Abstract
Polychlorinated biphenyls (PCBs) are a family of toxicants that persist in measurable quantities in human and wildlife tissues, despite their ban in production in 1977. Some PCB mixtures can act as endocrine disrupting chemicals (EDCs) by mimicking or antagonizing the actions of hormones in the brain and periphery. When exposure to hormonally active substances such as PCBs occurs during vulnerable developmental periods, particularly prenatally or in early postnatal life, they can disrupt sex-specific patterning of the brain, inducing permanent changes that can later be manifested as improper sexual behaviors. Here, we investigated the effects of prenatal exposure to the PCB mixture Aroclor (A) 1221 on adult female reproductive behaviors in a dose-response model in the Sprague-Dawley rat. Using a paced mating paradigm that permits the female to set the timing of mating and control contact with the male during copulation, we were able to uncover significant differences in female-typical sexual activities in A1221-exposed females. Specifically, A1221 causes significant effects on mating trial pacing, vocalizations, ambulation and the female’s likelihood to mate. The results further demonstrate that the intermediate treatment group has the greatest number of disrupted endpoints, suggestive of non-linear dose responses to A1221. These data demonstrate that the behavioral phenotype in adulthood is disrupted by low, ecologically relevant exposures to PCBs, and the results have implications for reproductive success and health in wildlife and women.
Keywords: Aroclor 1221, Paced mating, PCB, Female reproductive behavior, Endocrine disruption
Polychlorinated biphenyls (PCBs) were used in industry as inflammable coolants and lubricants and as components of paints and plastics. Banned in 1977 in response to dawning public awareness of their estrogenic and potentially toxic effects on humans and wildlife, PCBs continue to leach into soil, air and groundwater via retired industrial equipment, and from old factories and buildings. PCBs may have variable degrees of impact depending on which congeners or congener mixtures are involved, the organism’s age at exposure, the sex of the individual, the degree of exposure and the availability of compensatory diet or social buffering to counteract those effects. An accurate evaluation of ecologically relevant xenobiotic exposure depends on the close examination of all PCBs at a variety of low doses (Battershill, 1994; Brouwer et al., 1999).
The neuroendocrine system serves as an interface between the central nervous system and peripheral endocrine organs, and thus represents a prime target for endocrine disruption by PCBs (Patisaul et al., 2006). PCBs and their metabolites can act at multiple nodes of the neuroendocrine axis: they may serve as hormone mimics (Connor et al., 1997), alter circulating hormone levels (Desaulniers et al., 1999), change patterns of estrous cyclicity (Meerts et al., 2004; Buitenhuis et al., 2004), disrupt hormone metabolism (Gregoraszczuk et al., 2005; Kester et al., 2000; Yamane et al., 1975), influence endocrine-related and hypothalamic gene expression (Aluru et al., 2004; Bansal et al., 2005; Colciago et al., 2005; Flouriot et al., 1995; Gore et al., 2002; Pravettoni et al., 2005; Salama et al., 2003), interfere with hormone binding proteins (Brouwer and van den Berg, 1986; Chauhan et al., 2000), alter neuronal signaling to endocrine regions of the brain (Khan and Thomas, 2001; Morse et al., 1996; Seegal et al., 1985; Seegal et al., 1990) or indirectly affect steroid receptor availability via molecular crosstalk (Brunnberg et al., 2003; Pearce et al., 2004).
The behavioral phenotype is perhaps the most sensitive and salient measure of PCB disruption of the neuroendocrine system because reproductive success hinges upon the normal complement of reproductive behaviors. Previously, PCBs and their metabolites were shown to impact neurotransmitter and steroid hormone systems underlying reproductive function (Khan and Thomas, 2001; Ptak et al., 2005; Seegal et al., 1985; Seegal et al., 2002; Tsai et al., 1997). These changes in turn are likely to have profound effects on reproductive behaviors. Moreover, the timing of exposure to PCBs is key to the severity of the reproductive phenotype. In particular, exposure during the critical period of brain sexual differentiation is potentially detrimental. This critical period in rats has been proposed to begin in the third trimester of pregnancy and end shortly after birth, from approximately embryonic day 16 to postnatal day (P) 5 in rats (Becu-Villalobos et al., 1997; Breedlove, 1992; Dohler, 1991; Rhees et al., 1990; Tobet and Fox, 1989; Wagner et al., 1998), although a revisitation of brain sensitivity to steroid hormones suggests that the critical period may last longer into postnatal life than previously thought (Primus and Kellogg, 1990; Romeo, 2003). Nevertheless, it is clear that late gestation, the time during which we administered PCBs in our current study, is an important critical window in the organization of sex-typical behaviors (Perakis and Stylianopoulou, 1986). It represents a period during which hypothalamic GnRH neurons are developing (Aubert et al., 1985), hypothalamic and preoptic area estrogen receptor alpha expression increases (Pasterkamp et al., 1996) and sexually dimorphic progesterone receptor expression is determined (Quadros et al., 2002; Chung et al., 2001; Wang et al., 2002).
In the present study, we investigated the effects of prenatal exposure to the PCB mixture Aroclor (A) 1221 on adult female reproductive behaviors, using a dose-response model encom-passing ecologically relevant exposures, and a paced mating paradigm to uncover feminine reproductive behaviors in the Sprague-Dawley rat. Our results show specific impairments in several feminine sexual behaviors that have implications for reproductive success.
Methods
Animals
All experimental procedures were performed following protocols approved by the Institutional Animal Care and Use Committee at the University of Texas at Austin. Timed pregnant Sprague-Dawley rats were purchased from the University of Texas Animal Resources Center and were housed individually under a 12:12 light cycle. Animals were fed low-phytoestrogen rat chow (Harlan Teklad Global Diet 2019) and water ad libitum. Pregnant dams were intraperitoneally injected with 0.1 ml of vehicle (dimethyl sulfoxide, DMSO) or Aroclor 1221 (Accustandard #C-221N-50MG; Lot#072-202, reconstituted in DMSO) at one of three doses (0.1, 1 or 10 mg/kg), on E16 and E18, the third trimester of pregnancy in rats. Intraperitoneal injection was chosen as a mode of administration to eliminate possible variability in gastrointestinal absorption via oral exposures, as per the experimental methods of other published studies of PCB effects (Chung and Clemens, 1999; Gillette et al., 1987; Murugesan et al., 2005).
PCBs administered to the dam are not fully transferred to offspring, either during gestation or via lactation, and amounts transferred to each pup are estimated to be approximately 500× less than maternal exposure (Takagi et al., 1986). Therefore, we estimate that our pups were exposed to a maximum of 0.2, 2 and 20 g/kg, in the range of estimated human exposures (Lackmann, 2002; Stellman et al., 1998). Impregnated dams were handled throughout the first few days of gestation to minimize stress of handling during injections, and a two-person method of injection was used to ensure accurate localization of the injection and to decrease stress. For this method, one person gently held the rat and a second person administered the drug; rats remained calm through the procedure due to extensive handling experience. Nesting materials were provided on gestational day 20, and the day of birth was recorded as P0. Pups were not handled until P1 in order to reduce stress to the dams and pups, and to avoid the possibility of interrupting parturition.
On P1, litters were culled to 4 female pups per litter (or fewer if litters had fewer females) in order to minimize inter-litter variability. Whereas culling to single-sex litters may impact the development of normal sociosexual behaviors (Moore and Morelli, 1979; Pellis and Pellis, 1997; Sharpe, 1975), dams show suckling and grooming preferences that differ depending upon sex ratio (Crews et al., 2006; Szyf et al., 2005). Rats were weaned on P22 to 3-4 littermates/cage. Daily vaginal smears were conducted to determine estrous cyclicity following puberty. The control (vehicle), 0.1 mg/kg, 1 mg/kg and 10 mg/kg A1221 treatment groups contained, respectively, 11, 11, 10 and 10 litters, and 3-4 females were used per litter for behavioral tests described below.
Paced mating chamber
Female-typical sexual behaviors were tested using a paced mating protocol (Coopersmith and Erskine, 1994; Erskine, 1985; Paredes and Vazquez, 1999). Paced mating cages were constructed of a 30-in. long×12-in. wide×17-in. tall Plexiglas aquarium fitted with a clear Plexiglas panel bisecting the cage lengthwise. Two 1.75-in. diameter openings at the base of this panel allow the female to pass between the two chambers; however, the males were too large to fit through the openings. The side of the cage that restricts the male is hereby referred to as the “mating chamber” and the other side is called the “escape chamber”.
Criteria, preparation for and analysis of trials
Paced mating experiments commenced when the females reached P50. Experimental trials were conducted starting 5 h after lights out under dim red lighting, on the evening of proestrus, a time when females are sexually receptive (Barfield and Lisk, 1970). Sexually experienced but otherwise experimentally untreated males (7-11 months) were used. Rats were habituated to the mating cage at least twice prior to the experiment for 20 min (males) or 10 min each (females). One hour prior to each experiment, activity levels (ambulation) of experimental females were tested by allowing the female to roam freely throughout the mating cage for 10 min. The baseline ambulation rate was determined by counting the number of times the female crossed the panel during the 10-min period. This pretrial ambulation value was later used to normalize the mating trial ambulation. Males were placed into the mating chamber for at least 20 min directly preceding each mating trial, after which a solid opaque divider was placed between the chambers. The experimental female was then introduced to the escape chamber, and the opaque divider was lifted to begin the trial. Paced mating parameters were scored for statistical purposes through seven intromissions (Edmonds et al., 1972; Erskine et al., 2004). This choice was made because female rats receiving between 5 and 10 intromissions are most sensitive to mating trial pacing in the establishment of pregnancy (Erskine et al., 2004). As the number of intromissions to ejaculation was variable, we chose seven as a standard number for most analyses, and it falls within this range of 5 to 10. In addition, trials were allowed to proceed to the first ejaculation so that postejaculatory behaviors could be recorded, but non-ejaculatory events following the seventh intromission were not included in analysis, or in calculating trial length. After each trial, the male and female were placed in a housing cage and allowed to continue mating overnight to reinforce the behavior for the males.
Females that did not show lordosis within 20 min in the presence of a sexually active male were permitted 3 additional mating trial opportunities that always occurred on the evening of proestrus. After four unsuccessful mating trials, females were eliminated from the experiment and were excluded from analyses. In order to eliminate any effect of the male, the same male animals were mated at different times with females from control and all three doses of PCB-treated groups. Males were given 2- to 3-day intervals between mating trials to prevent fatigue. The day following a successful mating trial, vaginal smears were conducted to determine the presence of sperm, and vaginal cytology was recorded. Females were then weighed and euthanized via decapitation.
Trials were recorded on videotape for later review by the experimenter, who was blind to the rats’ identities. Following completion of mating trial event logs, treatments were decoded and analysis was performed on the following parameters (described in Table 1): male-typical behaviors, mating trial pacing, avoidance/rejection behaviors, ambulation and receptivity. When calculating total time for the mating trial, post-ejaculatory refractory period was not included. Female vocalizations were recorded as the number of audible vocalizations per minute. When a female failed to vocalize during a mating trial, she was given a score of 0, which was factored in for statistical analysis.
Table 1.
Paced mating behaviors
| Mating trial pacing | |
| Mount-return latency | Mean time between a mount and the female’s return to the mating chamber |
| Intromission-return latency | Mean time between an intromission and the female’s return to the mating chamber |
| Post-ejaculatory-return latency | Mean time between an ejaculation and the female’s return to the mating chamber |
| Avoidance and rejection behaviors | |
| Percent time in escape chamber | Time in the escape chamber/trial length |
| Rejection quotient | (No. of lateral kicks+No. of face-to-face’s)/No. of mounts |
| Vocalizations/time | No. of audible vocalizations/trial length |
| Ambulation | |
| Pretrial activity level | No. of crossings of empty mating cage/10 min period |
| Trial activity level | No. of crossings of mating cage/trial length |
| Normalized activity levels | Trial activity level/pretrial activity level |
| Receptivity and proximity behaviors | |
| Lordosis quotient (LQ) | No. of lordosis/No. of mounts |
| Percent exits after mount | No. of times female leaves mating chamber after a mount/No. of mounts |
| Percent exits after intromission | No. of times female leaves mating chamber after an intromission/No. of intromissions |
| No. of attempts | No. of mating trial attempts |
| Male-typical behaviors | |
| Mount frequency | No. of mounts/trial length |
| Intromission frequency | No. of intromissions/trial length |
Statistics
The number of animals tested in the control, 0.1, 1 and 10 mg/kg groups were 35, 40, 32 and 35, respectively. We first determined whether each endpoint was normally or non-parametrically distributed, and then carried out the following analysis: with the statistical analysis software SAS (Littell et al., 1999), we completed a simple linear mixed model ANOVA analysis including a fixed treatment effect and a random dam (treatment) effect. In addition, we included a fixed covariate of the log transform of mating trial length to control for mating time. These models were estimated using restricted maximum likelihood and statistical significance was determined by a z-score or an F-value for the random and fixed terms, respectively. A number of the phenotypes were non-normally distributed and so were transformed using a log (1+y) transformation. For very highly skewed traits, we employed a non-parametric permutation testing procedure (Cassell, 2002). In this case, the phenotypes were randomized with respect to the experimental effects 1000 times and the analyses were completed as above. Here, the distribution of the test statistics under the null hypothesis was empirically determined with alpha set at 0.05. If significant treatment effects were observed, we used a series of post hoc tests for all combinations of treatments. Here we controlled for multiple tests with a Tukey-Kramer adjustment. For data presentation, raw data are shown, and statistically significant differences calculated as above are indicated.
Results
Mating trial pacing
Results on latencies for females to return to the mating chamber after mounts, intromissions, and ejaculations are shown in Fig. 1. Of the behaviors scored, mount-return latency (F(3, 38)=3.53, p<0.05) and the post-ejaculatory interval (F(3, 38)=3.23, p<0.05) were significantly affected by A1221 treatment (p<0.05 for both). Post hoc analyses of mount-return latency revealed that the 1 mg/kg group had a longer mount-return latency than the 0.1 mg/kg group (p<0.05). For post-ejaculatory return interval, post hoc analysis showed that the 1 mg/kg group had a longer post-ejaculatory interval than the 10 mg/kg treatment group. No groups were significantly different from control.
Fig. 1.
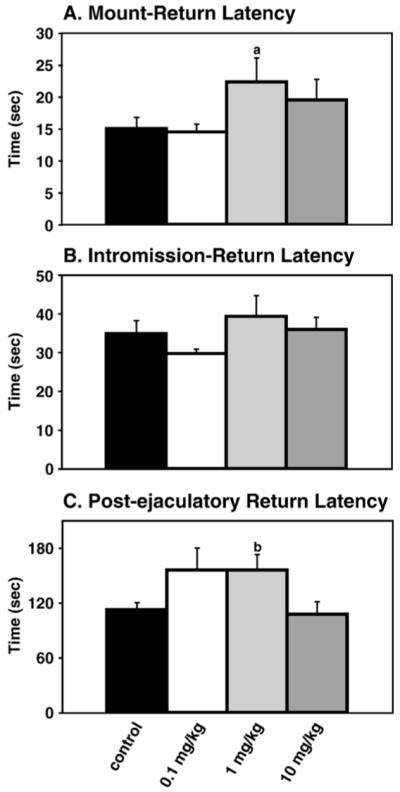
Mating trial pacing. (A) Mount-return latency: A significant effect of treatment was found (p<0.05), with significant differences between the 1 mg/kg and the 0.1 mg/kg group (p<0.05), designated by (a). (B) Intromission-return latency: No significant differences were detected. (C) Post-ejaculatory-return latency: A1221 significant altered this parameter (p<0.03), with post hoc analysis confirming significance between the 1 mg/kg and 10 mg/kg group (p<0.03), designated by (b). Data shown in this and subsequent figures are mean±SEM.
Receptivity and proximity behaviors
Neither lordosis quotient (Fig. 2A) nor the percentage of mounts and intromissions followed by the female leaving the mating chamber (percent exits after mount and percent exits after Intromission, respectively) differed between treatment groups (Figs. 2B and C). By contrast, when effects of A1221 on the number of trials required for a proestrous female to exhibit receptivity was quantified, a highly significant effect was found (F(3, 38)=4.84, p<0.005; Fig. 2D). Post hoc analyses showed that rats in the 1 mg/kg A1221 dose required significantly more trials to mate successfully compared to control rats (p<0.005). In addition, rats in both the 1 mg/kg and 10 mg/kg groups required significantly more mating trials than the 0.1 mg/kg group (p<0.01; p<0.05, respectively).
Fig. 2.
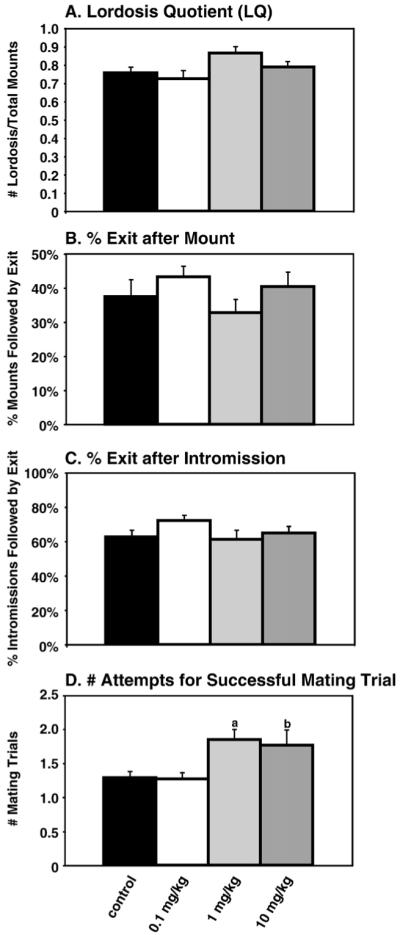
Receptivity. (A) Lordosis quotient: No significant effects were found. (B) Percent exits after mount: No differences among groups were detected. (C) Percent exits after intromission: There were no significant differences among groups. (D) Number of attempts required for a successful mating trial: There was a significant difference between groups at p<0.005. Post hoc analysis showed that the 1 mg/kg group required significantly more mating trials before being receptive to a male compared to control (p<0.005) and 0.1 mg/kg treated rats (p<0.01). These differences are designated by (a). The 10 mg/kg group also required more mating trials than the 0.1 mg/kg group (p<0.05, designated by (b)).
Avoidance/rejection behaviors
Three behaviors were evaluated as avoidance/rejection behaviors: the percent time the female spent away from the male in the escape chamber, the numbers of kicks and face-to-face behaviors and the number of vocalizations in the audible range, the latter a potential index of stress (Han et al., 2005). There were no significant differences between groups for the percentage of time in escape chamber (Fig. 3A) or for rejectionquotient (Fig. 3B). A statistically significant effect was found for female audible vocalizations (F(3, 38)=2.53, p<0.05;Fig. 3C), and post hoc analysis found that the 1 mg/kg group vocalized significantly less than either the control or the 0.1 mg/kg groups (p<0.05 for both comparisons).
Fig. 3.
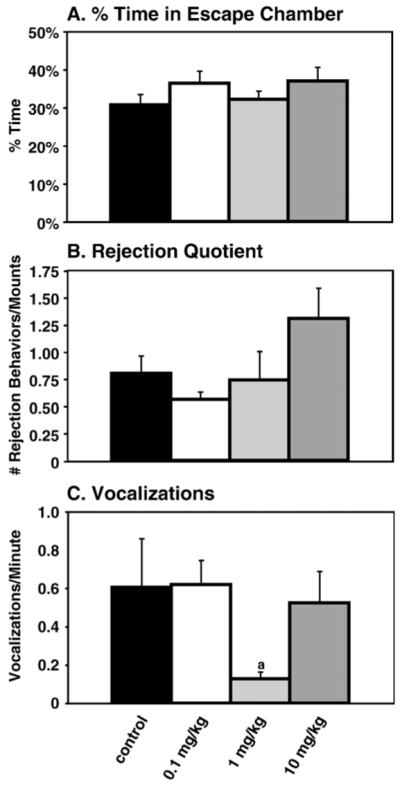
Avoidance/rejection behaviors. (A) Percent time in escape chamber: All of the treatment groups spent approximately the same percentage of the mating trial away from the male in the escape chamber. (B) Rejection quotient: The number of face-to-face and lateral kicks in a mating trial divided by total mounts, did not differ among groups. (C) Vocalizations: Statistical analyses showed an overall main effect of treatment (p<0.05). Post hoc analysis demonstrated that the 1 mg/kg had significantly fewer vocalizations than control (p<0.05) and 0.1 mg/kg (p<0.05); these differences are designated by (a).
Ambulation
Although pretrial ambulation levels did not differ significantly among groups (Fig. 4A), when mating trial ambulation was normalized to pretrial levels, a significant difference was detected (F(3, 38)=3.01, p<0.05). However, post hoc analyses found no significant interactions, although the 10 mg/kg group showed a non-significant trend for reduced activity compared to the 0.1 mg/kg group (p<0.07; Fig. 4B).
Fig. 4.
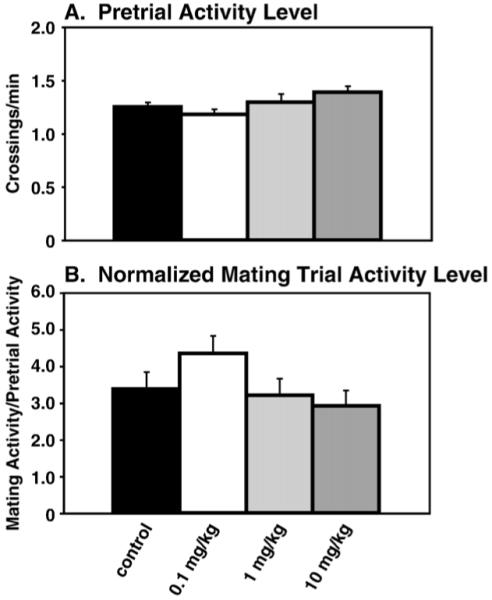
Ambulation. (A) Pretrial ambulation: Female activity levels were measured at least 1 h prior to mating trial commencement in a paced mating cage in the absence of the male. No differences were detected. (B) Normalized mating trial ambulation level: Mating trial activity was normalized to pretrial values, and a significant main effect was found (p<0.05). However, post hoc analysis found no significant differences between groups.
Male-typical behaviors
The same males were rotated through mating trials across all groups of females to minimize any effects of inter-male variability. We observed no statistically significant differences between any combination of treatment groups for mount frequency or intromission frequency of males towards females among the different treatment groups (Table 2).
Table 2.
Male-typical behaviors
| Control | 0.1 mg/kg | 1 mg/kg | 10 mg/kg | |
|---|---|---|---|---|
| Mount frequency | 1.40±0.08 | 1.39±0.13 | 1.41±0.16 | 1.28±0.13 |
| Intromission frequency | 0.68±0.05 | 0.75±0.05 | 0.80±0.09 | 0.71±0.06 |
Mount frequency and intromission frequency are shown as number of events per minute. Neither parameter varied significantly among treatment groups. Data shown are mean±SEM.
Discussion
We investigated the effects of prenatal PCB exposures on adult female sexual behaviors and observed several endpoints that were significantly altered by A1221 exposure. Overall, we observed the greatest number of effects of the intermediate(1 mg/kg) dosage of A1221, exposure to which affected adult mating trial pacing, receptivity/proximity behaviors, and audible vocalizations compared to control and lower or higher doses of A1221. This suggests the possibility of a non-linear dose response curve common to endocrine disruption studies (Calabrese and Baldwin, 2001; reviewed in Gore et al., 2006), wherein an intermediate dose level elicits the greatest effects. Thus, the paced mating paradigm reveals that small, ecologically relevant exposures at a precise developmental stage can permanently alter specific aspects of adult female-typical sexual behaviors.
In 1999 and 2001, two papers were published reporting that perinatal (embryonic day (E) 14, P0 and P10; Chung and Clemens, 1999), but not postnatal (P1-7; Chung et al., 2001) exposure of rat dams to the commercial PCB mixture A1221 alters sexual behaviors in the female offspring in adulthood. Our current study follows up upon and extends the work of Chung and Clemens in several novel ways. First, we focus on effects of prenatal A1221 administered to the dams during the third trimester of gestation to clarify the role of this developmental window on latent reproductive behaviors. Second, we use gonadally intact as opposed to ovariectomized females. Third, we use a slightly lower and broader range of exposures (0.1, 1 and 10 mg/kg) to approximate human and wildlife exposure levels and to investigate the possibility of a non-linear dose response curve (Gore et al., 2006; Weltje et al., 2005). By comparison, the dosages used by Clemens’ laboratory ranged from approximately 8 to 42 mg/kg. Fourth, and most importantly, we investigated some endpoints that, to our knowledge, have not been investigated by other laboratories. Our most robust novel findings were of significant effects of A1221 on audible vocalizations, a potential index of stress during mating, and on the number of trials required to mate successfully. These latter results and their implications are discussed in more detail below.
Properties of A1221 as an endocrine-disrupting chemical
A1221 has previously been shown to exert actions on endocrine systems. It alters numbers of estrogen receptor beta immunoreactive cell numbers in the anteroventral periventricular nucleus of the female rat brain, a region important for ovulation in rats (Salama et al., 2003). In addition, A1221 interferes with other endocrine systems and functions including, but not limited to, the thyroid neuroendocrine axis (Kilic et al., 2005); aromatase activity in vitro (Woodhouse and Cooke, 2004); it acts as an androgen receptor antagonist in vitro (Schrader and Cooke, 2003); it is estrogenic in estrogen-sensitive cell preparations (Shekhar et al., 1997); and it can inhibit fertilization of the mouse oocyte (Kholkute et al., 1994). Together, these data suggest the potential for a wide spectrum of A1221 actions on endocrine systems.
A1221 is a very lightly chlorinated PCB mixture containing primarily mono- and ortho-substituted congeners. A1221 is more volatile than more heavily chlorinated PCB mixtures and exerts more transient effects (Thomas et al., 1998), making it difficult to reliably measure in biological samples (Frame, 1997). Although to our knowledge tissue burden analysis has not been conducted specifically for the commercial mixture A1221, half-life analysis has been conducted on other lightly chlorinated PCBs, supporting a positive correlation between degree of chlorination and half-life (Matthews and Anderson, 1975), with the shortest half lives associated with the lowest chlorinated congeners, being in the range of several days (Tanabe et al., 1981). Our experimental animals were treated in utero with low doses of PCBs approximately 5 days prior to birth and were euthanized at approximately 60 days, making it highly unlikely that detectable PCBs persist. Nevertheless, our results demonstrate a long-lasting effect following developmental exposures. This is most likely due to interference in normal developmental patterning during the late embryonic sensitive period for neuroendocrine sexual differentiation.
As reported previously for A1221 and other PCBs, effects on endocrine systems are often non-linear (reviewed in Gore et al., 2006), and our results support such findings. For the two endpoints that differed between A1221 and control rats, namely, the number of opportunities required for successful mating, and audible vocalizations, we observed the greatest effect on the intermediate dose of A1221, 1 mg/kg, compared to the control group. In addition, we observed several other significant differences between treatment groups. These latter observations demonstrate how employing a range of dosages is informative in revealing complex effects of environmental endocrine-disrupting chemicals.
A1221 effects on paced mating behaviors
The paced mating paradigm enables the female rather than the male to control the pattern of mating. Not only is this paradigm considered most “rewarding” for females as measured by place preference for the location where paced mating occurred (Paredes and Vazquez, 1999), but it also increases fecundity as evidenced by the average number of pups/litter (Coopersmith and Erskine, 1994). Here, we observed several significant effects of prenatal exposure to A1221 on female mating trial pacing that varied by dosage, with the intermediate 1 mg/kg dosage of A1221 exerting the strongest effects. One of our most robust findings was that females exposed prenatally to A1221 at 1 mg/kg required a greater number of opportunities before they would mate than either the control or the 0.1 mg/kg groups. The 10 mg/kg treatment group was also affected, requiring more mating trials than the 0.1 mg/kg group to mate successfully.
Proximity (event-return) behaviors, which measure the female’s choice to remain with a male after a mating event and are revealed in the paced mating model, were significantly affected in A1221 rats, specifically for mount-return and post-ejaculatory-return latencies, which were longest in the 1 mg/kg group. Event-return latencies are believed to be a function of the intensity of vaginal stimulation by the male’s pelvis and penis (Erskine, 1992; Wersinger et al., 1993; Meredith et al., 1998), and PCBs can potentially alter vaginocervical sensation by affecting peripheral nerve conduction, as has been shown in humans (Chen et al., 1985). A longer event-return latency may thus be an indicator of altered vaginal sensitivity in the intermediate A1221 group. Our results suggest that A1221 may alter the amount of time that females choose to spend with males after a mating event. Consistent with this, other reports on PCBs have shown effects on other sensory and motor parameters in many systems, including audition (Crofton et al., 2000), sensory and motor nerve conduction velocities (Chen et al., 1985) and vision (Kremer et al., 1999).
Not all aspects of the suite of mating behaviors were significantly affected by early PCB treatment. We did not detect significant differences in lordosis quotient (LQ) among treatment groups, although there was a non-significant trend for the highest LQ in the 1 mg/kg group. Similarly, percent exits after mounts and intromissions were unaffected. These results indicate that only specific behaviors, and presumably specific neural circuits underlying these behaviors, are altered by fetal A1221 exposure.
Mating trial pacing by the female rat is directly related to successful impregnation (Erskine et al., 2004). Our finding that A1221 significantly alters specific aspects of the timing of paced mating behaviors has relevance to wildlife exposed to endocrine-disrupting chemicals, as such animals may not get a second chance to mate and reproduce in order to ensure transmission of their genes to subsequent generations.
Effects of A1221 on audible vocalizations
Rat vocalizations in the human frequency range are indicative of stress or pain, especially at increased temporal frequencies (Han et al., 2005). An unexpected outcome of our study was that the 1 mg/kg PCB group vocalized significantly less than the control and 0.1 mg/kg groups, suggestive of a decreased stress response. PCBs are associated with decreased glucocorticoid levels in rat (Durham and Brouwer, 1990), a human in situ system (Li and Wang, 2005), an avian model (American Kestrel) (Love et al., 2003), polar bears (Oskam et al., 2004) and rainbow trout (Aluru and Vijayan, 2006). Our results suggest that A1221 may be decreasing stress responsiveness in the 1 mg/kg exposed females. Interestingly, this is the same group that required more trials to successfully mate. The two apparently divergent effects of A1221 on the decreased likelihood to mate and decreased stress response of the female are likely not part of a cause-effect relationship, but rather, due to differential effects of PCBs on the hypothalamic-pituitary-gonadal and hypothalamic-pituitary-adrenal neuroendocrine axes.
A1221 effects on other behaviors
We tested ambulation in the mating cage, both prior to mating and during mating trials. This variable relates possible neurodevelopmental effects of prenatal PCB exposure on hyper/hypoactivity. Although treatment did not affect activity prior to the mating trial, activity during the trial itself was significantly different between groups; post hoc analyses did not reveal specific differences between groups, although there was a trend for increased activity in the 0.1 mg/kg group. The overall effect of treatment on activity is consistent with previous reports on PCB exposure in rhesus monkey and rats (Bowman et al., 1981; Holene et al., 1995; Kuriyama and Chahoud, 2004; Lilienthal et al., 1990). Effects of A1221 on time spent in the escape chamber did not differ among groups, nor was there any effect on rejection quotient. Thus, again, effects of A1221 appear to be specific to subsets of behaviors.
Conclusion
Studies in humans and wildlife reveal that environmental exposures to polychlorinated biphenyls continue to be ubiquitous across a broad geographical range. This study on Sprague-Dawley female rats demonstrates that exposure to low levels of A1221 during a prenatal developmental window of brain sexual differentiation is sufficient to induce latent changes in a subset of adult reproductive behaviors. Specifically, at PCB exposures approximating those of wildlife and humans, the number of trials required for successful mating was compromised. In addition, abnormal audible vocalization behavior during mating may suggest that the stress system may be affected in PCB-exposed females. These results have potential implications for reproductive disorders of women and endangered animals.
Acknowledgments
We acknowledge generous support from the PhRMA Foundation (Predoctoral Fellowship to RMS) and the NIEHS (ES12272, ES07784 to ACG). We are grateful to Drs. Marilyn McGinnis and Mary Erskine for valuable advice on the paced mating regime.
References
- Aluru N, Vijayan MM. Aryl hydrocarbon receptor activation impairs cortisol response to stress in rainbow trout by disrupting the rate-limiting steps in steroidogenesis. Endocrinology. 2006;147(4):1895–1903. doi: 10.1210/en.2005-1143. [DOI] [PubMed] [Google Scholar]
- Aluru N, Jorgensen EH, Maule AG, Vijayan MM. PCB disruption of the hypothalamus-pituitary-interrenal axis involves brain glucocorticoid receptor downregulation in anadromous Arctic charr. Am. J. Physiol., Regul. Integr. Comp. Physiol. 2004;287(4):R787–R793. doi: 10.1152/ajpregu.00091.2004. [DOI] [PubMed] [Google Scholar]
- Aubert ML, Begeot M, Winiger BP, Morel G, Sizonenko PC, Dubois PM. Ontogeny of hypothalamic luteinizing hormone-releasing hormone (GnRH) and pituitary GnRH receptors in fetal and neonatal rats. Endocrinology. 1985;116(4):1565–1576. doi: 10.1210/endo-116-4-1565. [DOI] [PubMed] [Google Scholar]
- Bansal R, You SH, Herzig CT, Zoeller RT. Maternal thyroid hormone increases HES expression in the fetal rat brain: an effect mimicked by exposure to a mixture of polychlorinated biphenyls (PCBs) Brain Res. Dev. Brain Res. 2005;156(1):13–22. doi: 10.1016/j.devbrainres.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Barfield MA, Lisk RD. Advancement of behavioral estrus by subcutaneous injection of progesterone in the 4-day cyclic rat. Endocrinology. 1970;87(5):1096–1098. doi: 10.1210/endo-87-5-1096. [DOI] [PubMed] [Google Scholar]
- Battershill JM. Review of the safety assessment of polychlorinated biphenyls (PCBs) with particular reference to reproductive toxicity. Hum. Exp. Toxicol. 1994;13(9):581–597. doi: 10.1177/096032719401300901. [DOI] [PubMed] [Google Scholar]
- Becu-Villalobos D, Gonzalez Iglesias A, Diaz-Torga G, Hockl P, Libertun C. Brain sexual differentiation and gonadotropins secretion in the rat. Cell. Mol. Neurobiol. 1997;17(6):699–715. doi: 10.1023/a:1022542221535. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Heironimus MP, Barsotti DA. Locomotor hyperactivity in PCB-exposed rhesus monkeys. Neurotoxicology. 1981;2(2):251–268. [PubMed] [Google Scholar]
- Breedlove SM. Sexual dimorphism in the vertebrate nervous system. J. Neurosci. 1992;12(11):4133–4142. doi: 10.1523/JNEUROSCI.12-11-04133.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer A, van den Berg KJ. Binding of a metabolite of 3,4,3′,4′-tetrachlorobiphenyl to transthyretin reduces serum vitamin A transport by inhibiting the formation of the protein complex carrying both retinol and thyroxin. Toxicol. Appl. Pharmacol. 1986;85(3):301–312. doi: 10.1016/0041-008x(86)90337-6. [DOI] [PubMed] [Google Scholar]
- Brouwer A, Longnecker MP, Birnbaum LS, Cogliano J, Kostyniak P, Moore J, Schantz S, Winneke G. Characterization of potential endocrine-related health effects at low-dose levels of exposure to PCBs. Environ. Health Perspect. 1999;107(Suppl 4):639–649. doi: 10.1289/ehp.99107s4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunnberg S, Pettersson K, Rydin E, Matthews J, Hanberg A, Pongratz I. The basic helix-loop-helix-PAS protein ARNT functions as a potent coactivator of estrogen receptor-dependent transcription. Proc. Natl. Acad. Sci. U. S. A. 2003;100(11):6517–6522. doi: 10.1073/pnas.1136688100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buitenhuis C, Cenijn PC, van Velzen M, Lilienthal H, Malmberg T, Bergman A, Gutleb AC, Legler J, Brouwer A. Effects of prenatal exposure to hydroxylated PCB metabolites and some brominated flame retardants on the development of rats. Organohalog. Compd. 2004;66:3586–3592. [Google Scholar]
- Calabrese EJ, Baldwin LA. U-shaped dose-responses in biology, toxicology, and public health. Annu. Rev. Public Health. 2001;22:15–33. doi: 10.1146/annurev.publhealth.22.1.15. [DOI] [PubMed] [Google Scholar]
- Cassell DL. A randomized-test wrapper for SAS PROCs. SUGI. 2002;27:1–4. [Google Scholar]
- Chauhan KR, Kodavanti PR, McKinney JD. Assessing the role of ortho-substitution on polychlorinated biphenyl binding to transthyretin, a thyroxine transport protein. Toxicol. Appl. Pharmacol. 2000;162(1):10–21. doi: 10.1006/taap.1999.8826. [DOI] [PubMed] [Google Scholar]
- Chen RC, Tang SY, Miyata H, Kashimoto T, Chang YC, Chang KJ, Tung TC. Polychlorinated biphenyl poisoning: correlation of sensory and motor nerve conduction, neurologic symptoms, and blood levels of polychlorinated biphenyls, quaterphenyls, and dibenzofurans. Environ. Res. 1985;37(2):340–348. doi: 10.1016/0013-9351(85)90114-8. [DOI] [PubMed] [Google Scholar]
- Chung YW, Clemens LG. Effects of perinatal exposure to polychlorinated biphenyls on development of female sexual behavior. Bull. Environ. Contam. Toxicol. 1999;62(6):664–670. doi: 10.1007/s001289900925. [DOI] [PubMed] [Google Scholar]
- Chung YW, Nunez AA, Clemens LG. Effects of neonatal polychlorinated biphenyl exposure on female sexual behavior. Physiol. Behav. 2001;74(3):363–370. doi: 10.1016/s0031-9384(01)00579-0. [DOI] [PubMed] [Google Scholar]
- Colciago A, Celotti F, Pravettoni A, Mornati O, Martini L, Negri-Cesi P. Dimorphic expression of testosterone metabolizing enzymes in the hypothalamic area of developing rats. Brain Res. Dev. Brain Res. 2005;155(2):107–116. doi: 10.1016/j.devbrainres.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Connor K, Ramamoorthy K, Moore M, Mustain M, Chen I, Safe S, Zacharewski T, Gillesby B, Joyeux A, Balaguer P. Hydroxylated polychlorinated biphenyls (PCBs) as estrogens and antiestrogens: structure-activity relationships. Toxicol. Appl. Pharmacol. 1997;145(1):111–123. doi: 10.1006/taap.1997.8169. [DOI] [PubMed] [Google Scholar]
- Coopersmith C, Erskine MS. Influence of paced mating and number of intromissions on fertility in the laboratory rat. J. Reprod. Fertil. 1994;102(2):451–458. doi: 10.1530/jrf.0.1020451. [DOI] [PubMed] [Google Scholar]
- Crews D, Lou W, Fleming A, Ogawa S. From gene networks underlying sex determination and gonadal differentiation to the development of neural networks regulating sociosexual behavior. Brain Res. 2006;1126(1):109–121. doi: 10.1016/j.brainres.2006.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofton KM, Kodavanti PR, Derr-Yellin EC, Casey AC, Kehn LS. PCBs, thyroid hormones, and ototoxicity in rats: cross-fostering experiments demonstrate the impact of postnatal lactation exposure. Toxicol. Sci. 2000;57(1):131–140. doi: 10.1093/toxsci/57.1.131. [DOI] [PubMed] [Google Scholar]
- Desaulniers D, Leingartner K, Wade M, Fintelman E, Yagminas A, Foster WG. Effects of acute exposure to PCBs 126 and 153 on anterior pituitary and thyroid hormones and FSH isoforms in adult Sprague Dawley male rats. Toxicol. Sci. 1999;47(2):158–169. doi: 10.1093/toxsci/47.2.158. [DOI] [PubMed] [Google Scholar]
- Dohler KD. The pre- and postnatal influence of hormones and neurotransmitters on sexual differentiation of the mammalian hypothalamus. Int. Rev. Cytol. 1991;131:1–57. doi: 10.1016/s0074-7696(08)62016-1. [DOI] [PubMed] [Google Scholar]
- Durham SK, Brouwer A. 3,4,3′,4′-Tetrachlorobiphenyl distribution and induced effects in the rat adrenal gland. Lab. Invest. 1990;62(2):232–239. [PubMed] [Google Scholar]
- Edmonds S, Zoloth SR, Adler NT. Storage of copulatory stimulation in the female rat. Physiol. Behav. 1972;8(2):161–164. doi: 10.1016/0031-9384(72)90354-x. [DOI] [PubMed] [Google Scholar]
- Erskine MS. Effects of paced coital stimulation on estrus duration in intact cycling rats and ovariectomized and ovariectomized-adrenalectomized hormone-primed rats. Behav. Neurosci. 1985;99(1):151–161. doi: 10.1037//0735-7044.99.1.151. [DOI] [PubMed] [Google Scholar]
- Erskine MS. Pelvic and pudendal nerves influence the display of paced mating behavior in response to estrogen and progesterone in the female rat. Behav. Neurosci. 1992;106(4):690–697. doi: 10.1037//0735-7044.106.4.690. [DOI] [PubMed] [Google Scholar]
- Erskine MS, Lehmann ML, Cameron NM, Polston EK. Co-regulation of female sexual behavior and pregnancy induction: an exploratory synthesis. Behav. Brain Res. 2004;153(2):295–315. doi: 10.1016/j.bbr.2004.01.026. [DOI] [PubMed] [Google Scholar]
- Flouriot G, Pakdel F, Ducouret B, Valotaire Y. Influence of xenobiotics on rainbow trout liver estrogen receptor and vitellogenin gene expression. J. Mol. Endocrinol. 1995;15(2):143–151. doi: 10.1677/jme.0.0150143. [DOI] [PubMed] [Google Scholar]
- Frame GM. A collaborative study of 209 PCB congeners and 6 Aroclors on 20 different HRGC columns. Fresenius J. Anal. Chem. 1997;357:714–722. [Google Scholar]
- Gillette DM, Corey RD, Lowenstine LJ, Shull LR. Comparative toxicology of tetrachlorobiphenyls in mink and rats. Fundam. Appl. Toxicol. 1987;8(1):15–22. doi: 10.1016/0272-0590(87)90096-0. [DOI] [PubMed] [Google Scholar]
- Gore AC, Wu TJ, Oung T, Lee JB, Woller MJ. A novel mechanism for endocrine-disrupting effects of polychlorinated biphenyls: direct effects on gonadotropin-releasing hormone neurones. J. Neuroendo-crinol. 2002;14(10):814–823. doi: 10.1046/j.1365-2826.2002.00845.x. [DOI] [PubMed] [Google Scholar]
- Gore AC, Heindel JJ, Zoeller RT. Endocrine disruption for endocrinologists (and others) Endocrinology. 2006;147(6 Suppl):S1–S3. doi: 10.1210/en.2005-1367. [DOI] [PubMed] [Google Scholar]
- Gregoraszczuk EL, Zemla M, Ptak A, Grabic R. The action of low- and high-chlorinated biphenyl mixture on prepubertal porcine ovary: steroid secretion and cells apoptosis. Endocr. Regul. 2005;39(2):33–41. [PubMed] [Google Scholar]
- Han JS, Bird GC, Li W, Jones J, Neugebauer V. Computerized analysis of audible and ultrasonic vocalizations of rats as a standardized measure of pain-related behavior. J. Neurosci. Methods. 2005;141(2):261–269. doi: 10.1016/j.jneumeth.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Holene E, Nafstad I, Skaare JU, Bernhoft A, Engen P, Sagvolden T. Behavioral effects of prenatal and postnatal exposure to individual polychlorinated biphenyl congeners in rats. Environ. Toxicol. Chem. 1995;14(6):967–976. [Google Scholar]
- Kester MH, Bulduk S, Tibboel D, Meinl W, Glatt H, Falany CN, Coughtrie MW, Bergman A, Safe SH, Kuiper GG, Schuur AG, Brouwer A, Visser TJ. Potent inhibition of estrogen sulfotransferase by hydroxylated PCB metabolites: a novel pathway explaining the estrogenic activity of PCBs. Endocrinology. 2000;141(5):1897–1900. doi: 10.1210/endo.141.5.7530. [DOI] [PubMed] [Google Scholar]
- Khan IA, Thomas P. Disruption of neuroendocrine control of luteinizing hormone secretion by Aroclor 1254 involves inhibition of hypothalamic tryptophan hydroxylase activity. Biol. Reprod. 2001;64(3):955–964. doi: 10.1095/biolreprod64.3.955. [DOI] [PubMed] [Google Scholar]
- Kholkute SD, Rodriguez J, Dukelow WR. Effects of polychlorinated biphenyls (PCBs) on in vitro fertilization in the mouse. Reprod. Toxicol. 1994;8(1):69–73. doi: 10.1016/0890-6238(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Kilic N, Sandal S, Colakoglu N, Kutlu S, Seyran A, Yilmaz B. Endocrine disruptive effects of polychlorinated biphenyls on the thyroid gland in female rats. Tohoku J. Exp. Med. 2005;206(4):327–332. doi: 10.1620/tjem.206.327. [DOI] [PubMed] [Google Scholar]
- Kremer H, Lilienthal H, Hany J, Roth-Harer A, Winneke G. Sex-dependent effects of maternal PCB exposure on the electroretinogram in adult rats. Neurotoxicol. Teratol. 1999;21(1):13–19. doi: 10.1016/s0892-0362(98)00030-0. [DOI] [PubMed] [Google Scholar]
- Kuriyama SN, Chahoud I. In utero exposure to low-dose 2,3′,4,4′,5-pentachlorobiphenyl (PCB 118) impairs male fertility and alters neurobehavior in rat offspring. Toxicology. 2004;202(3):185–197. doi: 10.1016/j.tox.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Lackmann GM. Polychlorinated biphenyls and hexachlorobenzene in full-term neonates. Reference values updated. Biol. Neonate. 2002;81(2):82–85. doi: 10.1159/000047188. [DOI] [PubMed] [Google Scholar]
- Li LA, Wang PW. PCB126 induces differential changes in androgen, cortisol, and aldosterone biosynthesis in human adrenocortical H295R cells. Toxicol. Sci. 2005;85(1):530–540. doi: 10.1093/toxsci/kfi105. [DOI] [PubMed] [Google Scholar]
- Lilienthal H, Neuf M, Munoz C, Winneke G. Behavioral effects of pre- and postnatal exposure to a mixture of low chlorinated PCBs in rats. Fundam. Appl. Toxicol. 1990;15(3):457–467. doi: 10.1016/0272-0590(90)90032-f. [DOI] [PubMed] [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS Systems for Mixed Models. SAS Institute Inc.; Cary, NC.: 1999. [Google Scholar]
- Love OP, Shutt LJ, Silfies JS, Bortolotti GR, Smits JE, Bird DM. Effects of dietary PCB exposure on adrenocortical function in captive American kestrels (Falco sparverius) Ecotoxicology. 2003;12(14):199–208. doi: 10.1023/a:1022502826800. [DOI] [PubMed] [Google Scholar]
- Matthews H, Anderson M. Effect of chlorination on the distribution and excretion of polychlorinated biphenyls. Metab. Dispos. 1975;3(5):371–380. [PubMed] [Google Scholar]
- Meerts HA, Lilienthal H, Hoving S, vand den Berh JH, Weijers BM, Bergman A, Koeman JH, Brouwer A. Developmental exposure to 4-hydroxy-2,3,3′,4′,5-pentachlorobiphenyl (4-OH-CB107): Long-term effects on brain development, behavior, and brain stem auditory evoked potential in rats. Toxciol. Sci. 2004;82:207–218. doi: 10.1093/toxsci/kfh252. [DOI] [PubMed] [Google Scholar]
- Meredith JM, Moffatt CA, Auger AP, Snyder GL, Greengard P, Blaustein JD. Mating-related stimulation induces phosphorylation of dopamine- and cyclic AMP-regulated phosphoprotein-32 in progestin receptor-containing areas in the female rat brain. J. Neurosci. 1998;18(23):10189–10195. doi: 10.1523/JNEUROSCI.18-23-10189.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C, Morelli G. Mother rats interact differently with male and female offspring. J. Comp. Physiol. Pychol. 1979;93(4):677–684. doi: 10.1037/h0077599. [DOI] [PubMed] [Google Scholar]
- Morse DC, Seegal RF, Borsch KO, Brouwer A. Long-term alterations in regional brain serotonin metabolism following maternal polychlorinated biphenyl exposure in the rat. Neurotoxicology. 1996;17(34):631–638. [PubMed] [Google Scholar]
- Murugesan P, Kanagaraj P, Yuvaraj S, Balasubramanian K, Aruldhas MM, Arunakaran J. The inhibitory effects of polychlorinated biphenyl Aroclor 1254 on Leydig cell LH receptors, steroidogenic enzymes and antioxidant enzymes in adult rats. Reprod. Toxicol. 2005;20(1):117–126. doi: 10.1016/j.reprotox.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Oskam I, Ropstad E, Lie E, Derocher A, Wiig O, Dahl E, Larsen S, Skaare JU. Organochlorines affect the steroid hormone cortisol in free-ranging polar bears (Ursus maritimus) at Svalbard, Norway. J. Toxicol. Environ. Health, A. 2004;67(12):959–977. doi: 10.1080/15287390490443731. [DOI] [PubMed] [Google Scholar]
- Paredes RG, Vazquez B. What do female rats like about sex? Paced mating. Behav. Brain Res. 1999;105(1):117–127. doi: 10.1016/s0166-4328(99)00087-x. [DOI] [PubMed] [Google Scholar]
- Pasterkamp RJ, Yuri K, Visser DT, Hayashi S, Kawata M. The perinatal ontogeny of estrogen receptor-immunoreactivity in the developing male and female rat hypothalamus. Brain Res. Dev. Brain Res. 1996;91(2):300–303. doi: 10.1016/0165-3806(95)00185-9. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Fortino AE, Polston EK. Neonatal genistein or bisphenol-A exposure alters sexual differentiation of the AVPV. Neurotoxicol. Teratol. 2006;28:111–118. doi: 10.1016/j.ntt.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Pearce ST, Liu H, Radhakrishnan I, Abdelrahim M, Safe S, Jordan VC. Interaction of the aryl hydrocarbon receptor ligand 6-methyl-1,3,8-trichlorodibenzofuran with estrogen receptor alpha. Cancer Res. 2004;64(8):2889–2897. doi: 10.1158/0008-5472.can-03-1770. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Pellis VC. The prejuvenile onset of play fighting in laboratory rats (Rattus norvegicus) Dev. Psychobiol. 1997;31(3):193–205. [PubMed] [Google Scholar]
- Perakis A, Stylianopoulou F. Effects of a prenatal androgen peak on rat brain sexual differentiation. J. Endocrinol. 1986;108(2):281–285. doi: 10.1677/joe.0.1080281. [DOI] [PubMed] [Google Scholar]
- Pravettoni A, Colciago A, Negri-Cesi P, Villa S, Celotti F. Ontogenetic development, sexual differentiation, and effects of Aroclor 1254 exposure on expression of the arylhydrocarbon receptor and of the arylhydrocarbon receptor nuclear translocator in the rat hypothalamus. Reprod. Toxicol. 2005;20(4):521–530. doi: 10.1016/j.reprotox.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Primus RJ, Kellogg CK. Gonadal hormones during puberty organize environment-related social interaction in the male rat. Horm. Behav. 1990;24(3):311–323. doi: 10.1016/0018-506x(90)90012-m. [DOI] [PubMed] [Google Scholar]
- Ptak A, Ludewig G, Lehmler HJ, Wojtowicz AK, Robertson LW, Gregoraszczuk EL. Comparison of the actions of 4-chlorobiphenyl and its hydroxylated metabolites on estradiol secretion by ovarian follicles in primary cells in culture. Reprod. Toxicol. 2005;20(1):57–64. doi: 10.1016/j.reprotox.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Quadros PS, Pfau JL, Goldstein AY, De Vries GJ, Wagner CK. Sex differences in progesterone receptor expression: a potential mechanism for estradiol-mediated sexual differentiation. Endocrinology. 2002;143(10):3727–3739. doi: 10.1210/en.2002-211438. [DOI] [PubMed] [Google Scholar]
- Rhees RW, Shryne JE, Gorski RA. Onset of the hormone-sensitive perinatal period for sexual differentiation of the sexually dimorphic nucleus of the preoptic area in female rats. J. Neurobiol. 1990;21(5):781–786. doi: 10.1002/neu.480210511. [DOI] [PubMed] [Google Scholar]
- Romeo RD. Puberty: a period of both organizational and activational effects of steroid hormones on neurobehavioural development. J. Neuroendocrinol. 2003;15(12):1185–1192. doi: 10.1111/j.1365-2826.2003.01106.x. [DOI] [PubMed] [Google Scholar]
- Salama J, Chakraborty TR, Ng L, Gore AC. Effects of polychlorinated biphenyls on estrogen receptor-beta expression in the anteroventral periventricular nucleus. Environ. Health Perspect. 2003;111(10):1278–1282. doi: 10.1289/ehp.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader TJ, Cooke GM. Effects of Aroclors and individual PCB congeners on activation of the human androgen receptor in vitro. Reprod. Toxicol. 2003;17(1):15–23. doi: 10.1016/s0890-6238(02)00076-x. [DOI] [PubMed] [Google Scholar]
- Seegal RF, Bush B, Brosch KO. Polychlorinated biphenyls induce regional changes in brain norepinephrine concentrations in adult rats. Neurotoxicology. 1985;6(3):13–23. [PubMed] [Google Scholar]
- Seegal RF, Bush B, Shain W. Lightly chlorinated ortho-substituted PCB congeners decrease dopamine in nonhuman primate brain and in tissue culture. Toxicol. Appl. Pharmacol. 1990;106(1):136–144. doi: 10.1016/0041-008x(90)90113-9. [DOI] [PubMed] [Google Scholar]
- Seegal RF, Okoniewski RJ, Brosch KO, Bemis JC. Polychlorinated biphenyls alter extraneuronal but not tissue dopamine concentrations in adult rat striatum: an in vivo microdialysis study. Environ. Health Perspect. 2002;110(11):1113–1117. doi: 10.1289/ehp.021101113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe RM. The influence of the sex of litter-mates on subsequent maternal behaviour in Rattus norvegicus. Anim. Behav. 1975;23(3):551–559. doi: 10.1016/0003-3472(75)90132-3. [DOI] [PubMed] [Google Scholar]
- Shekhar PV, Werdell J, Basrur VS. Environmental estrogen stimulation of growth and estrogen receptor function in preneoplastic and cancerous human breast cell lines. J. Natl. Cancer Inst. 1997;89(23):1774–1782. doi: 10.1093/jnci/89.23.1774. [DOI] [PubMed] [Google Scholar]
- Stellman SD, Djordjevic MV, Muscat JE, Gong L, Bernstein D, Citron ML, White A, Kemeny M, Busch E, Nafziger AN. Relative abundance of organochlorine pesticides and polychlorinated biphenyls in adipose tissue and serum of women in Long Island, New York. Cancer Epidemiol., Biomarkers Prev. 1998;7(6):489–496. [PubMed] [Google Scholar]
- Szyf M, Weaver IC, Champagne FA, Diorio J, Meaney MJ. Maternal programming of steroid receptor expression and phenotype through DNA methylation in the rat. Front. Neuroendocrinol. 2005;26(34):139–162. doi: 10.1016/j.yfrne.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Takagi Y, Aburada S, Hashimoto K, Kitaura T. Transfer and distribution of accumulated (14C)polychlorinated biphenyls from maternal to fetal and suckling rats. Arch. Environ. Contam. Toxicol. 1986;15(6):709–715. doi: 10.1007/BF01054917. [DOI] [PubMed] [Google Scholar]
- Tanabe S, Nakagawa Y, Tatsukawa R. Absorption efficiency and biological half-life of individual chlorobiphenyls in rats treated with kanechlor products. Agric. Biol. Chem. 1981;45(3):717–726. [Google Scholar]
- Thomas G, Sweetman AJ, Ockenden WA, Mackay D, Jones KC. Air-pasture transfer of PCBs. Environ. Sci. Technol. 1998;32:936–942. [Google Scholar]
- Tobet SA, Fox TO. Sex- and hormone-dependent antigen immunoreactivity in developing rat hypothalamus. Proc. Natl. Acad. Sci. U. S. A. 1989;86(1):382–386. doi: 10.1073/pnas.86.1.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai ML, Webb RC, Loch-Caruso R. Increase of oxytocin-induced oscillatory contractions by 4-hydroxy-2′,4′,6′-trichlorobiphenyl is estrogen receptor mediated. Biol. Reprod. 1997;56(2):341–347. doi: 10.1095/biolreprod56.2.341. [DOI] [PubMed] [Google Scholar]
- Wagner CK, Nakayama AY, De Vries GJ. Potential role of maternal progesterone in the sexual differentiation of the brain. Endocrinology. 1998;139(8):3658–3661. doi: 10.1210/endo.139.8.6223. [DOI] [PubMed] [Google Scholar]
- Wang XQ, Fang J, Nunez AA, Clemens LG. Developmental exposure to polychlorinated biphenyls affects sexual behavior of rats. Physiol. Behav. 2002;75(5):689–696. doi: 10.1016/s0031-9384(02)00673-x. [DOI] [PubMed] [Google Scholar]
- Weltje L, vom Saal FS, Oehlmann J. Reproductive stimulation by low doses of xenoestrogens contrasts with the view of hormesis as an adaptive response. Hum. Exp. Toxicol. 2005;24(9):431–437. doi: 10.1191/0960327105ht551oa. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Baum MJ, Erskine MS. Mating-induced FOS-like immunoreactivity in the rat forebrain: a sex comparison and a dimorphic effect of pelvic nerve transection. J. Neuroendocrinol. 1993;5(5):557–568. doi: 10.1111/j.1365-2826.1993.tb00522.x. [DOI] [PubMed] [Google Scholar]
- Woodhouse AJ, Cooke GM. Suppression of aromatase activity in vitro by PCBs 28 and 105 and Aroclor 1221. Toxicol. Lett. 2004;152(1):91–100. doi: 10.1016/j.toxlet.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Yamane T, Fukuda N, Inaba J, Nishimura Y. Effect of polychlorinated biphenyls (PCB) on metabolism of thyroid hormone in Wistar rats. Nippon Eiseigaku Zasshi. 1975;30(4):497–502. doi: 10.1265/jjh.30.497. [DOI] [PubMed] [Google Scholar]


