Abstract
During CNS injury and diseases, NO is released at a high flux rate leading to formation of peroxynitrite (ONOO-) and other reactive nitrogenous species (RNS), which nitrate tyrosines of proteins to form 3-nitrotyrosine (3-NY), leading to cell death. Previously, we have found that motor neurons exposed to low levels of NO become resistant to subsequent cytotoxic NO challenge; an effect dubbed induced adaptive resistance (IAR). Here we report IAR mitigates, not only cell death, but 3NY formation in response to cytotoxic NO. Addition of an NO scavenger before NO challenge duplicates IAR, implicating RNS in cell death. Addition of uric acid (a peroxynitrite scavenger) before cytotoxic NO challenge, duplicates IAR, implicating peroxynitrite, with subsequent 3-NY formation, in cell death, and abrogation of this pathway as a mechanism of IAR. IAR is dependent on the heme-metabolizing enzyme, heme oxygenase-1 (HO1), as indicated by the elimination of IAR by a specific HO1 inhibitor, and by the finding that neurons isolated from HO-1 null mice have increased NO sensitivity with concomitant increased 3-NY formation. This data indicates that IAR is an HO1-dependent mechanism that prevents peroxynitrite-mediated NO toxicity in motor neurons, thereby elucidating therapeutic targets for the mitigation of CNS disease and injury.
Keywords: nitric oxide, peroxynitrite, nitrotyrosine, hemoxygenase 1, resistance, motor neurons
Introduction
The free radical nitric oxide (NO) is actively synthesized by many mammalian cells and utilized for a variety of functions. At low levels, NO effects intercellular signaling in vascular relaxation, neurotransmission, and cellular differentiation (Brenman & Bredt, 1996, Feelisch et al., 1994, Hobbs & Ignarro, 1996, Packer et al., 2003, Stamler et al., 1997, Stuehr,1999). During normal physiological processes, usually during induction of nNOS and eNOS, NO can reach steady state concentrations from ∼50nM to ∼500nM (Clough et al., 1998, Huk et al., 1998, Pacher et al.,2007). It has been demonstrated that neurons release around 33nM of NO during normal activity (Leonard et al.,2001). Clearly NO at this range carries out many beneficial functions.
At high levels, NO causes toxicity and thus is employed as a weapon in the immune system. NO also plays a role in neuronal injury and in the pathology of various diseases, such as Parkinson, disease (PD), Alzheimer disease (AD), multiple sclerosis (MS), and amyotrophic lateral sclerosis (ALS) ( Hall et al.,1998, Huk et al., 1998, Kawase et al., 1996, MackMicking,Panahian & Maines, 2001, Pacher et al.,2007, Xie & Nathan, 1997, Vaziri et al., 2004). During pathological states, activated microglial and astrocytes, through activation of iNOS, release bursts of NO at high steady state concentrations of up to 1uM (Hall et al.,1998, Kawase et al., 1996, Pacher et al.,200, Stuehr,1999,Tominaga et al.,1994). It is important to note that in a variety of insults NO release can increase up to 10x above the concentrations seen before the insult (Clough et al., 1998, Huk et al., 1998, Pacher et al.,2007). NO at these ranges is toxic to the cell.
During pathological processes, such as spinal injury, MS, and ALS, nitric oxide damages essentially all the critical biological macromolecules. Of particular importance are NO and its reactive nitrogenous species (RNS), such as peroxynitrite (ONOO-), which go on to damage proteins (Beckman, 1996, Cassina et al. 2002, Ischiropoulos & Beckman,2003, Pacher et al., 2007,Tamir et al., 1993). NO-dependent nitration of tyrosine residues (forming 3nitrotyrosine; 3NY) disrupts protein structure and function, thereby interrupting or altering cell signaling (Bishop et al., 2003, 2005,2006, Cassina et al. 2002,, Ischiropoulos & Beckman,2003, Pacher et al.,2007, Tamir et al., 1993). 3NY formation can be the result of the traditional peroxynitrite mediated pathway, the predominate pathway, ( Ischiropoulos & Beckman,2003, Pacher et al., 2007) or by the less well explored iron / peroxidase mediated pathway (Espey et al.,2002,Pfeiffer et al.,1997, Thomas et al., 2002). 3NY formation is found in the CNS of patients with spinal injury, Parkinsons Disease, Alzheimers Disease, MS and ALS,and is considered a marker for RNS mediated damage in the cell (Ischiropoulos & Beckman,2003, Kawase et al., 1996, Kuljis.& Schelper,1996, , McDonald, 1999, Pacher et al.,200, Sharma et al.,1996). Clearly 3NY formation can prove quite useful as a marker for NO damage.
The heme metabolizing enzyme, HO1, is linked to cellular resistance to oxidants such as heavy metals and peroxide (Fung et al., 1999, Kitamura et al., 2003, Maines, 1997), and NO (Bishop et al., 1998, 2003, 2004, 2005, 2006). HO1 metabolizes the heme freed from proteins during normal turnover and during NO stress (Bishop et al., 1998, 2003, 2004, 2005,2006, Fung et al., 1999, Kitamura et al., 2003 Maines, 1997) releasing the end products, CO (Maines, 1997, Soares et al.,2002), bilirubin (Fung et al., 1999,Kitamura et al., 2003, Maines, 1997) and iron (Juckett, et al.,1998), all of which have been implicated as antioxidants. Increased HO1 levels are found in AD and spinal injury, and correlate with markers of free radical damage (Fukuda et al., Kitamura et al., 2003, Schipper,1995,). HO1, unlike its isozyme, HO2, is inducible in response to a variety of oxidants, suggesting its importance in saving cells from RNS.
Previously we have found that motor neurons could be primed by a subtoxic dose of NO (∼25nM/s) to mount a robust resistance to a subsequent toxic dose of NO (∼300nM/s for most experiments), and that this induced adaptive resistance (IAR) is dependent on HO1 (Bishop et al.,1999, 2003, 2004, 2005, 2006). Here, we use more physiologically relevant doses to elicit IAR to explore resistance to cytotoxic NO challenge (administered by the rapid NO donor, spermine-NONOate). We found this both in a motor neuron cell line and in primary mouse motor neurons. In addition we use specific inhibitors to determine whether the NO toxicity is due to NO or to the RNS, peroxynitrite. Finally, in HO1 null mice we investigate the role of HO1 in IAR and its relationship with RNS with subsequent 3NY formation. Our findings elucidate the motor neurons normal resistance processes and offer possible therapies for CNS injury and diseases.
Methods
Vertebrate animals
The use of mice for this work was essential and conformed to all institutional and government regulations. The protocol, with measures against pain and suffering, was approved by our Institutional Animal Care and Use Committee (IACUC)-assurance number 07-001-R. The mice (Mus musculus) wildtype were derived from a 129 SV/BalbC strain.
Primary motor neurons from mice that are HO1 wildtype, homozygous HO1 null, and heterozygous, HO1 null
Mice heterozygous for an HO1 knockout allele were mated (Bishop et al., 2004) and on day E13 the pregnant females were euthanized by CO2. Death or unconsciousness was assessed by unresponsiveness to tail pinch. The mouse was sterilized by ethanol wash, the uteri were extracted, and the embryos harvested for cells isolated from the spinal cord, after which the mother’s abdominal aorta was cut to guarantee death.
Genotyping of null, heterozygous and wildtype mouse embryos
PCR for genotyping was performed on DNA samples purified from each embryo as described, using primers specific for the disrupted HO1 gene and the wildtype HO1 gene. This was done after NO testing to assure objectivity.
The primers for detecting the wild-type HO1 gene were 5′-GGTGACAGAAGAGGCTAAG-3′ and 5′-CTGTAACTCCACCTCCAAC-3′.
For the HO1knockout allele, the primers were 5′-TCTTGACGAGTTCTTCTGAG-3′ and 5′-ACGAAGTGACGCCATCTGT-3′.
The PCR products were resolved on 1-2% agarose gels and visualized by staining with ethidium bromide as described (Bishop et al., 2004).
Isolation and maintenance of pure primary motor neurons
The embryos harvested and spinal cords (cervical and thoracic portion) were isolated from each embryo as per the protocol of Dr. Alvaro Estevez (Bishop et al., 1999, 2004, Estevez et al., 1998, 2000.,Schnaar & Schnaffner, 1981). The meninges layers were dissected away and the dorsal roots were removed. The ventral part of the spinal cords were dissected away from the dorsal region and were minced. The minced cords were separated initially by BSA gradient and then by Optiprep gradient into fractions that were enriched for motor neurons. This was repeated several times to “enrich” further for motor neuronal cell types (Bishop et al., 1999, 2004, Estevez et al., 1998, 2000) followed by immunopanning. The motor neurons were plated on flasks coated with a mixture of laminin and poly-D-lysine at a density of 2×106 cells per flask and cultured under 37°C, 5% CO2 in minimal Eagle’s Medium supplemented with D-glucose, L-glutamine and 5% fetal bovine serum. (Bishop et al., 1999, 2004, Estevez et al., 1998, 2000).
Growth and maintenance of NSC34 cells
Growth and maintenance of the NSC 34 cells was performed as described (Bishop et al., 1999, 2004). NSC34 cells were made from a fusion of primary mouse spinal cord motor neurons with spinal neuroblastoma cells which have many of the characteristics of motor neurons assayed for thus far and are an accepted model of motor neurons (Bishop et al., 1998,2004, Cashman et al., 1992, Durham et al.,1993, Eggett et al.,2000, Matsumoto et al.,1995,). The cells were grown in a humidified 5% CO2 environment in plastic T25 flasks in Dulbecco’s modified Eagle’s medium (Atlanta Biologicals, Atlanta) without sodium pyruvate and supplemented with 10% heat-inactivated, fetal bovine serum (Bishop et al., 1999,2004).
NO Treatment
We employed diazenium-diolate NO-donor compounds as described (Bishop et al., 1999,2004,). For low NO doses in pretreatment, we used the compound, (z)-1-[2-Aminoethyl)-N-(2-ammonioethyl)amino]diazen-1-ium-1,2-diolate] DETA-NONOate, (Bishop et al., 1999,2004, Bouton & Demple, 2000). DETA-NONOate has a relatively slow decay rate (half-life ∼16 h) and releases lower, steadier NO fluxes allowing us a more “physiological” NO exposure than other NO donors (Bishop et al., 1999,2004, Bouton & Demple, 2000, Tamir et al., 1993,). For cytotoxic NO challenge we used spermine-NONOate. With a half-life of ∼40 min, it releases NO at a high rate in a burst mimicking the “pathological” burst a cell sees during toxic NO release by macrophages and microglia (Bishop et al., 1999,2004, Bouton & Demple, 2000, Tamir et al., 1993,).The advantages of NONOate NO donors are easy application and predictable NO release, depending on the concentration of the NO donor, pH, and temperature of the media, and duration of exposure (Bishop et al., 1999,2004, Bouton & Demple, 2000, Tamir et al., 1993,).
Previous standard curves were established indicating how much NO was released under pH7.4, 37°C within a certain time period (Bishop et al., 1999, 2004, , Bouton & Demple, 2000, Estevez, et al., Tamir et al., 1993). In addition we confirmed the NO released over time with a colorimetric NO assay kit (Bishop et al., 1999, 2004). Spent NO donor was used in untreated (UT) controls.
Addition of test agents
The NO scavenger, 10μM carboxy-PTIO, the specific HO1 inhibitor, 20μM zinc protoporphyrin (ZnPPIX) (Akins et al., 2004, Yang et al., 2001), or the peroxynitrite scavenger, 10μM uric acid (Dickhout et a., 2005, Hooper et al., 2000, Pacher et al., 2007), or 10μM CORM-A1 carbon monoxide donor (Zimmermann et al., 2007), were added to cells undergoing an NO protocol. Cells were incubated with the test agent alone to control for toxicity of the test agent in question.
Assay of cell survival
Percent cell survival was assayed by % healthy cells remaining after treatment. Healthy cells were defined as those bearing neurites and those that excluded Trypan blue (Bishop et al., 1999, 2004). Any cells that detached from the plate were also assayed; >99%% were found to no longer exclude trypan blue (Bishop et al., 1999, 2004). Medium containing spent NO donor was used as a negative control (untreated-UT) in all experiments.
Isolation of protein from treated cells and measurement of protein concentration
We lysed the cells in Sigma cell lysis buffer with protease inhibitor cocktail. To generate a positive control for nitrotyrosine immunoblotting, a cell extract was treated with a peroxynitrite standard solution. Protein molecular weight standards were also treated with peroxynitrite in vitro to generate additional markers (Bishop et al.,2004) as was bovine serum albumin (Bishop et al.,2004). Non nitrated molecular weight markers were used as a negative control. Protein concentrations in the cell extracts were assayed using the BioRad Protein Assay Kit..
ELISA assay
The indicated amounts of cell extract protein were loaded, along with a standard curve of 3NY albumin, on a 96well plate. Even loading was assured by measurement of protein content by Biorad assay. The 96 well plates were incubated with rabbit primary anti-3NY (a kind gift of Dr. Alvaro Estevez). The bound antibody was detected using goat anti-rabbit IgG conjugated with horseradish peroxidase, and the absorbance read and compared to the standard curve. The ELISA was a kit from Promega.
Western Blot/Immunoblot analysis of nitrated proteins
The indicated amounts of cell extract protein were loaded evenly, with the same ug of protein for each lane, and were run on a 12% SDS polyacrylamide gel at 100 V for 2 hr (Bishop et al.,2004). For convenience, some gels were stopped sooner. The gels were electroblotted onto nitrocellulose membranes. The blots were washed, blocked with BSA solution, incubated with rabbit primary anti-3NY (a kind gift of Dr. Alvaro Estevez), and the bound antibody was detected using goat anti-rabbit IgG conjugated with horseradish peroxidase or chemiluminescence. The 3NY signal was quantified by densitometry. The amount of 3NY detected was divided by the amount of protein loaded to yield a normalized estimate for 3NY. The Western blots were checked for even loading of proteins by BIORAD assay and for even transfer by Ponceau staining. Non nitrated molecular weight markers were used as a negative control. Peroxynitrite treated protein extract was used as a positive control (Bishop et al., 1999, 2004).
Statistical analysis
All experiments were performed a minimum of four times for at least four independent data points. For any data point involving cell counting, a minimum of 200 cells were counted from at least 5 randomly chosen fields. The mean of the data points were taken and the standard error of the mean was calculated. Each data point was compared to the control values, or to a value from a comparison group to determine whether there was a significant difference. The data was analyzed by the Student two tailed t test and significance (p value) calculated. P<.01 was determined to be significant.
Results
More physiologically relevant induced adaptive resistance (IAR) protocol
We formulated a protocol to ask if IAR can be demonstrated by lower, more physiologically relevant doses of NO. We chose pretreatment and challenge doses closer to the range of NO concentrations seen by cells during physiological and pathological conditions respectively (Clough et al.,1998, Hall et al.,1998, Kawase et al., 1996, Pacher et al., 2007, Stuehr,1999, Tominaga et al.,1994). For NO donors we used DETA-NONOate which donates only NO (Dickhout et al.,2005) at a lower flux rate of ∼4 nM/s, which is equivalent to 4pmols/s with a total release of 14nmoles (Figure 1A low NO). For the challenge NO dose, we used spermine-NONOate, which donates NO (Cornish et al., 2002) at a higher rate of ∼77-110nM/s at our high dose concentration, which is equivalent to 110 pmoles/s for a total release of 400nmoles for (Fig.1A cytotoxic). It has been demonstrated that NO at a high flux produces RNS with resultant 3NY formation (Tamir et al., 1993, 1996), and that spermine-NONOate, unlike DETA-NONOate, does donate NO at a high flux (Cornish et al., 2002). We asked if pretreatment of motor neurons with low dose NO induced NO resistance to normally cytotoxic doses of NO (Fig 1A). This IAR protocol detailed in Figure 1A not only mimics physiological and pathological conditions (Pacher et al., 2007), but also functions as a tool which enables manipulation and study of cellular resistance mechanisms.
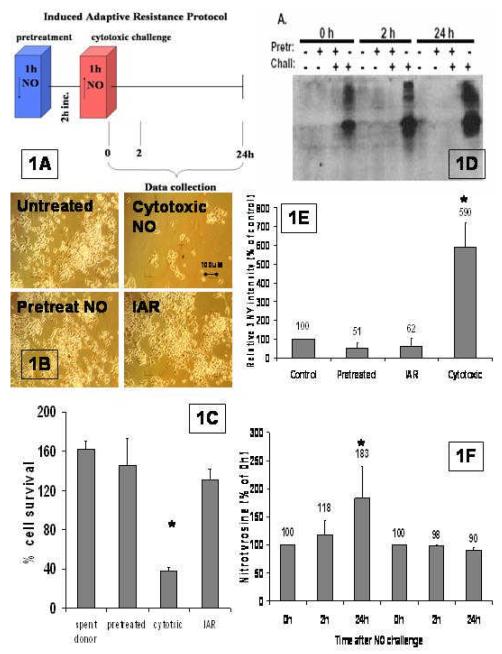
Fig. 1. Induced adaptive resistance.
A.) Diagram of NO treatment protocol for IAR. Cells are pretreated with low dose of NO (2pmoles/s) for 1 hour (PT), incubated for 2 hours and then treated with the cytotoxic dose of NO (∼110pmoles/s) (CT) after which cells are assayed for acquired NO resistance (IAR). B.) Demonstration of IAR. UT = Non-pretreated and non-challenged cells, PT = NO-pretreated cells not challenged, CT = Non-pretreated cells challenged with NO, IAR = NO-pretreated cells challenged with NO. Photos at 100X mag. C) Cell survival after 24 hours. Cells were plated evenly and the number of cells 24h after the treatment was divided by the cells in that same well right before treatment to normalize for any unintended differences between each well. For all samples standard error of the mean was calculated. The IAR group was compared to the CT group with an asterisk designating a significance of P<.01.D). Western blot probed for nitrotyrosine. The protein extracts were isolated from cells that were untreated--those treated with spent NO donor [with (–) in both rows)], pretreated with low dose NO (∼2pmoles/s) [with (+) in pretreated row and (–) in challenge row ], or pretreated incubated then NO challenged (∼110pmoles/s) [IAR cells adapted with (+) in both rows], or challenged with high NO dose alone (∼110pmoles/s) [cytotoxic with (–) in pretreated row and (+) in challenged row], and assayed 0hrs,2hrs,24hrs after completion of treatment protocol. In these experiments we ran the westerns for a very short time for convenience. E). Quantification of densitometry. Densitometry values were divided by amount of protein loaded or band intensity of Ponceau staining-both of which were equal in all lanes. The four treatment groups are untreated (UT), pretreated (pretreated), IAR (adapted), and cytotoxic levels of NO (cytotoxic) at 24hrs. This was done for multiple experiments (n=6), averaged and an SEM was calculated. The 3NY levels in the CT and IAR group was compared, and significance of (p<.01) was designated by an asterisk. F). 3NY increase over 24h. Quantification by densitometry was measured for 24 hours post treatment to assay long term disposition of 3NY levels in the cell. SEM was calculated, the difference between each time point and 0h was calculated, and the significance determined (p<.05) and designated by an asterisk.
IAR in motor neurons
Figure 1B are micrographs where it can be easily seen that IAR is still robust even at the more physiologically relevant doses of pretreatment of (∼4 pmoles/s) against a challenge of (∼110 pmoles/s). When analyzed, 24 hours post treatment, non-IAR cells have a cell survival of 38% ± 3 SEM (n=12) as compared to IAR cells with a cell survival of 130% ± 11 SEM (n=12) (Fig. 1C). This difference in cell survival is significant (p<.01). The cell survival of IAR cells did not differ significantly from either untreated or pretreated cells (Figure 1C). Often after treatment, we have rounding and lifting off of dead cells. When we measured the lifted off cells with trypan blue we found that >99%% were dead..
3NY formation in NO challenged versus IAR cells
We asked if the cell killing challenge dose of NO administered by spermine-NONOate results in protein nitration (3NY), and if in IAR cells there is a concomitant mitigation of 3NY formation. To answer this question, we treated cells with pretreatment doses of DETA-NONOate, or we treated the cells with cytotoxic doses of NO of spermine-NONOate, or the IAR protocol, as detailed in Figure 1A. After each of the NO regimens the cells were incubated at 37°C, and at 0, 2, or 24 hours protein lysates were taken and analyzed by Western blot (Figure 1 D,E,F). Figure 1D is a Western blot of the 24 hour protein lysates. We found that both untreated cells and cells pretreated with “low” NO have very little formation of 3NY (Figure 1E), which indicates that the low dose NO administered by DETA-NONOate results in very little NO-mediated cellular damage. In response to a cytotoxic NO dose (∼77 pmoles/s) administered by spermine-NONOate, non-IAR cells have significantly more than 10-fold 3-NY formation (n=6) as compared to untreated controls (p<.01) (Figure 1D&E). The adapted cells, (IAR), have <1 fold 3-NY formation as compared to that in untreated or pretreated cells (Figure 1D,E). Interestingly, the amount of nitrotyrosine in the non-adapted cells appears to increase as time goes on while there is no increase in 3NY formation in IAR cells (Figure 1F). This difference in accumulation in IAR vs CT is not extremely significant (p<.05), but it does exhibit a strong trend.
This data establishes nitration as an indicator of NO-mediated damage, thereby implicating heme-mediated and/or peroxynitrite-mediated 3NY formation as the pathological agent in cytotoxic NO challenge. In addition, the data establishes a sharp decrease in the level of nitrated tyrosines as a marker for successfully adapted cells. Clearly, induced resistance works by preventing or lessening the formation of nitrated proteins, thus raising the possibility that this is the mechanism by which IAR prevents NO-mediated cell death.
The addition of a general NO scavenger, PTIO, in the presence of NO challenge
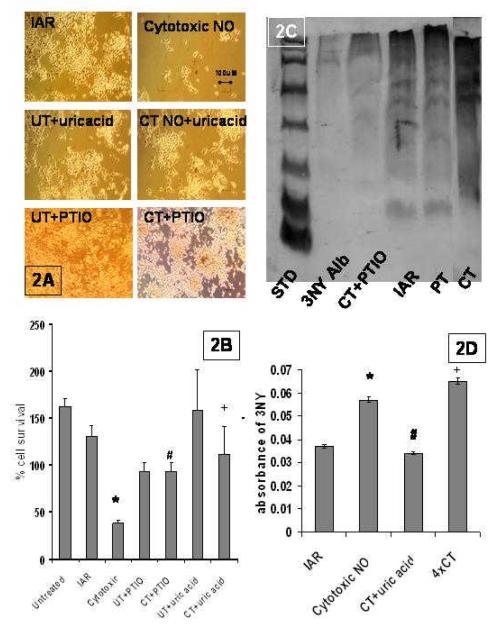
PTIO scavenges high flux NO (Dickhout et al.,2005, Pacher et al.,2007) thereby inhibiting all NO mediated RNS with subsequent 3NY formation (Espey et al.,2002, Pacher et al., 2007,Pfeiffer et al.,1997, Thomas et al., 2002). Asking if this NO scavenger protects cells from cytotoxic NO insult will elucidate the effects from heme-mediated protein nitration as well as from the predominant peroxynitrite-mediated protein nitration (Espey et al.,2002, Pfeiffer et al.,1997, Thomas et al, 2002), both of which culminate in cell death (Dickhout et al.,2005, Espey et al.,2002, Pacher et al., 2007, Pfeiffer et al.,1997, Thomas et al, 2002). In Figure 2A there are four panels of interest for PTIO treatment: UT + PTIO(untreated cells with PTIO to control for any toxicity); CT (challenge alone with cytotoxic NO); IAR; CT+PTIO (challenged with cytotoxic NO in the presence of PTIO). When we compare cell survival after cytotoxic NO with cells preincubated with PTIO, we see a significant difference ( Fig. 2A & B CT cell survival of 38%±3 vs CT+PTIO cell survival of 93%±10 (n=12) p<.01). It is clear that when PTIO is added to cells exposed to cytotoxic doses of NO that cell survival is increased to that equal to IAR cells as seen in Fig 2A &B. When we compare the IAR % survival, 130% ± 11 SEM vs CT+PTIO % survival, 93%±10 % (n=12), we find that the actions of PTIO are sufficient to duplicate induce adaptive resistance. Most importantly the extensive 3NY formation (∼10fold) seen in cells exposed to cytotoxic doses of NO is mitigated in cells pretreated with the NO scavenger PTIO (Fig. 2C). This finding adds to our evidence that high flux NO induces 3NY formation, and links the mitigation of heme-mediated and/or peroxynitrite-mediated 3NY formation to cellular NO resistance.
Figure 2. PTIO and uric acid mitigates NO toxicity.
A. IAR with peroxynitrite inhibitors: IAR are cells pretreated for 60 min with ∼2pmols/sec NO, incubated 2h to allow gene expression and challenged with ∼110pmols/sec NO for 60 min.; CT is non-pretreated cells, challenged with cytotoxic levels of NO (∼110pmols/sec NO for 60 min.); UT + UA were cells treated with uric acid alone; CT + UA are cells pretreated with uric acid and then challenged with cytotoxic doses of NO (∼110pmols/sec NO for 60 min.); UT + PTIO are control cells treated with PTIO alone; and CT + PTIO are cells pretreated with PTIO before being challenged with cytotoxic doses of NO (micrographs at 100x mag). B. % cell survival 24 hours after treatment. The values are the mean + SEM of 6 experiments performed in duplicate and a comparison between the CT group and IAR group was made and determined to be significant (p<.001) designated by an asterisk. A comparison between CT and CT+PTIO was determined and significance (p<.001) was designated by a #. CT vs CT+Uric acid (p<.01) was designated by a +. C. Western blots probed for nitrotyrosine. The bands were all 3NY positive, so the entire lane was taken into account for the densitometry in gels where equal loading of proteins has been assured by Ponceau staining or by Biorad assay. D. ELISA assays: ELISAs were run on cell extracts and were probed for 3NY content. Absorbance values subtracting controls for background were graphed. IAR vs CT was compared and found to be significant (p<.01) and designated with an asterisk. CT vs CT +uric acid was compared and found to be significant (p<.01) and designated by a #. 4×CT were cells exposed to 4x the CTNO dose and the 3NY formation was significantly greater than that of IAR (p<.01) designated with a +.
The addition of a specific peroxynitrite scavenger, uric acid
To determine the role that peroxynitrite / peroxynitrite-mediated 3NY formation plays in NO-mediated cytotoxicity, we asked if the addition of the peroxynitrite scavenger, uric acid, before cytotoxic NO challenge, saved cells, and if the cell saving equaled that seen by the addition of PTIO. If yes then this would imply that cell death is due to predominantly peroxynitrite-mediated cytotoxicity. In fact, there is a cell saving effect with addition of uric acid before challenge with cytotoxic NO, and it equals that of PTIO, indicating that most, if not all, of PTIO cell saving effect is due to mitigation of peroxynitrite mediated 3NY formation ( Fig 2A & B, compare % cell survival of CT +PTIO 93% ± 10 SEM, vs CT + uric acid 112% ± 29 (n=4)). With pretreatment with uric acid there is complete restoration of cell survival in the face of an NO challenge, to that of untreated cells (cells incubated with uric acid alone), which indicates that most, perhaps all, of the cytotoxicity is due to peroxynitrite mediated events (Fig 2A & B, compare % cell survival of CT +uric acid 112% ± 29 SEM vs. UT + UA 158% ± 43 ). When we compare % cell survival in Fig 2A & B (CT cell survival of 38% ± 3 SEM (n=12), vs CT + uric acid cell survival of 112% ± 29 SEM (n=4) p<.001) we have yet another piece of evidence indicating that most of the cellular cytotoxicity is due to peroxynitrite. We then asked if the IAR cell saving effect is also due primarily to abrogation of peroxynitrite-mediated 3NY formation. In fact it is. The IAR effect is duplicated by the addition of uric acid before cytotoxic challenge (Figure 2A&B, compare % cell survival of IAR of 130% ± 11 SEM (n=12) vs CT + uric acid of 112% ± 29 (n=4)) with no significant difference.
When we look at ELISA assays of 3NY formation we see that cells challenged with cytotoxic NO have significantly increased 3NY formation as compared to IAR cells, however if we treat cells challenged with cytotoxic NO with uric acid we see an abrogation of 3NY such that the levels are the same as that seen in IAR cells (Figure 1D). The findings that the addition of uric acid before cytotoxic challenge duplicates induced adaptive resistance without a low NO dose pretreatment, and the fact that the uric acid more than duplicates the ameliorating effects of PTIO, with the abrogation of 3NY formation seen in our ELISA studies, indicates scavenging of peroxynitrite, with subsequent mitigation of the peroxynitrite-mediated 3-NY formation, is the mechanism underlying IAR.
The link between HO1 activity, IAR, and RNS mediated 3NY formation
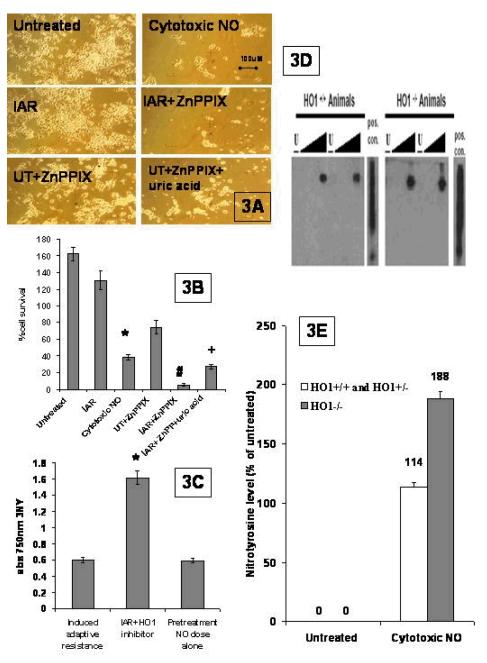
We asked if there is a link between the HO1 activity necessary for IAR and abrogation of 3NY formation, thereby linking HO1, IAR and peroxynitrite mediated 3NY formation. To clarify the mechanisms of how HO1 activity might exert IAR with the more physiologically relevant doses we use here, we wanted to establish if HO1 activity is still necessary for IAR. There was abrogation of IAR with the more selective HO1 inhibitor, ZnPP, in Figure 3A&B, as indicated by a cell survival for IAR + ZnPPIX of 5.59% ± 1.71 SEM, (n=4) vs the IAR value of 130% ±11 (n=12) p<.01. In fact, when the IAR protocol is carried out in the presence of the HO1 inhibitor, it brings cell survival below that of the cytotoxic NO challenge alone (Fig. 3 A & B CT cell survival of 38%±3 SEM vs for IAR + ZnPPIX of 5.59% ± 1.71 SEM, n=4). When we add the peroxynitrite scavenger to the ZnPPIX-inhibited cells, their ability to mount NO resistance is restored significantly (Figure 3A&B IAR + ZnPPIX cell survival of 5.6% ± 1.71 SEM, (n=4) vs IAR + ZnPPIX + uric acid cell survival of 27% ± 3,p<.01 ). However, the addition of uric acid to ZPPIX cells subject to IAR does not return their NO resistance to IAR % cell survival. Perhaps the double addition of uric acid and ZnPPIX is too cumbersome technically to yield results. Finally, in Figure 3C, with the ELISA results for 3NY formation, we see that 3NY formation seen with IAR is increased significantly when the IAR cells are preincubated with the specific HO1 inhibitor linking HO1 activity with 3NY formation.
Fig. 3. A more specific HO1 inhibitor abrogates IAR.
A. Micrographs of motor neurons: NSC34 cells were subjected to the IAR protocol(IAR), the IAR protocol in the presence of the HO1 inhibitor (IAR+ZnPP), the untreated cells incubated with ZnPPIX (UT+ZnPPIX), and the IAR in the presence of the HO1 inhibitor and uric acid (IAR + ZnPP + uric acid). (100×magnification) B. Quantification of cell survival and HO1 inhibition: The experiments were repeated, the cell counts were reported, and a comparison between IAR and cytotoxic NO was determined as significant (p<.001) and designated by an asterisk. A comparison between IAR and IAR +ZnPPIX was determined as significant (p<.001) and designated by a #. A comparison between IAR +ZnPPIX and IAR+ ZnPPIX +uric acid was determined to be significant (p<.01) and designated by +. C. ELISA analysis of 3NY levels. 3NY levels in IAR and HO1 inhibited IAR cells were measured and the difference was determined to be significant (p<.001): D. Western of 3NYin HO1 wildtype and HO1 null cells: Primary motor neurons were isolated from wildtype, heterozygous, or HO1 null mice. The groups of cells were untreated (u), or treated with a low NO dose (l) or medium NO dose (m) or high NO dose (h). Protein was isolated and westerns run and probed for nitrotyrosine. E. Quantification of 3NY levels: The protein levels were checked for even loading and nitrotyrosine levels were quantified against controls by densitometry. This was repeated, averaged, an SEM was calculated, and significance determined.
3NY formation in neurons from HO1 null mice
To establish a true genetic test of the necessity of HO1, we used mice that were made null for HO1 (see methods). These transgenic mice do not express HO1 mRNA, nor HO1 protein (Bishop et al., 2004). We have found that motor neurons from HO1 null mice have profound NO sensitivity as compared to motor neurons from wildtype or even heterozygous mice (Bishop et al., 2004). We assayed primary neurons from these HO1 null mice for 3NY formation in response to cytotoxic NO challenge to determine, mechanistically, if there was a link between HO1 activity and 3NY formation. The HO1 null cells were so NO sensitive as to render the IAR protocol untenable, so we instead did a simple dose response curve to compare NO sensitivity of both groups as well as 3NY formation. Note in Figure 3D&E that none of the cells, neither wildtype cells nor HO1 null cells, have undergone the induced adaptive resistance protocol. Therefore, in neither do we see almost complete abrogation of 3NY formation as we do in IAR cells, which is to be expected. Protein was extracted and levels of nitrotyrosine were assayed by Western blot (Fig 3D). In the primary motor neurons the absence of HO1 significantly exacerbates 3NY formation in neurons exposed to toxic doses of NO (see Figure 3D &E compare the thickest part of the wedge depicting NO exposure for +/+ wildtype (w.t.) animals to the thickest part of the wedge for -/- HO1null animals.). This suggests that HO-1 is involved in mitigation of protein nitration (3NY), which is the chief mechanism of NO mediated damage.
This correspondence of the inhibition of HO1 activity with IAR and 3NY abrogation , as well as HO1 null cells having increased 3NY formation after NO challenge does link HO1, IAR ,and IAR abrogation of 3NY formation. Clearly HO1 is involved.
Discussion
The fact that our low flux NO donated by DETANONOate does not form 3NY confirms that physiological NO is not damaging, and adds to the literature of the Janus nature of NO where low dose is actually beneficial (Brenman & Bredt, 1996, Feelisch et al., 1994, Hobbs & Ignarro, 1996, Packer et al., 2003, Stamler et al., 1997, Stuehr, 1999, Bishop, Demple, Peunova, Bredt). Our finding, that the spermine NONOate releases high flux NO that goes on to form 3NY, adds to the literature that describes the pathology of NO which leads to the 3NY seen in spinal injury, MS, ALS, and even AD (Bishop et al., 2003, 2005,2006, Cassina et al. 2002,, Ischiropoulos & Beckman,2003, Pacher et al.,2007, Tamir et al., 1993). Our finding of complete abrogation of 3NY formation by IAR, is the first to demonstrate that, in the CNS, low dose NO has a priming effect on subsequent cellular sensitivity to cytotoxic NO, and that it exerts a quantifiable effect on the pathology, namely 3NY formation.
Our data also suggest that the signal transduction system that abrogates NO mediated 3NY formation involves HO1. Our ELISA studies illustrate that inhibition of HO1 activity, which abrogates IAR, augments NO mediated 3NY formation. In addition, motor neurons isolated from the profoundly NO sensitive HO1 null mice have more 3NY formation in response to NO challenge than do motor neurons isolated from mice that are wildtype, or heterozygous for HO1. This mechanistic study performed by pharmacological and genetic manipulation of cellular resistance mechanisms has elucidated an HO1 dependent signal transduction cascade that prevents 3NY formation and subsequent cell death.
The question raised by our studies is whether HO1 abrogates cell death and 3NY formation by acting on the peroxynitrite RNS or other (heme mediated) RNS, both of which go on to form 3NY and cause cell death. In light of the fact that HO1 activity is needed for IAR, and that the primary function of HO1 is to break down heme, one might suppose that the heme-mediated RNS with subsequent 3NY formation would be a major player in our system. Since we found that the cell saving effects of the more general NO scavenger (PTIO) can be duplicated totally by the addition of uric acid (a peroxynitrite scavenger) we can conclude that HO1 exerts IAR by peroxynitrite scavenging. When we take into account the fact that NO challenged cells pretreated with uric acid are as protected from death as those that have undergone the IAR protocol, that peroxynitrite yields 3NY reliably, and that 3NY abrogation is a hallmark of IAR and HO1 activity, we can safely assume that it is the peroxynitrite-mediated 3NY formation that is the toxic pathway. Thus, 3NY is the central factor in NO-mediated cellular pathology, and its mitigation is the key to IAR.
Since our studies indicate that the 3NY formation is peroxynitrite-mediated we should ask how high flux NO can generate 3NY in a peroxynitrite-dependent manner since peroxyntirite is a product of superoxide and NO. We have to ask how superoxide is produced in these cells. There are many mechanisms by which high flux NO can damage the cell in such a way that superoxide is released and goes on to combine with NO, leading to peroxyntrite production with subsequent 3NY formation (Estevez et al.1998,2000,, Pacher et al., 2007). NO can bind to cytochrome c and other complexes in the mitochondria reducing efficiency of the oxidative phosphorylation pathway with subsequent release of superoxide (Pacher et al., 2007). Another possible pathway for superoxide production in the cell during an NO insult is that NMDA receptors can be activated leading to excitotoxicity with further release of NO and superoxide. Both our cells, NSC34s, and, of course, primary motor neurons, express NMDA receptors. In short, all of these processes and others, during a pathological event, will release superoxide which, with NO, can form ONOO and 3NY (Pacher et al., 2007). These many processes offer directions for further studies of the mechanisms of IAR.
A question raised is why, in IAR cells, there is little 3NY formation while in cytotoxic cells there is a significant amount? We now know from our studies that the DETA-NONOate used to donate low flux NO does not form RNS, while the cytotoxic dose of NO donated by spermine-NONOate does. However, spermine-NONOate is used in both challenged cells and in IAR cells. Perhaps, in IAR cells, where HO1 is upregulated by pretreatment, exposure to toxic NO does not result in 3NY formation because the already upregulated HO1 is primed and ready to metabolize the freed heme that would lead to 3NY formation. With this model, non IAR cells are caught unprepared, in that large amounts of heme are released from NO-damaged proteins without HO1 already having been upregulated, and therefore are available for 3NY formation. If we invoke a classic peroxynitrite-mediated event (which is supported by our studies) then perhaps pretreatment upregulates HO1 so it is present and ready to metabolize the heme released by NO challenge, with the active protective agents being the heme metabolites, such as CO, which is a proven antioxidant in many systems (Zimmermann et al., 2007, Pacher et al.,2007). Each of the HO1 metabolites (CO, bilirubin and iron) and the heme itself are good candidates for participating in the HO1-mediated signal transduction cascade and we have begun studies to investigate this.
Two key recent studies implicate other gene products in neuroprotection which gives us additional targets for our future research. One group found that when cortical neurons were deprived of glucose and oxygen for a brief period they were more resistant to death from ischemia reperfusion via increased activity of nNOS with downstream ERK cascade followed by an increase MnSOD activity (Scorziello et al.,2007). In another study it has been demonstrated that transfection of PC12 cells with glucose-6-phosphate dehydrogenase (G6PD) induced NADPH accumulation with concomitant NO resistance (Garci et al., 2003). .Our studies tracing the second messenger cascade utilized by the motor neurons for protection against NO stress will provide additional, critical information that may lead to possible therapeutic strategies for successful intervention in NO pathology seen in spinal injury, MS and ALS.
Acknowledgments
We gratefully acknowledge the expertise and assistance of Dr. Bruce Demple, Dr. Neil R. Cashman and his kind gift of the NSC34 cells, the support of NASA and Dr. Robert R. Richmond-NASA Biology Directorate. Our work was supported by NASA, Louis Stokes Alliance for Minority Participation, & UAH Young Faculty Award, NIH R15.
Footnotes
JNC-W-2008-1468R1: In response to the referees’ critiques I have added a paragraph in the discussion of future directions of my research within which I cited the two suggested journal articles which were very informative. To clarify the discussion I corrected a typo and deleted repetitive sentences. I made minor changes to the labeling of the figures, such as clearer axis labeling, text boxes and size designations on the micrographs. For greater clarity I also eliminated the CORM figure as requested as I agree with the referee that it is really the beginning of another study and distracts from this one. I added the two references to my reference list and also formatted all of the references properly. I then read the manuscript over and checked for any typos, etc. Thanks to the referees for their valuable comments.
Literature Cited
- Akins R, Jr., McLaughlin T, Boyce R, Gilmour L, Gratton K. Exogenous Metalloporphyrins Alter the Organization and Function of Cultured Neonatal Rat Heart Cells Via Modulation of Heme Oxygenase Activity. Journal of Cellular Physiology. 2004;201:26–34. doi: 10.1002/jcp.20040. [DOI] [PubMed] [Google Scholar]
- Beckman JS. Oxidative damage and tyrosine nitration from peroxynitrite. Chem. Res. Toxicol. 1996;9:836–44. doi: 10.1021/tx9501445. [DOI] [PubMed] [Google Scholar]
- Bishop A, Marquis JC, Cashman NR, Demple B. Adaptive resistance to nitric oxide in motor neurons. Free Radical Biology & Medicine. 1999;26(7-8):978–986. doi: 10.1016/s0891-5849(98)00284-6. [DOI] [PubMed] [Google Scholar]
- Bishop A, Cashman Neil R. Induced adaptive resistance to oxidative stress in the CNS: Discussion of possible mechanisms and their therapeutic potential. Current Drug Metabolism. 2003;4(2):171–184. doi: 10.2174/1389200033489514. [DOI] [PubMed] [Google Scholar]
- Bishop A, Fung-Yet Shaw, Perrella Mark J., Lee Arthur M., Cashman Neil R., Demple Bruce. Decreased resistance to nitric oxide in motor neurons of HO-1 null mice. BBRC. 2004;325:3–9. [Google Scholar]
- Bishop A, Anderson James. NO signaling in the CNS:from the physiological to the pathological. Toxicology. 2005;(208):193–205. doi: 10.1016/j.tox.2004.11.034. [DOI] [PubMed] [Google Scholar]
- Bouton C, Demple B. Nitric oxide-inducible expression of heme oxygenase-1 in human cells. Translation-independent stabilization of the mRNA and evidence for direct action of nitric oxide. J Biol Chem. 2000;275:32688–93. doi: 10.1074/jbc.275.42.32688. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Hwang PM, Glatt CE, Lowenstein C, Reed RR, Snyder SH. Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature. 1991;351:714–718. doi: 10.1038/351714a0. [DOI] [PubMed] [Google Scholar]
- Brenman JE, Bredt DS. Nitric oxide signaling in the nervous system. Meth. Enzymol. 1996;269:119–129. doi: 10.1016/s0076-6879(96)69014-4. 1996. [DOI] [PubMed] [Google Scholar]
- Cashman NR, Durham HD, Blusztajn JK, Oda K, Tabira T, Shaw IT, Dahrouge S, Antel JP. Neuroblastoma × spinal cord (NSC) hybrid cell lines resemble developing motor neurons. Devel. Dynamics. 1992;194:209–221. doi: 10.1002/aja.1001940306. [DOI] [PubMed] [Google Scholar]
- Cassina P, Peluffo H, Pehar M, Martinez-Palma L, Ressia A, Beckman JS, Estevez AG, Barbeito L. Peroxynitrite triggers a phenotypic transformation in spinal cord astrocytes that induces motor neuron apoptosis. J.Neurosci.Res. 2002;67:21–29. doi: 10.1002/jnr.10107. [DOI] [PubMed] [Google Scholar]
- Clough GF, Bennett AR, Church MK. Measurement of nitric oxide concentration in human skin in vivo using dermal microdialysis. Exp Physiol. 1998;83(3):431–4. doi: 10.1113/expphysiol.1998.sp004126. [DOI] [PubMed] [Google Scholar]
- Cornish AS, Jijon H, Yachimec C, Madsen KL. Peroxynitrite enhances the ability of Salmonella Dublin to invade T84 monolayers. Shock. 2002;18(1):93–96. doi: 10.1097/00024382-200207000-00017. [DOI] [PubMed] [Google Scholar]
- Dickhout JG, Hossain GS, Pozza LM, Zhou J, Lhoták S, Austin RC. Vascular Endothelium: Implications in Atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2005;25:2623–2629. doi: 10.1161/01.ATV.0000189159.96900.d9. [DOI] [PubMed] [Google Scholar]
- Durham HD, Dahrouge S, Cashman NR. Evaluation of the spinal cord neuron × neuroblastoma hybrid cell line NSC-34 as a model for neurotoxicity testing. Neurotoxicol. 1993;14:387–395. [PubMed] [Google Scholar]
- Eggett CJ, Crosier S, Manning P, Cookson MR, Menzies FM, McNeil CJ, Shaw PJ. Development and characterisation of a glutamate-sensitive motor neuron cell line. J. Neurochemistry. 2000;74:1895–1902. doi: 10.1046/j.1471-4159.2000.0741895.x. [DOI] [PubMed] [Google Scholar]
- Espey MG, Xavier S, Thomas DD, Miranda KM, Wink WD. Direct real-time evaluation of nitration with green fluorescent protein in solution and within human cells reveals the impact of nitrogen dioxide vs. peroxynitrite mechanisms. PNAS. 2002;99:3481–3486. doi: 10.1073/pnas.062604199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estevez AG, Spear N, Manuel SM, Radi R, Henderson CE, Barbieto L, Beckman JS. Nitric oxide and superoxide contribute to motor neuron apoptosis induced by trophic factor deprivation. J. Neurosci. 1998;18(3):923–931. doi: 10.1523/JNEUROSCI.18-03-00923.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estevez AG, Sampson JB, Zhuang Y-X, Spear N, Richardson GJ, Crow JP, Tarpey MM, Barbeito L, Beckman JS. Liposome-delivered superoxide dismutase prevents nitric oxidedependent motor neuron death induced by trophic factor withdrawal. Free Radic. Biol. Med. 2000;28:437–446. doi: 10.1016/s0891-5849(99)00261-0. [DOI] [PubMed] [Google Scholar]
- Feelisch M, te Poel M, Zamora R, Deussen A, Moncada S. Understanding the controversy over the identity of EDRF. Nature. 1994;368:62–65. doi: 10.1038/368062a0. [DOI] [PubMed] [Google Scholar]
- Fung-Yet S, Perrella MA, Layne MD, Hsieh CM, Maemura K, Kobzik L, Weisel P, Christou H, Kourembanas S, Lee Mu-En. Hypoxia induces severe right ventricular dilation and infarction in heme oxygenase-1 null mice. J. Clinical Investigation. 1999;103:R23–9. doi: 10.1172/JCI6163. (Arthur) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Nogales P, Angeles A, Bolanos JP. Peroxynitrite Protects Neurons against Nitric Oxide-mediated Apoptosis: A Key Role for Glucose-6-Phospate dehydrogenase activity in neuroprotection. The Journal of Biological Chemistry. 2003;278(2):874–874. doi: 10.1074/jbc.M206835200. [DOI] [PubMed] [Google Scholar]
- Hall ED, Oostveen JA, Gurney ME. Relationship of microglial and astrocytic activation to disease onset and progression in a transgenic model of familial ALS. Glia. 1998;23(3):249–256. doi: 10.1002/(sici)1098-1136(199807)23:3<249::aid-glia7>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Hobbs AJ, Ignarro LJ. Nitric oxide-cyclic GMP signal transduction system. Meth. Enzymol. 1996;269:134–148. doi: 10.1016/s0076-6879(96)69016-8. [DOI] [PubMed] [Google Scholar]
- Hooper DC, Scott GS, Zborek A, Mikheeva T, Kean RB, Koprowski H, Spitsin SV. Uric Acid, A PerOxynitrite Scavenger, Inhibits CNS Inflammation, Blood-CNS Barrier Permeability Changes, And Tissue Damage In A Mouse Model Of MS. FASEB J. 2000;14(5):691–698. doi: 10.1096/fasebj.14.5.691. [DOI] [PubMed] [Google Scholar]
- Huk I, Brovkovych V, Vili J. Nanobash, Weigel G, Neumayer Ch., Partyka L, Patton S, Malinski T. Bioflavonoid quercetin scavenges superoxide and increases nitric oxide concentration in ischaemia-reperfusion injury: an experimental study. British Journal of Surgery. 1998;85(8):1080–1085. doi: 10.1046/j.1365-2168.1998.00787.x. [DOI] [PubMed] [Google Scholar]
- Ischiropoulos H, Beckman JS. Oxidative stress and nitration in neurodegeneration: Cause, effect, or association? J. Clin. Invest. 2003;111:163–169. doi: 10.1172/JCI17638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juckett M, Zheng Y, Yuan H, Pastor T, Antholine W, Weber M, Vercellotti G. Heme and the endothelium. Effects of nitric oxide on catalytic iron and heme degradation by heme oxygenase. J Biol Chem. 1998;273:23388–97. doi: 10.1074/jbc.273.36.23388. [DOI] [PubMed] [Google Scholar]
- Kawase M, Kinouchi H, Kato I, Akabane A, Kondo T, Arai S, Fujimura M, Okamoto H, Yoshimoto T. Inducible nitric oxide synthase following hypoxia in rat cultured glial cell. Brain Research. 1996;738:319–322. doi: 10.1016/s0006-8993(96)00924-9. [DOI] [PubMed] [Google Scholar]
- Kitamura Y, Furakawa M, Matsuoka Y, Tooyama I, Kimura H, Nomura Y, Taniguchi T. In vitro and in vivo induction of heme oxygenase-1 in rat glial cells-possible involvement of nitric oxide production from inducible nitric oxide synthase. Glia. 1998;22:138–148. [PubMed] [Google Scholar]
- Kitamura Y, Ishida Y, Takata K, Mizutani H, Kakimura J, Inden M, Nakata J, Taniguchi T, Tsukahara T, Akaike A, Shimohama S. Hyperbilirubinemia protects against focal ischemia in rats. J. Neurosci. Res. 2003;71:544–550. doi: 10.1002/jnr.10514. [DOI] [PubMed] [Google Scholar]
- Kuljis RO, Schelper RL. Alterations in nitrogen monoxide-synthesizing cortical neurons in amyotrophic lateral sclerosis with dementia. J. Neuropathology & Experimental Neurology. 1996;55(1):25–35. doi: 10.1097/00005072-199601000-00003. [DOI] [PubMed] [Google Scholar]
- Leonard CS, Michaelis EK, Mitchell KM. Activity-Dependent Nitric Oxide Concentration Dynamics in the Laterodorsal Tegmental Nucleus In Vitro. J Neurophysiol. 2001;86:2159–2172. doi: 10.1152/jn.2001.86.5.2159. [DOI] [PubMed] [Google Scholar]
- MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annual Review of Pharmacology and Toxicology. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Yoshino H, Yuki N, Hara Y, Cashman NR, Handa S, Miyatake T. Ganglioside characterization of a cell line displaying motor neuron-like phenotype: GM2 as a possible major ganglioside in motor neurons. J. Neurol. Sci. 1995;131:111–118. doi: 10.1016/0022-510x(95)00101-7. [DOI] [PubMed] [Google Scholar]
- McDonald JW, the Research Consortium of the Christopher Reeve Paralysis Foundation Repairing the damaged spinal cord. Scientific American. 1999:64–73. doi: 10.1038/scientificamerican0999-64. [DOI] [PubMed] [Google Scholar]
- Pacher P, Beckman JS, Liaudet L. Nitric Oxide and Peroxynitrite in Health and Disease Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer MA, Stasiv Y, Benraiss A, Chmieinicki E, Grinberg A, Westphal H, Goldman SA, Enikolopov G. Nitric oxide negatively regulates mammalian adult neurogenesis. PNAS. 2003;100(16):9566–9571. doi: 10.1073/pnas.1633579100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panahian N, Maines MD. Site of injury-directed induction of heme oxygenase-1 and -2 in experimental spinal cord injury: differential functions in neuronal defense mechanisms? J. Neurochem. 2001;76:539–554. doi: 10.1046/j.1471-4159.2001.00023.x. [DOI] [PubMed] [Google Scholar]
- Pfeiffer S, Leopold E, Hemmens B, Schmidt K, Werner ER, Mayer B. Interference of carboxy-PTIO with nitric oxide- and peroxynitrite-mediated reactions. Free Radic Biol Med. 1997;22(5):787–94. doi: 10.1016/s0891-5849(96)00407-8. [DOI] [PubMed] [Google Scholar]
- Schipper HM, Cisse S, Stopa E. Expression of heme oxygenase-1 in the senescent and Alzheimer-diseased brain. Ann. Neurol. 1995;37:758–768. doi: 10.1002/ana.410370609. [DOI] [PubMed] [Google Scholar]
- Schnaar RI, Schaffner AE. Separation of cell types from embryonic chicken and rat spinal cord: characterization of motoneuron-enriched fractions. J Neurosci. 1981;1(2):204–17. doi: 10.1523/JNEUROSCI.01-02-00204.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorziello A, Santillo M, Adornetto A, Dell’Aversano C, Sirabella R, Damiano S, Canzoniero LMT, Di Renzo GF, Annunziato L. NO-induced neuroprotection in ischemic preconditioning stimulates mitochondrial Mn-SOD activity and expression via RAS/ERK1/2 pathway. Journal of Neurochemistry. 2007;103(4):1472–1480. doi: 10.1111/j.1471-4159.2007.04845.x. [DOI] [PubMed] [Google Scholar]
- Sharma HS, Westman J, Olsson Y, Alm P. Involvement of nitric oxide in acute spinal cord injury: an immunocytochemical study using light and electron microscopy in the rat. Neuroscience Research. 1996;24(4):373–384. doi: 10.1016/0168-0102(95)01015-7. [DOI] [PubMed] [Google Scholar]
- Soares MP, Usheva A, Brouard S, Berberat PO, Gunther L, Tobiasch E, Bach FH. Modulation of endothelial cell apoptosis by heme oxygenase-1-derived carbon monoxide. Antioxid Redox Signal. 2002;4:321–9. doi: 10.1089/152308602753666370. [DOI] [PubMed] [Google Scholar]
- Stamler JS, Jia L, Eu JP, Mcmahon TJ, Demchenko IT, Bonaventura J, Gernert K, Piantadosi CA. Blood flow regulation by S-nitrosohemoglobin in the physiological oxygen gradient. Science. 1997;276:2034–2037. doi: 10.1126/science.276.5321.2034. [DOI] [PubMed] [Google Scholar]
- Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- Stuehr DJ. Mammalian nitric oxide synthases. Biochim Biophys Acta. 1999;1411(2-3):217–30. doi: 10.1016/s0005-2728(99)00016-x. [DOI] [PubMed] [Google Scholar]
- Tamir S, Burney S, Tannenbaum SR. DNA damage by nitric oxide. Chem. Res. Toxicol. 1996;9:821–7. doi: 10.1021/tx9600311. [DOI] [PubMed] [Google Scholar]
- Tamir S, Lewis RS, de Rojas Walker T, Deen WM, Wishnok JS, Tannenbaum SR. The influence of delivery rate on the chemistry and biological effects of nitric oxide. Chem. Res. Toxicol. 1993;6:895–9. doi: 10.1021/tx00036a021. [DOI] [PubMed] [Google Scholar]
- Thomas DD, Espey MG, Vitek MP, Miranda KM, Wink D. A Protein nitration is mediated by heme and free metals through Fenton-type chemistry: An alternative to the NO/O2-reaction. PNAS. 2002;99(20):12691–12696. doi: 10.1073/pnas.202312699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga T, Sato S, Ohnishi T, Ohnishi ST. Electron paramagnetic resonance (EPR) detection of nitric oxide produced during forebrain ischemia of the rat. J Cereb Blood Flow Metab. 1994;14:715–722. doi: 10.1038/jcbfm.1994.92. [DOI] [PubMed] [Google Scholar]
- Vaziri ND, Lee YS, Lin CY, Lin VW, Sindhu RK. NAD(P)H oxidase, superoxide dismutase, catalase, glutathione peroxidase and nitric oxide synthase expression in subacute spinal cord injury. Brain Res. 2004;995(1):76–83. doi: 10.1016/j.brainres.2003.09.056. [DOI] [PubMed] [Google Scholar]
- Yang G, Nguyen X, Ou J, Rekulapelli P, Stevenson DK, Dennery PA. Unique effects of zinc protoporphyrin on HO-1 induction and apoptosis. Blood. 2001;97:1306–1313. doi: 10.1182/blood.v97.5.1306. [DOI] [PubMed] [Google Scholar]
- Zimmermann A, Leffler CW, Tcheranova D, Fedinec AL, Parfenova H. Cerebroprotective effects of the CO-releasing molecule CORM-A1 against seizure-induced neonatal vascular injury. Am J Physiol Heart Circ Physiol. 2007;293(4):H2501–H2507. doi: 10.1152/ajpheart.00354.2007. [DOI] [PubMed] [Google Scholar]