grade | 15 (2.1%) | 10 (2.3%) | 2 (1.5%) | 0.003 |
| grades 9–11 | 41 (5.8%) | 21 (4.8%) | 13 (9.6%) | |
| completed high school | 152 (21.3%) | 102 (23.4%) | 23 (16.9%) | |
| some college | 272 (38.1%) | 182 (41.7%) | 41 (30.1%) | |
| bachelors degree or higher | 232 (32.5%) | 120 (27.5%) | 57 (41.9%) | |
|
| ||||
| Income [n (%)] | ||||
| <$15,000 | 55 (7.7%) | 31 (7.1%) | 15 (11.2%) | 0.123 |
| 15,000–29,999 | 89 (12.5%) | 57 (13.1%) | 16 (11.9%) | |
| 30,000–49,000 | 149 (20.9%) | 103 (23.6%) | 21 (15.7%) | |
| 50,000+ | 409(57.4%) | 241 (55.3%) | 81 (61.2%) | |
| Missing | 11 (1.5%) | 4 (0.9%) | 3 (2.2%) | |
|
| ||||
| Weight (kg) | ||||
| Mean ± SD (range) | 96.9 ± 20.8 (48.4–169.8) | 97.0 ± 20.3 | 96.2 ± 20.9 | 0.689 |
|
| ||||
| Height (m) | ||||
| Mean ± SD (range) | 1.68 ± .10 (1.4–1.9) | 1.67 ± 0.10 | 1.68 ± 0.10 | 0.744 |
|
| ||||
| BMI | ||||
| Mean ± SD (range) | 34.3 ± 6.5 (20.0–59.5) | 34.5 ± 6.3 | 34.1 ± 6.7 | 0.558 |
|
| ||||
| Fibrosis [n (%)] | ||||
| none | 167 (23.4%) | 28 (6.4%) | 94 (69.1%) | <0.001 |
| 1a, mild, zone 3 perisinusoidal, | 109 (15.3%) | 68 (15.6%) | 9 (6.6%) | |
| 1b, moderate, zone 3, perisinusoidal | 73 (10.2%) | 64 (14.7%) | 1 (0.7%) | |
| 1c, portal/periportal only | 29 (4.1%) | 2 (0.5%) | 15 (11.0%) | |
| zone 3 and periportal | 138 (19.4%) | 107 (24.5%) | 4 (2.9%) | |
| bridging | 131 (18.4%) | 118 (27.1%) | 2 (1.5%) | |
| cirrhosis | 66 (9.3%) | 49 (11.2%) | 11 (8.1) | |
P-value is from comparison (t-test or Chi-Square test) between participants with definite NASH and participants with NAFLD but without NASH
Baseline Characteristics, No NASH vs. NASH
There was a significantly (P = 0.006) greater proportion of females in the group with NASH (67%) than in those with NAFLD but without NASH (54%). Type 2 diabetes was more prevalent among adults with NASH than in those without NASH (32% vs. 19%, P = 0.005). There were no significant differences between adults with and without NASH in regards to age, race, marital status, employment status, or BMI (Table 1).
Baseline QOL Scores
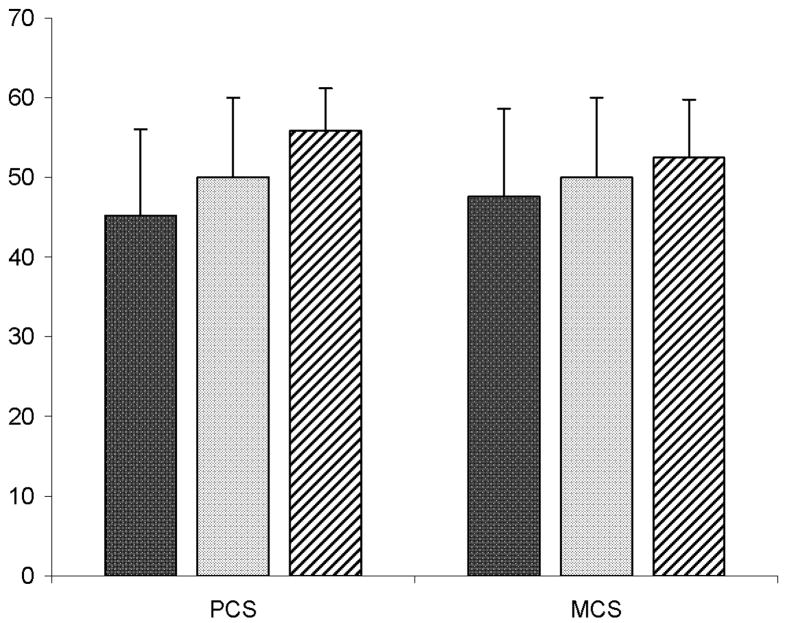
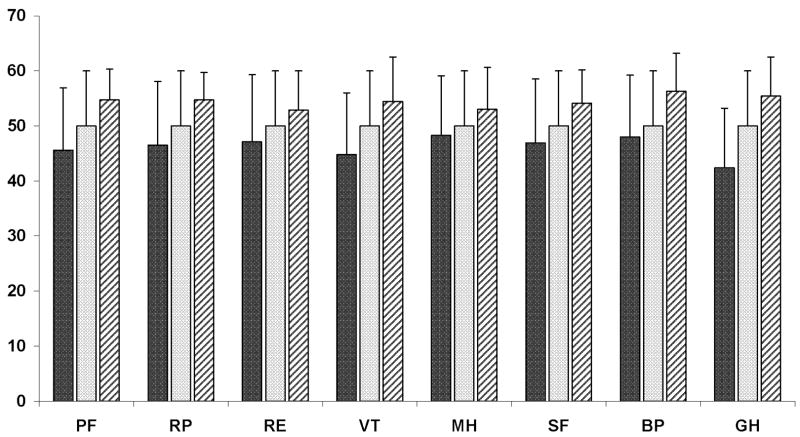
Individuals with NAFLD had significantly (P < 0.001) lower QOL for both physical (45.2) and mental health (47.6) than the general healthy U.S. population, (mean PCS score = 55.8, MCS score = 52.5). Individuals with NAFLD also had significantly (P < 0.001) lower QOL scores compared to the general U.S. population (including individuals with chronic illnesses; mean PCS and MCS scores = 50) (Figure 1). Age and gender adjustment of the U.S. normative sample did not change these results. All SF-36 subscale scores were also lower for adults with NAFLD compared to the U.S. general and healthy populations. Among adults with NAFLD, the lowest (worst) subscale mean scores were in general health (42.4), vitality (44.8), and physical functioning (45.6) (Figure 2).
Figure 1.
Mean SF-36 PCS and MCS scores of adults enrolled in NASH CRN (n=713) and US normative sample with (n=1,982) and without (n=571) chronic illness. Error bars display standard deviations. Black bar = Mean scores of Individuals with NAFLD. Gray bar: Mean scores of general U.S. reference population. White bar with black diagonal lines: Mean scores of healthy (i.e., no chronic illness) reference sample. Mean PCS and MCS scores of NAFLD sample are significantly (P < 0.001) lower than PCS and MCS scores of general U.S. population and healthy sample
Figure 2.
Mean SF-36 sub-scale scores of adults enrolled in NASH CRN (n=713) and US normative sample with (n=1,982) and without (n=571) chronic illness. Error bars display standard deviations. Black bar = Mean scores of Individuals with NAFLD. Gray bar: Mean scores of general U.S. reference population. White bar with black diagonal lines: Mean scores of healthy (i.e., no chronic illness) reference sample.
PF: Physical Function; RP: Role-Physical; RE: Role-Emotional; VT: Vitality; MH: Mental Health; SF: Social Function; BP: Bodily Pain; GH: General Health. Mean subscale scores of NAFLD sample are significantly (P ≤ 0.001) lower than subscales scores of general U.S. population and healthy sample.
Comparison of QOL between Subjects with and without NASH
The mean PCS of participants with NAFLD but not NASH was 47.1, which was significantly (p = 0.001) lower than the normative U.S. population. Notably, adults with definite NASH (PCS 44.5) reported significantly (P = 0.02) poorer physical health compared to adults with no NASH (PCS 47.1). For SF-36 subscale scores, adults with NASH reported significantly poorer vitality (44.3 vs. 46.6 for subjects with and without NASH, respectively, P = 0.04), general health (41.8 vs. 44.2 for subjects with and without NASH, respectively, P = 0.02), bodily pain (47.7 vs. 50.0 for subjects with and without NASH, respectively, P = 0.04) and role limitations due to physical health (45.9 vs. 48.3 for subjects with and without NASH, respectively, P = 0.04). Individuals with and without NASH did not significantly differ in mental health (Table 2).
Table 2.
QOL of Participants in the NASH CRN with and without NASH
| All subjects N=713 | NASH N=436 | No NASH N=136 | P-Value* | |
|---|---|---|---|---|
| SF-36 PCS (standardized) | ||||
|
| ||||
| Mean ± sd (range) | 45.2 ± 10.9 (15.7 – 63.8) | 44.5 ± 11.0 | 47.1 ± 10.4 | 0.018 |
|
| ||||
| SF-36 MCS (standardized) | ||||
|
| ||||
| Mean ± sd (range) | 47.6 ± 11.0 (9.2 – 68.7) | 47.5 ± 10.9 | 48.6 ± 11.3 | 0.342 |
|
| ||||
| SF-36 Sub-Scales(standardized) | Mean ± SD | Mean ± SD | Mean ± SD | |
|
| ||||
| Physical functioning | 45.6 ± 11.3 | 44.9 ± 11.7 | 47.0 ± 11.0 | 0.066 |
| Role limitations due to physical health | 46.5 ± 11.6 | 45.8 ± 11.6 | 48.3 ± 11.4 | 0.036 |
| Role limitations due to emotional problems | 47.1 ± 12.2 | 46.9 ± 12.1 | 48.6 ± 12.0 | 0.154 |
| Vitality | 44.8 ± 11.2 | 44.4 ± 11.1 | 46.6 ± 11.5 | 0.043 |
| Mental Health | 48.3 ± 10.8 | 48.0 ± 10.7 | 49.1 ± 11.7 | 0.336 |
| Social functioning | 46.9 ± 11.6 | 46.9 ± 11.3 | 48.0 ± 12.0 | 0.328 |
| Bodily pain | 48.0 ± 11.2 | 47.7 ± 11.2 | 50.0 ± 11.4 | 0.043 |
| General Health | 42.4 ± 10.8 | 41.8 ± 10.9 | 44.2 ± 10.9 | 0.023 |
P-value is from comparison (t-test) between participants with definite NASH and participants with NAFLD but without NASH
Factors Associated With QOL in Subjects With NAFLD
In univariable analyses, the following characteristics were significantly (P < 0.05) associated with poorer physical health: age greater than 40 years, female gender, type 2 diabetes, whether or not there NASH was present, income less than $15,000, divorced, separated or widowed marital status, and BMI of ≥ 40. Race and level of education were not associated with physical health score.
In multivariable analysis, characteristics significantly associated with poorer physical health, after adjusting for all other variables in the model, included: older age (vs. age 18–30 yrs. age 41–50 yrs., B: −3.34, P = 0.031; age 51–60 yrs., B: −4.69, P = 0.003; age 61–76 yrs., B: −4.85, P = 0.004), female gender (B: −4.67, P < 0.001), type 2 diabetes (B: −4.11, P < 0.001), and lower income ($30K–49.4K vs. <$15,000, B: 4.65, P = 0.002; ≥50K vs. <$15,000, B: 7.24, P < 0.001). The presence of NASH as determined by liver histology was not independently associated with poorer physical health.
Characteristics significantly (P < 0.05) associated with poorer mental health in univariate analysis included: female gender, type 2 diabetes, income of less than $15,000, less than high school diploma, and divorced, separated or widowed marital status. Age, presence of NASH, race, and BMI were not associated with mental health score.
In multivariable analysis, characteristics significantly associated with poorer mental health, after adjusting for all other variables in the model, included: female gender (B: −2.40, P = 0.007), age 18–30 yrs (vs. 61–76 yrs., B: −4.91, P = 0.010), type 2 diabetes (B: −2.12, p=0.028), less than high school diploma (B: −3.94, P = 0.020), and annual household lower income (≥ $50K vs. <15,000, B: 5.36, P = 0.001).
Gender Stratified Multivariable Analyses
Since gender was a significant independent predictor of both MCS and PCS, multivariable models were re-run stratified by gender. Older age, type 2 diabetes and low household income were associated with poorer physical health in both men and women. Divorced, separated or widowed marital status (vs. married) was associated with worse mental health in men; low household income and less than high school diploma were associated with worse mental health in women.
QOL by degree of fibrosis
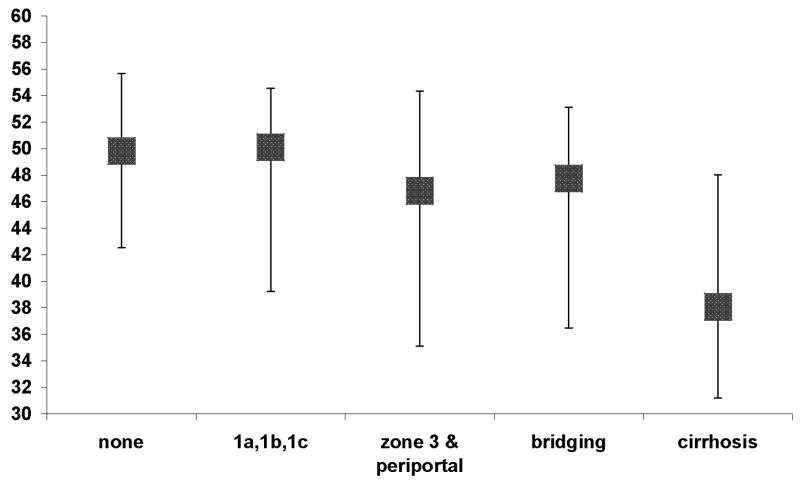
As shown in Figure 3, there was a significant (P < 0.001) overall difference in physical health score between the fibrosis groups; scores tended to worsen as the amount of fibrosis increased. Tukey’s multiple comparison tests demonstrated that participants with cirrhosis reported significantly worse physical health compared to each of the other groups (all P-values = 0.001). After adjusting for age, gender, race, marital status, education, annual household income, BMI, and type 2 diabetes, presence of cirrhosis was independently associated with significantly worse physical health score compared to no fibrosis (B= −5.06, P < 0.001). If the participants with cirrhosis, however, were removed from analysis, those participants with NAFLD but not cirrhosis had a PCS score of 45.9 which remains significantly (p<0.001) lower than the normative reference population. The difference in mental health scores did not significantly differ between the fibrosis categories.
Figure 3.
Median PCS scores and interquartile range by degree of fibrosis. ANOVA and Tukey’s post-hoc analysis demonstrated that PCS score for individuals with cirrhosis was significantly (P < 0.001) lower than each of the other groups.
DISCUSSION
These data demonstrate that individuals with the full spectrum of NAFLD have reduced QOL. Impairment in QOL is most evident in physical health while mental health is affected to a lesser degree. Furthermore, there was an inverse relationship between severity of NAFLD and lower QOL, and increased fibrosis portended a decrease in physical QOL. Factors independently associated with lower QOL included older age, female gender, presence of type 2 diabetes, and poverty.
QOL was particularly low in individuals with cirrhosis; after adjustment for other characteristics cirrhosis remained a significant independent predictor of lower physical health score. Previous studies have reported lower QOL in cirrhotic patients compared to normal controls and liver disease patients without cirrhosis. However, the majority of those studies were limited by small sample sizes (19,24,25), use of single center samples (13, 26–30), disease-specific measures (31,32), and/or the inclusion of univariable analysis only (9, 26–28, 33). Studies that have utilized surrogate measures of cirrhosis, i.e., presence of splenomegaly or patient self-report of cirrhosis, suggested that cirrhosis may be independently associated with lower QOL, in a variety of other liver diseases (34,35). In this study there was a strong association between presence of cirrhosis and lower QOL in individuals with NAFLD.
Subjects with NAFLD reported physical QOL scores that were an average of 4.9 points lower than the general U.S. reference population while mental health scores were on average 2.4 points lower. The reference population includes individuals with chronic diseases, possibly including NAFLD - 22% of the sample reported presence of type 2 diabetes and 27% reported hypertension, two conditions associated with NAFLD. Comparing the NAFLD sample to a subset of the reference population that excluded individuals with chronic disease (23), the physical scores of the NAFLD sample were an average of 10.7 points lower, and mental health scores were an average of 4.9 points lower. Discussions surrounding what constitutes a clinically significant difference in QOL scores are longstanding (36). A difference in scores of at least half of a standard deviation (i.e., 5 points) is generally believed to be a conservative estimate of a clinically significant effect size (37). In this study, physical health scores were a half standard deviation lower than the U.S. general population and a full standard deviation lower than individuals without chronic health conditions. Conversely, the mental health scores of individuals with NAFLD are likely clinically significantly lower than individuals without chronic health conditions, but not significantly different than the U.S. general population.
Although QOL is a relatively new concept to the study of NAFLD, QOL research itself is not new. Standard methods in QOL research are to detect if QOL differences exist, and, if so, to then identify what factors underlie the difference. By identifying and intervening on modifiable factors that are found to be associated with decreased QOL, improvements in QOL can be realized. While NAFLD is generally asymptomatic, fatigue is one symptom shown to be common in individuals with NAFLD (38), and to negatively affect QOL of individuals with other types of liver disease (9, 24, 39–42). Thus, NAFLD-associated symptoms, in particular fatigue, may contribute to decreased QOL. A recent study demonstrated that fatigue was significantly associated with a decrease in all domains of the Chronic Liver Disease Questionnaire in individuals with NAFLD (11). In the present study, vitality was one of the QOL dimensions on which individuals with NAFLD reported the lowest scores.
The current data suggest that individuals with NAFLD have a greater degree of limitation in physical health than patients with other types of liver disease. Using a liver disease-specific instrument, patients with NAFLD reported greater systemic symptoms and greater degrees of limitations in activity compared to patients with HBV or HCV (11). It is notable that the PCS scores of individuals with NAFLD in this study are comparable to the PCS scores reported for patients with HBV who had decompensated cirrhosis (13). From the cross-sectional studies done to date one cannot determine whether poor physical function contributes to NAFLD and NAFLD severity, or conversely, if presence of NAFLD results in poor physical function.
Obesity (43–46) and diabetes (23) are two common co-morbidities in individuals with NAFLD (41), and are also associated with decreased QOL. Moreover, the presence of co-morbid illness has been associated with decreased QOL in patients with other chronic liver diseases (47,48). In the current study, patients with severe obesity had significantly lower QOL than patients who were normal weight, although there was not an independent relationship between BMI and QOL score after adjustment for other covariates. Conversely, the presence of type 2 diabetes had a significant independent negative association with both physical and mental QOL scores. However, cirrhosis was associated with lower QOL physical health score after adjusting for presence of type 2 diabetes. Thus, type 2 diabetes does not fully explain the decreased QOL in NAFLD.
Assessment of QOL is useful because this measure provides information about the overall burden of NAFLD from the individual’s perspective. In addition to the negative impact poor physical health may have on an individual personally, there may be other important implications of poor self-reported physical health. A recent study found that NAFLD is associated with significant increases in medical costs and health care utilization over time (49). The degree that health perception influences health care seeking behavior is unknown but it can be hypothesized that poor physical health perception may impact health care utilization in individuals with NAFLD. Productivity may also be affected by poor physical health perception. One study found that lower SF-36 physical health score significantly differentiated between individuals who returned to work following liver transplantation and those who did not, despite the fact that all participants reported good health status as measured by a performance status scale (50). Poor QOL also has implications for mortality. In numerous studies, including those in patients with a variety of chronic and acute disease states, (e.g., type 2 diabetes (51), arthritis (52), COPD (53), hemodialysis patients (54)) low QOL scores have been associated with increased mortality risk (55). Thus, poor subjective physical health has important implications in terms of increased health care utilization, productivity losses, and mortality.
The multi-center design of the NASH CRN and the recruitment of subjects from a variety of settings make these results generalizable to adults in the U.S. with a clinical diagnosis of NAFLD. However, these data may not fully reflect the larger population of all individuals with NAFLD, especially those with unrecognized NAFLD. A particular strength of this study was the inclusion of liver histology on all patients with a rigorous, standardized, central biopsy review. A limitation was the cross sectional nature of the study, thus we cannot determine cause and effect from these data. Longitudinal studies of NAFLD are warranted to clarify the nature and direction of associations identified from cross sectional data. In addition, there are domains not captured by the SF-36 which may also contribute to QOL (e.g., sleep, cognition, sex, family, self-esteem, eating, hobbies, and communication); thus a broader examination of such factors should be incorporated into future studies.
In summary, individuals with NAFLD have lower QOL than the U.S. general population, with and without chronic illness. Decrement in physical health is particularly apparent. Future research should focus on identifying modifiable factors that affect QOL and can be targeted for improvement. The independent inverse relationship between cirrhosis and physical health is novel and merits further evaluation. The association between diabetes and NAFLD severity, and between diabetes and QOL in adults with NAFLD warrants additional attention as these patients will increasingly be co-managed by internists, endocrinologists, and hepatologists.
Acknowledgments
NAFLD Database and PIVENS trial are supported by the National Institute of Diabetes and Digestive and Kidney Disease. Additional funding to conduct PIVENS trial is provided by the Takeda Pharmaceuticals North America, Inc. through a Cooperative Research and Development Agreement (CRADA) with the National Institutes of Health.
The following members of the Nonalcoholic Steatohepatitis Clinical Research Network have been instrumental in the design and conduct of the NAFLD Database and PIVENS trial. Baylor College of Medicine, Houston, TX: Stephanie Abrams, MD; Diana Arceo, Denise Espinosa, Leanel Fairly
Case Western Reserve University Cleveland, OH: MetroHealth Medical Center: Arthur McCullough, MD, PI; Diane Bringman, RN, BSN; Srinivasan Dasarathy, MD; Carol Hawkins, RN; Yao-Chang Liu, MD; Nicholette Rogers, PhD, PA-C; Margaret Stager, MD; Cleveland Clinic Foundation: Arthur McCullough, MD, PI; Srinivasan Dasarathy, MD; Kevin Edwards, NP; Ruth Sargent, LPN
Children’s Hospital & Regional Medical Center, Seattle, WA: Melissa Coffey, Karen Murray, MD; Melissa Young
Children’s National Medical Center, Washington DC: Parvathi Mohan, MD; Kavita Nair Duke University Medical Center, Durham, NC: Manal Abdelmalek, MD; Anna Mae Diehl, MD; Marcia Gottfried, MD; Cynthia Guy, MD; Paul Killenberg, MD; Samantha Kwan, Yi-Ping Pan, Dawn Piercy, FNP; Melissa Smith
Indiana University School of Medicine, Indianapolis, IN: Prajakta Bhimalli, Naga Chalasani, MD; Oscar W. Cummings, MD; Lydia Lee, Linda Ragozzino, Raj Vuppalanchi, MD Johns Hopkins Hospital, Baltimore, MD: Ann Scheimann, MD; Michael Torbenson, MD Riley Hospital for Children, Indianapolis, IN: Ann Klipsch, RN; Jean Molleston, MD; Girish Subbarao, MD
St Louis University, St Louis, MO: Sarah Barlow, MD (2002–2007); Jose Derdoy, MD (2007-); Joyce Hoffmann, Debra King, RN; Joan Siegner, RN; Susan Stewart, RN; Brent A. Tetri, MD; Judy Thompson, RN
University of California San Diego, San Diego, CA: Cynthia Behling, MD; Manual Celedon, Lisa Clark, Janis Durelle, Tarek Hassanein, MD; Joel E. Lavine, MD, PhD; Susana Mendoza, Jeffrey B. Schwimmer, MD; Claude Sirlin, MD; Tanya Stein, Allison Tobin
University of California San Francisco, San Francisco, CA: Kiran Bambha, MD, Nathan M. Bass, MD, PhD; Linda D. Ferrell, MD; Danuta Filipowski, Raphael Merriman, MD (2002–2007); Mark Pabst, Monique Rosenthal, Philip Rosenthal, MD; Tessa Steel
Virginia Commonwealth University, Richmond, VA: Sherry Boyett, RN, Daphne Bryan, MD; Melissa J. Contos, MD; Michael Fuchs, MD; Martin Graham, MD; Amy Jones, Velimir AC Luketic, MD; Bimalijit Sandhu, MD; Arun J. Sanyal, MD; Carol Sargeant, RN, MPH; Kimberly Selph, Melanie White, RN
Virginia Mason Medical Center, Seattle, WA: Grace Gyurkey, Kris V. Kowdley, MD; Jody Mooney, MS; James Nelson, PhD; Sarah Roberts, Cheryl Saunders, MPH; Alice Stead, Chia Wang, MD; Matthew Yeh, MD, PhD
Washington University, St. Louis, MO: Elizabeth Brunt, MD
Resource Centers
National Cancer Institute, Bethesda, MD: David Kleiner, MD, PhD
National Institute of Child Health and Human Development, Bethesda, MD: Gilman D. Grave, MD; Terry TK Huang, PhD, MPH
National Institute of Diabetes, Digestive and Kidney Diseases, Bethesda, MD: Edward Doo, MD; James E. Everhart, MD, MPH; Jay H. Hoofnagle, MD; Patricia R. Robuck, PhD (Project Scientist); Leonard Seeff, MD
Johns Hopkins University, Bloomberg School of Public Health (Data Coordinating Center), Baltimore, MD: Patricia Belt, BS; Fred Brancati, MD, MHS; Jeanne Clark, MD, MPH; Ryan Colvin, MPH; Michele Donithan, MHS; Mika Green, MA; Rosemary Hollick (2003–2005), Milana Isaacson, Wana Kim, Alison Lydecker, Laura Miriel, Alice Sternberg, ScM; James Tonascia, PhD; Aynur Ünalp-Arida, MD, PhD; Mark Van Natta, MHS; Laura Wilson, ScM; Katherine Yates, ScM
Grant support: The Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN) is supported by the National Institute of Diabetes and Digestive and Kidney Diseases grants U01DK061718, U01DK061728, U01DK061731, U01DK061732, U01DK061734, U01DK061737, U01DK061738, U01DK061730, U01DK061713, and the National Institute of Child Health and Human Development. This study is supported in part by the Intramural Research Program of the National Cancer Institute. Other grant support include following National Institutes of Health General Clinical Research Centers or Clinical and Translational Science Awards: UL1RR024989, M01RR000750, RR02413101, M01RR000827, UL1RR02501401, M01RR000065.
Abbreviations
- NAFLD
Nonalcoholic fatty liver disease
- NASH
Nonalcoholic steatohepatitis
- QOL
quality of life
- HCV
Hepatitis C virus
- HBV
Hepatitis B virus
- NASH CRN
Nonalcoholic Steatohepatitis Clinical Research Network
- NIDDK
National Institute of Diabetes & Digestive & Kidney Diseases
- BMI
Body mass index
- SF-36
Short form 36
- PCS
Physical component score
- MCS
Mental component score
- ANOVA
Analysis of variance
Contributor Information
Kristin David, Email: kdavid@ucsd.edu.
Kris V. Kowdley, Email: Kris.Kowdley@vmmc.org.
Aynur Unalp, Email: aunalp@jhsph.edu.
Fasiha Kanwal, Email: Fasiha.Kanwal@va.gov.
Elizabeth M. Brunt, Email: EBrunt@path.wustl.edu.
Jeffrey B. Schwimmer, Email: jschwimmer@ucsd.edu.
References
- 1.American Gastroenterological Association medical position statement: nonalcoholic fatty liver disease. Gastroenterology. 2002;123:1702–1704. doi: 10.1053/gast.2002.36569. [DOI] [PubMed] [Google Scholar]
- 2.Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98:960–967. doi: 10.1111/j.1572-0241.2003.07486.x. [DOI] [PubMed] [Google Scholar]
- 3.Kim WR, Brown RS, Jr, Terrault NA, El-Serag H. Burden of liver disease in the United States: summary of a workshop. Hepatology. 2002;36:227–242. doi: 10.1053/jhep.2002.34734. [DOI] [PubMed] [Google Scholar]
- 4.Ioannou GN, Boyko EJ, Lee SP. The prevalence and predictors of elevated serum aminotransferases activity in the United States in 1999–2002. Am J Gastroenterol. 2006;101:76–82. doi: 10.1111/j.1572-0241.2005.00341.x. [DOI] [PubMed] [Google Scholar]
- 5.Bell BP, Manos MM, Zaman A, Terrault N, Thomas A, Navarro VJ, et al. The Epidemiology of Newly Diagnosed Chronic Liver Disease in Gastroenterology Practices in the United States: Results From Population-Based Surveillance. Am J Gastroenterol. 2008;103:2727–2736. doi: 10.1111/j.1572-0241.2008.02071.x. [DOI] [PubMed] [Google Scholar]
- 6.Browning JD, et al. Prevalence of hepatic steatosis in an urban population in the United States: Impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 7.Wanless IR, Lentz JS. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology. 1990;12:1106–1110. doi: 10.1002/hep.1840120505. [DOI] [PubMed] [Google Scholar]
- 8.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202–1219. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- 9.Younossi ZM, Boparai N, Price LL, Kiwi ML, McCormick M, Guyatt G. Health-related quality of life in chronic liver disease: the impact of type and severity of disease. Am J Gastroenterol. 2001;96:2199–2205. doi: 10.1111/j.1572-0241.2001.03537.x. [DOI] [PubMed] [Google Scholar]
- 10.Younossi ZM, Boparai N, McCormick M, Price LL, Guyatt G. Assessment of Utilities and Health-Related Quality of Life in Patients With Chronic Liver Disease. Am J Gastroenterol. 2001;96:579–583. doi: 10.1111/j.1572-0241.2001.03537.x. [DOI] [PubMed] [Google Scholar]
- 11.Dan AA, Kallman JB, Wheeler A, Younoszai Z, Collantes R, Bondini S, et al. Health-related quality of life in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2007;26:815–820. doi: 10.1111/j.1365-2036.2007.03426.x. [DOI] [PubMed] [Google Scholar]
- 12.Spiegel BM, Younossi ZM, Hays RD, Revicki D, Robbins S, Kanwal F. Impact of hepatitis C on health related quality of life: a systematic review and quantitative assessment. Hepatology. 2005;41:790–800. doi: 10.1002/hep.20659. [DOI] [PubMed] [Google Scholar]
- 13.Ong SC, Mak B, Aung MO, Li SC, Lim SG. Health-related quality of life in chronic hepatitis B patients. Hepatology. 2008;47:1108–1117. doi: 10.1002/hep.22138. [DOI] [PubMed] [Google Scholar]
- 14.Younossi ZM, Kiwi ML, Boparai N, Price LL, Guyatt G. Cholestatic liver diseases and health-related quality of life. Am J Gastroenterol. 2000;95:497–502. doi: 10.1111/j.1572-0241.2000.01774.x. [DOI] [PubMed] [Google Scholar]
- 15.Nonalcoholic Steatohepatitis Clinical Research Network. Hepatology. 2003;35:244. doi: 10.1002/hep.510370203. [DOI] [PubMed] [Google Scholar]
- 16.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 17.Marchesini G, Bianchi G, Amodio P, Salerno F, Merli M, Panella C, et al. Factors associated with poor health-related quality of life of patients with cirrhosis. Gastroenterology. 2001;120:170–178. doi: 10.1053/gast.2001.21193. [DOI] [PubMed] [Google Scholar]
- 18.Cordoba J, Flavia M, Jacas C, Sauleda S, Esteban JI, Vargas V, et al. Quality of life and cognitive function in hepatitis C at different stages of liver disease. J Hepatol. 2003;39:231–238. doi: 10.1016/s0168-8278(03)00189-2. [DOI] [PubMed] [Google Scholar]
- 19.Chong CAKY, Gulamhussein A, Heathcote EJ, Lilly L, Sherman M, Naglie G, et al. Health-state utilities and quality of life in hepatitis C patients. Am J Gastroenterol. 2003;98:630–638. doi: 10.1111/j.1572-0241.2003.07332.x. [DOI] [PubMed] [Google Scholar]
- 20.Younossi ZM, Guyatt G. Quality-of life assessments and chronic liver disease. Am J Gastroenterol. 1998;93:1037–1041. doi: 10.1111/j.1572-0241.1998.00325.x. [DOI] [PubMed] [Google Scholar]
- 21.Foster GR, Goldin RD, Thomas HC. Chronic hepatitis C virus infection causes a significant reduction in quality of life in the absence of cirrhosis. Hepatology. 1998;27:209–212. doi: 10.1002/hep.510270132. [DOI] [PubMed] [Google Scholar]
- 22.Ware JE, jr, Kosinski M. SF-36© Health Survey & Interpretation Guide. Lincoln, RI: QualityMetric Incorporated; 1993, 2000. [Google Scholar]
- 23.Ware JE, jr, Kosinski M, Gamdek B. SF-36© Physical & Mental Health Summary Scales: A Manual For Users of Version 1. 2. Lincoln, RI: QualityMetric Incorporated; 2001. [Google Scholar]
- 24.Sogolow ED, Lasker JN, Short LM. Fatigue as a major predictor of quality of life in women with autoimmune liver disease: the case of primary biliary cirrhosis. Womens Health Issues. 2008;18:336–342. doi: 10.1016/j.whi.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Poupon RE, Chrétien Y, Chazouillères O, Poupon R, Chwalow J. Quality of life in patients with primary biliary cirrhosis. Hepatology. 2004;40:489–494. doi: 10.1002/hep.20276. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki K, Suzuki K, Koizumi K, Takada H, Nishiki R, Ichimura H, et al. Effect of symptomatic gastroesophageal reflux disease on quality of life of patients with chronic liver disease. Hepatol Res. 2008;38:335–339. doi: 10.1111/j.1872-034X.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- 27.Estraviz B, Quintana JM, Valdivieso A, Bilbao A, Padierna A, de Urbina JO, et al. Factors influencing change in health-related quality of life after liver transplantation. Clin Transplant. 2007;21:481–499. doi: 10.1111/j.1399-0012.2007.00672.x. [DOI] [PubMed] [Google Scholar]
- 28.Bao ZJ, Qiu DK, Ma X, Fan ZP, Zhang GS, Huang YQ, et al. Assessment of health-related quality of life in Chinese patients with minimal hepatic encephalopathy. World J Gastroenterol. 2007;13:3003–3008. doi: 10.3748/wjg.v13.i21.3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalaitzakis E, Simrén M, Olsson R, Henfridsson P, Hugosson I, Bengtsson M, et al. Gastrointestinal symptoms in patients with liver cirrhosis: associations with nutritional status and health-related quality of life. Scand J Gastroenterol. 2006;41:1464–1472. doi: 10.1080/00365520600825117. [DOI] [PubMed] [Google Scholar]
- 30.Rowan PJ, Al-Jurdi R, Tavakoli-Tabasi S, Kunik ME, Satrom SL, El-Serag HB. Physical and psychosocial contributors to quality of life in veterans with hepatitis C not on antiviral therapy. J Clin Gastroenterol. 2005;39:731–736. doi: 10.1097/01.mcg.0000173860.08478.a6. [DOI] [PubMed] [Google Scholar]
- 31.Sumskiene J, Sumskas L, Petrauskas D, Kupcinskas L. Disease-specific health-related quality of life and its determinants in liver cirrhosis patients in Lithuania. World J Gastroenterol. 2006;12:7792–7797. doi: 10.3748/wjg.v12.i48.7792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dias Teixeira MC, de Fátima Gomes de Sá Ribeiro M, Strauss E. A new insight into the differences among non-cirrhotic and cirrhotic patients using the liver disease quality of life instrument (LDQOL) Ann Hepatol. 2005;4:264–271. [PubMed] [Google Scholar]
- 33.Younossi ZM, Guyatt G, Kiwi M, Boparai N, King D. Development of a disease specific questionnaire to measure health related quality of life in patients with chronic liver disease. Gut. 1999;45:295–300. doi: 10.1136/gut.45.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonkovsky HL, Snow KK, Malet PF, Back-Madruga C, Fontana RJ, Sterling RK, et al. HALT-C Trial Group. Health-related quality of life in patients with chronic hepatitis C and advanced fibrosis. J Hepatol. 2007;46:420–431. doi: 10.1016/j.jhep.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Plas SM, Hansen BE, de Boer JB, Stijnen T, Passchier J, de Man RA, et al. Generic and disease-specific health related quality of life in non-cirrhotic, cirrhotic and transplanted liver patients: a cross-sectional study. BMC Gastroenterol. 2003;17(3):33. doi: 10.1186/1471-230X-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lydick E, Epstein RS. Interpretation of quality of life changes. Qual Life Res. 1993;2:221–226. doi: 10.1007/BF00435226. [DOI] [PubMed] [Google Scholar]
- 37.Sloan JA, Cella D, Hays RD. Clinical significance of patient-reported questionnaire data: another step towards consensus. J Clin Epidemiol. 2005;58:1217–1219. doi: 10.1016/j.jclinepi.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 38.Newton JL, Jones DEJ, Henderson, Kane L, Wilton K, Burt AD, et al. Fatigue in non-alcoholic fatty liver disease (NAFLD) is significant and associates with inactivity and excessive daytime sleepiness but not with liver disease severity or insulin resistance. Gut. 2008;57:807–813. doi: 10.1136/gut.2007.139303. [DOI] [PubMed] [Google Scholar]
- 39.Stanca CM, Bach N, Krause C, Tandon N, Freni MA, Gutierrez JA, et al. Evaluation of fatigue in U.S. patients with primary biliary cirrhosis. Am J Gastroenterol. 2005;100:1104–1109. doi: 10.1111/j.1572-0241.2005.41315.x. [DOI] [PubMed] [Google Scholar]
- 40.Gutteling JJ, De Man RA, Van Der Plas SM, Schalm SW, Busschbach JJ, Darlington ASE. Determinants of quality of life in chronic liver patients. Aliment Pharmacol Ther. 2006;23:1629–1635. doi: 10.1111/j.1365-2036.2006.02934.x. [DOI] [PubMed] [Google Scholar]
- 41.Clark JM. The epidemiology of nonalcoholic fatty liver disease in adults. J Clin Gastroenterol. 2006;40(Suppl):S5–10. doi: 10.1097/01.mcg.0000168638.84840.ff. [DOI] [PubMed] [Google Scholar]
- 42.Häuser W, Holtmann G, Grandt D. Determinants of health-related quality of life in patients with chronic liver diseases. Clin Gastroenterol Hepatol. 2004;2:157–163. doi: 10.1016/s1542-3565(03)00315-x. [DOI] [PubMed] [Google Scholar]
- 43.Fontaine KR, Cheskin LJ, Barofsky I. Health-related quality of life in obese persons seeking treatment. J Fam Pract. 1996;43:265–270. [PubMed] [Google Scholar]
- 44.Kortt MA, Clarke PM. Estimating Utility Values for Health States of Overweight and Obese Individuals Using the SF-36. Qual Life Res. 2005;14:2177–2185. doi: 10.1007/s11136-005-8027-6. [DOI] [PubMed] [Google Scholar]
- 45.Doll HA, Petersen SEK, Stewart-Brown SL. Obesity and Physical and Emotional Well-Being: Associations between Body Mass Index, Chronic Illness, and the Physical and Mental Components of the SF-36 Questionnaire. Obesity Research. 2000;8:160–170. doi: 10.1038/oby.2000.17. [DOI] [PubMed] [Google Scholar]
- 46.Katz DA, McHorney CA, Atkinson RL. Impact of obesity on health-related quality of life in patients with chronic illness. J Gen Internal Med. 2000;15:789–796. doi: 10.1046/j.1525-1497.2000.90906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Häuser W, Holtmann G, Grandt D. Determinants of health-related quality of life in patients with chronic liver diseases. Clin Gastroenterol Hepatol. 2004;2:157–163. doi: 10.1016/s1542-3565(03)00315-x. [DOI] [PubMed] [Google Scholar]
- 48.Hussain KB, Fontana RJ, Moyer CA, Su GL, Sneed-Pee N, Lok AS. Comorbid illness is an important determinant of health-related quality of life in patients with chronic hepatitis C. Am J Gastroenterol. 2001;96:2737–2744. doi: 10.1111/j.1572-0241.2001.04133.x. [DOI] [PubMed] [Google Scholar]
- 49.Baumeister SE, Volzke H, Marschall P, John U, Schmidt CO, Flessa S, et al. Impact of Fatty Liver Disease on Health Care Utilization and Costs in a General Population: A 5-Year Observation. Gastroenterology. 2008;134:85–94. doi: 10.1053/j.gastro.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 50.Hunt C, Tart JS, Dowdy E, Bute BP, Williams DM, Clavien PA. Effect of orthotopic liver transplantation on employment and health status. Liver Transplantation. 2005;2:148–153. doi: 10.1002/lt.500020211. [DOI] [PubMed] [Google Scholar]
- 51.Kleefstra N, Landman GW, Houweling ST, Ubink-Veltmaat LJ, Logtenberg SJ, Meyboom-de Jong B, et al. Prediction of mortality in type 2 diabetes from health-related quality of life (ZODIAC-4) Diabetes Care. 2008;31:932–933. doi: 10.2337/dc07-2072. [DOI] [PubMed] [Google Scholar]
- 52.Singh JA, Nelson DB, Fink HA, Nichol KL. Health-related quality of life predicts future health care utilization and mortality in veterans with self-reported physician-diagnosed arthritis: the veterans arthritis quality of life study. Semin Arthritis Rheum. 2005;34:755–765. doi: 10.1016/j.semarthrit.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 53.Fan VS, Curtis JR, Tu SP, McDonell MB, Fihn SD Ambulatory Care Quality Improvement Project Investigators. Using quality of life to predict hospitalization and mortality in patients with obstructive lung diseases. Chest. 2002;122:429–436. doi: 10.1378/chest.122.2.429. [DOI] [PubMed] [Google Scholar]
- 54.Mapes DL, Lopes AA, Satayathum S, McCullough KP, Goodkin DA, Locatelli F, et al. Health-related quality of life as a predictor of mortality and hospitalization: the Dialysis Outcomes and Practice Patterns Study (DOPPS) Kidney Int. 2003;64:339–349. doi: 10.1046/j.1523-1755.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 55.Elwing JE, Lustman PJ, Wang HL, Clouse RE. Depression, anxiety, and nonalcoholic steatohepatitis. Psychosom Med. 2006;68:563–569. doi: 10.1097/01.psy.0000221276.17823.df. [DOI] [PubMed] [Google Scholar]