Abstract
Background
The segment of the vein mobilized for arterial anastomosis in the creation of an arteriovenous fistula (AVF) is the swing segment. This segment may experience turbulent flow and altered shear mechanical stress that result in stenosis. We sought to determine the frequency of stenotic lesions in the swing segment.
Study Design
Case series.
Settings & Participants
From January 31, 2003, to June 30, 2005, records of all patients referred to an outpatient hemodialysis vascular access center for AVF dysfunction were reviewed (n = 484). Of these, 278 patients had angiographically documented stenosis (any degree of luminal narrowing) on their first visit.
Outcomes & Measurements
Distribution of stenoses in different segments of the AVF. Swing-segment stenoses were classified as proximal (outflow into axillary vein system), distal or juxta-anastomotic (adjacent to the anastomosis), and the cephalic arch.
Results
Overall prevalence of angiographically documented swing segment stenosis (proximal, distal or juxta-anastomotic, and cephalic arch) was 45.7% (127 of 278 patients), whereas the remaining stenoses (151 of 278 patients) were distributed among the puncture zone, arterial, arterial anastomosis, and central veins. The most frequent location of the swing-segment stenosis was juxta-anatomosis (63%; 80 of 127 patients), followed by cephalic arch (19%; 24 of 127 patients) and proximal swing segment (18%; 23 of 127 patients). The distribution of swing-segment stenosis (n = 127) was equivalent among the various fistulas (brachial-cephalic, 35.4%; radial-cephalic, 33.9%; and brachial-basilic, 30.7%). Eighty-three percent of swing-segment stenoses were significant (>50% luminal narrowing) and underwent percutaneous transluminal angioplasty, with a 93% success rate.
Limitations
Retrospective nature of the study and potential selection bias.
Conclusion
In our population, swing-segment stenosis is the most common lesion in dysfunctional AVFs; juxta-anastomotic stenosis is the predominant lesion independent of fistula type. Whether the occurrence of swing-segment stenosis is caused by mobilization of the vein during surgery is not clear.
Keywords: Swing segment, stenosis, arteriovenous fistula (AVF), angioplasty, prevalence
Arteriovenous fistulas (AVFs) currently are regarded as the gold standard for dialysis access because of their superior long-term patency and lower intervention and infection rates compared with other types of dialysis accesses.1-3 However, AVFs are prone to early failure because of poor maturation and subsequent thrombosis resulting from several factors, including inadequate inflow or limited venous outflow.4
The Fistula First project was initiated by the Centers for Medicare & Medicaid Services with the goal of AVF rates of 50% in incident and 40% in prevalent hemodialysis patients.5 With this increasing trend toward predialysis fistula placement and imminent increase in problems, early identification of lesions associated with this preferred type of vascular access becomes a necessity.
Swing-segment lesions4,6,7 predispose to early fistula failure, defined by some as failure of a new AVF to mature within 3 months of creation. A distal swing segment is the segment of the native vein that is mobilized during radialcephalic, brachial-cephalic, and transposed brachial-basilic fistula creation. It is located about 2 to 3 cm above the anastomotic region known as the juxta-anastomotic segment. A proximal swing segment occurs in the basilic vein near the axillary region during transposition surgery. A naturally occurring swing segment occurs in the arch of the cephalic vein or cephalic arch segment as it drains into the axillary vein. This segment of the vein may experience turbulent flow and altered shear mechanical stress because it forms the outflow conduit for autogenous radial-cephalic and brachial-cephalic fistulas.8 These swing segment stenoses (Fig 1) were described previously,6,7 and here we determine their prevalence and anatomic locations in a large population of patients with end-stage renal disease with dysfunctional AVFs.
Figure 1.
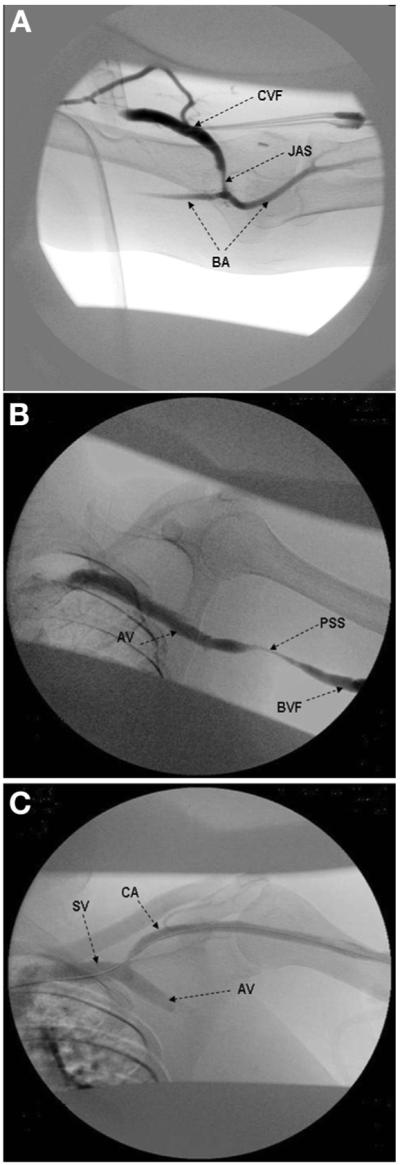
Digital subtraction angiography of swing segments in arteriovenous fistulas: A. juxta-anastomotic stenosis (JAS), cephalic vein fistula (CVF), and brachial artery (BA); B. proximal swing-segment (PSS) stenosis, the adjacent basilic vein fistula (BVF), and axillary vein (AV); and C. the cephalic arch (CA), which joins the axillary vein (AV) to form the subclavian vein (SV).
METHODS
Study Population
Between January 31, 2003, and June 30, 2005, all records of patients referred to an outpatient hemodialysis vascular access center for AVF fistula dysfunction were reviewed (n = 484). Eligibility criteria included end-stage renal disease, treatment with hemodialysis, AVF as access type, referral to the access center for the first time for AVF dysfunction, and adequate medical records. Patients were excluded if they had no evidence of stenosis on fistulography or physical examination of the fistula had normal findings precluding fistulography. Only patients with angiographically documented stenosis of any degree were included in the final analysis. Reasons for referral included evaluation for AVF maturation, arm swelling, difficult cannulation, decreased Kt/V, clot aspiration during access cannulation, increased venous pressure, clotted access, prolonged bleeding from puncture sites, and decrease in access flow rate greater than 20% from baseline or absolute access flow rate less than 600 mL/min by means of ultrasound dilution technique (Transonic Flow-QC; Transonic Systems, Ithaca, NY). Lesions were considered angiographically significant if there was stenosis greater than 50% of the vascular luminal diameter and percutaneous transluminal angioplasty was performed. The first episode of stenosis was recorded for analysis.
Procedural Technique
After physical examination and determination of the site of cannulation, a diagnostic fistulogram was obtained down to the central veins by using the digital subtraction technique, and the site and severity of stenosis were determined by using radiopaque contrast. A retrograde arteriogram was obtained to evaluate the artery, arterial anastomosis, and juxta-anastomosis. Conscious sedation before balloon angioplasty or thrombectomy was achieved by using intravenous fentanyl citrate, 50 μg, and midazolam, 1 mg. Patients were monitored by using blood pressure, electrocardiography, and pulse oximetry throughout the procedure. Any lesion greater than 50% of the vascular luminal diameter underwent angioplasty with an appropriately sized noncompliant balloon catheter advanced over the wire through a corresponding sheath, using a hand syringe assembly. The success of angioplasty was defined as residual stenosis less than 30%. Balloon sizes used were 8 mm × 4 cm, 6mm × 2 cm, or 4 mm × 2 cm, depending on the site and severity of the lesion. Heparin was administered at a dose of 5,000 units in the case of a thrombosed fistula by means of a mechanical thromboaspiration technique using a Fogarty catheter. After interventions, balloon catheters and sheaths were withdrawn and hemostasis was obtained with manual compression.
Data Collection
Data collected include demographics (age, sex, and race), comorbidities (diabetes, hypertension, peripheral vascular disease, heart disease, human immunodeficiency virus, stroke, contrast allergy, and history of smoking), previous history of a failed access, access type and location, site and severity of stenosis, and procedure outcomes. The study was approved by the Institutional Review Board at Emory University (Atlanta, GA).
Statistical Analysis
Descriptive analysis was used for continuous variables, and frequency analysis, for categorical variables. Results are expressed as mean ± SD and percentages, respectively. Data was analyzed using SPSS, version 13.0 (SPSS Inc, Chicago, IL).
RESULTS
A total of 484 patients were reviewed, 278 of whom had angiographically documented stenosis on their first visit. The remaining patients (n = 206) were excluded from analysis because they had either no stenosis on a fistulogram or initial clinical examination findings that did not suggest a need for a fistulogram. Characteristics of the study population are listed in Table 1. Most patients were African American (93%), mean age was 55 ± 18 years, and 64% were men. Upper-arm brachial-cephalic fistulas comprised 42%; forearm radial-cephalic fistulas, 37%; upper-arm transposed brachial-basilic fistulas, 21%; and Gracz fistulas (anastomosis of the brachial artery or beginning of the radial artery to the perforating vein in the elbow), 1%. As listed in Table 2, access evaluation, cannulation difficulties, and prolonged bleeding were the most frequent reasons for referral.
Table 1. Baseline Characteristics of the Study Population.
| Mean age (y) | 58 ± 8 |
| Sex | |
| Men | 64 (178) |
| Women | 36 (100) |
| Race | |
| African American | 93 (258) |
| White | 6 (17) |
| Hispanic | 1 (3) |
| Cause of ESRD | |
| Hypertension | 31 (86) |
| Diabetes | 28 (78) |
| Polycystic kidney disease | 2 (6) |
| Glomerulonephritis | 1 (3) |
| Human immunodeficiency virus | 3 (8) |
| Other | 2 (6) |
| Unknown | 33 (91) |
| AVF type | |
| Radial-cephalic | 37 (103) |
| Brachial-cephalic | 42 (117) |
| Brachial-basilic | 21 (58) |
Note: N = 278. Values expressed as mean ± SD or percent (number).
Abbreviations: ESRD, end-stage renal disease; AVF, arteriovenous fistula.
Table 2. Reasons for Referral.
| Prolonged bleeding | 12.2 (59) |
| Clotted access | 10.8 (52) |
| Arm swelling | 4.1 (20) |
| Increased venous pressure | 10.4 (50) |
| Decreased transonic flow | 11.1 (53) |
| Difficult cannulation | 17.6 (85) |
| Decreased Kt/V | 6.4 (31) |
| Evaluate AVF maturation | 25.9 (125) |
| Clot aspiration during cannulation | 1.5 (7) |
Note: Values expressed as percent (number).
Abbreviation: AVF, arteriovenous fistula.
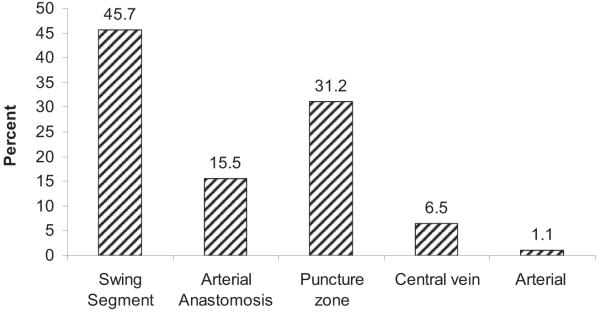
Figure 2 shows frequencies of the various types of stenoses seen on angiography. These are presented as percentages, which represent number of stenoses. Most stenoses were in the swing segment, accounting for 45.7%, followed by the puncture zone (area of cannulation, usually middle third of the fistula) at 31.2%. Arterial anastomosis stenosis comprised 15.5%; central venous stenosis, 6.5%; and arterial stenosis, 1.1%. Of all swing-segment stenoses (Fig 3), juxtaanastomotic lesions accounted for the majority (63%), whereas cephalic arch and proximal swing-segment lesions accounted for 18% and 19%, respectively. These swing segments as well as other segments of AVF anatomy are schematically illustrated in Fig 4.
Figure 2.
Distribution of stenosis in the study population (n = 278). Percentage refers to stenosis. Stenosis in the swing segment accounted for 45.7% (n = 127); arterial anastomosis, 15.5% (n = 43); puncture zone, 31.2% (n = 87); central veins, 6.5% (n = 18); and arterial anastomosis, 1.1% (n = 3).
Figure 3.
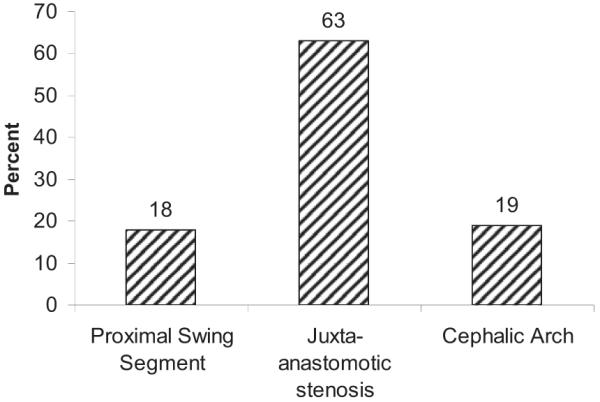
Distribution of stenoses among the types of swing segments (n = 127). Percentage refers to stenosis. Stenosis of the proximal swing segment accounted for 18% (n = 23); juxta-anastomotic segment, 63% (n = 80); and cephalic arch, 19% (n = 24).
Figure 4.
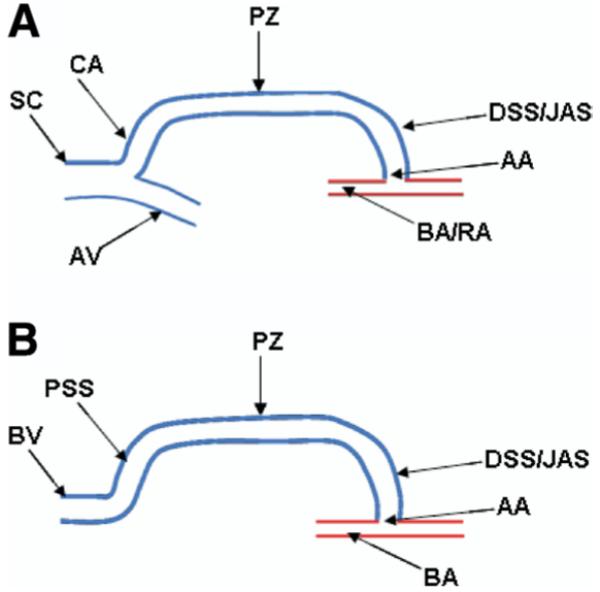
Schematic diagram of arteriovenous fistula anatomy. A. Brachial or radial artery (BA/RA) cephalic fistula shows the arterial anastomosis (AA), distal swing segment or juxta-anastomotic segment (DSS/JAS), puncture zone (PZ), cephalic arch (CA), and axillary and subclavian veins (AS and SC). B. Brachial-basilic transposition fistula and proximal swing segment (PSS). Abbreviation: BV, basilic vein.
The distribution of swing-segment stenoses was equivalent among the various fistulas: 35.4% in brachial-cephalic fistulas, 33.9% in radialcephalic fistulas, and 30.7% in transposed brachial-basilic fistulas. Furthermore, the severity of stenosis (<50%, 50% to 70%, 70% to 90%, and >90%) was equivalent among the various swing segments (chi-square = 2.7; P = 0.8).
Most patients referred for arm swelling, increased venous pressure, and prolonged bleeding had stenosis involving the proximal swing segment and/or central outflow veins, whereas most patients referred for access evaluation and decreased Kt/V typically had lesions involving the juxta-anastomosis swing segment and arterial anastomosis. The majority of patients with swing segment stenosis (83%) underwent balloon angioplasty, with a 93% technical success rate. There were patients (1.1%) with major complications after balloon angioplasty. One complication was a grade 2 rupture that resulted in fistula loss, whereas the other 2 complications were grade 1 ruptures that were successfully salvaged. There was no incidence of symptomatic arterial embolization or clinical signs of pulmonary embolism.
DISCUSSION
We observed that the lesions most frequently identified in patients referred for a dysfunctional AVF were located at the swing segment or swing point, most commonly in the juxta-anastomotic region. Our study examines the largest patient population referred to an outpatient hemodialysis vascular access center and provides greater understanding of the frequency and location of AVF stenotic lesions.
In previous work, Beathard et al4 found that the juxta-anastomotic segment accounted for 43% of stenoses in 100 patients with early fistula failure in 2004, and Turmel-Rodrigues et al9 reported that 55% of first symptomatic stenoses occurred at or near the anastomosis in a study involving 209 forearm fistulas. Clark et al10 reported a 47% prevalence of stenosis involving the initial 2 cm of the anastomosis (juxtaanastomosis) in a prospective study involving 25 patients with radial-cephalic fistulas followed up from the time of fistula creation by using serial duplex and color flow ultrasound. Sivanesan et al11 found that stenosis developed at or near the anastomosis within 3 months in all patients. Progressive narrowing of the stenosis occurred in 13 patients, with resultant fistula loss in 6 patients.11 Interestingly, this lesion, the juxtaanastomosis stenosis, is more common in forearm than upper-arm AVFs. Up to 77%7,11 of forearm AVFs have a juxta-anastomosis stenosis compared with 56% of upper-arm AVFs. In our patient population, we experienced a juxtaanastomotic stenosis rate of 54% in dysfunctional forearm AVFs compared with 46% in upper-arm AVFs.
A similar observation was made regarding cephalic arch stenosis. Rajan et al8 reported a 15% prevalence of cephalic arch stenosis in a cohort of 177 patients with dysfunctional AVFs in 2003. They observed a greater prevalence of cephalic arch stenosis (39%) in brachial-cephalic fistulas compared with radial-cephalic fistulas.
Reasons for the differences in cephalic arch stenosis between brachial-cephalic and radialcephalic AVFs are unclear. Rajan et al8 proposed that the proximity of the cephalic arch to the anastomosis in a brachial-cephalic fistula compared with a radial-cephalic fistula might predispose this segment of the cephalic arch to greater flow and pressures. They also noted that venous drainage at the level of the elbow in forearm fistulas potentially enabled additional drainage through the basilic and brachial veins, thereby increasing the venous capacitance of radialcephalic AVFs and decreasing the degree of turbulence encountered. Similarly, brachial-basilic AVFs were prone to proximal swingsegment stenosis at the venous outflow region, where mobilization of the vein occurred during transposition surgery.
A cause is yet to be defined for these lesions. Mobilization of the swing segment of the vein during surgery may disrupt the vasa vasorium, leading to ischemic changes, coupled with intimal hyperplasia resulting from exposure to high blood flow in the region. One school of thought proposed a low wall shear stress and/or flow separation hypothesis, in which areas with decreased local flow and low wall shear stress were prone to intimal thickening. Several reports supporting decreased local flow as a predisposing factor to stenosis were published.12-15 The juxtaanastomotic segment was described as an area of lower wall shear stress.10 Ojha et al16 used an in vitro model of an end-to-side 45° anastomosis similar to a radial-cephalic anastomosis and found high correlation between areas of low wall shear stress and intimal hyperplasia. They admitted that this low wall shear stress and/or flow separation pathogenesis might not explain events in the arterial end, which seems to be subjected to a complex hemodynamic environment. High-flow mechanics leading to intimal hyperplasia resulting in low-flow states and resultant stenosis is a possibility.
Hofstra et al17 characterized cellular proliferation patterns within stenotic segments of dysfunctional AVFs of 12 patients after resection and placement of an interposition bypass using ultrasound. Areas of intimal proliferation correlated with areas of low shear stress. The presence of endothelial cells covering the stenosis was also an associated finding with increased intimal thickening.17 The report also indicated that approximately 90% of proliferating cells in the subendothelial area of the intima were possibly vascular smooth muscle cells; a fraction of proliferating cells in the stenotic area was macrophages and endothelial cells covering microvessels. However, several inhibitory pharmacological agents of vascular smooth muscle cell proliferation did not show much success in humans, as reported in animal models.18-20 This suggests a varied pattern of cell injury and proliferation and opens the door for more histologic studies involving the stenotic segments of hemodialysis AVFs, particularly swing segments.
Our study is limited by its retrospective nature. Selection bias may be present because only patients referred for a dysfunctional AVF were included in analyses. Therefore, results cannot be generalized to all patients with an AVF.
We conclude that in patients referred for AVF dysfunction, stenosis most commonly occurs at the swing segment, with the juxta-anastomotic region the most common site. Although the cause of these lesions is poorly understood, nephrologists should be aware of common and likely locations of lesions to render early diagnosis, referral, and appropriate interventions to prevent early or late AVF failure.
ACKNOWLEDGEMENTS
Abstract presented at the National Kidney Foundation Spring Clinical Meeting, Chicago, IL, April 19 to 23, 2006
Footnotes
Support: None.
Financial Disclosure: None.
REFERENCES
- 1.National Kidney Foundation K/DOQI Clinical Practice Guidelines for Hemodialysis Adequacy: Update 2000. Am J Kidney Dis. 2001;37(suppl 1):S7–S64. doi: 10.1016/s0272-6386(01)70005-4. [DOI] [PubMed] [Google Scholar]
- 2.Ascher E, Gade P, Hingorani A, et al. Changes in the practice of angioaccess surgery: Impact of Dialysis Outcome and Quality Initiative recommendations. J Vasc Surg. 2000;31:84–92. doi: 10.1016/s0741-5214(00)70070-x. [DOI] [PubMed] [Google Scholar]
- 3.Gibson KD, Gillen DL, Caps MT, Kohler TR, Sherrard DJ, Stehman-Breen CO. Vascular access survival and incidence of revisions: A comparison of prosthetic grafts, simple autogenous fistulas, and venous transposition fistulas from the United States Renal Data System Dialysis Morbidity and Mortality Study. J Vasc Surg. 2001;34:694–700. doi: 10.1067/mva.2001.117890. [DOI] [PubMed] [Google Scholar]
- 4.Beathard GA, Arnold P, Jackson J, Litchfield T. Aggressive treatment of early fistula failure. Kidney Int. 2003;64:1487–1494. doi: 10.1046/j.1523-1755.2003.00210.x. [DOI] [PubMed] [Google Scholar]
- 5.Grogan J, Castilla M, Lozanski L, Griffin A, Loth F, Bassiouny H. Frequency of critical stenosis in primary arteriovenous fistulae before hemodialysis access: Should duplex ultrasound surveillance be the standard of care? J Vasc Surg. 2005;41:1000–1006. doi: 10.1016/j.jvs.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 6.Falk A, Teodorescu V, Lou WY, Uribarri J, Vassalotti JA. Treatment of “swing point stenoses” in hemodialysis arteriovenous fistulae. Clin Nephrol. 2003;60:35–41. doi: 10.5414/cnp60035. [DOI] [PubMed] [Google Scholar]
- 7.Vassalotti JA. Arteriovenous fistula stenosis: New terminology. Semin Dial. 2004;17:243. doi: 10.1111/j.0894-0959.2004.17315.x. [DOI] [PubMed] [Google Scholar]
- 8.Rajan DK, Clark TW, Patel NK, Stavropoulos SW, Simons ME. Prevalence and treatment of cephalic arch stenosis in dysfunctional autogenous hemodialysis fistulas. J Vasc Interv Radiol. 2003;14:567–573. doi: 10.1097/01.rvi.0000071090.76348.bc. [DOI] [PubMed] [Google Scholar]
- 9.Turmel-Rodrigues L, Pengloan J, Baudin S, et al. Treatment of stenosis and thrombosis in haemodialysis fistulas and grafts by interventional radiology. Nephrol Dial Transplant. 2000;15:2029–2036. doi: 10.1093/ndt/15.12.2029. [DOI] [PubMed] [Google Scholar]
- 10.Clark TW, Hirsch DA, Jindal KJ, Veugelers PJ, LeBlanc J. Outcome and prognostic factors of restenosis after percutaneous treatment of native hemodialysis fistulas. J Vasc Interv Radiol. 2002;13:51–59. doi: 10.1016/s1051-0443(07)60009-8. [DOI] [PubMed] [Google Scholar]
- 11.Sivanesan S, How TV, Bakran A. Sites of stenosis in AV fistulae for haemodialysis access. Nephrol Dial Transplant. 1999;14:118–120. doi: 10.1093/ndt/14.1.118. [DOI] [PubMed] [Google Scholar]
- 12.Zarins CK, Bomberger RA, Glagov S. Local effects of stenoses: Increased flow velocity inhibits atherogenesis. Circulation. 1981;64(Pt 2):II221–II227. [PubMed] [Google Scholar]
- 13.Sterpetti AV, Cucina A, Santoro L, Cardillo B, Cavallaro A. Modulation of arterial smooth muscle cell growth by haemodynamic forces. Eur J Vasc Surg. 1992;6:16–20. doi: 10.1016/s0950-821x(05)80088-x. [DOI] [PubMed] [Google Scholar]
- 14.Kohler TR, Jawien A. Flow affects development of intimal hyperplasia after arterial injury in rats. Arterioscler Thromb. 1992;12:963–971. doi: 10.1161/01.atv.12.8.963. [DOI] [PubMed] [Google Scholar]
- 15.Kohler TR, Kirkman TR, Kraiss LW, Zierler BK, Clowes AW. Increased blood flow inhibits neointimal hyperplasia in endothelialized vascular grafts. Circ Res. 1991;69:1557–1565. doi: 10.1161/01.res.69.6.1557. [DOI] [PubMed] [Google Scholar]
- 16.Ojha M, Ethier CR, Johnston KW, Cobbold RS. Steady and pulsatile flow fields in an end-to-side arterial anastomosis model. J Vasc Surg. 1990;12:747–753. doi: 10.1067/mva.1990.24365. [DOI] [PubMed] [Google Scholar]
- 17.Hofstra L, Tordoir JH, Kitslaar PJ, Hoeks AP, Daemen MJ. Enhanced cellular proliferation in intact stenotic lesions derived from human arteriovenous fistulas and peripheral bypass grafts. Does it correlate with flow parameters? Circulation. 1996;94:1283–1290. doi: 10.1161/01.cir.94.6.1283. [DOI] [PubMed] [Google Scholar]
- 18.Liu MW, Roubin GS, King SB., III Restenosis after coronary angioplasty. Potential biologic determinants and role of intimal hyperplasia. Circulation. 1989;79:1374–1387. doi: 10.1161/01.cir.79.6.1374. [DOI] [PubMed] [Google Scholar]
- 19.McBride W, Lange RA, Hillis LD. Restenosis after successful coronary angioplasty. Pathophysiology and prevention. N Engl J Med. 1988;318:1734–1737. doi: 10.1056/NEJM198806303182606. [DOI] [PubMed] [Google Scholar]
- 20.Ferrell M, Fuster V, Gold HK, Chesebro JH. A dilemma for the 1990s. Choosing appropriate experimental animal model for the prevention of restenosis. Circulation. 1992;85:1630–1631. doi: 10.1161/01.cir.85.4.1630. [DOI] [PubMed] [Google Scholar]