Abstract
Wounds in fetal skin heal without scar, however the mechanism is unknown. We identified a novel group of E-cadherin positive cells in the blood of fetal and adult mice and named them “Dot cells”. The percentage of Dot cells in E16.5 fetal mice blood is more than twenty times higher compared to adult blood. Dot cells also express integrin β1, CD184, CD34, CD13low and Sca1 low, but not CD45, CD44, and CD117. Dot cells have a tiny dot shape between one to seven μm diameters with fast proliferation in vitro. Most of Dot cells remain positive for E-cadherin and integrin β1 after one month in culture. Transplantation of Dot cells to adult mice heals skin wounds with less scar due to reduced smooth muscle actin and collagen expression in the repair tissue. Tracking GFP-positive Dot cells demonstrates that Dot cells migrate to wounds and differentiate into dermal cells, which also express strongly to FGF-2, and later lose their GFP expression. Our results indicate that Dot cells are a group of unidentified cells that have strong wound healing effect. The mechanism of scarless wound healing in fetal skin is due to the presence of large number of Dot cells.
Introduction
Scarring can be caused by traumatic injury, surgery and fibrotic disease. Patients with large scars have life long psychological and physical burdens since no proven therapy for scarring exists. However, observations that fetuses heal skin wounds without scar have been reported in human, monkey, sheep and rodents [1–3]. Until now, the mechanism of fetal scarless healing is not clear. In mice, the scarless wound healing time period is before E17.5, when hair follicles are not fully developed and have no bulge region ready either [4].
In recent years, studies of skin stem cells suggest a potential treatment for scarring. However most studies focus on postnatal epidermal stem cells, as these can regenerate hair or epidermis. The niche for postnatal epidermal stem cells is suggested to be in the bulge region of hair follicles [5–7]. However, the niche in fetal skin is unknown, as the bulge region within hair follicles is not developed. Some researchers report that epidermal stem cells are in the follicular epithelium in fetal skin, and relocate to the bulge area when the skin has fully developed [8]. Cell surface markers that identify epidermal stem cells include integrin β1, CD34, integrin α6, P63 and keratin19 [5, 7, 9, 10]. Other skin-derived precursor cells have been isolated from neonatal and adult skin. These cells express nestin, fibronectin and βIII tubulin and can differentiate into both neural and mesodermal cell types [11]. One group also reported that epithelial stem cells, isolated from the fetal dermis express E-cadherin, cytokeratin-8, -18, -19, p63 and integrin β1 [12]. In addition, participation of bone marrow (BM)- or blood-derived hematopoietic stem cells (HSCs) have been demonstrated in tissue development and regeneration [13, 14]. Unfractionated bone marrow cells can regenerate myocytes, neurons, hepatocytes, smooth muscle cells, and other tissues specific cells [15–18], indicating the presence of stem cells in BM. Most researchers believe that BM contains two types of stem cells: HSCs and stromal stem cells. HSCs express c-kit(+) lin(−) sca-1(+) and only differentiate to hematopoietic tissues [19–21]. Stromal stem cells undergo differentiation towards osteogenic, adipogenic, myogenic and chondrogenic lineages. Stromal stem cells include multipotent adult progenitor cells (MAPCs) which have been characterized as CD34(−) CD44(−) CD45(−) c-kit(−) [22], marrow-isolated adult multi-lineage inducible (MIAMI) cells characterized as CD29(+), CD63(+), Cd81(+), CD122(+), CD164(+), Cd34(−), Cd36(−), Cd45(−) and c-kit(−) [23], unrestricted somatic stem cells (USSC) that express CD34(low) CD45(−) c-kit(low) [24], and amniotic fluid-derived stem (AFS) cells that express similar surface markers as USSCs [25]. Although attempts to isolate HSCs with multi-lineage potential have been made for years, the results remain unclear or controversial [25, 26]. The tissue repair effects of HSCs have been found through either lineage differentiation or cellular fusion[18, 27].
E-cadherin is a transmembrane protein that is mainly expressed on epithelial cells [28]. However, it is also expressed in mast cells, brain endothelial cells, and skin Langerhans cells [29–31]. In addition, E-cadherin is expressed on embryos at the two-cell stage. E-cadherin null embryos die due to failed implantation [32], suggesting a critical function of E-cadherin during development. In addition, over expression of E-cadherin also repress TGF-β induced epithelia-mesenchymal transition [33]. Here we provide evidence that a group of blood-derived E-cadherin positive cells, Dot cells, are found in fetal dermal blood with their highest numbers on E16.5, when scarless wound healing occurs. We also identified Dot cells from the blood of human and mice, however, with much lower ratio of total blood cells compared to that in fetal mice. Dot cells migrate to wounds and repair the damaged tissues through cellular differentiation. Transplantation of isolated Dot cells to wounded adult mice induces scarless healing, suggesting Dot cells are the fetal cells that responsible for scarless repair. Our data are the first to describe the Dot cells and their function during tissue repair.
Material and methods
Animals and materials
Time dated sixteen-day (E16.5) pregnant Balb/C mice and GFP (FVB.Cg-Tg(ACTB-EGFP)B5Nagy/J, Jackson Lab) mice were bred and maintained in the Stanford Animal Care Laboratory. Mice received food and water ad libitum. All procedures with animals were conducted in accordance with university-approved protocols according to NIH guidelines. E-cadherin, integrin β1, PECAM-1, and c-kit antibodies were from Santa Cruz biotechnology (Santa Cruz, CA). CD34 antibody was from Abcam. CD45, Sca-1 and c-kit antibodies were from BD Pharmingen (San Diego, CA). Alexa Fluor goat anti-rabbit IgG was from Molecular Probes (Eugene, OR). Rhodamine–labeled y-chromosome and FITC-labeled x-chromosome probes were from ID Labs (Ontario, Canada). Anti-rabbit IgG-conjugated magnetic beads and columns were from Miltenyi Biotech Inc. (Auburn, CA). Other chemicals were from Sigma Chemical Co., (St. Louis, MI).
Cell isolation using magnetic bead sorting
E16.5 time-dated fetuses of Balb/C or GFP mice were collected. After removing the head, limbs and intestinal organs, whole E16.5 fetuses were minced with scissors and the mixture was then washed with PBS, centrifuged for 5 min at 35 × g to remove large tissues, and filtered through 70 μm cell strainers. In pilot testing, flow cytometry-sorted cells were used. However, for all reported data, cells isolated by magnetic bead sorting were used. Magnetic bead cell sorting was followed per manufacturer’s instructions. Briefly, isolated cells were blocked with blocking buffer, then reacted with anti-E-cadherin antibody for 30 min and then incubated in separation buffer for 30 min before incubation with anti-rabbit IgG-conjugated magnetic beads for 30 min at 4°C. After labeling, cells in the 0.5 ml separation buffer were passed through a magnetic field MACS separation MS column followed by 3 washes with 1 × PBS before elution. About 0.4% of total cells was positive and consistently obtained each time. Postnatal Dot cells were obtained by blood collection from 4-week old mice through cardiac puncture and followed by cell sorting as described above. The sorting efficiency was further confirmed by fluorescent activated cell sorting (FACS), and more than 85% cells were E-cadherin positive.
Fluorescent cell sorting (FACS)
Dot cells from either freshly sorted or collected from culture conditions were washed with PBS and blocked with 1% normal horse serum (NHS) for one hour. Then, cells were labeled with different antibodies in the dilution from 1:50 or 1:100 in 100 μl PBS with 1% NHS for 30 min and followed with washes in PBS with 1% NHS and reacted with secondary antibody conjugated with either FITC (fluorescien isothiocyanate) or PE for 30 min on ice. After washing, the ratio of positive labeling was analyzed using the Vantage SE or DiVa Vantoo FACS machines at the Stanford Shared FACS Facility. Flow cytometry data was then acquired with CellQuest software (BD Biosciences) and analyzed with FlowJo software (FlowJo, Palo Alto, CA). Isotope antibody labeled cells was used for non-specific labeling control.
Wound creation and cell transplantation
Eight to ten week-old female Balb/C mice were anesthetized. After shaving the hair, dorsal skin was cleaned before two 0.6 cm diameter excisional wounds were made with a biopsy punch. Five hundred thousand sorted Dot cells in 100 μl normal saline were then injected with 26-gauge needle through tail-vein in each wounded mouse. The control groups were composed of tail-vein saline injection, same number of fetal fibroblasts or same number of E-cadherin positive cells sorted from postnatal mice skin.
Immunohistochemistry and immunofluorescent staining
Fetuses aged from E13.5 to E18.5 were removed from the uteri and immediately fixed in 4% paraformadehyde. All tissues were embedded in paraffin. Seven μm thickness paraffin sections were de-waxed and re-hydrated, washed three times with PBS, and treated with proteinase-k. Wounds collected from GFP-cell transplantation animals were embedded in OCT for frozen section. After washing, sections were blocked and reacted with anti-E-cadherin (1:100 dilution) overnight at 4°C and followed by VECTASTAIN ABC kit, per instructions, and then counterstained with hematoxylin and mounted. Staining was visualized and pictured by an Axioplan 2 microscope (Zeiss). For fluorescent studies, sections were fixed, washed, blocked and then reacted with the different antibodies at dilutions ranging between 1:50 and 1:200 in blocking buffer over night at 4°C. After washing, sections were counterstained with DAPI and photographed by confocal microscopy (Leica DM IRE2) or standard fluorescent microscopy (Axioplan 2 microscope, Zeiss). The control group was treated without primary antibody. The scanned confocal images were further analyzed with Velocity software for 3 dimensional and 360-degree rotation movie images.
Protein preparation and immunoblot
Normal or wounded skin tissues were collected and stored immediately in liquid nitrogen until protein analysis. Tissues were homogenized in lysis buffer. Total protein was purified by centrifugation of tissue lysates at 12,000 g for 30 minutes at 4°C. After protein concentration was determined, thirty μg of protein from each tissue was heated at 100°C for 5 minutes with loading buffer, then separated by 10% SDS-polyacrylamide gel and blotted onto a PVDF membrane. After reaction with anti-SMA antibody at 4°C over night, membranes were washed and reacted with HRP-conjugated secondary antibodies. After washing, bands were visualized by blot exposure to X-ray film after 5 min reaction with ECL Western blotting detection reagents.
Electron microscopy
Dot cells were fixed in 2% glutaraldehyde and 4% paraformadehyde in sodium cacodylate buffer, pH 7.3, for 30 min at room temperature. After washing with in sodium cacodylate buffer, ice cold, 1% osmium tetroxide in distilled water was added to the cell pellet, followed with gentle shaking at 4°C for 2 hours. After washing with water, 1% uranyl acetate was added to the cell pellet overnight. Cells were then dehydrated with serial ethanol dilutions and embedded in Epon at 65°C for 24 hours. Ultra-thin sections were cut and doubly stained with uranyl acetate and lead citrate followed by electron microscopy examination.
Results
Identification of Dot cells in fetal mouse skin
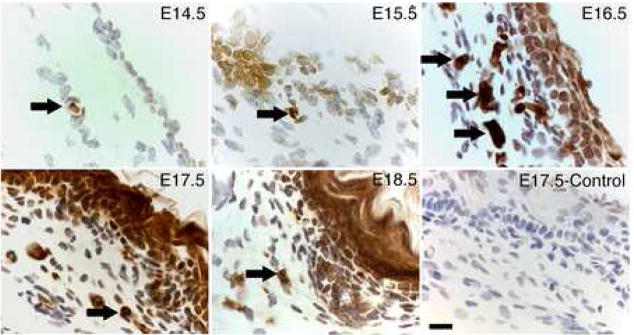
We found a group of small cells that heavily express E-cadherin and are located in the dermis of fetal mice. Figure 1 shows small cells that express E-cadherin in the dorsal skin of embryonic day E14.5 to E18.5 mice. On E14.5 and E15.5, only a few scattered small cells stained densely for E-cadherin in the dermal area (arrows). On E16.5, these small cells grouped together in clustered patterns (arrows) without clear cell-cell boundaries with dense E-cadherin staining in the dermis. Meanwhile, epithelial cells also expressed E-cadherin, but relatively weak. By E18.5, only few E-cadherin positive cells were detected in the dermal area, but epithelial cells were now strongly expressing E-cadherin.
Figure 1.
Localization of E-cadherin positive cells in fetal mouse skin. Dorsal skin was collected from E14.5 to E18.5 fetal mice and the expression of E-cadherin was analyzed by IHC. The control section was stained without primary antibody. Arrows indicate E-cadherin-positive cells, with the highest number of dermal E-cadherin cells occurring on E16.5 compared to the other ages. By E18.5, E-cadherin was expressed strong in epithelial cells. Bars = 40 μm.
Dot cells are located in blood
Using a whole E16.5 fetal section (Figure 2A), we found that clustered cells which strongly express E-cadherin were located in the dermal area (1), muscle areas (2) and adjacent to cartilage (3). To further identify the location of the dermal E-cadherin positive cells, immunofluorescent labeling for both E-cadherin and PECAM-1, a marker for endothelial cells, was examined using confocal microscopy. Figure 2B shows the merged images of immunofluorescent staining for E-cadherin and PECAM-1 in dermis of E16.5 skin section. E-cadherin positive cells (red, arrows) were present in a clustered pattern with small nuclei located adjacent to PECAM labeled blood vessel cells (green). The nucleus size of E-cadherin positive cells was significantly smaller compared to other unstained cells.
Figure 2.
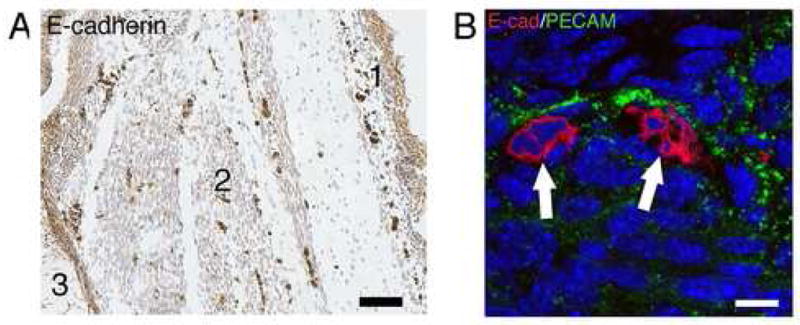
Dermal E-cadherin positive cells are blood-derived. 2A shows E-cadherin immuno-staining on an E16.5 fetal mouse whole section. The epidermis had weak staining, however, strongly stained and clustered E-cadherin positive cells were scattered in the dermis (1), muscle (2) and in areas adjacent to cartilage (3). 2B shows E-cadherin and PECAM-1 immunofluorescent staining with examination by confocal microscopy. The merged image shows that the clustered small E-cadherin positive (red) cells (arrows) in the dermis are located next to PECAM-1 (green) positive cells. Bar = 300μm in A; Bar = 25 μm in B.
Blood derived E-cadherin positive cells have a “Dot” shape
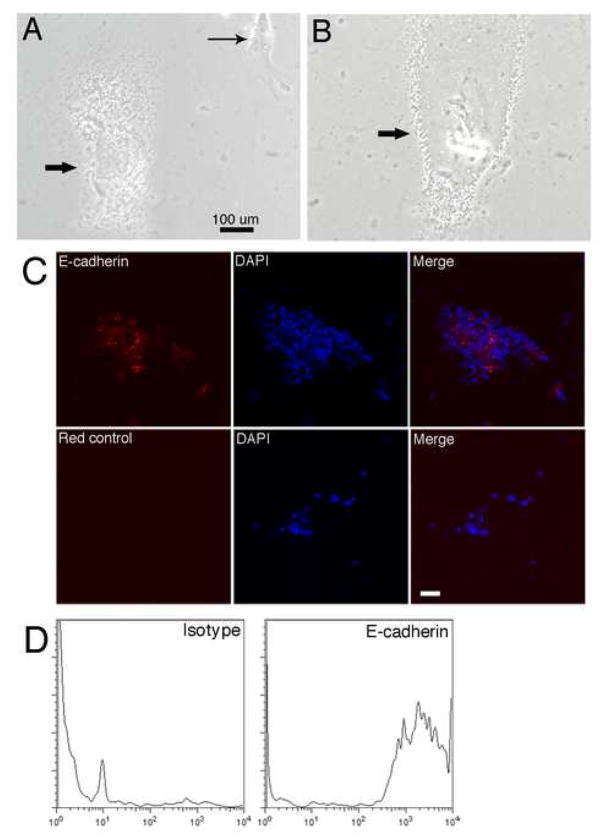
Subsequently, these previously unidentified, blood-derived E-cadherin positive cells were cultured in non-feeder-layer conditions. Figure 3A shows that Dot cells were in tiny dots shape in culture. Their sizes ranged between two to seven μm in vitro. Dot cells formed tight cell-cell connections inside colonies. After one month in culture, more than seventy percent of cultured Dot cells were still expressing E-cadherin. Figure 3B shows E-cadherin staining of confocal merged images of cultured Dot cells. FACS analysis for E-cadherin on cultured cells also confirmed that more than seventy percent cells were still positive for E-cadherin after one month in culture (Figure 3C).
Figure 3.
In vitro culture and characterization of Dot cells. Dot cells sorted with anti-E-cadherin antibody and magnetic beads were isolated from E16.5 and 4-week old mouse blood. Sorted cells were cultured on collagen-coated plates in α-MEM with 20% FBS and antibiotics. Figure 3A and 3B show the cultured tiny dot-shaped cells imaged by inverted microscopy using phase contract light. In 3A, a fibroblast (thin arrow) is identified for Dot cell size comparison. 3B shows Dot cells forming tight cell-cell connection in monolayer with an undistinguishable cell membrane boundary. Figure 3C shows E-cadherin staining of cultured Dot cells. Cultured Dot cells were fixed and immuno-stained for E-cadherin (red), and examined by confocal microscopy. E-cadherin localizes both on the cell membrane and intracellularly. 3D shows FACS analysis for E-cadherin expression in cultured Dot cells. More than 70% of cells were positive for E-cadherin in four separate experiments after one month in culture (N ≥ 4). Bar = 100 μm in A and B; Bar = 10 μm in C.
Dot cells are present in blood of both postnatal mice and humans
To further confirm the presence of Dot cells in blood, we collected blood through cardiovascular puncture and bone marrow from 4-week old adult mice. E-cadherin sorted cells were cultured, and Dot cells were identified with inverted microscopy. In addition, human blood was collected from a 48 year-old volunteer man to examine the presence of Dot cells in human blood. Figure 4 shows that Dot cells were also present in the blood (m-Blood) and bone marrow (m-BM) of 4-week old mice. Dot cells were found in human blood (h-Blood). We further compared the ratio of Dot cells in E16.5 fetal, 4-week-old and 34 week-old mouse blood. The chart in Figure 4 shows the fold of ratio of Dot cells in E16.5 fetal mice and 4-week-old mice compared to 34-week old mice. The isolation rate of Dot cells in the total blood of E16.5 fetal mice (0.46±0.029% of the total cells, n≥6) was about twenty times higher than that in 4-week old mice (0.023±0.002% of the total cells, n=4) and 25 times higher than that in 34-week old adult mice (0.02±0.001% of the total cells, n=3).
Figure 4.
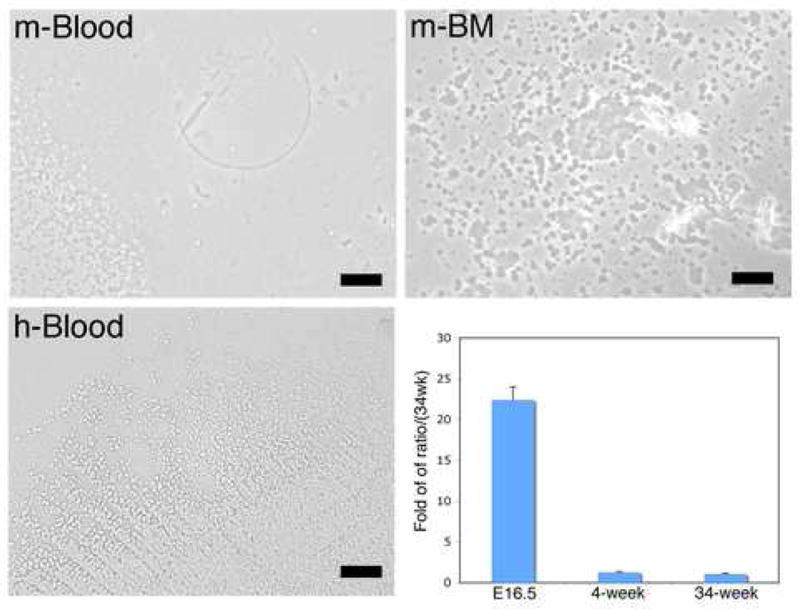
Dot cells are present in peripheral blood and bone marrow of adult mice as well as in humans. Adult mouse blood was collected through cardiac puncture. Bone marrow from adult mice was collected through long bone perfusion followed with centrifugation. In addition, human peripheral blood was collected from a 48-year old man. Dot cells were sorted and cultured and images taken using an inverted microscopy. Dot cells are present in adult mouse blood (m-blood), mouse bone marrow (m-BM) and human blood (h-blood). The chart in figure 4 shows the comparison ratios of Dot cells in E16.5 fetal mouse, 4-week old and 34-week old mouse blood. The ratio of Dot cells in E16.5 fetal blood compared to 34-week old blood is more than twenty times greater (ratio of 34-weeks was calculated as 1).
Dot cells also express integrin β1, CD34 and CD184
To further characterize the surface markers of Dot cells, sorted Dot cells were examined by FACS analysis. Figure 5A shows that Dot cells also expressed integrin β1 (CD29), partly CD34 (50–60%), CD184. About 15% of Dot cells also expressed CD13 and few of them (~5%) expressed Sca1. However, Dot cells did not express CD45, CD44 and CD117 (c-kit). Dot cells were cultured on cover slides and their surface marker expression was further examined using immunofluorescent staining and a confocal microscopy. Figure 5B shows an electron microscopy image of a Dot cell that was freshly sorted. This cell was about 5 μm in diameter and had a large nucleus with few cellular organelles and almost no cytoplasmic components. Cultured Dot cells were stained with integrin β1, CD34 and CD184 (Figure 5C). Integrin β1 stained as rode shape on Dot cells.
Figure 5.
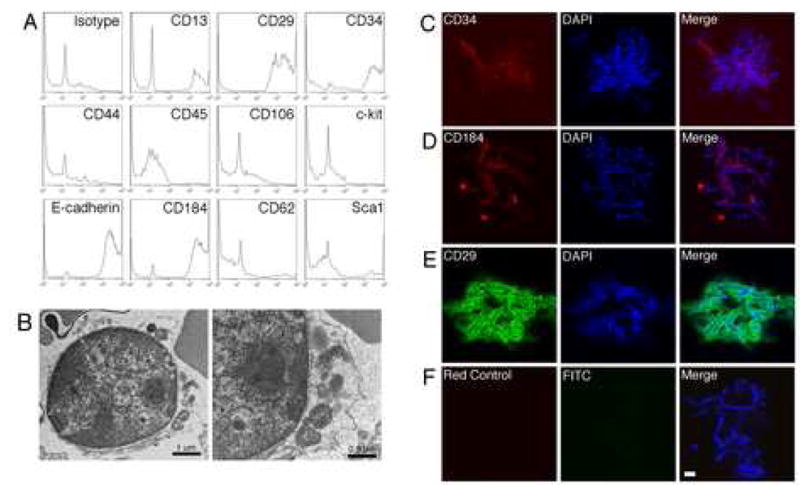
Dot cells express stem cell markers. 5A shows FACS analysis of stem cell markers on Dot cells. Freshly sorted Dot cells with E-cadherin were reacted with other stem cell marker antibodies as described in the methods section. The expression of each marker was analyzed using FlowJo software. More than 85% of Dot cells express integrin β1 and CD184. About 50% express CD34, and 15% express CD13. The detailed morphology of Dot cells was also examined by electron microscopy. 5B shows a sorted Dot cell with a 5 μm diameter size, having a large nucleus but small cytoplasm. This cell also has some intracellular organelles, but no significant number of cytoplasm components. The stem cell marker expression on Dot cells was also examined using fluorescent-staining by a confocal microscopy (Figure 5C). Dot cells express positive to CD34, CD184 and integrin β1. Bar = 10 μm.
Transplantation of Dot cells to wounded adult mice reduces scar
The effects of Dot cells on postnatal wound repair were examined. Five hundred thousand freshly sorted E16.5 Dot cells in 100 μl saline were injected to each wounded postnatal mouse through its tail vein. Figure 6 shows trichrome stained wound sections collected on day 5 (A), day 7 (C), day 15 (E) and day 20 (G) after Dot cell-transplantation. The wounds in saline injected control group were collected on the same days (B, D, E, H). Images were taken with a conventional microscopy with bright field light. Significantly less scar tissue was observed in the Dot cell-transplanted group. In addition, control E16.5, passage 3, dermal fibroblasts were transplanted via tail vein injection into wounded adult mice. This group had similar scarring as the saline control group (data not shown).
Figure 6.
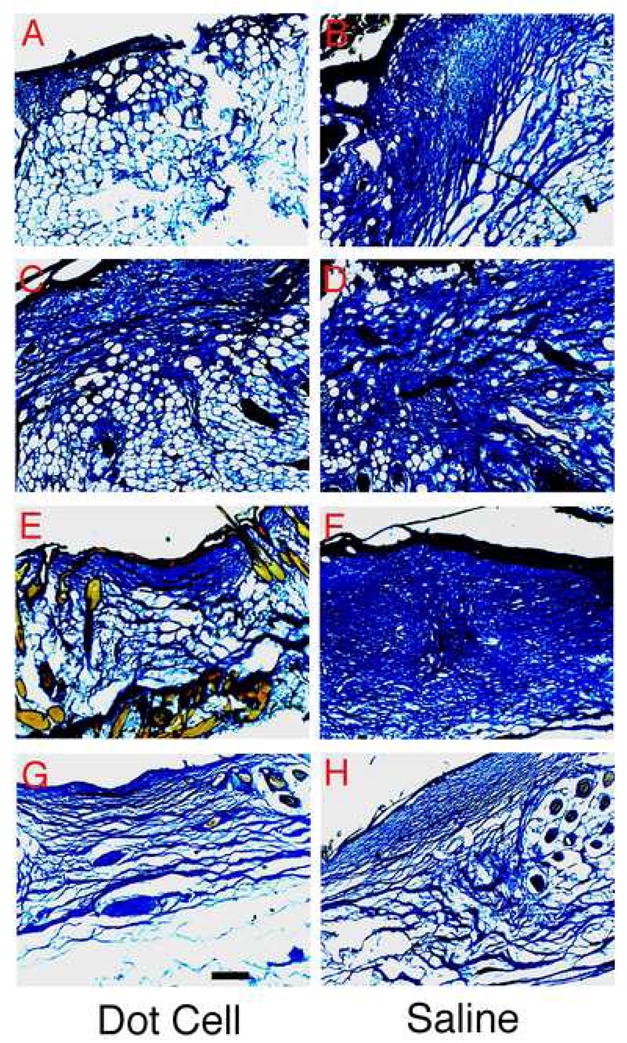
Wound repair function of Dot cells. Five hundred thousand freshly sorted Dot cells in 100 μl saline were injected by tail vein into mouse with fresh dorsal skin wounds. Wounds were collected on day 5 (A), day 7 (C), day 15 (E) and day 20 (G). Control saline-injection wounds were collected on the same days (B, D, E, H). Fibrotic tissue expression in wounds was examined using trichrome stain, and images were taken using conventional microscopy with bright field light. Bar = 400 μm.
Dot cell-transplanted wounds express less type-I collagen and smooth muscle actin
Five hundred thousand epithelial E-cadherin positive cells isolated by FACS from postnatal mice skin were transplanted to each wounded adult mouse, and compared to Dot cell-transplanted wounds. Figure 7A shows both H&E stain and immunofluorescent-labeled collagen expression on 8-day wounds. Less fibrotic tissue and less collagen were observed in the Dot cell-transplanted wounds compared to the epithelial E-cadherin positive cell transplanted wounds; the repaired dermal tissue formed a reticular network structure in the Dot cell-transplanted wounds. Because smooth muscle actin (SMA) is expressed by myofibroblasts that release type-I collagen and exhibit strong contraction ability and scarring [34, 35], the expression of SMA in the wounded tissue was examined using immunoblot in wounds collected from E16.5 Dot cell-transplanted (E1–E7) and fibroblast-transplanted (F1–F7) groups (Figure 7B). Lower SMA expression in Dot cell-transplanted wounds was detected on day 7 in two separated groups (Figure 7C). Also postnatal Dot cells, collected from adult mouse blood, were transplanted to wounded mice. The expression of SMA in wounds from Dot cell-transplanted (DC) was compared to saline-injected groups (con), and significantly decreased SMA expression was detected from 8 day and later (Figure 7D).
Figure 7.
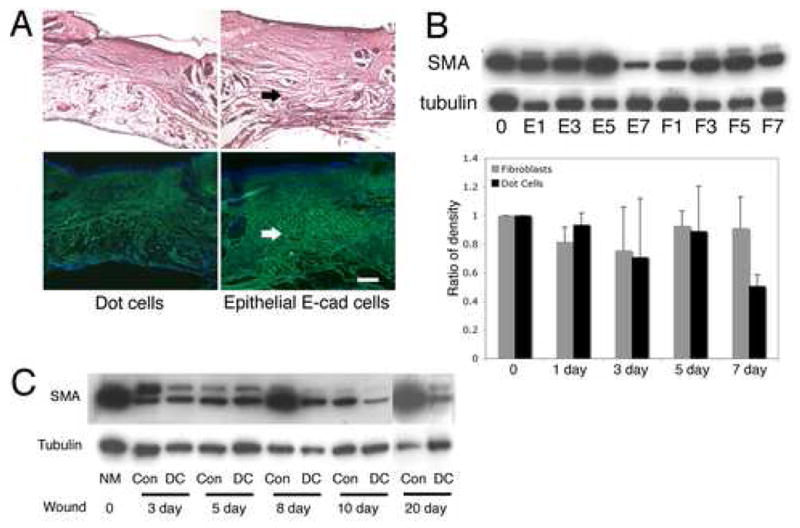
Expression of type-I collagen and SMA in wounds. Five hundred thousand E-cadherin positive cells sorted from postnatal mouse skin or the same number of Dot cells were transplanted via tail vein injection into each wounded mouse. Wounds were collected and sections were stained with H&E or type-I collagen antibody. 7A shows H&E staining (upper panel) and fluorescent FITC-labeled collagen expression (lower panel). Less fibrotic tissue and less collagen expression was observed in Dot cell transplanted wounds (left panel) compared to postnatal skin E-cadherin positive cell-transplanted wounds (right panel). Bar = 400 μm. 7B shows an immunoblot of SMA expression in the Dot cell-transplanted group from day 1 to day 7 (E1–E7) wounds compared to the same number of E16.5 fetal fibroblast-transplanted wounds (F1–F7). Decreased SMA was detected in the Dot cell-transplanted group on day 7 (E7). The densitometry analysis of two separate immunoblot experiments demonstrated a decreased expression of SMA in Dot cell transplanted wounds on day 7. Figure 7C shows an immunoblot of SMA expression in wounds collected after transplantation of Dot cells from adult mouse blood (DC) compared to the control saline injected group (Con). Reduced SMA expression in the adult Dot cell transplanted wounds was observed from day 8 and thereafter.
Dot cells restore dermal tissue and blood vessels
The location of transplanted Dot cells in wounds was examined using E16.5 Dot cells freshly isolated from GFP-transgenic mice (Figure 8). On day 1, GFP-labeled cells were observed at both sides of the wound (8A–8D). The majority of bright GFP-staining cells are small in size. However, some bright GFP stained large cells can also observed in wounds, indicating these cells are differentiate dermal cells. On day 3, GFP cells were detected in the dermal wound bed (8E–8H) and a GFP-positive circle could also be detected (arrow in 8G), indicating Dot cells also can differentiate to blood vessel in wounds. On day 7, wounds had reepithelialized, and the newly formed dermis had a reticular cellular network structure; GFP cells were found mainly at the center of the wound bed, indicating the center area was not fully repaired (8I–8L). By day 15 (8M–8P), when the wound defects were totally repaired, few small GFP-labeled Dot cells were detected. However, the majority of differentiated dermal cells in wounds still weakly express GFP.
Figure 8.
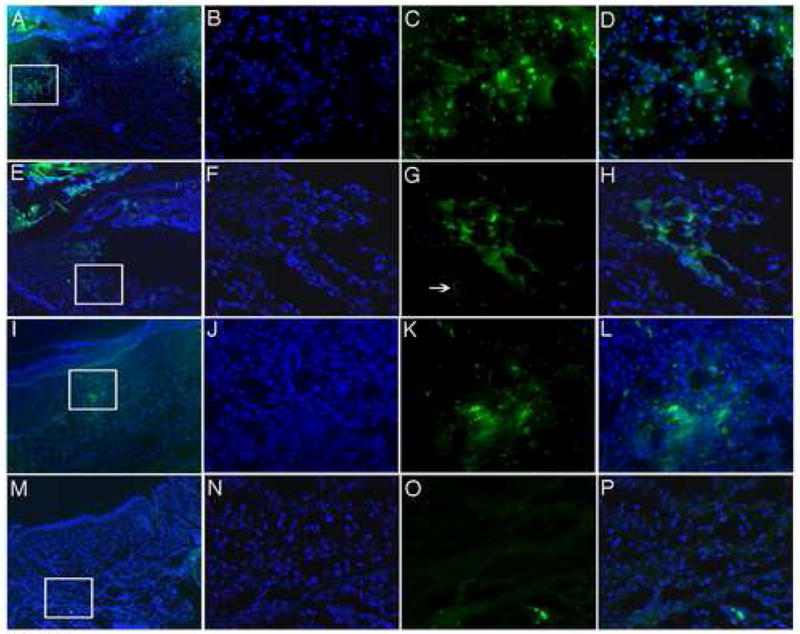
Dot cells repair wounded dermal tissue. Five hundred thousand Dot cells freshly sorted from E16.5 GFP fetuses were transplanted via tail vein injection to each wounded Balb/C adult mouse. Wounds were collected on day 1 (A–D), day 3 (E–H), day 7 (I–L) and day 15 (M–P). The images in B–D, F–H, J–L and N–P are the enlargement of the square images in A, E, M, and I respectively. In 1 and 3-day wounds, GFP cells were located at the edge of the damaged area. By day 7, when the reepithelialization was finished and the majority of the dermal structure was restored, GFP cells still located in the center of the wound bed in order to restore the dermal structure. After 15 days, only a small number of GFP positive cells remained, and the restored tissue showed very week GFP expression.
Differentiated Dot cells express FGF-2
The scarless healing mechanism of Dot cells was further examined through their FGF-2 expression. FGF-2 is an antagonist to fibrosis through its function of inducing cell proliferation and decreasing cell differentiation. Figure 9 shows FGF-2 (red) immunofluorescent staining in 7-day wound sections. These wounds were collected from GFP-labeled Dot cell-transplanted mice. Some brightly labeled GFP smaller cells do not express FGF-2. However, differentiated larger cells with relatively weaker GFP labeling also co-express FGF-2. These data indicate that some dermal repair cells were derived from differentiation of Dot cells, which appear to express FGF-2 only after they have differentiated in the microenvironment.
Figure 9.
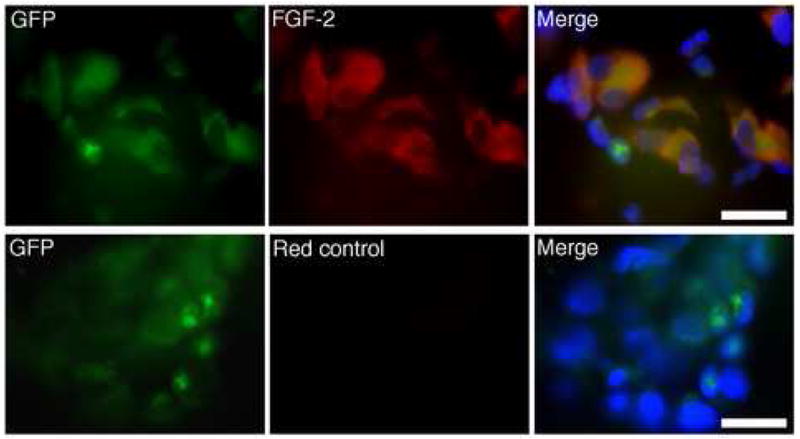
Dermal cells derived from Dot cells express FGF-2. FGF-2 expression was examined using fluorescent staining on 7-day wounds after GFP-labeled Dot cell-transplantation. Smaller, but bright GFP positive cells did not show FGF-2 expression. However, the majority of large differentiated cells expressed both FGF-2 (red) and relatively weaker GFP. Bars = 100 μm.
Discussion
In the present study, we introduced a new group of small cells, Dot cells that were detected within both of fetal and adult mouse blood. We believe that Dot cells are a previously unidentified group of cells since no other reports have described a similar cell morphology or marker profile. Dot cells strongly express to E-cadherin, integrin β1, CD184. They have weaker to CD34, CD13 and low to Sca1 expression. Because E-cadherin is expressed mainly by epithelial cells, and integrin β1 is a well-known epidermal stem cell marker, the strong expression of both E-cadherin and integrin β1 by Dot cells in unwounded E16.5 mouse skin are likely targeted to epidermal development. We believe that Dot cells are relatively primitive cells or stem cells due to their unusually small size and the fact that they express stem cell markers such as E-cadherin, integrin β1 and CD34.
Our data show that Dot cells have a strong homing effect to sites of injury for tissue regeneration. They specifically migrate to wounds and differentiate into dermal cells, which release less interstitial collagen and reduce scar. We believe that the mechanism for Dot cell migration to the damaged area could be due to the presence of CD184, a seven-transmembrane G-protein coupled receptor, which functions as a receptor for stromal cell-derived factor-1 (SDF-1), an important chemokine for heart development [36] and stem cell migration [37]. The release of SDF-1 is upregulated at sites of tissue injury. The increased SDF-1, in turn, leads to the homing of circulating stem cells [38]. The fibroblast-transplanted group did not show the same scarless repair effect as seen in the Dot cell-transplanted group. This indicates that transplanted fibroblasts do not home to damaged tissue. This phenomenon has also been described by other reporters [39].
Our data also show one mechanism of Dot cell tissue repair occurs by differentiation of Dot cells within wounds to dermal and endothelial cells. The differentiation of Dot cells is a rapid process that results the repairing tissues derived all from Dot cells differentiation (Figure 8). We used Dot cell-transplantation method instead of topic application of Dot cells to the wound bed in our experiments. Because Dot cells are blood-derived and very small, so it is easier and more physiological to apply them through circulation. In addition, blood-transplantation is easier to control the cell numbers without loosing cells that could be happened in wound topic application. One mechanism of Dot cell-induced scarless healing could be their lack of some cytokine expression. For example, the differentiated Dot cells strongly express FGF-2, which function to increase proliferation of the Dot cell-derived fibroblasts. Their rapid proliferation may have anti-differentiation effects, such as reduction of latent local TGF-β, since TGF-β is pro-fibrotic. FGF-2 may function to reduce fibroblasts differentiation into myofibroblasts, the cells that release large amounts of interstitial collagens and induce strong tissue contraction, i.e. resulting scarring. Because all wounds in the present study were made in 6 mm diameter, we only detected only a small of accelerated wound healing by Dot cells. However, we believe that Dot cells can also increase healing speed, and my help in the healing of diabetic wounds. These hypothesis need to be investigated in the future studies.
Dot cells function during early stages of repair, which may be requisite to scarless healing. We observed that the scar on saline control wounds remodels after day 20 when there were no significant differences of scaring between Dot cells and saline injected wounds. The recovery of saline-injected group by 20 days could be explained by host stem cells in saline-injected mice also migrate to the wounds and repair tissue, however, in a slower way, due to the smaller number of stem cells in saline-injected mice compared to the Dot cell-transplanted wounds.
The ratio of Dot cells in E16.5 fetal mouse blood is more than twenty times higher than that of adult mice, suggesting Dot cells may be the key for scarless healing. To determine if the higher Dot cell number enhances tissue repair, we transplanted 500,000 isolated Dot cells into each wounded adult mouse. The Dot cell number for transplantation was calculated as 40% of the total number Dot cells in one recipient animal, i.e. 1.5 (ml of blood in one mouse) × 4 ×109 (total blood cells per ml) × 0.02% (the ratio of adult Dot cells within total blood cells) = 500,000. Our data suggest that a 40% increase in the number of Dot cells can induce scarless healing. The ratio of Dot cells in E16.5 fetal mice is twenty times higher than in adult mice, the high number of Dot cells in fetus maybe the key for scarless wound healing.
In our transplantation model, we found that GFP-labeled Dot cells lose their GFP expression in wounds after the dermal structure is fully restored. This suggests that the self-renew of Dot cells population is well regulated, which effectively limit their proliferation rate in vivo. Dys-regulation or other damage during self-renewal could cause hyperproliferation of the transplanted stem cells, and thus cause them to become ‘cancer stem cells’ in recipients [40, 41]. We believe that after wound has healed, Dot cells stay in the circulation in the number as same as the normal unwounded mouse.
Our data provide evidence that Dot cells have a major role in repairing wounded dermis to reduce scar formation. We believe the high number of circulating Dot cells in fetal blood is the key for fetal scarless wound healing. In contrast, scarring in adults could be due to the lower number of Dot cells in blood and BM in adults. However, the mechanism of tissue repair by Dot cells is still unclear and further studies on the skin progenitor effects of Dot cells need to be performed.
Acknowledgments
This work was supported by Oak foundation and NIH grant RO1-GM041343. The authors thank John Perrino for electron microscope image processing. The authors thank Dr. Edward P. Buchanan for editing the manuscript and thank Bryan W. Lin for histology data analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adzick NS, Harrison MR, Glick PL, Beckstead JH, Villa RL, Scheuenstuhl H, Goodson WH., 3rd Comparison of fetal, newborn, and adult wound healing by histologic, enzyme-histochemical, and hydroxyproline determinations. J Pediatr Surg. 1985;20:315–9. doi: 10.1016/s0022-3468(85)80210-4. [DOI] [PubMed] [Google Scholar]
- 2.Longaker MT, Whitby DJ, Adzick NS, Crombleholme TM, Langer JC, Duncan BW, Bradley SM, Stern R, Ferguson MW, Harrison MR. Studies in fetal wound healing, VI. Second and early third trimester fetal wounds demonstrate rapid collagen deposition without scar formation . J Pediatr Surg. 1990;25:63–8. doi: 10.1016/s0022-3468(05)80165-4. discussion 68–9. [DOI] [PubMed] [Google Scholar]
- 3.Lorenz HP, Adzick NS. Scarless skin wound repair in the fetus. West J Med. 1993;159:350–5. [PMC free article] [PubMed] [Google Scholar]
- 4.Soo C, Beanes SR, Hu FY, Zhang X, Dang C, Chang G, Wang Y, Nishimura I, Freymiller E, Longaker MT, Lorenz HP, Ting K. Ontogenetic transition in fetal wound transforming growth factor-beta regulation correlates with collagen organization. Am J Pathol. 2003;163:2459–76. doi: 10.1016/s0002-9440(10)63601-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones PH, Watt FM. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell. 1993;73:713–24. doi: 10.1016/0092-8674(93)90251-k. [DOI] [PubMed] [Google Scholar]
- 6.Rendl M, Lewis L, Fuchs E. Molecular dissection of mesenchymal-epithelial interactions in the hair follicle. PLoS Biol. 2005;3:e331. doi: 10.1371/journal.pbio.0030331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–63. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levy V, Lindon C, Harfe BD, Morgan BA. Distinct stem cell populations regenerate the follicle and interfollicular epidermis. Dev Cell. 2005;9:855–61. doi: 10.1016/j.devcel.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Trempus CS, Morris RJ, Bortner CD, Cotsarelis G, Faircloth RS, Reece JM, Tennant RW. Enrichment for living murine keratinocytes from the hair follicle bulge with the cell surface marker CD34. J Invest Dermatol. 2003;120:501–11. doi: 10.1046/j.1523-1747.2003.12088.x. [DOI] [PubMed] [Google Scholar]
- 10.Yang A, Kaghad M, Caput D, McKeon F. On the shoulders of giants: p63, p73 and the rise of p53. Trends Genet. 2002;18:90–5. doi: 10.1016/s0168-9525(02)02595-7. [DOI] [PubMed] [Google Scholar]
- 11.Toma JG, McKenzie IA, Bagli D, Miller FD. Isolation and characterization of multipotent skin-derived precursors from human skin. Stem Cells. 2005;23:727–37. doi: 10.1634/stemcells.2004-0134. [DOI] [PubMed] [Google Scholar]
- 12.Medina RJ, Kataoka K, Takaishi M, Miyazaki M, Huh NH. Isolation of epithelial stem cells from dermis by a three-dimensional culture system. J Cell Biochem. 2006 doi: 10.1002/jcb.20757. [DOI] [PubMed] [Google Scholar]
- 13.Shizuru JA, Negrin RS, Weissman IL. Hematopoietic stem and progenitor cells: clinical and preclinical regeneration of the hematolymphoid system. Annu Rev Med. 2005;56:509–38. doi: 10.1146/annurev.med.54.101601.152334. [DOI] [PubMed] [Google Scholar]
- 14.Wagers AJ, Christensen JL, Weissman IL. Cell fate determination from stem cells. Gene Ther. 2002;9:606–12. doi: 10.1038/sj.gt.3301717. [DOI] [PubMed] [Google Scholar]
- 15.Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, Mavilio F. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279:1528–30. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- 16.Mezey E, Chandross KJ, Harta G, Maki RA, McKercher SR. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science. 2000;290:1779–82. doi: 10.1126/science.290.5497.1779. [DOI] [PubMed] [Google Scholar]
- 17.Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman IL, Grompe M. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000;6:1229–34. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- 18.Sata M, Saiura A, Kunisato A, Tojo A, Okada S, Tokuhisa T, Hirai H, Makuuchi M, Hirata Y, Nagai R. Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nat Med. 2002;8:403–9. doi: 10.1038/nm0402-403. [DOI] [PubMed] [Google Scholar]
- 19.Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297:2256–9. doi: 10.1126/science.1074807. [DOI] [PubMed] [Google Scholar]
- 20.Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–73. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 21.Roybon L, Ma Z, Asztely F, Fosum A, Jacobsen SE, Brundin P, Li JY. Failure of transdifferentiation of adult hematopoietic stem cells into neurons. Stem Cells. 2006;24:1594–604. doi: 10.1634/stemcells.2005-0548. [DOI] [PubMed] [Google Scholar]
- 22.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–9. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 23.D’Ippolito G, Diabira S, Howard GA, Menei P, Roos BA, Schiller PC. Marrow-isolated adult multilineage inducible (MIAMI) cells, a unique population of postnatal young and old human cells with extensive expansion and differentiation potential. J Cell Sci. 2004;117:2971–81. doi: 10.1242/jcs.01103. [DOI] [PubMed] [Google Scholar]
- 24.Kogler G, Sensken S, Airey JA, Trapp T, Muschen M, Feldhahn N, Liedtke S, Sorg RV, Fischer J, Rosenbaum C, Greschat S, Knipper A, Bender J, Degistirici O, Gao J, Caplan AI, Colletti EJ, Almeida-Porada G, Muller HW, Zanjani E, Wernet P. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med. 2004;200:123–35. doi: 10.1084/jem.20040440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiavegato A, Bollini S, Pozzobon M, Callegari A, Gasparotto L, Taiani J, Piccoli M, Lenzini E, Gerosa G, Vendramin I, Cozzi E, Angelini A, Iop L, Zanon GF, Atala A, De Coppi P, Sartore S. Human amniotic fluid-derived stem cells are rejected after transplantation in the myocardium of normal, ischemic, immuno-suppressed or immuno-deficient rat. J Mol Cell Cardiol. 2007;42:746–59. doi: 10.1016/j.yjmcc.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 26.Holden C. Stem cells. Controversial marrow cells coming into their own? Science. 2007;315:760–1. doi: 10.1126/science.315.5813.760. [DOI] [PubMed] [Google Scholar]
- 27.Rizvi AZ, Swain JR, Davies PS, Bailey AS, Decker AD, Willenbring H, Grompe M, Fleming WH, Wong MH. Bone marrow-derived cells fuse with normal and transformed intestinal stem cells. Proc Natl Acad Sci U S A. 2006;103:6321–5. doi: 10.1073/pnas.0508593103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zavadil J, Bottinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 2005;24:5764–74. doi: 10.1038/sj.onc.1208927. [DOI] [PubMed] [Google Scholar]
- 29.Nishida M, Kawai K, Tanaka M, Tegoshi T, Arizono N. Expression of E-cadherin in human mast cell line HMC-1. Apmis. 2003;111:1067–74. doi: 10.1111/j.1600-0463.2003.apm1111109.x. [DOI] [PubMed] [Google Scholar]
- 30.Abbruscato TJ, Davis TP. Protein expression of brain endothelial cell E-cadherin after hypoxia/aglycemia: influence of astrocyte contact. Brain Res. 1999;842:277–86. doi: 10.1016/s0006-8993(99)01778-3. [DOI] [PubMed] [Google Scholar]
- 31.Borkowski TA, Van Dyke BJ, Schwarzenberger K, McFarland VW, Farr AG, Udey MC. Expression of E-cadherin by murine dendritic cells: E-cadherin as a dendritic cell differentiation antigen characteristic of epidermal Langerhans cells and related cells. Eur J Immunol. 1994;24:2767–74. doi: 10.1002/eji.1830241129. [DOI] [PubMed] [Google Scholar]
- 32.Larue L, Ohsugi M, Hirchenhain J, Kemler R. E-cadherin null mutant embryos fail to form a trophectoderm epithelium. Proc Natl Acad Sci U S A. 1994;91:8263–7. doi: 10.1073/pnas.91.17.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, Garcia De Herreros A. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–9. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- 34.Boor PJ, Ferrans VJ. Ultrastructural alterations in allylamine cardiovascular toxicity. Late myocardial and vascular lesions. Am J Pathol. 1985;121:39–54. [PMC free article] [PubMed] [Google Scholar]
- 35.Hu K, Wu C, Mars WM, Liu Y. Tissue-type plasminogen activator promotes murine myofibroblast activation through LDL receptor-related protein 1-mediated integrin signaling. J Clin Invest. 2007;117:3821–32. doi: 10.1172/JCI32301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–8. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 37.Mohle R, Bautz F, Rafii S, Moore MA, Brugger W, Kanz L. The chemokine receptor CXCR-4 is expressed on CD34+ hematopoietic progenitors and leukemic cells and mediates transendothelial migration induced by stromal cell-derived factor-1. Blood. 1998;91:4523–30. [PubMed] [Google Scholar]
- 38.Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106:1901–10. doi: 10.1182/blood-2005-04-1417. [DOI] [PubMed] [Google Scholar]
- 39.Schenk S, Mal N, Finan A, Zhang M, Kiedrowski M, Popovic Z, McCarthy PM, Penn MS. Monocyte chemotactic protein-3 is a myocardial mesenchymal stem cell homing factor. Stem Cells. 2007;25:245–51. doi: 10.1634/stemcells.2006-0293. [DOI] [PubMed] [Google Scholar]
- 40.Caussinus E, Gonzalez C. Induction of tumor growth by altered stem-cell asymmetric division in Drosophila melanogaster. Nat Genet. 2005;37:1125–9. doi: 10.1038/ng1632. [DOI] [PubMed] [Google Scholar]
- 41.Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer. 2003;3:895–902. doi: 10.1038/nrc1232. [DOI] [PubMed] [Google Scholar]