Abstract
Objectives
The primary objective was to evaluate the effect of omega-3 fatty acids (omega-3 FA) on matrix metalloproteinase-9 (MMP-9) production by immune cells in multiple sclerosis (MS). Quality of life, fatty acid levels, and safety were also evaluated.
Materials and Methods
Ten participants with relapsing-remitting MS (RRMS) received omega-3 FA supplementation (9.6 grams/day fish oil) in an open-label study. Participants were evaluated at four time points, baseline, after 1-month of omega-3 FA supplementation, after 3-months of omega-3 FA supplementation, and after a 3-month wash out.
Results
Immune cell secretion of MMP-9 decreased by 58% after 3-months of omega-3 FA supplementation when compared to baseline levels (p < 0.01). This effect was coupled with a significant increase in omega-3 FA levels in red blood cell membranes.
Conclusions
Omega-3 FA significantly decreased MMP-9 levels in RRMS and may act as immune-modulator that has potential therapeutic benefit in MS patients.
1. Introduction
Multiple sclerosis (MS) is an inflammatory disease of the central nervous system (CNS) in which T lymphocytes, macrophages, and antibodies are believed to be involved in disease pathogenesis1-2. Although the cause of MS is unknown, there is general agreement that MS results from an acquired immune-dysregulation and aberrant activation leading to T cell driven inflammatory processes in the CNS that result in demyelination and axonal damage. About 85% of patients begin with a relapsing-remitting clinical course in which there are relapses or attacks of MS lasting days to weeks followed by improvement and stability lasting months to years. About 50% of patients with relapsing-remitting MS (RRMS) develop secondary progressive MS in which there is progressive worsening of the disease. Although there are heterogeneous mechanisms to disease pathology, active inflammatory processes in the CNS are more highly associated with RRMS than with the progressive types of MS3.
Current pharmacological therapies for MS include: interferon beta, glatiramer acetate, natalizumab, and mitoxantrone. These are immunomodulatory therapies and act by altering the T cell driven inflammatory processes in the CNS4-7. In large randomized, double-blind, placebo-controlled trials, these therapies decrease the frequency and severity of relapses and have a modest effect on lengthening time to disability4-9. Although the availability of these therapies has improved the disease course in MS, they all require injection rather than an oral route of administration and there are significant side effects which include, injection site reactions, flu-like symptoms, and muscle aches10. A subset of MS patients produce anti-interferon-beta neutralizing antibodies in response to interferon beta (IFN-beta) therapy which decreases the effectiveness of this therapy and can increase the risk of subsequent relapses in these patients11. The high cost of MS immunomodulatory therapies, which can cost $25,000 or more per year, may prohibit their use in some patients. Considering the limitations of current MS immunomodulatory therapies, identifying a cost effective oral therapy that has the potential to alter the MS disease course with minimal side effects is warranted.
The immunomodulatory effects of omega-3 fatty acids have been well documented. Numerous studies have reported a decrease in protein levels of inflammatory cytokines including, tumor necrosis factor- α (TNF-α), interferon- γ (IFN-γ), interleukin-1 (IL-1), interleukin-2 (IL-2), and vascular cell adhesion molecule-1 (VCAM-1)12. Omega-3 fatty acids (omega-3 FA) have been evaluated in a number of inflammatory diseases such as rheumatoid arthritis and multiple sclerosis with proven benefit13, 14. In an open-label study (n=20 MS and 15 healthy participants), Gallai et al. found a significant decrease from baseline, in the levels of interleukin-1β (p<0.03), TNF-α (p<0.02), IL-2 (p<0.002), and IFN-γ (p<0.01) produced from unstimulated and stimulated peripheral blood mononuclear cells (PBMC) after 3-months of omega-3 FA supplementation containing 3.0 grams/day of eicosapentaenoic acid (EPA) and 1.8 grams/day docosahexaenoic acid (DHA)15. Weinstock-Guttman et al. conducted a double-blind, placebo-controlled study in RRMS patients (n=31) and found a significant improvement in quality of life (physical and mental health components) favoring participants receiving a low saturated fat diet and omega-3 FA supplementation at six months. This study also examined changes in plasma inflammatory cytokine levels and found no difference between groups16. The difference in outcomes on inflammatory cytokine levels between these two studies may reflect differences in the concentrations of EPA and DHA evaluated (Weistock-Guttman et al. evaluated a lower concentration than Gallai et al.) and differences in cytokine measures (plasma versus immune cell secreted). Despite these differences, both studies reported positive effects of omega-3 FA supplementation in MS.
In addition to the omega-3 FA effect on decreasing proinflammatory cytokines levels, there is evidence supporting an omega-3 FA effect in decreasing matrix metalloproteinase (MMP) levels. There are a number of published in vitro studies that report a significant omega-3 FA effect in decreasing genetic expression, protein levels, and activity of MMP-2, -3, -9, -1317-19. One in vitro study reports a significant and dose-dependent decrease in MMP-9 protein levels secreted from LPS activated microglial cells that were incubated with either fish oil or an omega-3 FA mixture (53% EPA and 27% DHA)20.
In MS, MMP-9 is thought to have a significant role in the transmigration of inflammatory cells into the CNS by aiding in the disruption of the blood brain barrier21. Several studies have reported higher MMP-9 levels in MS subjects when compared to control subjects22-24. RRMS patients are reported to have an increase in immune cell expression of MMP-9 (measured by messenger ribonucleic acid (mRNA) levels), compared to healthy controls25. Interferon beta has the ability to inhibit MMP-9 levels produced from T-lymphocytes and CD4+ T cells26-28 which is thought to be one mechanism by which this therapy acts to alter the disease course23. To date the immunomodulatory effects of omega-3 FA on MMP-9 levels in MS patients have not been evaluated.
The primary objective of the present study was to evaluate the ability of omega-3 FA supplementation in decreasing MMP-9 protein levels secreted from peripheral blood mononuclear cells (PBMC) and from serum in RRMS.
2. Materials and Methods
This was an open-label study in ten relapsing remitting MS patients to determine the immunomodulatory effects of omega-3 FA supplemenatation. The study was approved by the Institutional Review Board at Oregon Health & Science University (OHSU). All participants gave informed consent before entering the study. The study duration was six months with three months of omega-3 FA supplementation followed by a three month wash out period. After baseline assessments, all ten participants were given omega-3 FA in the form of fish oil concentrate (9.6 grams/day containing 2.9 grams EPA and 1.9 grams DHA) (Vital Nutrients, Middletown, CT, USA). To account for pretreatment variations in MMP-9 levels, blood was collected at three baseline time points at one-week intervals. Once omega-3 FA supplementation was started only one MMP-9 blood draw was performed to accurately reflect treatment timepoints, after 1-month of omega-3 FA supplementation; after 3-months of omega-3 FA supplementation; after a 3-month wash out.
2.1 Inclusion and exclusion criteria
Participants with a definite diagnosis of MS using the McDonald criteria29; relapsing-remitting type of MS; ages 18-65 years were enrolled. Exclusions to participation included the following: other types of MS; omega-3 FA supplementation within 30 days of enrollment; eating more than 6 ounces per week of fish within 30 days of enrollment; pregnancy; MS exacerbation or corticosteroid treatment within 60 days of enrollment; significant health condition (e.g. coronary heart disease, uncontrolled diabetes mellitus, liver disease). Participants were allowed to continue their MS disease modifying therapies, therapies for MS symptoms, and dietary supplements other than omega-3 fatty acids.
2.2 Primary outcome measure
The primary outcome measure was MMP-9 levels secreted from PBMC and MMR-9 levels in serum. Blood samples for PBMC isolation were collected by venipuncture into herparinized Vacutainer® tubes. PBMC were isolated within 4 hours of blood draw (Lymphocyte separation media; Mediatech) and were incubated in PBS at 2 × 106 cells/ml at 37°C for 48hrs. Cell supernatants were collected and stored at −80°C until assayed. Blood samples for serum were collected by venipuncture into untreated Vacutainer® tubes. Serum was separated from the clot by centrifugation, aliquoted (0.5 ml) and stored at −80°C until assayed. MMP-9 levels from cell supernatants and serum were measured by enzyme-linked immunosorbent assay (R&D Systems Inc, Minneapolis, MN).
2.3 Secondary outcome measure
Quality of life was assessed by the MS Quality of Life Inventory (MSQLI) as a secondary outcome measure. The MSQLI is a modular MS-specific health-related quality of life (HRQL) instrument consisting of the Short Form-36 questions (SF-36) supplemented by nine symptom specific measures (fatigue, pain, bladder function, bowel function, emotional status, perceived cognitive function, visual function, sexual satisfaction, and social relationships)30.
2.4 Other measures
Incorporation of fatty acids into cell membranes was measured by red blood cell (RBC) membrane fatty acid analysis. Erythrocytes were separated from plasma by centrifugation. Lipids were extracted from erythrocytes and fatty acids were measured by capillary gas/liquid chromatography. This assay was performed at Oregon Health & Science University's Lipid Laboratory using methods that have been validated and previously published31, 32.
Neurologic impairment was measure by the Expanded Disability Status Scale (EDSS). This is an ordinal scale giving a measure between 0 (normal) to 10.0 (dead). Patients with scores in the 0.5-4.0 range have mild impairment and are able to walk at least 500 meters without aid or rest. Patients with scores in the 4.5-7.0 range have increasing limitations in their ability to walk. Patients with scores of ≥ 7.5 cannot walk and have upper extremity dysfunction33.
Safety was assessed monthly by adverse event reports and by laboratory measures (comprehensive metabolic panel and prothrombin time). Compliance was measured by pill count.
2.5 Data analysis
Statistical analysis was performed using SPSS version 14.0. Descriptive statistics was used to summarize participant characteristics information. Linear mixed model was used to compare baseline MMP-9 levels from PBMC and serum to the three other time points (1-month of omega-3 FA supplementation, 3-months of omega-3 FA supplementation, and after a 3-month wash out). This model was used to adjust for increased variability in the baseline measurement as a result of comparing three baseline measurements for MMP-9 levels to one measurement at each time of the other time points. PBMC or serum MMP-9 levels were entered as the dependent variable and intervention time (baseline, 1-month of omega-3 FA, 3-months of omega-3 FA, 3-months of wash out) was entered as both fixed and random variables. Age, education, MS duration, disability (EDSS), and MS disease-modifying therapy use were entered into the model to evaluate their effects on MMP-9 levels. Repeated measures were used to compare EPA and DHA levels and quality of life scores.
3. Results
All ten subjects completed the six-month study. Subject characteristics are summarized in Table 1. All of the subjects were Caucasian, most were female (90%) with an education level of high school or greater (70%). Mean age was 47.6 years, mean MS duration was 15.3 years with a mean EDSS of 3.2. Half of the subjects were on a MS immunomodulatory therapy (IFN-beta or glatiramer acetate).
Table 1.
Subject Characteristics (n=10)
| Characteristic | Mean (SD) or % |
|---|---|
| Age | 47.6 (9.0) |
| Gender | |
| Female | 90% |
| Male | 10% |
| Race | |
| Caucasian | 100% |
| Education | |
| High School Grad/Some college | 70% |
| Bachelors degree or greater | 30% |
| MS duration in years | 15.3 (9.4) |
| *EDSS | 3.2 (0.23) |
| **MS Drug Therapy use | 50% |
Expanded Disability Status Scale
Disease Modifying Therapy (IFN-beta or glatiramer acetate)
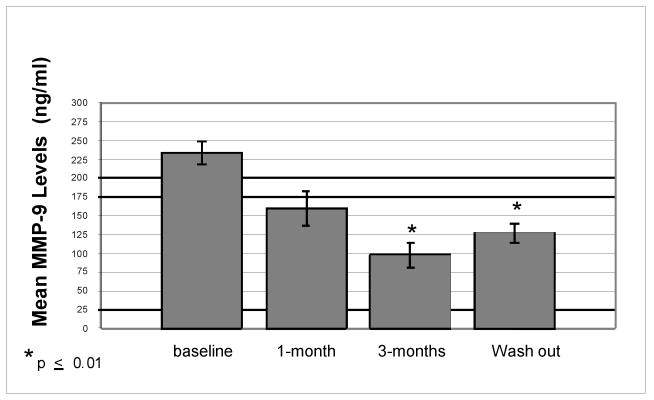
There was a 58% decrease from mean baseline MMP-9 levels secreted from PBMC after 3-months of omega-3 FA supplementation (p=0.002) and a 45% decrease from mean baseline levels after 3-months of wash out (p=0.01). The decrease from mean baseline MMP-9 levels was significant at both time points (Figure 1). This difference persisted even after adjusting for age, education level, MS duration, disability, and IFN-beta and glatiramer acetate use. All ten subjects showed a decrease in MMP-9 levels after 3-months of omega-3 FA supplementation (Figure 2). There was no significant difference from mean baseline MMP-9 levels in serum after 1-month or 3-months of omega-3 FA supplementation (p=0.61, p=0.68) or after a 3-month wash out (p=0.95) (Table 2).
Figure 1. Mean MMP-9 Levels Secreted by Unstimulated PBMC: Comparison to Baseline.
Mean MMP-9 levels measured from unstimulated PBMC at baseline, after 1-month of omega-3 FA supplementation, after 3-months of omega-3 FA supplementation, and after a 3-month wash out.
Figure 2. Change in MMP-9 Levels (PBMC Secreted) from Baseline to 3-months of Omega-3 FA Supplementation in All Ten MS Subjects.
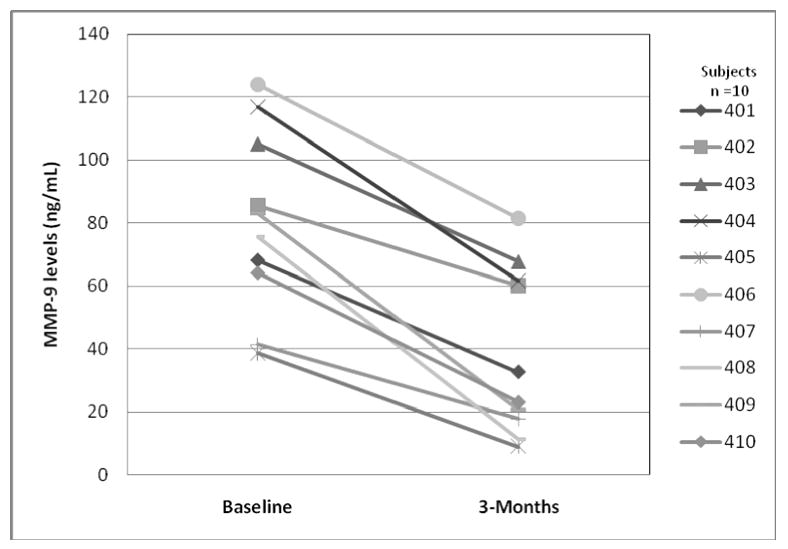
Change in unstimulated PBMC secreted MMP-9 levels from baseline to 3-months of omega-3 FA supplementation in all ten relapsing remitting MS subjects. Baseline MMP-9 levels reflect the mean of three baseline measures.
Table 2.
Mean Serum MMP-9 Levels in MS Subjects: Comparison to Baseline (n=10)
| Timepoint | Mean | Standard error | p-value |
|---|---|---|---|
| Baseline | 532.3 | 18.7 | |
| 1-Month | 562.7 | 30.6 | 0.61 |
| 3-Months | 553.2 | 38.3 | 0.68 |
| Wash out | 527.1 | 61.4 | 0.95 |
Mean serum MMP-9 levels measured at baseline, after 1-month of omega-3 FA supplementation, after 3-months of omega-3 FA supplementation, and after a 3-month wash out.
After 3-months of omega-3 FA supplementation, there was a 6.3 fold increase from mean baseline levels in RBC membrane EPA levels (p=0.001) and a 1.7 fold increase in DHA levels (p=0.04) (Figure 3). Although the mean EPA and DHA levels had decreased after a 3-month wash out, they remained significantly higher than baseline levels, EPA (p=0.02), DHA (p=0.05).
Figure 3. Mean EPA and DHA Levels in MS Subjects Measured from RBC Membranes:Comparison to Baseline.
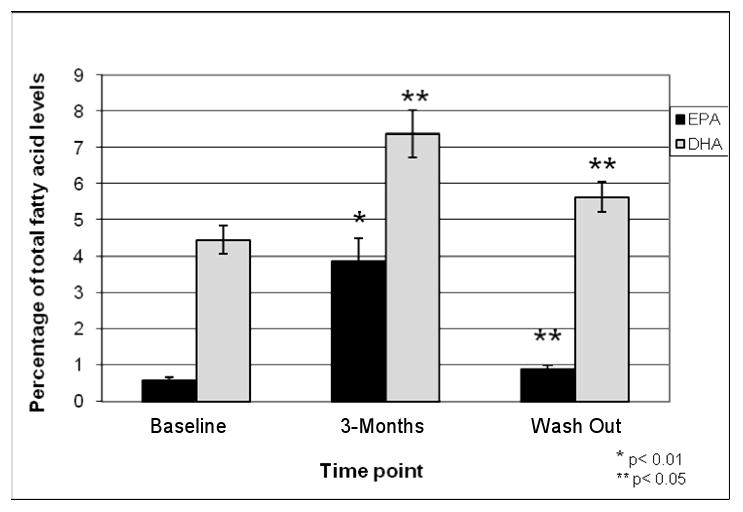
Mean EPA and DHA levels measured from red blood cell membranes at baseline, after 3-months of omega-3 FA supplementation and after a 3-month wash out.
3.1 Quality of life
No significant differences were found from mean baseline physical or mental components scores of the SF-36 or from mean baseline values on any of the MS-specific components of the MSQLI (data not shown, all p-values > 0.20).
3.2 Safety and Compliance
All of the subjects (10/10) reported “fish burps” and 3/10 subjects reported fatigue within the first month of omega-3 FA supplementation (two of these subjects reported that hot weather was bothering them). No MS relapses occurred during the study. All mean baseline measures on the comprehensive metabolic panel were within normal limits and there was no significant change from mean baseline levels on any of these measures. Mean baseline prothrombin levels were 0.96 INR (SD 0.05) and mean levels after 3-months of omega-3 FA supplementation were 0.99 INR (SD 0.08). There was no significant difference between these two time points (p=0.308). Mean percent compliance by pill count was 88.6% with a compliance range of 75%-100%.
4. Discussion
This is the first study to demonstrate the ability of omege-3 FA supplementation to alter MMP-9 production by immune cells in RRMS. The study demonstrated that omega-3 FA with 9.6 grams of fish oil concentrate per day (containing 2.9 grams EPA and 1.9 grams DHA) resulted in a significant decrease in MMP-9 levels secreted from PBMC in people with MS after three months of supplementation. This outcome was independent of age, MS duration, neurologic impairment (EDSS), and IFN-beta or glatiramer acetate use. All ten subjects showed a decrease in immune cell secreted MMP-9 levels after three months of supplementation which suggests that the observed effect may be independent of IFN-beta use, glatiramer acetate use, or over the counter supplement use (Figure 2). Omega-3 FA at this dose was well tolerated and did not significantly alter basic metabolic function or prothrombin time over a period of three months. Our study did not show a significant effect of omega-3 FA supplementation on serum MMP-9 levels. As increased MMP-9 levels may have an impact on MS disease pathogenesis, the ability of omega-3 FA to decrease the levels secreted by immune cells is a significant observation.
In the periphery, MMP-9 is produced by several cells types including monocytes, macrophages, T-lymphocytes, neutrophils and endothelial cells34. Our study was interested in examining the ability of omega-3 FA in modulating MMP-9 secretion from PBMC because the migration of activated T-lymphocytes into the CNS is one mechanism of disease pathogenesis. PBMC are composed of both T-lymphocytes (CD4+ and CD8+), B cells, and natural killer cells (NK cells). PBMC from MS patients are reported to have significantly elevated MMP-9 mRNA levels when compared to healthy controls, the increased MMP-9 expression in PBMC may enable these cells to migrate into the CNS35, 36. Stuve et al observed that activated T-lymphocytes isolated from untreated MS patients were able to migrate through a fibronectin barrier due to increased MMP-9 activity. T-lymphocytes treated with IFN-beta decreased the migratory ability of these cells27. Dressel et al. observed that the migration of CD4+ T cells through a fibronectin barrier, but not CD8+ cells, was enhanced in untreated MS patients compared with controls and that CD4+ migration and MMP-9 production was reduced by IFN-beta treatment26. Decreasing MMP-9 expression and MMP-9 protein levels in T-lymphocytes is one proposed mechanism for the therapeutic action of IFN-beta therapy in MS37. The results from our study suggest that omega-3 FA may act by a similar mechanism.
How omega-3 FA decrease MMP-9 production in immune cells remains uncertain. It is possible that the effects of omega-3 FA on MMP-9 levels are secondary to decreasing proinflammatory cytokine levels. Release of MMP-9 by monocytes can be induced by autocrine secretion of IL-1 or TNF-α and it has been reported that both proinflammatory cytokines increase MMP-9 mRNA levels38. Omega-3 FA are reported to decrease both IL-1 and TNF-α in MS subjects and healthy controls 15 and may decrease MMP-9 levels by decreasing levels of both cytokines. Omega-3 FA also have the ability to decrease nuclear factor-kappa B (NF-κB) and activator protein-1 (AP-1) binding activity, both of which have binding sites on the MMP-9 gene promotor38-41. Omega-3 FA may inhibit MMP-9 expression by modulating NF-κB and AP-1 binding activity which could lead to decreased MMP-9 protein levels.
Our study did not evaluate the effect of omega-3 FA on TIMP-1 and TIMP-2 levels as a possible mechanism for regulating MMP-9 levels in RRMS. TIMP-1 and TIMP-2 form complexes with soluble MMP-9 that result in an inhibition of MMP-9 activity. There are five pilot studies that have reported significant increases in TIMP-1 levels in MS patients after IFN-beta therapy42-46. Collectively these studies suggest that one mechanism through which IFN-beta therapy may act is by simultaneously decreasing MMP-9 while significantly increasing TIMP-1 levels. In vitro, there is limited evidence that omega-3 FA have the ability to decrease MMP levels while increasing TIMP levels. Renal cell carcinoma cells (Caki-1) exposed to EPA, but not arachidonic acid (an omega-6 fatty acid) showed a dose-related decrease in MMP-2 expression and a dose related increase in TIMP-2 expression19. It will be of interest in future studies to determine if omega-3 FA have the ability to increase TIMP-1 levels while decreasing MMP-9 levels in MS.
A small sample size and not having a placebo-control group limits the interpretation of the study results. Although our study had a small sample size, all ten subjects showed a decrease in MMP-9 levels after three months of omega-3 FA supplementation, decreasing the likelihood that the effect was a random event. In support of the presents study's findings, our group has observed a significant decrease in MMP-9 activity in PMBC treated with EPA or DHA at a concentration of 5 mg/dl with no effect observed in PBMC treated with oleic acid at the same concentration (unpublished in vitro data). The in vitro concentration of EPA and DHA at 5 mg/dl would be achievable in whole blood following a daily oral dose of 0.6 grams EPA and 0.50 grams DHA47, an oral dose that is significantly lower than the dose used in our clinical study (daily dose of 2.9 grams EPA and 1.9 grams DHA). Our unpublished in vitro results suggest that it may be possible to decrease MMP-9 levels secreted from immune cells at a lower oral dose of omega-3 FA than used in our clinical study and that evaluation of lower doses of omega-3 FA in MS should be explored. Although not done in the present study, future studies evaluating the effects of omega-3 FA supplementation on MMP-9 levels in MS warrant the inclusion of a placebo-control group. Olive oil contains up to 75% oleic acid and may serve as a good placebo for future clinical studies evaluating omega-3 FA effects on MMP-9 in MS as oleic acid did not affect MMP-9 activity in vitro (our unpublished data). Careful attention to blinding in the design of omega-3 FA supplementation studies is also warranted, as all ten participants in our study reported ‘fish burps’ during omega-3 FA supplementation.
No changes in quality of life as measured by the MSQLI from baseline values after 3-months of omega-3 FA supplementation or after a 3-month washout were observed. Because MS patients score significantly lower on health related quality of life measures (HRQL) than those in the general population48, 49 and drug therapy side affects can decrease HRQL in MS50, it is important to identify therapies that are effective but do not significantly decrease quality of life. Omega-3 FA was given at a fairly high dose, 9.6 grams of fish oil concentrate per day for 3 months, yet quality of life was not significantly decreased.
5. Conclusions
Our data showed that omega-3 FA has the ability to significantly decrease immune cell secreted MMP-9 levels and was well tolerated at a dose of 9.6 grams/day over three months. Because increased MMP-9 levels may facilitate the migration of activated immune cells into the CNS, omega-3 FA may have a potential therapeutic role in RRMS patients. Future studies evaluating the effects of omega-3 FA on MMP-9 and TIMP-1 levels and the evaluation of longer-term effects of omega-3 FA supplementation on clinical outcomes in MS are warranted.
Acknowledgments
This research was funded by National Institutes of Health grant P50 AT00066-01, the Department of Veterans Affairs, and the Nancy Davis Center Without Walls. The authors wish to thank Vital Nutrients, Middletown, CT, for supplying the fish oil concentrate.
This research was support by the National Institutes of Health P50 AT00066-01, the Department of Veterans Affairs, and the Nancy Davis Center Without Walls.
Footnotes
None of the authors have anything to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hafler DA, Weiner HL. Immunologic mechanisms and therapy in multiple sclerosis. Immunol Rev. 1995;144:75–107. doi: 10.1111/j.1600-065x.1995.tb00066.x. [DOI] [PubMed] [Google Scholar]
- 2.Martin R, McFarland HF, McFarlin DE. Immunological aspects of demyelinating diseases. Annu Rev Immunol. 1992;10:153–87. doi: 10.1146/annurev.iy.10.040192.001101. [DOI] [PubMed] [Google Scholar]
- 3.Morales Y, Parisi JE, Lucchinetti CF. The pathology of multiple sclerosis: evidence for heterogeneity. Adv Neurol. 2006;98:27–45. [PubMed] [Google Scholar]
- 4.The IFNB Multiple Sclerosis Study Group. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. Clinical results. Neurology. 1993;43:655–61. doi: 10.1212/wnl.43.4.655. [DOI] [PubMed] [Google Scholar]
- 5.Johnson KP, Brooks BR, Cohen JA, et al. Copolymer 1 reduces relapse rate and improves disability in relapsing- remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial. The Copolymer 1 Multiple Sclerosis Study Group. Neurology. 1995;45(7):1268–76. doi: 10.1212/wnl.45.7.1268. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs LD, Cookfair DL, Rudick RA, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG) Ann Neurol. 1996;39(3):285–94. doi: 10.1002/ana.410390304. [DOI] [PubMed] [Google Scholar]
- 7.PRISMS Group. Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group. Lancet. 1998;352(9139):1498–504. [PubMed] [Google Scholar]
- 8.Rudick RA, Lee JC, Simon J, Fisher E. Significance of T2 lesions in multiple sclerosis: A 13-year longitudinal study. Ann Neurol. 2006;60(2):236–42. doi: 10.1002/ana.20883. [DOI] [PubMed] [Google Scholar]
- 9.Niino M, Bodner C, Simard ML, et al. Natalizumab effects on immune cell responses in multiple sclerosis. Ann Neurol. 2006;59(5):748–54. doi: 10.1002/ana.20859. [DOI] [PubMed] [Google Scholar]
- 10.Mohr DC, Likosky W, Boudewyn AC, et al. Side effect profile and adherence to in the treatment of multiple sclerosis with interferon beta-1a. Mult Scler. 1998;4(6):487–89. doi: 10.1177/135245859800400605. [DOI] [PubMed] [Google Scholar]
- 11.Farrell R, Kapoor R, Leary S, et al. Neutralizing anti-interferon beta antibodies are associated with reduced side effects and delayed impact on efficacy of Interferon-beta. Mult Scler. 2008;14(2):212–18. doi: 10.1177/1352458507082066. [DOI] [PubMed] [Google Scholar]
- 12.Calder PC. Dietary modification of inflammation with lipids. Proc Nutr Soc. 2002;61(3):345–58. doi: 10.1079/pns2002166. [DOI] [PubMed] [Google Scholar]
- 13.Babcock T, Helton WS, Espat NJ. Eicosapentaenoic acid (EPA): an antiinflammatory omega-3 fat with potential clinical applications. Nutrition. 2000;16(1112):1116–8. doi: 10.1016/s0899-9007(00)00392-0. [DOI] [PubMed] [Google Scholar]
- 14.Cleland LG, James MJ. Fish oil and rheumatoid arthritis: antiinflammatory and collateral health benefits. J Rheumatol. 2000;27(10):2305–7. [PubMed] [Google Scholar]
- 15.Gallai V, Sarchielli P, Trequattrini A, et al. Cytokine secretion and eicosanoid production in the peripheral blood mononuclear cells of MS patients undergoing dietary supplementation with n-3 polyunsaturated fatty acids. J Neuroimmunol. 1995;56(2):143–53. doi: 10.1016/0165-5728(94)00140-j. [DOI] [PubMed] [Google Scholar]
- 16.Weinstock-Guttman B, Bayer M, Park Y, et al. Low fat dietary intervention with omega-3 fatty acid supplementation in multiple sclerosis patients. Prostaglandins Leukot Essent Fatty Acids. 2005;73(5):397–404. doi: 10.1016/j.plefa.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 17.Curtis CL, Rees SG, Little CB, et al. Pathologic indicators of degradation and inflammation in human osteoarthritic cartilage are abrogated by exposure to n-3 fatty acids. Arthritis Rheum. 2002;46(6):1544–53. doi: 10.1002/art.10305. [DOI] [PubMed] [Google Scholar]
- 18.Harris MA, Hansen RA, Vidsudhiphan P, et al. Effects of conjugated linoleic acids and docosahexaenoic acid on rat liver and reproductive tissue fatty acids, prostaglandins and matrix metalloproteinase production. Prostaglandins Leukot Essent Fatty Acids. 2001;65(1):23–9. doi: 10.1054/plef.2001.0283. [DOI] [PubMed] [Google Scholar]
- 19.McCabe AJ, Wallace J, Gilmore WS, et al. The effect of eicosapentanoic acid on matrix metalloproteinase gene expression. Lipids. 1999;34(Suppl):S217–8. doi: 10.1007/BF02562295. [DOI] [PubMed] [Google Scholar]
- 20.Liuzzi GM, Latronico T, Rossano R, et al. Inhibitory effect of polyunsaturated fatty acids on MMP-9 release from microglial cells--implications for complementary multiple sclerosis treatment. Neurochem Res. 2007;32(12):2184–93. doi: 10.1007/s11064-007-9415-9. [DOI] [PubMed] [Google Scholar]
- 21.Leppert D, Lindberg RL, Kappos L, Leib SL. Matrix metalloproteinases: multifunctional effectors of inflammation in multiple sclerosis and bacterial meningitis. Brain Res Brain Res Rev. 2001;36(23):249–57. doi: 10.1016/s0165-0173(01)00101-1. [DOI] [PubMed] [Google Scholar]
- 22.Waubant E, Goodkin DE, Gee L, et al. Serum MMP-9 and TIMP-1 levels are related to MRI activity in relapsing multiple sclerosis. Neurology. 1999;53(7):1397–401. doi: 10.1212/wnl.53.7.1397. [DOI] [PubMed] [Google Scholar]
- 23.Trojano M, Avolio C, Liuzzi GM, et al. Changes of serum sICAM-1 and MMP-9 induced by rIFNbeta-1b treatment in relapsing-remitting MS. Neurology. 1999;53(7):1402–8. doi: 10.1212/wnl.53.7.1402. [DOI] [PubMed] [Google Scholar]
- 24.Lee MA, Palace J, Stabler G, et al. Serum gelatinase B, TIMP-1 and TIMP-2 levels in multiple sclerosis. A longitudinal clinical and MRI study. Brain. 1999;122(Pt 2):191–97. doi: 10.1093/brain/122.2.191. [DOI] [PubMed] [Google Scholar]
- 25.Bar-Or A, Nuttall RK, Duddy M, et al. Analyses of all matrix metalloproteinase members in leukocytes emphasize monocytes as major inflammatory mediators in multiple sclerosis. Brain. 2003;126(Pt 12):2738–49. doi: 10.1093/brain/awg285. [DOI] [PubMed] [Google Scholar]
- 26.Dressel A, Mirowska-Guzel D, Gerlach C, et al. Migration of T-cell subsets in multiple sclerosis and the effect of interferon-beta1a. Acta Neurol Scand. 2007;116(3):164–68. doi: 10.1111/j.1600-0404.2007.00829.x. [DOI] [PubMed] [Google Scholar]
- 27.Stuve O, Dooley NP, Uhm JH, et al. Interferon beta-1b decreases the migration of T lymphocytes in vitro: effects on matrix metalloproteinase-9. Ann Neurol. 1996;40(6):853–63. doi: 10.1002/ana.410400607. [DOI] [PubMed] [Google Scholar]
- 28.Leppert D, Waubant E, Burk MR, et al. Interferon beta-1b inhibits gelatinase secretion and in vitro migration of human T cells: a possible mechanism for treatment efficacy in multiple sclerosis. Ann Neurol. 1996;40(6):846–52. doi: 10.1002/ana.410400606. [DOI] [PubMed] [Google Scholar]
- 29.McDonald W, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: Guidelines from the international panel on the diagnosis of multiple sclerosis. Annals of Neurology. 2001;50(1):121–27. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- 30.Fischer JS, LaRocca NG, Miller DM, et al. Recent developments in the assessment of quality of life in multiple sclerosis (MS) Mult Scler. 1999;5(4):251–59. doi: 10.1177/135245859900500410. [DOI] [PubMed] [Google Scholar]
- 31.Ruyle M, Connor WE, Anderson GJ, Lowensohn RI. Placental transfer of essential fatty acids in humans: venous-arterial difference for docosahexaenoic acid in fetal umbilical erythrocytes. Proc Natl Acad Sci U S A. 1990;87(20):7902–6. doi: 10.1073/pnas.87.20.7902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson GJ, Connor WE, Corliss JD, et al. Rapid modulation of the n-3 docosahexaenoic acid levels in the brain and retina of the newly hatched chick. J Lipid Res. 1989;30(3):433–41. [PubMed] [Google Scholar]
- 33.Kurtzke J. Rating neurological impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–52. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 34.Avolio C, Giuliani F, Liuzzi GM, et al. Adhesion molecules and matrix metalloproteinases in Multiple Sclerosis: effects induced by Interferon-beta. Brain Res Bull. 2003;61(3):357–64. doi: 10.1016/s0361-9230(03)00098-4. [DOI] [PubMed] [Google Scholar]
- 35.Lichtinghagen R, Seifert T, Kracke A, et al. Expression of matrix metalloproteinase-9 and its inhibitors in mononuclear blood cells of patients with multiple sclerosis. J Neuroimmunol. 1999;99(1):19–26. doi: 10.1016/s0165-5728(99)00094-6. [DOI] [PubMed] [Google Scholar]
- 36.Ozenci V, Rinaldi L, Teleshova D, et al. Metalloproteinases and their tissue inhibitors in multiple Sclerosis. J Autoimmun. 1999;12(4):297–303. doi: 10.1006/jaut.1999.0285. [DOI] [PubMed] [Google Scholar]
- 37.Markowitz CE. Interferon-beta: mechanism of action and dosing issues. Neurology. 2007;68(24) 4:S8–S11. doi: 10.1212/01.wnl.0000277703.74115.d2. [DOI] [PubMed] [Google Scholar]
- 38.St-Pierre Y, Van Themsche C, Esteve PO. Emerging features in the regulation of MMP-9 gene expression for the development of novel molecular targets and therapeutic strategies. Curr Drug Targets Inflamm Allergy. 2003;2(3):206–15. doi: 10.2174/1568010033484133. [DOI] [PubMed] [Google Scholar]
- 39.Zhao G, Etherton TD, Martin KR, et al. Anti-inflammatory effects of polyunsaturated fatty acid in THP-1 cells. Biochem Biophys Res Commun. 2005;336(3):909–17. doi: 10.1016/j.bbrc.2005.08.204. [DOI] [PubMed] [Google Scholar]
- 40.Chen W, Esselman WJ, Jump DB, et al. Anti-inflammatory effect of docosahexaenoic acid on cytokine-induced adhesion molecule expression in human retinal vascular endothelial cells. Invest Ophthalmol Vis Sci. 2005;46(11):4342–7. doi: 10.1167/iovs.05-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao Y, Chen LH. Eicosapentaenoic acid prevents lipopolysaccharide-stimulated DNA binding of activator protein-1 and c-Jun N-terminal kinase activity. J Nutr Biochem. 2005;16(2):78–84. doi: 10.1016/j.jnutbio.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 42.Boz C, Ozmenoglu M, Velioglu S, et al. Matrix metalloproteinase-9 (MMP-9) and tissue inhibitor of matrix metalloproteinase (TIMP-1) in patients with relapsing-remitting multiple sclerosis treated with interferon beta. Clin Neurol Neurosurg. 2006;108(2):124–28. doi: 10.1016/j.clineuro.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 43.Avolio C, Filippi M, Tortorella C, et al. Serum MMP-9/TIMP-1 and MMP-2/TIMP-2 ratios in multiple sclerosis: relationships with different magnetic resonance imaging measures of disease activity during IFN-beta-1a treatment. Mult Scler. 2005;11(4):441–46. doi: 10.1191/1352458505ms1193oa. [DOI] [PubMed] [Google Scholar]
- 44.Karabudak R, Kurne A, Guc D, et al. Effect of interferon beta-1a on serum matrix metalloproteinase-9 (MMP-9) and tissue inhibitor of matrix metalloproteinase (TIMP-1) in relapsing remitting multiple sclerosis patients. One year follow-up results. J Neurol. 2004;251(3):279–83. doi: 10.1007/s00415-004-0285-7. [DOI] [PubMed] [Google Scholar]
- 45.Waubant E, Goodkin D, Bostrom A, et al. IFNbeta lowers MMP-9/TIMP-1 ratio, which predicts new enhancing lesions in patients with SPMS. Neurology. 2003;60(1):52–57. doi: 10.1212/wnl.60.1.52. [DOI] [PubMed] [Google Scholar]
- 46.Waubant E, Gee L, Miller K, et al. IFN-beta1a may increase serum levels of TIMP-1 in patients with relapsing-remitting multiple sclerosis. J Interferon Cytokine Res. 2001;21(3):181–85. doi: 10.1089/107999001750133230. [DOI] [PubMed] [Google Scholar]
- 47.Marsen TA, Pollok M, Oette K, Baldamus CA. Pharmacokinetics of omega-3-fatty acids during ingestion of fish oil preparations. Prostaglandins Leukot Essent Fatty Acids. 1992;46(3):191–96. doi: 10.1016/0952-3278(92)90069-u. [DOI] [PubMed] [Google Scholar]
- 48.Wu N, Minden SL, Hoaglin DC. Quality of life in people with multiple sclerosis: data from the Sonya Slifka Longitudinal Multiple Sclerosis Study. J Health Hum Serv Adm. 2007;30(3):233–67. [PubMed] [Google Scholar]
- 49.Nortvedt MW, Riise T. The use of quality of life measures in multiple sclerosis research. Mult Scler. 2003;9(1):63–72. doi: 10.1191/1352458503ms871oa. [DOI] [PubMed] [Google Scholar]
- 50.Filippini G, Munari L, Incorvaia B, et al. Interferons in relapsing remitting multiple sclerosis: a systematic review. Lancet. 2003;361(9357):545–52. doi: 10.1016/S0140-6736(03)12512-3. [DOI] [PubMed] [Google Scholar]