Abstract
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD), a known disruptor of B-cell differentiation and a ligand for the aryl hydrocarbon receptor (AhR), induces binding of the AhR to dioxin responsive elements (DRE) in sensitive genes. The Ig heavy chain (IgH) gene is a sensitive target of TCDD and may be transcriptionally inhibited by TCDD through inhibition of the 3′IgH transcriptional regulatory region (3′IgHRR). While the 3′IgHRR contains binding sites for several transcription factors, two DRE motifs were also identified which may be responsible for TCDD-induced inhibition of 3′IgHRR activation and may implicate the AhR as an important regulator of IgH expression. The objectives of the present study were to determine if 3′IgHRR modulation is limited to TCDD or if structurally diverse chemicals (AhR ligands and non-AhR ligands) from environmental, industrial, dietary or pharmaceutical origin are also capable of modulating the 3′IgHRR and to verify a correlation between effects on a stable 3′IgHRR reporter and the endogenous IgH protein. Utilizing a CH12.LX mouse B-cell line that stably expresses a 3′IgHRR-regulated transgene, we identified an inhibition of both 3′IgHRR activation and IgH protein expression by the non-dioxin AhR activators indolo(3,2-b)carbazole, primaquine, carbaryl, and omeprazole which followed a rank order potency for AhR activation supporting a role of the AhR in the transcriptional regulation of the 3′IgHRR and IgH expression. However, modulation of the 3′IgHRR and IgH expression was not limited to AhR activators or to suppressive effects. Hydrogen peroxide and terbutaline had an activating effect and benzyl isothiocyanate was inhibitory. These chemicals are not known to influence the AhR signaling pathway but have been previously shown to modulate humoral immunity and/or transcription factors that regulate the 3′IgHRR. Taken together these results implicate the 3′IgHRR as a sensitive immunological target and are the first to identify altered 3′IgHRR activation by a diverse range of chemicals.
Keywords: 3′IgHRR, immunoglobulin, aryl hydrocarbon receptor, B-cell line, in vitro
INTRODUCTION
2,3,7,8-Tetracholordibenzo-p-dioxin (TCDD) is a potent and persistent environmental toxicant, which produces a variety of biological effects in animal and cellular models (Birnbaum and Tuomisto, 2000). Of these effects, immune suppression including inhibition of B-lymphocyte differentiation into antibody secreting cells is one of the most sensitive consequences of TCDD exposure (Kerkvliet, 2002). Inhibition of Ig expression and secretion in B lymphocytes has been well documented with several studies supporting the involvement of the aryl hydrocarbon receptor (AhR) signaling pathway; however, the specific mechanism remains unclear (Holsapple et al., 1991; Sulentic et al., 1998; Sulentic et al., 2000; Vorderstrasse et al., 2001).
The AhR and its dimerization partner AhR nuclear translocator (ARNT) are believed to regulate transcription by binding dioxin responsive elements (DREs) in regulatory regions of dioxin-sensitive genes (for review, see Okey, 2007). The CH12.LX B-cell line has provided a useful model in studying the effects of TCDD on Ig expression in B cells and has lead to the identification of a novel transcriptional target of TCDD: the Ig heavy chain (IgH) locus (Sulentic et al., 2000; Sulentic et al., 2004b). Regulation of the murine IgH locus is governed through a complex interaction of several regulatory elements. One such element, the 3′IgH regulatory region (3′IgHRR), appears to mediate processes late in B-cell differentiation such as up-regulation of IgH expression as well as class switch recombination (Manis et al., 1998; Pinaud et al., 2001). The 3′IgHRR is a large, approximately 40 kb region that lies downstream of the IgH constant regions. The 3′IgHRR is most often associated with four enhancer domains (hs3A; hs1,2; hs3B; and hs4) which contain DNA binding sites for several transcription factors that appear to be important regulators of enhancer activity (reviewed by Khamlichi et al., 2000). The AhR may also contribute to the regulation of the 3′IgHRR since at least two functional DRE sites (i.e., TCDD-induced AhR and ARNT binding) have been identified within the hs1,2 and hs4 enhancers (Sulentic et al., 2000). Correspondingly, in the CH12.LX cell line TCDD profoundly inhibited LPS activation of a luciferase reporter construct regulated by the 3′IgHRR (Sulentic et al., 2004b), correlating with the significant inhibition of LPS-induced IgH gene expression and antibody secretion by TCDD and other polychlorinated dibenzo-p-dioxin congeners that followed a structure activity relationship for AhR-mediated effects (Sulentic et al., 1998; Sulentic et al., 2000). Interestingly, the incidence and/or severity of the human diseases, IgA nephropathy and Celiac disease, have been associated with altered regulation of the 3′IgHRR (Aupetit et al., 2000; Frezza et al., 2004). Therefore, chemical-induced modulation of the 3′IgHRR likely has significant implications regarding the potential for altered Ig regulation and impaired immunity.
The most widely studied exogenous AhR ligands are the highly persistent halogenated aromatic hydrocarbons (HAHs), of which TCDD is considered the prototype, and the less persistent polycyclic aromatic hydrocarbons (PAHs). However, it is becoming increasingly evident that exogenous activators of the AhR signaling pathway are not limited to HAHs or PAHs but also comprise a diverse array of chemicals including those of dietary or therapeutic origin; and studies have also identified indoles, tetrapyroles, and arachidonic acid metabolites as potential endogenous, albeit low affinity, ligands for the AhR (reviewed in (Denison and Nagy, 2003; Nguyen and Bradfield, 2008). Therefore an objective of the present study was to determine if the suppression of 3′IgHRR transcriptional activity and Ig expression was dioxin-specific or also sensitive to non-dioxin AhR ligands. However, the 3′IgHRR is clearly regulated by many transcription factors and signaling pathways and consequently may be a sensitive toxicological and clinical target of a broader range of chemicals. Indeed, an additional objective of this study was to validate the utility of our 3′IgHRR model in identifying chemicals that can alter 3′IgHRR activity. Therefore, we expanded our evaluation to chemicals not known to modulate the AhR signaling pathway but shown to modulate the antibody response or signaling pathways involved in 3′IgHRR activity.
Using the well-characterized CH12.LX cell line and both transiently and stably expressed 3′IgHRR reporters, we found that modulation of 3′IgHRR activity was not limited to the AhR ligand TCDD but was sensitive to diverse AhR activators as well as chemicals that influence non-AhR signaling pathways implicated in 3′IgHRR regulation. Additionally, effects on 3′IgHRR activity correlated well with effects on endogenous IgH protein expression. Collectively, these results support the hypothesis that the 3′IgHRR is a sensitive immunological target of a diverse range of chemicals and that altered 3′IgHRR activity will alter IgH expression and antibody-mediated immune function. Furthermore, these results support a role of the AhR in 3′IgHRR transcriptional activity but also underscore the complexity of 3′IgHRR regulation and the diversity of chemicals that may alter 3′IgHRR activity.
MATERIALS AND METHODS
Chemicals and Reagents
TCDD, 1-monochlorodibenzo-p-dioxin (MCDD), and 2,2′,3,5′,6-pentachlorobiphenyl (PCB95) in 100% dimethyl sulfoxide (DMSO) were purchased from AccuStandard Inc. (New Haven, CT). Hydrogen peroxide solution (30 wt. % in water) and the neat form of carbaryl, primaquine, omeprazole, terbutaline, and carbachol were purchased from Sigma Aldrich (Milwaukee, WI). The certificates of product analysis stated the purity of TCDD, MCDD, PCB95, primaquine, carbaryl, omeprazole, terbutaline, and carbachol to be 99.1%, 100%, 100%, 98%, 98.9%, 100%, 99% and 99%, respectively. Indolo(3,2,b)carbazole (ICZ) was generously provided by Dr. Leonard F. Bjeldanes (University of California, Berkely, CA). ICZ, carbaryl, primaquine, and omeprazole were dissolved in DMSO. Terbutaline and carbachol were dissolved in water. Benzyl isothiocyanate (BITC) dissolved in a 50% DMSO phosphate buffered saline (PBS) solution was generously provided by Dr. Sanjay Srivastava (Texas Tech University) and Dr. Thomas Brown (Wright State University). See Table 1 for a list of the chemicals tested and a summary of their purity, vehicle, final vehicle concentration, and range of concentrations tested. Lipopolysaccharide (LPS, Escherichia coli) and DMSO were purchased from Sigma Aldrich.
Table 1. Test chemicals.
Non-dioxin AhR ligands include ICZ, primaquine, carbaryl, and omeprazole. Negative controls for AhR binding include 1-monochlorodibenzo-p-dioxin and the non-coplanar PCB95 both of which have no affinity for the AhR. Chemicals not known to modulate the AhR signaling pathway but shown to modulate the antibody response or signaling pathways involved in 3′IgHRR activity include hydrogen peroxide, terbutaline, carbachol, and BITC. DMSO vehicle concentrations varied according to the solubility and effective concentration range of the chemical. ND, not determined; NA, not applicable.
| Chemical | Purity | Vehicle | Final vehicle concentration | Concentration Range Tested |
|---|---|---|---|---|
| 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) | 99.1% | 100% DMSO | 0.01% or 0.019% DMSO | 0.01–30 nM |
| Indolo(3,2,b)carbazole ICZ | ND | 100% DMSO | 0.01% DMSO | 0.5–500 nM |
| Primaquine (PMQ) | 98% | 100% DMSO | 0.01% DMSO | 6.25–100 μM |
| Carbaryl (CBRL) | 98.9% | 100% DMSO | 0.1% DMSO | 12.5–100 μM |
| Omeprazole (OME) | 100% | 100% DMSO | 0.1% DMSO | 12.5–62.5 μM |
| 1-Monochlorodibenzo- p-dioxin (MCDD) | 100% | 100% DMSO | 0.01% or 0.1% | 30 and 300 nM |
| Polychlorinated biphenyl 95 (PCB95) | 100% | 100% DMSO | 0.01% or 0.1% | 30 and 300 nM |
| Hydrogen Peroxide (H2O2) | ND | water | NA | 20–50 μM |
| Terbutaline (TERB) | 99% | water | NA | 0.1–100 μM |
| Carbachol (CBOL) | 99% | water | NA | 0.01–100 μM |
| Benzyl Isothiocyanate (BITC) | ND | 50% DMSO 1x PBS | 0.05% DMSO | 0.5- 5.0 μM |
Cell Lines
The CH12.LX B cell line derived from the murine CH12 B cell lymphoma (Arnold et al., 1983), which arose in B10.H-2aH-4bp/Wts mice (B10.A × B10.129), has been previously characterized by Bishop and Haughton (Bishop and Haughton, 1986) and was a generous gift from Dr. Geoffrey Haughton (University of North Carolina, Chapel Hill, NC). Since its initial characterization, the CH12.LX cell line has been extensively utilized to study a variety of cellular processes specific to B cells and has provided a useful model in studying the effects of TCDD on B-cell differentiation. Employing the γ2b mini-locus model which was developed and generously provided by Dr. Laurel Eckhardt (Hunter College, The University of New York City, NY) (Shi and Eckhardt, 2001), we generated the CH12.γ2b-3′IgH cell line. The CH12.γ2b-3′IgH cell line is a subclone isolated from CH12.LX cells that were under antibiotic selection to induce the stable expression of a transgene (γ2b Ig heavy chain gene) regulated by the 3′IgHRR. Inducible expression of the γ2b transgene in the CH12.γ2b-3′IgH was confirmed by Real-Time RT-PCR and Enzyme-Linked Immunosorbent Assay (ELISA) analysis (data not shown and Fig. 2). Analysis of the CH12.γ2b-3′IgH cells by flow cytometry and ELISA identified them as IgA expressing B cells and genomic analysis identified insertion of one copy of the γ2b transgene (Fig. 5 and 7 and data not shown). The CH12.γ2b-3′IgH cells do not endogenously express γ2b IgH (major component of an IgG2b antibody) or secrete IgG2b (data not shown). Cells were grown in RPMI 1640 media (MediaTech, Herndon, VA) supplemented with 10% bovine calf serum (Hyclone, Logan, UT), 13.5mM HEPES, 23.8 mM sodium bicarbonate, 100 units/ml penicillin, 100 μg/ml streptomycin, 2 mM L-glutamine, 0.1 mM nonessential amino acids, 1.0 mM sodium pyruvate, and 50 μM β-mercaptoethanol. Cells were maintained at 37°C in an atmosphere of 5% CO2.
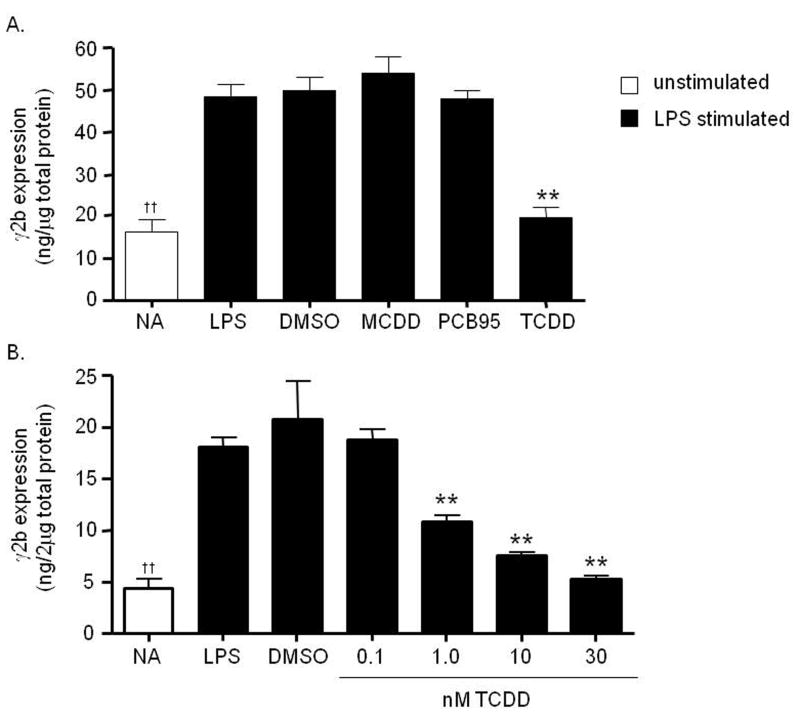
Figure 2. Concentration-dependent inhibition of the 3′IgHRR-regulated γ2b transgene by TCDD but not by MCDD or PCB95.
Stably transfected CH12.γ2b-3′IgH cells were treated for 48 hr, in the presence of LPS (3 μg/ml) stimulation, with vehicle (0.019% DMSO) or A) 30 nM concentrations of MCDD, PCB95, or TCDD; or B) varying concentrations of TCDD. In contrast to the AhR high affinity ligand TCDD, the MCDD congener and PCB95 have no affinity for the AhR. γ2b protein expression (mean ± SE, n=3) in the cell lysate was determined by sandwich ELISA and standardized to ng γ2b per 1 or 2μg of total protein as shown on the y-axis. NA denotes the unstimulated naïve control. Significance was determined by a 1-way ANOVA followed by a Dunnett’s post-hoc test. A “**” denotes significance from the DMSO control at p<0.01. A “††” denotes significance of the NA control from the LPS control at p<0.01.
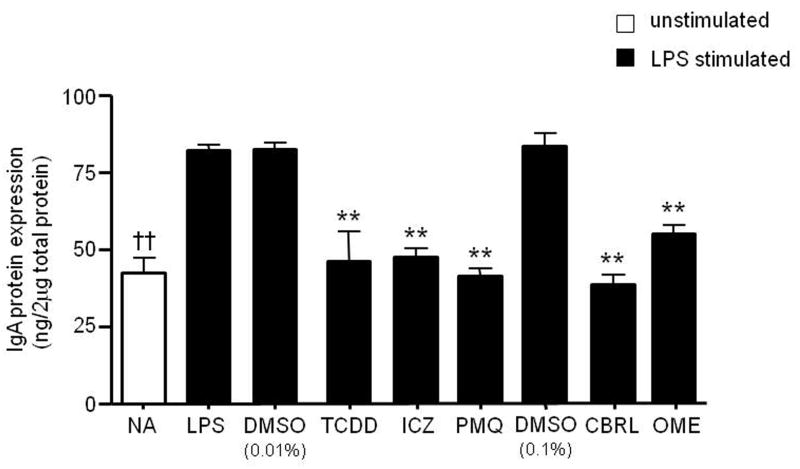
Figure 5. Inhibition of endogenous IgH protein expression by TCDD and non-dioxin AhR activators.
CH12.γ2b-3′IgH cells were treated for 24 hr with vehicle (0.01% DMSO for TCDD, ICZ and primaquine or 0.1% DMSO for carbaryl and omeprazole) or specific concentrations of TCDD (10 nM), ICZ (500nM), primaquine (PMQ; 50μM), carbaryl (CBRL; 100μM), or omeprazole (OME; 62.5μM), in the presence of LPS (3 μg/ml). Concentrations that produced a maximum inhibition of LPS-induced 3′IgHRR activity were used. IgA protein expression (mean ± SE, n=3) in the cell lysate was determined by standard ELISA and standardized to ng IgA/2μg of total protein. NA denotes the unstimulated naïve control. Significance was determined by a 1-way ANOVA followed by a Dunnett’s post-hoc test. A “**” denotes significance from the DMSO control at p<0.01. A “††” denotes significance of the NA control from the LPS control at p<0.01.
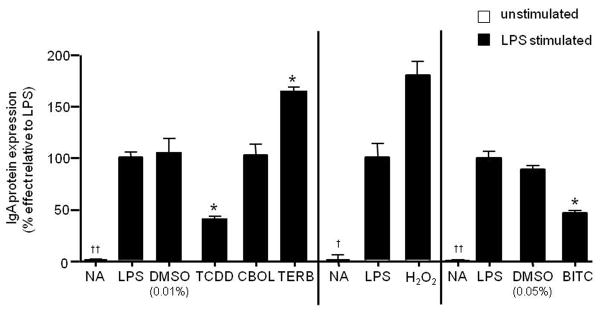
Figure 7. Altered IgA secretion correlated with effects on 3′IgHRR activity.
CH12.γ2b-3′IgH cells were treated with a specific concentration of TCDD (10nM), carbachol (CBOL; 100μM), terbutaline (TERB; 100μM), hydrogen peroxide (H2O2; 30μM), or BITC (5μM), or the appropriate vehicle control (VH, 0.01% DMSO for TCDD or 0.05% DMSO for BITC) in the presence of LPS (3 μg/ml) stimulation. IgA protein expression (mean ± SE, n=3) in the cell lysate was determined by sandwich ELISA and standardized to ng IgA/2μg of total protein. Three experiments (separated by the vertical lines) are graphed together and for comparative purposes. IgA protein expression was normalized to background and transformed to percent effect with the LPS control set to 100% as shown on the y-axis. NA denotes the unstimulated naïve control. Significance was determined by a 1-way ANOVA followed by a Dunnett’s post-hoc test. A “*” denotes significance from the appropriate LPS or vehicle (0.01% DMSO for TCDD and 0.05% DMSO for BITC) control at p<0.05. A “†” or “††” denotes significance of the NA control from the LPS control at p<0.05 or p<0.01, respectively.
Transient Transfection
Luciferase reporter plasmids were generously provided by Dr. Robert Roeder (Rockefeller University, New York, NY) and included the 5-kb reporter with a variable heavy chain (VH) promoter upstream of the luciferase gene which served as a promoter alone control and the 12.4-kb reporter with the upstream VH promoter and the 3′IgHRR downstream of the luciferase gene. Plasmids were constructed using a pGL3 basic luciferase reporter construct (Promega, Madison, WI) as described previously (Ong et al., 1998).
Transient transfections were performed as follows. CH12.LX cells (2.2×107) were resuspended in 440 μl of culture media with 22 μg of plasmid (3′IgHRR or VH promoter alone) and 200 μl transferred into two 2-mm gap electroporation cuvettes (Molecular BioProducts, San Diego, CA). Cells were electroporated using an electro cell manipulator (ECM 630; BTX, San Diego, CA) with the voltage at 250 V, the capacitance at 150 μF, and the resistance at 75 ohms. For each plasmid, multiple transfections were pooled then cells aliquoted into 12-well plates at 2.0×105 cells/ml.
Luciferase Assay
Immediately after transfection, CH12.LX cells, in the absence or presence of LPS stimulation, were treated for 48 hr with TCDD, ICZ, carbaryl, primaquine, omeprazole, terbutaline, carbachol, hydrogen peroxide, or BITC or the appropriate vehicle control (see Table 1). Between chemicals the DMSO vehicle concentrations varied according to the effective concentration range of the chemical and the stock concentration. Based on initial studies, 48 hr is optimum for LPS to maximally induce the 3′IgHRR luciferase reporter; therefore, this time point was selected to evaluate the effects of the test chemicals on LPS-induced 3′IgHRR activation. Notably, a similar profile of TCDD-induced inhibition of 3′IgHRR activation occurs following a 24-hr incubation period but the activation by LPS is not as pronounced or dependable which may relate to the relatively low transfection efficiency of the large 12.4 kb 3′IgHRR plasmid (unpublished observations and (Sulentic et al., 2004b). After the 48-hr incubation period, cells were washed with 1x PBS and then lysed with a 1x reporter lysis buffer (Promega). Samples were immediately frozen at −80°C. Treatments were either in triplicate or quadruplicate. To measure luciferase enzyme activity, samples were thawed at room temperature and then 20 μl of sample lysate was mixed with 100 μl of luciferase assay reagent (Promega) using an autoinjector. Luciferase activity or luminescence was measured with a luminometer (Berthold detection systems; Oak Ridge, TN) and represented as relative light units or percent effect with the LPS control set to 100% effect.
Protein Isolation for γ2b and IgA Analysis
CH12.LX cells or CH12.γ2b-3′IgH cells, in the absence or presence of LPS stimulation, were treated with TCDD, ICZ, carbaryl, primaquine, omeprazole, terbutaline, carbachol, hydrogen peroxide, or BITC or the appropriate vehicle control (see Table 1) then plated into 12-well plates. For γ2b protein isolation, CH12.γ2b-3′IgH cells were plated at a concentration of 2.5×104 cells/well and incubated for 48 hr. For IgA protein isolation, CH12.LX cells were plated at a concentration 2.0×105 cells/ml and incubated for 24 to 48 hr. Following the incubation period, cells were centrifuged at 3000 rpm, lysed with mild lysis buffer (1% NP-40, 150 mM NaCl, 10 mM NaPO4, 2 mM EDTA) containing freshly added protease inhibitors (Complete Mini Protease Inhibitor Cocktail; Roche Diagonostics, Indianapolis, IN). Lysed cells were centrifuged at 14,000 rpm then supernatants were collected and stored at −80 °C until analysis. To measure γ2b or IgA, cell lysates were thawed on ice and protein concentrations were determined by a Bradford assay (Bio-Rad Laboratories, Hercules, CA). Samples were then diluted to the lowest sample concentration and 1 or 2 μg of total protein was analyzed for γ2b or IgA by ELISA.
Enzyme-Linked Immunosorbent Assay (ELISA)
Cell lysates were analyzed for IgA or γ2b by sandwich ELISA as described previously (Sulentic et al., 2000). Colorimetric detection was performed every minute over a 1-h period using a Spectramax plus 384 automated microplate reader with a 405-nm filter (Molecular Devices, Sunnyvale, CA). The SOFTmax PRO analysis software (Molecular Devices) calculated the concentration of IgA or γ2b in each sample from a standard curve generated from the kinetic rate of absorption for known IgA or γ2b concentrations, respectively. Results are represented as the mean ng IgA or γ2b per 2 μg of total protein ± S.E. (n=3 to 4) or the mean percent effect ± S.E. (n=3 to 4) with the LPS control set to 100% effect.
Statistical Analysis of Data
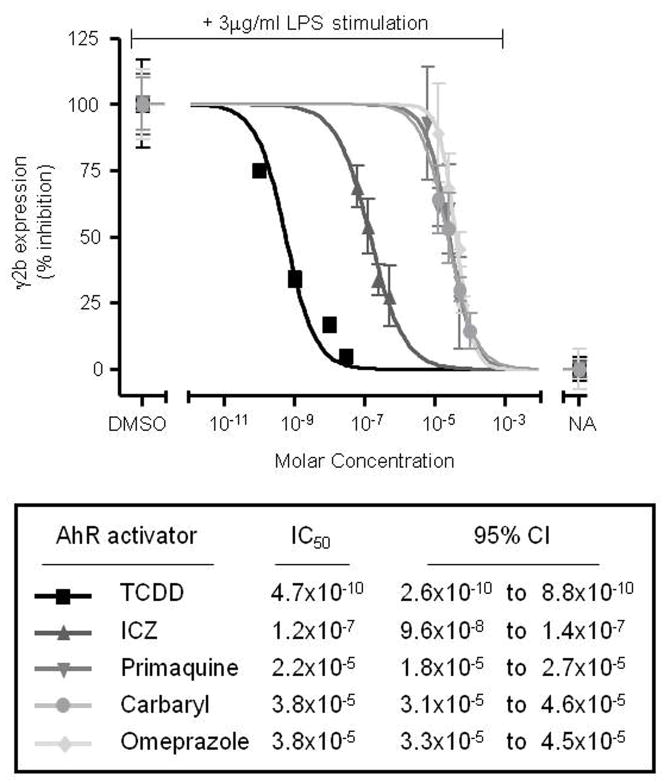
Mean ± S.E. (n=3 to 4) was determined for each treatment group of a given experiment. For figures comparing the concentration response of multiple chemicals, the data was normalized to background and transformed to percent effect with the LPS control set to 100% effect. A statistical difference in the untransformed data (i.e. prior to transformation to percent effect) between treatment groups and the vehicle controls was determined by Dunnett’s two-tailed t test. For IC50 generation, a complete concentration-response curve for each chemical was determined. Concentration-response curves were fit by a four-parameter logistic concentration-response equation given as Y = Bottom + (Top-Bottom)/(1+10^((LogIC50-X)*Hill Slope)). X is the logarithm of concentration. Y is the response. The derived IC50 (concentration generating half-maximal response) and 95% confidence interval for each AhR activator were expressed as a molar (M) concentration and were generated from the best-fit value for all data sets (n=3 to 4) (Fig. 4).
Figure 4. Inhibition of the 3′IgHRR-regulated γ2b transgene follows a rank order potency.
Percent inhibition was calculated from 3 to 4 separate concentration-response curves for each AhR activator (one representative curve shown) and fit to a four-parameter logistic concentration-response equation to generate the average 50% inhibitory concentration (IC50) and 95% confidence interval for each AhR activator. The vehicle control is 0.01% DMSO for ICZ and primaquine; 0.019% DMSO for TCDD; and 0.1% DMSO for carbaryl and omeprazole. NA denotes the unstimulated naïve control.
RESULTS
AhR ligands inhibit LPS-induced 3′IgHRR activation in CH12.LX B cells
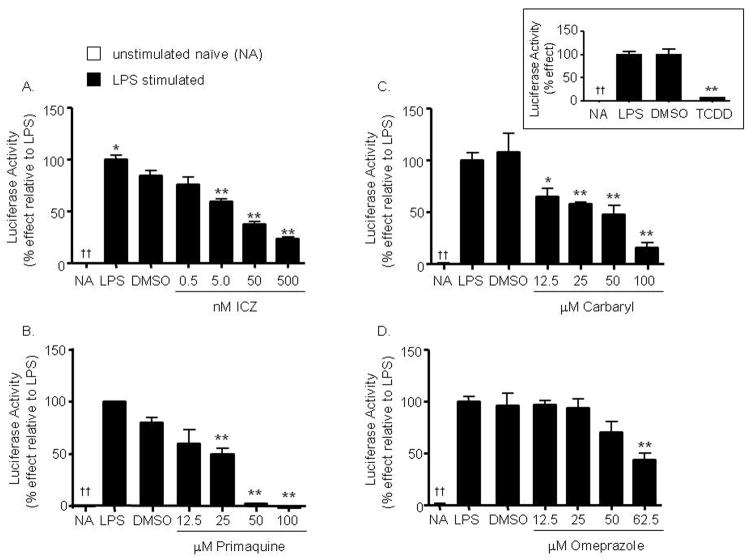
Though we have previously identified AhR binding within the 3′IgHRR and AhR-dependent transcriptional regulation of the hs4 enhancer, the role of the AhR in mediating TCDD-induced inhibition of the 3′IgHRR is unknown. Further, TCDD represents a large class of chemicals, those known and those yet to be discovered, that activate the AhR signaling pathway and therefore may modulate 3′IgHRR activity. Notably, the effect of chemicals in general on 3′IgHRR activity has been largely unexplored. Therefore, to determine if the effect of TCDD on 3′IgHRR activity is truly representative of all AhR activators including non-dioxin AhR ligands, we examined the effect of four structurally diverse, non-dioxin chemicals (Table 1) on a luciferase reporter regulated by the 3′IgHRR that was transiently transfected into CH12.LX B cells. As previously demonstrated, the polyclonal B-cell activator and toll-like receptor-4 (TLR-4) ligand LPS significantly activated the 3′IgHRR (Fig. 1) with optimum induction occurring at 48 hr of stimulation (unpublished observations). Similar to the effect seen with TCDD (Fig. 1, inset and (Sulentic et al., 2004b), we identified a concentration-dependent inhibition, at concentrations that did not affect cell viability (data not shown), of LPS-induced 3′IgHRR activity by the following activators of the AhR signaling pathway: the dietary metabolite ICZ; the antimalarial drug primaquine; the pesticide carbaryl; and the proton pump inhibitor omeprazole (Prilosec) (Fig. 1, A–D). Each of these chemicals has been shown to either bind the AhR and/or induce AhR/DRE binding and Cyp1a1 activation and previous reports have determined their potency in relation to TCDD (Chen et al., 1995; Werlinder et al., 2001; Backlund and Ingelman-Sundberg, 2004; Bohonowych and Denison, 2007) suggesting a rank order potency of TCDD > ICZ > carbaryl ≥ primaquine > omeprazole. Indeed μM concentrations of carbaryl, primaquine, and omeprazole were required to significantly inhibit LPS-induced 3′IgHRR activation; whereas nM concentrations of ICZ and TCDD resulted in inhibition. Additionally, the VH promoter control which showed little activity alone was activated by LPS but to a lesser degree than the 3′IgHRR and was not modulated by ICZ, primaquine, carbaryl, or omeprazole at concentrations that had produced a marked inhibition of LPS-induced 3′IgHRR activation suggesting that these chemicals target the 3′IgHRR versus the VH promoter (data not shown). Furthermore, as expected these AhR activators had no effect on 3′IgHRR in the absence of LPS stimulation (data not shown).
Figure 1. AhR activators inhibit LPS-induced 3′IgHRR luciferase reporter activity.
CH12.LX cells were transiently transfected with the 3′IgHRR reporter plasmid then treated for 48 hr with vehicle (0.01% DMSO for ICZ and primaquine; 0.019% for TCDD; or 0.1% DMSO for carbaryl and omeprazole); or varying concentrations of indolo(3,2,b)carbazole (ICZ) (A), primaquine (B), carbaryl (C), or omeprazole (D); or 30nM TCDD (inset figure) in the presence of LPS (3 μg/ml) stimulation. Luciferase enzyme activity (mean ± SE, n=3) was measured in relative light units (RLU) then normalized to background and transformed to percent effect with the LPS control set to 100% as shown on the y-axis. NA denotes the unstimulated naïve control. Significance was determined by a 1-way ANOVA followed by a Dunnett’s post-hoc test. A “*” or “**” denotes significance from the DMSO control at p<0.05 or p<0.01, respectively. A “††” denotes significance of the NA control from the LPS control at p<0.01, respectively.
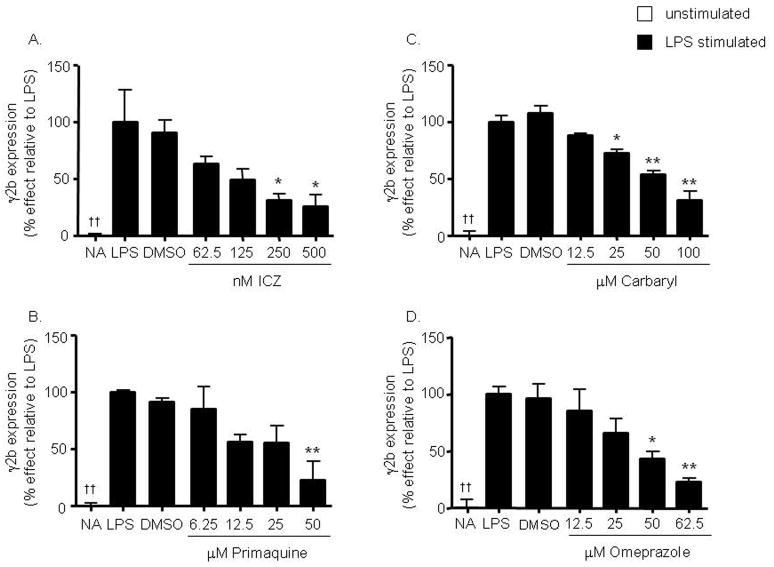
To more closely approximate the situation on the IgH chromosome and to avoid the many limitations of transient transfection experiments, we generated an experimental model (CH12.γ2b-3′IgH) with the CH12.LX cells that stably expresses a γ2b heavy chain transgene under the regulation of the 3′IgHRR (Shi and Eckhardt, 2001). Analysis of the CH12.γ2b-3′IgH cells by flow cytometry and ELISA analysis identified them as IgA expressing B cells and genomic analysis suggested the insertion of one copy of the γ2b transgene (data not shown). As determined by Real-Time RT-PCR and ELISA analysis, the CH12.γ2b-3′IgH cells do not endogenously express γ2b IgH (major component of an IgG2b antibody) or secrete IgG2b (data not shown). Similar to our 3′IgHRR transient reporter results, TCDD, ICZ, carbaryl, primaquine, and omeprazole inhibit LPS-induced γ2b transgene expression in a concentration-dependent manner (Fig. 2 and Fig. 3A–D) which followed a rank order potency for AhR activation, i.e. TCDD > ICZ > primaquine > carbaryl = omeprazole (Fig. 4). Additionally, the dioxin congener MCDD and the non-coplanar PCB95 congener, which have no affinity for the AhR, had no effect on γ2b expression (Fig. 2A) even at concentrations as high as 300 nM (data not shown), supporting a role of the AhR in the above rank order potency and verifying the specificity of this response. Furthermore, since the 3′IgHRR appears to play a significant role in IgH expression (Manis et al., 1998; Pinaud et al., 2001; Shi and Eckhardt, 2001), we verified that the effects on the γ2b transgene reflected the effects on endogenous IgA protein expression. Indeed, TCDD, ICZ, primaquine, carbaryl, and omeprazole significantly inhibited IgA protein expression in the parental CH12.LX cells (data not shown) and in the CH12.γ2b-3′IgH cells (Fig. 5). MCDD, similar to previous results (Sulentic et al., 2000), and PCB95 had no effect on IgA protein expression (data not shown). Although TCDD has long been known to inhibit Ig expression and secretion in B cells, the specific transcriptional target(s) has been less clear. These results along with previous studies (Sulentic et al., 1998; Sulentic et al., 2000; Sulentic et al., 2004a; Sulentic et al., 2004b) implicate an inhibitory mechanism involving the AhR and targeting the transcriptional activity of the 3′IgHRR.
Figure 3. Concentration-dependent inhibition of the 3′IgHRR-regulated γ2b transgene by non-dioxin AhR activators.
Stably transfected CH12.γ2b-3′IgH cells were treated for 48 hr with vehicle (0.01% DMSO for ICZ and primaquine and 0.1% DMSO for carbaryl and omeprazole) or varying concentrations of ICZ (A), primaquine (B), carbaryl (C), or omeprazole (D), in the presence of LPS (3 μg/ml). γ2b protein expression (mean ± SE, n=3) in the cell lysate was determined by sandwich ELISA and standardized to ng γ2b/2μg of total protein. γ2b protein expression was then normalized to background and transformed to percent effect with the LPS control set to 100% as shown on the y-axis. NA denotes the unstimulated naïve control. Significance was determined by a 1-way ANOVA followed by a Dunnett’s post-hoc test. A “*” or “**” denotes significance from the DMSO control at p<0.05 or p<0.01, respectively. A “††” denotes significance of the NA control from the LPS control at p<0.01.
Modulation of LPS-induced 3′IgHRR activation by non-AhR ligands in CH12.LX B cells
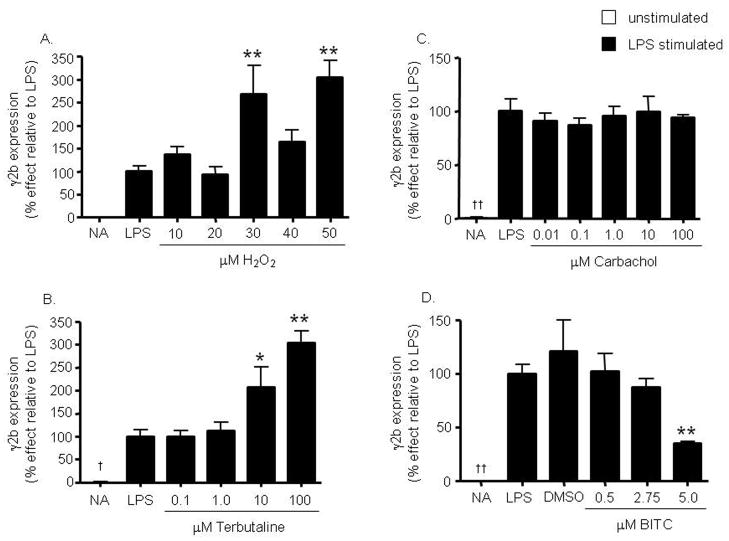
The above results are the first to support an inhibition of the 3′IgHRR by structurally diverse, non-dioxin AhR ligands; however, the 3′IgHRR is known to be regulated by many transcription factors and signaling pathways including but not limited to NF-κB, Oct, and OCA-B (Michaelson et al., 1996; Khamlichi et al., 2000; Podojil et al., 2004). Consequently, it is likely that the 3′IgHRR can also be modulated by a diverse range of chemicals in addition to ligands for the AhR as identified above. As mentioned earlier the effect of chemicals in general on 3′IgHRR activity has not been previously explored and if 3′IgHRR activity can be altered by a wide array of chemicals through differing mechanisms, the 3′IgHRR may be a highly relevant toxicological or pharmacological target due to its proposed role in Ig expression and B-cell function. Therefore, we expanded the evaluation of our model to include chemicals not known to modulate the AhR signaling pathway but shown to modulate the antibody response or signaling pathways involved in 3′IgHRR activity. We initially tested hydrogen peroxide, terbutaline, and carbachol which are known to influence transcription factors that regulate the 3′IgHRR and/or to alter the antibody forming response. Hydrogen peroxide is a well known activator of the NF-κB signaling pathway (reviewed in Gloire et al., 2006), which is an important pathway in B-cell activation. Terbutaline is an agonist for the β2-adrenergic receptor and has been previously shown to induce OCA-B binding to the 3′IgHRR and to increase serum IgG1 levels in mice as well as Igγ1 transcripts in primary mouse splenic B cells following in vivo administration of terbutaline (Podojil et al., 2004). Carbachol is a cholinergic agonist for the muscarinic and nicotinic receptors and has been previously shown to enhance the T cell-dependent antibody-forming cell response in mouse splenocytes (Pruett et al., 1992). Both hydrogen peroxide and terbutaline enhanced LPS-induced expression of the γ2b transgene (Fig. 6A and B) and 3′IgHRR luciferase reporter (data not shown); whereas, carbachol had no affect on γ2b expression (Fig. 6C) or 3′IgHRR luciferase activity (data not shown). The effect of terbutaline was concentration-dependent as opposed to hydrogen peroxide which consistently demonstrated a spike in activation that resolved with increasing concentration (Fig. 6A and B). Additionally, terbutaline and carbachol did not affect VH promoter activity in LPS-stimulated cells nor did they affect basal activity of the VH promoter or the 3′IgHRR in the absence of LPS stimulation (data not shown). Furthermore, we again verified that the effects on the γ2b transgene reflected the effects on endogenous IgA protein expression. Correspondingly, terbutaline significantly enhanced LPS-induced IgA protein expression in the CH12.γ2b-3′IgH cells; carbachol had no affect (Fig. 7). However, for hydrogen peroxide there was some variability in inducing the enhancement of IgA expression which may relate to the unstable nature of ROS and/or the antioxidant defense capacity of the CH12.LX B cells (Fig. 7).
Figure 6. Inhibition of the 3′IgHRR-regulated γ2b transgene by non-AhR ligands.
Stably transfected CH12.γ2b-3′IgH cells were treated for 48 hr with varying concentrations of hydrogen peroxide (H2O2) (A), terbutaline (B), carbachol (C), and BITC (D) in the presence of LPS (3 μg/ml) stimulation. γ2b protein expression (mean ± SE, n=3) in the cell lysate was determined by sandwich ELISA and standardized to ng γ2b/2μg of total protein. γ2b protein expression was then normalized to background and transformed to percent effect with the LPS control set to 100% as shown on the y-axis. NA denotes the unstimulated naïve control. Significance was determined by a 1-way ANOVA followed by a Dunnett’s post-hoc test. A “*” or “**” denotes significance from the appropriate LPS or vehicle (0.05% DMSO for BITC) control at p<0.05 or p<0.01, respectively. A “†” or “††” denotes significance of the NA control from the LPS control at p<0.05 or p<0.01, respectively.
We also evaluated the isothiocyanate BITC which has been shown to alter NF-κB signaling and the production of ROS (Srivastava and Singh, 2004; Jakubikova et al., 2006) and to induce anti-carcinogenic effects in animal models (reviewed in (Hecht, 2000). Isothiocyanates are highly bioavailable dietary metabolites that are released during the normal consumption of cruciferous vegetables (Hecht, 2000). BITC modulated 3′IgHRR activity by producing a concentration-dependent inhibition of LPS-induced expression of the γ2b transgene (Fig. 6D) and of the 3′IgHRR luciferase reporter (data not shown). Consistent with the 3′IgHRR reporters, BITC also inhibited endogenous IgA protein expression in LPS-stimulated cells (Fig. 7).
These results support our hypothesis that the 3′IgHRR is sensitive to a wide range of chemicals (a question previously unexplored) which may have toxicological significance. This broad modulation is not entirely surprising given the number of transcription factors and signaling pathways thought to be involved in 3′IgHRR activity. Although the exact mechanism for the effects of the above chemicals on 3′IgHRR activity is unknown each chemical was selected based on previous reports demonstrating either an inhibition of Ig expression or modulation of a signaling pathway implicated in 3′IgHRR regulation; therefore these chemicals were considered likely to modulate 3′IgHRR activity. Furthermore, this study is the first to evaluate the effect of several diverse chemicals on 3′IgHRR activity and to compare these results with effects on endogenous IgH expression. Results support a direct correlation between 3′IgHRR activity and IgH expression and underscore the sensitivity of the 3′IgHRR to chemical-induced modulation.
DISCUSSION
The 3′IgHRR appears to mediate processes late in B-cell differentiation such as up-regulation of IgH expression as well as class switch recombination; both are critical processes in immunoglobulin or antibody production (Manis et al., 1998; Pinaud et al., 2001). Our past studies have identified the 3′IgHRR as a sensitive target of TCDD and have supported a role of the AhR signaling pathway in TCDD-induced inhibition of 3′IgHRR activation (Sulentic et al., 2000; Sulentic et al., 2004b). Therefore, a major focus of the present studies was to determine if inhibition of 3′IgHRR activation applied to a diverse range of non-dioxin AhR activators. To this end, we chose to evaluate the following structurally diverse, non-dioxin AhR activators: the dietary metabolite ICZ; the antimalarial drug primaquine; the pesticide carbaryl; and the proton pump inhibitor omeprazole.
In the current studies, we utilized the CH12.LX B-cell line that has been extensively utilized to study a variety of cellular processes specific to B cells and has been well-characterized in regards to TCDD-induced inhibition of B-cell function and to the AhR signaling pathway (Sulentic et al., 2004b). Using a transient luciferase reporter and a stably integrated γ2b reporter, both under the regulation of the 3′IgHRR, we identified an inhibition of LPS-induced 3′IgHRR activity that followed a rank order potency for AhR activation. These results suggest that the AhR is a significant regulatory element in the 3′IgHRR and that the 3′IgHRR is a significant target of chemicals that modulate the AhR signaling pathway. Indeed, we have previously characterized two DRE-like motifs within the hs1,2 and hs4 enhancers of the 3′IgHRR and identified AhR/ARNT binding to these motifs (Sulentic et al., 2000). Interestingly, the DRE motif is in close proximity to or overlapping a κB motif in the hs1,2 and hs4 enhancers, respectively; and mutational analysis identified a dependence on protein binding to both the DRE and κB motifs in the transcriptional effect of TCDD on hs4 enhancer activity in LPS-activated CH12.LX cells (Sulentic et al., 2000; Sulentic et al., 2004a). A direct association between the AhR and the NF-κB/Rel subunits RelA and RelB has been previously demonstrated in other cellular models (Tian et al., 1999; Kim et al., 2000; Vogel et al., 2007). Therefore it is possible that the AhR modulates 3′IgHRR transcriptional activity directly through DRE binding and/or indirectly by associating with specific NF-κB/Rel proteins and influencing the composition of NF-κB/Rel dimers binding to the κB site. In support of this possibility, κB binding within the 3′IgHRR has been shown to play either an inhibitory or activating role in the transcriptional activity of the hs1,2 enhancer which was dependent on the B-cell activation state (Michaelson et al., 1996) and is perhaps mediated by binding of activating versus inhibitory NF-κB/Rel protein dimers as seen in the germline γ1 promoter (Lin et al., 1998). Therefore, ongoing studies are focused on testing the hypothesis that the inhibitory effect of TCDD on 3′IgHRR activation is mediated through an AhR-dependent shift in the NF-κB/Rel protein complexes binding to κB motifs within the hs1,2 and hs4 enhancers. Lastly, if the 3′IgHRR is a significant transcriptional regulator of the IgH gene then altered 3′IgHRR activity should directly influence IgH transcription leading to altered Ig protein levels. Correspondingly, the AhR activators used in this study inhibited endogenous IgH protein expression in a similar manner to the 3′IgHRR further supporting a critical role of the 3′IgHRR in IgH expression. Moreover these results extend our previous studies in which we demonstrated an AhR structure activity relationship for the inhibition of IgH mRNA expression and Ig secretion by chlorinated dibenzo-p-dioxin congeners (Sulentic et al., 2000). In fact for these endpoints the generated IC50’s for TCDD-induced inhibition correlated well with the IC50 generated here for TCDD-induced inhibition of 3′IgHRR activity.
A second objective of these studies was to validate the use of the CH12.γ2b-3′IgH cell line that stably expresses the γ2b-3′IgHRR reporter as an appropriate model to evaluate chemical-induced modulation of 3′IgHRR activity. For the AhR activators, there is a general concordance between the transient luciferase and stable 3′IgHRR reporters suggesting that both are sensitive indicators of 3′IgHRR modulation. Additionally, the IC50’s generated for a particular AhR activator using either the transient or stable reporter did not substantially differ and fell within or just outside the best fit 95% confidence interval for either reporter. Significantly, modulation of the 3′IgHRR in our reporters correlated with the effect of these chemicals on endogenous Ig protein expression. This correlation between effects on the transient and stable 3′IgHRR reporters and endogenous Ig protein expression also occurred with non-AhR activators. This is particularly important since the 3′IgHRR has been shown to be regulated by multiple transcription factors including but not limited to NF-κB, OCA-B, and Oct-2. Thus, it is likely that the 3′IgHRR can also be modulated by a diverse array of chemicals in addition to AhR agonists and the ability to alter 3′IgHRR activity through differing mechanisms suggests that the 3′IgHRR may be a highly relevant toxicological or pharmacological target due to its role in Ig expression and B-cell function. Therefore, we expanded the evaluation of our model to include chemicals (terbutaline, hydrogen peroxide, carbachol, and BITC) which have been shown to influence these transcription factors and/or to alter B-cell differentiation into antibody-secreting cells.
In vivo administration of terbutaline has been previously shown to increase serum IgG1 levels and mature Igγ1 transcripts in mice treated with the B-cell stimulants IL-4 and antibodies against the B-cell surface signaling receptors, CD40 and CD86 (Podojil et al., 2004). This effect appears to be mediated by the activation of β2-adrenergic receptors and CD86 resulting in an increase in the expression of OCA-B and Oct-2, respectively, and increased binding of the OCA-B/Oct-2 dimer to octomer motifs within both the hs1,2 and hs4 enhancers of the 3′IgHRR; however, 3′IgHRR activity was not evaluated (Podojil et al., 2004). Here, we demonstrate a terbutaline-induced enhancement of 3′IgHRR activity (transient and stable reporters) and of IgH protein expression in CH12.LX B-cells stimulated with the TLR4 ligand LPS. Taken together terbutaline enhanced 3′IgHRR activity and Ig expression in B cells activated with stimuli (LPS versus anti-CD86, anti-CD40 and IL-4) that activate different surface receptors and signaling pathways.
Low concentrations of hydrogen peroxide also enhanced LPS-induced 3′IgHRR activity (transient and stable reporters) and IgH protein expression though the effect on IgH tended to be more variable in its magnitude and significance which may reflect the unstable nature of ROS and/or the antioxidant defense capacity of CH12.LX cells. Notably, hydrogen peroxide and other ROS have been shown to induce NF-κB/Rel activation (reviewed by Gloire et al., 2006) which may, at least in part, mediate hydrogen peroxide’s effects on 3′IgHRR activity due to the importance of the NF-κB signaling pathway in B-cell activation and 3′IgHRR activity. Additionally, several reports support a physiological role of ROS in lymphocyte activation. Specifically, hydrogen peroxide increased phosphorylation and Ca2+ mobilization in B cells that was similar to antigen-induced receptor activation (reviewed in Verweij and Gringhuis, 2002). Clearly the concentration and duration of the redox signal will be an important factor in the ultimate effect on cell function and it has been proposed that chronic oxidative stress will result in cellular damage while acute oxidative stress will induce activation signals akin to receptor activation by antigen (Verweij and Gringhuis, 2002).
We also evaluated the effect of the muscarinic and nicotinic agonist carbachol. Previous studies with carbachol demonstrated an increase in the IgM response to the T-cell dependent antigen sheep red blood cell, which requires signals from T cells and macrophages to induce Ig expression and antibody secretion in B cells (Pruett et al., 1992). The cellular target and molecular mechanism for this effect is unknown and carbachol did not alter LPS-induced 3′IgHRR activation or endogenous IgH protein expression in our model. Although there could be several reasons for this including a lack of functional cholinergic receptors, perhaps the most likely conclusion is that carbachol does not directly target B cells but instead targets T-cell and/or macrophage functions. The CH12.γ2b-3′IgH cellular model is limited to chemicals that directly target B-cell function as opposed to indirect effects mediated by altered T-cell and/or macrophage function.
Isothiocyanates including BITC are highly bioavailable dietary metabolites released during the normal consumption of cruciferous vegetables and appear to have anti-carcinogenic properties that may be related to their modulation of NF-κB/Rel activity, ROS production, and/or Phase I and II metabolic enzyme activity (Hecht, 2000; Srivastava and Singh, 2004; Jakubikova et al., 2006). While the effects of BITC on Ig expression and secretion have not been previously evaluated, due to its previously reported effects on NF-κB/Rel activity and ROS production and its high bioavailability, BITC seemed a good candidate to alter 3′IgHRR activity. Indeed, BITC inhibited both the 3′IgHRR transient and stable reporters as well as endogenous Ig expression. Although the exact mechanism for this effect is unclear and may be mediated through modulation of NF-κB/Rel activity, our model has identified BITC as a direct modulator of 3′IgHRR activity and Ig expression and due to its reported bioavailability during normal consumption of fruits and vegetables, BITC may alter B-cell function in humans.
Clearly, the 3′IgHRR and IgH expression in our cellular model is sensitive to modulation by exogenous chemicals which is a novel observation and may have important implications to human health particularly considering the multiple signaling pathways and transcription factors that appear to regulate 3′IgHRR activity and the diversity of chemicals, known and yet to be identified, that alter these pathways. In assessing the potential risk to human health several biokinetic factors need to be considered including bioavailability and distribution, effective concentrations, and half-life which will determine if target cells (i.e. B cells in the lymph nodes, spleen, and gastrointestinal tract) will be exposed to sufficient concentrations to mediate an effect. There is also the question of whether a synergistic, additive, or antagonistic effect will result from the simultaneous exposure to multiple chemicals in the environment, diet, and therapeutics that modulate the same or different signaling pathways involved in 3′IgHRR activity (i.e. NF-κB versus AhR versus β-adrenergic receptors, etc).
Interestingly, non-persistent AhR activators demonstrate AhR-induced Cyp1a1 induction without dioxin-like toxicities which are thought to be mediated by the sustained activation of the AhR (reviewed in Okey, 2007). Whether weak and/or non-persistent AhR activators could sufficiently modulate Ig expression and B-cell function in vivo and the relative risk to human immunocompetence is uncertain. Hu and coworkers identified six marketed drugs including omeprazole that are weak, nonpersistent AhR ligands and noted that there have been no reports of dioxin-like toxicities in rats and no evidence of immunosuppression in exposed humans (Hu et al., 2007). However, we propose that non-persistent AhR activators (and other modulators of the 3′IgHRR) directly target IgH transcription much like Cyp1a1 and will likely produce subtle effects on B-cell function and immunocompetence which may not have been directly evaluated or may be difficult to even identify in humans. Furthermore, the etiology for many immune ailments from increased infections to autoimmunity is not clearly defined and probably depends on both environmental factors and genetics.
It is intriguing that the 3′IgHRR has been associated to date with several human immune-related disorders including Celiac disease, IgA nephropathy, Burkitt’s lymphoma, and more recently systemic sclerosis, plaque psoriasis, psoriatic arthritis, and dermatitis herpetiformis (Aupetit et al., 2000; Frezza et al., 2004; Wang and Boxer, 2005; Frezza et al., 2007; Cianci et al., 2008). Interestingly, disease incidence and/or severity of these diseases (except Burkitt’s lymphoma) appears to correlate with a polymorphism within the hs1,2 enhancer of the 3′IgHRR. This polymorphism is characterized by varying repeats of a 38 to 44 bp invariant sequence containing potential binding sites for NF-κB, AP-1, Sp-1, and NF-1 (Denizot et al., 2001); we have also identified a DRE-like site that is fairly well conserved with the functional DRE we have previously evaluated in the mouse hs1,2 and like the mouse hs1,2 the DRE-like site is in close proximity to a NF-κB binding site (Sulentic et al., 2000). It is tempting to speculate that altered 3′IgHRR activity by chemicals could modulate the occurrence and severity of these diseases and that the hs1,2 polymorphism may confer an increased sensitivity to AhR ligands and other modulators of the 3′IgHRR. The AhR offers an appealing avenue to explore for understanding mechanisms of altered immunocompetence and future therapeutic interventions.
Acknowledgments
This work was supported in part by funds from the Colgate-Palmolive Grants for Alternative Research, the Boonshoft School of Medicine at WSU, and the National Institute of Environmental Health Sciences grant R01ES014676. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding organizations acknowledged above.
We wish to acknowledge the contributions of Ms. Dilini Ranatunga in the generation of the CH12.γ2b-3′IgH cell line. We are also thankful for the generous contributions from Dr. Geoffrey Haughton for the CH12.LX cells, Dr. Laurel Eckhardt for the γ2b mini-locus plasmid, Dr. Robert Roeder for the luciferase reporter plasmids, Dr. Leonard Bjeldanes for the ICZ, and Drs. Sanjay Srivastava and Thomas Brown for the BITC. We particularly wish to acknowledge the multifaceted contributions and support provided by Dr. Norbert Kaminski and members of his lab including Robert Crawford. We also greatly appreciate Dr. Michael Denison’s insightful and thought-provoking critique of this manuscript.
Nonstandard abbreviations
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
- AhR
aryl hydrocarbon receptor
- DRE
dioxin responsive element
- IgH
immunoglobulin heavy chain
- 3′IgHRR
3′IgH regulatory region
- ARNT
AhR nuclear translocator
- HAHs
halogenated aromatic hydrocarbons
- PAHs
polycyclic aromatic hydrocarbons
- DMSO
dimethyl sulfoxide
- ICZ
indolo(3,2,b)carbazole
- BITC
benzyl isothiocyanate
- PBS
phosphate buffered saline
- LPS
lipopolysaccharide
- RT-PCR
reverse transcription-polymerase chain reaction
- ELISA
enzyme-linked immunosorbent assay
- VH
variable heavy chain
- IC50
50% inhibitory concentration
- NF-κB
nuclear factor-κB
- Oct
octamer-binding factor
- OCA-B
Oct-1-associated coactivator
- AP-1
activator protein 1
- NF-1
neurofibromatosis type 1
Footnotes
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnold LW, LoCascio NJ, Lutz PM, Pennell CA, Klapper D, Haughton G. Antigen-induced lymphomagenesis: identification of a murine B cell lymphoma with known antigen specificity. J Immunol. 1983;131:2064–2068. [PubMed] [Google Scholar]
- Aupetit C, Drouet M, Pinaud E, Denizot Y, Aldigier JC, Bridoux F, Cogne M. Alleles of the alpha1 immunoglobulin gene 3′ enhancer control evolution of IgA nephropathy toward renal failure. Kidney Int. 2000;58:966–971. doi: 10.1046/j.1523-1755.2000.00253.x. [DOI] [PubMed] [Google Scholar]
- Backlund M, Ingelman-Sundberg M. Different structural requirements of the ligand binding domain of the aryl hydrocarbon receptor for high- and low-affinity ligand binding and receptor activation. Mol Pharmacol. 2004;65:416–425. doi: 10.1124/mol.65.2.416. [DOI] [PubMed] [Google Scholar]
- Birnbaum LS, Tuomisto J. Non-carcinogenic effects of TCDD in animals. Food Addit Contam. 2000;17:275–288. doi: 10.1080/026520300283351. [DOI] [PubMed] [Google Scholar]
- Bishop GA, Haughton G. Induced differentiation of a transformed clone of Ly-1+ B cells by clonal T cells and antigen. Proc Natl Acad Sci U S A. 1986;83:7410–7414. doi: 10.1073/pnas.83.19.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohonowych JE, Denison MS. Persistent Binding of Ligands to the Aryl Hydrocarbon Receptor. Toxicol Sci. 2007;98:99–109. doi: 10.1093/toxsci/kfm085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YH, Riby J, Srivastava P, Bartholomew J, Denison M, Bjeldanes L. Regulation of CYP1A1 by indolo[3,2-b]carbazole in murine hepatoma cells. J Biol Chem. 1995;270:22548–22555. doi: 10.1074/jbc.270.38.22548. [DOI] [PubMed] [Google Scholar]
- Cianci R, Giambra V, Mattioli C, Esposito M, Cammarota G, Scibilia G, Magazzu G, Orlando A, Sandri G, Bianchi L, Gasbarrini GB, Pandolfi F, Frezza D. Increased frequency of Ig heavy-chain HS1,2-A enhancer *2 allele in dermatitis herpetiformis, plaque psoriasis, and psoriatic arthritis. J Invest Dermatol. 2008;128:1920–1924. doi: 10.1038/jid.2008.40. [DOI] [PubMed] [Google Scholar]
- Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol. 2003;43:309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- Denizot Y, Pinaud E, Aupetit C, Le Morvan C, Magnoux E, Aldigier JC, Cogne M. Polymorphism of the human alpha1 immunoglobulin gene 3′ enhancer hs1,2 and its relation to gene expression. Immunology. 2001;103:35–40. doi: 10.1046/j.1365-2567.2001.01217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frezza D, Giambra V, Cianci R, Fruscalzo A, Giufre M, Cammarota G, Martinez-Labarga C, Rickards O, Scibilia G, Sferlazzas C, Bartolozzi F, Starnino S, Magazzu G, Gasbarrini GB, Pandolfi F. Increased frequency of the immunoglobulin enhancer HS1,2 allele 2 in coeliac disease. Scand J Gastroenterol. 2004;39:1083–1087. doi: 10.1080/00365520410007999. [DOI] [PubMed] [Google Scholar]
- Frezza D, Giambra V, Tolusso B, De Santis M, Bosello S, Vettori S, Triolo G, Valentini G, Ferraccioli G. Polymorphism of immunoglobulin enhancer element HS1,2A: allele *2 associates with systemic sclerosis. Comparison with HLA-DR and DQ allele frequency. Ann Rheum Dis. 2007;66:1210–1215. doi: 10.1136/ard.2006.066597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloire G, Legrand-Poels S, Piette J. NF-kappaB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol. 2006;72:1493–1505. doi: 10.1016/j.bcp.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Hecht SS. Inhibition of carcinogenesis by isothiocyanates. Drug Metab Rev. 2000;32:395–411. doi: 10.1081/dmr-100102342. [DOI] [PubMed] [Google Scholar]
- Holsapple MP, Morris DL, Wood SC, Snyder NK. 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced changes in immunocompetence: possible mechanisms. Annu Rev Pharmacol Toxicol. 1991;31:73–100. doi: 10.1146/annurev.pa.31.040191.000445. [DOI] [PubMed] [Google Scholar]
- Hsing Y, Bishop GA. Requirement for nuclear factor-kappaB activation by a distinct subset of CD40-mediated effector functions in B lymphocytes. J Immunol. 1999;162:2804–2811. [PubMed] [Google Scholar]
- Hu W, Sorrentino C, Denison MS, Kolaja K, Fielden MR. Induction of Cyp1a1 is a Non-Specific Biomarker of Aryl Hydrocarbon Receptor Activation: Results of Large Scale Screening of Pharmaceuticals and Toxicants In Vivo and In Vitro. Mol Pharmacol. 2007 doi: 10.1124/mol.106.032748. [DOI] [PubMed] [Google Scholar]
- Jakubikova J, Sedlak J, Bod’o J, Bao Y. Effect of isothiocyanates on nuclear accumulation of NF-kappaB, Nrf2, and thioredoxin in caco-2 cells. J Agric Food Chem. 2006;54:1656–1662. doi: 10.1021/jf052717h. [DOI] [PubMed] [Google Scholar]
- Kerkvliet NI. Recent advances in understanding the mechanisms of TCDD immunotoxicity. Int Immunopharmacol. 2002;2:277–291. doi: 10.1016/s1567-5769(01)00179-5. [DOI] [PubMed] [Google Scholar]
- Khamlichi AA, Pinaud E, Decourt C, Chauveau C, Cogne M. The 3′ IgH regulatory region: a complex structure in a search for a function. Adv Immunol. 2000;75:317–345. doi: 10.1016/s0065-2776(00)75008-5. [DOI] [PubMed] [Google Scholar]
- Kim DW, Gazourian L, Quadri SA, Romieu-Mourez R, Sherr DH, Sonenshein GE. The RelA NF-kappaB subunit and the aryl hydrocarbon receptor (AhR) cooperate to transactivate the c-myc promoter in mammary cells. Oncogene. 2000;19:5498–5506. doi: 10.1038/sj.onc.1203945. [DOI] [PubMed] [Google Scholar]
- Lin SC, Wortis HH, Stavnezer J. The ability of CD40L, but not lipopolysaccharide, to initiate immunoglobulin switching to immunoglobulin G1 is explained by differential induction of NF-kappaB/Rel proteins. Mol Cell Biol. 1998;18:5523–5532. doi: 10.1128/mcb.18.9.5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manis JP, van der Stoep N, Tian M, Ferrini R, Davidson L, Bottaro A, Alt FW. Class switching in B cells lacking 3′ immunoglobulin heavy chain enhancers. J Exp Med. 1998;188:1421–1431. doi: 10.1084/jem.188.8.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelson JS, Singh M, Snapper CM, Sha WC, Baltimore D, Birshtein BK. Regulation of 3′ IgH enhancers by a common set of factors, including kappa B-binding proteins. J Immunol. 1996;156:2828–2839. [PubMed] [Google Scholar]
- Nguyen LP, Bradfield CA. The search for endogenous activators of the aryl hydrocarbon receptor. Chem Res Toxicol. 2008;21:102–116. doi: 10.1021/tx7001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okey AB. An aryl hydrocarbon receptor odyssey to the shores of toxicology: the Deichmann Lecture, International Congress of Toxicology-XI. Toxicol Sci. 2007;98:5–38. doi: 10.1093/toxsci/kfm096. [DOI] [PubMed] [Google Scholar]
- Ong J, Stevens S, Roeder RG, Eckhardt LA. 3′ IgH enhancer elements shift synergistic interactions during B cell development. J Immunol. 1998;160:4896–4903. [PubMed] [Google Scholar]
- Pinaud E, Khamlichi AA, Le Morvan C, Drouet M, Nalesso V, Le Bert M, Cogne M. Localization of the 3′ IgH locus elements that effect long-distance regulation of class switch recombination. Immunity. 2001;15:187–199. doi: 10.1016/s1074-7613(01)00181-9. [DOI] [PubMed] [Google Scholar]
- Podojil JR, Kin NW, Sanders VM. CD86 and beta2-adrenergic receptor signaling pathways, respectively, increase Oct-2 and OCA-B Expression and binding to the 3′-IgH enhancer in B cells. J Biol Chem. 2004;279:23394–23404. doi: 10.1074/jbc.M313096200. [DOI] [PubMed] [Google Scholar]
- Pruett SB, Han Y, Munson AE, Fuchs BA. Assessment of cholinergic influences on a primary humoral immune response. Immunology. 1992;77:428–435. [PMC free article] [PubMed] [Google Scholar]
- Shi X, Eckhardt LA. Deletional analyses reveal an essential role for the hs3b/hs4 IgH 3′ enhancer pair in an Ig-secreting but not an earlier-stage B cell line. Int Immunol. 2001;13:1003–1012. doi: 10.1093/intimm/13.8.1003. [DOI] [PubMed] [Google Scholar]
- Srivastava SK, Singh SV. Cell cycle arrest, apoptosis induction and inhibition of nuclear factor kappa B activation in anti-proliferative activity of benzyl isothiocyanate against human pancreatic cancer cells. Carcinogenesis. 2004;25:1701–1709. doi: 10.1093/carcin/bgh179. [DOI] [PubMed] [Google Scholar]
- Sulentic CE, Holsapple MP, Kaminski NE. Aryl hydrocarbon receptor-dependent suppression by 2,3,7, 8-tetrachlorodibenzo-p-dioxin of IgM secretion in activated B cells. Mol Pharmacol. 1998;53:623–629. [PubMed] [Google Scholar]
- Sulentic CE, Holsapple MP, Kaminski NE. Putative link between transcriptional regulation of IgM expression by 2,3,7,8-tetrachlorodibenzo-p-dioxin and the aryl hydrocarbon receptor/dioxin-responsive enhancer signaling pathway. J Pharmacol Exp Ther. 2000;295:705–716. [PubMed] [Google Scholar]
- Sulentic CE, Kang JS, Na YJ, Kaminski NE. Interactions at a dioxin responsive element (DRE) and an overlapping kappaB site within the hs4 domain of the 3′alpha immunoglobulin heavy chain enhancer. Toxicology. 2004a;200:235–246. doi: 10.1016/j.tox.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Sulentic CE, Zhang W, Na YJ, Kaminski NE. 2,3,7,8-tetrachlorodibenzo-p-dioxin, an exogenous modulator of the 3′alpha immunoglobulin heavy chain enhancer in the CH12.LX mouse cell line. J Pharmacol Exp Ther. 2004b;309:71–78. doi: 10.1124/jpet.103.059493. [DOI] [PubMed] [Google Scholar]
- Tian Y, Ke S, Denison MS, Rabson AB, Gallo MA. Ah receptor and NF-kappaB interactions, a potential mechanism for dioxin toxicity. J Biol Chem. 1999;274:510–515. doi: 10.1074/jbc.274.1.510. [DOI] [PubMed] [Google Scholar]
- Verweij CL, Gringhuis SI. Oxidants and tyrosine phosphorylation: role of acute and chronic oxidative stress in T-and B-lymphocyte signaling. Antioxid Redox Signal. 2002;4:543–551. doi: 10.1089/15230860260196344. [DOI] [PubMed] [Google Scholar]
- Vogel CF, Sciullo E, Li W, Wong P, Lazennec G, Matsumura F. RelB, a New Partner of Aryl Hydrocarbon Receptor-Mediated Transcription. Mol Endocrinol. 2007;21:2941–2955. doi: 10.1210/me.2007-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorderstrasse BA, Steppan LB, Silverstone AE, Kerkvliet NI. Aryl hydrocarbon receptor-deficient mice generate normal immune responses to model antigens and are resistant to TCDD-induced immune suppression. Toxicol Appl Pharmacol. 2001;171:157–164. doi: 10.1006/taap.2000.9122. [DOI] [PubMed] [Google Scholar]
- Wang J, Boxer LM. Regulatory elements in the immunoglobulin heavy chain gene 3′-enhancers induce c-myc deregulation and lymphomagenesis in murine B cells. J Biol Chem. 2005;280:12766–12773. doi: 10.1074/jbc.M412446200. [DOI] [PubMed] [Google Scholar]
- Werlinder V, Backlund M, Zhukov A, Ingelman-Sundberg M. Transcriptional and post-translational regulation of CYP1A1 by primaquine. J Pharmacol Exp Ther. 2001;297:206–214. [PubMed] [Google Scholar]