Abstract
Phosphatidylinositol 4,5-bisphosphate (PIP2) is a minority phospholipid of the inner leaflet of plasma membranes. Many plasma membrane ion channels and ion transporters require PIP2 to function and can be turned off by signaling pathways that deplete PIP2. This review discusses the dependence of ion channels on phosphoinositides and considers possible mechanisms by which PIP2 and analogues regulate ion channel activity.
Keywords: Ion channel, phosphoinositides, PLC, lipid kinase, potassium channel, TRP channel, KCNQ, PIP3
INTRODUCTION TO PHOSPHOINOSITIDES
Phosphoinositides are acidic phospholipids of cell membranes with myo-inositol in the head group. The parent compound phosphatidylinositol (PI) can become phosphorylated on the 3, 4, and 5 positions of the inositol ring in every combination, giving rise to the seven low-abundance poly-phosphoinositides. These lipids, found primarily in the cytoplasmic leaflet, mark the identity of specific subcellular membrane compartments, serve as membrane recognition sites for specific cytoplasmic proteins, and act as membrane-delimited second messengers modulating the activities of some membrane proteins. The latter function is the focus here. We consider the question, how does membrane phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2, commonly called PIP2) bind to and activate many ion channels.
PIP2 is found principally in the plasma membrane. Although it is the most abundant poly-phosphoinositide there, it is still only 1% of the acidic lipid in the whole cell (27, 52, 91). The total PIP2 would be equivalent to a 4–10 μM solution if dissolved in cytoplasm and to 5,000–10,000 molecules μm−2 of plasma membrane. PIP2 has three phosphate groups, one of which is in a phosphodiester linkage, and a net charge near −4 at neutral pH (52). Starting in the late 1970s, PIP2 received a lot of attention as the substrate for cleavage by the enzyme phospholipase C (PLC), a reaction that produces the two classical second messengers, soluble inositol 1,4,5-trisphosphate (IP3) and membrane-delimited diacylglycerol (DAG). In turn, IP3 and DAG were discovered to release Ca2+ from intracellular stores and to recruit and activate protein kinase C (PKC), respectively, and thus the cleavage products of PIP2 are components of two major signaling pathways. Only in the 1990s was a signaling role for PIP2 itself and for the other phosphoinositides recognized. The feature we will emphasize here is that intact PIP2 is needed for the operation of certain ion channels and transporters in the plasma membrane, and temporary depletion of PIP2 during signaling by PLC transiently shuts down these functions. For example, PIP2 is clearly known to regulate the activity of inwardly rectifying K+ (Kir) channels, KCNQ channels, transient receptor potential (TRP) channels, and ion transporters such as the Na+-Ca2+ exchanger. In a number of these cases the PIP2 sensitivity is established but the physiological relevance is still under investigation. More widely in cell biology, PIP2 also serves as a targeting anchor for proteins that catalyze endocytosis and exocytosis, for small molecular weight GTPases, and for components of the actin cytoskeleton.
DEMONSTRATING PIP2 DEPENDENCE OF MEMBRANE PROTEINS
The first paper in this field, Hilgemann and Ball (28) concluded correctly that the plasma membrane Na+-Ca2+ exchanger and the KATP channels of guinea pig cardiac myocytes need PIP2 to function. The function of these proteins was monitored by the ionic currents they generated in excised giant inside-out membrane patches. The basic observation was that the currents ran down (decayed) strongly within a minute after excision of the patch but could be restored by application of Mg/ATP to the cytoplasmic face of the membrane. Currents could not be restored by Mg/ATP if a phosphoinositide-specific PLC enzyme had been applied to the membrane to hydrolyze phosphoinositides generally, but even then function returned if PIP2-containing vesicles were applied. The deduction was that rundown is caused by dephosphorylation of the PIP2 in the inner leaflet of the membrane (by lipid phosphatases) in the absence of normal cytoplasm, and that Mg/ATP is a missing ingredient to fuel lipid kinases that continually remake the PIP2 by phosphorylating the inositol ring. Polyvalent cations like penta-lysine and Al3+ that were expected to form complexes with PIP2 in the membrane also reduced the currents.
This study was soon confirmed in other laboratories and set the tone for the field. The function of a membrane protein would be monitored electrically while still embedded in the bilayer and treatments are applied to an excised patch or within the whole cell that increase or decrease the amount of available PIP2. Many common manipulations are outlined in Table 1. Most do not need further explanation. Some take advantage of the ease with which solutions can be applied to the cytoplasmic side of an inside-out patch, and some overcome the diminished and slower access one has with whole-cell recording in intact cells. Shorter-chain PIP2 analogs such as dioctanoyl PIP2 (diC8 PIP2) are convenient because they are more water soluble and much easier to wash out of membranes. The lipid kinase inhibitors wortmannin and phenylarsine oxide and the dimerizer rapamycin are highly membrane permeant so they can be applied from outside.
We now mention some problems with these approaches and some quantitative considerations. Most methods manipulate PIP2 down or up and look for predicted changes of ionic currents. The more different ways you can change the PIP2, the stronger the evidence becomes. However, these methods suffer from being indirect. None of them asks if the change in current results from binding or unbinding of PIP2 from the channel itself. Does PIP2 act on other proteins that signal to the channels? Does the manipulation initiate other intracellular signals that mediate the change in current? For example, with activation of PLC are there Ca2+ rises or actions of downstream products of the phosphoinositides that are doing the job or that are co-activators of the overall signal? Some of these objections are answered by comparing manipulations that do and don’t give rise to specific ancillary signals. The experiments with isolated patches might seem especially direct, but it is well known that excised patches include a significant sample of cytoplasm and organelles that might support local cascades of signals involving enzymes and small-molecule intermediates (16). We require new in vitro approaches with purified components. Direct measures of binding can use immunoprecipitation and Western blot (34), pulldown with beads (32), surface plasmon resonance (30), binding to immobilized lipids (24, 36). A procedural difficulty in these assays is to use an appropriate protein. Channels are not soluble proteins, so one might have to make fusion proteins with shorter pieces of the channel. However, as we discuss in the next section, “binding sites” may result from folding together widely separated residues in a way that is hard to reproduce with a fragment from one part of the protein.
Another approach, also indirect, is to mutate residues that might interact with PIP2 and to show that the effects of PIP2 are altered. Positively charged (basic) arginines, lysines, and potentially histidines are candidates for electrostatic interaction with the multiple negatively charged phosphates of PIP2 or other phosphoinositides. Indeed such mutations, described later, do reduce apparent PIP2 affinity strongly in several ion channels. Often it is tempting to then declare that this residue is the binding site for the lipid. Formally, this is subject to the general criticism that perhaps the mutation initiates a conformational change that allosterically alters some PIP2 binding site elsewhere. But more likely, this residue is only part of a greater story. The salient features of the PIP2 head group can be recognized only by having 5–10 atomic contacts with the protein. Each basic residue can contribute only one or two of several interactions that account for the total negative free energy of binding. The energies sum, and the affinities change exponentially. If each contact contributed, for example, −2.3 kT to the attraction energy, removing any one of them would decrease the affinity 10-fold. That might make the mutated residue seem uniquely important, but it is possible that mutations of other contacting residues would give an identical result once they are identified.
Whether a reduction or increase in PIP2 affinity will have much functional effect depends on the relative affinity of the protein for PIP2. When the dissociation constant for PIP2 is below physiological ambient PIP2 concentrations, binding will be saturated and some loss of affinity may have little effect. Such proteins are said to have a high affinity for PIP2. When the dissociation constant for PIP2 is above physiological PIP2 concentrations, binding is only partial and some loss of affinity will have a large effect. Such proteins are said to have a low affinity for PIP2. Further, the channels with low apparent affinity for PIP2 will be those that would be most susceptible to regulation by physiological changes of PIP2. The apparent affinity of ion channels is conveniently measured in excised patches by constructing a dose-response curve measuring the size of current at different concentrations of soluble diC8 PIP2. This is a good measure for comparisons, although the result might depend on other factors such as the concentration of endogenous full-length PIP2. Another commonly used measure of affinity uses the time taken for inhibition or recovery. The reasoning goes as follows: During manipulations that deplete or sequester PIP2, low-affinity proteins will lose their PIP2 before high-affinity proteins do, and after elevation of PIP2, high-affinity proteins will be the first to recover PIP2. In such experiments, significant decreases in apparent affinity are observed when relevant basic residues of the protein are mutated to neutral amino acids (see later), and the results usually agree with those from dose-response measurements. This approach would be theoretically clearer if one could use a reproducible slow ramp of decrease and increase of PIP2 to try to remain near equilibrium in the binding process. In practice, the change is probably fairly quick, and during the decrease the result could be dominated by the dissociation rate of the PIP2 complex and during the increase it could be dominated by the association rate.
STRUCTURAL BASIS FOR RECOGNITION OF PIP2 BY PROTEINS
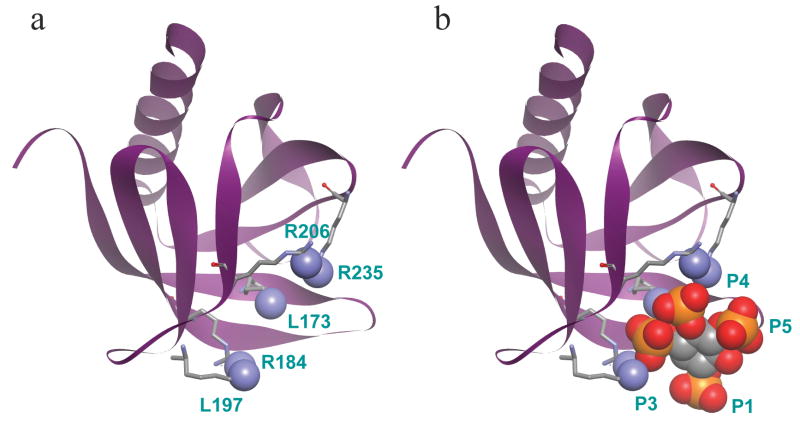
What might phosphoinositide binding sites in proteins look like? Fortunately several have been described. We begin with pleckstrin homology domains (PH domains) as informative examples. PH domains comprise a fold about 100 residues long found in at least 250 human proteins--typically cytoplasmic proteins. Their sequences are quite divergent, but a number of them present basic amino acids in a pocket that binds the phosphorylated head groups of poly-phosphoinositides electrostatically with a specificity that depends on the PH domain. Several other consensus protein domains also form phosphoinositide binding sites in cytoplasmic proteins (38). Because of such domains, the specific phosphoinositide composition of different subcellular membranes dynamically determines which cytoplasmic proteins are attracted to that membrane. A few PH domains have been co-crystallized with acidic head-group surrogates such as free inositol polyphosphates so we can see their atomic interactions. Figure 1 shows two views of the structure of the PH domain of an adapter protein DAPP1 (dual adaptor for phosphotyrosine and 3-phosphoinositides 1) co-crystallized with I(1,3,4,5)P4, the head group of PI(3,4,5)P3 (19). The protein forms a seven-stranded β sandwich and an α-helix. Five basic residues that make direct contact with the bound I(1,3,4,5)P4, are shown with their contacting nitrogen atoms filled in as blue spheres (Figure 1a). Several other uncharged residues contribute additional specific hydrogen-bonding interactions that are not shown. The DAPP1 protein binds PI(3,4,5)P3 and PI(3,4)P2 equally and hardly binds PI(4,5)P2, a preference that can be explained by the presence of basic residues in the positions that contact the 3 and the 4 phosphates on the inositol ring but not the 1 or 5 phosphates (Figure 1b). Other PH domains that place basic groups in different patterns have different specificities (19).
Figure 1.
Crystal structure of the PH domain of DAPP1 (residues 162–261) bound to I(1,3,4,5)P4. a, The protein component alone, with the main chain drawn as a ribbon and five basic residues drawn in stick form. Their contacting nitrogens are drawn as CPK balls. b, The full complex with I(1,3,4,5)P4 represented in space-filling CPK form. The 1, 3, 4, and 5 phosphates are labeled. Colors: Blue nitrogen, red oxygen, orange phosphorus. Coordinates from (19).
Two points can be made from this discussion. (i) In the DAPP1 PH domain the cationic residues Lys173, Arg184, Lys197, Arg206, and Arg235 are brought together in space by the protein fold and are not adjacent in the sequence (Figure 2). A similar conclusion is drawn for other PH domains like that of the commonly used PIP2-specifec PH domain of PLCδ1 (Figure 2; see 20) as well as for PX, FYVE, and ENTH domains that bind poly-phosphoinositides in a similar specific 1:1 fashion (38). The relevant basic residues may be widely spaced in the sequence but they fold together in space in an organized manner. (ii) For these domains, the strength and specificity of binding depend on numerous directed electrostatic and other interactions contributing to the total interaction energy, and small variations of the position and nature of basic residues in the sequence and of the fold would make a significant change. Nevertheless, at present it is a challenge to predict lipid specificity a priori from such protein sequences.
Figure 2.
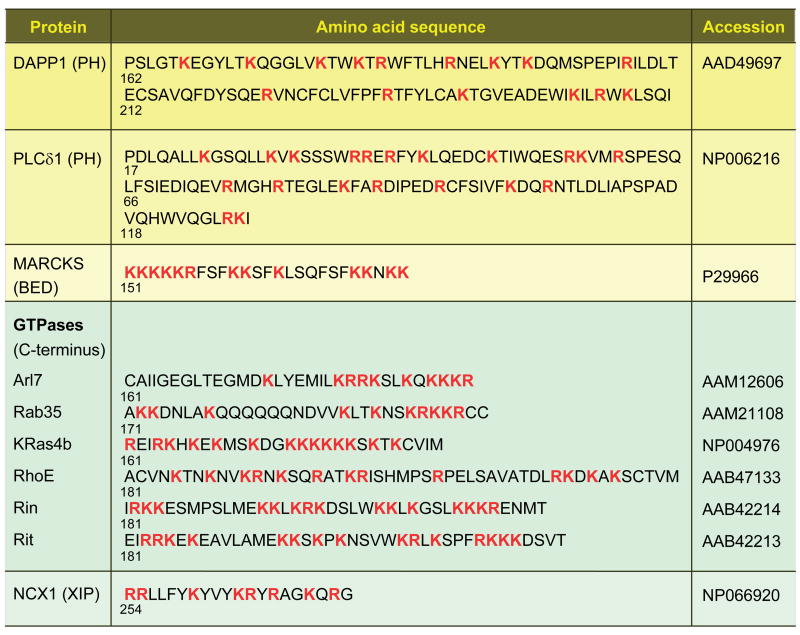
Amino acid sequences of some putative phosphoinositide-binding domains. All basic residues are bold and red. Shown are the PH domains of DAPP1 and PLCδ1, the BED domain of MARCKS; the C-terminus of several GTPases, and the XIP domain of the Na+-Ca2+ exchanger (NCX1). Numbers below each sequence indicate the initial residue number.
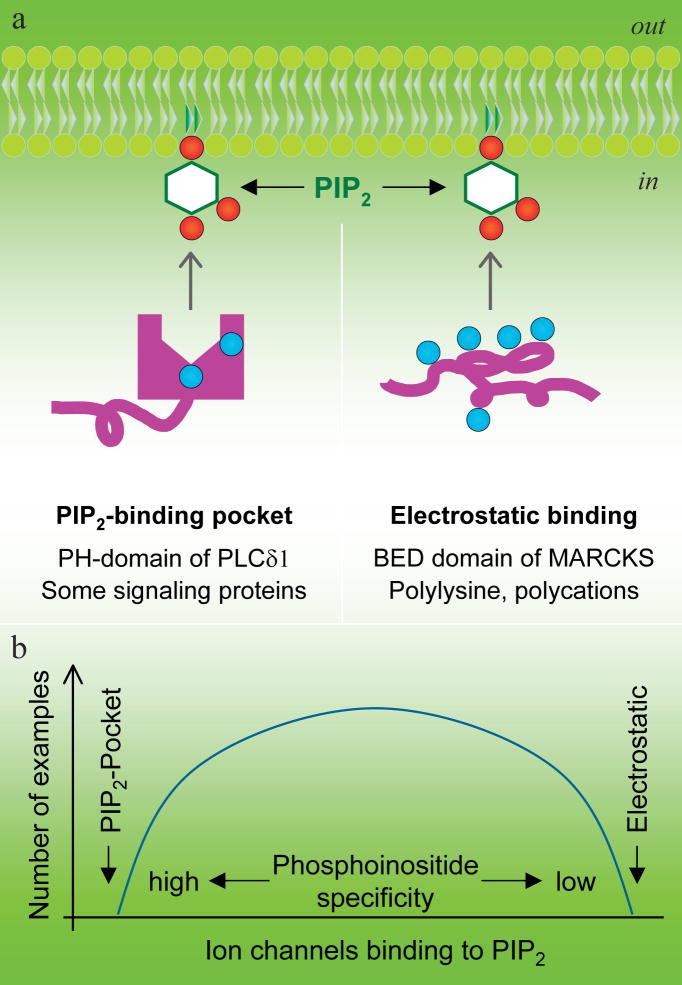
A quite different model is usually used in discussions of the PIP2-sequestering molecule MARCKS (myristoylated alanine-rich C kinase substrate). This 331-residue protein is predominantly quite acidic except in the region from residues 151–175 where there are 13 clustered basic residues (Figure 2). That region is variously called the “phosphorylation-site domain” because of three serine residues that are targets of PKC or the “basic effector domain” (BED) because it is the target site of PKC phosphorylation and of calmodulin binding and it sequesters PIP2. MARCKS is a kind of PIP2 buffer. The protein and its basic effector domain are localized to the plasma membrane by three factors: myristoylation provides an N-terminal lipid anchor, the local negative surface potential of the bilayer cytoplasmic leaflet attracts the basic residues of the effector domain, and potentially, the hydrophobic bilayer attracts five hydrophobic phenylalanine residues also clustered in the effector domain. When not phosphorylated or interacting with calmodulin, the effector domain is capable of binding the acidic head groups of several PIP2 molecules electrostatically, sequestering them laterally from the rest of the bilayer (87). When phosphorylated or interacting with calmodulin (92), MARCKS may release PIP2 molecules so they can move about in the bilayer and interact with other proteins. MARCKS is considered natively unfolded (83) and the effector peptide is called unstructured, although its shape is well resolved when co-crystallized with calmodulin (92). The operating concept is that the basic residues are already bunched together by sequence and do not present a structurally selective binding site. Rather they form a strong local positive cloud of electrostatic potential that would attract any acidic lipid, strongly favoring those with multiple negative charges. PIP 2 would be the principal target because it is the most abundant multiply-phosphorylated lipid of the plasma membrane.
We have presented two extreme models, one of a carefully constructed 3-dimensional binding pocket that binds phosphoinositides selectively and another of a cloud of positivity that attracts polyanionic molecules with little selectivity (Figure 3a). Probably each is an exaggeration, an extreme view not accurately realized by any protein, and real proteins fall between. For example, somewhat more in the MARCKS model than in the PH domain model, many small molecular weight GTPases of the Ras superfamily have clusters of basic residues in the C-terminus, but there are also short gaps between them (Figure 2). A number of these GTPases, some of which are also C-terminally geranylated, show plasma membrane localization and are attracted to poly-phosphoinositides. This localization is essential for their cell biological function (13). The GTPases leave the plasma membrane when PIP2 is hydrolyzed--or if some of their basic or hydrophobic residues are mutated (26). In some few tested examples, PI(3,4,5)P3 can be as effective as PIP2. Other GTPases target other cellular membranes containing different phosphoinositides. The Na+-Ca2+ exchanger is believed to acquire its PIP2 dependence from a polybasic exchange-inhibitory peptide (XIP) domain of the large third intracellular loop (Figure 2), which has 8 basic residues in a 20-residue sequence (24).
Figure 3.
Two extreme models for selective versus diffuse binding of poly-phosphoinositides. a, Specific PIP2 interaction in a structured PIP2 binding pocket structure and nonselective electrostatic PIP2 attraction by polybasic peptides or polycations. b, A hypothesis suggesting a correlation between strong PIP2 binding and high PIP2 selectivity. The curve representing the number of channels in each category is completely hypothetical.
MODULATION OF ION CHANNELS BY PIP2
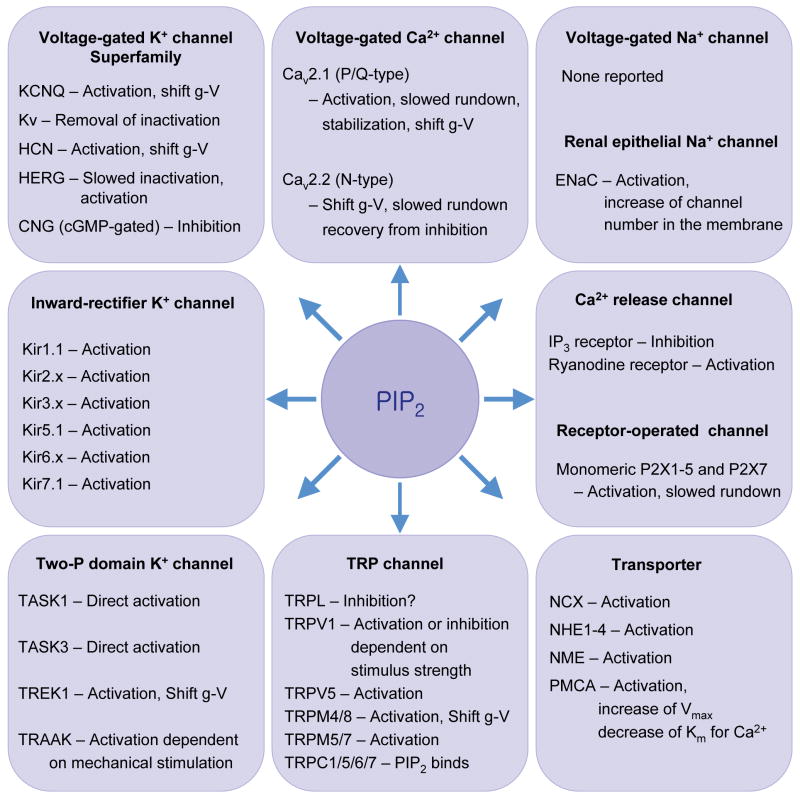
We turn now to ion channels that need PIP2 as a cofactor. The list is surprisingly large (Figure 4). Many were described in earlier reviews (29, 79). To the question, what advantage does PIP2 dependence confer we offer two simple answers. The first is that since PIP2 is found almost exclusively in the plasma membrane, a dependence on PIP2 keeps these channels inactive whenever they are not in the plasma membrane (29). Thus during trafficking from synthesis in the ER through the Golgi and on to the plasma membrane, they could remain silent until they arrive, and during recycling or endocytosis they can be resilenced when they leave. This concept has not been tested directly. Second, since plasma membrane PIP2 can be transiently depleted by neurotransmitters activating PLC, the activity of this group of channels can be regulated by the incoming signals. This concept is well demonstrated.
Figure 4.
Ion channels and transporters sensitive to PIP2. They include: Kir, inward rectifier K+ channel; Kv, voltage-gated K+ channel; HERG, human ether-à-go-go-related gene K+ channel; HCN channel, hyperpolarization-activated, CNG, cyclic nucleotide-regulated channel; Cav, voltage-gated Ca2+ channel; TRP, transient receptor potential; TrpL, TRP-like; CNG, cyclic nucleotide-gated channel; ENaC, epithelial Na+ channel; NCX, Na+-Ca2+ exchanger; NHE, Na+-H+ exchanger; NME, Na+-Mg2+ exchanger; PMCA, plasma membrane Ca2+ ATPase. Activation means increase of open probability, prevention of run down, or recovery from desensitization. Shift g-V means that the conductance-voltage relation is shifted along the voltage axis by PIP2.
In the following we focus on a few channels. Assuming that PIP2 acts directly on them, how do they bind the PIP2? Here we are at a disadvantage compared to other proteins. Channels are not soluble proteins. We lack crystal structures of most of them. Channel proteins are often very large and only a few basic residues have been looked at for possible relevance in phosphoinositide binding. Recall that the poly-phosphoinositol head group extends away from the hydrophobic bilayer into the cytoplasm (up to 17 Å for PIP2, 72), and in the examples considered so far, proteins approach the phosphoinositide from the cytoplasmic side. We will hypothesize that the relevant parts of an ion channel also approach phosphoinositides from the cytoplasmic side, which means that the principal determinants of the binding site would lie in the N- and C-termini and in cytoplasmic loops. They could be very close to transmembrane segments, but need not be. The sequences of ion channels do not show the extreme density of basic residues found in MARCKS, but they do show clustered and isolated basic residues often with nearby hydrophobic residues in cytoplasmic domains (Figure 5) that are candidates for electrostatic and hydrophobic contributions to interactions with acidic lipids. We will conclude that the PH-domain model describes them better than the MARCKS model.
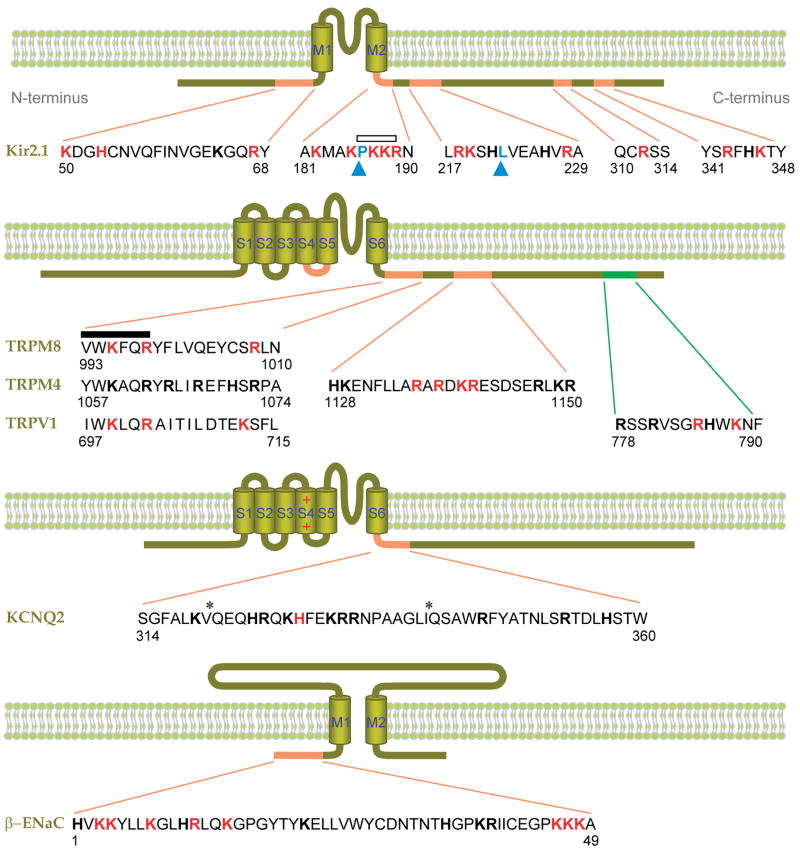
Figure 5.
Putative PIP2-interacting residues of ion channels. Shown are Kir2.1 (32, 45), β-ENaC (48, 94), KCNQ2 (95), and several TRP channels (5, 54, 69, 64). Basic amino acids experimentally implicated in PIP2 binding are red, and other basic residues are shown in block bold. Hydrophobic residues implicated in PIP2 binding are indicated by blue arrowheads (76, 96). The distal C-terminal sequence of TRPV1 implicated in inhibition by PIP2 is green (64). Asterisks denote putative calmodulin binding sites. An open bar indicates a conserved PKKR domain in Kir channels involved in PIP2 binding, and the closed bar indicates the highly conserved TRP box (XWK(F/X)QR). Numbers indicate the amino acid positions. M1–2 and S1–6 denote the transmembrane domains.
Kir channels
Kir6.x (KATP) channels were the first channels recognized as PIP2 dependent (28). They are members of the larger superfamily of inward rectifier channels all of which may require PIP2 for function (Figure 4). Some mutant channels associated with inherited diseases have increased susceptibility to modulation by stimuli that decrease membrane PIP2 levels, exacerbating the disease phenotype (61, 73). In general, Kir channels run down if PIP2 is depleted and reactivate if PIP2 liposomes are applied. PIP2 stabilizes the open state. C-terminal fusion proteins made from Kir1.1 (ROMK1), Kir2.1 (IRK1), Kir3.1 (GIRK1), or Kir3.2 (GIRK2) channels bind PIP2 directly (32). In Kir2.1, there is a cluster of basic residues just after the second transmembrane segment (Figure 5), including arginine R189, which is conserved among most members of the Kir family. Mutating the homologous arginine in Kir1.1, Kir6.2, and Kir5.1 reduces the apparent affinity for PIP2 (1, 32, 93). Other basic and hydrophobic residues in the N-terminus and distal C-terminus are also implicated in PIP2 binding to the channels (45, 76, 96). They may enhance the specificity and affinity towards PIP2 in Kir2.1 compared to other types of Kir channels (67).
The apparent affinity for PIP2 is itself subject to regulation by other signals. Thus, phosphorylation of Kir1.1 channels by protein kinase A (PKA) increases the apparent affinity for PIP2, making the current less sensitive to inhibition by PIP2 antibody (40). Similarly, the apparent affinity of Kir3.1/4 (GIRK1/4) channels for PIP2 is increased by the Gβγ subunits of GTP-binding proteins and by intracellular Na+ (32). On the other hand, the PIP2 interaction with Kir6.x channels is reduced by intracellular ATP, reducing the probability of opening (1, 15, 74).
Fortunately we are just approaching a time when the three-dimensional structure of PIP2 binding sites in Kir channels is determined. The initial leads were a crystal structure of a prokaryotic Kir channel KirBac1.1 that is inhibited by PIP2 and PIP3 (35), and structures of cytoplasmic domains of Kir3.1 and Kir 2.1 (57, 59). From these, Logothetis et al. (43) developed a homology model for the Kir3.1 channel and concluded that residues implicated in PIP2 binding and residues implicated in the actions of several other modulatory agents (Na+, Gβγ) cluster in the same region of the channel in space. Presumably this region closely affects the balance between open and closed channels and coordinates the actions of many gating modifiers. Analogously, Haider et al. (22; 72) made a homology model of Kir6.2 and successfully docked inositol phosphates as surrogates for phosphoinositide head groups with the model. Finally, Nishida et al. (56) obtained the crystal structure of a chimera of the membrane portion of KirBac1.1 and the cytoplasmic portion of Kir3.1. They highlight a ring-like cloud of basic residues a small distance from the putative bilayer that might bind to phosphoinositides. The homology model for Kir6.1 is the most stimulating at present since it predicts the position of PIP2 when bound to the channel. In the model there are four PIP2 binding sites, each at the interface between homotetrameric subunits where gating conformational shifts might occur. The phosphoinositide would be bound by contact with six basic residues (K39, K67, R176, R177, R301 from one subunit and R54 from the neighboring subunit) and some hydrogen-bond interactions (22). The phosphoinositide head group is near its maximum distance from the hydrophobic bilayer, which might imply that it pulls like a spring on the structure (near the slide helix) and this strain assists channel opening. This is reminiscent of old predictions (18).
Mammalian TRP Channels
PIP2 dependence is also evident in the TRP channels (70), channels that are activated by many exogenous and endogenous ligands and physical stimuli (65). It is proposed that dependence on phosphoinositide is a physiological pathway for desensitization or sensitization to sensory stimuli in response to calcium entry or in response to stimulation of G-protein coupled and tyrosine kinase receptors. Some, mammalian TRP channels reported to be regulated by PIP2 are listed in Figure 4. Quite likely, other subtypes of TRP channel are PIP2 sensitive as well.
Surprisingly, one of the most studied examples, the acid-, capsaicin-, and heat-activated TRPV1 channel, is presently the most controversial. Two initial studies (10, 64) reported inhibition by PIP2, activation by PIP2 antibodies, and upregulation upon stimulation of G protein-coupled receptors or receptor tyrosine kinases, and linked these effects to a polybasic distal C-terminal region (residues 777–792; Figure 5). This was followed by reports that some of the receptor-mediated enhancement of TRPV1 currents actually resulted from phosphorylation of the channels (97), that lipid kinase-mediated resynthesis of PIP2 is required for the TRPV1 channel recovery from desensitization (41), that PIP2 applied to excised patches activates TRPV1 currents and phosphatidylinositide 3-kinase is associated with channels and helps their trafficking (77), and that PIP2 both activates and inhibits channels, perhaps at separate sites and PIP3 activates currents as well (46). Phosphoinositides clearly have large effects on this channel, but detailed understanding will need a more nuanced explanation. One paper develops a homology model for TRPV1 similar to those discussed for Kir channels and docks PIP2 in a site formed by convergence of basic residues of the proximal C-terminal “TRP box” and basic residues of the cytoplasmic linker between S4 and S5 (Figure 5) (5).
TRPM7 is inhibited by receptor-stimulated PIP2 depletion (71). The recovery is slowed by PI 4-kinase inhibitors and accelerated by dialyzing diC8 PIP2 through the pipette. The channel co-immunoprecipitates with PLCβ2, suggesting that the channel might be regulated by local PIP2 hydrolysis. Residues contributing to PIP2 binding have not been reported. A study of TRPM8, a cold-sensing ion channel (50, 66), proposed a conserved region in the proximal C-terminus, known as the TRP-domain, as a general PIP2-binding pocket in TRP channels (69). Figure 5 shows an alignment of the TRP-box of the TRP-domain for TRPM8, TRPM4, and TRPV1. In TRPM8, mutation of basic residues in that region induced decreases in apparent PIP2 affinity, including a 100-fold loss in affinity with the R1008Q mutation. This mutation also lowered the apparent menthol affinity (69). For TRPM8 and TRPM4, PIP2 shifts the voltage range of activation toward negative potentials (69, 54). TRPM4 and TRPM5 are nonselective, Ca2+ impermeable cation channels that are activated by PIP2 and by cytoplasmic Ca2+ elevation (42, 98). Mutation of basic residues in the TRP domain of TRPM4 did not have a dramatic effect on PIP2-dependent current recovery after inhibition, although the apparent sensitivity to PIP2 was decreased (54). Instead, a cluster of positive charges more distally in the C-terminus of TRPM4 seemed to contribute more to PIP2 binding. Mutations of basic residues in this region decreased the apparent affinity for PIP2 and the activity of the channels (54). PIP2 effects on TRPC channels have not been explored, but the C-terminus of several TRPC channels binds to immobilized PIP2 and PIP3 (36).
KCNQ channels
The Kv7 or KCNQ family of voltage-gated K+ channels regulates neuronal excitability, cardiac pacemaking, and hearing. Of all the channels in Figure 4, the KCNQ channels are the ones whose regulation by membrane PIP2 is most obviously tied to physiological functions (11). Their current is suppressed by activation of Gq/11-coupled receptors through the activation of PLC and depletion of PIP2 (11, 80). The suppression by Gq/11-coupled receptors (31, 81) or by inducible translocation of PIP2 5-phosphatase (82) takes only 10 seconds, which is comparable to the estimated time course for the depletion of membrane PIP2 by these maneuvers. This measurement means that PIP2 takes no more than a few seconds to dissociate from the channel protein. The recovery of current from inhibition needs the resynthesis of PIP2 from PI by the sequential actions of PI 4-kinase and PIP 5-kinase (78, 89). Current recovers in ~100–200 seconds, which is consistent with estimates of the slow resynthesis of membrane PIP2. Direct application of PIP2 to excised membrane patches increases the channel open probability and slows rundown (39).
As in other channels, a polybasic domain in the C-terminus close to the last transmembrane segment (S6) might be involved in the recognition of membrane PIP2. A point mutation of the region (H328C) significantly reduces sensitivity to PIP2 and increases susceptibility to bradykinin receptor-induced inhibition (95). Several candidate basic residues around that histidine are untested (Figure 5). Interestingly they are very near or overlap with two putative calmodulin binding sites, a theme reported for other channels (36). Thus KCNQ channel coupling to PIP2 might also be regulated by calmodulin binding to the channels (21, 36, 88).
PIP2 may be the only phosphoinositide for KCNQ channel activation in intact cells. Selective depletion of PIP2 using an engineered chemical dimerization system almost completely suppressed the current, whereas the activation of PIP 5-kinase augmented the current (82). PIP and PIP3 might not be able to activate the current, as there is still complete inhibition in the rapamycin system when they are elevated by action of PIP2 5-phosphatase and 3-kinase, respectively. PI(3,4)P2 has little effect on the current in excised patches (39).
ENaC Channels
Amiloride-sensitive epithelial Na+ channels (ENaC) are heteromeric channels consisting of α, β, and γ subunits (6). Each subunit has two transmembrane domains and a large extracellular region (Figure 5). Modulation of ENaC activity plays an essential role in Na+ absorption across apical membranes in the distal nephron for regulation of body Na+ homeostasis and blood pressure (33, 75). Several lines of evidence suggest that removal of PIP2 decreases channel activity (48, 94) and elevation of PIP3 increases it (62, 84). Thus in excised, inside-out patches, applied PIP2 enhanced the open probability of ENaC, whereas PIP2 antibodies and polylysine accelerated current run-down. In part decreases of PIP2 may underlie the depression of current during activation of P2Y receptors or EGF receptors, and increases of PIP3 may underlie the enhancement of current by aldosterone seen in the kidney (25, 34, 85). Several lysine and arginine residues are found in the cytoplasmic N-terminus of β-ENaC (Figure 5) and γ-ENaC from human, rat, mouse, and Xenopus (47). Mutation or deletion of this N-terminal region of β-ENaC dramatically reduces channel activity without affecting surface expression (7, 34). It may contribute to a binding site for PIP2 (34, 94). Proximal basic residues of the C-terminus of β- and γ-ENaC may bind to PIP3 and bestow PIP3 sensitivity to the channel (62). Membrane PIP2 and PIP3 may also control ENaC activity by additional mechanisms related to membrane trafficking and independent of direct phosphoinositide binding to channels (63).
Other channels
PIP2 regulation of many other types of ion channels is reported (Figure 4; 79), but their PIP2-binding properties are little explored. The list includes Kv channels (58), HERG channels (2, 3), CNG channels (23, 90), and Ca2+ release channels such as IP3 receptors and ryanodine receptors. Recent studies have added additional channels. Current in two-pore domain K+ (K2P) channels can be suppressed by depleting PIP2 in excised patches (44) or by activating Gq-coupled receptors. Although the channels clearly respond to PIP2, the action of receptors might be mostly via a direct inhibition of channels by activated Gαq subunits (8, 49). The P2X receptors are trimeric cation channels gated by extracellular ATP. Seven vertebrate P2X subunits (P2X1–7) have been cloned. PIP2 is said to activate the current of all homomeric P2X receptors through direct binding to the proximal C-terminal basic region (99). P2X current is significantly inhibited by PDGF-mediated PIP2 depletion in oocytes. The activity of hyperpolarization-activated, cyclic nucleotide-regulated (HCN) channels also is enhanced by membrane phosphoinositides, which increase the open probability and shift the activation curve by up to 20 mV to more positive voltages (60, 101). PIP2 is the most effective but PI(3,4)P2, PI(3,4,5)P3, and even PI(4)P act. P/Q-type and N-type voltage-gated Ca2+ channels are regulated by PIP2 changes (12, 53). PIP2 retards the rundown and shifts the voltage-dependence of both channels. A point mutation (I1520H) of the intracellular end of IIIS6 in the α subunit of P/Q-type Ca2+ channels greatly attenuated the current rundown and increased the apparent affinity for PIP2, probably through an allosteric effect (100).
PHOSPHOINOSITIDE SPECIFICITY
In introducing PIP2 binding proteins, we contrasted PH domains having a structured binding pocket with MARCKS presenting a positively charged cloud (Figure 3a). Proteins with a binding pocket have the potential to discriminate among the different phosphoinositides, including between similar isomers such as PI(4,5)P2 and PI(3,4)P2. Some but not all PH domains can do that (37). Molecules such as MARCKS presenting only an unstructured positive cloud could attract anything negative with preference for high charge, but could not discriminate isomers (51, 86). Some channel proteins seem to interact with PIP2 through a structured PIP2-binding pocket, since the apparent affinity for PIP2 is higher than for PI(3,4)P2 or PI(3,5)P2 (55, 98). Other channels show little selectivity among poly-phosphoinositides sometimes accompanied by general low affinity for PIP2 (14, 68).
There may be a partial correlation between phosphoinositide specificity and the apparent PIP2 binding affinity measured in functional experiments on ion channels. The additional interactions that would give specificity would also strengthen binding. This hypothesis is diagramed as a graph in Figure 3b. For example, Kir2.1 channels, which have a high apparent affinity for PIP2, bind only to PIP2, whereas Kir2.3 channels, which have a lower apparent affinity for PIP2, also bind PIP3 (14). In addition, Kir2.3 channels are more sensitive to regulation by various modulators than Kir2.1 (9, 14). Mutation of Kir2.1 channels to make a low-affinity channel results in lowered phosphoinositide specificity (68). Kir3.x and Kir1.1 channels, reported to be activated preferentially by PI(4,5)P2 and to a lesser extent by PI(3,4,5)P3 and PI(3,4)P2, have a moderate affinity for PIP2. Kir6.2 channels have the lowest apparent PIP2 affinity and the least phosphoinositide specificity. They can be activated by PI(4,5)P2, PI(3,4)P2, and PIP3 about equally (68) and by higher concentrations of PI(4)P, PI (18), phosphatidic acid (17), and even long-chain acyl-coenzyme A (4). Among TRP channels, TRPM8 channels are activated best by PI(4,5)P2, less well by PI(3,4)P2 and PI(3,4,5)P3, and only 1/10th as well by PI(4)P (69). Tests of the phosphoinositide specificity of TRPM4 channels (54, 98) reveal comparable effects of PI(4,5)P2, PI(3,4)P2 and PI(3,4,5)P3. A recent study of TRPV1 channels says they can be equally activated by PIP2 and PI(4)P at high capsaicin concentration, and are inhibited by PI(4)P more potently than PIP2 at low concentration of capsaicin (46). The apparent affinity for PIP2 in TRP channels seems to fall between those for Kir2.1 and Kir6.2.
CONCLUDING THOUGHTS
A requirement for PIP2 is clearly established for many ion channels and transporters. The next steps will be to clarify the molecular mechanisms and to define the scope of this phenomenon.
Mechanisms
Models say that PIP2 binds in a cytoplasmic pocket near components of the gating machinery. Very soon we should expect to have plausible mechanical descriptions of the conformational changes that underlie gating in channels and completed atomic models of PIP2 binding sites. Together, these advances should allow us to understand the forces that underlie PIP2 actions on channels. We have shown that the selectivity for PIP2 is sometimes not great, and other phosphoinositides may act as well. Does this have a biological function? Does it allow the channels to function in several compartments? Do other phosphoinositides act exactly like PIP2 when bound, or does each confer a subtly unique phenotype?
Scope
How general are these phenomena? There have been no systematic searches for membrane proteins that depend on PIP2 or other phosphoinositides. It is easy to see how generality could be explored with other plasma membrane ion channels and ion transporters. One would repeat the protocols already in use, focusing on protocols that leave the fewest alternative explanations of what is observed and always using multiple lines of evidence. Presumably of the several hundred channels known, many additional ones are influenced by the lipid and phosphoinositide environment. Asking the same question about ion channels of intracellular compartments will be harder. Both the channel assay and the phosphoinositide manipulation need new thinking. Finally determining phosphoinositide influences on membrane enzymes is unexplored. If PIP2 affects channels and transporters, it most probably affects many membrane enzymes in interesting ways.
Cell biology
For PIP2 and other phosphoinositides there remain many cell biological and biochemical questions. Where and when is each one made and how are they replenished? How much movement occurs between compartments? Are the lipid kinases and phosphatases highly regulated? Thus, when PIP2 is depleted, is there a feedback signal that hastens its resynthesis? How uniform is the phosphoinositide distribution in each membrane? Do breakdown and synthesis generate local microdomains, and is this a significant component of receptor signaling?
SUMMARY POINTS
Many cytoplasmic proteins bind phosphoinositides in cell membranes using a collection of basic residues for major electrostatic interactions and additional hydrophobic and hydrogen-bonding residues.
Many ion channels and ion transporters need PIP2 to function, and numerous basic and other residues in the C-terminus and the N-terminus are implicated in PIP2 recognition.
Because ion channels bind phosphoinositides with partial specificity, they must fold the interacting residues into a structured binding pocket.
For the tetrameric K+-channel-like superfamily, existing models suggest one binding pocket per subunit that is formed by both C-and N-terminal residues possibly at the interface between neighboring subunits.
Attachment to PIP2 may draw this region towards the membrane and exert tension that facilitates or is permissive of opening of gates.
Phosphoinositide dependence may restrict the activity of membrane proteins to the appropriate subcellular compartment(s) and offers a route for receptor-mediated regulation of protein activities via phosphoinositide metabolism.
New approaches will be needed to extend such ideas to membrane proteins in general and to membranes other than the plasma membrane.
Acknowledgments
We thank Lea M Miller for technical help, Sharona E. Gordon for comments on the manuscript, and the National Institutes of Health for grant support: NS08174 and GM083913.
Contributor Information
Byung-Chang Suh, Email: bcs@u.washington.edu.
Bertil Hille, Email: hille@u.washington.edu.
LITERATURE CITED
- 1.Baukrowitz T, Schulte U, Oliver D, Herlitze S, Krauter T, et al. PIP2 and PIP as determinants for ATP inhibition of KATP channels. Science. 1998;282:1141–4. doi: 10.1126/science.282.5391.1141. [DOI] [PubMed] [Google Scholar]
- 2.Bian J, Cui J, McDonald TV. HERG K+ channel activity is regulated by changes in phosphatidylinositol 4,5-bisphosphate. Circ Res. 2001;89:1168–76. doi: 10.1161/hh2401.101375. [DOI] [PubMed] [Google Scholar]
- 3.Bian JS, Kagan A, McDonald TV. Molecular analysis of PIP2 regulation of HERG and IKr. Am J Physiol Heart Circ Physiol. 2004;287:H2154–63. doi: 10.1152/ajpheart.00120.2004. [DOI] [PubMed] [Google Scholar]
- 4.Branstrom R, Leibiger IB, Leibiger B, Corkey BE, Berggren PO, et al. Long chain coenzyme A esters activate the pore-forming subunit (Kir6.2) of the ATP-regulated potassium channel. J Biol Chem. 1998;273:31395–400. doi: 10.1074/jbc.273.47.31395. [DOI] [PubMed] [Google Scholar]
- 5.Brauchi S, Orta G, Mascayano C, Salazar M, Raddatz N, et al. Dissection of the components for PIP2 activation and thermosensation in TRP channels. Proc Natl Acad Sci USA. 2007;104:10246–51. doi: 10.1073/pnas.0703420104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, et al. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature. 1994;367:463–7. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- 7.Chalfant ML, Denton JS, Langloh AL, Karlson KH, Loffing J, et al. The NH2 terminus of the epithelial sodium channel contains an endocytic motif. J Biol Chem. 1999;274:32889–96. doi: 10.1074/jbc.274.46.32889. [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Talley EM, Patel N, Gomis A, McIntire WE, et al. Inhibition of a background potassium channel by Gq protein α-subunits. Proc Natl Acad Sci USA. 2006;103:3422–7. doi: 10.1073/pnas.0507710103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chuang H, Jan YN, Jan LY. Regulation of IRK3 inward rectifier K+ channel by m1 acetylcholine receptor and intracellular magnesium. Cell. 1997;89:1121–32. doi: 10.1016/s0092-8674(00)80299-8. [DOI] [PubMed] [Google Scholar]
- 10.Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, et al. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411:957–62. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- 11.Delmas P, Brown DA. Pathways modulating neural KCNQ/M (Kv7) potassium channels. Nat Rev Neurosci. 2005;6:850–62. doi: 10.1038/nrn1785. [DOI] [PubMed] [Google Scholar]
- 12.Delmas P, Coste B, Gamper N, Shapiro MS. Phosphoinositide lipid second messengers: new paradigms for calcium channel modulation. Neuron. 2005;47:179–82. doi: 10.1016/j.neuron.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–7. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- 14.Du X, Zhang H, Lopes C, Mirshahi T, Rohacs T, et al. Characteristic interactions with phosphatidylinositol 4,5-bisphosphate determine regulation of Kir channels by diverse modulators. J Biol Chem. 2004;279:37271–81. doi: 10.1074/jbc.M403413200. [DOI] [PubMed] [Google Scholar]
- 15.Enkvetchakul D, Loussouarn G, Makhina E, Shyng SL, Nichols CG. The kinetic and physical basis of KATP channel gating: toward a unified molecular understanding. Biophys J. 2000;78:2334–48. doi: 10.1016/S0006-3495(00)76779-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ertel EA. Excised patches of plasma membrane from vertebrate rod outer segments retain a functional phototransduction enzymatic cascade. Proc Natl Acad Sci USA. 1990;87:4226–30. doi: 10.1073/pnas.87.11.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan Z, Gao L, Wang W. Phosphatidic acid stimulates cardiac KATP channels like phosphatidylinositols, but with novel gating kinetics. Am J Physiol Cell Physiol. 2003;284:C94–102. doi: 10.1152/ajpcell.00255.2002. [DOI] [PubMed] [Google Scholar]
- 18.Fan Z, Makielski JC. Anionic phospholipids activate ATP-sensitive potassium channels. J Biol Chem. 1997;272:5388–95. doi: 10.1074/jbc.272.9.5388. [DOI] [PubMed] [Google Scholar]
- 19.Ferguson KM, Kavran JM, Sankaran VG, Fournier E, Isakoff SJ, et al. Structural basis for discrimination of 3-phosphoinositides by pleckstrin homology domains. Mol Cell. 2000;6:373–84. doi: 10.1016/s1097-2765(00)00037-x. [DOI] [PubMed] [Google Scholar]
- 20.Ferguson KM, Lemmon MA, Schlessinger J, Sigler PB. Structure of the high affinity complex of inositol trisphosphate with a phospholipase C pleckstrin homology domain. Cell. 1995;83:1037–46. doi: 10.1016/0092-8674(95)90219-8. [DOI] [PubMed] [Google Scholar]
- 21.Gamper N, Shapiro MS. Calmodulin mediates Ca2+-dependent modulation of M-type K+ channels. J Gen Physiol. 2003;122:17–31. doi: 10.1085/jgp.200208783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haider S, Tarasov AI, Craig TJ, Sansom MS, Ashcroft FM. Identification of the PIP2-binding site on Kir6.2 by molecular modelling and functional analysis. EMBO J. 2007;26:3749–59. doi: 10.1038/sj.emboj.7601809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He F, Mao M, Wensel TG. Enhancement of phototransduction G protein-effector interactions by phosphoinositides. Biol Chem. 2004;279:8986–90. doi: 10.1074/jbc.M311488200. [DOI] [PubMed] [Google Scholar]
- 24.He Z, Feng S, Tong Q, Hilgemann DW, Philipson KD. Interaction of PIP2 with the XIP region of the cardiac Na/Ca exchanger. Am J Physiol Cell Physiol. 2000;278:C661–6. doi: 10.1152/ajpcell.2000.278.4.C661. [DOI] [PubMed] [Google Scholar]
- 25.Helms MN, Liu L, Liang YY, Al-Khalili O, Vandewalle A, et al. Phosphatidylinositol 3,4,5-trisphosphate mediates aldosterone stimulation of epithelial sodium channel (ENaC) and interacts with γ-ENaC. J Biol Chem. 2005;280:40885–91. doi: 10.1074/jbc.M509646200. [DOI] [PubMed] [Google Scholar]
- 26.Heo WD, Inoue T, Park WS, Kim ML, Park BO, et al. PI(3,4,5)P3 and PI(4,5)P2 lipids target proteins with polybasic clusters to the plasma membrane. Science. 2006;314:1458–61. doi: 10.1126/science.1134389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hilgemann DW. Local PIP2 signals: when, where, and how? . Pflugers Arch. 2007 doi: 10.1007/s00424-007-0280-9. [Epub ahead of print] Complete when published. [DOI] [PubMed] [Google Scholar]
- 28.Hilgemann DW, Ball R. Regulation of cardiac Na+, Ca2+ exchange and KATP potassium channels by PIP2. Science. 1996;273:956–9. doi: 10.1126/science.273.5277.956. [DOI] [PubMed] [Google Scholar]
- 29.Hilgemann DW, Feng S, Nasuhoglu C. The complex and intriguing lives of PIP2 with ion channels and transporters. Sci STKE. 2001;2001:RE19. doi: 10.1126/stke.2001.111.re19. [DOI] [PubMed] [Google Scholar]
- 30.Hirose K, Kadowaki S, Tanabe M, Takeshima H, Iino M. Spatiotemporal dynamics of inositol 1,4,5-trisphosphate that underlies complex Ca2+ mobilization patterns. Science. 1999;284:1527–30. doi: 10.1126/science.284.5419.1527. [DOI] [PubMed] [Google Scholar]
- 31.Horowitz LF, Hirdes W, Suh BC, Hilgemann DW, Mackie K, Hille B. Phospholipase C in living cells: activation, inhibition, Ca2+ requirement, and regulation of M current. J Gen Physiol. 2005;126:243–62. doi: 10.1085/jgp.200509309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang CL, Feng S, Hilgemann DW. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gβγ. Nature. 1998;391:803–6. doi: 10.1038/35882. [DOI] [PubMed] [Google Scholar]
- 33.Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev. 2002;82:735–67. doi: 10.1152/physrev.00007.2002. [DOI] [PubMed] [Google Scholar]
- 34.Kunzelmann K, Bachhuber T, Regeer R, Markovich D, Sun J, et al. Purinergic inhibition of the epithelial Na+ transport via hydrolysis of PIP2. FASEB J. 2005;19:142–3. doi: 10.1096/fj.04-2314fje. [DOI] [PubMed] [Google Scholar]
- 35.Kuo A, Gulbis JM, Antcliff JF, Rahman T, Lowe ED, et al. Crystal structure of the potassium channel KirBac1.1 in the closed state. Science. 2003;300:1922–6. doi: 10.1126/science.1085028. [DOI] [PubMed] [Google Scholar]
- 36.Kwon Y, Hofmann T, Montell C. Integration of phosphoinositide- and calmodulin-mediated regulation of TRPC6. Mol Cell. 2007;25:491–503. doi: 10.1016/j.molcel.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lemmon MA, Ferguson KM, O’Brien R, Sigler PB, Schlessinger J. Specific and high-affinity binding of inositol phosphates to an isolated pleckstrin homology domain. Proc Natl Acad Sci USA. 1995;92:10472–6. doi: 10.1073/pnas.92.23.10472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lemmon MA. Phosphoinositide recognition domains. Traffic. 2003;4:201–13. doi: 10.1034/j.1600-0854.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- 39.Li Y, Gamper N, Hilgemann DW, Shapiro MS. Regulation of Kv7 (KCNQ) K+ channel open probability by phosphatidylinositol 4,5-bisphosphate. J Neurosci. 2005;25:9825–35. doi: 10.1523/JNEUROSCI.2597-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liou HH, Zhou SS, Huang CL. Regulation of ROMK1 channel by protein kinase A via a phosphatidylinositol 4,5-bisphosphate-dependent mechanism. Proc Natl Acad Sci USA. 1999;96:5820–5. doi: 10.1073/pnas.96.10.5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu B, Zhang C, Qin F. Functional recovery from desensitization of vanilloid receptor TRPV1 requires resynthesis of phosphatidylinositol 4,5-bisphosphate. J Neurosci. 2005;25:4835–43. doi: 10.1523/JNEUROSCI.1296-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu D, Liman ER. Intracellular Ca2+ and the phospholipid PIP2 regulate the taste transduction ion channel TRPM5. Proc Natl Acad Sci USA. 2003;100:15160–5. doi: 10.1073/pnas.2334159100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Logothetis DE, Lupyan D, Rosenhouse-Dantsker A. Diverse Kir modulators act in close proximity to residues implicated in phosphoinositide binding. J Physiol. 2007;582:953–65. doi: 10.1113/jphysiol.2007.133157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lopes CM, Rohacs T, Czirjak G, Balla T, Enyedi P, et al. PIP2 hydrolysis underlies agonist-induced inhibition and regulates voltage gating of two-pore domain K+ channels. J Physiol. 2005;564:117–29. doi: 10.1113/jphysiol.2004.081935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lopes CM, Zhang H, Rohacs T, Jin T, Yang J, et al. Alterations in conserved Kir channel-PIP2 interactions underlie channelopathies. Neuron. 2002;34:933–44. doi: 10.1016/s0896-6273(02)00725-0. [DOI] [PubMed] [Google Scholar]
- 46.Lukacs V, Thyagarajan B, Varnai P, Balla A, Balla T, et al. Dual regulation of TRPV1 by phosphoinositides. J Neurosci. 2007;27:7070–80. doi: 10.1523/JNEUROSCI.1866-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma HP, Eaton DC. Acute regulation of epithelial sodium channel by anionic phospholipids. J Am Soc Nephrol. 2005;16:3182–7. doi: 10.1681/ASN.2005040434. [DOI] [PubMed] [Google Scholar]
- 48.Ma HP, Saxena S, Warnock DG. Anionic phospholipids regulate native and expressed epithelial sodium channel (ENaC) J Biol Chem. 2002;277:7641–4. doi: 10.1074/jbc.C100737200. [DOI] [PubMed] [Google Scholar]
- 49.Mathie A. Neuronal two-pore-domain potassium channels and their regulation by G protein-coupled receptors. J Physiol. 2007;578:377–85. doi: 10.1113/jphysiol.2006.121582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–8. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 51.McLaughlin S, Murray D. Plasma membrane phosphoinositide organization by protein electrostatics. Nature. 2005;438:605–11. doi: 10.1038/nature04398. [DOI] [PubMed] [Google Scholar]
- 52.McLaughlin S, Wang J, Gambhir A, Murray D. PIP2 and proteins: interactions, organization, and information flow. Annu Rev Biophys Biomol Struct. 2002;31:151–75. doi: 10.1146/annurev.biophys.31.082901.134259. [DOI] [PubMed] [Google Scholar]
- 53.Michailidis IE, Zhang Y, Yang J. The lipid connection-regulation of voltage-gated Ca2+ channels by phosphoinositides. Pflugers Arch. 2007 doi: 10.1007/s00424-007-0272-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 54.Nilius B, Mahieu F, Prenen J, Janssens A, Owsianik G, et al. The Ca2+-activated cation channel TRPM4 is regulated by phosphatidylinositol 4,5-biphosphate. EMBO J. 2006;25:467–78. doi: 10.1038/sj.emboj.7600963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nilius B, Prenen J, Janssens A, Voets T, Droogmans G. Decavanadate modulates gating of TRPM4 cation channels. J Physiol. 2004;560:753–65. doi: 10.1113/jphysiol.2004.070839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nishida M, Cadene M, Chait BT, Mackinnon R. Crystal structure of a Kir3.1-prokaryotic Kir channel chimera. EMBO J. 2007 doi: 10.1038/sj.emboj.7601828. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nishida M, MacKinnon R. Structural basis of inward rectification: cytoplasmic pore of the G protein-gated inward rectifier GIRK1 at 1.8 A resolution. Cell. 2002;111:957–65. doi: 10.1016/s0092-8674(02)01227-8. [DOI] [PubMed] [Google Scholar]
- 58.Oliver D, Lien CC, Soom M, Baukrowitz T, Jonas P, et al. Functional conversion between A-type and delayed rectifier K+ channels by membrane lipids. Science. 2004;304:265–70. doi: 10.1126/science.1094113. [DOI] [PubMed] [Google Scholar]
- 59.Pegan S, Arrabit C, Zhou W, Kwiatkowski W, Collins A, et al. Cytoplasmic domain structures of Kir2.1 and Kir3.1 show sites for modulating gating and rectification. Nat Neurosci. 2005;8:279–87. doi: 10.1038/nn1411. [DOI] [PubMed] [Google Scholar]
- 60.Pian P, Bucchi A, Robinson RB, Siegelbaum SA. Regulation of gating and rundown of HCN hyperpolarization-activated channels by exogenous and endogenous PIP2. J Gen Physiol. 2006;128:593–604. doi: 10.1085/jgp.200609648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Plaster NM, Tawil R, Tristani-Firouzi M, Canun S, Bendahhou S, et al. Mutations in Kir2.1 cause the developmental and episodic electrical phenotypes of Andersen’s syndrome. Cell. 2001;105:511–9. doi: 10.1016/s0092-8674(01)00342-7. [DOI] [PubMed] [Google Scholar]
- 62.Pochynyuk O, Staruschenko A, Tong Q, Medina J, Stockand JD. Identification of a functional phosphatidylinositol 3,4,5-trisphosphate binding site in the epithelial Na+ channel. J Biol Chem. 2005;280:37565–71. doi: 10.1074/jbc.M509071200. [DOI] [PubMed] [Google Scholar]
- 63.Pochynyuk O, Tong Q, Staruschenko A, Ma HP, Stockand JD. Regulation of the epithelial Na+ channel (ENaC) by phosphatidylinositides. Am J Physiol Renal Physiol. 2006;290:F949–57. doi: 10.1152/ajprenal.00386.2005. [DOI] [PubMed] [Google Scholar]
- 64.Prescott ED, Julius D. A modular PIP2 binding site as a determinant of capsaicin receptor sensitivity. Science. 2003;300:1284–8. doi: 10.1126/science.1083646. [DOI] [PubMed] [Google Scholar]
- 65.Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol. 2006;68:619–47. doi: 10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- 66.Reid G, Flonta ML. Cold current in thermoreceptive neurons. Nature. 2001;413:480. doi: 10.1038/35097164. [DOI] [PubMed] [Google Scholar]
- 67.Rohacs T, Chen J, Prestwich GD, Logothetis DE. Distinct specificities of inwardly rectifying K+ channels for phosphoinositides. J Biol Chem. 1999;274:36065–72. doi: 10.1074/jbc.274.51.36065. [DOI] [PubMed] [Google Scholar]
- 68.Rohacs T, Lopes CM, Jin T, Ramdya PP, Molnar Z, et al. Specificity of activation by phosphoinositides determines lipid regulation of Kir channels. Proc Natl Acad Sci USA. 2003;100:745–50. doi: 10.1073/pnas.0236364100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rohacs T, Lopes CM, Michailidis I, Logothetis DE. PI(4,5)P2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nat Neurosci. 2005;8:626–34. doi: 10.1038/nn1451. [DOI] [PubMed] [Google Scholar]
- 70.Rohacs T. Regulation of TRP channels by PIP2. Pflugers Arch. 2007;453:753–62. doi: 10.1007/s00424-006-0153-7. [DOI] [PubMed] [Google Scholar]
- 71.Runnels LW, Yue L, Clapham DE. The TRPM7 channel is inactivated by PIP2 hydrolysis. Nat Cell Biol. 2002;4:329–36. doi: 10.1038/ncb781. [DOI] [PubMed] [Google Scholar]
- 72.Sansom MS, Bond PJ, Deol SS, Grottesi A, Haider S, et al. Molecular simulations and lipid-protein interactions: potassium channels and other membrane proteins. Biochem Soc Trans. 2005;33:916–20. doi: 10.1042/BST20050916. [DOI] [PubMed] [Google Scholar]
- 73.Schulte U, Hahn H, Konrad M, Jeck N, Derst C, et al. pH gating of ROMK (Kir1.1) channels: control by an Arg-Lys-Arg triad disrupted in antenatal Bartter syndrome. Proc Natl Acad Sci USA. 1999;96:15298–303. doi: 10.1073/pnas.96.26.15298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shyng SL, Nichols CG. Membrane phospholipid control of nucleotide sensitivity of KATP channels. Science. 1998;282:1138–41. doi: 10.1126/science.282.5391.1138. [DOI] [PubMed] [Google Scholar]
- 75.Snyder PM. The epithelial Na+ channel: cell surface insertion and retrieval in Na+ homeostasis and hypertension. Endocr Rev. 2002;23:258–75. doi: 10.1210/edrv.23.2.0458. [DOI] [PubMed] [Google Scholar]
- 76.Soom M, Schönherr R, Kubo Y, Kirsch C, Klinger R, Heinemann SH. Multiple PIP2 binding sites in Kir2.1 inwardly rectifying potassium channels. FEBS Lett. 2001;490:49–53. doi: 10.1016/s0014-5793(01)02136-6. [DOI] [PubMed] [Google Scholar]
- 77.Stein AT, Ufret-Vincenty CA, Hua L, Santana LF, Gordon SE. Phosphoinositide 3-kinase binds to TRPV1 and mediates NGF-stimulated TRPV1 trafficking to the plasma membrane. J Gen Physiol. 2006;128:509–22. doi: 10.1085/jgp.200609576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Suh BC, Hille B. Recovery from muscarinic modulation of M current channels requires phosphatidylinositol 4,5-bisphosphate synthesis. Neuron. 2002;35:507–20. doi: 10.1016/s0896-6273(02)00790-0. [DOI] [PubMed] [Google Scholar]
- 79.Suh BC, Hille B. Regulation of ion channels by phosphatidylinositol 4,5-bisphosphate. Curr Opin Neurobiol. 2005;15:370–8. doi: 10.1016/j.conb.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 80.Suh BC, Hille B. Regulation of KCNQ channels by manipulation of phosphoinositides. J Physiol. 2007;582:911–6. doi: 10.1113/jphysiol.2007.132647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Suh BC, Horowitz LF, Hirdes W, Mackie K, Hille B. Regulation of KCNQ2/KCNQ3 current by G protein cycling: the kinetics of receptor- mediated signaling by Gq. J Gen Physiol. 2004;123:663–83. doi: 10.1085/jgp.200409029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Suh BC, Inoue T, Meyer T, Hille B. Rapid chemically induced changes of PtdIns(4,5)P2 gate KCNQ ion channels. Science. 2006;314:1454–7. doi: 10.1126/science.1131163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tapp H, Al-Naggar IM, Yarmola EG, Harrison A, Shaw G, et al. MARCKS is a natively unfolded protein with an inaccessible actin-binding site: evidence for long-range intramolecular interactions. J Biol Chem. 2005;280:9946–56. doi: 10.1074/jbc.M414614200. [DOI] [PubMed] [Google Scholar]
- 84.Tong Q, Gamper N, Medina JL, Shapiro MS, Stockand JD. Direct activation of the epithelial Na+ channel by phosphatidylinositol 3,4,5-trisphosphate and phosphatidylinositol 3,4-bisphosphate produced by phosphoinositide 3-OH kinase. J Biol Chem. 2004;279:22654–63. doi: 10.1074/jbc.M401004200. [DOI] [PubMed] [Google Scholar]
- 85.Tong Q, Stockand JD. Receptor tyrosine kinases mediate epithelial Na+ channel inhibition by epidermal growth factor. Am J Physiol Renal Physiol. 2005;288:F150–61. doi: 10.1152/ajprenal.00261.2004. [DOI] [PubMed] [Google Scholar]
- 86.Wang J, Gambhir A, Hangyas-Mihalyne G, Murray D, Golebiewska U, et al. Lateral sequestration of phosphatidylinositol 4,5-bisphosphate by the basic effector domain of myristoylated alanine-rich C kinase substrate is due to nonspecific electrostatic interactions. J Biol Chem. 2002;277:34401–12. doi: 10.1074/jbc.M203954200. [DOI] [PubMed] [Google Scholar]
- 87.Wang J, Gambhir A, McLaughlin S, Murray D. A computational model for the electrostatic sequestration of PI(4,5)P2 by membrane-adsorbed basic peptides. Biophys J. 2004;86:1969–86. doi: 10.1016/S0006-3495(04)74260-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wen H, Levitan IB. Calmodulin is an auxiliary subunit of KCNQ2/3 potassium channels. J Neurosci. 2002;22:7991–8001. doi: 10.1523/JNEUROSCI.22-18-07991.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Winks JS, Hughes S, Filippov AK, Tatulian L, Abogadie FC, et al. Relationship between membrane phosphatidylinositol-4,5-bisphosphate and receptor-mediated inhibition of native neuronal M channels. J Neurosci. 2005;25:3400–13. doi: 10.1523/JNEUROSCI.3231-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Womack KB, Gordon SE, He F, Wensel TG, Lu CC, et al. Do phosphatidylinositides modulate vertebrate phototransduction? J Neurosci. 2000;20:2792–99. doi: 10.1523/JNEUROSCI.20-08-02792.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xu C, Watras J, Loew LM. Kinetic analysis of receptor-activated phosphoinositide turnover. J Cell Biol. 2003;161:779–91. doi: 10.1083/jcb.200301070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yamauchi E, Nakatsu T, Matsubara M, Kato H, Taniguchi H. Crystal structure of a MARCKS peptide containing the calmodulin-binding domain in complex with Ca2+-calmodulin. Nat Struct Biol. 2003;10:226–31. doi: 10.1038/nsb900. [DOI] [PubMed] [Google Scholar]
- 93.Yang Z, Xu H, Cui N, Qu Z, Chanchevalap S, et al. Biophysical and molecular mechanisms underlying the modulation of heteromeric Kir4.1-Kir5.1 channels by CO2 and pH. J Gen Physiol. 2000;116:33–45. doi: 10.1085/jgp.116.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yue G, Malik B, Yue G, Eaton DC. Phosphatidylinositol 4,5- bisphosphate (PIP2) stimulates epithelial sodium channel activity in A6 cells. J Biol Chem. 2002;277:11965–9. doi: 10.1074/jbc.M108951200. [DOI] [PubMed] [Google Scholar]
- 95.Zhang H, Craciun LC, Mirshahi T, Rohacs T, Lopes CM, et al. PIP2 activates KCNQ channels, and its hydrolysis underlies receptor-mediated inhibition of M currents. Neuron. 2003;37:963–75. doi: 10.1016/s0896-6273(03)00125-9. [DOI] [PubMed] [Google Scholar]
- 96.Zhang H, He C, Yan X, Mirshahi T, Logothetis DE. Activation of inwardly rectifying K+ channels by distinct PtdIns(4,5)P2 interactions. Nat Cell Biol. 1999;1:183–8. doi: 10.1038/11103. [DOI] [PubMed] [Google Scholar]
- 97.Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005;24:4211–23. doi: 10.1038/sj.emboj.7600893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang Z, Okawa H, Wang Y, Liman ER. Phosphatidylinositol 4,5-bisphosphate rescues TRPM4 channels from desensitization. J Biol Chem. 2005;280:39185–92. doi: 10.1074/jbc.M506965200. [DOI] [PubMed] [Google Scholar]
- 99.Zhao Q, Yang M, Ting AT, Logothetis DE. PIP2 regulates the ionic current of P2X receptors and P2X7 receptor-mediated cell death. Channels. 2007;1:46–55. [PubMed] [Google Scholar]
- 100.Zhen XG, Xie C, Yamada Y, Zhang Y, Doyle C, et al. A single amino acid mutation attenuates rundown of voltage-gated calcium channels. FEBS Lett. 2006;580:5733–8. doi: 10.1016/j.febslet.2006.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zolles G, Klocker N, Wenzel D, Weisser-Thomas J, Fleischmann BK, et al. Pacemaking by HCN channels requires interaction with phosphoinositides. Neuron. 2006;52:1027–36. doi: 10.1016/j.neuron.2006.12.005. [DOI] [PubMed] [Google Scholar]