Abstract
Nuclear factor-κB provides an adaptive response to protect cancer cells against cytotoxicity induced by redox active therapeutics. RelB is uniquely expressed at a high level in prostate cancer with high Gleason scores. Recently, we showed that the level of RelB rapidly increases in androgen-independent prostate cancer cells after exposure to ionizing radiation (IR), leading to a reduction in intrinsic radiosensitivity. Here, we show that interaction of 1α,25-dihydroxyvitamin ,D3 [1α,25-(OH)2D3] with the vitamin D receptor significantly enhances radiosensitivity of prostate cancer cells at clinically relevant radiation doses. The radiosensitization effect of 1α,25-(OH)2D3 is mediated, at least in part, by selectively suppressing IR-mediated RelB activation, leading to a reduced expression of its target gene MnSOD, a primary antioxidant enzyme in mitochondria. These results suggest that suppression of manganese superoxide dismutase is a mechanism by which 1α,25-(OH)2D3 exerts its radiosensitization effect and that 1α,25-(OH)2D3 may serve as an effective pharmacologic agent for selectively sensitizing prostate cancer cells to IR via suppression of antioxidant responses in mitochondria.
Introduction
Prostate cancer is the most common malignancy in North American countries and the second leading cause of cancer deaths in U.S. men (1). Effective treatment options for the early stages of prostate cancer include surgery and localized radiation therapy. Androgen ablation therapy initially controls the advanced stages of the disease, but eventually, in nearly all patients, the disease develops to the more aggregative, androgen-independent prostate cancer forms that are resistant to hormone manipulations (2). Although ionizing radiation (IR) is one of the most commonly used therapies to treat many malignancies, its therapeutic efficacy decreases when cancer cells develop adaptive responses to resist IR. Novel therapeutic strategies are therefore needed for the control of prostate cancer that have developed resistance to radiation therapy.
Epidemiologic data suggest that low exposure to sunlight and vitamin D deficiency may be risk factors for development of prostate cancer (3, 4). Substantial experimental studies indicate that secosteroid hormone 1α,25-dihydroxyvitamin D3 [1α,25-(OH)2D3], the active form of vitamin D, exerts both antiproliferative and prodifferentiating effects in many normal and malignant cells including prostate cancer (5, 6). The effects of 1α,25-(OH)2D3 on the growth of human prostate cancer cells vary widely. For example, LNCaP, an androgen-dependent prostate cancer cell line, is responsive to 1α,25-(OH)2D3 (7, 8). Androgen-independent prostate cancer cell lines, such as PC-3 cells, are generally less responsive (9, 10), whereas vitamin D receptor (VDR)–deficient prostate cancer cell line DU-145 exhibits no change in expression of antiapoptotic proteins when exposed to 1α,25-(OH)2D3 (11). The biological actions of 1α,25-(OH)2D3 are primarily mediated by the VDR that binds to the VDR response elements either alone or by forming heterodimers with a retinoid X receptor, resulting in regulation of target genes (12). Several mechanisms have been proposed for the antiproliferative effect of 1α,25-(OH)2D3 in prostate cancer cells, including promotion of cell cycle arrest, induction of apoptosis, inhibition of growth factor, and modulation of kinase pathways (11, 13–18). However, the precise molecular mechanism(s) associated with the antiproliferative effects of 1α,25-(OH)2D3 is not fully elucidated.
Reactive oxygen species–mediated nuclear factor-κB (NF-κB) activation has been implicated in radiation resistance of cancers (19–21). It has been shown that NF-κB activation confers to cancer cells a resistance to IR-induced cell death by up-regulating antiapoptotic proteins, such as bcl-2, bcl-xl (22–24). NF-κB is composed of homodimers or heterodimers formed by five family members [e.g., RelA (p65), RelB, c-Rel, NF-κB1 (p50/p105), and NF-κB2 (p52/p100)]. The NF-κB dimers are inactive when bound by IκB inhibitors in cytoplasm. Transactivation on of NF-κB is mediated by targeting IκB through phosphorylation ubiquitination, and degradation on by proteasome after exposure to many types of stimuli including IR (25). Lessard et al. (26) showed recently that the nuclear localization of RelB correlates to a prostate cancer patient’s Gleason scores. This clinical finding is consistent with our recent study, which showed that IR induces RelB activation in androgen-independent prostate cancer PC-3 cells (27). In the present study, we show that 1α,25-(OH)2D3 sensitizes prostate cancer cells to IR by selectively suppressing expression of RelB, leading to reduction of manganese superoxide dismutase (MnSOD), a mitochondria-located primary antioxidant protein. Transcriptional analysis with NF-κB binding assay indicates that IR-induced NF-κB activation in PC-3 cells is largely mediated by RelB, whose transactivation is blocked by 1α,25-(OH)2D3. Functional studies of the NF-κB target genes reveal that MnSOD is rapidly induced by IR but suppressed by 1α,25-(OH)2D3. Thus, our results provide a potential approach for enhancing the radiosensitivity of androgen-independent prostate cancer using 1α,25-(OH)2D3 coupled to an inhibition of the antioxidant response mechanism and identify mitochondria as a target for the radiosensitization effect of 1α,25-(OH)2D3 in prostate cancer cells.
Materials and Methods
Cell Culture and Treatments
This study used human prostate carcinoma/adenocarcinoma cell lines, including LNCaP and PC-3 and DU-145, which were obtained from the American Type Culture Collection. American Type Culture Collection’s recommended media were used to grow and maintain the appropriate cell lines. 1α,25-(OH)2D3 was purchased from Sigma and dissolved in ethanol at a concentration of 10−2 mol/L. The cultured cells were pretreated with 1α,25-(OH)2D3 by replacing the media containing 1α,25-(OH)2D3 at concentrations of 10−10 to 10−5 mol/L or with ethanol as a control followed by irradiation treatment using a 100 kV X-ray machine (Philips) at a range of 0.5 to 6 Gy.
Cell Survival Analyses
For colony survival analyses, the cells were plated in six-well well plates at low densities and then pretreated with 1α,25-(OH)2D3 for 24 h before radiation treatment. The cells were cultured until colonies formed. The colonies were washed with 1× PBS and stained with a crystal violet dye. The surviving fraction was calculated as the ratio of the number of colonies formed to the number of cells efficiently plated. Trypan blue exclusion assay was used to determine the effects of transfected RelB and VDR short interference RNA (siRNA) on radiosensitization. The cells were plated at a concentration of 105 cells per well. After treatment, the cells were stained with a 0.4% trypan blue dye and counted using a Vi-Cell cell viability analyzer (Beckman Coulter). To estimate the he intake of 1α,25-(OH)2D3 into cells, 7 days after 1α ,25-(,25-(OH)2D3 treatment, media were collected. Concentrations of 1α,25-(OH)2D3 in the collected media were extracted and measured using a 1α,25-dihydroxyvitamin D enzyme immunoassay kit (IDS, Inc.) according to the manufacturer’s protocol. In addition to the two controls provided by the kit, an it, additional control of 1α,25-(OH)2D3 dilution (10−10 mol/L) was included. Testing samples and controls of 1α,25-(OH)2D3 were purified by immunoextraction and then quantified by enzyme immunoassay. Because the sensitivity of the assay is accurate within 5 to 550 pmol/L, a series of dilutions was made to ensure that the level of 1α,25-(OH)2D3 in each sample fell within this linear range. The final concentrations of 1α,25-(OH)2D3 in the samples were calculated using MultiCalc data reduction software (Perkin-Elmer) and normalized by 1α,25-(OH)2D3 dilution control.
Plasmid Construction and Cell Transfection
To study the role of NF-κB in radiosensitization of prostate cancer cells, tandem ligations of four NF-κB motifs were cloned between SacI and XhoI sites of SV40/pG L3 (Promega). Sequences of NF-κB oligonucleotides tagged with SacI and XhoI at their terminals were 5′-CAGTTCAGGGGACTTTCCCAGGC-3′ (upper strand) and 5′-TCGAGGCCTGGGAAAGTCCCCTCAACTGAGCT-3′(lower strand). The construct contains multiple NF-κB elements up-stream of the SV40 promoter-driven luciferase reporter. The generated construct was cotransfected into 1α,25-(OH)2D3–pretreated or unpretreated PC-3 cells with a β-galactosidase expression construct using LipofectAMINE (Invitrogen) according to the manufacturer’s protocol. Transfected cell cells were treated with IR at 6 Gy. Luciferas and β-galactosidase activities were measured as described previously (28). In addition on, expression constructs for the human RelB gene (America Type Culture Collection) and siRNA for targeting RelB and VDR (Santa Cruz Biotechnology) were transfected to modulate their expression levels in the PC-3 cells.
NF-κB Binding Assay
Nuclear extracts from the treated or untreated PC-3 cells were prepared as described previously (29). Binding activities of five members of the NF-κB family were measured using an ELISA-based TransAM NF-κB Family kit (Active Motif)) according to the manufacturer’s protocol.
Chromatin Immunoprecipitation
A ChIP-IT system (Active Motif) was used to study 1α,25-(OH)2D3–mediated transcriptional regulation, according to the manufacturer’s protocol. PC-3 chromatin was pulled down using a p50 antibody, and the NF-κB enhancer region of the MnSOD gene was quantified by PCR. PCR primer sequences were 5′-CGGGGTTATGAAATTTGTTGAGTA-3′ (upper strand) and 5′-CCACAAGTAAAGGACTGAAATTAA-3′ (lower strand). The MnSOD exon 2 was amplified as an untargeted control. Primer sequences were 5′-TGACCGGGCTGTGCTTTCTCG-3′ (upper strand) and 5′-ACTGCCTCCCGCCGCTCAGCC-3′ (lower strand). In addition, Western blots were done to quantify RelB in the chromatin immunoprecipitation preparations.
Reverse Transcription-PCR
mRNA was isolated from the treated and untreated PC-3 cells using a Micro-Fast Track 2.0 mRNA Isolation kit (Invitrogen) and then analyzed using a SuperScript First-Strand Synthesis System for reverse transcription-PCR (RT-PCR; Invitrogen) with gene-specific primers. Primers for amplification of the human RelA and RelB genes were purchased from Santa Cruz Biotechnology. Primers for MnSOD were 5′-AGCATGTTGAGCCGGGCAGT-3′ (forward) and 5′-AGGTTGTTCACGTAGGCCGC-3′ (reverse); primers for bcl-xl were 5′-CCCAGAAAGGATACAGCTGG-3′ (forward) and 5′-GCGATCCGACTCACCAATAC-3′ (reverse); and primers for β-actin were 5′-TGATGATATCGCCGCGCTCGTCGT-3′ (forward) and 5′-CACAGCCTGGATAGCAACGTACAT-3′ (reverse).
RNA Interference
siRNA was used to selectively knock down RelB or VDR in PC-3 cells. siRNA targeting RelB or VDR (0.1 µmol/L) mol/was transfected into the PC-3 cells using LipofectAMINE 2000 within a serum-reduced Opti-MEM (Invitrogen) and followed by 6 Gy IR treatment. Reduction of RelB and VDR and effects on radiosensitization in the cells were quantified by Western blots and trypan blue exclusion assay, respectively.
Immunoblotting Analysis
Total cellular extracts were prepared from treated and untreated PC-3 cells as described previously (27). To quantify levels of the NF-κB family and its target gene, 100 µg of cellular extracts were fractionated by a SDS-PAGE, 8% (w/v) polyacrylamide gel, and then transferred onto a nitrocellulose membrane and blotted with antibodies to RelA, RelB, MnSOD, bcl-x, 24-hydroxylase (CYP24), and β-actin. With the exception of the MnSOD antibody obtained from Upstate Biotechnology, all antibodies were purchased from Santa Cruz Biotechnology. A goat anti-rabbit IgG-horseradish peroxidase–conjugated secondary antibody (Santa Cruz Biotechnology) was used to detect all proteins with the exception that a goat anti-mouse IgG-horseradish peroxidase–conjugated secondary antibody (Santa Cruz Biotechnology) was used to detect β-actin. Immunoblots were visualized by an enhanced chemiluminescence detection system (Amersham Pharmacia Biotech).
SOD Activity Gels
To quantify MnSOD activity, 100 µg of cellular extracts were separated on SDS-PAGE gels. After electrophoresis, the gels were stained with 2.3 mmol/L nitroblue tetrazolium solution for 20 min and with 280 mmol/L TEMED solution for 15 min. After washing with distilled water, SOD activity bands were detected by exposing the gels to fluorescent light.
Quantitative and Statistical Data Analyses
Multiple independent experiments were done. Data of PCR, RT-PCR, Western blots, and SOD activity were quantified using an imaging quantitative software, Quantity One (Bio-Rad). Statistical significances between treatments and controls were analyzed using one-way ANOVA and Tukey’s multiple comparison test followed by data analysis with GraphPad Prism version 4.0. Differences in the comparison tests lower than P < 0.01 level were considered to be significant.
Results
1α,25-(OH)2D3 Enhances Radiosensitivity of Prostate Cancer Cells
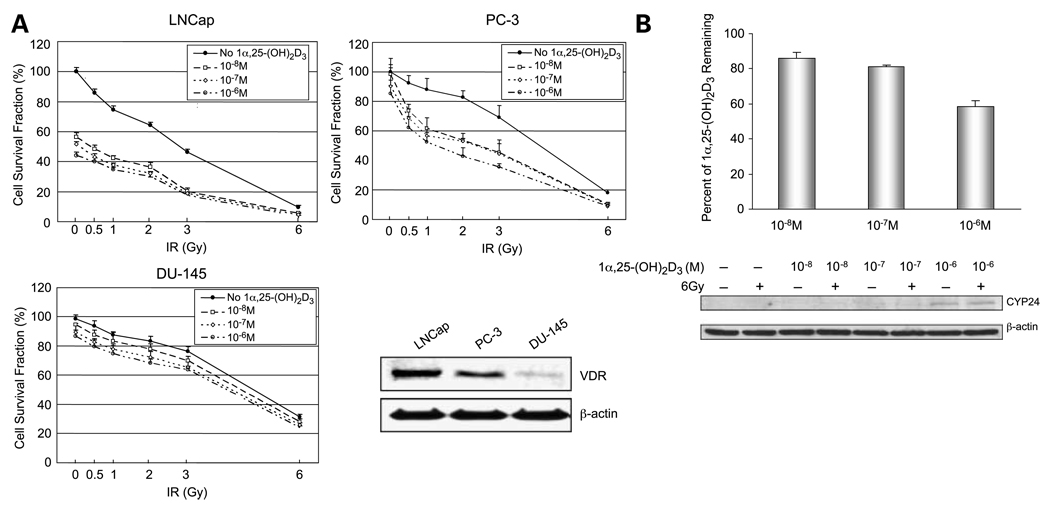
To determine the effect of 1α,25-(OH)2D3 on radiation sensitivity of prostate cancer cells, prostate cancer cells were treated with 1α,25-(OH)2D3 before IR treatment. As shown in Fig. 1A, although 6 Gy IR significantly kills all three cell lines, the androgen-independent prostate cancer cells PC-3 and DU-145 are more resistant to low doses of IR than the androgen-dependent prostate cancer LNCaP cells are. Pretreatment with 1α,25-(OH)2D3 enhances the radiosensitivity of both LNCaP and PC-3 cells, which express a high level of VDR. Importantly, pretreatment with 1α,25-(OH)2D3 significantly enhances the sensitivity of PC-3 as the radiation dose range between 0.5 and 3.0 Gy. In contrast to PC-3 cells, DU-145 cells, which are deficient in VDR, do not show increased radiosensitivity when pretreated with 1α,25-(OH)2D3. These results suggest that 1α,25-(OH)2D3 exerts its radiosensitization effect on prostate cancer cells through VDR.
Figure 1.
Radiosensitization of prostate cancer cells by 1α,25-(OH)2D3. A, three prostate cancer cell lines were treated with different concentrations of 1α,25-(OH)2D3 and then treated with different doses of IR as indicated. Effect of 1α,25-(OH)2D3 on IR-induced cell death was determined by colony survival analysis. Cellular extract from each cell line was used to determine level of VDR by Western blots normalized with β-actin. B, PC-3 (105 cells per well) were treated with different concentrations of 1α,25-(OH)2D3 (10−8−10−6 mol/L) for 7 d. 1α,25-(OH)2D3 remaining in media was extracted and quantified using a 1α,25-(OH)2D3 ELISA kit (top). Concentrations of 1α,25-(OH)2D3 in the media were measured based on a standard curve and normalized with controls provided in the kit. Percentage of 1α,25-(OH)2D3 in the media was calculated by subtracting background levels in the untreated controls and then dividing by the amounts initially added into the media. CYP24 in the cells was detected as a 53-kDa protein by Western blots, which was normalized with β-actin(bottom).
A high dose of 1α,25-(OH)2D3 (10−5 mol/L) is significantly toxic to prostate cancer cells, but not to DU-145 cells, and low doses of 1α,25-(OH)2D3 (<10−9 mol/L) do not enhance radio diosensitivities of prostate cancer cells (data not shown). When concentrations of 1α,25-(OH)2D3 between 10−8 to 10−6 mol/L were added to the media, significant toxicity of PC-3 cells does not occur, but their radiation sensitivity is enhanced (Fig. 1A). Because the levels of 1α,25-(OH)2D3 added to the media may have been excessive, we determined 1α,25-(OH)2D3 remaining in media after 7 days of incubation. As shown in Fig. 1B, the majority of 1α,25-(OH)2D3 was found in the media portions. It has been shown that the major 1α,25-(OH)2D3 catabolic enzyme, CYP24, can be induced in prostate cancer cells, leading to a reduction of 1α,25-(OH)2D3 antiproliferative effects (30, 31). Our finding that 1α,25-(OH)2D3 enhances CYP24 levels in PC-3 cells in a dose-dependent manner (Fig. 1B) agrees with earlier studies. Our results suggest that biological levels of 1α,25-(OH)2D3 effectively enhance radiation sensitivity of prostate cancer cells at a level 10-fold lower than the amount added to the media. Because PC-3 has functional VDR and represents a radiation-resistant androgen-independent prostate cancer, it was used in subsequent experiments as a model cell line to investigate the molecular mechanism for 1α,25-(OH)2D3–mediated radiosensitization.
1α,25-(OH)2D3 Inhibits RelB Expression in Prostate Cancer Cells
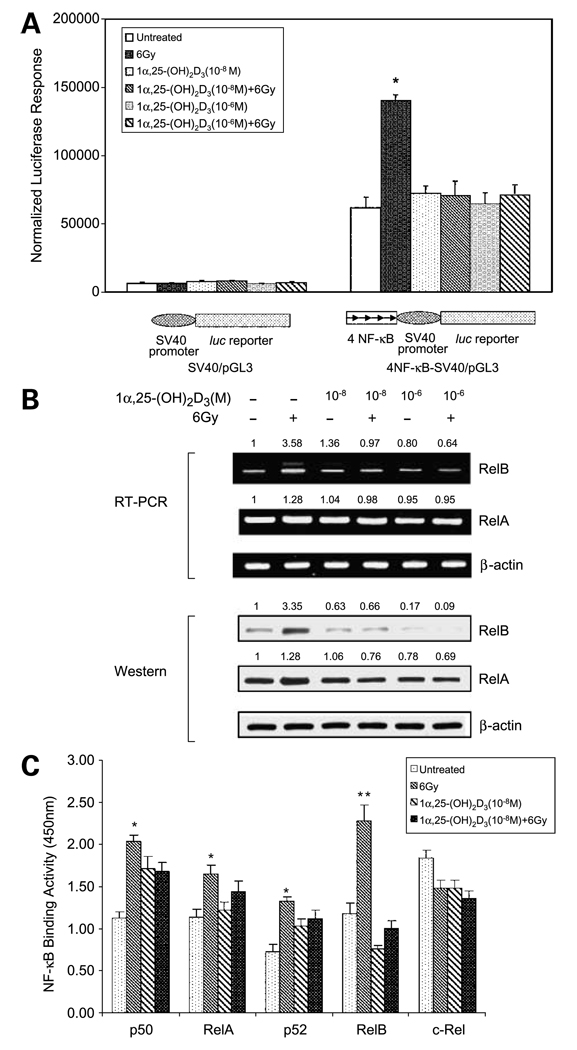
NF-κB activation has been implicated in resistance to IR in many types of cancer including prostate cancer (19–21). As an initial step to examine whether 1α,25-(OH)2D3–induced radiosensitization is mediated through suppression of IR-induced NF-κB activation, a NF-κB–driven luciferase reporter plasmid was used as a probe. The results show that NF-κB elements are necessary for IR-induced luciferase activity but that radiation-induced NF-κB activation was abolished by 1α,25-(OH)2D3 (Fig. 2A). To confirm that 1α,25-(OH)2D3 regulates NF-κB signal transduction, mRNA isolated from PC-3 cells treated with 1α,25-(OH)2D3 were quantified by RT-PCR. The results indicate that IR increases the level of RelB mRNA, and pretreatment with 1α,25-(OH)2D3 blunts this is response. The mRNA level of RelA is only slightly altered by IR, 1α,25-(OH)2D3, or , by a combination of the two. Consistent with the results of RT-PCR, Western blots show that IR causes higher increases of RelB than of RelA in PC-3 cells, but IR-mediated RelB induction is suppressed by 1α,25-(OH)2D3 (Fig. 2B). To further verify the effect of 1α,25-(OH)2D3 on NF-κB family members, a NF-κB binding assay was done for all known members of the NF-κB family. An oligonucleotide containing NF-κB consensus sequence was bound with nuclear extracts from the treated and untreated PC-3 cells and then incubated with antibodies against each of the five NF-κB family members. The binding activities were quantified by ELISA assay. The results confirm that IR can activate all members of the NF-κB family, with the exception of c-rel, in PC-3 cells. Importantly, 1α,25-(OH)2D3 selectively blocks locks IR-induced RelB activation (Fig. 2C). These results suggest that RelB activation may be a major contributor to NF-κB–mediated radiation resistance in prostate cancer cells and that 1α,25-(OH)2D3 enhances radiosensitization through selectively suppressing RelB activation.
Figure 2.
Association on of 1α,25-(OH)2D3–mediated radiosensitivity of PC-3 cells and RelB suppression. A, luciferase reporter constructs shown were cotransfected into PC-3 cells with a β-galactosidase expression construct. Positions and orientations of four NF-κB elements are indicated by arrows . The transfected cells were treated with 1α,25-(OH)2D3 and IR as described in Fig. 1. After 24 h, activities of luciferase and β-galactosidase were measured. Transcription regulated by the NF-κB was estimated by β-galactosidase–normalized luciferase activity. *, significant difference compared with both untreated group and 1α,25-(OH)2D3–pretreated groups. B, mRNA and total proteins were extracted from the treated an and untreated treated PC-3 cells. Effect of IR alone or 1α,25-(OH)2D3 with IR on RelA or RelB expression was determine determined by RT-PCR (top) and by Western blots (bottom). β-actin serves as a control to normalize RelA and RelB signals. The relative sign signals in the treated groups were further normalized by untreated controls. Fold increases or decreases are indicated above corresponding bands. C, 24 h after treatment, nuclear clear extracts from the treated or untreated PC-3 cells were subjected to the NF-κB binding assay kit. Binding activity of each member of the NF-κB family was determined by ELISA analysis. *, significant differences between the untreated groups and the IR-treated groups; **, significant difference of the IR-treated group compared with both the untreated and the combined treatment of 1α,25-(OH)2VD31α,25-(OH)2D3 and IR.
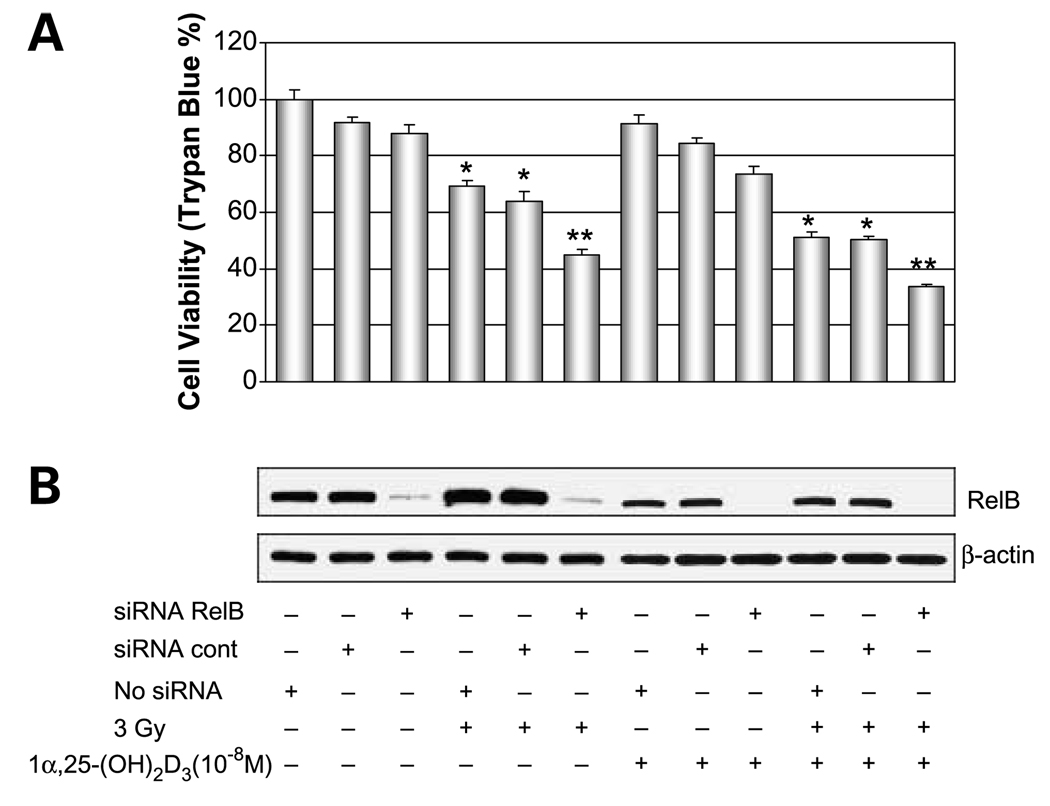
Reduction of RelB Is Critical for 1α,25-(OH)2D3-Enhanced Radiosensitization
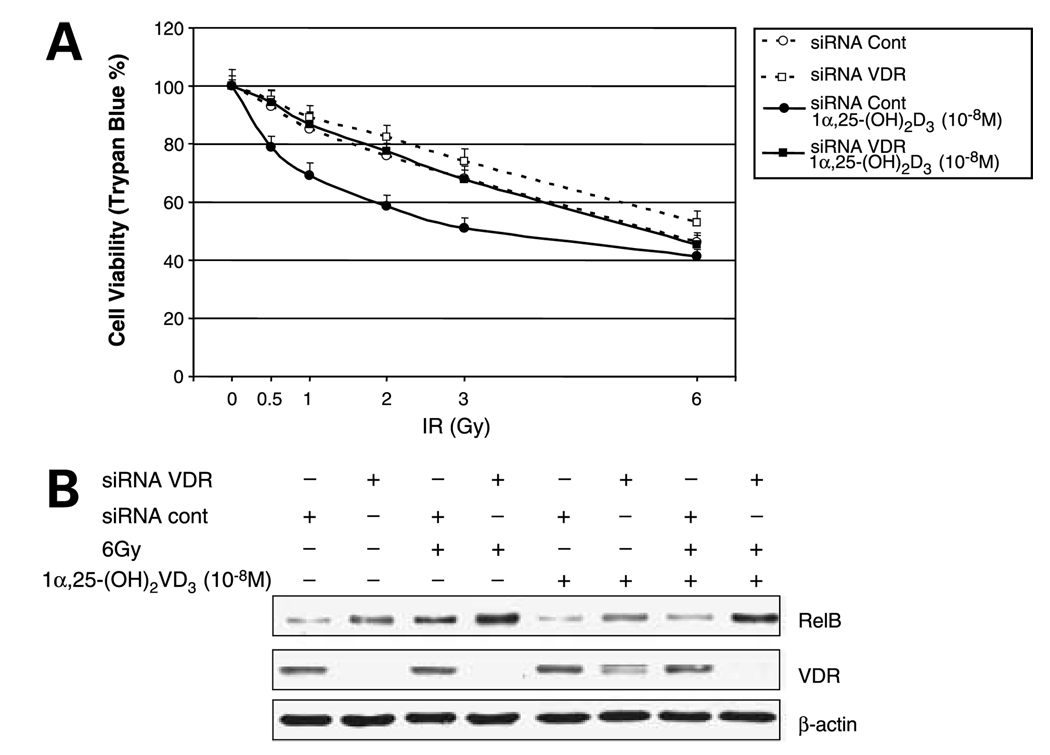
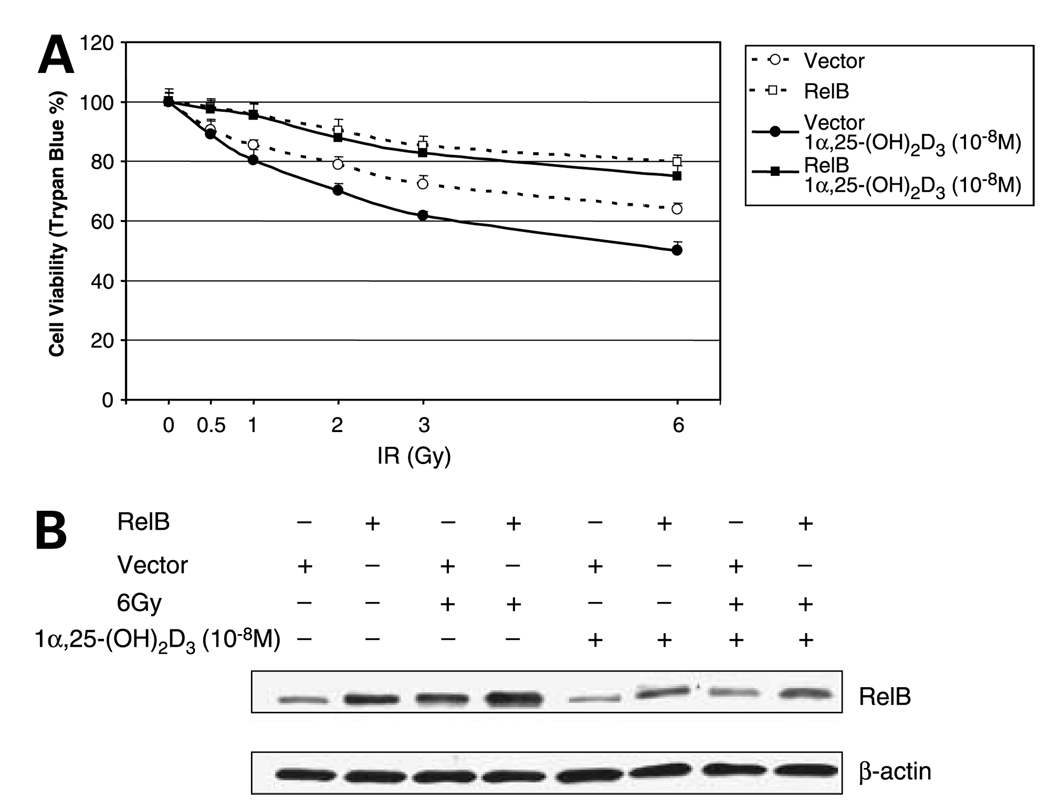
RelB is a member of the NF-κB family that is associated with advanced stages of prostate cancer (26). To verify that 1α,25-(OH)2D3 selectively suppresses RelB expression leading to enhanced radiosensitization, three approaches were used. Because 1α,25-(OH)2D3 exerts its cytotoxic effect via VDR, in the first approach, a VDR siRNA was transfected into the 1α,25-(OH)2D3–pretreated PC-3 cells to selectively knock down expression of VDR. As expected, VDR siRNA-transfected cells become resistant to IR (Fig. 3A). Western blots confirmed that expression of VDR is reduced by the targeted siRNA but not by the siRNA control. Consistent with reduced VDR, the level of RelB is increased in the siRNA-targeted cells (Fig. 3B). In the second approach, RelB was overexpressed in PC-3 cells. The human RelB gene is driven by the cytomegalovirus promoter that lacks a VDR element; thus, expression of RelB by this his construct is not affected by 1α,25-(OH)2D3. Cell viability of transfected cells shows that the increased RelB confers cellular resistance to IR compared with the vector only control (Fig. 4A). Western blots confirmed that RelB is ectopically expressed in 1α,25-(OH)2D3–pretreated PC-3 cells but is not reduced by 1α,25-(OH)2D3 (Fig. 4B). In the third approach, to further establish the role of RelB in mediating 1α,25-(OH)2D3– enhanced radiosensitization, we did experiments using siRNA-based knocked-down RelB in the presence and absence of 1α,25-(OH)2D3. RelB siRNA was transfected into1α,25-(OH)2D3–pretreated or untreated PC-3 cells and then treated with IR. Similar to the effect of 1α,25-(OH)2D3 treatment, the siRAA-based down-regulation of RelB expression enhances radiosensitization (Fig. 5A). Western blots verified that the transfected RelB siRNA significantly reduces RelB expression on levels in both 1α,25-(OH)2D3 –pretreated and unpretreated cells (Fig. 5B). The combined suppressive effects of iRNA targeting and 1α,25-(OH)2D3 result in the lowest RelB expression level in 1α,25-(OH)2 VD3–pretreated cells. These results further support they hypothesis that suppression of RelB by 1α,25-(OH)2D3 is a mechanism for radiosensitizing prostate cancer cells.
Figure 3.
Radioresistance of PC-3 cells by siRNA targeting VDR. A, VDR siRNA and siRNA control (Cont) were transfected into 1α,25-(OH)2D3–pretreated or unpretreated PC-3 cells before the IR treatment. After 24 h, effects of transfected siRNAs on cell survival were determined by trypan blue assay. B, knocked-down expression levels of VDR and resulting increases in RelB expression by the siRNA target were confirmed by Western blots normalized with β-actin.
Figure 4.
Radioresistance of PC-3 cells by overexpression of RelB. A, a RelB expression construct was transfected into 1α,25-(OH)2D3–pretreated or unpretreated PC-3 cells before the IR treatment. After 24 h, effect of the expressed RelB on cell survival was determined by trypan blue assay. B, ectopically expressed RelB was confirmed by Western blots normalized with β-actin.
Figure 5.
Radiosensitization of PC-3 cells by siRNA targeting RelB. Control siRNA and RelB siRNA were transfected into 1α,25-(OH)2D3–pretreated and unpretreated PC-3 cells before the IR treatment. No siRNA transfection was added for control. After treatment for 24 h, effect of the knocked-down RelB level on cell survival was determined by trypan blue assay (A) and suppression of RelB by siRNA targeting was confirmed by Western blots normalized with β-actin (B). *, significant differences compared with non – IR-treated groups; **, significant differences compared red with the siRNA control groups in either non – IR-treated or IR-treated groups.
1α,25-(OH)2D3 Suppresses MnSOD, a RelB-Regulated Primary Antioxidant Protein
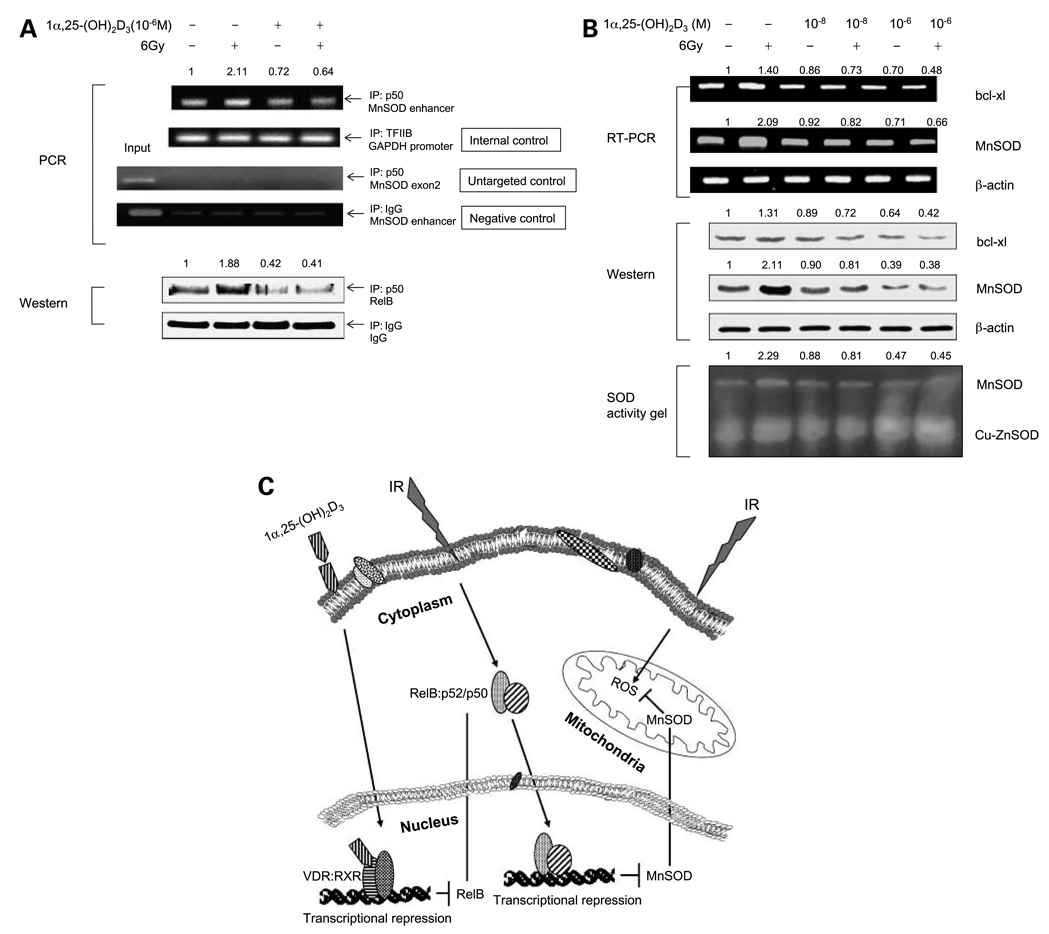
Reactive oxygen species–induced toxicity has been implicated in IR-mediated cell death (19–21). An important mitochondrial antioxidative protein, MnSOD, is rapidly induced by reactive oxygen species through NF-κB–mediated transcriptional activation (27, 28). To identify RelB target genes responsible for the 1α,25-(OH)2D3–enhanced radiosensitization, we examined the effects of RelB on the transcription of the human MnSOD gene. Chromatin immunoprecipitation assay was done to detect RelB binding to the intronic NF-κB enhancer region of the gene. A p50 antibody was used to precipitate chromatin, and DNA was analyzed by PCR using the gene-specific primers. The results shown in Fig. 6A indicate that IR increases the amount of the enhancer region pulled down by the p50 antibody, but pretreatment with 1α,25-(OH)2VD3 reduces the PCR products. As an internal control, a transcription factor IIB–associated glyceraldehyde-3-phosphate dehydrogenase promoter fragment was precipitated by the transcription factor IIB antibody, which was not affected by the treatments. A fragment (459–612) located in the MnSOD exon 2 was amplified as an untargeted control and chromatin pulled down by an IgG served as a negative antibody control. The expected fragments were amplified using input DNA templates, and no PCR product was observed from these controls. In addition, Western blots showed that the amount of RelB in chromatin is increased by IR but reduced by 1α,25-(OH)2D3, whereas , the IgG control does not respond to the treatments.
Figure 6.
1α,25-(OH)2D3 – mediated suppression of RelB target genes. A, chromatin from the treated and untreated PC-3 cells was precipitated using p50, transcription factor IIB (TFIIB), or IgG antibody. The NF-κB enhancer region of the human MnSOD gene was analyzed by PCR (top). Fragments of the exon 2 of the human MnSOD gene and the glyceraldehyde-3-phosphate dehydrogenase promoter region were amplified as an untargeted targeted control and an internal control, respectively. IgG-precipitated product served as a negative antibody control. RelB in the precipitated chromatin was measured by Western blots normalized with IgG (bottom). B, 24 h after treatment, mRNA and total proteins from the untreated or treated PC-3 cells were quantified for expression on levels of MnSOD and bcl-xl by RT-PCR (top), by Western blots (middle), and by SOD activity gel (bottom). The expression levels of the target genes shown in RT-PCR and Western blots were normalized by β-actin.MnSOD activity was compared with copper/zinc SOD (Cu-ZnSOD) activity. PCR products of the MnSOD enhancer region and RelB amounts in (A) and expression of MnSOD shown in the three panels of (B) were normalized by the relating controls, and fold increases or decreases were calculated by normalizing amounts in the treated groups with the untreated controls as described in Fig. 2B. C, a model showing the effect of IR on RelB and various steps where the expression of MnSOD SOD can be affected by 1α,25-(OH)2D3 in prostate cancer cells.
To verify that MnSOD is a major or RelB target involved in the modulation of radiosensitization by 1α,25-(OH)2D3, mRNA and cellular proteins were extracted from PC-3 cells after treatments and then quantified by RT-PCR using the gene-specific primers and by Western blots with the protein-specific antibodies. To further verify that the effect of 1α,25-(OH)2D3 on MnSOD suppression leads to a reduction of MnSOD activity, the same amounts of cellular extracts used for Western blots were also quantified by analysis of SOD activity gels. The results, shown in the three panels of Fig. 6B, are consistent with that IR significantly induces MnSOD expression but is blocked by 1α,25-(OH)2D3, whereas bcl-xl is less responsive to the treatment, suggesting that MnSOD is a major or target of RelB in response to IR. Thus, removal of MnSOD induction by 1α,25-(OH)2D3 likely facilitates radiosensitization, as shown in Fig. 6C
Discussion
NF-κB has been shown to respond rapidly to a variety of stimuli by selectively activating prosurviving genes to prevent stimulus-induced apoptosis (32). In general, cancer cells express high levels of constitutive NF-κB compared with their normal counterparts (33). Importantly, NF-κB transactivation is induced in many types of cancer by therapeutics including radiation (21, 34, 35). Thus, inhibition of the NF-κB pathway is being used to search for novel anticancer drugs that selectively kill tumor cells. Accumulated evidence shows that combinations of certain effective NF-κB inhibitors with conventional radiotherapeutic or chemotherapeutic treatments can improve the efficiency of standard therapies (34–36). Studies to inhibit NF-κB as a means to enhance cancer therapy have traditionally focused on the canonical dimer p50/RelA because it is the best-known dimer of the NF-κB family for being responsive to cytokine-mediated NF-κB transactivation. In fact, inhibition of p65 and ablation of IκB kinase β are beneficial for enhancing chemosensitivity and radiosensitivity, respectively, (35, 37, 38). However, among NF-κB family members, the nuclear level of RelB in prostate cancer patients correlates with Gleason scores (26). Recently, we showed that the noncanonical dimer p52/RelB is more important in protecting prostate cancer cells against IR than p50/RelA is (27). The present study extends our previous findings to show that IR induces RelB in prostate cancer cells to a greater extent than other members of the NF-κB family and that 1α,25-(OH)2D3 selectively inhibits radiation-induced RelB in prostate cancer cells. Thus, 1α,25-(OH)2D3 may be effective for enhancing the susceptibility of prostate cancer cells with high Gleason scores to IR.
1α,25-(OH)2D3, a , member of a steroid hormone family, is known to regulate calcium homeostasis and bone formation. Several studies have shown its significant antiproliferative activity when administered to several types of cancer in vitro and in vivo (5, 6, 39, 40). In particular, the association between vitamin D deficiencies with prostate carcinogenesis has provided a rational basis for clinical trials to use 1α,25-(OH)2D3 and its analogues in the control of prostate cancer (40, 41). However, little is known about the potential effect of 1α,25-(OH)2D3 on sensitization of prostate cancer cells to radiation therapy. 1α,25-(OH)2D3 exerts its biological actions through nuclear receptor-dependent ligand transcriptional regulation of target genes. Our results, which indicate that androgen-dependent prostate cancer LNCaP cells with a high level of VDR are sensitive to both 1α,25-(OH)2D3 and IR, are consistent with our previous studies that showed lower levels of RelB and MnSOD in LNCaP cells than in androgen-independent prostate cancer cells (27). In contrast to LNCaP cells, PC-3 cells, which express VDR with a high level of RelB, are less sensitive to IR, indicating that RelB may play an important role in protecting prostate cancer cells against IR. 1α,25-(OH)2D3 consistently and efficiently suppresses RelB and significantly enhances the radiosensitivity of PC-3 cells, suggesting that inhibition of RelB may serve as a mechanism for 1α,25-(OH)2D3 –mediated radiosensitization. Additionally, VDR-deficient androgen-independent prostate cancer DU-145 cells are refractory to the radiosensitization effect of 1α,25-(OH)2D3, which , is consistent with the requirement of VDR in order for 1α,25-(OH)2D3 to mediate radiosensitization. Recent epidemiologic studies of prostate cancer have shown that risks associated with VDR polymorphisms are significantly reduced in the presence of high sun exposure (4, 42), suggesting that for most prostate cancer patients, functional VDR for 1α,25-(OH)2D3 has a biological effect. It is noteworthy that the radiosensitization effect of 1α,25-(OH)2D3 is observed with doses of IR as low as 2 Gy. This observation may be clinically important because 2 Gy is used as the standard daily dose of fractionated external radiation. It should also be noted that the range of 1α,25-(OH)2D3 concentrations added to the media is ~ 10 times higher than the accumulated intracellular level of 1α,25-(OH)2D3. Thus, a cellular level of 1α,25-(OH)2D3 in the nanomolar range is needed to effectively sensitize radiation-resistant prostate cancer cells. This level of 1α,25-(OH)2D3, coupled , to the fact that the major 1α,25-(OH)2D3 catabolizing enzyme, CYP24 (43), is increased in a 1α,25-(OH)2D3 concentration-dependent manner, suggests that further development of dosing methods and/or 1α,25-(OH)2D3 analogues is needed to fully determine the usefulness of vitamin D for sensitization of radiation therapy. Recently, several clinical trials using intermittent dosing in dose escalation studies have suggested that a peak blood level of 1α,25-(OH)2D3 in the nanomolar range is achievable in patients (44, 45). Alternative routes of administration have been used in patients with hepatocellular carcinoma (46). Although the effectiveness of regional administration of 1α,25-(OH)2D3 remains to be proven, it is interesting to note that the prostate is one organ most amendable to such an approach. These possibilities, coupled to our finding that the radiosensitization effect of 1α,25-(OH)2D3 is, at least in part, mediated by the suppression of RelB, suggest that further development of vitamin D analogues that can effectively block lock RelB transcription may be particularly useful for radiosensitization of radiation-resistant prostate cancer cells.
We have shown previously that activation of NF-κB is important for induction of MnSOD, a primary antioxidant enzyme that removes superoxide radicals generated in mitochondria (28, 47). Our previous results show that RelB plays an important role in IR-induced MnSOD expression in prostate cancer cells (27). It has been reported that 1α,25-(OH)2D3 transcriptionally represses the human and mouse RelB genes through interaction with VDR that specifically binds to VDR response elements located in the RelB promoter regions (48, 49). The results from the present study further show that 1α,25-(OH)2VD3 represses IR-induced RelB transcription leading to suppression of RelB-mediated radioprotection. Together, these findings identify a potential approach to enhance the efficacy of IR therapy in prostate cancer cells by targeting RelB. As depicted in Fig. 6C, IR activates NF-κB members, RelB in particular, leading to induction of MnSOD and resulting in protection against IR-induced cell death. 1α,25-(OH)2D3 acts to repress RelB transcription through interaction with VDR that at binds to VDR response elements. Down-regulation of RelB by 1α,25-(OH)2VD3, in , turn, blocks RelB-mediated MnSOD induction by IR.
In addition to MnSOD, bcl-xl, a NF-κB target antiapoptotic protein, whose response is widely thought to be involved in both chemoresistance and radioresistance(22–24), is also changed by modulating RelB level in cells. However, our results suggest that induction of bcl-xl may not be the primary response to IR. Response to IR occurs in MnSOD as early as 3 h after IR treatment, but in bcl-xl, response is detectable after 12 h (data not shown). It is possible that IR induces expression of MnSOD and bcl-xl in prostate cancer cells to sequentially protect the cells against IR. The ability of MnSOD to remove reactive oxygen species necessitates a rapid induction of MnSOD by IR, whereas the function of bcl-xl to inhibit apoptosis initiated by reactive oxygen species renders further protection. Because apoptosis is not the main mechanism for the death of cancer cells in response to radiation (50), inhibition of MnSOD may participate in the red reduction of primary and/or secondary adaptive responses by IR, which could efficiently enhance the efficacy of radiotherapy.
Acknowledgments
Grant support: University of Kentucky Research Foundation and NIH grant CA 49797.
References
- 1.Gronberg H. Prostate cancer epidemiology. Lancet. 2003;361:859–864. doi: 10.1016/S0140-6736(03)12713-4. [DOI] [PubMed] [Google Scholar]
- 2.Santen R. Clinical review 37: endocrine treatment of prostate cancer. J Clin Endocrinol Metab. 1992;75:685–689. doi: 10.1210/jcem.75.3.1517354. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz GG, Hulka BS. Is vitamin D deficiency a risk factor for prostate cancer? (Hypothesis) Anticancer Res. 1990;10:1307–1311. [PubMed] [Google Scholar]
- 4.John EM, Schwartz GG, Koo J, et al. Sun exposure, vitamin D receptor gene polymorphisms, and risk of advanced prostate cancer. Cancer Res. 2005;65:5470–5479. doi: 10.1158/0008-5472.CAN-04-3134. [DOI] [PubMed] [Google Scholar]
- 5.Mehta RG, Mehta RR. Review, vitamin D and cancer. J Nutr Biochem. 2002;13:252–264. doi: 10.1016/s0955-2863(02)00183-3. [DOI] [PubMed] [Google Scholar]
- 6.Stewart LV, Weigel NL. Vitamin D and prostate cancer. Exp Biol Med. 2004;229:277–284. doi: 10.1177/153537020422900401. [DOI] [PubMed] [Google Scholar]
- 7.Miller GJ, Stapleton GE, Ferrara JA, et al. The human man prostatic carcinoma cell line LNCaP expresses biologically active, specific receptors for 1 α,25-dihydroxyvitamin D3. Cancer Res. 1992;52:515–520. [PubMed] [Google Scholar]
- 8.Blutt SE, Polek TC, Stewart LV, et al. A calcitriol analogue, EB1089, iinhibits the growth of LNCaP tumors in nude mice. Cancer Res. 2000;60:779–782. [PubMed] [Google Scholar]
- 9.Skowronski RJ, Peehl DM, Feldman D. Vitamin D and prostate cancer: 1,25 dihydroxyvitamin D3 receptors and actions in human prostate cancer cell lines. Endocrinology. 1993;132:1952–1960. doi: 10.1210/endo.132.5.7682937. [DOI] [PubMed] [Google Scholar]
- 10.Ikezoe T, Gery S, Yin D, et al. CCAAT/enhancer-binding protein δ: a molecular target of 1,25-dihydroxyvitamin D3 in androgen-responsive prostate cancer LNCaP cells. Cancer Res. 2005;65:4762–4768. doi: 10.1158/0008-5472.CAN-03-3619. [DOI] [PubMed] [Google Scholar]
- 11.Guzey M, Kitada S, Reed JC. Apoptosis induction by 1α25-dihydroxyvitamin D3 in prostate cancer. Mol Cancer Ther. 2002;1:667–677. [PubMed] [Google Scholar]
- 12.Carlberg C, Bendik I, Wyss A, et al. Two nuclear signalling pathways for vitamin D. Nature. 1993;361:657–660. doi: 10.1038/361657a0. [DOI] [PubMed] [Google Scholar]
- 13.Guzey M, Luo J, Getzenberg RH. Vitamin D3 modulated gene expression on patterns in human primary normal and cancer prostate cells. J Cell Biochem. 2004;93:271–285. doi: 10.1002/jcb.20182. [DOI] [PubMed] [Google Scholar]
- 14.Bao BY, Hu YC, Ting HJ, Lee YF. Androgen signaling is required for the vitamin D-mediated growth inhibition in human prostate cancer cells. Oncogene. 2004;23:3350–3360. doi: 10.1038/sj.onc.1207461. [DOI] [PubMed] [Google Scholar]
- 15.Yang ES, Burnstein KL. Vitamin D inhibits G1 to S progression in LNCaP prostate cancer cells through p27Kip1 stabilization and Cdk2 mislocalization to the cytoplasm. J BiolChem. 2003;278:46862–46868. doi: 10.1074/jbc.M306340200. [DOI] [PubMed] [Google Scholar]
- 16.Polek TC, Stewart LV, Ryu EJ, et al. p53 is required for 1,25-dihydroxyviitamin D3-induced G0 arrest but is not required for G1 accumulation or apoptosis of LNCaP prostate cancer cells. Endocrinology. 2003;144:50–60. doi: 10.1210/en.2001-210109. [DOI] [PubMed] [Google Scholar]
- 17.Matilainen M, Malinen M, Saavalainen K, Carlberg C. Regulation on of multiple insulin-like grow factor binding protein genes by 1α,25-dihydroxyviitamin D3. Nucleic Acids Res. 2005;33:5521–5532. doi: 10.1093/nar/gki872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nonn L, Peng L, Feldman D, Peehl DM. Inhibition of p38 by vitamin in D reduces interleukin-6 production in normal prostate cells via mitogen-activated activated protein kinase phosphatase 5: implications for prostate cancer prevention by vitamin D. Cancer Res. 2006;66:4516–4524. doi: 10.1158/0008-5472.CAN-05-3796. [DOI] [PubMed] [Google Scholar]
- 19.Kimura K, Bowen C, Spiegel S, Gelmann EP. Tumor necrosis factor-α sensitizes prostate cancer cells to γ-irradiation-induced apoptosis. Cancer Res. 1999;59:1606–1614. [PubMed] [Google Scholar]
- 20.Criswell T, Leskov K, Miyamoto S, et al. Transcription factors activated in mammalian cells after clinically relevant doses of ionizing radiation. Oncogene. 2003;22:5813–5827. doi: 10.1038/sj.onc.1206680. [DOI] [PubMed] [Google Scholar]
- 21.Magne N, Toillon RA, Bottero V, et al. NF-κB modulation and ionizing radiation: mechanisms and future directions for cancer treatment. Cancer Lett. 2006;231:158–168. doi: 10.1016/j.canlet.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 22.Findley HW, Gu L, Yeager AM, Zhou M. Expression and regulation on of Bcl-2 Bcl-xl, and Bax correlate with p53 status and sensitivity to apoptosis in childhood acute lymphoblastic leukemia. Blood. 1997;89:2986–2993. [PubMed] [Google Scholar]
- 23.Lee JU, Hosotani R, Wada M, et al. Role of Bcl-2 family proteins (Bax, Bcl-2, and Bcl-X) on cellular susceptibility to radiation in pancreatic cancer cells. Eur J Cancer. 1999;35:1374–1380. doi: 10.1016/s0959-8049(99)00134-3. [DOI] [PubMed] [Google Scholar]
- 24.Su ZZ, Lebedeva IV, Sarkar D, et al. Ionizing radiation enhances therapeutic activity of mda-7/IL-24: overcoming radiation- and mda-7/IL-24-resistance in prostate cancer cells overexpressing the antiapoptotic proteins bcl-xL or bcl-2. Oncogene. 2006;25:2339–2348. doi: 10.1038/sj.onc.1209271. [DOI] [PubMed] [Google Scholar]
- 25.Ghosh S, Karin M. Missing pieces in the NF-κB puzzle. Cell. 2002;109:81–96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 26.Lessard L, Begin LR, Gleave ME, et al. Nuclear localisation of nuclear clear factor-κB transcription factors in prostate cancer: an immunohistochemical study. Br J Cancer. 2005;93:1019–1023. doi: 10.1038/sj.bjc.6602796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Josson S, Xu Y, Fang F, et al. RelB regulates manganese superoxide dismutase gene and resistance to ionizing radiation of prostate cancer cells. Oncogene. 2006;25:1554–1559. doi: 10.1038/sj.onc.1209186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu Y, Kiningham KK, Devalaraja MN, et al. An intronic NF-κB element is essential for induction of the human manganese superoxide dismutase gene by tumor necrosis factor-α and interleukin-1β. DNA Cell Biol. 1999;18:709–722. doi: 10.1089/104454999314999. [DOI] [PubMed] [Google Scholar]
- 29.Xu Y, Porntadavity S, St Clair DK. Transcriptional regulation of the human manganese superoxide dismutase gene: the role of specificity protein 1 (Sp1) and activating protein-2 (AP-2) Biochem J. 2002;362:401–412. doi: 10.1042/0264-6021:3620401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skowronski RJ, Peehl DM, Feldman D. Actions of vitamin D3, analogs on human prostate cancer cell lines: comparison with 1,25-dihydroxyvitamin in D3. Endocrinology. 1995;136:20–26. doi: 10.1210/endo.136.1.7530193. [DOI] [PubMed] [Google Scholar]
- 31.Lou YR, Tuohimaa P. Androgen enhances the antiproliferative activity of vitamin D3 by suppressing 24-hydroxylase expression in LNCaP cells. J Steroid Biochem Mol Biol. 2006;99:44–49. doi: 10.1016/j.jsbmb.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 32.Aggarwal BB. Nuclear clear factor-κB: the enemy within. Cancer Cell. 2004;6:203–208. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 33.Karin M. Nuclear factor-κB in cancer development and progression. Nature. 2006;44:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 34.Baldwin AS. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-κB. J Clin Invest. 2001;107:241–246. doi: 10.1172/JCI11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo J, Verma UN, Gaynor RB, et al. Enhanced chemosensitivity to irinotecan by RNA interference-mediated down-regulation of the nuclear factor-κB p65 subunit. Clin Cancer Res. 2004;10:3333–3341. doi: 10.1158/1078-0432.CCR-03-0366. [DOI] [PubMed] [Google Scholar]
- 36.Lin A, Karin M. NF-κB in cancer: a marked target. Semin Cancer Biol. 2003;13:107–114. doi: 10.1016/s1044-579x(02)00128-1. [DOI] [PubMed] [Google Scholar]
- 37.Egan LJ, Eckmann L, Greten FR, et al. IκB-kinaseβ-dependent NF-κB activation provides radioprotection to the intestinal epithelium. Proc Natl Acad Sci U S A. 2004;101:2452–2457. doi: 10.1073/pnas.0306734101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luo JL, Kamata H, Karin M. IKK/NF-κB signaling: balancing life and death—a new approach to cancer therapy. J Clin Invest. 2005;115:2625–2632. doi: 10.1172/JCI26322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krishnan AV, Peehl DM, Feldman D. The role of vitamin D in prostate cancer. Recent Results Cancer Res. 2003;164:205–221. doi: 10.1007/978-3-642-55580-0_15. [DOI] [PubMed] [Google Scholar]
- 40.Krishnan AV, Peehl DM, Feldman D. Inhibition on of prostate cancer growth by vitamin D: regulation of target gene expression. J Cell Biochem. 2003;88:363–371. doi: 10.1002/jcb.10334. [DOI] [PubMed] [Google Scholar]
- 41.Vijayakumar S, Mehta RR, Boerner PS, et al. Clinical trials involving vitamin D analogs in prostate cancer. Cancer J. 2005;11:362–373. doi: 10.1097/00130404-200509000-00002. [DOI] [PubMed] [Google Scholar]
- 42.Price DK, Franks ME, Figg WD. Genetic variations in the vitamin D receptor, androgen receptor, and enzymes that regulate androgen meta metabolism. J Urol. 2004;171:S45–S49. doi: 10.1097/01.ju.0000108402.60404.48. [DOI] [PubMed] [Google Scholar]
- 43.Toman M, Tenenhouse HS, Jones G. 1, 25-Dihydroxyvitamin D3-inducible catabolism of vitamin D metabolites in mouse intestine. Am J Physiol. 1990;258:G557–G563. doi: 10.1152/ajpgi.1990.258.4.G557. [DOI] [PubMed] [Google Scholar]
- 44.Beer TM, Myrthue A. Calcitrol in cancer treatment: from the lab to the clinic. Mol Cancer Ther. 2004;3:373–381. [PubMed] [Google Scholar]
- 45.Masuda S, Jones G. Promise of vitamin D analogues in the treatment of hyperproliferative conditions. Mol Cancer Ther. 2006;5:797–808. doi: 10.1158/1535-7163.MCT-05-0539. [DOI] [PubMed] [Google Scholar]
- 46.Morris DL, Jourdan JL, Finlay I, et al. Pourgholami MH Hepatic intra arterial injection of 1, 25-dihydroxyvitamin D3 in lipiodol: pilot study in patients with hepatocellular carcinoma. Int J Oncol. 2002;21:901–906. doi: 10.3892/ijo.21.4.901. [DOI] [PubMed] [Google Scholar]
- 47.Dhar SK, Lynn BC, Daosukho C, St Clair DK. Identification of nucleophosmin as an NF-κB co-activator for the induction of the human sod2 gene. J BiolChem. 2004;279:28209–28219. doi: 10.1074/jbc.M403553200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dong X, Craig T, Xing N, et al. Direct transcriptional regulation of RelB by 1α,25-dihydroxyvitamin D3 and its analogs: physiologic and therapeutic implications for dendritic cell function. J Biol Chem. 2003;278:49378–49385. doi: 10.1074/jbc.M308448200. [DOI] [PubMed] [Google Scholar]
- 49.Dong X, Lutz W, Schroeder TM, et al. Regulation of relB in dendritic cells by means of modulated association of vitamin in D receptor and histone deacetylase 3 with the promoter. Proc Natl Acad Sci U S A. 2005;102:16007–16012. doi: 10.1073/pnas.0506516102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brown JM, Attardi LD. The role of apoptosis is in cancer development and treatment response. Nat Rev Cancer. 2005;5:231–237. doi: 10.1038/nrc1560. [DOI] [PubMed] [Google Scholar]