Abstract
Objective
There is a paucity of level-one evidence comparing STN and GPi DBS. Our aim in this prospective blinded randomized trial was to compare the cognitive and mood effects of unilateral subthalamic nucleus (STN) vs. unilateral globus pallidus interna (GPi) deep brain stimulation (DBS) in patients with Parkinson disease (PD).
Methods
Fifty-two subjects with moderate-to-advanced PD were randomized to either unilateral STN or GPi DBS. Right or alternatively left sided stimulation was chosen to address the side of the body with the most bothersome symptoms. The co-primary outcome measures were the change in the 8 subscales of the Visual Analog Mood Scale (VAMS), and the change in the 2 versions of verbal fluency (i.e. semantic and letter), at 7 months post-DBS in the optimal setting compared to the pre-DBS state. In addition, at 7 months post-DBS, after subjects underwent initial evaluation off medications and on optimized DBS therapy, they were tested in four randomized and counterbalanced conditions (optimal DBS, ventral DBS, dorsal DBS, and off DBS) while remaining off medication. Secondary outcome measures then compared the differences in the VAMS items and verbal fluency subscales within the 4 DBS conditions at 7 months, and the change in the VAMS items and verbal fluency subscales from the pre-DBS state to the other 3 DBS conditions (ventral, dorsal and off ) at 7 months.
Results
Forty-five subjects (23 GPi and 22 STN) completed the protocol. The study revealed no significant difference between STN and GPi DBS in the change of co-primary mood and cognitive outcomes from pre- to post-DBS in the optimal setting (Hotelling's T2 test: p=0.16 and 0.08 respectively). When comparing the 4 DBS conditions at 7 months, subjects in both targets were less “happy”, less “energetic” and more “confused” when stimulated ventrally to the optimal stimulation site. When comparing the other 3 DBS conditions (ventral, dorsal and off DBS) to the pre-DBS state, the STN group showed a larger deterioration of letter verbal fluency scores than the GPi group, especially in the off DBS state. A 12-point mean improvement in the UPDRS motor subscale was seen post DBS, but there was no significant difference between targets.
Interpretations
There were no significant differences in in the co-primary outcome measures of mood and cognition between STN and GPi in the optimal DBS state.. However, adverse mood effects were noted when stimulating ventrally to the optimal site in both targets. Furthermore, a worsening for letter verbal fluency was noted in the 3 non-optimal post-DBS states in the STN target only. The persistence of deterioration in verbal fluency in the off DBS state at 7 months is, suggestive of a surgical rather than a stimulation-induced effect at the STN target. STN and GPi DBS resulted in similar motor improvement.
Keywords: GPi, STN, DBS, Mood, Cognition, Side Effects, verbal fluency, motor, UPDRS
Introduction
There is a paucity of level one evidence comparing subthalamic nucleus (STN) and internal globus pallidus (GPi) deep brain stimulation (DBS) for advanced cases of Parkinson disease (PD)1, 2. Most available comparative data are non-randomized 3, 4, have small sample sizes 5, 6, and primarily focus on motor improvement1 without careful assessment of the effects of DBS on non-motor function. PD is associated with relatively high rates of mood and cognitive dysfunction 7, 8. Deep brain stimulation of STN and GPi have each been associated with mild improvements in mood as measured by the Beck Depression Inventory, and mild cognitive decline, particularly in verbal fluency tasks9-15.
The goals of this study were to characterize and compare mood and cognitive changes associated with unilateral STN or GPi DBS. We had two major hypotheses. First, based on pilot data16, we felt it likely that both brain targets would be associated with changes in mood and cognition, and we hypothesized this was due to the spread of current to non-motor areas within these nuclei, as well as due to the spread of current to adjacent pathways mediating non-motor functions16-18. Second, we hypothesized that stimulation within specific regions of the STN or GPi would have different effects based on the known neuroanatomy of limbic and associative basal ganglia. The four contacts on each implanted DBS lead provided us an experimental paradigm to test the effects of regional stimulation, especially in locations dorsal and ventral to the region of optimal motor benefit. We chose the Visual Analogue Mood Scale (VAMS) because it was a validated scale that was ideal for our study design, and had been utilized in our previous pilot study16. Verbal Fluency was chosen because at the time of study inception it was the most frequently reported cognitive deficit following DBS19. Finally, the unilateral nature of the surgical protocol allowed us to examine the relative effects of hemispheric laterality on mood and cognition for each target.
The study was a NIH-sponsored, single-center, prospective, randomized, patient- and rater-blind, parallel-group trial that aimed to compare the effects of unilateral STN and unilateral GPi DBS on mood and cognitive function in patients with advanced PD. Motor outcome measures were included as a secondary aim. This paper aims to present the results of only primary mood and cognitive outcome measures as well as results of motor measures.
Methods
Patient Population
Subjects were required to meet UK PD Brain Bank Criteria 20-22, be 30−75 years old, have an adequate response to levodopa (i.e. an improvement of >30% on the Unified Parkinson Disease Rating Scale (UPDRS) motor subscale in the on compared to the off medication state), be right-handed, and have disabling motor fluctuations or dyskinesias. Sixty-two patients were recruited and ten patients failed initial screening. Fifty-two patients were randomized to STN or GPi DBS. Forty-five subjects completed the study while seven subjects (four from the STN group, and three from the GPi group) terminated prematurely (Figure 1).
Study Protocol and Randomization
Fifty-two subjects were consented according to University and Federal guidelines. The sample size was determined such that, when the change of VAMS mood scores within each group has 12 points standard deviation and there is a difference of 10 points between the two stimulation sites (STN and DBS), the overall power equals to 83%at the type-I error level 0.025 for a two-sample t-test.
Prior to surgery, baseline neuropsychological and psychiatric evaluation, as well as on versus off medication function were performed. Patients were off medications overnight (>12 hours) for all pre-operative mood and motor testing, and also for all follow-up testing at 7 months post-DBS. Pre-operative neuropsychological testing (performed in the on medication state) was performed on a different day than the motor testing. Patients were then enrolled and randomized to receive either unilateral STN or GPi DBS to address the side of the body with the most bothersome symptoms (Figure 1). All surgeries were performed by a single neurosurgeon (K.D.F.)/neurologist (M.S.O.) team with multiple pass microelectrode mapping 23-26. The DBS devices were activated one month after intracranial lead implantation. Following initial DBS activation, repeated follow-up evaluations were performed, as needed, until the optimal chronic stimulation parameters and adjunctive PD medication regimen were determined. The average time to achieve optimization of DBS therapy was 134.4 days (SD±25.2) and did not vary significantly between the two target groups. All patients were kept stable on their optimized DBS setting and medication regimen for a minimum of 30 days (mean = 75.7±28.3 days) before repeat mood, cognitive or motor evaluation was performed.
Post-DBS Baseline and Evaluation of STN and GPi Regional Settings (Optimal,Ventral, Dorsal, Off)
Approximately seven months following DBS surgery (210.1±37.8 days), subjects were admitted to the General Clinical Research Center (GCRC) to participate in a 2-day standardized testing protocol to examine the mood, cognitive, and motor effects of DBS. For each testing day, dopaminergic medications were withheld beginning at 10 PM the night prior (i.e., 12 hour washout period). While still in the off medication state, testing began on the first day with an initial Baseline evaluation in which patients remained on stimulation at their empirically derived optimal DBS setting in order to assess baseline effects of chronic DBS therapy on mood scales and the motor section of the UPDRS. This was followed by testing across four randomized conditions (see Figure 1): stimulation at the contact associated with the optimal clinical effect (Optimal), stimulation at contact points deep and superficial adjacent to the optimal site (Ventral and Dorsal), and DBS stimulation turned off (Off). Testing in these four conditions (Optimal, Ventral, Dorsal, Off) were randomized and counterbalanced across STN and GPi groups, and took place over two days – with one stimulation condition tested per half day. The change in the DBS condition from Baseline to the experimental setting (Ventral, Dorsal, Optimal, Off) was performed by a DBS programmer who was blinded to target location (GPi, STN). The programmer slowly increased the voltage in 0.1−0.2 volt aliquots to a maximum that did not exceed the optimal voltage condition. The frequency and pulse width were held steady and matched the chronic clinic settings. For the optimal DBS condition the voltage was slowly increased to match the chronic clinic setting. For the dorsal and ventral settings the voltage was increased to optimal levels unless a side effect was encountered. Transient side effects (i.e. <30 seconds) were considered acceptable, however if the side effect was persistent, the DBS device was programmed to a voltage just below the side effect threshold. Once programmed, the setting was maintained for 10 minutes before any testing ensued.
Test Measures and Outcome Variables
Descriptions of the primary and secondary outcome measures are shown in supplementary table 1, available online. The co-primary outcome variables were the 8 subscales of the VAMS, a measure of acute emotional state, and the 2 versions of Verbal Fluency (semantic and letter), a cognitive measure involving speeded word retrieval with letter cues (i.e., “f”) or semantic cues (i.e., animals). The significance level for the co-primary outcome variables was set at p<.025 for each measure. Other secondary outcome measures, except for the motor symptom severity (UPDRS-motor subscale) and the 4 experimental DBS settings at 7 months, will be reported in detail in a separate paper, but are listed in supplementary table 1. All testing was administered by the same individual (i.e., neuropsychology graduate student) who was blinded as to the DBS condition and target. Alternate versions of the tests were given for verbal fluency. While the sequence of the four stimulation settings over 2 days were randomized and counter-balanced, all primary and secondary outcome measures under each setting were administered in the same order.
Procedure to Localize DBS Lead Location
Precise localization of implanted leads was performed as follows: a high resolution CT scan was acquired one month after lead implantation to allow complete resolution of procedure-related brain shift and pneumocephalus. This post-operative CT was then carefully fused to the pre-operatively acquired, high resolution MRI and an orthogonal Cartesian coordinate system was set up with its origin at the mid-commissural point (MCP). The lead location was ascertained using the MCP-based (x,y,z) coordinate of the center of the ventral aspect of the deepest contact. With appropriate windowing, this point is readily identified on a CT scan with greater precision than is possible on postoperative MRI. Using this point, along with the measured linear trajectory of the ventral aspect of the lead and the fixed lead geometry, vector calculations produced the MCP-based (x,y,z) coordinates of the center of each of the four contacts. The optimal contact for stimulation was determined through an algorithm-based, systematic programming technique that did not take into account the measured contact locations. The optimal contact was chosen based on the best motor response obtained in the clinic. The dorsal or ventral contacts during follow-up testing were located 3mm superior or inferior to the optimal contact respectively. The center of the active contact (cathode) was used for localization.
Statistical Analyses
Descriptive statistics were used to describe the baseline characteristics of the two groups. Analysis was performed using Hotelling's T2 test to compare STN and GPi DBS on change scores (post-DBS at optimal contact testing minus pre-DBS). This test was conducted for both co-primary outcome measures at a type-I error level of 0.025 (Bonferroni adjustment), followed by t-tests for individual subscales. Also we tested whether there were significant changes over the time period when the two groups were combined.
In our secondary analyses, to compare the four DBS settings (ventral, dorsal, optimal, off), a repeated measures analysis using a mixed model was used. The dependent variables were the measures at the four conditions for each mood and cognitive outcome, whereas the independent variables included the DBS target (STN, GPi), side of stimulation (right, left), stimulation setting (ventral, dorsal, optimal, off), testing sequence (1st to 4th), timing of observation (immediate vs. delayed for mood outcomes only). Patient age and gender were included as covariates. To compare the pre-DBS state to the 3 other DBS settings at 7 months (ventral, dorsal, and off), we used paired t-test. All statistical analyses other than the Hotelling's T2 test for the two co-primary outcomes were secondary, thus no further correction for multiple comparison was applied.
Results
1. Patient General Characteristics
There were no significant differences between the STN and GPi groups in the general characteristics, mean UPDRS motor subscale scores, or on the pre-operative mood and cognitive states. However, more subjects in the STN group had a Hoehn and Yahr stage of four or higher in the off state (Table 1).
Table 1. Comparison of Pre-operative Characteristics Between the STN and GPi.
*Analysis of the baseline characteristics was also performed on all 52 subjects and no significant differences between groups was identified.
| Variable | Overall (N=45) | STN (N=22) | GPi (N=23) | p value |
|---|---|---|---|---|
| Age (mean(sd)) | 60.0(8.2) | 59.8(10.0) | 60.2(6.2) | 0.8729 |
| Gender (male%) | 67.3 | 69.2 | 65.4 | 0.7675 |
| Ethinicity (whie%) | 94.2 | 96.2 | 92.3 | 0.5520 |
| Disease Duration (Years) | 12.9(3.8) | 13.3(4.0) | 12.5(3.6) | 0.5437 |
| LED Before Surgery | 1054.9(517.1) | 935.9(373.9) | 1168.3(611.8) | 0.1527 |
| LED at 6 Month Visit | 1088.1(668.8) | 916.6(426.5) | 1259.5(820.0) | 0.0892 |
| Hoehn and Yahr off stage | ||||
| 2 (%) | 16.3 | 8.3 | 24.0 | 0.0082 |
| 2.5 (%) | 18.4 | 29.2 | 8.0 | |
| 3 (%) | 51.0 | 37.5 | 64.0 | |
| 4 (%) | 12.2 | 25.0 | 0.0 | |
| 5 (%) | 2.0 | 0.0 | 4.0 | |
| Preop off UPDRS III score | 42.9(11.3) | 45.2(12.6) | 40.6(9.5) | 0.1475 |
| Preop on UPDRS III score | 21.6(7.6) | 22.5(8.2) | 20.7(7.1) | 0.4014 |
| Mini-Mental State Exam | 28.3(1.6) | 28.0(1.8) | 28.5(1.3) | 0.2764 |
| Dementia Rating Scale (raw) | 137.6(5.9) | 136.5(7.0) | 138.8(4.4) | 0.1782 |
| Beck Depression Inventory | 11.2(6.1) | 10.4(5.9) | 11.9(6.3) | 0.3937 |
| State-Trait Anxiety Inventory | ||||
| State Anxiety (raw) | 37.4(10.8) | 37.2(10.9) | 37.5(11.0) | 0.9236 |
| Trait Anxiety (raw) | 35.5(11.0) | 35.9(10.6) | 35.1(11.6) | 0.8075 |
| Visual Analogue Mood Scale | ||||
| Afraid (Tscore) | 55.9(16.1) | 56.3(17.8) | 55.5(14.8) | 0.8573 |
| Angry (Tscore) | 47.7(8.2) | 48.4(10.0) | 47.5(6.4) | 0.8588 |
| Confused (TScore) | 50.2(9.8) | 51.1(11.7) | 49.4(7.9) | 0.5559 |
| Energetic (TScore) | 39.6(11.6) | 40.4(11.1) | 38.8(12.2) | 0.6290 |
| Happy (TScore) | 42.0(12.0) | 42.6(13.2) | 41.4(10.9) | 0.7359 |
| Sad (TScore) | 53.8(14.6) | 54.9(17.8) | 52.7(11.2) | 0.6105 |
| Tense (TScore) | 63.9(16.3) | 61.7(16.8) | 66.6(16.0) | 0.3697 |
| Tired (TScore) | 57.0(11.0) | 58.5(11.0) | 55.6(11.1) | 0.3501 |
| Verbal Fluency Tasks | ||||
| Letter Fluency (raw) | 38.0(13.1) | 38.1(11.7) | 37.8(14.5) | 0.9254 |
| Animal fluency(raw) | 18.3(5.1) | 18.3(4.7) | 18.4(5.5) | 0.9226 |
Table 2 depicts the mean chronic optimized DBS parameters and lead locations for the active contacts in the STN and GPi groups. As shown, the two groups did not differ in terms of surgical characteristics. Forty-three out of the 45 leads were programmed in a single contact monopolar setting, while two (in the STN group) required bipolar settings. A higher voltage was required in the GPi target.
Table 2. Summary of Stimulation Parameters, Day in Optimized DBS State, Measured Lead Locations, and Microelectrode Passes Comparison of stimulation parameters between the STN and GPi.
This table summarizes the side of stimulation, the days spent in an optimized state prior to testing (to assure changes did not occur as a result of changing programming parameters), the number of microelectrode/macroelectrode passes, the measured lead locations by CT-MRI fusion, and the mean chronic DBS parameters used in all patients.
| Variables | Overall (N=45) | STN (N=22) | GPi (N=23) | p_value |
|---|---|---|---|---|
| Stimulation side (left%) | 57.77 | 63.63 | 52.17 | 0.4364 |
| Days spent optimizing stimulation parameters | 134.4(25.2) | 130.3(18.7) | 138.3(30.2) | 0.2950 |
| Days maintained in optimized state | 75.7(28.3) | 73.5(30.4) | 77.9(26.7) | 0.6043 |
| Number of microelectrode passes | 4.0(1.2) | 4.1(1.4) | 4.0(1.1) | 0.8129 |
| Number of macroelectrode passes | 1.6(1.0) | 1.5(1.3) | 1.7(0.8) | 0.6528 |
| Lateral location of the active DBS contact | 16.7(5.7) | 11.5(2.8) | 21.7(1.7) | |
| Antero-posterior location of the active DBS contact | 1.7(3.5) | −0.1(3.8) | 3.6(1.7) | |
| Axial location of the active DBS contact | −0.5(2.5) | −1.1(3.0) | 0.0(1.7) | |
| Mean voltage of stimulation | 2.7(0.5) | 2.4(0.6) | 2.9(0.4) | 0.0053 |
| Mean frequency of stimulation | 146.4(17.2) | 141.1(13.1) | 151.5(19.3) | 0.0424 |
| Mean pulse width of stimulation | 89.3(17.5) | 94.0(19.1) | 84.7(14.7) | 0.0741 |
2. Pre- vs Post-DBS Changes in Mood and Cognition
To examine the influence of DBS surgery per se, we compared the pre-DBS to post-DBS performance at 7 months when patients were on their optimal stimulation setting, but off medication. In the sections below and statistics presented in Table 3, we describe mood and cognitive changes for the DBS group as a whole followed by the differential findings for the STN versus GPi subgroups. The dependent variables in all analyses were difference scores (i.e., Post-DBS Optimal Stimulation condition minus Pre-DBS condition).
Table 3. Changes in Mood and Cognition.
Table 3 summarizes the changes in mood and cognition between targets.
| Variable | Overall (N=45)1 | STN (N=22) | GPi (N=23) | p_value |
|---|---|---|---|---|
| Visual Analogue Mood (VAMS) | ||||
| Afraid (T) | −0.5(14.9) | 1.3(16.9) | −2.2(13.0) | 0.4325 |
| Angry (T) | 2.4(8.4) | 5.3(10.2) | −0.1(5.3) | 0.0270 |
| Confused (T) | 3.4(13.3) | 5.7(15.4) | 1.3(11.0) | 0.2745 |
| Energetic (T) | 0.4(15.9) | −1.4(12.3) | 2.0(18.7) | 0.4708 |
| Happy (T) | 4.0(15.4) | 2.9(17.2) | 5.0(13.8) | 0.6511 |
| Sad (T) | −1.7(15.3) | −2.4(17.8) | −1.−(13.0) | 0.7543 |
| Tense (T) | −5.4(18.9) | −2.0(17.5) | −8.5(20.0) | 0.2591 |
| Tired (T) | −5.1(13.1) | −8.5(12.0) | −2.−(13.5) | 0.0999 |
| Beck Depression Inventory (raw) | −3.7(5.9) | −2.8(6.3) | −4.6(5.4) | 0.3043 |
| State-Trait Anxiety (STAI) | ||||
| State Anxiety (Raw) | −1.4(13.4) | −3.3(13.4) | 0.3(13.6) | 0.3778 |
| Trait Anxiety (raw) | 0.4(11.4) | −0.2(11.3) | 1.1(11.7) | 0.6864 |
| Verbal Fluency Tasks | ||||
| Category Fluency (raw) | 0.7(5.5) | 0.2(4.7) | 1.2(6.3) | 0.5664 |
| Letter Fluency (raw) | −2.6(9.3) | −5.6(6.7) | 0.3(10.7) | 0.0322 |
2A. Mood Changes
Comparison of GPi vs STN subgroups on Mood
There was no significant difference between STN and GPi DBS in changes in the 8 mood items in the VAMS from pre-DBS to post-DBS performance at 7 months in the optimal setting (Hotelling's T2 test, p=0.16). However, an exploratory analysis using t-tests revealed the mean change in the VAMS “angry” item for the STN group to be larger than the GPi group (p=0.027, see Table 3).
Overall Influence of DBS Surgery on Mood (Combined STN and GPi Groups)
There was a significant reduction in “tiredness” ratings on the VAMS (p = .013), as well as a trend (p < .10) toward higher scores in the following subscales: “happy”, “tense”, “angry”, and “confused.” These results suggested that patients tended to be happier and less tense following DBS surgery, but also more angry/irritable and more confused.
2B. Cognitive Changes
Comparison of GPi vs STN subgroups on Cognitive Tasks
There was no significant difference between STN and GPi DBS in changes of the combined letter and semantic verbal fluency from pre-DBS to post-DBS performance at 7 months in the optimal setting (Hotelling's T2 test, p=0.08). However, the STN subgroup did exhibit a greater decline on the letter verbal fluency task than the GPi subgroup (p = .03), but this did not reach the predefined p<.025 level of significance. On average, the STN subgroup produced 5.6±6.7 fewer words following DBS than before, and this contrasted with minimal changes in letter fluency for the GPi subgroup (0.4±10.7). Both groups exhibited no changes on the semantic verbal fluency task (p = .57).
Overall Influence of DBS Surgery on Cognition (STN and GPi Groups Combined)
While a trend toward reduction in letter fluency (p = .07) following DBS surgery was noted, further analyses revealed no significant changes post-DBS in semantic (category) fluency (p = .36).
3. Pre-Post DBS Changes in Motor Symptoms
We compared the changes on the motor subscale of the UPDRS before and after DBS. Patients were off medication during both testing periods, but on optimal DBS setting during post-DBS testing.
Comparison of GPi vs STN Subgroups on Changes in Motor Symptoms
No difference was noted between the STN and GPi subgroups on the UPDRS motor subscale improvement (p=0.64), with a mean percent improvement of 29.9% for the STN and 26.6% for the GPi subgroup. In terms of specific motor domains, there were no group differences for improvements in bradykinesia or tremor. However, the STN subgroup exhibited a greater improvement in rigidity compared to the GPi group (−5.6±2.8 vs. −2.9±3.1, p=0.01).
Overall Influence of DBS Surgery on Changes in Motor Symptoms
In both targets, the DBS patients showed a significant improvement in motor symptoms. An average of 11.8±9.9 point reduction on the UPDRS motor subscale was noted between pre-operative versus post-operative testing (p<0.01). Significant improvements occurred across each of the following motor domains: rigidity (−4.2 ± 3.2 points, p<0.01), bradykinesia (−3.0 ± 5.1 points, p < 0.01), and tremor (−2.6 ± 2.9 points, p<0.01).
4. Mood and Cognitive Measures during Optimal, Ventral, Dorsal, and Off DBS Testing Conditions
To examine the regional effects of DBS activation within the STN vs GPi, we compared the four DBS testing conditions across mood and cognitive measures. The “optimal condition” was used as the reference condition. In addition, we compared the 3 other DBS settings (ventral, dorsal, and off) at 7 months to the pre-DBS state. We report all these as secondary outcomes.
4a. Mood Measures
For both the STN and GPi groups, subjects rated themselves as more “confused” (p=0.04), less “energetic” (p<.01), less “happy” (p=0.03) and more “sad” (p=0.05) on the VAMS items when stimulation was delivered ventral to the optimal stimulation site. Additionally, subjects were less “energetic” at dorsal DBS (p=0.02) and off DBS (p<.01) when compared to the optimal DBS setting. In addition, subjects who received stimulation on the left side were significantly less “tired” than those who received stimulation on the right (p=0.01). However, no unique differences between the GPi and STN subgroups were found.
4b. Cognitive Measures
Unlike the mood items, there was no significant difference in cognitive measures within the 4 stimulation settings at 7 months in each of the targets. However, when comparing the 3 DBS stimulations settings (ventral, dorsal and off) to the pre-DBS state, the mean letter verbal fluency scores in the STN group decreased more than the GPi group: −5.8±10.0; −3.6±14.9; −6.6±10.3 words respectively in STN group, compared to changes of −3.1±7.6; −1.1±12.7 and 0.6±9.2 words in the GPi group (p<0.05). In other words, letter verbal fluency worsened regardless of stimulation setting in the STN group.
5. Adverse Events
The adverse events are summarized in supplementary table 2 (Randomized Stimulation Setting-Specific Adverse Events), supplementary table 3 (Post-Surgical Mood and Cognitive Adverse Events), and supplementary table 4 (General Post-surgical Adverse Events). The number of adverse events during randomized testing of the four stimulation settings were similar between targets (199 STN to 201 GPi), and were mostly mild and transient. However, the number of post-surgical mood and cognitive adverse events were higher in the STN group when compared to the GPi group (75 vs. 45). More patients in the STN group experienced, anxiety, confusion, irritability, aggressiveness, obsessive compulsive symptoms, manic symptoms and decreased confidence/motivation. Moreover, the number of general post-surgical adverse events was higher in the STN group (95 vs. 67). Overall, serious AE's included pneumonia/death (STN n=1), symptomatic hemorrhage (STN n=1, GPi n=1), delayed venous hemorrhage with full resolution (GPi n=2), and asymptomatic hemorrhage (STN n=2). The complete adverse events tables (supplementary tables 2−4) are available as web based supplements.
Discussion
The data from this prospective double-blinded randomized study revealed no significant difference in the primary mood and cognitive outcomes between STN and GPi DBS. However, exploratory secondary investigation of the eight VAMS mood subscales suggested that there was increased “anger” with STN DBS only. The potentially increased anger seen in the STN target was consistent with previously reported cases of STN DBS-induced anger, aggressiveness 27, 28, and impulsivity29. When both groups were combined, the VAMS “tired” scores significantly improved post-DBS. In our secondary analyses, when comparing all 4 DBS stimulation settings (ventral, dorsal, optimal and off) at 7 months, the ventral stimulation settings often worsened many VAMS mood items across both targets (more confused, less energetic, less happy and more sad).
The primary cognitive outcome comparing the pre-DBS state to the optimal DBS setting at 7 months revealed a trend for worsening letter verbal fluency in STN, but not with GPi DBS (p<.03), since we set the level of our p value to be significant at < 0.025. Furthermore, in our secondary analyses, when comparing the pre-DBS state to the 3 other DBS stimulation setting at 7 months (ventral, dorsal, and off), this impairment in letter verbal fluency in the STN group remained constant. The persistence of this finding, including the off stimulation setting at 7 months, collectively suggests an insertion or lesion effect as a possible underlying mechanism.
There was no difference between the two targets in motor function improvement, similar to the findings of a previous smaller comparative study5. Mood, cognitive and general surgical adverse events occurred at a higher frequency in the STN target. This information may be useful in understanding differences between surgical targets for PD.
Prior to randomization we hypothesized, based on our pilot work16, that both brain targets would be associated with changes in mood and cognition, and that these changes would likely result from spread of current into non-motor portions of the nuclei, as well as from spread into adjacent pathways mediating non-motor functions16-18. Indeed, many investigators have reported changes in mood and cognition with either target9-11, 13, 15, 19, 27, 30-35, and we believed prior to inception of the study that although less numbers of GPi DBS had been performed worldwide, this target could provide a safer architectural environment to protect against mood and cognitive issues (due to the significantly larger volume of the structure). The STN (∼158 mm3) is a smaller nucleus than the GPi (∼478 mm3)17, 18, and its motor, associative and limbic circuits contain multiple fiber pathways within a very compact area. Rothlind and colleagues also recently reported declines in verbal fluency with only unilateral STN and GPi DBS, although they did not perform on/off DBS blinded testing to directly compare the targets36. Although this architecture could provide an ideal single locus for neuromodulation, the region of interest is located in such a tiny neuronal complex that its disruption may more easily lend itself to increasing the risk for post-operative cognitive and behavioral issues. The verbal fluency findings from the secondary outcome data in this trial suggest that the structural damage from insertion of the DBS lead likely had a large role in the cognitive dysfunction post-DBS in the smaller STN target. All of the factors however, that may potentially lead to verbal fluency decline in unilateral or bilateral DBS remain to be better and more completely characterized.
The design of the study made it ideal to attempt to delineate regions associated with mood and cognitive changes resulting from STN or GPi DBS (particularly dorsal or ventral to the active optimal DBS contact). The territories of STN have been anatomically divided into a dorsolateral sensorimotor region, a ventromedial associative region, and a medially located limbic region17, 18. Similarly, the non-motor regions of GPi have been described as being located anterior and medial, with a rich plexus of neurotransmitters situated in a more ventral position. We hypothesized that ventral and medial stimulation would preferentially affect non-motor circuits within the STN. Similarly we supposed that anterior and medial stimulation would lead to more adverse issues with GPi DBS. The data were revealing in that ventral stimulation was worse for STN, but it also demonstrated similar worsening for the GPi group. The study was less effective in the evaluation of the medial-lateral issues as the careful microelectrode mapping resulted in mean lead locations well within the sensorimotor and not limbic/cognitive regions (Table 2).
Finally, we aimed to assess the relative effects of right versus left DBS. Unilateral DBS rather than the more common bilateral simultaneous implantation was chosen because the unilateral staged approach has been standard at our center, and also because a unilateral approach offered a cleaner method for examination of the effects of laterality. The sample size for this analysis was reasonably adequate, and there was a roughly equal distribution of right versus left-sided leads. Laterality in the lesion literature had been suggested to play a role in both mood and in cognition. Right hemispheric lesions had been reported to be associated with euphoria, and left sided lesions with depression37-40. Our data did not reveal any laterality effect with the exception of decreased tiredness with left-sided stimulation. One potential shortcoming of our study was that ethically we did not have the equipoise to randomize right versus left stimulation which would have greatly strengthened the study design.
This study provides level one evidence supporting no general difference between the mood, cognitive and motor effects of unilateral STN versus GPi DBS. Many previous studies, although not randomized, revealed positive benefits, mainly in motor function, with less clear data on mood and cognitive changes. Our data revealed that unilateral STN and unilateral GPi DBS when taken together may have mild mood elevating effects, and trended toward verbal fluency issues. Our secondary analyses showed STN had a worsened verbal fluency on the letter task, and overall also had an increased amount of mood/cognitive/surgical adverse events. The strengths of this study included the single surgical team performing all procedures, blinding, and randomization, as well as the use of unilateral stimulation to assess laterality effects. An additional strength was the complete and prospective recording of adverse events which were not insignificant for this study and highlight risks of DBS surgery. The findings however, were limited by a number of important factors. The power analysis was based on only one of the co-primary outcome variables and therefore lack of difference may have reflected a power issue. In clinical practice, more centers perform DBS in a bilateral simultaneous fashion than the unilateral staged approach. Additionally, medication reduction which trended in favor of STN DBS (Table 1) in our study, has been previously shown to be robust for bilateral STN DBS 4, 14. Our experiment focused on a small number of relevant outcomes in order to limit the effects of fatigue and testing order. Additionally, it is important to note the confound of testing off medication which may reveal the effects of stimulation only. The aggregate “DBS” effects reported were therefore the cumulative impact of being off medications and on DBS. It should be noted that 3/52 (∼5%) subjects could not complete the protocol due to death or hemorrhage which is higher than other reported studies. Finally, there may have been other differences missed as a result of the sharp focus of our testing (e.g. dyskinesia, dystonia, quality of life, etc.), and we have yet to report the numerous secondary outcome variables.
Based on our findings, and other available data in the literature, there is emerging evidence that the DBS target choice may be tailored to individual patient needs. If cognitive or behavioral issues are of concern, GPi stimulation should be potentially considered. If medication reduction is an important goal, then bilateral STN DBS may prove in future studies to be the best choice. As data from comparative studies, and particularly bilateral studies, becomes more available, hopefully it will enable DBS practitioners to tailor target selection and programming based on each patient's therapeutic need and “risk profiles1.” Finally, when identifying the optimal stimulation settings in either target, more ventral contacts may need to be avoided when cognitive and mood effects are encountered. The safety of staging operations (unilateral staged versus bilateral simultaneous), as well as the clinical relevance of fluency issues will need to be addressed in future studies.
Supplementary Material
Figure 1A.
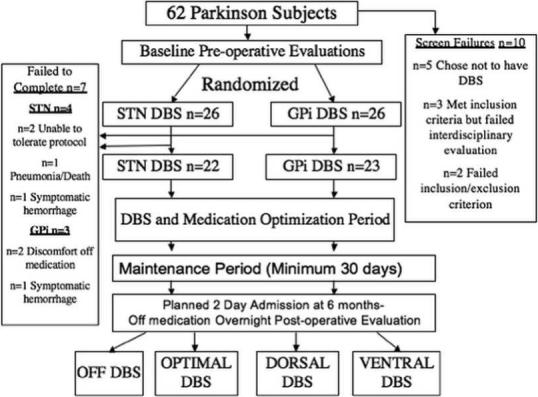
reveals the flow chart for the study; and figure 1B a picture representation of the DBS testing procedures in the four blinded conditions examined in the general clinical research center (CRC).
Figure 1B.
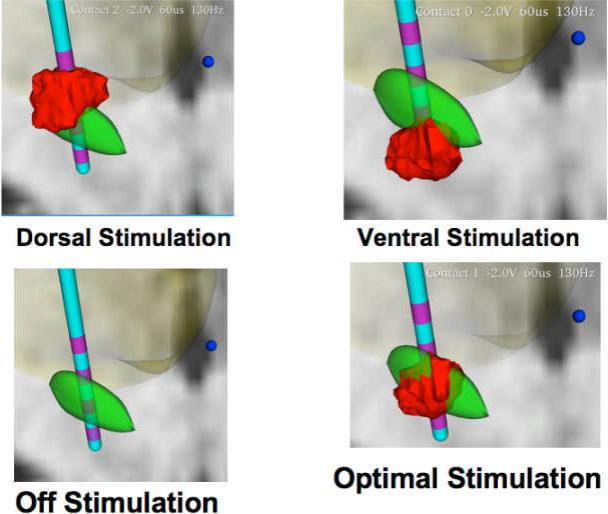
reveals four examples of field modeling of DBS during the four acute simulation conditions. The STN is shown in green, the contacts on the DBS lead in purple, and the field model in red. The top left panel reveals an example of dorsal stimulation, or stimulation one contact superior to the optimal contact utilized for DBS. The top right panel reveals an example of the stimulation field when moving the contact to a more ventral setting. The bottom left panel shows the DBS lead when the voltage is turned off. The panel on the right bottom reveals an example of stimulation at the optimal DBS contact. This figure reveals to the reader the types of changes that were made in each patient's DBS during the course of the study. Field models were generated using the Stim-Explorer software package were provided by the McIntyre laboratory (Cleveland Clinic).
Grant Support/Acknowledgements
This work was supported by a grant from NIH/NINDS K23NS044997 (PI: Okun), as well as supported by the National Parkinson Foundation UF Center of Excellence, the General Clinical Research Center (GCRC), the McKnight Brain Institute, Shands Hospital, and the UF College of Medicine. We would also like to thank the advisory support of Jerry Vitek, Mahlon DeLong, Joseph Friedman and Kenneth Heilman. We would like to thank Cameron McIntyre for help on constructing the field modeling for the figure. Finally we would like thank Elaine Whidden, ARNP and Eric Adkisson, B.S. for their assistance with data collection.
Role of the Funding Source: The NIH had an independent panel of experts review the grant prior to funding and make suggestions as to study design and procedures. The authors of this paper modified the study design and procedures prior to the onset of the trial based on the comments of the expert review panel. Appropriate revision and resubmission of protocols is a standard operating procedure for NIH funding of a clinical trial.
Footnotes
This trial was registered with NIH Clinical Trials.gov: Registration Number: NCT00360009
This submission is not under review at any other publication.
Conflict of Interest: This study was industry independent and completely funded by the NIH. Dr. Okun serves as a consultant to the National Parkinson Foundation (National Medical Director), and Dr. Foote and Dr. Okun receive honoraria for DBS fellows and for physician teaching from the Medtronic company.
References
- 1.Okun MS, Foote KD. Subthalamic nucleus vs globus pallidus interna deep brain stimulation, the rematch: will pallidal deep brain stimulation make a triumphant return? Arch Neurol. 2005;62:533–536. doi: 10.1001/archneur.62.4.533. [DOI] [PubMed] [Google Scholar]
- 2.Pahwa R, Factor SA, Lyons KE, et al. Practice Parameter: treatment of Parkinson disease with motor fluctuations and dyskinesia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;66:983–995. doi: 10.1212/01.wnl.0000215250.82576.87. [DOI] [PubMed] [Google Scholar]
- 3.Deep-brain stimulation of the subthalamic nucleus or the pars interna of the globus pallidus in Parkinson's disease. N Engl J Med. 2001;345:956–963. doi: 10.1056/NEJMoa000827. [DOI] [PubMed] [Google Scholar]
- 4.Weaver F, Follett K, Hur K, et al. Deep brain stimulation in Parkinson disease: a metaanalysis of patient outcomes. J Neurosurg. 2005;103:956–967. doi: 10.3171/jns.2005.103.6.0956. [DOI] [PubMed] [Google Scholar]
- 5.Anderson VC, Burchiel KJ, Hogarth P, et al. Pallidal vs subthalamic nucleus deep brain stimulation in Parkinson disease. Arch Neurol. 2005;62:554–560. doi: 10.1001/archneur.62.4.554. [DOI] [PubMed] [Google Scholar]
- 6.Burchiel KJ, Anderson VC, Favre J, Hammerstad JP. Comparison of pallidal and subthalamic nucleus deep brain stimulation for advanced Parkinson's disease: results of a randomized, blinded pilot study. Neurosurgery. 1999;45:1375–1382. doi: 10.1097/00006123-199912000-00024. discussion 1382−1374. [DOI] [PubMed] [Google Scholar]
- 7.Lang AE, Obeso JA. Challenges in Parkinson's disease: restoration of the nigrostriatal dopamine system is not enough. Lancet Neurol. 2004;3:309–316. doi: 10.1016/S1474-4422(04)00740-9. [DOI] [PubMed] [Google Scholar]
- 8.Lang AE, Obeso JA. Time to move beyond nigrostriatal dopamine deficiency in Parkinson's disease. Ann Neurol. 2004;55:761–765. doi: 10.1002/ana.20102. [DOI] [PubMed] [Google Scholar]
- 9.Funkiewiez A, Ardouin C, Caputo E, et al. Long term effects of bilateral subthalamic nucleus stimulation on cognitive function, mood, and behaviour in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2004;75:834–839. doi: 10.1136/jnnp.2002.009803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Funkiewiez A, Ardouin C, Cools R, et al. Effects of levodopa and subthalamic nucleus stimulation on cognitive and affective functioning in Parkinson's disease. Mov Disord. 2006;21:1656–1662. doi: 10.1002/mds.21029. [DOI] [PubMed] [Google Scholar]
- 11.Funkiewiez A, Ardouin C, Krack P, et al. Acute psychotropic effects of bilateral subthalamic nucleus stimulation and levodopa in Parkinson's disease. Mov Disord. 2003;18:524–530. doi: 10.1002/mds.10441. [DOI] [PubMed] [Google Scholar]
- 12.Pillon B, Ardouin C, Damier P, et al. Neuropsychological changes between “off” and “on” STN or GPi stimulation in Parkinson's disease. Neurology. 2000;55:411–418. doi: 10.1212/wnl.55.3.411. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez RL, Miller K, Bowers D, et al. Mood and cognitive changes with deep brain stimulation. What we know and where we should go. Minerva Med. 2005;96:125–144. [PubMed] [Google Scholar]
- 14.Rodriguez-Oroz MC, Obeso JA, Lang AE, et al. Bilateral deep brain stimulation in Parkinson's disease: a multicentre study with 4 years follow-up. Brain. 2005;128:2240–2249. doi: 10.1093/brain/awh571. [DOI] [PubMed] [Google Scholar]
- 15.Saint-Cyr JA, Trepanier LL, Kumar R, et al. Neuropsychological consequences of chronic bilateral stimulation of the subthalamic nucleus in Parkinson's disease. Brain. 2000;123(Pt 10):2091–2108. doi: 10.1093/brain/123.10.2091. [DOI] [PubMed] [Google Scholar]
- 16.Okun MS, Green J, Saben R, et al. Mood changes with deep brain stimulation of STN and GPi: results of a pilot study. J Neurol Neurosurg Psychiatry. 2003;74:1584–1586. doi: 10.1136/jnnp.74.11.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sudhyadhom A, Bova FJ, Foote KD, et al. Limbic, associative, and motor territories within the targets for deep brain stimulation: potential clinical implications. Curr Neurol Neurosci Rep. 2007;7:278–289. doi: 10.1007/s11910-007-0043-1. [DOI] [PubMed] [Google Scholar]
- 18.Yelnik J. Functional anatomy of the basal ganglia. Mov Disord. 2002;17(Suppl 3):S15–21. doi: 10.1002/mds.10138. [DOI] [PubMed] [Google Scholar]
- 19.Woods SP, Rippeth JD, Conover E, et al. Statistical power of studies examining the cognitive effects of subthalamic nucleus deep brain stimulation in Parkinson's disease. Clin Neuropsychol. 2006;20:27–38. doi: 10.1080/13854040500203290. [DOI] [PubMed] [Google Scholar]
- 20.Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ. What features improve the accuracy of clinical diagnosis in Parkinson's disease: a clinicopathologic study. Neurology. 1992;42:1142–1146. doi: 10.1212/wnl.42.6.1142. [DOI] [PubMed] [Google Scholar]
- 21.Hughes AJ, Daniel SE, Blankson S, Lees AJ. A clinicopathologic study of 100 cases of Parkinson's disease. Arch Neurol. 1993;50:140–148. doi: 10.1001/archneur.1993.00540020018011. [DOI] [PubMed] [Google Scholar]
- 22.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hutchison WD, Allan RJ, Opitz H, et al. Neurophysiological identification of the subthalamic nucleus in surgery for Parkinson's disease. Ann Neurol. 1998;44:622–628. doi: 10.1002/ana.410440407. [DOI] [PubMed] [Google Scholar]
- 24.Vitek JL, Bakay RA, Hashimoto T, et al. Microelectrode-guided pallidotomy: technical approach and its application in medically intractable Parkinson's disease. J Neurosurg. 1998;88:1027–1043. doi: 10.3171/jns.1998.88.6.1027. [DOI] [PubMed] [Google Scholar]
- 25.Starr PA. Placement of deep brain stimulators into the subthalamic nucleus or Globus pallidus internus: technical approach. Stereotact Funct Neurosurg. 2002;79:118–145. doi: 10.1159/000070828. [DOI] [PubMed] [Google Scholar]
- 26.Starr PA, Christine CW, Theodosopoulos PV, et al. Implantation of deep brain stimulators into the subthalamic nucleus: technical approach and magnetic resonance imaging-verified lead locations. J Neurosurg. 2002;97:370–387. doi: 10.3171/jns.2002.97.2.0370. [DOI] [PubMed] [Google Scholar]
- 27.Bejjani BP, Houeto JL, Hariz M, et al. Aggressive behavior induced by intraoperative stimulation in the triangle of Sano. Neurology. 2002;59:1425–1427. doi: 10.1212/01.wnl.0000031428.31861.23. [DOI] [PubMed] [Google Scholar]
- 28.Sensi M, Eleopra R, Cavallo MA, et al. Explosive-aggressive behavior related to bilateral subthalamic stimulation. Parkinsonism Relat Disord. 2004;10:247–251. doi: 10.1016/j.parkreldis.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Frank MJ, Samanta J, Moustafa AA, Sherman SJ. Hold your horses: impulsivity, deep brain stimulation, and medication in parkinsonism. Science. 2007;318:1309–1312. doi: 10.1126/science.1146157. [DOI] [PubMed] [Google Scholar]
- 30.Bejjani BP, Damier P, Arnulf I, et al. Transient acute depression induced by high-frequency deep-brain stimulation. N Engl J Med. 1999;340:1476–1480. doi: 10.1056/NEJM199905133401905. [DOI] [PubMed] [Google Scholar]
- 31.Deuschl G, Herzog J, Kleiner-Fisman G, et al. Deep brain stimulation: postoperative issues. Mov Disord. 2006;21(Suppl 14):S219–237. doi: 10.1002/mds.20957. [DOI] [PubMed] [Google Scholar]
- 32.Berney A, Panisset M, Sadikot AF, et al. Mood stability during acute stimulator challenge in Parkinson's disease patients under long-term treatment with subthalamic deep brain stimulation. Mov Disord. 2007;22:1093–1096. doi: 10.1002/mds.21245. [DOI] [PubMed] [Google Scholar]
- 33.Berney A, Vingerhoets F, Perrin A, et al. Effect on mood of subthalamic DBS for Parkinson's disease: a consecutive series of 24 patients. Neurology. 2002;59:1427–1429. doi: 10.1212/01.wnl.0000032756.14298.18. [DOI] [PubMed] [Google Scholar]
- 34.Biseul I, Sauleau P, Haegelen C, et al. Fear recognition is impaired by subthalamic nucleus stimulation in Parkinson's disease. Neuropsychologia. 2005;43:1054–1059. doi: 10.1016/j.neuropsychologia.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 35.Witt K, Daniels C, Herzog J, et al. Differential effects of L-dopa and subthalamic stimulation on depressive symptoms and hedonic tone in Parkinson's disease. J Neuropsychiatry Clin Neurosci. 2006;18:397–401. doi: 10.1176/jnp.2006.18.3.397. [DOI] [PubMed] [Google Scholar]
- 36.Rothlind JC, Cockshott RW, Starr PA, Marks WJ., Jr. Neuropsychological performance following staged bilateral pallidal or subthalamic nucleus deep brain stimulation for Parkinson's disease. J Int Neuropsychol Soc. 2007;13:68–79. doi: 10.1017/S1355617707070105. [DOI] [PubMed] [Google Scholar]
- 37.Bolla-Wilson K, Robinson RG, Starkstein SE, et al. Lateralization of dementia of depression in stroke patients. Am J Psychiatry. 1989;146:627–634. doi: 10.1176/ajp.146.5.627. [DOI] [PubMed] [Google Scholar]
- 38.Fedoroff JP, Starkstein SE, Forrester AW, et al. Depression in patients with acute traumatic brain injury. Am J Psychiatry. 1992;149:918–923. doi: 10.1176/ajp.149.7.918. [DOI] [PubMed] [Google Scholar]
- 39.Starkstein SE, Robinson RG. Cerebral lateralization in depression. Am J Psychiatry. 1986;143:1631–1632. doi: 10.1176/ajp.143.12.1631. [DOI] [PubMed] [Google Scholar]
- 40.Starkstein SE, Robinson RG. Mechanism of disinhibition after brain lesions. J Nerv Ment Dis. 1997;185:108–114. doi: 10.1097/00005053-199702000-00007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


