Abstract
Background
ImmuBalance™ is a koji fungus (Aspergillus oryzae) and lactic acid fermented soybean product. This unique production process is believed to create a food supplement that helps to induce or maintain normal immune response.
Objective
To assess possible therapeutic effects of ImmuBalance™ on peanut hypersensitivity using a murine model of peanut allergy (PNA).
Methods
Peanut (PN) allergic C3H/HeJ mice were fed standard mouse chow containing 0.5% or 1.0% ImmuBalance (ImmuBalance 2X), radiation-inactivated 1.0% ImmuBalance (I-ImmuBalance 2X), or regular diet chow (sham) for 4 weeks, beginning 10 weeks after the initial PN sensitization, and then challenged with PN. Anaphylactic symptom scores, plasma histamine, serum peanut specific-IgE levels and splenocyte cytokine profiles were determined.
Results
While 100 % of sham-treated peanut-allergic mice developed anaphylactic reactions with a median score of 3.3 following peanut challenge, only 50% of ImmuBalance, 30% of ImmuBalance 2X and 40% of I-ImmuBalance 2X-treated mice developed allergic reactions with median scores of 1.0, 0.4, and 0.5 respectively, which were significantly less than that in the sham-treated mice( p<0.05). Plasma histamine and PN specific-IgE levels were also significantly less in all treated mice than in sham-treated mice (p<0.05). Furthermore, IL-4, IL-5 and IL-13 production by PN-stimulated splenocytes in vitro from ImmuBalance fed mice were markedly reduced compared to sham-treated mice, whereas IFN-γ production was moderately increased. TGF-β and TNF-α production were similar.
Conclusions
ImmuBalance protects against peanut-induced anaphylaxis when administered as a food supplement in this model. Protection was associated with down-regulation of Th2 responses. This supplement may provide a potential novel therapy for peanut allergy.
Introduction
Peanut allergy (PNA) has been the target of most research on food allergy therapy for several reasons: peanut (PN) is one of the most common foods leading to anaphylactic reactions including fatal and near-fatal food-anaphylactic reactions; (1–4) PNA is difficult to avoid because of its frequent presence in manufactured foods; PNA is a lifelong condition in the most affected subjects; and the prevalence of PNA is increasing in westernized countries.(5;6) Given the persistence of this condition and the lack of therapeutic modalities, allergen-specific immunotherapy (IT) is currently being re-explored as a treatment option. Previous attempts to use peanut-specific IT were unsuccessful because of significant adverse effects (7). Soybean seed storage proteins share considerable amino acid homology with PN allergens(8). Soy IT could have a lower risk of adverse reactions than classical PN IT, while providing some level of desensitization, and may provide a new therapeutic intervention for PNA(8). Pons et al (8) reported that soybean protein IT reduced peanut-allergic responses in the PNA mouse model. However, soy IT had no effect on PN-specific IgE, and there was an increase in TNF-α, a proinflammatory cytokine. Furthermore soy was administrated intraperitoneally, which is not a clinical option in man. Alternative soy-based IT may be useful in the management of peanut allergy.
Another novel approach, which appears to have potential therapeutic benefits for food allergy, is probiotic therapy. A recent Finnish trial found that high-risk infants fed Lactobacillus rhamnosus GG (LGG) early in life had a decreased incidence of IgE-associated dermatitis.(9) In another study, administration of LGG to allergic infants increased levels of IFN- in isolated blood cells suggesting a possible Th1 stimulatory effect, (10), which has been associated with the development of peanut tolerance (11). Although the term probiotic was classically defined as a “live microbial food supplement which beneficially affects the host animal by improving its intestinal microbial balance” (12), the definition has been further refined to “living microorganisms, which upon ingestion in certain numbers, exert health benefits beyond inherent basic nutrition” (13)More recently, the National Center for Complementary and Alternative Medicine of the National Institutes of Health established a more comprehensive description of probiotics. It states that “probiotics can be administered orally as dietary supplements and in yogurt, fermented and unfermented milk, infant formula, bacterial lyophilizates, juices, and even candy. They may be applied topically and as suppositories. Probiotics in dietary supplements or foods may be live, heat-treated, irradiated, spray-dried, or freeze-dried. Inactivated probiotics may be as effective as live probiotics in certain conditions and may be more favorable because of lower infectious risk (especially in infants whose gut defense barrier is immature) (14). Probiotic studies, however, are still in the exploratory stage, and the feasibility of treating severe food allergy such as PNA with probiotic materials has not been previously explored. Although, the previous Finnish study reported evidence for prevention of food allergy, evaluation of the effects of probiotic products on established food allergy, particularly PNA, is limited.
The probiotic, ImmuBalance™ (Nichimo Co., Ltd., Tokyo, Japan) is a proprietary koji fermentation product made by fermenting defatted soybeans with Aspergillus oryzae, and lactic acid bacteria (Pediococcus parvulus and Enterococcus faecium) according to a new Japanese fermentation technology. Koji molds have traditionally been used in Japanese fermentation technology to produce a number of foods, such as miso (fermented soybean paste), shoyu (soy sauce), and sake (alcoholic beverage)(15). Recently, consumption of fermented soy products has been suggested to be of possible benefit for allergic conditions. A cross-sectional study of the relationship between soy product intake and prevalence of allergic rhinitis in pregnant Japanese women showed a clear inverse linear trend between dietary miso intake and prevalence of allergic rhinitis(16). Another study reported that soy sauce has hypoallergenic and anti-allergic properties(17). Recently an open-label pilot study reported that oral administration of Immubalance daily for 3 months improved clinical symptoms of Japanese cedar pollinosis (18). Given the need for effective and practical methods to treat food allergy, especially PNA, and based on previous studies, we hypothesized that ImmuBalance may have an immunotherapeutic effect on PNA. Given the potential life-threatening reactions of PNA, animal models of peanut allergy, which closely mimic human PNA, have provided useful tools to investigate the potential therapies for PNA(19–22). In this study, we tested the effect of ImmuBalance in two different doses on an established murine model of PNA. Furthermore, since inactivated probiotics may be more desirable than live organisms for treatment of pediatric diseases, we also tested effects of irradiation sterilized ImmuBalance (I-ImmuBalance), which contains inactivated microorganisms. Standard mouse chow supplemented with ImmuBalance or I-ImmuBalance was employed as a means of administering the probiotics, thereby mimicking animal and human use of fermented soy products as food supplements. We found that PN challenged ImmuBalance-treated mice exhibited significantly reduced clinical symptoms as compared with control mice. I-ImmuBalance was comparable to ImmuBalance. ImmuBalance protection against PNA might be secondary to its suppressive effect on Th2 responses. These results suggest that ImmuBalance and perhaps I-ImmuBalance may have potential for developing a novel probiotic therapy for PNA and other food allergies
MATERIALS AND METHODS
Mice and reagents
Five-week-old female C3H/HeJ mice purchased from the Jackson Laboratory (Bar Harbor, ME) were maintained on PN-free chow under specific pathogen-free conditions. Standard guidelines for the care and use of animals were followed. Freshly ground, roasted, whole PN was employed as allergen. Crude PN extract (CPE) was prepared as described previously.(23) Cholera toxin was purchased from List Biological Laboratories, Inc (Campbell, CA). Concanavalin A (Con A) and albumin, human-dinatrophenyl (DNP-albumin) were purchased from Sigma (St Louis, MO). Antibodies for ELISAs were purchased from The Binding Site, Inc, (San Diego, CA) or PharMingen (San Diego, CA).
Preparation of ImmuBalance/I-ImmuBalance containing mouse Chow
ImmuBalance powders added to diet chow were products of Nichimo Co. Ltd., Japan. To manufacture this product, defatted soybean is fed into fermentation chamber. While the chamber mixes the defatted soybeans, water was sprayed over the defatted soybean uniformly until its moisture content became approximately 41%. The defatted soybean was steam sterilized at 100°C for 150 minutes and left in the chamber at ambient temperature until it reached room temperature. The defatted soybean was mixed again in the chamber while koji mold (a commercially available culture of Aspergillus oryzae) was added. The mixture was kept in the chamber at a controlled optimum growth temperature (40°C). As hyphae grow from the spores, the defatted soybean starts to clump. The defatted soybean was mixed again in the chamber until it was well separated and then left in the chamber at the controlled optimum growth temperature (40°C) for continued hyphae growth for 20 hours. The defatted soybean was mixed again in the chamber and clumps separated. The above cycle was repeated once until the fermentation process is completed. The defatted soybean is then transferred from the fermentation chamber to the hydration and degradation machine. Water is added to the defatted soybean in the hydration and degradation machine to increase its moisture content uniformly. After water is added, the defatted soybean is left for at least 24 hours at a controlled temperature at 40°C. The extent of hydrolysis of the defatted soybean by the enzymes of koji fungus is determined and the hydration and degradation time is adjusted. Enterococcus and Pediococcus begin to grow from koji incubation through hydrolysis. After enzymatic degradation, the fermented defatted soybean was transferred to a warm air dryer. Temperature and airflow are adjusted so that its moisture content becomes 13% or less. The dried fermented soybean is then fed into a crusher and pulverized at 80 mesh pass, and the moisture content becomes 6% or less. The pulverized fermented soybean is then packaged. The control data of final product, ImmuBalance are outlined in Table 1.
Table 1.
Composition of ImmuBalance
| Guaranteed Analysis (n=10) | A Range of Molecular Weight | ||||
|---|---|---|---|---|---|
| Crude Protein, not less than 40% | 55.6±1.9% | 10,000 above | 9% | ||
| Crude Fat, less than 5% | 1.5±0.4% | 3,000~10,000 | 31% | ||
| Crude Fiber, | 3.4±0.7% | 1,000~3,000 | 6% | ||
| Crude Ash | 7.1±0.3% | 500~1,000 | 6% | ||
| Nitrogen-free Extracts | 20.7±0.5% | 500 under | 48% | ||
| Energy | 337 kcal/100 g | ||||
|
| |||||
|
Typical Analysis
| |||||
| Vitamins: | mg/100 g | Amino Acids: | (n=3) | ||
| Thiamin(Vit B1) | 0.24±0.05 (n=4) | Arginine | 1.64~3.22% | ||
| Riboflavin(Vit B2) | 1.07±0.20 (n=4) | Lysine | 3.00~3.29% | ||
| Pantothenic Acid | 1.58 | Histidine | 1.23~1.50% | ||
| Niacin | 3.10 | Phenylalanine | 1.84~2.74% | ||
| Pyridoxine(Vit B6) | 0.83±0.12 (n=4) | Tyrosine | 0.99~1.90% | ||
| Folic Acid | 0.34 | Leucine | 3.02~4.41% | ||
| Vitamin A | not detected | Isoleucine | 1.85~2.60% | ||
| Vitamin B12 | not detected | Methionine | 0.41~0.78% | ||
| Vitamin D | not detected | Valine | 2.06~2.75% | ||
| Vitamin E | 0.80 | Alanine | 2.46~2.92% | ||
| Glycine | 2.05~2.45% | ||||
| Minerals: | mg/100 g | Proline | 2.57~2.89% | ||
| Sodium (Na) | 31.5 | Glutamine | 10.1~10.3% | ||
| Phosphorus (P) | 898 | Serine | 1.53~2.73% | ||
| Iron (Fe) | 10.8 | Threonine | 1.16~2.17% | ||
| Calcium (Ca) | 354 | Asparagine | 5.59~6.47% | ||
| Potassium (K) | 2.69 | Tryptophan | 0.45~0.75% | ||
| Magnesium (Mg) | 360 | Cystine | 0.53~0.84% | ||
| Chlorine (Cl) | 61.0 | ||||
|
| |||||
|
Bacterial Analysis
| |||||
| Before sterilizing | (Spring) | (Summer) | (Autumn) | (Winter) | Average |
| Lot No. | HA050411 | HA050714 | HA041122 | HA050131 | |
| Pediococcus, cfu/g | 1.6×109 | 1.2×109 | 2.2×109 | 2.0×109 | 1.75×109 |
| Enterococcus, cfu/g | 7.5×108 | 5.6×108 | 6.0×108 | 3.3×108 | 5.60×108 |
| After sterilizing | |||||
| Quantity of koji mold, mg/g | 5.6 | ||||
| Coliform bacteria, | not more than 103 cfu/g | ||||
| Salmonella sp., | negative | ||||
|
| |||||
|
Isoflavone and Saponin Contents
| |||||
| Isoflavone | mg/100 g | Saponin | mg/100 g | ||
| Daidzein | 81.0 | Saponin Ab | 252 | ||
| Genistein | 99.2 | Saponin Bb | 501 | ||
| Glycitein | 11.6 | Total saponins | 753 | ||
| Total isoflavones | 191.8 | ||||
Diet chow containing ImmuBalance used in this study were formulated by adding 0.5% or 1% of ImmuBalance to the standard rodent diet chow # 5053 (weigh, ImmuBalance vs chow) (Research Diets Inc., New Brunswick, NJ). In each ImmuBalance containing chow, 0.5% ImmuBalance chow was composed of 9.0 × 107/g of lactic acid bacteria and named ImmuBalance; 1.0% ImmuBalance chow was composed of 1.8 × 108/g lactic acid bacteria and was labeled ImmuBalance 2X. In addition, 1.0% ImmuBalance chow was irradiated by Co60 as previously described 24 for the purpose of inactivation of microorganisms, and was labeled I-ImmuBalance 2X”. Based on the daily mouse chow consumption (approximately 3.5 g), the daily dosage of lactic acid bacteria used in this study was 3.15 × 108–6.3 × 108, which was within the bacterial dose range previously used in murine models (25;26).
PN sensitization/challenge and treatment
Mice were sensitized intragastrically (i.g.) as shown in Figure 1A with ground PN (10 mg per mouse) plus cholera toxin (20 μg per mouse), and boosted at weeks 6 and 8 with PN. Treatment began at week 10 at which time PN hypersensitivity was established, as demonstrated previously. (27) At week 10, PN allergic mice were fed ImmuBalance, ImmuBalance 2X, or I-ImmuBalance 2X diets for four weeks. Sham-treated PN allergic mice and naïve mice (non-sensitized) were maintained on regular diet chow (# 5053). Twenty-four hours after completing the 4-week treatment, all mice were challenged i.g. with 200 mg of PN.
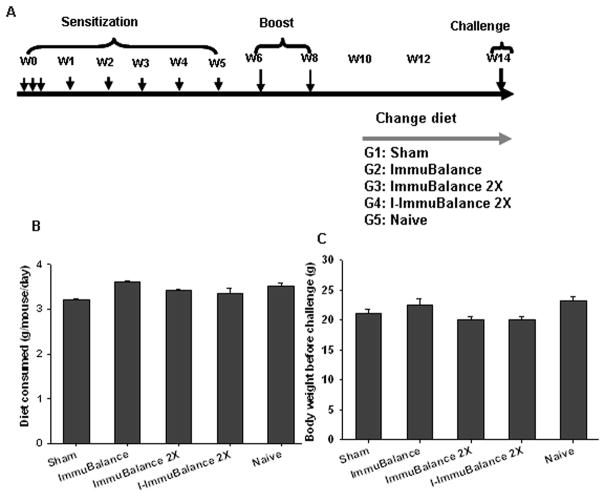
Fig 1. A. Experimental protocol.
C3H/HeJ mice were sensitized/boosted with PN and CT over 8 weeks as indicated. Different doses of ImmuBalance or I-ImmuBalance-containing chow were fed for 4 weeks commencing at week 10. All mice were challenged at week 14 (24 hours after completing the treatment). B. Chow consumption. Mouse chow consumption was monitored daily during the treatment. Data are mean± SEM of daily chow consumption of mouse in each group (N=10 in Sham, 8 in ImmuBalance, 10 in ImmuBalance 2X, 5 in I-ImmuBalance 2X and 10 in naïve) from 2 sets of in vivo experiments. C. Body weights. Body weights were measured at week 14, one day prior to challenge. Data are depicted as mean± SEM mouse body weight in each group as in Fig 2B.
Mouse chow consumption was monitored daily during the treatment. Body weight was measured at week 14, one day prior to the challenge.
Assessment of hypersensitivity reactions
Anaphylactic reactions were scored using a previously described scoring system-(21) 0, no symptoms; 1, scratching and rubbing around the snout and head; 2, puffiness around the eyes and snout, pilar erection, diarrhea, and reduced activity or standing still with an increased respiratory rate; 3, wheezing, labored respiration, and cyanosis around the mouth; 4, symptoms as in no; 3 with loss of consciousness, tremors, and/or convulsion; 5, death.
In addition, to obtain a more objective measure of anaphylaxis severity, rectal temperatures were measured 30 minutes after challenge using a thermal probe (Harvard Apparatus, Newark, NJ).
Measurement of plasma histamine levels and mast cell degranulation
Plasma was collected 30 minutes after challenge, and histamine levels were determined using an enzyme immunoassay kit (ImmunoTECH, Marseille, France), as previously described.(21)
Measurement of serum peanut-specific IgE, IgG1, and IgG2a levels
Venous blood samples were obtained from tail veins twice during the sensitization protocol (weeks 3 and 7), 1 day prior to treatment (week 10), 2 weeks after initiating treatment (week 12) and 1 day prior to challenge (week 14). Sera were collected and stored at −80°C until analyzed. Levels of peanut-specific IgE, IGg1 and IgG2a were determined by ELISA as previously described.(21)
Cell culture, proliferation and cytokine measurements
Splenocytes were prepared following the PN challenge as previously described.(8;27;28) Thirty minutes after challenge and recording of anaphylactic response scores, mice were sacrificed and spleens from each group were removed and pooled. Splenocytes were isolated by mincing the spleens between 2 sterile slides (Fisher Scientific), lysing of RBCs with lysing buffer (Sigma Chemical Co), and passaging through a cell strainer (Becton Dickinson and Co). After extensive washing, cells were suspended in complete cell culture medium (RPMI 1640 containing 10% FCS and 1% penicillin-streptomycin). For proliferation assays, splenocytes (1 × 106 cells per well in 0.2 mL) were cultured with CPE (200 μg/mL) in triplicate in 96-well plates. Unstimulated cells and Concanavalin (Con A, 2.5 μg/ml)-stimulated cells were used as negative and positive controls, respectively. Three days later, the cultures received an 18-hour pulse of 1 mCi [3H]thymidine per well. The cells were then harvested and the incorporated radioactivity counted in a β scintillation counter. The results were expressed as counts per minute (CPM) as described previously(29).
In parallel, cell suspensions were cultured in four replicates in 24-well plates (4×106/well/mL) in the presence of 200 μg/ml CPE, 2.5 μg/ml ConA, or medium alone. Supernatants were collected and pooled from each group after 72-hour culture and stored at −80°C until analyzed. Supernatants were collected after 72 hours of culture. Cytokine levels were determined by ELISA in triplicate according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN, for IL-13; PharMingen, San Diego, CA, for all others).
Acute toxicity study
To ensure the safety of the diets, we conducted acute toxicity studies utilizing large single oral doses of I-ImmuBalance in mice as described previously(30). Naive female C3H/HeJ mice (n=10) maintained on standard diet were fed I-ImmuBalance at 20 times the equivalent mouse daily dose (35 mg/day), and observed for 24 hours. Mice were then sacrificed for histological analyses. In addition, male and female Sprague Dawley rats (n=5 each group) were fed 1.25 g/kg, 2.5 g/kg, and 5 g/kg body weight of I-ImmuBalance followed by a 14 day observation period, and then sacrificed for histological analyses. The dose of 5 g/kg body weight of I-ImmuBalance tested in this study is 100 times the previously recommended human daily dose (50 mg/kg).
Microorganism-free ImmuBalance preparation
ImmuBalance was dissolved in saline (w:v=1:3), mixed and stored at 4°C for 24 h. After centrifugation at 3,000 rpm at 4°C for 10 min, the supernatant was collected and the remaining pellet was rinsed with saline, followed by centrifugation. The supernatants from the two centrifugations were combined, and freeze dried.
Determine ImmuBalance binding to PN–specific IgE antibodies in human sera
Since a number of previous publications have demonstrated that fermented soy products are hypoallergenic(31;32), and since our goal was to determine whether ImmnuBalance can be of benefit for the treatment of peanut allergy when used as a dietary supplement, we investigated whether there was any cross-reactivity between peanut and ImmuBalance. Sera from PN allergic patients (n=4) with greater than 100 kUA/L of peanut-specific IgE were obtained and diluted (1:50) in fish-gelatin PBST (0.5%). Immulon II 96-well plates (Dynatech Laboratories, Inc, Chantilly, VA) were coated with 2μg/mL CPE or microorganism free ImmuBalance (MF-IB) pretreated as above in coating buffer (Sigma, St Louis, MO). A 50 μl sample was added to each well in duplicate. After blocking and washing, anti-human IgE conjugated with HRP (KPL Gaithersburg, MD)(1:2000 dilutions) were added. Plates were then developed with TMB Microwell Peroxidase Substrate (KPL) and read on a microplate reader (Molecular Devices. Sunnyvale, CA) at 650 nM. The data are expressed as optimal density (OD).
Statistical analysis
Data were analyzed using SigmaStat 2.03 software (SPSS Inc. Chicago, IL). For studies with a single outcome measurement, we performed an overall Analysis of Variance (One way ANOVA) followed by pairwise testing using Bonferroni’s adjustment. For data that appeared skewed, i.e. non-normally distributed, differences between groups were analyzed by One Way Analysis of Variance on Ranks followed by all pairwise comparisons. p values =0.05 were considered significant
RESULTS
Measurements of daily chow consumption and body weight
Since mouse chow supplementation was used to administer ImmuBalance, daily mouse chow consumption was monitored during the treatment course to exclude the influence of variations in amounts of mouse chow consumption on the outcomes of ImmuBalance treatment. Body weights were obtained one day prior to the initiation of treatment and one day prior to the final challenge. Average daily mouse chow consumption was approximately 3.5 g, and there was no significant difference in chow consumption between the groups (Fig 1B). Furthermore, body weights did not differ between groups (Fig 1C).
Reduction of PN-induced anaphylactic reactions
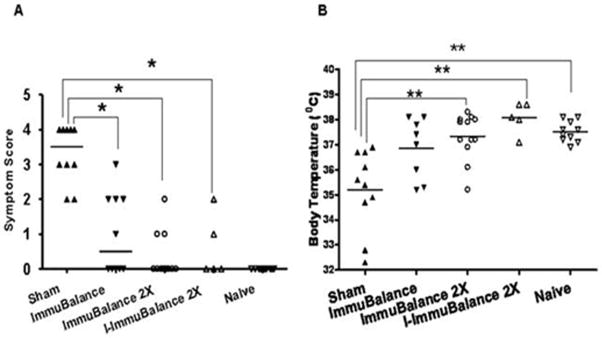
Anaphylactic symptom scores were determined 30 minutes after oral PN challenge. As shown in Fig 2 A, all mice in the sham-treated group exhibited symptoms such as puffiness around the eyes, swelling around the snout, and diarrhea (score 2, 20%); labored respiration (score 3, 30%); and near fatal reactions such as loss of consciousness or little activity despite gentle prodding (score 4, 50%). The median score was 3.2 (range: 2–4). In contrast, 50%, 30% and 40% of ImmuBalance, ImmuBalance 2X and I-ImmuBalance 2X treated-mice showed symptoms. The median scores in those treated groups were 1(range: 0–3); 0.4(range: 0–2); 0.5(range: 0–2) respectively, significantly lower than that seen in the sham-treated group (p<0.05). Naive mice showed no reaction to challenge (Fig 2 A). Protection against peanut-induced anaphylaxis appeared to be more pronounced in the double-dose ImmuBalance and I-ImmuBalance treated mice.
Fig. 2. Evaluation of anaphylactic reactions.
Thirty minutes following PN challenge, anaphylactic symptom scores (A) and rectal temperatures (B) were evaluated. Bars indicate median for symptom score and mean for temperature for mice in each group (same numbers of mice in each group as in Fig 1). *p<0.05 vs. Sham; **p<0.01 vs. Sham; # # p<0.01 vs. Naive
Since decreased core body temperatures reflect the severity of systemic anaphylaxis in mice, rectal temperatures were obtained 25 minutes after the PN challenge to objectively measure the severity of systemic anaphylaxis. Temperatures in the naive group ranged between 37° C and 38°C after oral PN challenge, whereas rectal temperatures in 8 of 10 mice in the sham-treated group were 1° to 4°C below normal. There was a significant difference in mean temperatures between sham and naïve groups (p<0.01). The mean temperatures of mice in all treated groups were higher than those of the sham-treated group, and ImmuBalance 2X-treated and I-ImmuBalance 2X-treated groups reached statistical significance (p < 0.05 vs sham). The temperatures in these groups were not significantly different from naïve mice (Fig 2 B). These results demonstrate that ImmuBalance was effective in protecting PN allergic mice from PN-induced anaphylactic reactions, and that a double dose of ImmuBalance enhanced the protection. Inactivation of microoganisms by irradiation in ImmuBalance did not significantly reduce its effectiveness.
Reduction of plasma histamine levels
Histamine is the major mediator of systemic anaphylactic reactions. Consistent with our previous findings(21), histamine levels in the sham-treated group were markedly elevated following peanut challenge. In contrast, histamine levels in all ImmuBalance-treated groups were significantly lower than those of the sham-treated group (Fig 3, p<0.05) being lowest in the ImmuBalance 2X-and I-ImmuBalance 2X-treated group.
Fig. 3. Plasma histamine levels.
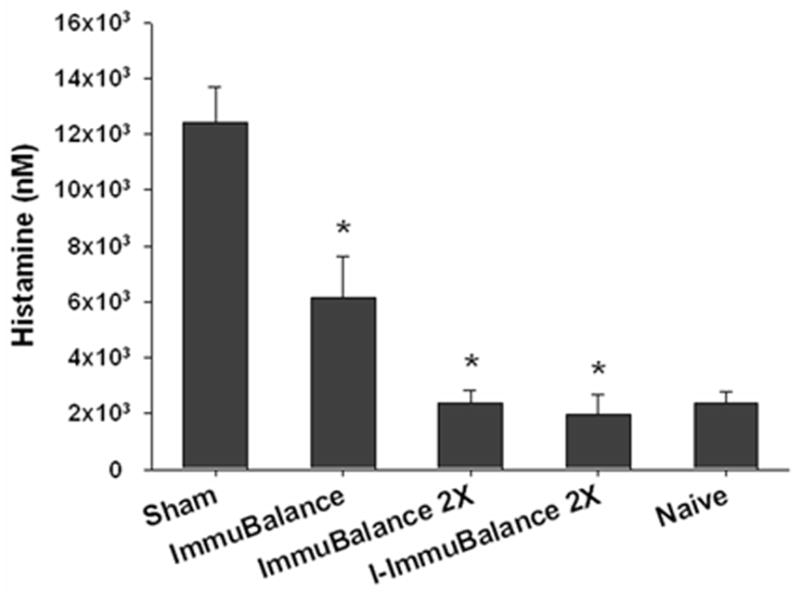
30 minutes following oral PN challenge, blood was collected and plasma histamine levels were measured using an enzyme immunoassay. Data are expressed as means ± SEM for mice from each group (same numbers of mice as in Fig 1). *P<0.05 vs sham.
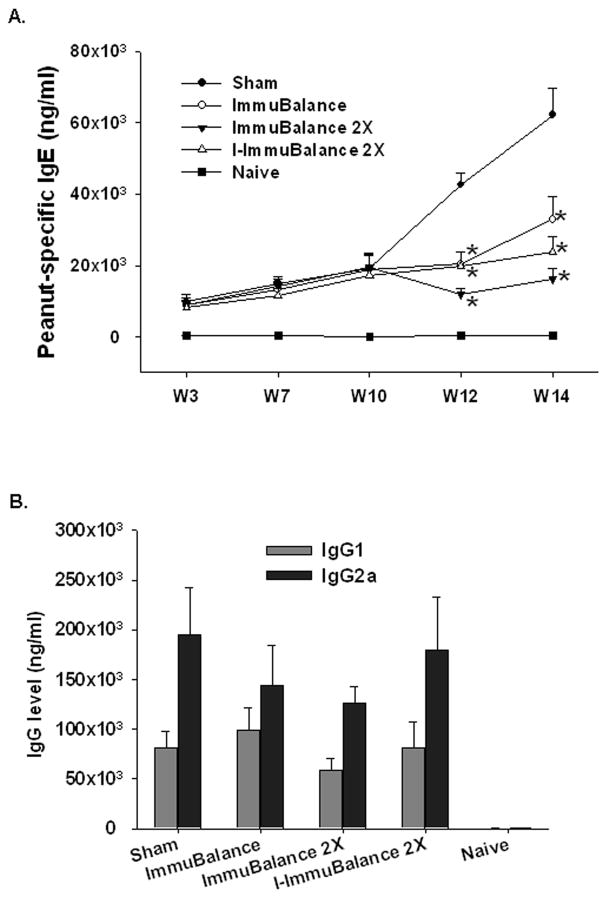
Reduction of PN-specific IgE levels
ImmuBalance treatment was initiated 10 weeks following the initial PN sensitization, at which time PN-specific IgE levels were markedly elevated. After two weeks treatment (week 12), IgE levels in all ImmuBalance-treated groups were significantly lower than those in the sham-treated groups (Fig 4A, p<0.05) and they remained significantly lower at the time of challenge (week 14). Reduction of IgE levels appeared to be more pronounced in the ImmuBalance 2X and I- ImmuBalance 2X groups. There were no significant differences in IgG1 or IgG2a levels between groups (Fig 4B). There results suggest that the effect of ImmuBalance on PNA was due at least in part to reduction of PN-specific IgE.
Fig 4. A. Serum PN-specific IgE levels.
Blood was collected from tail veins of each group of mice at the time points indicated, and sera were obtained. Serum PN-specific IgE levels were determined by ELISA. Data are expressed as means ± SEM (same numbers of mice as in Fig 1). *p<0.05 vs Sham. B. Serum PN specific IgG1 (gray bars) and IgG2a (solid bars) were determined by ELISA. Data are means ± SEM (same numbers of mice as in Fig 1) at week 14 one day before challenge.
Suppression of PN-specific T-cell proliferation and Th2 cytokine production
Consistent with our previous finding, splenocytes from sham-treated, PN-allergic mice showed a marked proliferative response to PN stimulation in vitro. PN-induced proliferative responses of splenocytes from ImmuBalance treated mice were significantly less than those from sham-treated mice (P < 0.01, Fig 5) and were essentially the same as splenocytes from naive mice. Interestingly, ImmuBalance treatment did not decrease the proliferative response to Con A stimulation, suggesting that the effect of ImmuBalance on T cells was PN antigen specific (data not shown).
Fig 5. Proliferation assay.
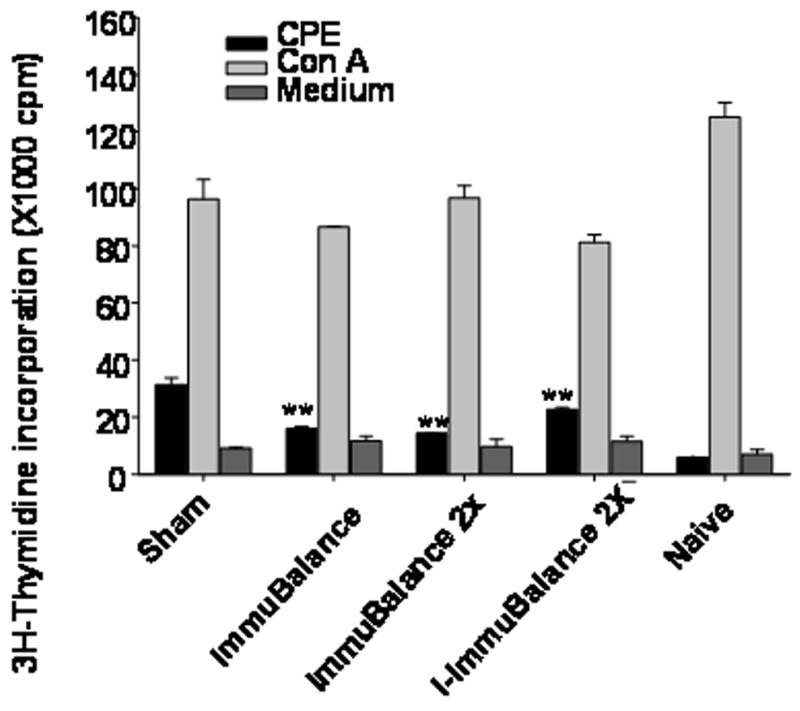
Immediately following evaluation of anaphylactic reactions, pooled splenocytes from each group of mice (n=5/group, from one set of in vivo experiment) were prepared and cultured in the presence or absence of CPE, or Con A. Data are expressed as Mean ± SEM of triplicates. **, p<0.01 vs sham.
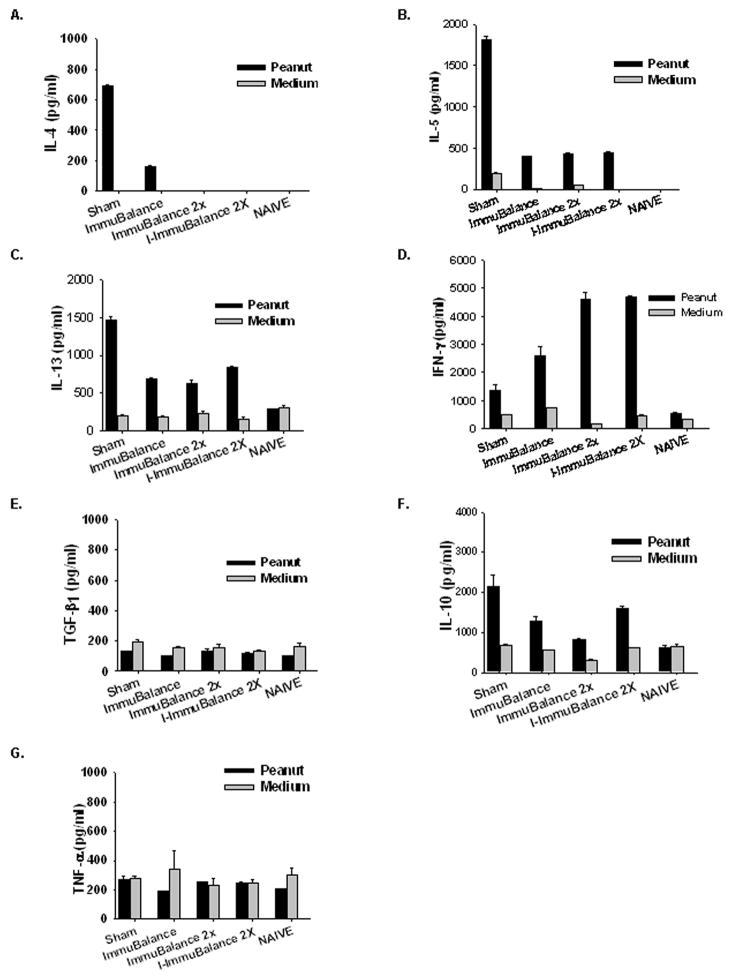
PN stimulated splenocytes from all ImmuBalance treated mice secreted less IL-4, IL-5, and IL-13 than splenocytes from sham-treated mice (Fig 6A–C), and ImmuBalance 2X and I-ImmuBalance 2X virtually abrogated IL-4 production. All treatments increased PN-stimulated IFN-γ zproduction (Fig 6D), and the higher dose appeared to be have a greater effect. No differences TGF-β and TNF-α production by splenocytes were detected between groups (Fig 6E and F) whereas IL-10 levels were moderately decreased (Fig 6G). These results show that ImmuBalance and I-ImmuBalance treatment resulted in specific suppression of both PN-induced T-cell proliferation and PN-activated Th2 cytokine secretion.
Fig 6. Effect of ImmuBalance on splenocyte cytokine production.
Splenocytes prepared as noted in Fig 5 were cultured in the presence or absence of CPE. Supernatants were harvested after 72 hours culture. Cytokine levels were measured by ELISA. Data are expressed as Mean ± SEM of duplicates of ELISA.
Acute toxicity evaluation
Like other fermented soy products, ImmuBalance is consumed by livestock and humans, and is considered to be safe. We found that no deaths occurred 24 hours after feeding 20 times the mouse daily dose of I-ImmuBalance to naïve C3H/HeJ mice. In addition, no deaths occurred over the 14 day observation period in Sprague Dawley rats fed the equivalent of 100 times the dose previously used in humans during 14 days observation. In addition, there were no abnormalities in histological analyses or body weight. These results together with the finding of no effect on body weights of PN-allergic mice show that I-ImmuBalance has a high margin of safety.
ImmuBalance does not bind to PN–specific IgE antibodies in human sera
Previous studies showed that fermented soy (Miso) is a hypo-allergenic soybean product(32) and that there is a surprisingly low rate of co-reactivity between PN and soy.(33) To further test potential ImmuBalance cross reactions in PN-allergic patients, we utilized an ELISA to test whether proteins in ImmuBalance were recognized by IgE antibodies in sera from PN-allergic patients. As shown in Fig 6, there was marked IgE binding in the PN coated wells, but no detectable IgE in the MF-IB coated wells. This result demonstrates that ImmuBalance does not bind to PN–specific IgE antibodies in human sera.
DISCUSSION
At the present time, strict avoidance of food allergens and ready access to self-injectable epinephrine is the standard of care for food allergy. Unfortunately, for a ubiquitous food such as peanut, the possibility of inadvertent ingestion is high. It is estimated that over 50% of PN allergic individuals will experience an accidental reaction to peanuts over a 2-year period.(34) Consequently there is a great need for effective and safe therapies for PNA.
In this study, we demonstrated that ImmuBalance significantly suppressed PNA in this model. ImmuBalance consumption significantly suppressed anaphylactic symptoms, which were related to a significant suppression of histamine release and peanut-specific IgE production. ImmuBalance also showed an immunomodulatory effect as evidenced by a reduction of Th2 and increase of Th1 responses.. In addition, we found that the therapeutic effect of I-ImmuBalance, which contains inactivated microorganisms, was comparable to ImmuBalance, which contained live organisms. Inactivated probiotics may carry a lower risk for children, who are more prone to food allergy than adults. Thus, I-ImmuBalance may provide additional advantages over live probiotics for food allergic children.
To further explore the immunotherapeutic effect of ImmuBalance or I-ImmuBalance on PNA in this model, we measured levels of TGF-β, a Th3 cytokine, and IL-10, a T regulatory cytokine. We found no differences in TGF-β production by splenocytes between treated and untreated PN-allergic mice, and only minor reductions in IL-10 in ImmuBalance or I-ImmuBalance treated mice, suggesting that ImmuBalance and I-ImmuBalance mediated protection against peanut-induced anaphylaxis in this model is not clearly associated with IL-10 or TGF-β regulatory cytokine levels. Alteration of Th1/Th2 responses may be an important mechanism underlying ImmuBalance or I-ImmuBalance therapeutic effect. However, in this study we did not rule out a potential role for regulatory cells involved in the clinical protection by ImmuBalance. Other potential mechanisms have not yet been explored.
Since soybeans and peanuts are both members of the legume family, potential cross-reactivity reactions of PN-allergic individuals to soy proteins must be considered. Clinical studies have shown a low rate of co-reactivity to PN and soy.(33;35–37) Furthermore, it was shown that fermented soy (Miso) is a hypoallergenic soybean product(32) and that about 80% of soybean-sensitive patients could ingest these soy products without any adverse reactions (27). Another study using a sandwich ELISA found that the soy allergen (Gly m Bd 28K) was not detected in fermented soybean products such as natto, soy sauce and miso, or in processed foods containing soybean protein isolate.(31) Our study found that ImmuBalance was not recognized by IgE antibodies in sera from peanut-sensitive patients. Probiotics are generally considered safe for dietary consumption, however, inactivated probiotics may be more desirable than live probiotics. (14) Cooked fermented soy products in the diet are common. However, one previous study reported that the use of inactivated LGG for the treatment of eczema was associated with adverse gastrointestinal symptoms and diarrhea (38). In our study, no abnormalities were observed in I-ImmuBalance-treated mice or ImmuBalance at the effective doses. We also tested acute toxicity of I-ImmuBalance in mice and rats, and no toxicity was observed at well above the effective dose, suggesting a high margin of safety. Taken together, ImmuBalance, particularly I-ImmuBalance, may prove to be a novel and effective therapy for PNA.
We are aware of the limitations of this study. While Immunobalance appears to have a significant therapeutic effect in this model, the product is a complex mixture of soy proteins and micro-organisms. We plan in the future to dissect the relative contributions of the Aspergillus fungus, the two lactic acid bacteria and modified soy protein, polysaccharides, and isoflavones etc. Some studies have demonstrated that Japanese-style fermented soy sauce, but not chemically fermented soy sauce promotes digestion. (39) A double-blind placebo controlled clinical trial involvning 51 subjects showed that shoyu polysacchrides from soy sauce improved quality of life for patients with seasonal allergic rhinitis(40). We suspect that probiotics in ImmuBalance may play a major role and that other components (microorganism-free fermented soy) may be necessary to generate a maximal effect of ImmuBalance on peanut allergy. Studies are underway to characterize pharmacological components in ImmuBalance product by comparing the efficacy and potency of inactivated Aspergillus oryzae, and lactic acid bacteria (Pediococcus parvulus and Enterococcus faecium) alone, and microorganism-free fermented soy from ImmuBalance on PNA using the same model system. In future studies, it is also important to address how much of the beneficial effect is due to TLR signaling versus immune tolerance. Furthermore, it may be a potential risk to use Immunobalance in peanut allergic patients who are also sensitized to Aspergillus antigens. We will address this question in future studies by determining if proteins in Immunobalance bind to Aspergillus specific IgE obtained from mould allergic patients.
In conclusion, this study demonstrated that ImmuBalance consumption has a therapeutic effect on PNA in a murine model of peanut anaphylaxis. Like other fermented soy products, ImmuBalance is hypoallergenic, and has an excellent safety profile. These findings suggest that ImmuBalance may be safe for PN-allergic patients. Since inactivated probiotics may be more desirable than live organisms for treating childhood food allergy, and I-ImmuBalance will be the focus of our future studies. Future studies will investigate the immunologically active components in I-ImmuBalance, whether I-ImmuBalance can provide long term protection, and whether it will block other food allergies.
Fig. 7. ImmuBalance does not bind to peanut –specific IgE antibodies in human sera.
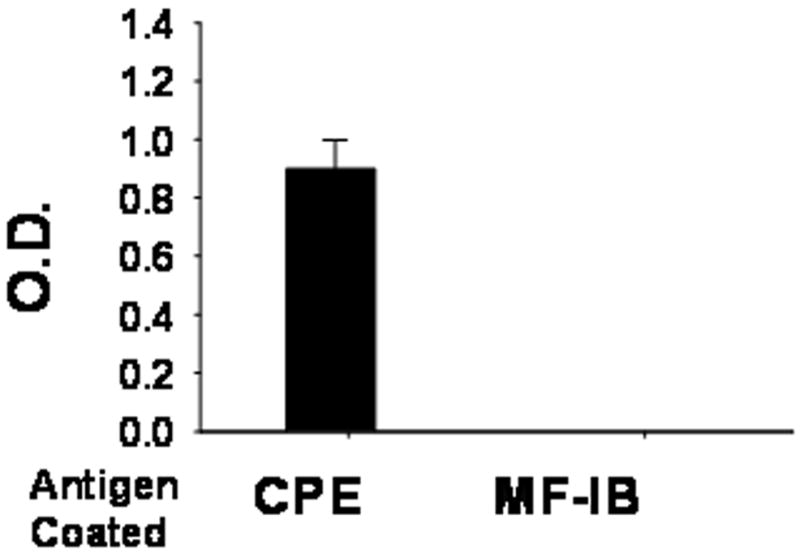
Sera from 4 peanut allergic patients with higher than 100 kUA/L peanut-IgE were diluted (1:50) and added to wells coated with either CPE or MF-IB. Binding to peanut-specific IgE antibodies was determined by ELISA. Results are expressed as mean ± SEM of O.D from 4 samples. CPE, Crude peanut extract; MF-IB, Microorganism-free ImmuBalance.
Acknowledgments
Funding source: This work was supported by National Institutes of Health grants # R01 AT001495 and Nichimo Co., Ltd., Tokyo, Japan.
Abbreviations
- PN
peanut (s)
- PNA
Peanut allergy
- ig
intragastric gavage
- CT
cholera toxin
- BF-IB
Microorganism-free ImmuBalance
References
- 1.Bock SA, Munoz-Furlong A, Sampson HA. Fatalities due to anaphylactic reactions to foods. J Allergy Clin Immunol. 2001;107(1):191–3. doi: 10.1067/mai.2001.112031. [DOI] [PubMed] [Google Scholar]
- 2.Sampson HA, Mendelson L, Rosen JP. Fatal and near-fatal anaphylactic reactions to food in children and adolescents. N Engl J Med. 1992;327:380–4. doi: 10.1056/NEJM199208063270603. [DOI] [PubMed] [Google Scholar]
- 3.Wang J, Sampson HA. Food anaphylaxis. Clin Exp Allergy. 2007;37(5):651–60. doi: 10.1111/j.1365-2222.2007.02682.x. [DOI] [PubMed] [Google Scholar]
- 4.Ramesh S. Food Allergy Overview in Children. Clin Rev Allergy Immunol. 2008;34(2):217–30. doi: 10.1007/s12016-007-8034-1. [DOI] [PubMed] [Google Scholar]
- 5.Nowak-Wegrzyn A, Sampson HA. Food allergy therapy. Immunol Allergy Clin North Am. 2004;24(4):705–25. viii. doi: 10.1016/j.iac.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Sampson HA. Update on food allergy. J Allergy Clin Immunol. 2004;113(5):805–19. doi: 10.1016/j.jaci.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 7.Oppenheimer JJ, Nelson HS, Bock SA, Christensen F, Leung DY. Treatment of peanut allergy with rush immunotherapy. J Allergy Clin Immunol. 1992;90(2):256–62. doi: 10.1016/0091-6749(92)90080-l. [DOI] [PubMed] [Google Scholar]
- 8.Pons L, Ponnappan U, Hall RA, Simpson P, Cockrell G, West CM, et al. Soy immunotherapy for peanut-allergic mice: modulation of the peanut-allergic response. J Allergy Clin Immunol. 2004;114(4):915–21. doi: 10.1016/j.jaci.2004.06.049. [DOI] [PubMed] [Google Scholar]
- 9.Kalliomaki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet. 2001;357(9262):1076–9. doi: 10.1016/S0140-6736(00)04259-8. [DOI] [PubMed] [Google Scholar]
- 10.Pohjavuori E, Viljanen M, Korpela R, Kuitunen M, Tiittanen M, Vaarala O, et al. Lactobacillus GG effect in increasing IFN-gamma production in infants with cow’s milk allergy. J Allergy Clin Immunol. 2004;114(1):131–6. doi: 10.1016/j.jaci.2004.03.036. [DOI] [PubMed] [Google Scholar]
- 11.Turcanu V, Maleki SJ, Lack G. Characterization of lymphocyte responses to peanuts in normal children, peanut-allergic children, and allergic children who acquired tolerance to peanuts. J Clin Invest. 2003;111(7):1065–72. doi: 10.1172/JCI16142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuller R. Probiotics in man and animals. J Appl Bacteriol. 1989;66(5):365–78. [PubMed] [Google Scholar]
- 13.Guarner F, Schaafsma GJ. Probiotics. Int J Food Microbiol. 1998;39(3):237–8. doi: 10.1016/s0168-1605(97)00136-0. [DOI] [PubMed] [Google Scholar]
- 14.http://grants.nih.gov/grants/guide/pa-files/PA-06-316.html. 1-15-0008.
- 15.Manabe M, Tanaka K, Goto T, Matsura S. Producing capability of kojic acid and aflatoxin by koji mould. Dev Food Sci. 1984;7:4–14. [Google Scholar]
- 16.Miyake Y, Sasaki S, Ohya Y, Miyamoto S, Matsunaga I, Yoshida T, et al. Soy, isoflavones, and prevalence of allergic rhinitis in Japanese women: the Osaka Maternal and Child Health Study. J Allergy Clin Immunol. 2005;115(6):1176–83. doi: 10.1016/j.jaci.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi M. Immunological functions of soy sauce: hypoallergenicity and antiallergic activity of soy sauce. J Biosci Bioeng. 2005;100(2):144–51. doi: 10.1263/jbb.100.144. [DOI] [PubMed] [Google Scholar]
- 18.Otsuka Y, Pan W. Effects of the novel symbiotic ImmuBalance as a food supplement in relieving clinical symptoms of Japanese cedar pollinosis: A pilot study. Clinical and Experimental Pharmacology and Physiology (2007) 2007;34:S73–S75. [Google Scholar]
- 19.Bashir ME, Louie S, Shi HN, Nagler-Anderson C. Toll-like receptor 4 signaling by intestinal microbes influences susceptibility to food allergy. J Immunol. 2004;172(11):6978–87. doi: 10.4049/jimmunol.172.11.6978. [DOI] [PubMed] [Google Scholar]
- 20.Helm RM. Food allergy animal models: an overview. Ann N Y Acad Sci. 2002;964:139–50. 139–50. doi: 10.1111/j.1749-6632.2002.tb04139.x. [DOI] [PubMed] [Google Scholar]
- 21.Li XM, Serebrisky D, Lee SY, Huang CK, Bardina L, Schofield BH, et al. A murine model of peanut anaphylaxis: T- and B-cell responses to a major peanut allergen mimic human responses. J Allergy Clin Immunol. 2000;106(1 Pt 1):150–8. doi: 10.1067/mai.2000.107395. [DOI] [PubMed] [Google Scholar]
- 22.Li XM. Beyond allergen avoidance: update on developing therapies for peanut allergy. Curr Opin Allergy Clin Immunol. 2005;5(3):287–92. doi: 10.1097/01.all.0000168796.20324.bd. [DOI] [PubMed] [Google Scholar]
- 23.Burks AW, Williams LW, Helm RM, Thresher W, Brooks JR, Sampson HA. Identification of soy protein allergens in patients with atopic dermatitis and positive soy challenges; determination of change in allergenicity after heating or enzyme digestion. Adv Exp Med Biol. 1991;289:295–307. 295–307. doi: 10.1007/978-1-4899-2626-5_22. [DOI] [PubMed] [Google Scholar]
- 24.Furuta M, Suwa T, Kuwabara Y, Otsuhata K, Takeda A. Electron-beam sterilization of laboratory animal diets--sterilizing effect of 10-MeV electrons from a linear accelerator. Exp Anim. 2002;51(4):327–34. doi: 10.1538/expanim.51.327. [DOI] [PubMed] [Google Scholar]
- 25.Lin WH, Yu B, Lin CK, Hwang WZ, Tsen HY. Immune effect of heat-killed multistrain of Lactobacillus acidophilus against Salmonella typhimurium invasion to mice. J Appl Microbiol. 2007;102(1):22–31. doi: 10.1111/j.1365-2672.2006.03073.x. [DOI] [PubMed] [Google Scholar]
- 26.Forsythe P, Inman MD, Bienenstock J. Oral treatment with live Lactobacillus reuteri inhibits the allergic airway response in mice. Am J Respir Crit Care Med. 2007;175(6):561–9. doi: 10.1164/rccm.200606-821OC. [DOI] [PubMed] [Google Scholar]
- 27.Qu C, Srivastava K, Ko J, Zhang TF, Sampson HA, Li XM. Induction of tolerance after establishment of peanut allergy by the food allergy herbal formula-2 is associated with up-regulation of interferon-gamma. Clin Exp Allergy. 2007;37(6):846–55. doi: 10.1111/j.1365-2222.2007.02718.x. [DOI] [PubMed] [Google Scholar]
- 28.Lee SY, Huang CK, Zhang TF, Schofield BH, Burks AW, Bannon GA, et al. Oral Administration of IL-12 Suppresses Anaphylactic Reactions in a Murine Model of Peanut Hypersensitivity. Clin Immunol. 2001;101(2):220–8. doi: 10.1006/clim.2001.5122. [DOI] [PubMed] [Google Scholar]
- 29.Srivastava K, Teper AA, Zhang TF, Li S, Walsh MJ, Huang CK, et al. Immunomodulatory effect of the anti-asthma Chinese herbal formula, MSSM-002 on Th2 cells. J Allergy Clin Immunol. 2004;113:268–76. doi: 10.1016/j.jaci.2003.10.062. [DOI] [PubMed] [Google Scholar]
- 30.Srivastava KD, Kattan JD, Zou ZM, Li JH, Zhang L, Wallenstein S, et al. The Chinese herbal medicine formula FAHF-2 completely blocks anaphylactic reactions in a murine model of peanut allergy. J Allergy Clin Immunol. 2005;115(1):171–8. doi: 10.1016/j.jaci.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Bando N, Tsuji H, Hiemori M, Yoshizumi K, Yamanishi R, Kimoto M, et al. Quantitative analysis of Gly m Bd 28K in soybean products by a sandwich enzyme-linked immunosorbent assay. J Nutr Sci Vitaminol (Tokyo) 1998;44(5):655–64. doi: 10.3177/jnsv.44.655. [DOI] [PubMed] [Google Scholar]
- 32.Ogawa A, Samoto M, Takahashi K. Soybean allergens and hypoallergenic soybean products. J Nutr Sci Vitaminol (Tokyo) 2000;46(6):271–9. doi: 10.3177/jnsv.46.271. [DOI] [PubMed] [Google Scholar]
- 33.Sicherer SH, Sampson HA, Burks AW. Peanut and soy allergy: a clinical and therapeutic dilemma. Allergy. 2000;55(6):515–21. doi: 10.1034/j.1398-9995.2000.00119.x. [DOI] [PubMed] [Google Scholar]
- 34.Sicherer SH, Munoz-Furlong A, Burks AW, Sampson HA. Prevalence of peanut and tree nut allergy in the US determined by a random digit dial telephone survey. J Allergy Clin Immunol. 1999;103(4):559–62. doi: 10.1016/s0091-6749(99)70224-1. [DOI] [PubMed] [Google Scholar]
- 35.Sampson HA, McCaskill CC. Food hypersensitivity and atopic dermatitis: evaluation of 113 patients. J Pediatr. 1985;107:669–75. doi: 10.1016/s0022-3476(85)80390-5. [DOI] [PubMed] [Google Scholar]
- 36.Bernhisel-Broadbent J, Sampson HA. Cross-allergenicity in the legume botanical family in children with food hypersensitivity. J Allergy Clin Immunol. 1989;83(2 Pt 1):435–40. doi: 10.1016/0091-6749(89)90130-9. [DOI] [PubMed] [Google Scholar]
- 37.Klemola T, Kalimo K, Poussa T, Juntunen-Backman K, Korpela R, Valovirta E, et al. Feeding a soy formula to children with cow’s milk allergy: the development of immunoglobulin E-mediated allergy to soy and peanuts. Pediatr Allergy Immunol. 2005;16(8):641–6. doi: 10.1111/j.1399-3038.2005.00326.x. [DOI] [PubMed] [Google Scholar]
- 38.Kirjavainen PV, Salminen SJ, Isolauri E. Probiotic bacteria in the management of atopic disease: underscoring the importance of viability. J Pediatr Gastroenterol Nutr. 2003;36(2):223–7. doi: 10.1097/00005176-200302000-00012. [DOI] [PubMed] [Google Scholar]
- 39.Kataoka S. Functional effects of Japanese style fermented soy sauce (shoyu) and its components. J Biosci Bioeng. 2005;100(3):227–34. doi: 10.1263/jbb.100.227. [DOI] [PubMed] [Google Scholar]
- 40.Kobayashi M, Matsushita H, Tsukiyama R, Saito M, Sugita T. Shoyu polysaccharides from soy sauce improve quality of life for patients with seasonal allergic rhinitis: a double-blind placebo-controlled clinical study. Int J Mol Med. 2005;15(3):463–7. [PubMed] [Google Scholar]